Abstract
Monoclonal antibodies (MAbs) were prepared against toxin-coregulated pili (TCP) isolated from Vibrio cholerae O1 El Tor. Despite their limited bactericidal potential, two MAbs were able to mediate biotype-specific protection against experimental cholera in infant mice. These MAbs were used in immunoblotting studies to assess seroconversion to El Tor TCP following cholera. Clear anti-pilus responses were observed in five of nine patients.
Cholera is caused by the gram-negative bacterium Vibrio cholerae. This noninvasive enteropathogen replicates on the intestinal surface, releasing a potent toxin that elicits a hypersecretory response from the underlying epithelial cells. Pathogenic isolates of V. cholerae produce either of two biotype-associated forms of the toxin-coregulated pilus (TCP), which plays a critical role in colonization (7, 20). Studies with the infant mouse cholera model (IMCM) have shown that this applies regardless of the serogroup (O1 or O139) or biotype (O1 classical or O1 El Tor) of the challenge strain (1, 20, 21). As a corollary, antibodies to TCP are sufficient to mediate protection of infant mice against a challenge with O1 or O139 isolates expressing the homologous pilus type (12, 15, 18, 24).
The IMCM is generally accepted as providing information relevant to human infection. For example, the relative significance of TCP and mannose-sensitive hemagglutinin pili as colonization factors was first elucidated by studies with this model (1, 21) and subsequently confirmed in human volunteer trials (19). The protective efficacy of antibodies to TCP in the IMCM suggests that such antibodies might also be of value in combating the human infection. Experience with enterotoxigenic Escherichia coli (ETEC) strains, which establish an infection very similar to cholera, is encouraging in this regard. Pilus colonization factors have been successfully exploited for vaccine development against ETEC pathogens of either veterinary or clinical significance (5, 9).
The vaccine potential of TCP remains uncertain, however, as these pili may be only weakly immunogenic compared with other virulence determinants such as lipopolysaccharide (LPS) and cholera toxin. Hall et al. (6) performed a retrospective analysis of samples collected from American volunteers and Indonesian cholera patients, seeking evidence of immune responses to TCP following induced or natural infection. None of 15 volunteers infected with a classical strain seroconverted to TCP, while only three of six cholera patients naturally infected with O1 El Tor strains showed marginal responses to pili. However, it is noteworthy that only a minority of the volunteer cohort seroconverted to LPS, despite its recognized immunogenicity. Moreover, the marginal responses of some cholera patients could be viewed as encouraging, given that pili isolated from a classical strain were used to assess immune responses following El Tor disease, thereby precluding detection of antibodies to biotype-restricted epitopes.
We now report a reevaluation of the immunogenicity of TCP in humans. Monoclonal antibodies (MAbs) have been prepared against El Tor TCP and used in immunoblotting analyses of sera collected from cholera patients. Our data indicate that cholera caused by O1 El Tor or O139 strains of V. cholerae induces an immune response to TCP.
O1 strain H1 (El Tor biotype), O139 strain AI-1838, and the respective tcpA mutant strains H1ΔtcpA (24) and AI-1838 tcpA::Kmr (1) were used for isolation of TCP and control antigen preparations. Bacteria were cultured under AKI conditions to promote TCP expression (23), and pili were isolated by a method based on that of Cowell et al. (4). Briefly, bacteria were harvested by centrifugation, washed in phosphate-buffered saline (PBS), and resuspended in PBS-10 mM EDTA. Shear forces were applied by passing the concentrated suspension sequentially through 22-gauge (twice) and then 26-gauge (four times) needles, the TCP-containing supernatants being retained after removal of bacteria (8,500 × g, 4°C, 15 min). After addition of ethanolamine to 0.125 M, the shearate was incubated with stirring at 4°C for 2 h, centrifuged as before, and dialyzed against PBS overnight. Protein was recovered by ammonium sulfate precipitation (4), and the pellet was resuspended in and dialyzed against PBS. Protein concentration was estimated with a bicinchoninic acid protein estimation kit (Pierce, Rockford, Ill.). Immunoblot analyses confirmed the absence of the TcpA pilus subunit from control preparations.
