Abstract
Muscle repair relies on coordinated activation and differentiation of satellite cells, a process that is unable to counterbalance progressive degeneration in sporadic inclusion body myositis (s-IBM). To explore features of myo regeneration, the expression of myogenic regulatory factors Pax7, MyoD and Myogenin and markers of regenerating fibers was analyzed by immunohistochemistry in s-IBM muscle compared with polymyositis, dermatomyositis, muscular dystrophy and age-matched controls. In addition, the capillary density and number of interstitial CD34+ hematopoietic progenitor cells was determined by double-immunoflourescence staining. Satellite cells and regenerating fibers were significantly increased in s-IBM similar to other inflammatory myopathies and correlated with the intensity of inflammation (R > 0.428). Expression of MyoD, visualizing activated satellite cells and proliferating myoblasts, was lower in s-IBM compared to polymyosits. In contrast, Myogenin a marker of myogenic cell differentiation was strongly up-regulated in s-IBM muscle. The microvascular architecture in s-IBM was distorted, although the capillary density was normal. Notably, CD34+ hematopoietic cells were significantly increased in the interstitial compartment. Our findings indicate profound myo-endothelial remodeling of s-IBM muscle concomitant to inflammation. An altered expression of myogenic regulatory factors involved in satellite cell activation and differentiation, however, might reflect perturbations of muscle repair in s-IBM.
Keywords: Sporadic inclusion body myositis, Myogenesis, Satellite cells, Microvascularization
1. Introduction
Sporadic inclusion body myositis (s-IBM) is an acquired myopathy that occurs mostly in patients above the age of 50 years [1]. Recently, the prevalence rate of s-IBM, age and sex-adjusted to the US census count in 2000, was estimated at 7.06 cases per 100,000 [2]. The disease manifests with progressive asymmetric weakness and atrophy of proximal and distal limb muscles [3] that are resistant to immunosuppressive treatment [4]. Current concepts on the underlying pathogenic mechanisms of s-IBM have focused on the unique combination of endomysial inflammation [5,6] and myo-degeneration related to impaired degradation and abnormal aggregation of amyloid and related proteins [7] that delineate s-IBM from other inflammatory myopathies (IM) [8]. The understanding of triggering events and pathogenic pathways that confer muscle fiber injury in s-IBM, however, remains incomplete [9].
Maintenance and repair of skeletal muscle relies mainly on the proliferative potential of satellite cells (SCs), a distinct population of committed myogenic progenitors also called adult muscle stem cells, that reside in sublaminal cell niches attached to mature myofibers [10]. In adult muscle, SCs represent approximately 5% of the total myonuclei [11,12] and remain in a quiescent state expressing the paired box transcription factor Pax7, necessary for their maintenance during postnatal life [13]. Muscle damage activates SCs to proliferate, which subsequently differentiate and form fusion-competent myoblasts in order to repair or replace damaged muscle fibers [11,14]. This highly coordinated myogenic pathway is controlled by myogenic regulatory factors Myf5, Mrf4, MyoD, and Myogenin, a group of nuclear transcription factors, which are sequentially expressed during muscle regeneration [15]. Following myoblast fusion, emerging muscle fibers transiently up-regulate developmental proteins such as embryonic and neonatal myosin heavy chains [15], the intermediate filament protein vimentin [16] and neural cell adhesion molecule (NCAM) that is widely expressed in SCs and regenerating as well as denervated muscle fibers [17]. Finally, maturation and functional re-integration of newly regenerated fibers critically depend on the reorganization of supporting structures in the extracellular compartment [15] and sufficient revascularization at the site of injury [18].
Muscle repair in s-IBM is unable to prevent progressive loss of muscle fibers, although induction of myogenesis has been indicated by the observation of an increased number of Pax7+ satellite cells [19] and regenerating fibers re-expressing developmental molecules in muscle biopsies from a small number of s-IBM patients [16,20]. In vitro studies, in contrast, demonstrated a reduced proliferation rate of s-IBM myoblasts versus age-matched controls and the authors proposed that a defective regeneration of s-IBM muscle might be a contributory factor to the complex pathophysiology of the disease [21]. The purpose of the present study was to explore the in situ expression of myogenic regulatory factors, the frequency and distribution of regenerating fibers and the pattern of microvascularisation in muscle biopsies from a large series of patients in order to analyze potential perturbations of the myogenic program and consecutive tissue remodeling in s-IBM skeletal muscle in an in vivo context. Results were compared with polymyositis and dermatomyositis, muscular dystrophies and normal aged muscle and were related to the age of patients, duration of the disease and to the extent of inflammation.
2. Materials and methods
2.1. Patients and biopsy specimens
Medical and pathological records from all patients with clinically suspected s-IBM who were seen at the Reference Center for Neuromuscular Diseases, Institut de Myologie, Hôpital Pitié-Salpetrière [4] and the Division for Neuromuscular Diseases at the Department of Neurology, Innsbruck Medical University between 1999 and 2008 were retrospectively reviewed. All patients had a muscle biopsy for diagnostic purposes after written informed consent. From 39 patients (19 females, 20 males) who fulfilled diagnostic criteria of definite s-IBM frozen muscle tissue stored at −80 °C was available for further analysis. Age at biopsy and duration of disease did not differ between male and female patients. Clinically, a typical proximal and distal asymmetric limb weakness was present in 34 patients, while 4 patients manifested with purely proximal and 1 patient with purely distal motor deficits at the time of biopsy; 21 patients developed dysphagia during the course of the disease. Eleven patients had received a treatment with either low-dose steroids alone (n = 5) or combined with azathrioprine or methotrexate (n = 6) before biopsy. The definitive diagnosis of s-IBM was based on established clinico-pathological criteria [5,22] and sarcoplasmic immunoreactivity for b-amyloid, phosphorylated tau, p62 [23] or TDP-43 protein [24] within >1% of muscle fibers. For comparison with other inflammatory myopathies, muscle biopsies from patients referred to the Reference Center for Neuromuscular Diseases, Institut de Myologie, Hôpital Pitié-Salpetrière who displayed treatment-responsive proximal limb muscle weakness and fulfilled immunopathological criteria for dermatomyositis (DM, n = 13) or polymyositis (PM, n = 13) as proposed by Dalakas and Hohlfeld [25] were analyzed. In addition, frozen muscle tissue from muscular dystrophies (MD, n = 10; 3 dysferlinopathies, 3 calpainopathies, 1 Bethlem myopathy, 3 unspecified cases of limb girdle MD) as well as 10 individuals, who underwent a muscle biopsy for diagnostic work-up of myalgia or fatigue but had no evidence of a myopathy, was retrieved from the tissue bank of the Unité de Morphologie Neuromusculaire, Institut de Myologie, and included in the study for control purposes. Demographic data of patients and controls are summarized in Table 1. The study was approved by the local ethics committee of the Medical University of Innsbruck, Austria (UN 3233_LEK) and the Hôpital Pitié-Salpêtrière, Paris, France.
