Summary
RNA-mediated transcriptional silencing prevents deleterious effects of transposon activity and controls the expression of protein-coding genes. It involves long noncoding RNAs (lncRNAs). In Arabidopsis thaliana, some of those lncRNAs are produced by a specialized RNA Polymerase V (Pol V). The mechanism by which lncRNAs affect chromatin structure and mRNA production remains mostly unknown. Here we identify the SWI/SNF ATP-dependent nucleosome-remodeling complex as a component of the RNA-mediated transcriptional silencing pathway. We found that SWI3B, an essential subunit of the SWI/SNF complex, physically interacts with a lncRNA-binding protein, IDN2. SWI/SNF subunits contribute to lncRNA-mediated transcriptional silencing. Moreover, Pol V mediates stabilization of nucleosomes on silenced regions. We propose that Pol V-produced lncRNAs mediate transcriptional silencing by guiding the SWI/SNF complex and establishing positioned nucleosomes on specific genomic loci. We further propose that guiding ATP-dependent chromatin-remodeling complexes may be a more general function of lncRNAs.
Graphical Abstract
Highlights
► The SWI/SNF nucleosome-remodeling complex indirectly associates with lncRNA ► SWI/SNF subunits are required for lncRNA-mediated transcriptional silencing ► lncRNA mediates nucleosome positioning
Introduction
Transposable elements and other classes of repetitive sequences are controlled by RNA-mediated transcriptional silencing (Girard and Hannon, 2008), which in plants is also known as RNA-dependent DNA methylation (RdDM) (Law and Jacobsen, 2010). This process not only prevents deleterious activities of transposons but is also believed to regulate the expression of protein-coding genes (Faulkner et al., 2009; Zheng et al., 2012).
RNA-mediated transcriptional silencing pathway involves two independent classes of noncoding RNA: small interfering RNA (siRNA) and long noncoding RNA (lncRNA) (Law and Jacobsen, 2010; Wierzbicki, 2012). In Arabidopsis thaliana, siRNA is produced by the activities of RNA Polymerase IV (Pol IV), RNA-DEPENDENT RNA POLYMERASE 2 (RDR2) and DICER-LIKE 3 (DCL3). It then incorporates into the ARGONAUTE4 (AGO4) protein and provides specificity toward genomic loci with the same sequence as siRNA (Law and Jacobsen, 2010). Despite being necessary for AGO4 to bind chromatin and mediate repressive chromatin modifications, siRNA is not sufficient for those events. Another required component is lncRNA, produced by RNA Polymerases II and V (Pol II and Pol V, respectively), which is necessary for siRNA to recognize its genomic target loci (Wierzbicki et al., 2008; Zheng et al., 2009; Wierzbicki, 2012).
Pol V is a DNA-dependent RNA polymerase with subunit composition similar to Pol II (Huang et al., 2009; Ream et al., 2009; Haag et al., 2012). Pol V-produced lncRNAs originating from silencing targets have been shown to be required for AGO4 association with chromatin, CHH DNA methylation, and transcriptional silencing (Wierzbicki et al., 2008, 2009; Zheng et al., 2012). These RNAs are believed to work as scaffolds for the assembly of silencing complexes, which establish DNA methylation and repressive histone modifications (Haag and Pikaard, 2011; Wierzbicki, 2012). They have also been proposed to be the primary factors guiding repressive chromatin modifications to gene regulatory regions (Zheng et al., 2012; Zhong et al., 2012). The mechanism connecting lncRNAs to the activities of chromatin-modifying enzymes is not well understood and only two lncRNA-binding proteins have been identified so far: AGO4 and SUPPRESSOR OF TY INSERTION 5-LIKE (SPT5L).
SPT5L is a homolog of the SPT5 Pol II elongation factor (Bies-Etheve et al., 2009; He et al., 2009; Huang et al., 2009). It binds chromatin at silenced loci independently of AGO4 and has been proposed to work together with AGO4 in the recruitment of chromatin-modifying enzymes (Rowley et al., 2011). lncRNAs were also hypothesized to interact with IDN2, an RNA-binding protein required for RNA-mediated transcriptional silencing, which has been shown to bind double-stranded RNA in vitro (Ausin et al., 2009, 2012; Zheng et al., 2010; Xie et al., 2012; Zhang et al., 2012). However, the in vivo function of IDN2 remains mysterious.
Besides DNA methylation and posttranslational histone modifications, chromatin status may also be affected by active nucleosome positioning by ATP-dependent chromatin-remodeling complexes, which control DNA accessibility by positioning, moving, or exchanging nucleosomes (Jiang and Pugh, 2009; Hargreaves and Crabtree, 2011). Although active chromatin remodeling has been shown to control nucleosome positioning and affect transcription throughout genomes (Sadeh and Allis, 2011), it remains unknown whether nucleosomes are controlled by the transcriptional silencing pathways. One of the families of ATP-dependent chromatin-remodeling complexes is known as SWI/SNF (Hargreaves and Crabtree, 2011). In Arabidopsis thaliana, SWI/SNF complexes are known to contain at least four core subunits, including an Snf2 family ATPase and two SWI3 proteins (Sarnowski et al., 2005; Knizewski et al., 2008).
In this work, we discovered a hitherto unidentified component of the RNA-mediated transcriptional silencing pathway. Looking for lncRNA-associated proteins, we identified SWI3B, a subunit of the SWI/SNF ATP-dependent chromatin-remodeling complex. SWI3B interacts with Pol V-produced lncRNAs indirectly with IDN2 dimers serving as adaptors. SWI3B is required for RNA-mediated transcriptional silencing as indicated by derepression of several known silenced loci and a significant overlap of genes misregulated in mutants defective in lncRNA production or SWI3B activity. Defects in silencing were also observed in mutants of other SWI/SNF subunits indicating that this process involves the whole ATP-dependent chromatin-remodeling complex. Consistently, elimination of Pol V-produced lncRNAs resulted in widespread changes in nucleosome positioning on silencing targets. Moreover, DNA methylation levels were partially reduced in the swi3b mutant. These results support a model, where lncRNA recruits IDN2 dimers, which interact with the SWI/SNF complex by its SWI3B subunit. The SWI/SNF complex positions nucleosomes, which affect Pol II transcription by facilitating DNA methylation and/or restricting protein access to DNA.
Results
Pol V-Produced lncRNAs Associate with IDN2
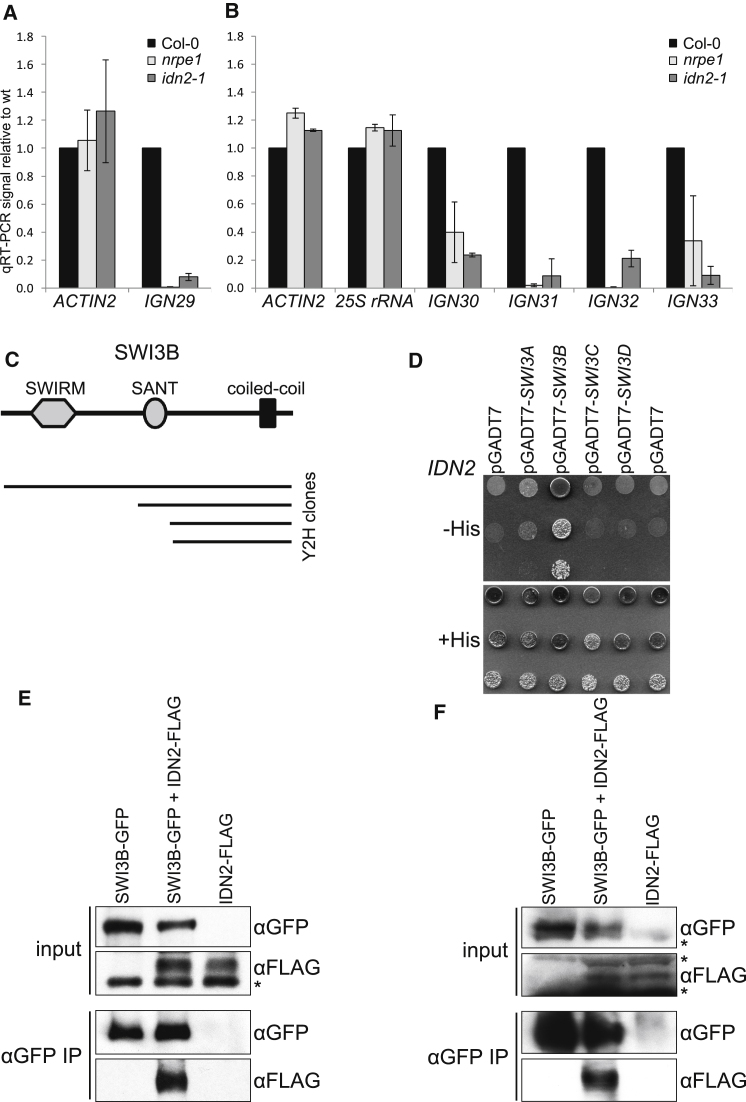
To test whether IDN2 physically interacts with Pol V-produced lncRNAs we performed RNA immunoprecipitation (RNA IP) with affinity-purified anti-IDN2 antibody and assayed the obtained samples using real time RT-PCR with primers specific for Pol V transcripts identified by Pol V ChIP-seq (Rowley et al., 2011; Wierzbicki et al., 2012). IDN2 pulled down RNA from Col-0 wild-type plants at much higher levels than from the idn2-1 mutant (Figures 1A and 1B and Figure S1A available online). RNA recovery was also strongly reduced in nrpe1, a mutant defective in the largest subunit of Pol V and unable to produce lncRNA (Wierzbicki et al., 2008), which suggests that pulled-down RNAs are Pol V-produced lncRNAs. Western blot demonstrated that the anti-IDN2 antibody was specific and that IDN2 stability was not affected in the nrpe1 mutant (Figure S1B). This demonstrates that IDN2 interacts at least with a subset of Pol V-produced lncRNAs. The idn2-1 mutant has a substitution followed by an 8 amino acid deletion in its XS domain, which adopts an RNA recognition motif fold and interacts with RNA (Ausin et al., 2009, 2012; Zhang et al., 2012). In contrast to the knockout T-DNA mutant allele idn2-2, idn2-1 line still accumulates IDN2, although at a lower level than the wild-type (Figure S1B). Because the idn2-1 mutant is defective in transcriptional silencing (Ausin et al., 2009) and unable to bind lncRNA (Figures 1A and 1B), this suggests that interaction of IDN2 with lncRNA may be important for its function in RdDM.
