Abstract
Tuberculosis (TB) is the most common opportunistic disease and a potentially fatal complication among immunocompromised individuals infected with human immunodeficiency virus (HIV). Effective vaccination against TB in persons with HIV has been considered unlikely because of the central role that CD4 cells play in controlling tuberculous infections. Here we show that the vaccination of CD8−/− mice with a TB DNA vaccine cocktail did not significantly enhance protective responses to a Mycobacterium tuberculosis infection. In contrast, immunization with a DNA vaccine cocktail or with the current TB vaccine, Mycobacterium bovis BCG, induced considerable antituberculosis protective immunity in immune-deficient mice lacking CD4 cells. In vaccinated CD4−/− animals, substantially reduced bacterial burdens in organs and much improved lung pathology were seen 1 month after an aerogenic M. tuberculosis challenge. Importantly, the postchallenge mean times to death of vaccinated CD4−/− mice were significantly extended (mean with DNA cocktail, 172 ± 7 days; mean with BCG, 156 ± 22 days) compared to that of naïve CD4−/− mice (33 ± 6 days). Furthermore, the treatment of DNA-vaccinated CD4−/− mice with an anti-CD8 or anti-gamma interferon (IFN-γ) antibody significantly reduced the effect of immunization, and neither IFN-γ−/− nor tumor necrosis factor receptor-deficient mice were protected by DNA immunization; therefore, the primary vaccine-induced protective mechanism in these immune-deficient mice likely involves the secretion of cytokines from activated CD8 cells. The substantial CD8-mediated protective immunity that was generated in the absence of CD4 cells suggests that it may be possible to develop effective TB vaccines for use in HIV-infected populations.
Tuberculosis (TB) remains a significant global threat to public health, with two million people dying from Mycobacterium tuberculosis infections each year and eight million cases of TB developing annually (6). The increasing linkage of TB with the human immunodeficiency virus (HIV) pandemic has magnified this tragedy in the past decade. The World Health Organization estimates that at least six million people worldwide are coinfected with M. tuberculosis and HIV (18). The HIV-M. tuberculosis coinfection rates exceed 5% in eight African countries, and in South Africa alone two million adults are coinfected (9). In these developing countries, TB is the most prevalent cause of morbidity and mortality for HIV-positive adults. In contrast to immunocompetent individuals, who have a 10% lifetime risk of disease following TB infection, persons coinfected with M. tuberculosis and HIV have a nearly 10% annual risk of developing disease.
A primary reason for the continued failure to curb the global TB epidemic is the absence of a highly effective vaccine. Although the current TB vaccine, Mycobacterium bovis BCG, has been widely used for decades, its efficacy in controlled clinical trials has been extremely variable and its value in protecting against the most prevalent form of the disease, adult pulmonary TB, is doubtful (7). Moreover, the effectiveness of BCG in preventing TB in HIV-infected individuals is uncertain. Since BCG is a live vaccine (attenuated but not avirulent) and since clinical cases of reactivated BCG have been reported for HIV-infected persons, BCG vaccination has not been indicated for immunocompromised individuals (33).
The development of a new vaccine against TB for use in HIV-positive persons has been considered unlikely because of the presumed essential roles that CD4 cells play in controlling TB infections. Mice that lack CD4 cells or that are aberrant in major histocompatibility complex class II presentation are extremely sensitive to a TB challenge and cannot effectively control acute TB infections (3, 13). The greatly enhanced susceptibility of HIV patients to both primary and reactivated disease argues that CD4 cells also play a prominent role in protective immune responses against human TB. The primary antituberculosis effector function of CD4+ T cells involves the production and secretion of cytokines which activate macrophages to control or eliminate the intracellular bacilli (13). In addition to this more direct role of CD4 cells in limiting tuberculous infections, CD4 cells also assist in the development of primary CD8 T-cell responses (23). CD4 cells stimulate professional antigen-presenting cells (APCs) primarily via CD40-CD40 ligand interactions; these activated APCs efficiently costimulate antigen-specific naïve CD8 T cells. Additionally, cytokine production from CD4 cells enhances the proliferation of primed CD8 cells.
Despite the critical immune functions of CD4 cells, recent studies have demonstrated that protective immunity to pathogens can be generated in the absence of CD4 cells. For instance, substantial CD8 T-cell responses to the influenza virus, lymphocytic choriomeningitis virus (LCMV), and pathogenic fungi can occur in mice lacking CD4 cells (17, 26, 34, 35). In a Listeria monocytogenes model, similar levels of activated CD8 T cells were detected in CD4−/− and wild-type (WT) C57BL/6 mice after an infection. Importantly, the epitope-specific CD8 T cells that were generated established long-term memory in CD4−/− mice and were capable of producing an effective recall response (21). Under circumstances in which CD4 help is not required for the generation of effective CD8 T-cell responses, alternate pathways for the activation of APCs exist. For influenza virus, a direct infection of dendritic cells results in the upregulation of costimulatory molecules. For bacteria, dendritic cells can be activated through recognition by Toll-like receptors (TLRs) of bacterial products such as peptidoglycan, glycolipids, and lipoproteins (2, 29, 30).
Based on these findings in other immune-deficient disease models, we evaluated whether cell-mediated antituberculosis protective immunity could be induced in mice lacking CD4 cells by immunization with a DNA vaccine cocktail or with BCG. Previously, members of our laboratory generated a DNA cocktail that expressed mycobacterial proteins fused at the N terminus to ubiquitin (UB), a eukaryotic intracellular targeting sequence (10, 11). These UB-conjugated proteins were designed to enhance major histocompatibility complex class I presentation. In studies using WT C57BL/6 mice, it was shown that vaccination with this combination was more effective than immunization with the individual single components and that the sustained protective immunity induced by the plasmid mixture was equivalent to the level of protection elicited by the BCG vaccine (10, 11). We report here that substantial antituberculosis protective immunity can be induced in the absence of CD4 cells. Immunization of CD4−/− mice with either the DNA vaccine cocktail or BCG leads to significantly decreased lung and spleen bacterial burdens and much improved lung pathology, relative to naïve controls, after an aerogenic M. tuberculosis infection. Most importantly, the survival of the vaccinated animals was substantially extended compared to nonimmunized CD4−/− controls.