Table 1.
Demographic data of patients.
| Disease | n | Median age at biopsy | Range | F/M | Median duration | Range |
|---|---|---|---|---|---|---|
| sIBM⁎ | 39 | 67 y | 40–89 y | 19/20 | 54 m | 6–198 m |
| PM | 13 | 43 y | 16–72 y | 11/2 | 5 m | 1–108 m |
| DM | 13 | 44 y | 17–79 y | 9/4 | 4 m | 1–8 m |
| MD | 10 | 43 y | 26–77 y | 7/3 | ||
| Co | 10 | 64 y | 55–74 y | 5/5 | ||
| Total | 85 |
n = number of patients, y = years, m = months.
Eleven patients with sIBM received a treatment with low-dose steroids (n = 5) or steroids combined with azathrioprine or methotrexate (n = 6) before biopsy. PM and DM patients had proximal limb weakness of less than 6 months duration with improvement after steroids, muscle biopsies fulfilled criteria of Dalakas and Hohlfeld [25].
2.2. Histological, enzyme histochemical, and immunohistochemical analyses
Muscle specimens from s-IBM patients processed for Gomori-Trichrome and Cytochrome-C-Oxidase reactions and Congo-red stain were re-examined for the presence of rimmed vacuoles, COX-negative fibers and Congo-red positive inclusions. For immunohistochemistry, sections were re-cut from stored frozen muscle (−80 °C), which comprised deltoid, biceps brachii and quadriceps muscles in most patients; in several s-IBM patients biopsy was performed from extensor muscles of the forearm (n = 8) or tibialis anterior muscle (n = 5). 8 mm thick cryo-sections were air-dried and fixed either in 4% formaldehyde (for MyoD, Myogenin, and b-amyloid) at room temperature or in cold acetone at −20 °C (for all other primary antibodies) for 10 min each. After washing in phosphate buffered saline (PBS), sections were incubated with 10% normal goat serum (G9023; Sigma) in antibody diluent (S3022; Dako) for 30 min to minimize unspecific binding. Primary antibodies (all mouse monoclonal; except against TDP-43 and p62: rabbit polyclonal) were directed against MHC-I (1:1000, Dako), CD4 (1:500, Dako), CD8 (1:200, Dako), CD68 (1:1000; Dako), phosphorylated tau (SMI-31; 1:1000; Covance), beta-amyloid (β-A4; 1:50; Dako), TDP-43 (1:2000; Proteintech Group), p62 (1:100; Santa Cruz Biotech), neuronal cell adhesion molecule (NCAM) (CD56; clone 123C3.D5; Ventana medical systems), vimentin (clone 3B4; Ventana medical systems), neonatal myosin (clone WB-MHCn; 1:20; Novocastra), Pax7 (clone Pax7; 1:200; R&D systems), MyoD (clone 5.8A; 1:100; Becton Dickinson (BD) Pharmingen) and Myogenin (clone F5D; 1:400; BD Pharmingen). Staining for MyoD, Myogenin and beta-amyloid was performed manually with incubation of primary antibodies over-night at 4 °C. Other stains were done with an automated immunostainer (BenchMark XT, Ventana medical systems). Binding of primary antibodies was detected with a peroxidase reaction and visualized with 3,3′-diaminobenzidine as chromogen (Dako Real™ Detection System (K5001; Dako) for manual stains; secondary reagents from Ventana medical systems for automated stains). For control purposes a mouse IgG1 isotype control (BD Pharmingen) was used instead of primary monoclonal mouse antibodies. Total IgG from rabbit serum (Sigma) was used to control for non-specific staining of polyclonal rabbit antibodies.
2.3. Fluorescence immunostaining
Density of capillaries and number of mononuclear interstitial cells positive for CD34, a marker expressed on mature endothelial cells as well as endothelial and hematopoietic progenitor cells [26], were assessed in 6 representative cases from each disease group and controls. Acetone-fixed cryo-sections were blocked with avidin/biotin blocking reagent (SP-2001, Vector Laboratories) for 15 min, briefly rinsed with PBS, exposed to 10% normal goat serum for 30 min and then incubated overnight at 4 °C with mouse monoclonal anti-CD34 (1:50; BD Pharmingen) and rabbit polyclonal anti-laminin (1:400; Dako). Further, sections from patients with s-IBM and PM and from controls were incubated overnight with mouse monoclonal anti-NCAM (IgG1; 1:20; Monosan) and rabbit polyclonal anti-TDP-43 (1:1000; Proteintech Group). Subsequently, sections were incubated with Alexa Fluor (AF) 555 goat-anti-mouse IgG (L + H) and AF 488 goat-anti-rabbit IgG (L + H) for 60 min at room temperature (all diluted 1:400; Invitrogen). Purified mouse IgG1 (BD Pharmingen) was included as an isotype control instead of primary monoclonal antibodies and total IgG from rabbit serum (Sigma) was used as control for polyclonal antibodies. Double-stained samples were covered with Vectashield mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI) (H-1200; Vector Laboratories) and analyzed using a flourescence microscope (Leica DFC300 FX).