Figure 1.
IDN2 Interacts with lncRNA and SWI3B
(A and B) IDN2 interacts with Pol V-produced lncRNA. RNA immunoprecipitation was performed with anti-IDN2 antibody in Col-0 wild-type, nrpe1 mutant, and idn2-1 mutant with a deletion in the RNA-binding XS domain. Recovered RNA was digested with DNase I and assayed by real-time RT-PCR (A) or reverse transcribed and amplified followed by real-time PCR (B). ACTIN2 signal serves as a loading control. Graphs show averages normalized to the wild-type and SD from four (A) or two (B) biological repeats. Input, no antibody and no RT controls as well as RNA IP results normalized to wild-type inputs are shown in Figure S1A.
(C) Domain structure of SWI3B and SWI3B clones identified with the yeast two-hybrid screen with IDN2 as bait.
(D) IDN2 interacts with SWI3B but not with its homologs, SWI3A, SWI3C, or SWI3D. Interaction of full-length SWI3A, SWI3B, SWI3C, and SWI3D with IDN2 was tested with a yeast two-hybrid assay. A series of three 10× dilutions is shown. Yeast growth on a plate with His is shown as a loading control.
(E) IDN2 interacts with SWI3B in tobacco. GFP-tagged SWI3B was coexpressed in tobacco leaves with FLAG-tagged IDN2. After immunoprecipitation with anti-GFP antibody the samples were analyzed by western blot with anti-FLAG antibody. Plants expressing only single construct were used as controls. Total protein extracts (inputs) were assayed by western blot to demonstrate comparable protein expression levels. The asterisk indicates a nonspecific band. Reciprocal coimmunoprecipitation is shown in Figure S1C.
(F) IDN2 interacts with SWI3B in Arabidopsis. GFP-tagged SWI3B and FLAG-tagged IDN2 under the control of their respective native promoters were transformed into Arabidopsis. Obtained transgenic lines were crossed and analyzed by coimmunoprecipitation with anti-GFP antibody and western blot with anti-FLAG antibody. Asterisks indicate nonspecific bands.
See also Figure S1.
IDN2 Interacts with SWI3B
Having established that IDN2 physically interacts with lncRNA, we used a yeast two-hybrid screen with IDN2 as a bait to identify proteins that might be indirectly associated with lncRNA. Among positive clones, we found several corresponding to SWI3B, a subunit of the SWI/SNF ATP-dependent chromatin-remodeling complex (Sarnowski et al., 2005) (Figure 1C). To validate this finding we used a targeted yeast two-hybrid test, which confirmed that IDN2 interacts with full-length SWI3B but not with other SWI3 homologs (SWI3A, SWI3C, or SWI3D; Figure 1D). This interaction was then confirmed in vivo by reciprocal coimmunoprecipitations of FLAG- and GFP-tagged IDN2 and SWI3B transiently overexpressed in tobacco leaves (Figures 1E and S1C) and by coimmunoprecipitation of SWI3B-GFP and IDN2-FLAG driven by their respective native promoters in stable Arabidopsis transformants (Figure 1F). This interaction was lost in IDN2 truncations eliminating predicted coiled-coil regions (Figure S1D) but not in a deletion mutant in the RNA-binding XS domain (Figures S1E and S1F) (Ausin et al., 2009), suggesting that IDN2 binds SWI3B using its coiled-coil domain. Together, these results indicate that SWI3B physically interacts with IDN2, and therefore SWI3B might be involved in lncRNA-mediated transcriptional silencing.
IDN2 Dimerization Is Required for Silencing but Not for Interaction with SWI3B
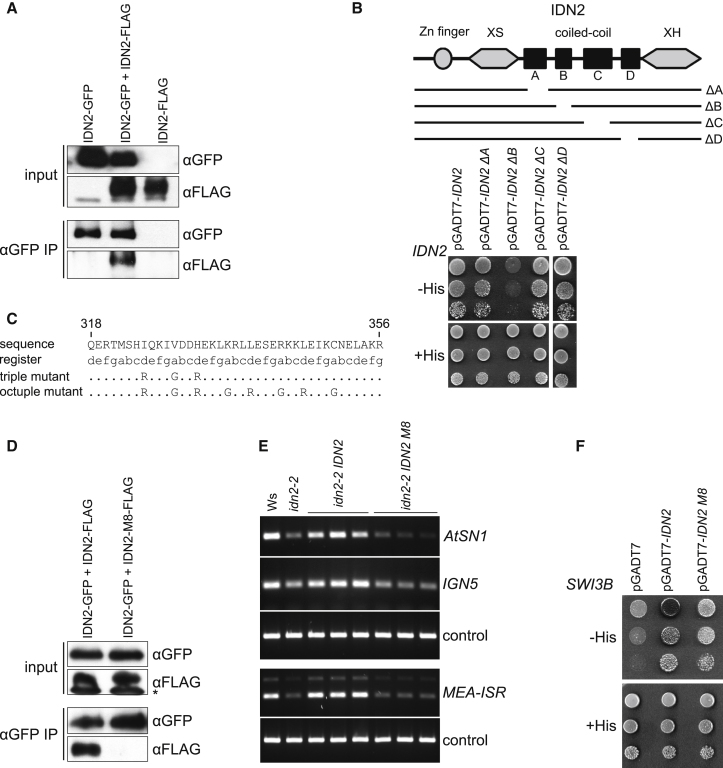
To check whether recently reported IDN2 dimerization (Ausin et al., 2012; Zhang et al., 2012) (Figure 2A) plays a role in the interaction with SWI3B, we identified a region within the IDN2 coiled-coil domain responsible for homodimerization (Figure 2B). We created the IDN2 M8 mutant with eight point mutations introduced within this region (Figure 2C) to disrupt homodimerization by specific interactions of the coiled-coil domain. The IDN2 M8 mutant was unable to dimerize in the yeast two-hybrid assay (Figure S2A) and in vivo coimmunoprecipitation in tobacco leaves (Figure 2D). We tested DNA methylation levels in idn2-2 knockout mutants transformed with wild-type IDN2 and IDN2 M8 by digesting genomic DNA with methylation-sensitive restriction endonucleases followed by PCR. DNA methylation levels on silencing targets AtSN1, IGN5, and MEA-ISR were reduced in the idn2-2 knockout mutant (Figures 2E and S2B). Transformation of the idn2-2 mutant with wild-type IDN2 restored DNA methylation to wild-type levels; however, IDN2 M8 was unable to restore DNA methylation (Figures 2E and S2B). This indicates that dimerization of IDN2 is required for the biological function of IDN2 in the transcriptional gene silencing pathway. We further tested whether IDN2 dimerization was disrupted by deletion in the XS domain present in the idn2-1 mutant. Yeast two-hybrid and coimmunoprecipitation in tobacco leaves revealed that deletion in the XS domain did not affect IDN2 dimerization (Figures S2C and S2D), suggesting that the XS domain is not needed for IDN2 dimerization. Together with our observation that deletion in the XS domain does not disrupt IDN2 interaction with SWI3B (Figures S1E and S1F), this suggests that deletion within the XS domain only disrupts IDN2 binding to RNA. This provides additional support for interaction with lncRNA being important for IDN2 function in RdDM. We further tested whether dimerization of IDN2 is required for its interaction with SWI3B. Yeast two-hybrid assay revealed that IDN2 M8 was still able to interact with SWI3B (Figure 2F). Although we cannot exclude that the M8 mutation affects other aspects of IDN2 function, these results suggest that IDN2 dimerization is required for RdDM but not for the interaction of IDN2 with SWI3B.
Figure 2.
IDN2 Homodimerization Is Required for Silencing but Not for the Interaction with SWI3B
(A) IDN2 dimerizes. GFP- and FLAG-tagged IDN2 were coexpressed in tobacco leaves. After immunoprecipitation with anti-GFP antibody, the sample was analyzed by western blot with anti-FLAG antibody.
(B) Subdomain B within coiled-coil region of IDN2 is responsible for IDN2 dimerization. Four coiled-coil subdomains were identified within IDN2 with Paircoil2 (McDonnell et al., 2006), and corresponding deletion mutants were tested by yeast two-hybrid assay for interaction with wild-type IDN2.