MATERIALS AND METHODS
DNA vaccine construction.
The construction of all of the plasmid constructs used in this study was described earlier (10, 11, 20). Briefly, all of the TB genes were amplified from M. tuberculosis H37Rv chromosomal DNA with either Vent polymerase (New England Biolabs, Beverly, Mass.) or Taq polymerase (Invitrogen, Carlsbad, Calif.), using primers designed from the M. tuberculosis genome sequence database. For preparation of the UB plasmid backbone, the mouse UB gene was amplified from a mouse cDNA library and cloned into the HindIII and NheI sites of the pJW4303 vector. The UB fusion constructs were generated by amplifying the TB genes by use of an NheI or XbaI restriction site adaptor and then by cloning the PCR products immediately downstream of the UB sequence in the pJW4303 plasmid. For these studies, UBGR constructs were evaluated. These UBGR fusion constructs expressed recombinant proteins conjugated to a 76-amino-acid UB moiety containing an additional C-terminal arginine residue. Earlier studies had shown that these UBGR constructs expressed highly unstable antigens that induced Th1-type immunity (11). Of these constructs, only the antigen 85B gene was cloned in its native form.
Evaluation of the effectiveness of the DNA cocktail against an aerogenic challenge with M. tuberculosis.
The TB DNA cocktail consisted of eight individual vaccines expressing the following TB antigens: antigen 85B, ESAT-6, KatG, MPT8.4, MPT12, MPT63, MPT64, and MPT83. In previous studies, each of these single vaccines induced significant protective responses in a mouse aerosol challenge model (20, 24). Endotoxin-free plasmid DNA was prepared for each individual construct by using an Endo-Free maxi kit (Qiagen, Valencia, Calif.). The eight-component DNA vaccine combination was prepared by combining equal amounts of the plasmids to a final concentration of 1 mg of DNA/ml in phosphate-buffered saline (PBS). For these experiments, pathogen-free WT C57BL/6, CD4-deficient (B6.129S2-Cd4tm1Mak), CD8-deficient (B6.129S2-Cd8tm1-Mak), gamma interferon (IFN-γ)-deficient (B6.129S7-Ifngtm1), and tumor necrosis factor (TNF) receptor (p55)-deficient (B6.129-Tnfrsflatm1) female mice were obtained from the Jackson Laboratory (Bar Harbor, Maine). Groups of mice were injected intramuscularly in all four limbs on days 1, 21, and 42 with 200 μg of the cocktail (25 μg of each individual constituent) or a single plasmid vaccine. The BCG Pasteur immunization was given subcutaneously on day 1 with 106 organisms. Five weeks after the final DNA vaccination, the mice were challenged aerogenically with 200 CFU of M. tuberculosis Erdman per mouse in a Middlebrook-type chamber (Glas-Col, Terre Haute, Ind.). Five mice were sacrificed after 24 h to confirm the size of the challenge dose.
For assessment of the bacterial growth in vivo, five mice per group were sacrificed and the lungs and spleens were removed aseptically. These organs were homogenized separately in 5 ml of 0.04% Tween 80-PBS in a Seward Stomacher 80 blender (Tekmar, Cincinnati, Ohio). The homogenates were diluted serially in the Tween-PBS solution, and aliquots were plated on Middlebrook 7H11 agar. The numbers of CFU in the infected organs were assessed after incubation of the plates for 14 to 21 days at 37°C in sealed plastic bags. For survival studies, 8 to 11 animals per group were maintained until they became moribund and had to be euthanized. The survival data and the CFU results were statistically evaluated by using either one-way analysis of variance or unpaired t test analyses provided by the GraphPad InStat program and the statistical analysis package of the Stat-View program.
Histopathology assessments.
For histopathologic analyses of infected animals, lung tissues were perfused, fixed with 10% formalin in PBS, and then embedded in paraffin for sectioning. The lung sections were stained with hematoxylin and eosin or with Ziehl-Neelsen acid-fast stain and were evaluated by light microscopy.
Antibody depletion studies.
For CD8+ and Thy-1.2+ cell depletion studies, CD4−/− mice were immunized with the DNA cocktail as described above and then were treated intraperitoneally (i.p.) with either anti-CD8 monoclonal antibody (clone 2.43) (Harlan Bioproducts, Indianapolis, Ind.) or anti-Thy-1.2 monoclonal antibody (clone 30-H12) (Harlan Bioproducts) at 0.2 and 0.5 mg per dose, respectively, for 2 days before the day of the tuberculous challenge and then twice per week until the mice were sacrificed. The effectiveness of the antibody treatments was confirmed by flow cytometry after staining of the peripheral blood and lung lymphocytes with Cy-chrome-conjugated rat anti-mouse CD8a (Ly-2) or allophycocyanin-conjugated rat anti-mouse CD90.2 (Thy-1.2) monoclonal antibody (Pharmingen, San Diego, Calif.). For IFN-γ depletion, vaccinated CD4−/− mice were treated i.p. with 0.5 mg of anti-IFN-γ monoclonal antibody (clone XMG-6) (Harlan Bioproducts), as described above.
Assessment of CD4 and CD8 T-cell cytokine responses.