2.4. Quantification
In order to examine a relationship between inflammation and myogenesis, the percentage of MHC-I positive muscle fibers and the extent of inflammatory cell infiltration were evaluated semiquantitatively on the entire cross-sectional area from each muscle biopsy; in all disease groups the diameter of cross sections varied between 5–10 mm. For MHC-I, grades 1–5 were applied when it was expressed on the sarcolemma of <10% (=1); 10–25% (=2); 25–75% (=3); 75–95% (=4), and >95% (=5) of the myofibers. CD4+ and CD8+ T-cell subsets and CD68+ mononuclear cells were graded as: 1 = single, 2 = sparse, 3 = moderate, 4 = dense. The percentage of non-necrotic myofibers immunoreactive for neonatal myosin, vimentin or NCAM and fibers containing cytoplasmic deposits of age-related proteins was determined on serial sections by counting 200 fibers in 20 randomly selected fields with a 40× objective. In analogy, values for Pax7+, MyoD+ and Myogenin+ nuclei were obtained as a percentage of the total myonuclei. Numbers of CD34+ capillaries and CD34+ interstitial mononuclear cells per mm2 were quantified in double-stained sections (CD34+/laminin) using Metavue image analysis software (Universal imaging, Downington, PA). Each biopsy was evaluated on coded sections by two independent researches blinded to the diagnosis; an average of the results was calculated and entered into statistical analysis.
2.5. Statistical analysis
Data are expressed as median values with interquartile range. Between-group comparisons of non-parametric data were done with Kruskall–Wallis and Dunn’s multiple comparison post hoc tests. P values < 0.05 were considered statistically significant. Spearmen bivariate correlation analysis was performed to identify interdependence of variables. The influence of age, disease duration, sex and pre-treatment on markers of regeneration in s-IBM muscle was analyzed using a general multivariate model after normalization of data. Statistical analysis was performed using SPSS (release 18.0, SPSS Inc., USA).
3. Results
3.1. Characteristics of s-IBM muscle biopsies
All patients fulfilled the Griggs’ criteria for definite s-IBM by the presence of endomysial inflammation and mononuclear cell invasion of non-necrotic muscle fibers, vacuolated muscle fibers and intracellular deposits of amyloid or related proteins (detected by electron microscopy or immunohistochemical staining for b-amyloid, phosphorylated tau, p62 or TDP-43 proteins), illustrated in Fig. 1A–C. The degree of myo-pathological alterations, i.e. frequency of rimmed vacuoles and congophilic deposits, extent of muscle fiber atrophy and endomysial fibrosis as well as intensity of inflammation varied considerably between individual patients. Evaluation of the percentage of MHC-I expressing muscle fibers and the extent of endomysial inflammatory infiltration by CD4+ T-cells, CD8+ T-cells and CD68+ monocytes/macrophages in s-IBM in comparison to other disease groups and controls is shown in Table 2.
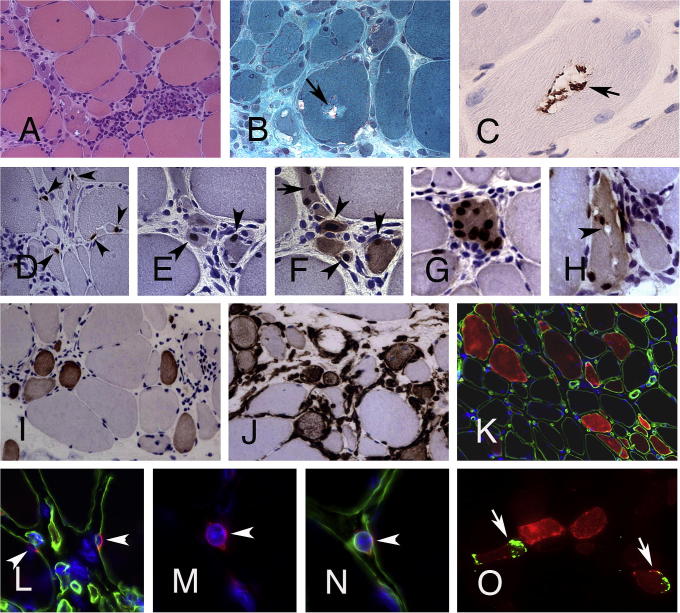
Fig. 1.
Features of myo-regeneration in s-IBM. (A) Inflammatory lesion in s-IBM with numerous small-sized muscle fibers (H&E, original magnification (OM) 200×). (B) Muscle fiber with rimmed vacuoles (Gomori Trichrome, OM 200×). (C) TDP-43+ deposits within a vacuole. (D) Numerous Pax7+ satellite cells (arrowheads) are closely attached to muscle fibers of different sizes (OM 400×). (E, F) Several small-diameter muscle fibers within an inflammatory lesion contain MyoD+ (E) and/or Myogenin+ (F) nuclei (arrowheads). (G) Muscle fiber with central Myogenin+ nuclei. (H) Abnormal fiber with Myogenin+ nuclei and vacuolization of the cytoplasm (arrowhead) (C–H, OM 400×). (I, J) Numerous small-diameter muscle fibers in inflammatory lesions in s-IBM express markers of early regeneration e.g. neonatal myosin (I) and vimentin (J) (OM 200×). (K) A high proportion of muscle fibers of different sizes in s-IBM muscle stains positive for NCAM (OM 200×). (L) Sublaminar NCAM+ satellite cells and (M, N) mononuclear NCAM+ myogenic cell covered by its own basal lamina (arrowhead); (K–N, double immunoflourescence for NCAM (red) and laminin (green), Dapi (blue) for nuclei; L–N OM 630×). (O) NCAM+ fibers (red) in s-IBM with TDP-43+ deposits (green, arrowheads) (double-immunoflourescence for NCAM (red) and TDP-43 (green), OM 400×).
Table 2.
Semiquantitative analysis of MHC-I expression and inflammatory infiltration.
| Median (Range) | s-IBM | PM | DM | MD | C | P-value# |
|---|---|---|---|---|---|---|
| MHC-I | 4 (1–5) | 4 (1–5) | 3 (3–5) | 1 (0–2) | 0 (0–1) | <0.0001 |
| CD8 | 2 (1–4) | 2 (1–3) | 1 (1–2)⁎ | 1 (0–1) | 0 (0–1) | <0.0001 |
| CD4 | 2 (1–4) | 3 (2–4) | 2 (1–2) | 0.5 (0–2) | 0 (0–1) | <0.0001 |
| CD68 | 3 (2–4) | 3 (2–4) | 2 (1–3) | 1.5 (1–3) | 1 (0–1) | <0.0001 |
Table 2 shows the extent of sarcolemmal MHC-I expression and endomysial infiltration by CD8+ and CD4+ T-cells and CD68+ monocytes/macrophages in muscle biopsies of patients and controls.
P-values determined by Kruskal–Wallis-test indicate significant differences among compared groups.