(C) Point mutants within subdomain B of the coiled-coil region of IDN2. Point mutants were designed to contain 3 (triple) or 8 (octuple, M8) amino acids within registers A and D of the coiled-coil alpha helix changed to arginines or glycines to disrupt interactions mediated by the coiled-coil with minimal impact on the alpha-helix of the coiled-coil domain (Tripet et al., 2000).
(D) Dimerization is lost in IDN2 with a mutated coiled-coil domain. GFP-tagged wild-type IDN2 was coexpressed in tobacco leaves with FLAG-tagged IDN2 M8. After immunoprecipitation with anti-GFP antibody the sample was analyzed by western blot with anti-FLAG antibody. FLAG-tagged wild-type IDN2 was used as a control. Total protein extracts (inputs) were assayed by western blot to demonstrate comparable protein expression levels. The asterisk indicates a nonspecific band.
(E) IDN2 dimerization is required for DNA methylation. idn2-2 knockout mutant Arabidopsis plants were transformed with wild-type IDN2 and IDN2 M8. Flowers of obtained transgenic plants were assayed for changes in DNA methylation by digesting with DNA methylation-sensitive restriction endonucleases (HaeIII for AtSN1 and IGN5, Sau3AI for MEA-ISR) followed by PCR. Sequences with no restriction sites were used as controls (ACTIN2 for HaeIII and JA35/JA36 for Sau3AI). More independent transgenic lines are shown in Figure S2B.
(F) IDN2 dimerization is not required for interaction with SWI3B. IDN2 M8 mutant within the subdomain B of the coiled-coil region was tested for interaction with SWI3B by yeast two-hybrid assay.
See also Figure S2.
SWI3B Contributes to Transcriptional Silencing
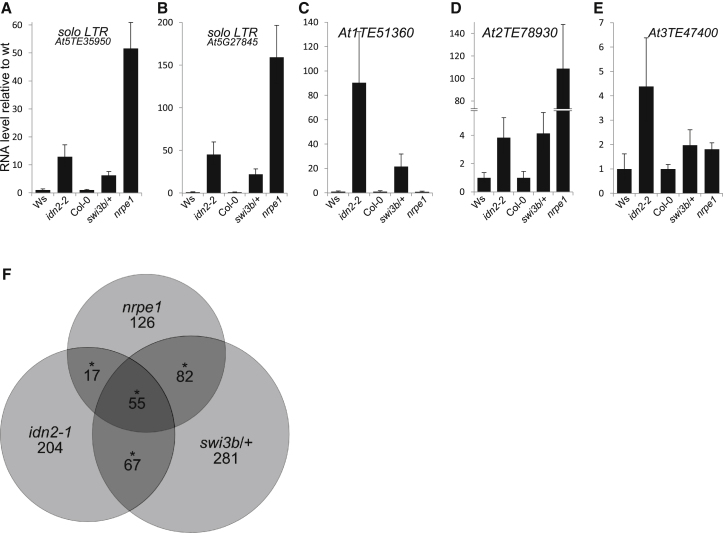
To test whether SWI3B is involved in RNA-mediated transcriptional silencing, we assayed accumulation of RNA produced from silencing targets in a swi3b mutant. Because homozygous knockout mutants of SWI3B are embryo lethal (Sarnowski et al., 2005), we used plants heterozygous for the swi3b-2 mutation (swi3b/+), which have been shown to have the expression level of SWI3B reduced to about 50% and display phenotypes attributed to SWI3B deficiency (Saez et al., 2008) (Figure S3A). Real-time RT-PCR revealed that two transposon-originating transcripts within the solo LTR region, a well-characterized target of RNA-mediated transcriptional silencing (Huettel et al., 2006; Wierzbicki et al., 2008), were derepressed in the swi3b/+ mutant as well as in the idn2 and nrpe1 mutants (Figures 3A and 3B). Derepression of solo LTR was more pronounced in the nrpe1 mutant than in the swi3b/+ and idn2 mutants, which is consistent with partial reduction of SWI3B in the swi3b/+ line (Saez et al., 2008) (Figure S3A) and with IDN2 having several potentially redundant homologs (Ausin et al., 2009; Zhang et al., 2012). Similar partial reactivation of RdDM targets in the swi3b/+ mutant was observed on several other RdDM targets (Figures 3C–3E and S3B–S3D). These results show that SWI3B contributes to RNA-mediated transcriptional silencing of at least a subset of silencing targets.
Figure 3.
SWI3B Contributes to RNA-Mediated Transcriptional Silencing
(A–E) Silencing targets are derepressed in the swi3b/+ mutant. RNA accumulation in flowers from solo LTR (A and B), At1TE51360 (C), At2TE78930 (D), and At3TE47400 (E) were assayed by real-time RT-PCR in idn2-2 mutant compared to Ws wild-type and in swi3b/+ and nrpe1 mutants compared to Col-0 wild-type. Graphs show averages normalized to ACTIN2 and SD from three biological repeats. More loci are shown in Figures S3B–S3E.
(F) SWI3B controls the expression levels of a significant subset of Pol V and IDN2 targets. Venn diagram showing genes identified by RNA-seq to be upregulated in nrpe1, idn2-1 and/or swi3b/+ mutants. RNA-seq was performed in three independent biological repeats in seedlings. Asterisks denote statistically significant enrichment of overlaps (see the main text and Figure S3G for details).
SWI3B Controls Expression of Genes Affected by Silencing
Interaction of SWI3B with lncRNA-binding protein IDN2 and requirement of SWI3B for transcriptional silencing suggest a functional relationship between lncRNA and SWI3B. To test this possibility we used a genome-wide approach to identify genes whose expression is affected in the swi3b/+, nrpe1, and idn2-1 mutants. RNA-seq from three biological repeats revealed that out of 280 genes significantly upregulated in the nrpe1 mutant, 137 (49%) were also upregulated in the swi3b/+ line (Figures 3F and S3G). This is significantly more than 1.8% expected by chance (p → 0, chi-square test). Similarly, out of 343 genes significantly upregulated in the idn2-1 mutant, 122 (36%) were also upregulated in the swi3b/+ line (Figures 3F and S3G). This is significantly more than 1.7% expected by chance (p → 0, chi-square test). Importantly, 55 genes were upregulated in all three mutants (Figure 3F), which is significantly more than less than one gene expected by chance (p → 0, chi-square test). A slightly smaller, although still highly significant, overlap was observed among genes downregulated in nrpe1, idn2, and swi3b/+ mutants (Figures S3F and S3G). In contrast, genes upregulated in one genotype and downregulated in another genotype were found at rates not significantly higher than expected by chance (Figure S3G). These results suggest that Pol V, IDN2, and SWI3B affect overlapping groups of genes. Although it is unknown which of those genes are direct targets of Pol V, IDN2, and SWI3B, these results are consistent with SWI3B being involved in the same gene regulatory pathway as Pol V and IDN2. Together with IDN2-lncRNA interaction, IDN2-SWI3B interaction and derepression of silencing targets in the swi3b/+ mutant, these data demonstrate that SWI3B is involved in the RNA-mediated transcriptional silencing pathway.
SWI/SNF Complex Contributes to Transcriptional Silencing
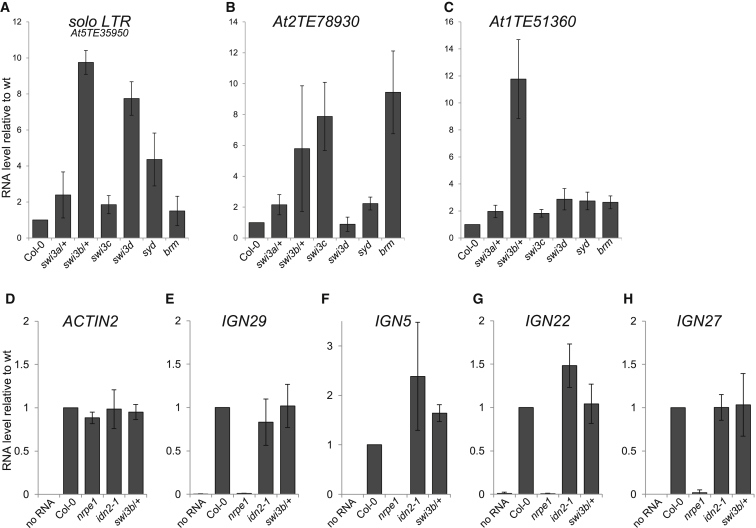
SWI3B is a subunit of a putative SWI/SNF ATP-dependent chromatin-remodeling complex (Sarnowski et al., 2005). Therefore, the involvement of SWI3B in RNA-mediated transcriptional silencing suggests that the SWI/SNF complex may be involved in this process. To test this possibility, we assayed transcriptional silencing in mutants defective in several known components of the SWI/SNF complexes, including four SWI3 proteins (Sarnowski et al., 2002, 2005) and two Snf2 family ATPases SPLAYED (SYD) (Wagner and Meyerowitz, 2002) and BRAHMA (BRM) (Farrona et al., 2004; Kwon et al., 2006; Knizewski et al., 2008). Solo LTR was significantly derepressed in the swi3b/+, swi3d, and syd mutants (Figure 4A), which shows that this locus is controlled by a SWI/SNF complex including SWI3B, SWI3D, and SYD. Another silencing target At2TE78930 was derepressed in swi3b/+, swi3c, and brm mutants (Figure 4B), indicating that this locus is also controlled by a SWI/SNF complex; however, the subunit composition of the complex acting on At2TE78930 is different than on solo LTR. Other tested loci also displayed locus-specific contributions of specific SWI/SNF subunits for transcriptional silencing (Figures 4C and S4A–S4C). These results indicate that SWI3B is not the only subunit of SWI/SNF required for transcriptional silencing. They further suggest that the SWI/SNF complex as a whole contributes to RNA-mediated transcriptional silencing, but subunit contributions may be locus specific.