For evaluation of vaccine-induced cytokine production, groups of vaccinated and control WT mice were sacrificed 14 days after a low-dose aerogenic challenge with M. tuberculosis. Splenocytes from four mice were pooled, and CD4 and CD8 T cells were isolated by magnetic cell sorting using CD4- or CD8a-specific MACS MicroBeads (Miltenyi Biotec, Auburn, Calif.) as described by the manufacturer. The purified CD4 and CD8 T-cell subsets were restimulated in vitro with mouse bone marrow macrophages infected with M. tuberculosis. The macrophage cultures were established by flushing the femurs of C57BL/6 mice and culturing the cells in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum (FBS) and 10% conditioned medium from cultured L929 cells. The bone marrow cells were infected with virulent M. tuberculosis (multiplicity of infection of 1 bacterium/cell) 24 h prior to the splenocyte incubation. Supernatants were collected 72 h later, and the amount of IFN-γ secreted was analyzed by a cytokine-specific enzyme-linked immunosorbent assay using an immunoglobulin specific for IFN-γ (Pharmingen) as described earlier (10).
For cytokine mRNA studies, lung cells were harvested by the incubation of minced lung tissue with dispase (2.0 mg/ml) (Invitrogen) in PBS plus 2% FBS (Invitrogen) for 1 h at 37°C. The cells were passed through a cell strainer, pelleted, and resuspended in ACK lysing buffer (Quality Biological Inc., Gaithersburg, Md.) to lyse the erythrocytes. After washing of the pellet in PBS-FBS, adherent cells were removed by incubation in Dulbecco's modified Eagle medium plus 5% FBS in a tissue culture flask for 1 h at 37°C. CD8 cells were removed by positive selection from the nonadherent cell population by the addition of magnetic microbeads conjugated to monoclonal rat anti-mouse CD8a (Ly-2) antibodies (Miltenyi Biotec) to the cells and then passaging of the cells through a MACS LS separation column (Miltenyi Biotec). After rinsing of the column with PBS-0.5% FBS, the CD8+ population was harvested by removing the column from the magnetic field, washing the column with PBS-FBS, and collecting the eluted cells. Total RNA from the isolated CD8 cells was purified by use of an RNAqueous-4PCR kit (Ambion Inc., Austin, Tex.) and was reverse transcribed by use of the Superscript first-strand synthesis system (Invitrogen). The cDNA was used as a template for real-time reverse transcription-PCR with a probe and primers specific for IFN-γ, as described previously (36). The PCR amplifications were completed with an ABI Prism 7000 sequence detection system (Applied Biosystems, Foster City, Calif.). Glyceraldehyde phosphate dehydrogenase (GAPDH) Taqman reagents (Applied Biosystems) were used to measure GAPDH mRNA levels as an internal standard, and the level of IFN-γ mRNA relative to GAPDH mRNA was calculated by using the following formula: relative mRNA expression = 2−(Ct of IFN-γ − Ct of GAPDH).
RESULTS
Immunization with a TB DNA cocktail stimulates CD4+ and CD8+ T cells.
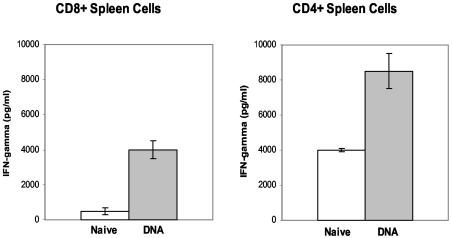
Our initial studies relating to the vaccination of immune-deficient mice examined the capacity of the TB DNA vaccine cocktail to induce both CD8 and CD4 T cells in WT mice. In these experiments, the vaccine effect postchallenge was measured by evaluating the secretion of IFN-γ, a critical cytokine in the control of M. tuberculosis infections (13). Fourteen days after an aerogenic challenge of vaccinated and naïve C57BL/6 mice, the splenic CD8 and CD4 T cells were isolated and stimulated in vitro with bone marrow macrophages infected with M. tuberculosis. As shown in Fig. 1, vaccine-induced cellular immune responses were detected in both the CD4 and CD8 T-cell populations after the TB challenge. Significantly more IFN-γ (P < 0.01) was secreted from the CD4 and CD8 splenocytes of mice immunized with the DNA vaccine combination than from splenic T cells from nonvaccinated mice.
FIG. 1.
CD8+ and CD4+ T cells from vaccinated mice produced significantly more IFN-γ than cells from naïve controls. Fourteen days after a low-dose aerosol infection with M. tuberculosis Erdman, splenic CD8 (left) and CD4 (right) cells from TB DNA cocktail-vaccinated or naïve WT C57BL/6 mice were isolated by magnetic cell sorting and then were restimulated in vitro with M. tuberculosis-infected bone marrow-derived macrophages. The amounts of IFN-γ that were secreted in these ex vivo cultures were determined by enzyme-linked immunosorbent assays.
Immunization with TB DNA vaccine cocktail protects CD4−/− mice against an aerogenic M. tuberculosis challenge.