Post-hoc analysis showed no differences among inflammatory myopathies except for values of CD8+ T-cells that are significantly lower in DM versus s-IBM (p = 0.002) and PM (p = 0.022).
3.2. Expression of myogenic regulatory factors and signs of myo-regeneration
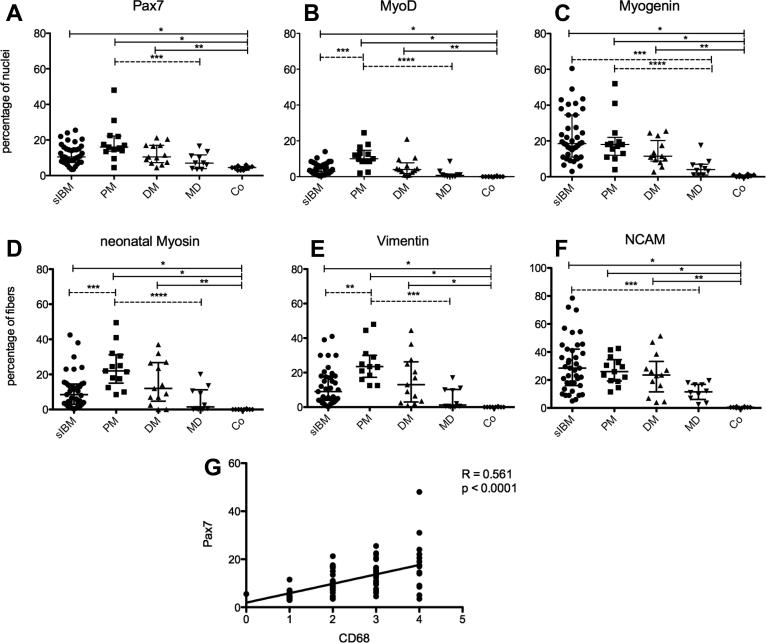
All IM subtypes displayed significantly increased numbers of Pax7+ satellite cells and nuclei expressing MyoD and Myogenin in comparison to controls (Fig. 2A–C). Most Pax7+ satellite cells were closely attached to muscle fibers (Fig. 1D) and were particularly numerous at sites of inflammation, although they were also found to be scattered throughout the specimen. Nuclear expression of MyoD and Myogenin was detectable in small round or spindle-shaped myogenic cells and small-diameter muscle fibers that preferentially clustered in the vicinity of inflammatory lesions (Fig. 1E–G). Median values of Pax7+ SCs and Myogenin+ nuclei did not differ between IM subtypes, but MyoD+ nuclei were significantly less numerous in s-IBM than in PM (p = 0.016, Fig. 2B). The lower frequency of MyoD+ nuclei in s-IBM contrasted with an excessive increase of Myogenin+ nuclei (Fig. 2C). Unlike PM, Myogenin+ nuclei in s-IBM were not confined to sites of active regeneration but were also seen in areas with advanced fibrosis within both atrophic and hypertrophic fibers, which occasionally displayed a vacuolization of the cytoplasm (Fig. 1H).
Fig. 2.
(A–C) Percentage of nuclei expressing MFRs. Plots show median values with interquartile range. Continuous bars show significant differences between disease groups and controls, dotted bars demonstrate variations between disease groups. (A) Pax7+ SCs, ∗p < 0.0001; ∗∗p = 0.003; ∗∗∗p = 0.016. (B) MyoD+ nuclei, ∗p < 0.0001; ∗∗p = 0.001; ∗∗∗p = 0.016; ∗∗∗∗p < 0.0001. (C) Myogenin+ nuclei, ∗p < 0.0001; ∗∗p = 0.008; ∗∗∗p = 0.001; ∗∗∗∗p = 0.018. (D–F) Percentage of muscle fibers expressing markers of regeneration. (D) Neonatal myosin+ fibers, ∗p < 0.0001; ∗∗p = 0.001; ∗∗∗p = 0.042, ∗∗∗∗p = 0.003. (E) Vimentin+ fibers, ∗p < 0.0001; ∗∗p = 0.031; ∗∗∗p = 0.001. (F) NCAM+ fibers, ∗p < 0.0001; ∗∗p = 0.001; ∗∗∗p = 0.024. (G) Significant correlation between the frequency of CD68+ mononuclear cells and Pax7+ SCs (R = 0.561).
In accordance with an increased expression of myogenic regulatory factors, the number of regenerating fibers expressing neonatal myosin, vimentin or NCAM was highly elevated in IMs compared to controls (p < 0.0001, Fig. 2D–F). Notably, early regenerating neonatal myosin+ and vimentin+ muscle fibers were numerous in s-IBM muscle in areas of inflammation (Fig. 1I and J), but the number diminished in areas of fiber atrophy and endomysial fibrosis resulting in overall lower median values of early regenerating fibers in s-IBM compared to PM (Fig. 2D and E). NCAM was widely expressed by small-diameter, normal appearing or hypertrophic muscle fibers (Fig. 1K) as well as sublaminar SCs (Fig. 1L) and mononuclear myogenic cells (Fig. 1M and N). NCAM+ fibers in s-IBM muscle excessively accumulated with advanced myo-pathological alterations and occasionally displayed sarcoplasmic deposits of TDP-43 (Fig. 1O).
3.3. Clinico-pathological associations
The satellite cell number, expression of myogenic regulatory factors and frequency of regenerating fibers significantly correlated with the percentage of MHC-I positive muscle fibers and the extent of inflammatory cell infiltration in all disease groups (R for all variables > 0.428; p < 0.0001, exemplified for Pax7 and CD68 in Fig. 2G). In s-IBM, expression of myogenic regulatory factors and markers of regeneration was not related to the degree of abnormal protein accumulation. Disease duration or age of s-IBM patients at the time of biopsy had no influence on any of the variables. Muscle from s-IBM patients who had received immunomodulatory treatment before biopsy showed a lower percentage of MHC-I positive muscle fibers (p = 0.019) and reduced infiltration by CD8+ T-cells (p = 0.026), while values of CD4+ T-cells and CD68+ mononuclear cells did not differ from untreated patients. Pretreatment had no effect on expression of myogenic regulatory factors and markers of myo-regeneration or deposition of abnormal proteins.