Figure 4.
RNA-Mediated Transcriptional Silencing Involves the SWI/SNF Complex, which Works Downstream of lncRNA Production
(A–C) Silencing targets are derepressed in mutants defective in SWI/SNF subunits. RNA accumulation in flowers from solo LTR (A), At2TE78930 (B), or At1TE51360 (C) was assayed by real-time RT-PCR in swi3a/+, swi3b/+, swi3c, swi3d, syd, and brm mutants compared to Col-0 wild-type. Graphs show averages normalized to ACTIN2 and SD from three biological repeats, normalized to Col-0 wild-type.
(D–H) IDN2 and SWI3B are not required for lncRNA production. Pol V-produced lncRNAs IGN29 (E), IGN5 (F), IGN22 (G), and IGN27 (H) were assayed in seedlings by real-time RT-PCR in idn2-1 and swi3b/+ mutants compared to Col-0 wild-type. nrpe1 mutant was used as a negative control. To check for potential DNA contaminations, no RT control was performed on ACTIN2 (Figure S4E) and, additionally, no RNA controls were performed for all primer pairs tested. ACTIN2 (D) is a loading control. Error bars show averages and SD from three biological repeats. More loci are shown in Figures S4F–S4I.
See also Figure S4.
SWI/SNF Functions Downstream of lncRNA Production
The physical interaction of a SWI/SNF subunit with lncRNA-binding protein IDN2 suggests that SWI/SNF functions downstream of Pol V and might be recruited by Pol V and IDN2 to specific targets in the genome in a similar fashion as AGO4 (Wierzbicki et al., 2009) and SPT5L (Rowley et al., 2011). Alternatively, SWI/SNF could work with the RDD complex (Law et al., 2010) and mediate Pol V binding to chromatin and/or Pol V transcription (Wierzbicki et al., 2008, 2009; Law et al., 2010). To distinguish between these possibilities, we assayed the accumulation of known Pol V transcripts (Wierzbicki et al., 2008, 2012; Rowley et al., 2011) in idn2 and swi3b/+ mutants. Because of low abundance, these transcripts were not detectable in our RNA-seq data sets and could only be assayed with targeted RT-PCR. We found levels of all tested Pol V transcripts to be unchanged in both idn2 and swi3b/+ mutants (Figures 4E–4H and S4F–S4I). This shows that IDN2 and SWI/SNF work either in parallel to Pol V transcription or downstream of Pol V-produced lncRNA. This was further confirmed by chromatin immunoprecipitation (ChIP) with anti-SWI3B antibody, which indicated that at least on the tested loci SWI3B binding to chromatin was reduced in the nrpe1 mutant (Figure S4J). Together with the IDN2-SWI3B interaction and the requirement of SWI/SNF for silencing, these results suggest that Pol V-produced lncRNAs guide the SWI/SNF complex to specific genomic loci with IDN2 being an intermediate adaptor protein.
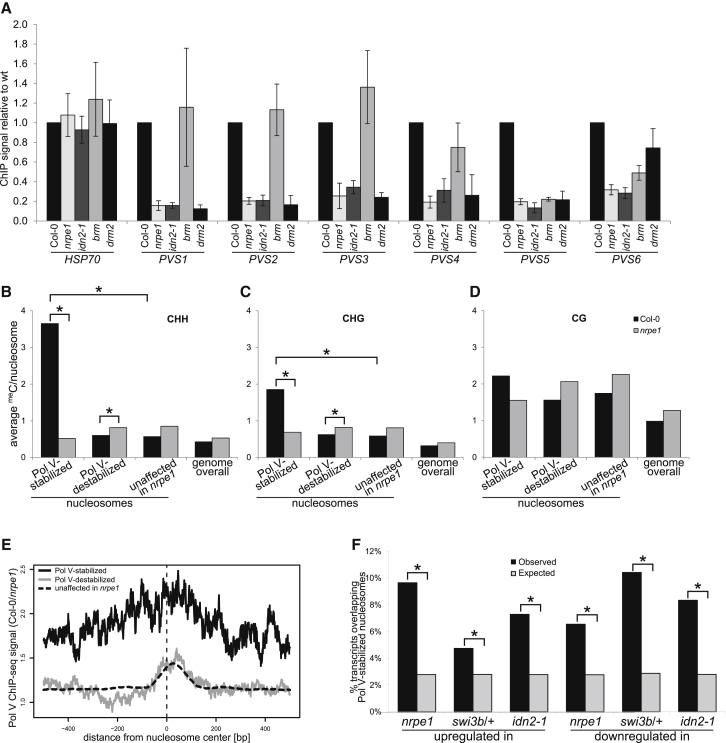
Pol V Affects Nucleosome Positioning
Because SWI/SNF is a putative ATP-dependent chromatin-remodeling complex, if it is guided to chromatin by lncRNA, it is expected to affect nucleosome positioning in an lncRNA-dependent manner. To test this prediction, we digested nuclei from Col-0 wild-type and nrpe1 mutant plants with micrococcal nuclease (MNase), which specifically cuts genomic DNA not protected by nucleosomes (Reeves and Jones, 1976) (Figure S5A). We sequenced mononucleosomal DNA and identified 2,544 nucleosomes that were significantly weaker in nrpe1 than Col-0 wild-type (“Pol V-stabilized”) and 2,362 nucleosomes that were significantly stronger in nrpe1 than Col-0 wild-type (“Pol V-destabilized”). We further narrowed down the list of differential nucleosomes by performing two biological repeats of ChIP-seq with anti-H3 antibody in Col-0 wild-type and nrpe1 mutant, although limited resolution of ChIP affects the ability to detect differential nucleosomes located in close proximity to well stabilized unaffected nucleosomes. This stringent approach yielded 108 Pol V-stabilized and 655 Pol V-destabilized high confidence nucleosomes. We validated several identified nucleosomes (Figure S5B) using a locus-specific assay, where MNase-digested chromatin is subject to ChIP with anti-H3 antibody followed by real time PCR (Figure 5A). These nucleosomes were also destabilized in the idn2-1 mutant (Figure 5A), which is consistent with IDN2 being and adaptor protein connecting Pol V-produced lncRNA to SWI/SNF. A subset of assayed nucleosomes was also affected in a knockout brm mutant (Figure 5A), which further suggests that at least a fraction of lncRNA-mediated nucleosome positioning is mediated by the SWI/SNF complex. Together, these data allow speculation that Pol V-produced lncRNAs mediate nucleosome positioning by guiding the SWI/SNF complex to specific genomic loci.
Figure 5.
Pol V Mediates Nucleosome Positioning
(A) Validation of Pol V-stabilized nucleosomes. Nucleosomes identified with genome-wide assays (Figure S5B) were assayed in seedlings and in case of brm in mature leaves with MNase digestion followed by H3 ChIP and real-time PCR. ChIP signal values were normalized to ACTIN2 and Col-0 wild-type. HSP70 is a negative control (Kumar and Wigge, 2010). Bars show averages and SD from three biological repeats.
(B–D) Nucleosomes stabilized by Pol V are enriched in Pol V-dependent DNA methylation. Published genome-wide DNA methylation data sets from Col-0 wild-type and nrpe1 mutant (Zhong et al., 2012) were used to calculate average DNA methylation levels on nucleosomes identified by MNase-seq and H3 ChIP-seq. Pol V-stabilized and Pol V-destabilized nucleosomes were compared to nucleosomes unaffected in nrpe1 and to the entire genome (genome overall). DNA methylation levels were independently calculated in CHH (B), CHG (C), and CG (D) contexts. Asterisks indicate significant enrichment (see the main text).
(E) Pol V-stabilized nucleosomes are enriched in Pol V binding. A published Pol V ChIP-seq data set (Wierzbicki et al., 2012) was used to calculate profiles of Pol V binding around the centers of nucleosomes identified using MNase-seq and H3 ChIP-seq, which are Pol V-stabilized, Pol V-destabilized, or unaffected in nrpe1.
(F) Pol V-stabilized nucleosomes are enriched on genes upregulated or downregulated in nrpe1 and swi3b/+ mutants. Nucleosomes identified with MNase-seq as Pol V-stabilized were overlapped with genes identified with RNA-seq as upregulated or downregulated in nrpe1 or swi3b. Permutations of gene sets were overlapped in parallel to calculate enrichment. Asterisks indicate significant enrichment (p < 0.02).