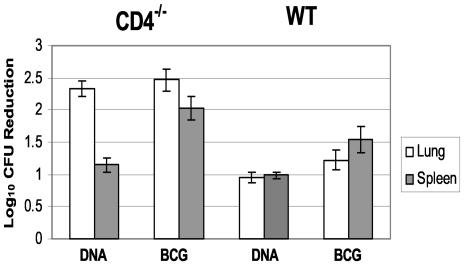
Since immunization with the TB DNA vaccine combination induced antituberculosis cytokine responses from both CD4 and CD8 T cells, we next investigated whether these responses could be generated in mice lacking either CD4 or CD8 cells. Specifically, we compared the effectiveness of immunization with the plasmid cocktail in protecting CD4−/− and CD8−/− mice against a tuberculous challenge with the protection induced in WT mice. As controls, CD4−/− and WT mice were also vaccinated with BCG. The effectiveness of the vaccine preparations was assessed by comparing the mycobacterial growth, lung pathology, and survival of immunized and control mice after the aerogenic challenge. Figure 2 shows the decreased mycobacterial burden in the lungs and spleens of vaccinated CD4−/− and WT mice, relative to naïve controls, at 1 month postchallenge. The WT CFU values were typical for this aerogenic infection model at this time point. An approximately 90% reduction (1 log10) in the bacterial burden in the lungs and spleens was seen for WT mice vaccinated with the TB DNA vaccine cocktail compared to nonimmunized controls. Additionally, a >1-log10 reduction in bacterial growth in the lungs and spleens (relative to naïve mice) was detected in BCG-vaccinated WT animals. The relative bacterial growth was more dramatically reduced at 1 month postchallenge in the vaccinated CD4−/− mice. Highly significant decreases in virulent organisms (relative to controls), of 2.3 to 2.5 log10 (>99%), were seen in the lungs for both the DNA cocktail and BCG vaccine groups. Furthermore, substantial reductions in splenic CFU (with the DNA cocktail, 1.2 log10; with BCG, 2.1 log10) were detected for both groups of vaccinated CD4−/− mice. Surprisingly, DNA immunization did not significantly impact mycobacterial growth in the CD8−/− mice. Similar lung bacterial CFU values were detected in both the DNA cocktail-immunized and naïve control groups (data not shown).
FIG. 2.
Vaccination with either BCG or the DNA cocktail resulted in a significant decline in lung and spleen bacterial burdens in CD4−/− mice (left) and WT mice (right). Organ bacterial CFU values were assessed 28 days after a low-dose aerosol challenge with M. tuberculosis Erdman. The vaccine-induced reduction in bacterial burden is represented as the difference between the CFU values (log10) of naïve controls and immunized mice. For this representative experiment, the CFU values (log10) for the CD4−/− mice were 8.6 (lung) and 6.6 (spleen) 28 days after the aerogenic infection. For the WT mice, the lung and spleen CFU values were 6.8 and 5.6, respectively.
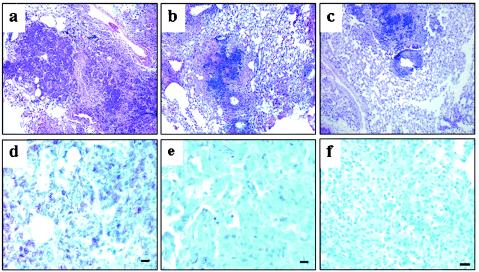
The relative lung pathology data at 1 month postchallenge paralleled the bacterial growth results. Substantive differences were seen in lung tissues taken from animals immunized with the plasmid cocktail or BCG compared to nonimmunized CD4−/− mice (Fig. 3). Hematoxylin and eosin staining of lung tissue showed that the aerogenic tuberculous infection caused severe acute multifocal suppurative pneumonia with extensive necrosis in the nonvaccinated CD4−/− controls (Fig. 3a). The inflammatory cells present in these lung sections were predominantly neutrophils; few macrophages and lymphocytes were detected. When these lung tissues from naïve CD4−/− mice were stained with the Ziehl-Neelsen stain to detect the infecting organisms, numerous acid-fast bacilli were seen (Fig. 3d). In contrast, the infection was subacute and much less severe in the CD4−/− mice that had been vaccinated with the TB DNA vaccine cocktail or BCG (Fig. 3b and c). For these mice, the lungs had much less inflammation and the moderately sized foci that were present contained predominantly lymphocytes, with fewer numbers of macrophages. Ziehl-Neelsen stains of lung tissue from these immunized CD4−/− mice revealed substantially smaller numbers of acid-fast bacilli than that in nonvaccinated controls (Fig. 3e and f).
FIG. 3.
Vaccination of CD4−/− mice with either BCG or the TB DNA cocktail resulted in a substantial improvement in lung histopathology and highly reduced numbers of acid-fast bacilli compared to naïve CD4−/− controls. Lung sections were fixed and stained with either hematoxylin and eosin (a, b, and c) or Ziehl-Neelsen reagent (d, e, and f) 28 days postchallenge. (a) Numerous necrotic, suppurative lesions containing many neutrophils and overall severe and acute coalescing inflammation with little functional tissue were seen in nonvaccinated CD4−/− mice. (d) An overwhelming number of acid-fast bacilli were also detected in the naïve controls. In contrast, lung sections from TB DNA cocktail (b and e)- and BCG (c and f)-vaccinated mice exhibited subacute lymphocytic granulomatous bronchopneumonia with no necrosis, and substantial amounts of intact functional tissue with few acid-fast bacilli were observed.
For the WT mice, vaccination yielded a similar but less dramatic effect on postchallenge lung pathology. In the nonimmunized WT controls, a low-dose aerosol infection with virulent M. tuberculosis caused pneumonia with significant inflammation at the 1-month time point. In contrast, vaccinated WT mice showed only mild signs of alveolitis and minimal consolidation at 1 month postchallenge (11; also data not shown). Interestingly, vaccination did not have an obvious effect on the relative postchallenge lung pathology of CD8−/− mice. For the vaccinated and control CD8−/− experimental groups, the aerosol infection caused a moderate bronchopneumonia to develop after 1 month (data not shown).