3.4. Microvascularization
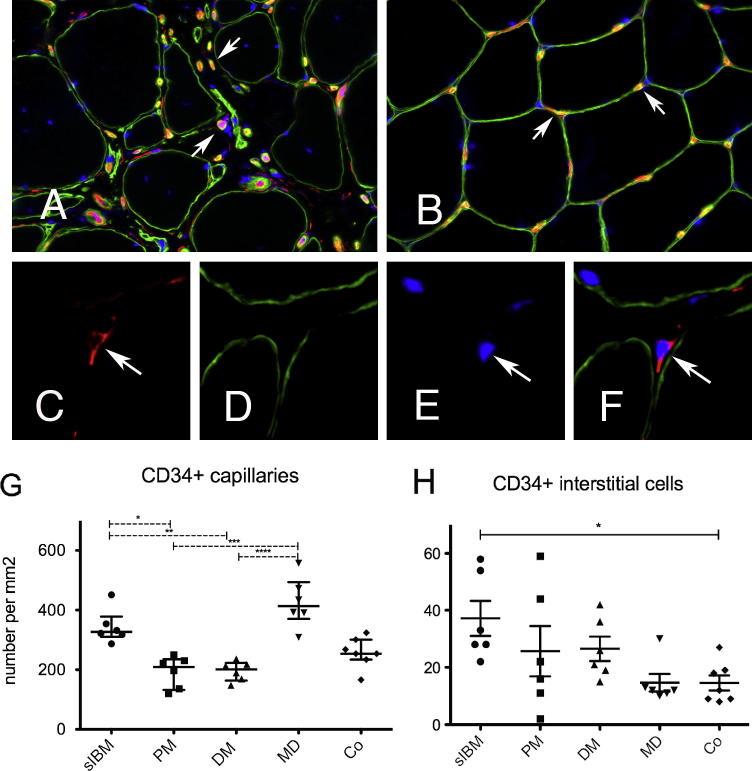
Visualization of microvessels in sections double-stained for CD34+ and laminin revealed marked irregularities of the capillary architecture in IMs. In s-IBM, the median number of capillaries per mm2 was not reduced (Fig. 3A and G), but the normal organization of 3–5 capillaries surrounding an individual muscle fiber (as seen in controls, Fig. 3B) was severely disrupted. Moreover, the distance between muscle fibers and capillaries was increased at sites of endomysial fibrosis. Morphometric analysis of CD34+ capillaries per mm2 showed similar results in s-IBM, MD and controls, while the capillary density in s-IBM and MD was higher than in PM and DM as shown in Fig. 3G. CD34+ cells within the interstitial compartment (Fig. 3C and F) were significantly increased in s-IBM muscle compared to controls (Fig. 3H, p = 0.034).
Fig. 3.
CD34+ capillaries and CD34+ interstitial cells in s-IBM: (A) Disorganization of the capillary architecture in s-IBM (OM 400×). (B) Regular capillary network in a healthy individual with 3–5 capillaries (arrows) surrounding a single fiber (OM 400×). (C) CD34+ mononuclear cell within the interstitial space (red, arrow, OM 630×). (D) Anti-laminin stain visualizing the basal lamina of muscle fibers (green). (E) Cell nucleus visualized by Dapi (arrow), (F) merged image (OM 630×). (G) Density of CD34+ capillaries per mm2, ∗p < 0.028; ∗∗p = 0.026; ∗∗∗p = 0.002; ∗∗∗∗p = 0.002. (H) CD34+ interstitial cells per mm2, ∗p < 0.034.
4. Discussion
Autoimmune T-cell mediated myo-cytotoxicity [5] and myofiber degeneration related to intracellular accumulation of multi-protein aggregates [7] are considered to be key mechanisms in the multifactorial pathogenesis of s-IBM. Muscle fiber damage activates compensatory myo-regeneration [14], but the in vitro proliferative activity of myoblasts derived from s-IBM muscle has been shown to be reduced suggesting that limitations in muscle repair mechanisms might contribute to progressive muscle wasting in s-IBM [21]. In the present study we have investigated in detail the frequency, distribution and morphological appearance of myogenic progenitor cells and regenerating fibers in their in vivo context in s-IBM muscle and we have explored potential alterations related to inflammation or degeneration. Quantitative analysis revealed significantly elevated numbers of Pax7+ SCs and myogenic cells containing MyoD+ and Myogenin+ nuclei in muscle specimens from s-IBM patients indicating enhanced myo-regenerative activity in s-IBM compared with age-matched healthy individuals. In comparison with other IMs we found a similar number of Pax7+ SCs in s-IBM, whereas the number of MyoD+ myogenic cells was significantly less frequent than in PM. MyoD regulates the activation of quiescent Pax7+ SCs and most of Pax7/MyoD co-expressing cells subsequently proceed to proliferation by down-regulation of Pax7, whereas some SCs maintain Pax7 and return to quiescence for replenishment of the resting SC pool [27]. Our observations argue against a depletion of the Pax7+ SC pool in s-IBM as a potential cause of impaired regeneration. Low expression of MyoD in s-IBM muscle, however, might indicate a delay and/or decrease of SC activation and myoblast proliferation, which may occur as a consequence of replicative senescence of SCs after repeated cycles of degeneration and regeneration [28]. This hypothesis is in agreement with the demonstration of significant telomere shortening and a reduced proliferation rate of sIBM myoblasts in vitro [21]. During muscle repair commitment of MyoD+ myoblasts to differentiation is regulated by the transient expression of Myogenin, which is down-regulated shortly after cells become incorporated into multinucleated muscle fibers [15]. In s-IBM, we have observed persistent nuclear expression of myogenin in numerous, both small and large diameter muscle fibers as well as vacuolated muscle fibers. These observations might reflect an impairment of both the differentiation of myoblasts [29] as well as maturation of emerging muscle fibers that are apparently vulnerable to undergo vacuolar degeneration.
Consistent with the expression pattern of myogenic regulatory factors in s-IBM, the frequency of muscle fibers expressing markers of regeneration remarkably increased in parallel with the extent of inflammation providing further evidence for a substantial attempt of spontaneous muscle repair in s-IBM, as suggested by previous reports [16,20]. In areas with advanced fiber atrophy and fibrosis, however, neonatal myosin+ and vimentin+ early regenerating fibers decreased, while the number of NCAM+ fibers remained high. NCAM, a cell-surface glycoprotein abundant in embryonic muscle, is lost during development but will be re-expressed in both regenerating and denervated muscle fibers [17]. Thus, the high proportion of NCAM+ fibers in s-IBM muscle might result from a delay or arrest of maturation of the newly regenerated fibers which maintain NCAM expression and display an increased susceptibility to protein aggregation as shown by the accumulation of TDP-43 protein in these fibers. Additionally, modifications of innervation [30] and/or a reaction to cellular stress [31] may contribute to the pronounced increase of NCAM+ fibers in s-IBM.