Pol V Mediates Nucleosome Stabilization on Silencing Targets
Nucleosomes have been shown to be generally correlated with DNA methylation (Chodavarapu et al., 2010). To test whether Pol V may contribute to this phenomenon, we compared published DNA methylation data (Zhong et al., 2012) to the nucleosomes affected by Pol V. We found that in Col-0 wild-type, Pol V-stabilized nucleosomes were significantly enriched in CHH methylation when compared to nucleosomes with no significant changes in nrpe1 (p < 10−9; t test; Figures 5B and S5C). Pol V-stabilized nucleosomes were also significantly, yet to a lesser extent enriched in CHG methylation (p < 10−4; t test; Figures 5C and S5D). We further compared DNA methylation on nucleosomes in Col-0 wild-type to the nrpe1 mutant. We found that DNA methylation on Pol V-stabilized nucleosomes was reduced in the nrpe1 mutant to levels comparable to those observed on nucleosomes unaffected in nrpe1 or throughout the entire genome (Figures 5B–5D). Observed reduction in DNA methylation was especially apparent in CHH (p < 10−9; t test) and CHG (p < 0.002; t test) contexts. This indicates that Pol V-stabilized nucleosomes overlap Pol V-mediated DNA methylation. In contrast, Pol V-destabilized nucleosomes showed no enrichment in wild-type DNA methylation compared to nucleosomes unaffected in nrpe1 or to the entire genome (Figures 5B–5D and S5C–S5E). In the nrpe1 mutant, DNA methylation on Pol V-destabilized nucleosomes was slightly increased (CHH, p < 0.002; CHG, p < 0.03; CG, p < 0.002; t test), which reflects similar effects on nucleosomes unaffected in nrpe1 or throughout the entire genome (Wierzbicki et al., 2012) (Figure 5B–5D). These findings indicate that Pol V-stabilized but not Pol V-destabilized nucleosomes are preferentially present on regions of Pol V-dependent DNA methylation. This is consistent with Pol V-produced lncRNAs mediating both DNA methylation and nucleosome positioning.
To further test whether Pol V-stabilized nucleosomes overlap direct Pol V targets, we used a published Pol V ChIP-seq data set (Wierzbicki et al., 2012) to calculate enrichment of Pol V binding on the three categories of nucleosomes. We found that Pol V-stabilized nucleosomes were enriched in Pol V binding compared to Pol V-destabilized nucleosomes or nucleosomes unaffected in nrpe1 (Figure 5E). Pol V binding and Pol V-dependent CHH methylation are also clearly visible on validated Pol V-stabilized nucleosomes (Figure S5B). These results show that Pol V-stabilized nucleosomes are present on Pol V targets. This suggests that, in addition to facilitating DNA methylation, Pol V mediates nucleosome positioning and further supports the model that Pol V-produced lncRNAs control nucleosome positioning on silencing targets.
Pol V-stabilized nucleosomes were also enriched on gene promoters and depleted on transcribed sequences (Figure S5F), suggesting that nucleosome remodeling may be involved in the control of gene expression. To test this possibility, we overlapped the long list of differential nucleosomes generated using only MNase-seq with genes affected in swi3b/+, nrpe1, and idn2-1 mutants. Pol V-stabilized nucleosomes were slightly, yet significantly enriched on genes upregulated or downregulated in all three mutants compared to corresponding permutations of genes (Figure 5F). Consistently, when we mapped nucleosomes affected in the nrpe1 mutant to chromosomes, Pol V-stabilized nucleosomes were present throughout gene-rich chromosome arms (Figure S5G). In contrast, Pol V-destabilized nucleosomes displayed a preference toward pericentromeric regions (Figure S5G), which is consistent with redistribution of silencing toward pericentromeric regions in the nrpe1 mutant (Wierzbicki et al., 2012). This shows that Pol V-stabilized nucleosomes can be correlated with Pol V- and SWI/SNF-controlled genes. This is further consistent with lncRNA-guided nucleosome positioning being an important factor in transcriptional silencing.
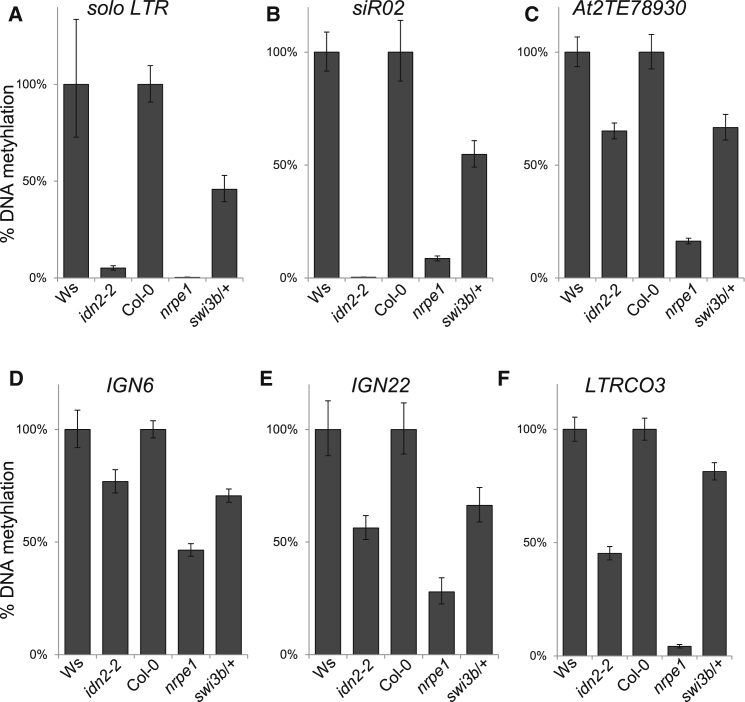
SWI/SNF Contributes to DNA Methylation
Correlation between Pol V-stabilized nucleosomes and Pol V-mediated DNA methylation may be explained by both nucleosome stabilization and DNA methylation being independently guided by lncRNA to the same genomic regions. Alternatively, preexisting DNA methylation may promote nucleosome stabilization or nucleosomes may be preferred targets for DNA methyltransferases. We tested these possibilities by assaying positioning of selected Pol V-stabilized nucleosomes in drm2, a mutant in de novo DNA methyltransferase. Five of the tested nucleosomes were destabilized in the drm2 mutant, while one nucleosome was not significantly affected (Figure 5A). This is consistent with DNA methylation promoting nucleosome positioning but only on a subset of loci. To test whether nucleosome positioning affects DNA methylation, we assayed DNA methylation on silenced loci in the swi3b/+ mutant. Solo LTR, siR02, and At2TE78930 all had CHH methylation levels reduced to around 50% in swi3b/+ (Figures 6A–6C). Less-pronounced reduction was observed at IGN6, IGN22, and LTRCO3 (Figures 6D–6F). This suggests that nucleosome positioning affects DNA methylation possibly by well-positioned nucleosomes being preferential targets for DNA methyltransferases; however, indirect effects cannot be excluded. Together, these results are consistent with a model where transcriptional silencing is established by Pol V-produced lncRNA guiding both positioned nucleosomes and DNA methylation, while maintenance of silencing is additionally facilitated by a mutual feedback loop between well positioned nucleosomes and DNA methylation.
Figure 6.
SWI/SNF Is Required for Wild-Type Levels of CHH Methylation
(A–F) DNA methylation was assayed in flowers with digestion with methylation-sensitive restriction endonucleases followed by real-time PCR amplification of solo LTR (A), siR02 (B), At2TE78930 (C), IGN6 (D), IGN22 (E) and LTRCO3 (F) (At3TE51910 [Huettel et al., 2006]). Graphs show average DNA methylation levels normalized to ACTIN2 and to wild-type. Error bars represent the SD from three biological repeats.
Discussion
Our results uncover an additional pathway connecting Pol V-produced lncRNA to the establishment of silent chromatin status. The initial step in this pathway is the association of lncRNA with IDN2 (Figures 1A and 1B), a process that may involve an entire IDN2-containing complex composed of an IDN2 dimer (Figure 2A) and two IDN2-related proteins (Ausin et al., 2012; Xie et al., 2012; Zhang et al., 2012). IDN2 has been shown in vitro to bind double-stranded RNA using its XS domain (Ausin et al., 2009, 2012; Zhang et al., 2012); therefore, IDN2 may bind lncRNA-siRNA duplexes or hairpin regions within lncRNA. It also implies that the IDN2-lncRNA association may be directed either by AGO4-siRNA complexes or IDN2 may bind lncRNA without the requirement for siRNA in a manner similar to SPT5L (Rowley et al., 2011).
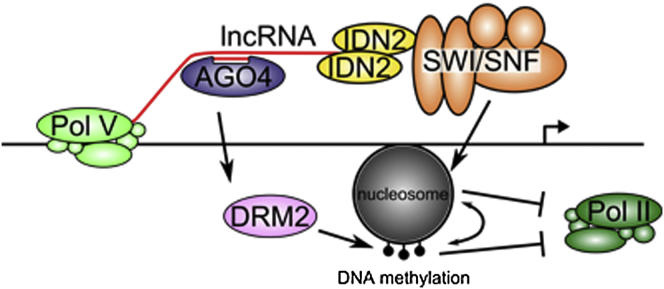
The next step in this pathway is the physical interaction between IDN2 and SWI3B, a subunit of the ATP-dependent chromatin-remodeling complex (Figures 1C–1F). Consistently, transcriptional silencing was partially lost on several known loci in the swi3b/+ mutant (Figures 3A–3E). Although it remains possible that IDN2-lncRNA complexes are distinct from the ones formed by IDN2 and SWI/SNF, our transcriptome analysis shows a significant overlap between genes affected by the nrpe1 mutant, idn2-1 mutant, in which IDN2 is unable to interact with lncRNA and the swi3b/+ mutant (Figure 3F). This is consistent with lncRNA, IDN2, and SWI/SNF working together and suggests that the silencing signal is transmitted from Pol V-produced lncRNA to SWI3B by IDN2 (Figure 7).