MAbs were produced essentially as described elsewhere (22). Spleen cells harvested from BALB/c mice immunized with a TCP preparation derived from the H1 strain were fused with Sp20 myeloma cells with polyethylene glycol. Hybridoma supernatants were initially screened by enzyme-linked immunosorbent assay (ELISA) against TCP-expressing bacteria of either biotype. Hybridomas producing supernatants with high titers against piliated bacteria but low titers against mutant bacteria were cloned. Resulting supernatants were analyzed by ELISA against TCP and control antigen preparations.
MAbs were examined for their potential to mediate complement-dependent lysis of H1 by using a tube-based assay described elsewhere (2). Since efficient bacteriolysis requires a temperature of 37°C, bacteria were cultured and assayed in AKI at 37°C; preliminary tests indicated that TCP expression was comparable to that seen at 30°C. For comparison, an immunoglobulin G (IgG) fraction of an absorbed rabbit anti-V. cholerae O1 serum was also tested; this reagent is specific for LPS and has complement-dependent lytic potential (2). For each antibody, a lytic endpoint was calculated as that dilution capable of killing 50% of the indicator bacteria (2).
The IMCM was used to assess the protective potential of MAbs to TCP. This was done as described previously (24), with AKI-grown H1 (AKI-H1) as the challenge bacteria. After centrifugation and resuspension in fresh culture medium, the bacteria were counted microscopically and diluted such that each mouse would receive an inoculum comprising ca. 20 50% lethal doses (ca. 2 × 105 bacteria) in 0.1 ml. Aliquots of bacterial suspensions were pretreated (15 min at 30°C) with dilutions of the test antibody and fed to groups of five or six mice; control animals received untreated bacteria. When the last control mouse died, survival data from the treatment groups were used to calculate a protective endpoint for the antibody under test; this 50% protective dose (24) represents the (theoretical) dilution that would protect 50% of the challenged mice.
A colonization experiment was also performed. Aliquots of the challenge suspension (AKI-H1) were pretreated with various MAbs and then fed to separate groups of mice. For comparison, other groups received either untreated bacteria or vibrios pretreated with an anti-LPS IgG fraction of an absorbed rabbit anti-V. cholerae O1 serum (2). The various antibodies were standardized by dilution to ca. 10 50% protective doses/ml of bacterial suspension (which had been diluted such that each animal received ca. 15 50% lethal doses). After 22 h, small intestines were excised and homogenized in PBS and various dilutions were plated to ascertain bacterial persistence.
Paired acute- and convalescent-phase blood samples were obtained from patients with bacteriologically confirmed O1 (El Tor) or O139 cholera as previously described (13). Acute-phase samples were collected 2 days after onset of disease (day 2), while convalescent-phase samples were collected on day 22 or 30. Paired samples were tested in immunoblotting analyses to seek evidence of seroconversion to TCP. Isolated pili or control antigen preparations were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis with precast 14% Novex Tris-glycine gels (Invitrogen). Proteins were transferred to a nitrocellulose membrane, which was then blocked (5% skim milk in PBS), washed, and cut into thin strips to allow screening of multiple patient sera for antibodies to TCP. Membrane strips were incubated with test sera overnight at room temperature; control strips were incubated with MAb 2:13 to denote the position of the TcpA pilin subunit. The membranes were then washed and incubated for a further 2 h with a secondary antibody (a mixture of horseradish peroxidase-conjugated anti-human IgG and IgA or, for control strips, horseradish peroxidase-conjugated anti-mouse IgG). Blots were developed by enhanced chemiluminescence (Boehringer-Mannheim ECL kit; 23). In later studies, binding of primary antibodies to nitrocellulose-bound proteins was performed in the presence of competing TCP or control antigen preparations (at 100 μg/ml) to verify the identity of the putative TcpA band. Preliminary experiments confirmed that at the concentrations used, the TCP preparations, but not the negative control antigens, blocked the recognition of TcpA by MAb 2:13.