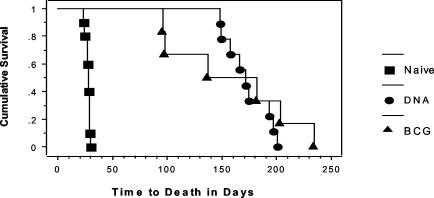
Survival studies provided the most compelling evidence for the protective immunity induced by the TB DNA vaccine cocktails in CD4−/− mice (Fig. 4). As expected from our bacterial growth and pathology data, the CD4−/− mice were extremely susceptible to aerogenic M. tuberculosis infection; the nonimmunized CD4−/− mice died about 1 month after the aerosol challenge (mean time to death [MTD] ± standard error of the mean = 28 ± 6 days). Injection of the vector alone into CD4−/− mice also did not substantially impact survival (MTD = 44 ± 11 days). However, vaccination with the DNA cocktail significantly extended the survival period for the CD4−/− mice (P < 0.001). A fivefold increase in the survival period was seen for CD4−/− mice that were immunized with the TB DNA plasmid cocktail (172 ± 7 days). Interestingly, immunization with live BCG also significantly increased the MTD for CD4−/− mice, to 156 ± 22 days (P < 0.01).
FIG. 4.
Kaplan-Meier plot of survival data. The plot shows that vaccination with BCG (▴) or the TB DNA cocktail (•) dramatically extended the survival time of CD4−/− mice after a low-dose aerogenic challenge with M. tuberculosis Erdman relative to that of naïve controls (▪).
The nonvaccinated CD8−/− mice were considerably less sensitive to the tuberculous challenge (MTD = 148 ± 19 days) than the corresponding CD4−/− group. Moreover, plasmid DNA vaccination did not significantly impact the survival period for CD8−/−, with only a modest increase in the survival period observed for the CD8−/− mice that were immunized with the TB DNA vaccine combination (173 ± 25 days). This survival result for CD8−/− mice was consistent with the minimal effect that vaccination had on organ bacterial burdens and lung pathology at 1 month postchallenge (as described above). For WT mice, the survival results were consistent with previous observations, in which it was shown that immunization with TB DNA vaccine cocktails extended survival times relative to controls (11). In these more recent experiments, the MTD for the nonvaccinated WT mice was 229 ± 13 days, while the survival period with the TB DNA vaccine combination was 311 ± 13 days (P < 0.01), relative to naïve controls.
Antituberculosis protective responses are mediated by CD8 cells.
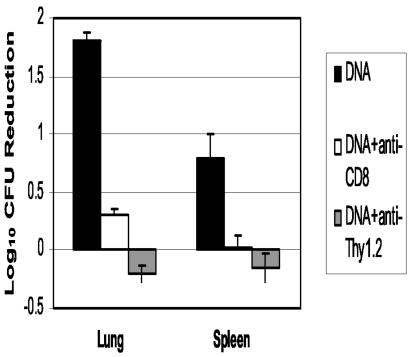
The effectiveness of these DNA vaccine combinations in protecting CD4−/− mice against a tuberculous challenge strongly suggests that CD8 T cells play a prominent role in antituberculosis protective immunity in mice lacking CD4 cells. For a more definitive demonstration of the importance of CD8 T cells in this protective response, CD4−/− mice that had been immunized with the DNA cocktail were treated with an anti-CD8 antibody and then were aerogenically challenged with M. tuberculosis. Flow cytometric analysis showed that the anti-CD8 antibody treatment decreased the concentrations of CD8 T cells in the lungs and peripheral blood to undetectable levels (not shown). When bacterial burdens were assessed postchallenge, high lung CFU values were detected and only a 0.3-log10 decrease in CFU relative to naïve mice was seen with the antibody-treated, vaccinated CD4−/− mice (Fig. 5). In contrast, a 1.8-log10 decrease in pulmonary CFU was detected in vaccinated CD4−/− mice that had not been depleted of CD8 cells. Similarly, a significant decrease in CFU in the spleen was seen with the vaccinated control mice (0.8 log10) relative to naïve mice, while no differences were observed between splenic CFU values for antibody-treated and naïve CD4−/− animals. The role of T cells in the protective immune response was confirmed by treating vaccinated CD4−/− mice with an anti-Thy-1.2 antibody and then challenging the mice aerogenically with M. tuberculosis Erdman. Twenty-eight days after the challenge, pulmonary bacterial burden determinations showed that repeated injections of the anti-Thy-1.2 antibody had completely abolished the protective effect of DNA vaccination (naïve CFU = 8.78 ± 0.25; antibody-treated vaccinated CFU = 8.96 ± 0.14). Similarly, the anti-Thy-1.2 treatment eliminated the reduced dissemination to the spleen that was detected with DNA-vaccinated CD4−/− mice (naïve CFU = 6.42 ± 0.41; anti-Thy-1.2-treated CFU = 6.58 ± 0.28).
FIG. 5.
Treatment of DNA-vaccinated CD4−/− mice with anti-CD8 or anti-Thy-1.2 antibody resulted in a significant reduction in lung CFU compared to nontreated CD4−/− vaccinated mice. CD4−/− mice vaccinated with the TB DNA cocktail were treated with 0.2 mg of anti-CD8 or anti-Thy-1.2 monoclonal antibody i.p. 2 days before, the day of, and twice per week after an aerogenic infection with M. tuberculosis Erdman. The protective response is represented as the reduction in CFU values (log10) for antibody-treated relative to untreated CD4−/− mice.