Figure 7.
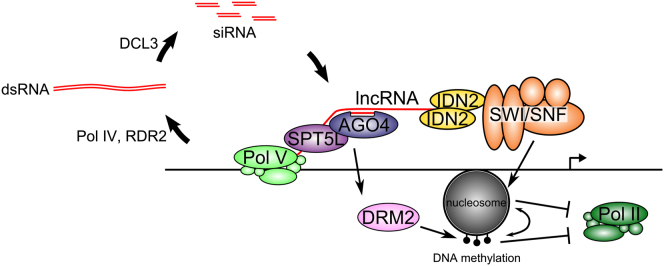
A Model of the Involvement of Nucleosome Positioning in lncRNA-Mediated Transcriptional Silencing
siRNA is produced by the activities of Pol IV, RDR2, and DCL3 to give AGO4 sequence specificity. Pol V produces lncRNAs, which are bound by IDN2 dimers. IDN2 recruits the SWI/SNF ATP-dependent chromatin-remodeling complex by physical interaction with SWI3B. The SWI/SNF complex positions nucleosomes. Positioned nucleosomes silence transcription directly or by facilitating DNA methylation by the de novo methyltransferase, DRM2. Maintenance of silencing is reinforced by a positive feedback between DNA methylation and nucleosome positioning.
Defects in transcriptional silencing observed in plant lines defective for the other tested subunits of the SWI/SNF complex (Figures 4A–4C) suggest that the involvement of SWI3B in transcriptional silencing reflects the involvement of the entire SWI/SNF complex. Interestingly, however, subunit composition of the SWI/SNF complex seems to be locus specific (Figures 4A–4C). This is consistent with the subunit variants being responsible for functional diversification of the SWI/SNF complexes (Sarnowski et al., 2005; Bezhani et al., 2007; Hargreaves and Crabtree, 2011). The high level of functional diversification of the Arabidopsis SWI/SNF complexes is further supported by the observation that mutants in SWI/SNF subunits have additional phenotypes, which are likely not associated with RNA-mediated transcriptional silencing (Sarnowski et al., 2005; Bezhani et al., 2007; Walley et al., 2008; Farrona et al., 2011; Wu et al., 2012). This indicates that SWI/SNF complexes also have other biological functions beyond RNA-mediated transcriptional silencing.
The following step in this pathway is nucleosome positioning, as demonstrated by the changes in nucleosome patterns in the nrpe1 mutant. Our findings are consistent with a model (Figure 7) in which these effects are mediated by the ATP-dependent chromatin-remodeling activity of SWI/SNF; however, alternative mechanisms cannot be excluded at this moment. Pol V-produced lncRNA does not seem to be involved in the establishment of conserved nucleosome patterns (Chodavarapu et al., 2010) on most protein-coding genes. Instead, it specifically mediates stabilization of nucleosomes on sequences enriched in Pol V-dependent non-CG DNA methylation (Figures 5B–5D), a hallmark of RNA-mediated transcriptional silencing (Law and Jacobsen, 2010). This is consistent with previously observed genome-wide correlation between positioned nucleosomes and DNA methylation in all sequence contexts (Chodavarapu et al., 2010). The Pol V-stabilized nucleosomes were also significantly enriched on genes upregulated in the nrpe1, idn2-1, and swi3b/+ mutants (Figure 5F), indicating that these nucleosomes may affect Pol II transcription of at least a subset of silencing targets.
The final step of the pathway is repression of Pol II transcription on silenced regions, demonstrated by reduction of transcriptional silencing in nrpe1, idn2, and swi3b/+ mutants (Figures 3A–3E). Locus-specific destabilization of nucleosomes in the drm2 mutant and partial reduction of DNA methylation in the swi3b/+ mutant (Figures 5A and 6A–6F) support a model in which transcriptional silencing is established by Pol V-produced lncRNA guiding both positioned nucleosomes and DNA methylation. On the other hand, maintenance of silencing is mediated by continuous action of lncRNA further reinforced by a mutual feedback loop between well-positioned nucleosomes and DNA methylation. Pol II is then repressed by DNA methylation of cis-regulatory regions and/or well-positioned nucleosomes directly affecting the ability of transcriptional machinery to bind DNA.
Our data show the role of nucleosome positioning in the final steps of the RNA-mediated transcriptional silencing pathway. It is however possible that active changes in nucleosome occupancy are also critical at other steps of the pathway and in other silencing pathways. Production of siRNA and Pol V-produced lncRNA has been shown to require two distinct putative chromatin remodelers CLASSY1 and DRD1 (Kanno et al., 2004; Smith et al., 2007; Wierzbicki et al., 2008; Law et al., 2011). Additionally, maintenance of DNA methylation requires the DDM1 protein, which has been shown to have a nucleosome-remodeling activity in vitro (Jeddeloh et al., 1999; Brzeski and Jerzmanowski, 2003). This indicates that nucleosome remodeling may play a multitude of roles in transcriptional silencing.
Generally, involvement of lncRNAs is a common theme in transcriptional regulation in various groups of organisms (Wang and Chang, 2011), and guiding protein factors to specific genomic loci is a function of lncRNA in several regulatory processes, including RNA-mediated transcriptional silencing (Cam et al., 2009). It is therefore possible that the recruitment of ATP-dependent chromatin-remodeling complexes and nucleosome positioning may be a general and conserved feature of lncRNA.
Overall, our results are consistent with a model in which lncRNAs produced by Pol V affect gene expression by mediating nucleosome positioning (Figure 7). Nascent Pol V transcripts physically interact with IDN2 dimers, which then recruit the SWI/SNF ATP-dependent chromatin-remodeling complex by the physical interaction with SWI3B. SWI/SNF positions nucleosomes, which affect transcriptional machinery. Additionally, maintenance of silencing is reinforced by a feedback loop between DNA methylation and nucleosome positioning.
Experimental Procedures
Plant Lines
Arabidopsis thaliana nrpe1 (nrpd1b-11) mutant was described previously (Pontes et al., 2006). swi3a-1 (SALK_035320), swi3c-2 (Koncz_3737), and swi3d-2 (Koncz_14259) were kindly provided by Tomasz Sarnowski (Sarnowski et al., 2005). swi3b-2 (Sarnowski et al., 2005) (GABI_302G08), syd-4 (SALK_149549), and brm-4 (Tang et al., 2008) (WiscDsLox 436E9) were obtained from the Arabidopsis Biological Resource Center. idn2-1 (Ausin et al., 2009) was kindly provided by Steve Jacobsen. idn2-2 (FLAG_550B05) was obtained from the French National Institute for Agricultural Research (INRA).
Protein-Protein Interaction Assays
Protein-protein interactions were assayed with yeast two-hybrid as well as coimmunoprecipitation of proteins expressed in tobacco leaves or in Arabidopsis (Su et al., 2011). Details are provided in the Supplemental Experimental Procedures.
Site-Directed Mutagenesis
Entry plasmids containing full-length genomic or complementary DNA (cDNA) clones were used to introduce deletions or mutations in IDN2 with the Quickchange Site-Directed Mutagenesis Kit (Stratagene).
RNA Immunoprecipitation
RNA IP with anti-IDN2 antibody was performed as described (Wierzbicki et al., 2008), except that IP was performed with 40 μl Dynabeads protein A at 4°C overnight and analyzed by real-time RT-PCR. Amplified cDNA was generated with the Ovation RNA-Seq System V2 (Nugen) according to the manufacturer’s protocol. Rabbit polyclonal anti-IDN2 antibody was raised against an N-terminal portion of the IDN2 protein (aa 4–201) expressed in bacteria and affinity purified.
Chromatin Immunoprecipitation
ChIP-seq was performed as described (Zheng et al., 2012). Library generation and Illumina sequencing were performed by the University of Michigan Sequencing Core. ChIP real-time PCR protocol was based on Wierzbicki et al. (2008) with an additional Micrococcal Nuclease (MNase) digestion prior to IP. We used anti-histone H3 antibody (ab1791, Abcam) or affinity-purified rabbit polyclonal anti-SWI3B antibody raised against a C-terminal portion of the SWI3B protein (aa 248–469). Details are provided in the Supplemental Experimental Procedures.
MNase-Seq
Nuclei were extracted from 2-week-old Arabidopsis seedlings as described (Wierzbicki et al., 2008) and were digested with Micrococcal Nuclease (MNase; NEB). Mononucleosomal DNA was gel purified and used for library generation and Illumina sequencing. Details are provided in the Supplemental Experimental Procedures.
Bioinformatic Analysis
RNA-seq reads from three independent biological repeats were aligned and processed to the Arabidopsis TAIR10 genome with the BOWTIE suite (BOWTIE, TOPHAT, and CUFFLINKS) (Langmead et al., 2009). Overlaps between sets of differentially expressed genes were determined based on gene ID while expected values were derived by the formula: set1(set2 / total genes); p values were generated from these values with a chi-square test.