Hybridomas obtained after immunization with purified El Tor TCP were initially screened by ELISA for reactivity with TCP-expressing bacteria of the classical or El Tor biotype. Subsequent ELISAs were performed with partially purified TCP preparations isolated from wild-type or (as a control) tcpA mutant vibrios of the El Tor biotype. After cloning, two MAbs (2:13 [IgG1] and 21:6 [IgG3]) that were evidently specific for El Tor TCP were obtained. These reacted strongly with TCP of the El Tor biotype but not with TCP of the classical biotype, as determined by ELISA (Table 1) and immunoblotting (data not shown). In both analyses, previously described MAb 20:2, which is specific for classical TCP (8), was included as a control.
TABLE 1.
Specificity and protective efficacy of MAbs to TCPa
| Antibody | ELISA titer vs:
|
Bactericidal titer vs El Tor V. cholerae | Protective titer vs El Tor V. cholerae | |||
|---|---|---|---|---|---|---|
|
V. cholerae
|
TCP+ antigen | TCP− antigen | ||||
| Classical | El Tor | |||||
| Serumb | 107,000 | 119,000 | 46,000 | 9,000 | 210,000 | 7,500 |
| El Tor MAb 2:13 | <10 | 3,300 | 10,000 | <10 | 440 | 4,300 |
| El Tor MAb 21:6 | <10 | 160 | 850 | <10 | 740 | 6,200 |
| Classical MAb 20:2 | 3,700 | <10 | NDc | ND | <40 | <40 |
ELISAs were performed against TCP-expressing bacteria of the classical or El Tor biotype or against TCP-positive or control (TCP-negative) antigen preparations. Bactericidal and protection tests were performed with TCP-expressing bacteria of the H1 strain. The titers shown are means of two or three estimates.
Serum used in ELISAs was from a mouse immunized with partially purified TCP, whereas for bactericidal and protection assays, it was an IgG fraction of an absorbed rabbit antiserum and was specific for LPS.
ND, not done.
Bactericidal assays revealed that the two MAbs specific for El Tor TCP displayed limited lytic potential (Table 1); even at high concentrations, lysis of a suspension of piliated bacteria was incomplete (data not shown). No activity was detected with MAb 20:2, for which the indicator bacteria were of heterologous biotype, whereas an anti-LPS reagent (prepared from a rabbit antiserum and also IgG) was highly active (Table 1). Both MAbs specific for El Tor TCP (2:13 and 21:6) were protective against a challenge with El Tor bacteria in the IMCM (Table 1). Neither MAb showed any protective potential against a challenge with classical V. cholerae (protective endpoint titers of <40), nor did MAb 20:2 confer protection against an El Tor strain (Table 1). These results are consistent with our earlier data (24) indicating that biotype-restricted pilus epitopes may be more relevant for the induction of protective immunity and therefore more important in the vaccine context. Although spleen cells from a total of six TCP-immunized mice have now been used for hybridoma production, no MAbs recognizing both biotypic forms of TCP have been isolated. It has therefore not been possible to directly evaluate the protective potential of antibodies against shared epitopes.
An experiment was performed to compare the capacities of the various MAbs to inhibit the colonization of TCP-expressing El Tor bacteria. The two MAbs directed against the homologous pilus type, as well as an anti-LPS IgG fraction, were similarly and highly effective. Each achieved an approximate 50-fold reduction in bacterial persistence, compared with the numbers of bacteria recovered from control animals receiving untreated bacteria (Table 2). Of the 15 mice in these three treatment groups, 14 had bacterial recoveries in the range of log10 4.57 to 6.22. In contrast, MAb 20:2 showed no protective effect against the heterologous challenge strain. Bacterial recoveries from these mice were very similar to those seen in controls; the range for the 10 mice in these groups was log10 6.92 to 7.46 (Table 2).
TABLE 2.
Inhibition of colonization by MAbs to TCPa
| Antibody (specificity) | Log10 bacterial recovery |
|---|---|
| MAb 2:13 (El Tor TCP) | 5.48 ± 0.68 (1.7%; P < 0.01) |
| MAb 21:6 (El Tor TCP) | 5.57 ± 0.48 (2.1%; P < 0.05) |
| MAb 20:2 (classical TCP) | 7.18 ± 0.15 (85%; NS) |
| Polyclonal IgG (LPS) | 4.65 ± 1.65b (0.25%; P < 0.01) |
| None | 7.25 ± 0.25 |
Various antibodies were tested for their potential to inhibit the colonization of TCP-expressing bacteria of the El Tor H1 strain. Values show log10 bacterial recoveries (geometric mean ± standard deviation; n = 5) at 22 h. Values in parentheses show percent recovery relative to the control group that received untreated bacteria, followed by a probability value (by one-way analysis of variance with Dunnett's post-test; NS, not significant).