The two important immune effector functions that have been described for CD8 T cells are cytokine production and cytotoxic activity (13). The role of cytokines in the protective response was initially investigated by evaluating the cytokine mRNA levels in vaccinated and naïve CD4−/− mice at 8 days postchallenge by real-time PCR assays. In three different experiments, a threefold elevation in the IFN-γ message levels was detected in pulmonary CD8 T cells from vaccinated mice relative to those in CD8 T cells from naïve controls. To further distinguish between these potential mechanisms of cell-mediated antituberculosis protective immunity, mice defective in IFN-γ production or TNF signaling were vaccinated with the DNA cocktail and were then aerogenically challenged with virulent M. tuberculosis. Immunization of IFN-γ−/− mice with the combination of DNA constructs did not significantly increase the survival period compared to nonvaccinated controls. All mice in this study died within 1 month of the tuberculous challenge (MTD for naïve mice = 27 ± 1 days; MTD with DNA cocktail = 29 ± 1 days). Moreover, vaccination of TNF receptor-deficient mice did not significantly impact the survival of these immunocompromised mice relative to naïve mice (MTD for naïve mice = 33 ± 1 days; MTD with DNA cocktail = 37 ± 3 days).
The role of IFN-γ in DNA vaccine-mediated protection was more directly evaluated by injecting an anti-IFN-γ antibody into immunized CD4−/− mice according to a previously defined schedule. In this experiment, the anti-IFN-γ treatment completely eliminated the survival enhancement seen after DNA vaccination (naïve MTD = 27 ± 2 days; anti-IFN-γ MTD = 26 ± 1 days). Moreover, the pulmonary bacterial burdens at 24 days postchallenge were not significantly different in the antibody-treated immunized mice and the naïve controls (naïve CFU = 8.53 ± 0.32; anti-IFN-γ-treated CFU = 8.84 ± 0.15). Taken together, these data suggest that the secretion of IFN-γ and TNF from CD8 cells is an important component of the protective response that is generated by immunization with the DNA vaccine cocktail.
DISCUSSION
The development of vaccines that prevent tuberculous disease in immunocompromised populations could reduce the enormous tragedy of HIV and M. tuberculosis coinfection. However, TB vaccine development in the context of HIV disease has been limited because of the prevailing paradigm that CD4 cells are absolutely required for the control of M. tuberculosis infections. For some pathogens, CD4 T-cell responses are critical for assisting the development of effective primary CD8 T-cell responses. For instance, the efficient control of Toxoplasma and Plasmodium infections requires CD4 help for the generation of proficient CD8-mediated immunity (4, 5). In contrast, effective CD8 T-cell responses to influenza, LCMV, Listeria, and pathogenic fungi have recently been shown to occur in the absence of CD4 help (21, 26, 29, 34, 35). In a Listeria model, the presence of CD4 T cells was not required for the induction of a primary CD8 T-cell immune response or the establishment and maintenance of CD8 memory cells (29). Additionally, Withrich et al. recently demonstrated that vaccination induced CD8 T-cell-mediated immunity against the pathogenic fungi Blastomyces dermatidis and Histoplasma capsulatum in CD4-deficient mice (34). Our results clearly support these emerging data. The substantially reduced mycobacterial growth in lungs and spleens, improved lung pathology, and significantly increased survival periods demonstrate that immunization with either the TB DNA cocktail or with BCG can provide considerable protection in mice lacking CD4 cells against a primary aerogenic challenge with virulent M. tuberculosis. Since the treatment of vaccinated CD4−/− mice with either anti-CD8 or anti-Thy-1.2 antibody virtually eliminated the antituberculosis protective response, the vaccine-induced activation of CD8 T cells is likely the primary protective mechanism. Three types of evidence suggest that cytokine secretion from these effector CD8 cells mediates the vaccine-induced protective immunity. First, immunization with the DNA cocktail did not extend the survival of IFN-γ−/− or TNF receptor-deficient mice after an aerosol M. tuberculosis infection. Furthermore, IFN-γ mRNA levels obtained from pulmonary CD8+ cells were elevated threefold in vaccinated CD4−/− mice compared to naïve mice at a critical early time point postchallenge. Finally, anti-IFN-γ treatment of vaccinated CD4−/− mice abolished the protective effect of immunization. This protective role of cytokine secretion from CD8 cells in CD4−/− mice is supported by a previous report which concluded that the priming and amplification of mycobacterial-specific IFN-γ-secreting CD8 T cells, but not CD8 cytotoxic cells, were efficiently performed without CD4 cells (27).
The mechanisms by which the APCs from vaccinated animals become stimulated in the absence of CD4 cells have not been defined, but likely involve TLRs. The binding of these unique pattern recognition receptors by bacterial products or vaccine components can upregulate the immunostimulatory molecules of APCs that support the development of a Th1-biased T-cell response. In our disease model, the direct stimulation of APCs by vaccine components via interactions with TLRs may alleviate the necessity for CD4 help. The recognition of the CpG motifs of DNA vaccines by TLR-9 and the binding of BCG-derived molecules by TLR-2 or TLR-4 could elevate the costimulatory signals of APCs that are needed to effectively prime naïve CD8 T cells (15, 25, 30). Current studies to examine the precise role of TLRs in the generation of vaccine-induced antituberculosis immunity in CD4−/− mice are ongoing.
In addition to possible TLR interactions, the high numbers of CD8 epitopes present in the eight-component DNA vaccine cocktail and in the BCG vaccine likely contribute to the effectiveness of these vaccination strategies in the CD4−/− mice (8, 14, 19). Previous reports have shown that CD8 cells can provide help for their own activation (independent of CD4 cells) if the CD8 precursor frequency is sufficiently elevated (22, 32). In preliminary studies, we have demonstrated that the DNA vaccine cocktail (which encodes at least eight CD8 epitopes) induces a significantly better protective response than equivalent doses of single vaccines expressing one to two antigens containing a limited number of murine CD8 epitopes (S. Derrick, unpublished results). Similarly, D'Souza et al. reported that immunization with a single vaccine did not protect mice lacking CD4 cells against M. tuberculosis challenge (12). Overall, these data suggest that vaccines designed for use against intracellular pathogens in an HIV-infected population will likely require multiple CD8 epitopes for optimal efficacy. In ongoing studies, we are evaluating combinations of DNA cocktails containing three to six different vaccines in order to determine the minimal DNA combinations required to elicit a strong protective response.