MNase-seq and ChIP-seq reads were aligned with BOWTIE’s default settings and nucleosomes were called using MNase-seq data as described (Weiner et al., 2010). Nucleosome lists were generated in Col-0 as well as in nrpe1 and the lists from both genotypes were combined. Reads corresponding to nucleosomes were counted in both genotypes, quantile normalization was applied and the highest 5% nucleosomes according to read depth were removed. The remaining nucleosomes were classified as either decreased in nrpe1 (Pol V-stabilized) or increased in nrpe1 (Pol V-destabilized) via a combination of 2-fold change cutoff and Poisson significance of p < 0.001; unchanged nucleosomes were classified as having less than 1.5-fold difference between genotypes. Nucleosomes were further filtered with enrichment scores from H3 ChIP-seq with a Poisson significance of p < 0.05. Published DNA methylation data (Zhong et al., 2012) were overlapped with the nucleosome list and methylation profiles were generated in 10 bp windows with a 5 bp sliding window for smoothing. Published Pol V ChIP-seq data (Wierzbicki et al., 2012) was similarly overlapped with nucleosomes and profiles were generated with Sitepro from the CEAS suite (Shin et al., 2009). Overlaps with differential transcripts were performed with 1,000 permutated gene sets to obtain expected numbers and p values.
Acknowledgments
We thank Ken Cadigan and David Engelke for critical reading of the manuscript, Eva Czarnecka-Verner, Steve Jacobsen, and Tomasz Sarnowski for reagents, and David Akey for helpful suggestions. We also thank Robert Lyons and Brendan Tarrier from the University of Michigan DNA Sequencing Core for performing next generation sequencing. This work was supported by National Science Foundation grant MCB 1120271 to A.T.W. and Austrian Science Fund (FWF) fellowship J3199-B09 to G.B. M.J.R. was supported by the NIH National Research Service Award number 5-T32-GM07544. Y.Z. initiated the yeast two-hybrid screen, performed all protein-protein interaction experiments, obtained and analyzed IDN2 M8 mutant and transgenic plants, performed RT-PCRs shown in Figures 3A–3E and 4A–4C, performed all DNA methylation assays, and generated anti-IDN2 and anti-SWI3B antibodies. M.J.R performed all ChIP and ChIP-seq experiments as well as all bioinformatic analyses. G.B. performed RNA IP shown in Figures 1A and 1B, RT-PCR shown in Figures 4D–4H, and prepared samples for RNA-seq and MNase-seq. A.T.W. wrote the manuscript.
Published: December 13, 2012
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, five figures, and two tables and can be found with this article online at http://dx.doi.org/10.1016/j.molcel.2012.11.011.
Accession Numbers
Next generation sequencing data reported in this manuscript have been deposited in the GEO under accession number GSE38464.
Supplemental Information
References
- Ausin I., Mockler T.C., Chory J., Jacobsen S.E. IDN1 and IDN2 are required for de novo DNA methylation in Arabidopsis thaliana. Nat. Struct. Mol. Biol. 2009;16:1325–1327. doi: 10.1038/nsmb.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausin I., Greenberg M.V.C., Simanshu D.K., Hale C.J., Vashisht A.A., Simon S.A., Lee T.-F., Feng S., Española S.D., Meyers B.C. INVOLVED IN DE NOVO 2-containing complex involved in RNA-directed DNA methylation in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2012;109:8374–8381. doi: 10.1073/pnas.1206638109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezhani S., Winter C., Hershman S., Wagner J.D., Kennedy J.F., Kwon C.S., Pfluger J., Su Y., Wagner D. Unique, shared, and redundant roles for the Arabidopsis SWI/SNF chromatin remodeling ATPases BRAHMA and SPLAYED. Plant Cell. 2007;19:403–416. doi: 10.1105/tpc.106.048272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bies-Etheve N., Pontier D., Lahmy S., Picart C., Vega D., Cooke R., Lagrange T. RNA-directed DNA methylation requires an AGO4-interacting member of the SPT5 elongation factor family. EMBO Rep. 2009;10:649–654. doi: 10.1038/embor.2009.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzeski J., Jerzmanowski A. Deficient in DNA methylation 1 (DDM1) defines a novel family of chromatin-remodeling factors. J. Biol. Chem. 2003;278:823–828. doi: 10.1074/jbc.M209260200. [DOI] [PubMed] [Google Scholar]
- Cam H.P., Chen E.S., Grewal S.I.S. Transcriptional scaffolds for heterochromatin assembly. Cell. 2009;136:610–614. doi: 10.1016/j.cell.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodavarapu R.K., Feng S., Bernatavichute Y.V., Chen P.-Y., Stroud H., Yu Y., Hetzel J.A., Kuo F., Kim J., Cokus S.J. Relationship between nucleosome positioning and DNA methylation. Nature. 2010;466:388–392. doi: 10.1038/nature09147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrona S., Hurtado L., Bowman J.L., Reyes J.C. The Arabidopsis thaliana SNF2 homolog AtBRM controls shoot development and flowering. Development. 2004;131:4965–4975. doi: 10.1242/dev.01363. [DOI] [PubMed] [Google Scholar]
- Farrona S., Hurtado L., March-Díaz R., Schmitz R.J., Florencio F.J., Turck F., Amasino R.M., Reyes J.C. Brahma is required for proper expression of the floral repressor FLC in Arabidopsis. PLoS ONE. 2011;6:e17997. doi: 10.1371/journal.pone.0017997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner G.J., Kimura Y., Daub C.O., Wani S., Plessy C., Irvine K.M., Schroder K., Cloonan N., Steptoe A.L., Lassmann T. The regulated retrotransposon transcriptome of mammalian cells. Nat. Genet. 2009;41:563–571. doi: 10.1038/ng.368. [DOI] [PubMed] [Google Scholar]
- Girard A., Hannon G.J. Conserved themes in small-RNA-mediated transposon control. Trends Cell Biol. 2008;18:136–148. doi: 10.1016/j.tcb.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haag J.R., Pikaard C.S. Multisubunit RNA polymerases IV and V: purveyors of non-coding RNA for plant gene silencing. Nat. Rev. Mol. Cell Biol. 2011;12:483–492. doi: 10.1038/nrm3152. [DOI] [PubMed] [Google Scholar]
- Haag J.R., Ream T.S., Marasco M., Nicora C.D., Norbeck A.D., Pasa-Tolic L., Pikaard C.S. In vitro transcription activities of Pol IV, Pol V, and RDR2 reveal coupling of Pol IV and RDR2 for dsRNA synthesis in plant RNA silencing. Mol. Cell. 2012 doi: 10.1016/j.molcel.2012.09.027. Published online November 8, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves D.C., Crabtree G.R. ATP-dependent chromatin remodeling: genetics, genomics and mechanisms. Cell Res. 2011;21:396–420. doi: 10.1038/cr.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X.-J., Hsu Y.-F., Zhu S., Wierzbicki A.T., Pontes O., Pikaard C.S., Liu H.-L., Wang C.-S., Jin H., Zhu J.-K. An effector of RNA-directed DNA methylation in arabidopsis is an ARGONAUTE 4- and RNA-binding protein. Cell. 2009;137:498–508. doi: 10.1016/j.cell.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Jones A.M.E., Searle I., Patel K., Vogler H., Hubner N.C., Baulcombe D.C. An atypical RNA polymerase involved in RNA silencing shares small subunits with RNA polymerase II. Nat. Struct. Mol. Biol. 2009;16:91–93. doi: 10.1038/nsmb.1539. [DOI] [PubMed] [Google Scholar]
- Huettel B., Kanno T., Daxinger L., Aufsatz W., Matzke A.J.M., Matzke M. Endogenous targets of RNA-directed DNA methylation and Pol IV in Arabidopsis. EMBO J. 2006;25:2828–2836. doi: 10.1038/sj.emboj.7601150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeddeloh J.A., Stokes T.L., Richards E.J. Maintenance of genomic methylation requires a SWI2/SNF2-like protein. Nat. Genet. 1999;22:94–97. doi: 10.1038/8803. [DOI] [PubMed] [Google Scholar]
- Jiang C., Pugh B.F. Nucleosome positioning and gene regulation: advances through genomics. Nat. Rev. Genet. 2009;10:161–172. doi: 10.1038/nrg2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno T., Mette M.F., Kreil D.P., Aufsatz W., Matzke M., Matzke A.J.M. Involvement of putative SNF2 chromatin remodeling protein DRD1 in RNA-directed DNA methylation. Curr. Biol. 2004;14:801–805. doi: 10.1016/j.cub.2004.04.037. [DOI] [PubMed] [Google Scholar]
- Knizewski L., Ginalski K., Jerzmanowski A. Snf2 proteins in plants: gene silencing and beyond. Trends Plant Sci. 2008;13:557–565. doi: 10.1016/j.tplants.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Kumar S.V., Wigge P.A. H2A.Z-containing nucleosomes mediate the thermosensory response in Arabidopsis. Cell. 2010;140:136–147. doi: 10.1016/j.cell.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Kwon C.S., Hibara K., Pfluger J., Bezhani S., Metha H., Aida M., Tasaka M., Wagner D. A role for chromatin remodeling in regulation of CUC gene expression in the Arabidopsis cotyledon boundary. Development. 2006;133:3223–3230. doi: 10.1242/dev.02508. [DOI] [PubMed] [Google Scholar]