This group contained one mouse with exceptionally low bacterial recovery; data for the other four mice were 5.34 ± 0.69 (1.2%; P < 0.01).
It has been suggested that TCP is unlikely to be a protective antigen in the context of human infection because of an inference that antibodies to these pili are not lytic in the presence of complement (6). This argument is based on the finding that the potential to induce serum bactericidal antibodies has proven to be the most consistent correlate of a vaccine's protective efficacy (11). Such antibodies are almost exclusively directed against LPS determinants, but it is generally accepted that the serum bactericidal response is simply a convenient indicator of a broader gut immune response to V. cholerae, with the latter mediating enteric defense (11). This presumably involves an antibody-mediated inhibition of bacterial colonization, but at least in the IMCM this can occur without involvement of the complement system (3, 16, 17). In the present studies, MAbs to El Tor TCP were very inefficient mediators of complement-dependent bacteriolysis compared with polyclonal anti-LPS antibodies of the same isotype. Despite this, the anti-TCP MAbs and the anti-LPS serum were similarly protective and achieved comparable reductions in bacterial colonization (Tables 1 and 2). These data suggest that antibodies to TCP protect by directly blocking colonization of the mucosal surface. This mechanism, rather than complement-dependent lysis, is also thought to underlie the protection mediated by antibodies specific for ETEC pili (5, 10, 14).
Immunoblotting analysis was used to compare the levels of antibodies to TcpA (the pilin monomer) in acute- and convalescent-phase sera from patients with O1 El Tor or O139 cholera. To avoid problems associated with recognition of LPS, patient samples were assessed against TCP and control antigens prepared from strains of heterologous serogroup. Initial immunoblots were difficult to interpret because of the presence of contaminating proteins similar in size to TcpA, prompting development of an immunoblotting inhibition approach. By blocking the binding of antibodies of other specificities, it became possible to detect anti-pilin responses.
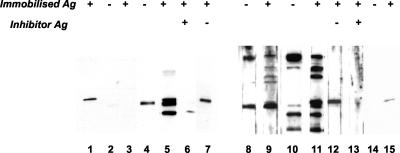
Figure 1 shows results obtained with two pairs of patient sera. In the left panel, the acute-phase serum did not react with any protein in either the TCP or control antigen preparation. The convalescent-phase serum recognized three proteins in the TcpA region of the gel, only one of which was present in the control antigen preparation (lanes 4 and 5). The control membrane incubated with MAb (lane 1) suggested that one of the proteins recognized by the convalescent-phase serum—the uppermost of the three bands—was TcpA. This was confirmed by the finding that binding to this protein was blocked by inclusion of TCP (lane 6)—but not control antigen (lane 7)—at the time of primary antibody binding. It was concluded that this patient had seroconverted to three proteins, including TcpA.
FIG. 1.
Immunoblotting detection of seroconversion to TCP. Recognition of proteins in immobilized TCP (+) and control antigen (Ag) (−) preparations by paired acute- and convalescent-phase serum samples obtained from patients with O1 (lanes 1 to 7) or O139 (lanes 8 to 15) disease. In some cases, the same antigens were included as inhibitors during the period of incubation with patient serum. (Left panel) Lanes: 1, MAb 2:13; 2 and 3, acute-phase serum at a 1 in 100 dilution; 4 to 7, convalescent-phase serum at a 1 in 100 dilution (for lanes 6 and 7, TCP or control antigen preparations were present during incubation with the primary antibody). (Right panel) Lanes: 8 and 9, acute-phase serum at a 1 in 100 dilution; 10 to 13, convalescent-phase serum at a 1 in 400 dilution (for lanes 12 and 13, control or TCP antigen preparations were present during incubation with the primary antibody); 14 and 15, MAb 2:13.