Pax7+ SCs, MyoD+ and Myogenin+ myogenic cells and early regenerating fibers preferentially co-localized with inflammatory lesions and we found a close correlation between the grade of MHC-I expression and inflammatory cell infiltration and the extent of the expression of myogenic regulatory factors as well as the frequency of regenerating fibers in all disease groups included in our study emphasizing an influence of inflammation on the process of myo-regeneration. Muscle injury evokes an influx of CD68+ macrophages engaged in the removal of cellular debris. Beside propagation of inflammation and cell lysis, experimental observations suggest that soluble factors released by CD68+ macrophages are also involved in the modulation of transcriptional activities of regenerative muscle cells [32]. Pro-inflammatory cytokines such as TNF-α, IL-1β and IL-6, that are strongly expressed in inflammatory myopathies [33], were reported to exert different effects on myogenesis [32]. While TNF-α and IL-6 were shown to promote proliferation of myoblasts in the early phase of regeneration [34,35], both TNF-α and IL-6 as well as IL-1β inhibit fusion of myogenic cells and transition to the phase of terminal differentiation via multiple pathways such as activation of NF-κB or impairment of insulin-like growth factor induced protein synthesis in myoblasts [35,36]. Infiltration by CD68+ macrophages was a prominent feature in all IM subtypes studied, but the effects on muscle repair may vary depending on the stage of muscle injury. While CD68+ macrophages stimulate the activation and proliferation of SCs in the acute stage of muscle damage, experimental prolongation of the presence of CD68+ macrophages is associated with a delay of myogenic differentiation [32]. In this sense, persistence of CD68+ macrophages in chronic lesions of s-IBM may exert inhibitory effects on the late phase of muscle regeneration and could in part explain the increased expression of Myogenin we have observed in s-IBM muscle.
Sufficient vascularization ensuring oxygen and nutrient supply is mandatory for the integration and survival of newly generated muscle fibers [15]. SCs organize close to capillaries suggesting a functional relationship between myogenesis and vasculogenesis [37]. Analyzing the density and distribution of capillaries we demonstrate pronounced irregularities of the microvascular architecture in s-IBM muscle, although median numbers of microvessels per square millimeter did not differ significantly from normal individuals. Sites of inflammation and muscle regeneration were accompanied by a focal increase of capillaries indicating vascular neo-formation in these areas. It has been shown that sprouts of endothelial cells emanating from pre-existing vessels may incorporate resident or circulating endothelial progenitors cells [38], but the precise mechanisms have not been elucidated. In s-IBM, the number of mononuclear CD34+ cells within the interstitial space was increased, in line with a previous study suggesting that circulating endothelial progenitor cells can colonize skeletal muscle in inflammatory conditions to support coordinated myo-vasculogenesis [19]. In areas with endomysial fibrosis, however, capillaries appeared reduced and were detached from muscle fibers, which might lead to insufficient perfusion and metabolic disturbance, hypoxia and oxidative stress in advanced stages of muscle pathology in s-IBM.
In conclusion our study demonstrates significantly enhanced myo-regenerative processes in s-IBM concomitant to inflammation and vascular reorganization. Although Pax7+ satellite cells may increase to a similar extent as in other inflammatory myopathies, an altered expression of myogenic regulatory factors that govern activation and differentiation of myogenic progenitor cells as well as morphological abnormalities of regenerating fibers suggest an impairment of the complex regulation of myogenesis and coordinated tissue repair in s-IBM.
Acknowledgements
This study (Project Grant No. V108-B05) was funded by the Austrian Science Fund (FWF). Further financial support was provided by the Association Française contre les Myopathies (AFM) MyoAge (EC 7th FP, contract 223576) ANR-Genopath In-A-Fib.
Prof. Valerie Askanas is kindly acknowledged for providing the protocole for p62 stain. We thank Prof. Markus Reindl for advices concerning statistical analysis.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.nmd.2012.09.003.
Appendix A. Supplementary data
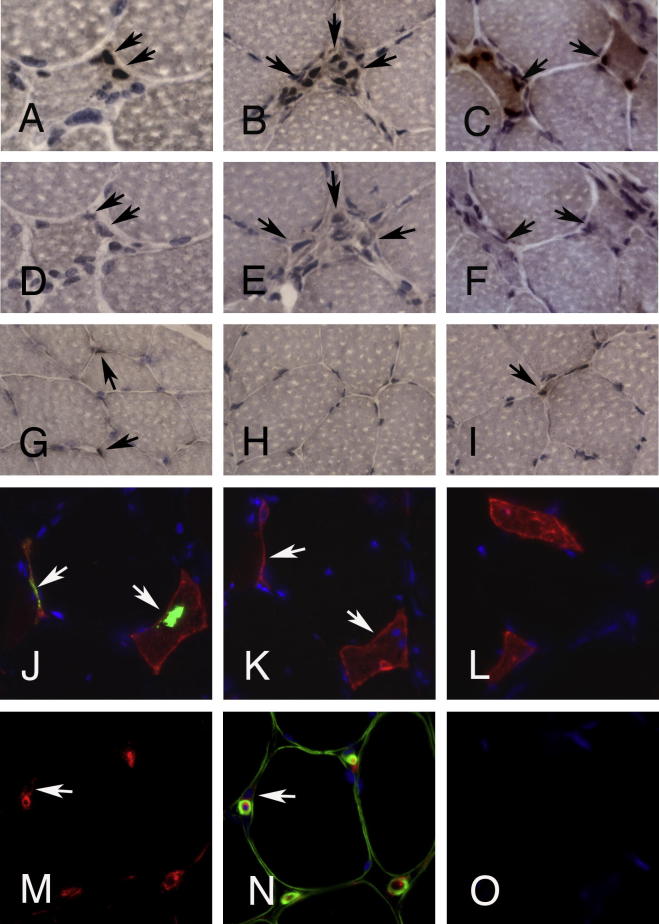
Supplementary Figure 1.