Our studies clearly demonstrate that substantial antituberculosis protective immunity can be induced in the absence of CD4 help and that our vaccination strategy produced sufficient protective immunity to control a primary challenge in CD4−/− mice. However, the data for the WT mice suggest that the protective responses could potentially be further amplified in the CD4−/− mice. For example, the mean survival period postchallenge for the vaccinated CD4-deficient mice was significantly less than the MTD for vaccinated and control WT mice. Specifically, the MTDs for the CD4−/− mice immunized with the DNA cocktail and BCG were 172 and 156 days, respectively, while the MTD for the vaccinated WT mice exceeded 300 days (P < 0.01). Even the nonvaccinated WT mice survived 2 to 3 months longer than vaccinated CD4−/− animals. These significant differences in survival cannot be solely attributed to relative bacterial numbers in the lungs since the pulmonary CFU in the vaccinated CD4−/− mice was actually lower 28 days after challenge (6 to 6.2 log10) than the lung CFU for the control WT mice (6.8 log10). Our failure to further extend the survival period of the CD4−/− mice in the chronic infection phase to WT levels is consistent with CD4 cells having an important role in maintaining long-lived CD8 cell functions. In LCMV studies, CD4 cells have been shown to be necessary to sustain CD8 cytotoxic T-lymphocyte responses, and CD8 memory responses become exhausted in the absence of CD4 T cells (21). Similarly, in a murine Toxoplasma gondii model, CD8 T-cell immunity was induced but not maintained in mice lacking CD4 cells (5). Overall, our data are generally consistent with the results from other vaccination schemes that have targeted CD8 immunity; these strategies have often yielded strong, early responses but failed to provide adequate long-term protection. Recent reports have suggested that the basis for these relatively inadequate long-term outcomes may be the inferior quality of the memory T cells generated in the absence of CD4 help (16, 17, 28, 31). Although CD8 memory T cells can clearly be produced without CD4 assistance, defective memory CD8 recall and proliferative responses have been detected in CD4−/− mice during a secondary encounter with a specific antigen. It is likely that CD4 T-cell interactions provide signals during memory CD8 T-cell differentiation which enhance the development of memory cells that are highly proliferative. For instance, the B7 pathway may provide crucial signals for high-quality memory T-cell development since CD28-B7 interactions are known to be important for naïve CD8 T-cell activation and proliferation during Listeria infections (21). In fact, the inclusion of B7 costimulatory molecules in HIV DNA vaccine preparations was recently shown to significantly enhance HIV-specific CD8 effector T-cell responses (1). The B7 studies strongly suggested that the molecular interactions and signals needed to produce long-lived CD8 memory cells need to be further defined. Understanding how these interactions help the maintenance of the proliferative potential of CD8 cells should facilitate the design of efficacious future vaccines targeting the CD8 T-cell subset, including vaccines that are developed to reduce tuberculous disease in HIV-infected individuals.
Editor: B. B. Finlay
REFERENCES
- 1.Boyer, J. D., M. Chattergoon, K. Muthumani, S. Kudchodkar, J. Kim, M. Bagarazzi, G. Pavlakis, R. Sekaly, and D. B. Weiner. 2002. Next generation DNA vaccines for HIV-1. J. Liposome Res. 12:137-142. [DOI] [PubMed] [Google Scholar]
- 2.Brightbill, H. D., D. H. Libraty, S. R. Krutzik, R. B. Yang, J. T. Belisle, J. R. Bleharski, M. Maitland, M. V. Norgard, S. E. Plevy, S. T. Smale, P. J. Brennan, B. R. Bloom, P. J. Bodowski, and R. L. Modlin. 1999. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science 285:732-736. [DOI] [PubMed] [Google Scholar]
- 3.Caruso, A., N. Serbina, E. Klein, K. Triebold, B. R. Bloom, and J. Flynn. 1999. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-γ, yet succumb to tuberculosis. J. Immunol. 162:5407-5416. [PubMed] [Google Scholar]
- 4.Carvalho, L. H., G. Sano, J. C. Hafalla, A. Morrot, M. A. Curotto de Lafaille, and F. Zavala. 2002. IL-4 secreting CD4+ T cells are crucial to the development of the CD8+ responses against malaria liver stages. Nat. Med. 8:166-170. [DOI] [PubMed] [Google Scholar]
- 5.Casciotti, C., K. Ely, M. Williams, and I. Khan. 2002. CD8+-T-cell immunity against Toxoplasma gondii can be induced but not maintained in mice lacking conventional CD4+ T cells. Infect. Immun. 70:434-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cegielski, J., D. P. Chin, M. A. Espinal, T. R. Frieden, C. R. Rodriguez, E. A. Talbot, D. E. Wiel, R. Zaleskis, and M. C. Raviglione. 2002. The global tuberculosis situation. Progress and problems in the 20th century, prospects for the 21st century. Infect. Dis. Clin. N. Am. 16:1-58. [DOI] [PubMed] [Google Scholar]
- 7.Colditz, G., T. F. Brewer, C. S. Berkey, M. E. Wilson, E. Burdick, H. B. Fineberg, and F. Mosteller. 1994. Efficacy of BCG vaccine in the prevention of tuberculosis. JAMA 271:698-702. [PubMed] [Google Scholar]