In the right panel of Fig. 1, the convalescent-phase serum was used at a dilution fourfold greater than that of the acute-phase sample to simplify the assessment of seroconversion. The day 2 serum gave bands with both antigen preparations in the TcpA region of the gel; a strong band was apparent just beneath a much weaker band that was only seen with the TCP antigen and was tentatively considered to be TcpA (lane 9). Again, the convalescent-phase serum indicated seroconversion to numerous proteins, two of which were only seen with the TCP antigen (lanes 10 and 11). One of the latter was an intense band in the TcpA position, suggestive of a strong anti-pilus response. The identity of this protein was confirmed by inhibition analysis, as its recognition was blocked by the presence of TCP (lane 13) but not by that of a control antigen (lane 12).
In all, five of nine serum pairs subjected to this inhibition immunoblotting analysis were scored as seroconverters to TCP. Two other individuals had comparable levels of antibodies to TCP in acute- and convalescent-phase sera. Since infection might precede presentation by several days, the presence of anti-TCP antibodies in acute-phase serum perhaps reflects the recent induction of an anamnestic response. The frequency of seroconversion to TCP is most encouraging and consistent with the finding of Hall et al. (6) that three of six Indonesian patients seroconverted to TCP. Together, these studies suggest that anti-TCP responses are common among cholera patients. If a way can be found to reliably induce such responses through vaccination, the significance of TCP as a vaccine antigen can be evaluated.
Acknowledgments
This study was funded by grant 16X-09084 from the Swedish Medical Research Council and grant INT-ICDDR,B-HN-01-AV from the Swedish Agency for Research Cooperation with Developing Countries.
We thank the Wenner-Gren Foundations of Stockholm for funding S.R.A.'s visits to Sweden and Liz Anderson for excellent technical assistance.
Editor: V. J. DiRita
REFERENCES
- 1.Attridge, S. R., P. A. Manning, J. Holmgren, and G. Jonson. 1996. Relative significance of mannose-sensitive hemagglutinin and toxin-coregulated pili in colonization of infant mice by Vibrio cholerae El Tor. Infect. Immun. 64:3369-3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attridge, S. R., F. Qadri, M. J. Albert, and P. A. Manning. 2000. Susceptibility of Vibrio cholerae O139 to antibody-dependent, complement-mediated bacteriolysis. Clin. Diagn. Lab. Immunol. 7:444-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Attridge, S. R., and D. Rowley. 1983. Prophylactic significance of the nonlipopolysaccharide antigens of Vibrio cholerae. J. Infect. Dis. 148:931-939. [DOI] [PubMed] [Google Scholar]
- 4.Cowell, J. L., J. M. Zhang, A. Urisu, A. Suzuki, A. C. Steven, T. Liu, T.-Y. Liu, and C. R. Manclark. 1987. Purification and characterization of serotype 6 fimbriae from Bordetella pertussis and comparison of their properties with serotype 2 fimbriae. Infect. Immun. 55:916-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaastra, W., and A.-M. Svennerholm. 1996. Colonization factors of human enterotoxigenic Escherichia coli (ETEC). Trends Microbiol. 4:444-452. [DOI] [PubMed] [Google Scholar]
- 6.Hall, R. H., G. Losonsky, A. P. D. Silveira, R. K. Taylor, J. J. Mekalanos, N. D. Witham, and M. M. Levine. 1991. Immunogenicity of Vibrio cholerae O1 toxin-coregulated pili in experimental and clinical cholera. Infect. Immun. 59:2508-2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herrington, D. A., R. H. Hall, G. A. Losonsky, J. J. Mekalanos, R. K. Taylor, and M. M. Levine. 1988. Toxin, toxin-coregulated pili, and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J. Exp. Med. 168:1487-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jonson, G., J. Holmgren, and A.-M. Svennerholm. 1991. Epitope differences in toxin coregulated pili produced by classical and El Tor Vibrio cholerae O1. Microb. Pathog. 11:179-188. [DOI] [PubMed] [Google Scholar]
- 9.Levine, M. M., J. A. Giron, and F. Noriega. 1994. Fimbrial vaccines, p. 255-270. In P. Klemm (ed.), Fimbriae: adhesion, biogenics, genetics and vaccines. CRC Press, Inc., Boca Raton, Fla.