Control stainings. (A–F) Staining for myogenic regulatory factors and corresponding IgG1 isotype controls on consecutive sections in a case with LGMD 2B (dysferlinopathy). (A) Pax7+ satellite cells attached to a muscle fiber (arrows; in (D) same fiber on the consecutive section exposed to IgG1 isotype control). (B) Mononuclear myogenic cells expressing MyoD (arrows). (C) Regenerating muscle fibers with Myogenin+ nuclei (arrows). (E and F) Corresponding IgG1 isotype controls on consecutive sections. (G–I) Muscle from a control individual without myopathy yielding few Pax7+ satellite cells (G, arrows) and a single Myogenin+ cell (H, arrow), while MyoD is not expressed (I). (A–F) original magnification 63×; (G–I) OM 40×. (J) Double-immunoflourescence stain for TDP-43 (green sarcoplasmic deposits indicated by arrows) and NCAM (red) in sIBM. (K) Consecutive section exposed to total IgG from rabbit serum instead of anti-TDP-43 antibody (both used at a concentration of 45 μg/150 ml) and NCAM excluding non-specific binding of the polyclonal antibody. (L) NCAM+ fibers in PM are negative for TDP-43 (double-immunoflourescence for TDP-43 and NCAM, Dapi for nuclei, OM 63×). (M) Anti-CD34 stain showing endothelial cells of capillaries and a CD34+ mononuclear cell (arrow) in case with PM. (N) Double stain for CD34 and laminin demonstrates the interstitial position of the CD34+ mononuclear cell, while capillaries are surrounded by a laminin+ basement membrane (double-immunoflourescence for CD34 and laminin, Dapi for nuclei, OM 63×). (O) Consecutive section exposed to IgG isotype control (Dapi for nuclei, OM 63×).
References
- 1.Needham M., Mastaglia F.L. Inclusion body myositis: current pathogenetic concepts and diagnostic and therapeutic approaches. Lancet Neurol. 2007;6:620–631. doi: 10.1016/S1474-4422(07)70171-0. [DOI] [PubMed] [Google Scholar]
- 2.Wilson F.C., Ytterberg S.R., St Sauver J.L., Reed A.M. Epidemiology of sporadic inclusion body myositis and polymyositis in Olmsted County, Minnesota. J Rheumatol. 2008;35:445–447. [PubMed] [Google Scholar]
- 3.Engel W.K., Askanas V. Inclusion-body myositis: clinical, diagnostic, and pathologic aspects. Neurology. 2006;66:S20–S29. doi: 10.1212/01.wnl.0000192260.33106.bb. [DOI] [PubMed] [Google Scholar]
- 4.Benveniste O., Guiguet M., Freebody J. Long-term observational study of sporadic inclusion body myositis. Brain. 2011;134:3176–3184. doi: 10.1093/brain/awr213. [DOI] [PubMed] [Google Scholar]
- 5.Dalakas M.C. Sporadic inclusion body myositis – diagnosis, pathogenesis and therapeutic strategies. Nat Clin Pract Neurol. 2006;2:437–447. doi: 10.1038/ncpneuro0261. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg S.A. Inflammatory myopathies: evaluation and management. Semin Neurol. 2008;28:241–249. doi: 10.1055/s-2008-1062267. [DOI] [PubMed] [Google Scholar]
- 7.Askanas V., Engel W.K., Nogalska A. Inclusion body myositis: a degenerative muscle disease associated with intra-muscle fiber multi-protein aggregates, proteasome inhibition, endoplasmic reticulum stress and decreased lysosomal degradation. Brain Pathol. 2009;19:493–506. doi: 10.1111/j.1750-3639.2009.00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalakas M.C. Review: an update on inflammatory and autoimmune myopathies. Neuropathol Appl Neurobiol. 2011;37:226–242. doi: 10.1111/j.1365-2990.2010.01153.x. [DOI] [PubMed] [Google Scholar]
- 9.Hohlfeld R. Update on sporadic inclusion body myositis. Brain. 2011;134:3141–3145. doi: 10.1093/brain/awr258. [DOI] [PubMed] [Google Scholar]
- 10.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhawan J., Rando T.A. Stem cells in postnatal myogenesis: molecular mechanisms of satellite cell quiescence, activation and replenishment. Trends Cell Biol. 2005;15:666–673. doi: 10.1016/j.tcb.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Renault V., Thornell L.E., Eriksson P.O., Butler-Browne G., Mouly V. Regenerative potential of human skeletal muscle during aging. Aging Cell. 2002;1:132–139. doi: 10.1046/j.1474-9728.2002.00017.x. [DOI] [PubMed] [Google Scholar]
- 13.Seale P., Sabourin L.A., Girgis-Gabardo A., Mansouri A., Gruss P., Rudnicki M.A. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 14.Wagers A.J., Conboy I.M. Cellular and molecular signatures of muscle regeneration: current concepts and controversies in adult myogenesis. Cell. 2005;122:659–667. doi: 10.1016/j.cell.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 15.Tajbakhsh S. Skeletal muscle stem cells in developmental versus regenerative myogenesis. J Intern Med. 2009;266:372–389. doi: 10.1111/j.1365-2796.2009.02158.x. [DOI] [PubMed] [Google Scholar]
- 16.Winter A., Bornemann A. NCAM, vimentin and neonatal myosin heavy chain expression in human muscle diseases. Neuropathol Appl Neurobiol. 1999;25:417–424. doi: 10.1046/j.1365-2990.1999.00178.x. [DOI] [PubMed] [Google Scholar]
- 17.Illa I., Leon-Monzon M., Dalakas M.C. Regenerating and denervated human muscle fibers and satellite cells express neural cell adhesion molecule recognized by monoclonal antibodies to natural killer cells. Ann Neurol. 1992;31:46–52. doi: 10.1002/ana.410310109. [DOI] [PubMed] [Google Scholar]
- 18.Tamaki T., Uchiyama Y., Okada Y. Functional recovery of damaged skeletal muscle through synchronized vasculogenesis, myogenesis, and neurogenesis by muscle-derived stem cells. Circulation. 2005;112:2857–2866. doi: 10.1161/CIRCULATIONAHA.105.554832. [DOI] [PubMed] [Google Scholar]