- Langmead B., Trapnell C., Pop M., Salzberg S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law J.A., Jacobsen S.E. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat. Rev. Genet. 2010;11:204–220. doi: 10.1038/nrg2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law J.A., Ausin I., Johnson L.M., Vashisht A.A., Zhu J.-K., Wohlschlegel J.A., Jacobsen S.E. A protein complex required for polymerase V transcripts and RNA- directed DNA methylation in Arabidopsis. Curr. Biol. 2010;20:951–956. doi: 10.1016/j.cub.2010.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law J.A., Vashisht A.A., Wohlschlegel J.A., Jacobsen S.E. SHH1, a homeodomain protein required for DNA methylation, as well as RDR2, RDM4, and chromatin remodeling factors, associate with RNA polymerase IV. PLoS Genet. 2011;7:e1002195. doi: 10.1371/journal.pgen.1002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell A.V., Jiang T., Keating A.E., Berger B. Paircoil2: improved prediction of coiled coils from sequence. Bioinformatics. 2006;22:356–358. doi: 10.1093/bioinformatics/bti797. [DOI] [PubMed] [Google Scholar]
- Pontes O., Li C.F., Costa Nunes P., Haag J., Ream T., Vitins A., Jacobsen S.E., Pikaard C.S. The Arabidopsis chromatin-modifying nuclear siRNA pathway involves a nucleolar RNA processing center. Cell. 2006;126:79–92. doi: 10.1016/j.cell.2006.05.031. [DOI] [PubMed] [Google Scholar]
- Ream T.S., Haag J.R., Wierzbicki A.T., Nicora C.D., Norbeck A.D., Zhu J.-K., Hagen G., Guilfoyle T.J., Pasa-Tolić L., Pikaard C.S. Subunit compositions of the RNA-silencing enzymes Pol IV and Pol V reveal their origins as specialized forms of RNA polymerase II. Mol. Cell. 2009;33:192–203. doi: 10.1016/j.molcel.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves R., Jones A. Genomic transcriptional activity and the structure of chromatin. Nature. 1976;260:495–500. doi: 10.1038/260495a0. [DOI] [PubMed] [Google Scholar]
- Rowley M.J., Avrutsky M.I., Sifuentes C.J., Pereira L., Wierzbicki A.T. Independent chromatin binding of ARGONAUTE4 and SPT5L/KTF1 mediates transcriptional gene silencing. PLoS Genet. 2011;7:e1002120. doi: 10.1371/journal.pgen.1002120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh R., Allis C.D. Genome-wide “re”-modeling of nucleosome positions. Cell. 2011;147:263–266. doi: 10.1016/j.cell.2011.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez A., Rodrigues A., Santiago J., Rubio S., Rodriguez P.L. HAB1-SWI3B interaction reveals a link between abscisic acid signaling and putative SWI/SNF chromatin-remodeling complexes in Arabidopsis. Plant Cell. 2008;20:2972–2988. doi: 10.1105/tpc.107.056705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnowski T.J., Swiezewski S., Pawlikowska K., Kaczanowski S., Jerzmanowski A. AtSWI3B, an Arabidopsis homolog of SWI3, a core subunit of yeast Swi/Snf chromatin remodeling complex, interacts with FCA, a regulator of flowering time. Nucleic Acids Res. 2002;30:3412–3421. doi: 10.1093/nar/gkf458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnowski T.J., Ríos G., Jásik J., Swiezewski S., Kaczanowski S., Li Y., Kwiatkowska A., Pawlikowska K., Koźbiał M., Koźbiał P. SWI3 subunits of putative SWI/SNF chromatin-remodeling complexes play distinct roles during Arabidopsis development. Plant Cell. 2005;17:2454–2472. doi: 10.1105/tpc.105.031203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H., Liu T., Manrai A.K., Liu X.S. CEAS: cis-regulatory element annotation system. Bioinformatics. 2009;25:2605–2606. doi: 10.1093/bioinformatics/btp479. [DOI] [PubMed] [Google Scholar]
- Smith L.M., Pontes O., Searle I., Yelina N., Yousafzai F.K., Herr A.J., Pikaard C.S., Baulcombe D.C. An SNF2 protein associated with nuclear RNA silencing and the spread of a silencing signal between cells in Arabidopsis. Plant Cell. 2007;19:1507–1521. doi: 10.1105/tpc.107.051540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su W., Liu Y., Xia Y., Hong Z., Li J. Conserved endoplasmic reticulum-associated degradation system to eliminate mutated receptor-like kinases in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2011;108:870–875. doi: 10.1073/pnas.1013251108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X., Hou A., Babu M., Nguyen V., Hurtado L., Lu Q., Reyes J.C., Wang A., Keller W.A., Harada J.J. The Arabidopsis BRAHMA chromatin-remodeling ATPase is involved in repression of seed maturation genes in leaves. Plant Physiol. 2008;147:1143–1157. doi: 10.1104/pp.108.121996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripet B., Wagschal K., Lavigne P., Mant C.T., Hodges R.S. Effects of side-chain characteristics on stability and oligomerization state of a de novo-designed model coiled-coil: 20 amino acid substitutions in position “d”. J. Mol. Biol. 2000;300:377–402. doi: 10.1006/jmbi.2000.3866. [DOI] [PubMed] [Google Scholar]
- Wagner D., Meyerowitz E.M. SPLAYED, a novel SWI/SNF ATPase homolog, controls reproductive development in Arabidopsis. Curr. Biol. 2002;12:85–94. doi: 10.1016/s0960-9822(01)00651-0. [DOI] [PubMed] [Google Scholar]
- Walley J.W., Rowe H.C., Xiao Y., Chehab E.W., Kliebenstein D.J., Wagner D., Dehesh K. The chromatin remodeler SPLAYED regulates specific stress signaling pathways. PLoS Pathog. 2008;4:e1000237. doi: 10.1371/journal.ppat.1000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K.C., Chang H.Y. Molecular mechanisms of long noncoding RNAs. Mol. Cell. 2011;43:904–914. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner A., Hughes A., Yassour M., Rando O.J., Friedman N. High-resolution nucleosome mapping reveals transcription-dependent promoter packaging. Genome Res. 2010;20:90–100. doi: 10.1101/gr.098509.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierzbicki A.T. The role of long non-coding RNA in transcriptional gene silencing. Curr. Opin. Plant Biol. 2012;15:517–522. doi: 10.1016/j.pbi.2012.08.008. [DOI] [PubMed] [Google Scholar]
- Wierzbicki A.T., Haag J.R., Pikaard C.S. Noncoding transcription by RNA polymerase Pol IVb/Pol V mediates transcriptional silencing of overlapping and adjacent genes. Cell. 2008;135:635–648. doi: 10.1016/j.cell.2008.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierzbicki A.T., Ream T.S., Haag J.R., Pikaard C.S. RNA polymerase V transcription guides ARGONAUTE4 to chromatin. Nat. Genet. 2009;41:630–634. doi: 10.1038/ng.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierzbicki A.T., Cocklin R., Mayampurath A., Lister R., Rowley M.J., Gregory B.D., Ecker J.R., Tang H., Pikaard C.S. Spatial and functional relationships among Pol V-associated loci, Pol IV-dependent siRNAs, and cytosine methylation in the Arabidopsis epigenome. Genes Dev. 2012;26:1825–1836. doi: 10.1101/gad.197772.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M.-F., Sang Y., Bezhani S., Yamaguchi N., Han S.-K., Li Z., Su Y., Slewinski T.L., Wagner D. SWI2/SNF2 chromatin remodeling ATPases overcome polycomb repression and control floral organ identity with the LEAFY and SEPALLATA3 transcription factors. Proc. Natl. Acad. Sci. USA. 2012;109:3576–3581. doi: 10.1073/pnas.1113409109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie M., Ren G., Costa-Nunes P., Pontes O., Yu B. A subgroup of SGS3-like proteins act redundantly in RNA-directed DNA methylation. Nucleic Acids Res. 2012;40:4422–4431. doi: 10.1093/nar/gks034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C.-J., Ning Y.-Q., Zhang S.-W., Chen Q., Shao C.-R., Guo Y.-W., Zhou J.-X., Li L., Chen S., He X.-J. IDN2 and its paralogs form a complex required for RNA-directed DNA methylation. PLoS Genet. 2012;8:e1002693. doi: 10.1371/journal.pgen.1002693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B., Wang Z., Li S., Yu B., Liu J.-Y., Chen X. Intergenic transcription by RNA polymerase II coordinates Pol IV and Pol V in siRNA-directed transcriptional gene silencing in Arabidopsis. Genes Dev. 2009;23:2850–2860. doi: 10.1101/gad.1868009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z., Xing Y., He X.-J., Li W., Hu Y., Yadav S.K., Oh J., Zhu J.-K. An SGS3-like protein functions in RNA-directed DNA methylation and transcriptional gene silencing in Arabidopsis. Plant J. 2010;62:92–99. doi: 10.1111/j.1365-313X.2010.04130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q., Rowley M.J., Böhmdorfer G., Sandhu D., Gregory B.D., Wierzbicki A.T. RNA polymerase V targets transcriptional silencing components to promoters of protein-coding genes. Plant J. 2012 doi: 10.1111/tpj.12034. Published online September 27, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X., Hale C.J., Law J.A., Johnson L.M., Feng S., Tu A., Jacobsen S.E. DDR complex facilitates global association of RNA polymerase V to promoters and evolutionarily young transposons. Nat. Struct. Mol. Biol. 2012;19:870–875. doi: 10.1038/nsmb.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.