- 10.Levine, M. M., D. R. Nalin, D. L. Hoover, E. J. Bergquist, R. B. Hornick, and C. R. Young. 1979. Immunity to enterotoxigenic Escherichia coli. Infect. Immun. 23:729-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levine, M. M., and C. O. Tacket. 1994. Recombinant live cholera vaccines, p. 395-413. In I. K. Wachsmuth, P. A. Blake, and O. Olsvik (ed.), Vibrio cholerae and cholera: molecular to global perspectives. ASM Press, Washington, D.C.
- 12.Osek, J., G. Jonson, A.-M. Svennerholm, and J. Holmgren. 1994. Role of antibodies against biotype-specific Vibrio cholerae pili in protection against experimental classical and El Tor cholera. Infect. Immun. 62:2901-2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qadri, F., C. Wenneras, M. J. Albert, J. Hossain, K. Mannoor, Y. A. Begum, G. Mohi, M. A. Salam, R. B. Sack, and A.-M. Svennerholm. 1997. Comparison of immune responses in patients infected with Vibrio cholerae O139 and O1. Infect. Immun. 65:3571-3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rutter, J. M., G. W. Jones, G. T. H. Brown, M. R. Burrows, and P. D. Luther. 1976. Antibacterial activity in colostrum and milk associated with protection of piglets against enteric disease caused by K88-positive Escherichia coli. Infect. Immun. 13:667-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma, D. P., C. Thomas, R. H. Hall, M. M. Levine, and S. R. Attridge. 1989. Significance of the toxin-coregulated pili as protective antigens of Vibrio cholerae in the infant mouse model. Vaccine 7:451-456. [DOI] [PubMed] [Google Scholar]
- 16.Steele, E. J., W. Chaicumpa, and D. Rowley. 1974. The isolation and biological properties of three classes of rabbit antibodies to V. cholerae. J. Infect. Dis. 130:93-103. [DOI] [PubMed] [Google Scholar]
- 17.Steele, E. J., W. Chaicumpa, and D. Rowley. 1975. Further evidence for cross-linking as a protective factor in experimental cholera: properties of antibody fragments. J. Infect. Dis. 132:175-180. [DOI] [PubMed] [Google Scholar]
- 18.Sun, D., J. J. Mekalanos, and R. K. Taylor. 1990. Antibodies directed against the toxin-coregulated pilus isolated from Vibrio cholerae provide protection in the infant mouse experimental cholera model. J. Infect. Dis. 161:1231-1236. [DOI] [PubMed] [Google Scholar]
- 19.Tacket, C. O., R. K. Taylor, G. Losonsky, Y. Lim, J. P. Nataro, J. B. Kaper, and M. M. Levine. 1998. Investigation of the roles of toxin-coregulated pili and mannose-sensitive hemagglutinin pili in the pathogenesis of Vibrio cholerae O139 infection. Infect. Immun. 66:692-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor, R. K., V. L. Miller, D. B. Furlong, and J. J. Mekalanos. 1987. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc. Natl. Acad. Sci. USA 84:2833-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thelin, K. H., and R. K. Taylor. 1996. Toxin-coregulated pilus, but not mannose-sensitive hemagglutinin, is required for colonization by Vibrio cholerae O1 El Tor biotype and O139 strains. Infect. Immun. 64:2853-2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vibould, G. I., N. Binsztein, and A.-M. Svennerholm. 1993. Characterization of monoclonal antibodies against putative colonization factors of enterotoxigenic Escherichia coli and their use in an epidemiological study. J. Clin. Microbiol. 31:558-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Voss, E., and S. R. Attridge. 1993. In vitro production of toxin-coregulated pili by Vibrio cholerae El Tor. Microb. Pathog. 15:255-268. [DOI] [PubMed] [Google Scholar]
- 24.Voss, E., P. A. Manning, and S. R. Attridge. 1996. The toxin-coregulated pilus is a colonization factor and protective antigen of Vibrio cholerae El Tor. Microb. Pathog. 20:141-153. [DOI] [PubMed] [Google Scholar]