- 19.Hollemann D., Budka H., Loscher W.N., Yanagida G., Fischer M.B., Wanschitz J.V. Endothelial and myogenic differentiation of hematopoietic progenitor cells in inflammatory myopathies. J Neuropathol Exp Neurol. 2008;67:711–719. doi: 10.1097/NEN.0b013e31817d8064. [DOI] [PubMed] [Google Scholar]
- 20.Arnardottir S., Borg K., Ansved T. Sporadic inclusion body myositis: morphology, regeneration, and cytoskeletal structure of muscle fibres. J Neurol Neurosurg Psychiatry. 2004;75:917–920. doi: 10.1136/jnnp.2003.018994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morosetti R., Broccolini A., Sancricca C. Increased aging in primary muscle cultures of sporadic inclusion-body myositis. Neurobiol Aging. 2010;31:1205–1214. doi: 10.1016/j.neurobiolaging.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Griggs R.C., Askanas V., DiMauro S. Inclusion body myositis and myopathies. Ann Neurol. 1995;38:705–713. doi: 10.1002/ana.410380504. [DOI] [PubMed] [Google Scholar]
- 23.Nogalska A., Terracciano C., D’Agostino C., King Engel W., Askanas V. p62/SQSTM1 is overexpressed and prominently accumulated in inclusions of sporadic inclusion-body myositis muscle fibers, and can help differentiating it from polymyositis and dermatomyositis. Acta Neuropathol. 2009;118:407–413. doi: 10.1007/s00401-009-0564-6. [DOI] [PubMed] [Google Scholar]
- 24.Salajegheh M., Pinkus J.L., Taylor J.P. Sarcoplasmic redistribution of nuclear TDP-43 in inclusion body myositis. Muscle Nerve. 2009;40:19–31. doi: 10.1002/mus.21386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dalakas M.C., Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971–982. doi: 10.1016/S0140-6736(03)14368-1. [DOI] [PubMed] [Google Scholar]
- 26.Asahara T., Kawamoto A. Endothelial progenitor cells for postnatal vasculogenesis. Am J Physiol Cell Physiol. 2004;287:C572–C579. doi: 10.1152/ajpcell.00330.2003. [DOI] [PubMed] [Google Scholar]
- 27.Zammit P.S., Golding J.P., Nagata Y., Hudon V., Partridge T.A., Beauchamp J.R. Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J Cell Biol. 2004;166:347–357. doi: 10.1083/jcb.200312007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bigot A., Jacquemin V., Debacq-Chainiaux F. Replicative aging down-regulates the myogenic regulatory factors in human myoblasts. Biol Cell. 2008;100:189–199. doi: 10.1042/BC20070085. [DOI] [PubMed] [Google Scholar]
- 29.Weise C., Dai F., Prols F. Myogenin (Myf4) upregulation in trans-differentiating fibroblasts from a congenital myopathy with arrest of myogenesis and defects of myotube formation. Anat Embryol (Berl) 2006;211:639–648. doi: 10.1007/s00429-006-0117-x. [DOI] [PubMed] [Google Scholar]
- 30.McFerrin J., Engel W.K., Askanas V. Impaired innervation of cultured human muscle overexpressing betaAPP experimentally and genetically: relevance to inclusion-body myopathies. Neuroreport. 1998;9:3201–3205. doi: 10.1097/00001756-199810050-00013. [DOI] [PubMed] [Google Scholar]
- 31.Muth I.E., Barthel K., Bahr M., Dalakas M.C., Schmidt J. Proinflammatory cell stress in sporadic inclusion body myositis muscle: overexpression of alphaB-crystallin is associated with amyloid precursor protein and accumulation of beta-amyloid. J Neurol Neurosurg Psychiatry. 2009;80:1344–1349. doi: 10.1136/jnnp.2009.174276. [DOI] [PubMed] [Google Scholar]
- 32.Tidball J.G., Villalta S.A. Regulatory interactions between muscle and the immune system during muscle regeneration. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1173–R1187. doi: 10.1152/ajpregu.00735.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Bleecker J.L., Meire V.I., Declercq W., Van Aken E.H. Immunolocalization of tumor necrosis factor-alpha and its receptors in inflammatory myopathies. Neuromuscul Disord. 1999;9:239–246. doi: 10.1016/s0960-8966(98)00126-6. [DOI] [PubMed] [Google Scholar]; Tews D.S., Goebel H.H. Cytokine expression profile in idiopathic inflammatory myopathies. J Neuropathol Exp Neurol. 1996;55:342–347. doi: 10.1097/00005072-199603000-00009. [DOI] [PubMed] [Google Scholar]; Lepidi H., Frances V., Figarella-Branger D., Bartoli C., Machado-Baeta A., Pellissier J.F. Local expression of cytokines in idiopathic inflammatory myopathies. Neuropathol Appl Neurobiol. 1998;24:73–79. doi: 10.1046/j.1365-2990.1998.00092.x. [DOI] [PubMed] [Google Scholar]
- 34.Li Y.P. TNF-alpha is a mitogen in skeletal muscle. Am J Physiol Cell Physiol. 2003;285:C370–C376. doi: 10.1152/ajpcell.00453.2002. [DOI] [PubMed] [Google Scholar]
- 35.Wang X., Wu H., Zhang Z. Effects of interleukin-6, leukemia inhibitory factor, and ciliary neurotrophic factor on the proliferation and differentiation of adult human myoblasts. Cell Mol Neurobiol. 2008;28:113–124. doi: 10.1007/s10571-007-9247-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Broussard S.R., McCusker R.H., Novakofski J.E. IL-1beta impairs insulin-like growth factor i-induced differentiation and downstream activation signals of the insulin-like growth factor i receptor in myoblasts. J Immunol. 2004;172:7713–7720. doi: 10.4049/jimmunol.172.12.7713. [DOI] [PubMed] [Google Scholar]
- 37.Christov C., Chretien F., Abou-Khalil R. Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol Biol Cell. 2007;18:1397–1409. doi: 10.1091/mbc.E06-08-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grenier G., Scime A., Le Grand F. Resident endothelial precursors in muscle, adipose, and dermis contribute to postnatal vasculogenesis. Stem Cells. 2007;25:3101–3110. doi: 10.1634/stemcells.2006-0795. [DOI] [PubMed] [Google Scholar]