- 8.Coler, R. N., A. Campos-Neto, P. Ovendale, F. H. Day, S. P. Fling, L. Zhu, N. Serbina, J. L. Flynn, S. G. Reed, and M. R. Alderson. 2001. Vaccination with the T cell antigen Mtb 8.4 protects against challenge with Mycobacterium tuberculosis. J. Immunol. 166:6227-6235. [DOI] [PubMed] [Google Scholar]
- 9.Corbett, E. L., C. J. Watt, N. Walker, D. Maher, B. G. Williams, M. C. Raviglione, and C. Dye. 2003. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch. Intern. Med. 163:1009-1021. [DOI] [PubMed] [Google Scholar]
- 10.Delogu, G., A. Howard, F. Collins, and S. Morris. 2000. DNA vaccination against tuberculosis: expression of a ubiquitin-conjugated tuberculosis protein enhances antimycobacterial immunity. Infect. Immun. 68:3097-3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delogu, G., A. Li, C. Repique, F. Collins, and S. Morris. 2002. DNA vaccine combinations expressing either tissue plasminogen activator signal sequence fusion proteins or ubiquitin-conjugated antigens induce sustained protective immunity in a mouse model of pulmonary tuberculosis. Infect. Immun. 70:292-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Souza, S., O. Denis, T. Scorza, F. Nzabintwali, H. Verschueren, and K. Huygen. 2000. CD4+ T cells contain Mycobacterium tuberculosis infection in the absence of CD8+ T cells in mice vaccinated with DNA encoding Ag85A. Eur. J. Immunol. 30:2455-2459. [DOI] [PubMed] [Google Scholar]
- 13.Flynn, J., and J. Chan. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19:93-129. [DOI] [PubMed] [Google Scholar]
- 14.Geluk, A., K. E. van Meijgaarden, K. L. Franken, J. W. Drijfhout, S. D'Souza, A. Necker, K. Huygen, and T. H. Ottenhoff. 2000. Identification of major epitopes of Mycobacterium tuberculosis AG85B that are recognized by HLA-A*0201-restricted CD8+ T cells in HLA-transgenic mice and humans. J. Immunol. 165:6463-6471. [DOI] [PubMed] [Google Scholar]
- 15.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740-745. [DOI] [PubMed] [Google Scholar]
- 16.Janssen, E. M., E. E. Lemmens, T. Wolfe, U. Christen, M. G. von Herrath, and S. P. Schoenberger. 2003. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 421:852-855. [DOI] [PubMed] [Google Scholar]
- 17.Kaesh, S. M., and R. Ahmed. 2003. CD8 T cells remember with a little help. Science 300:263-265. [DOI] [PubMed] [Google Scholar]
- 18.Leonard, M. K., N. Larsen, H. Drechsler, H. Blumberg, J. L. Lennox, M. Arrellano, J. Filip, and C. R. Horsburgh, Jr. 2002. Increased survival of persons with tuberculosis and human immunodeficiency virus infection, 1991-2000. Clin. Infect. Dis. 34:1002-1007. [DOI] [PubMed] [Google Scholar]
- 19.Lewinsohn, D. A., R. A. Lines, and D. M. Lewinsohn. 2002. Human dendritic cells presenting adenovirally expressed antigen elicit Mycobacterium tuberculosis-specific CD8+ T cells. Am. J. Respir. Crit. Care Med. 166:843-848. [DOI] [PubMed] [Google Scholar]
- 20.Li, Z., A. Howard, C. Kelley, G. Delogu, F. Collins, and S. Morris. 1999. Immunogenicity of DNA vaccines expressing tuberculosis proteins fused to tissue plasminogen activator signal sequences. Infect. Immun. 67:4780-4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matloubian, M., R. J. Concepion, and R. Ahmed. 1994. CD4+ T cells are required to sustain CD8+ cytotoxic T cell responses during a chronic viral infection. J. Virol. 68:8056-8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mintern, J. D., G. M. Davey, G. T. Belz, F. R. Carbone, and W. R. Heath. 2002. Precursor frequency affects the helper dependence of cytotoxic T cells. J. Immunol. 168:977-980. [DOI] [PubMed] [Google Scholar]
- 23.Mogues, T., M. E. Goodrich, L. Ryan, R. LaCourse, and R. J. North. 2001. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J. Exp. Med. 193:271-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris, S., C. Kelley, A. Howard, Z. Li, and F. Collins. 2000. The immunogenicity of single and combination DNA vaccines against tuberculosis. Vaccine 18:2155-2163. [DOI] [PubMed] [Google Scholar]
- 25.Reiling, N., C. Holscher, A. Fehrenbach, S. Kroger, C. J. Kirschning, S. Goyert, and S. Ehlers. 2002. Cutting edge: Toll-like receptor (TLR) 2- and TLR4-mediated pathogen recognition in resistance to airborne infection with Mycobacterium tuberculosis. J. Immunol. 169:3480-3484. [DOI] [PubMed] [Google Scholar]
- 26.Riberty, J. M., J. P. Christensen, K. Branum, and P. C. Doherty. 2000. Diminished primary and secondary influenza virus-specific CD8+ T-cell responses in CD4-depleted Ig−/− mice. J. Virol. 74:9762-9777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Serbina, N., V. Lazarevic, and J. Flynn. 2001. CD4+ T cells are required for the development of cytotoxic CD8+ T cells during Mycobacterium tuberculosis infection. J. Immunol. 167:6991-7000. [DOI] [PubMed] [Google Scholar]
- 28.Shedlock, D. J., and H. Shen. 2003. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science 300:337-339. [DOI] [PubMed] [Google Scholar]
- 29.Shedlock, D. J., J. K. Whitmire, J. Tan, A. S. MacDonald, R. Ahmed, and H. Shen. 2003. Role of CD4 help and costimulation in CD8 T cell responses during Listeria monocytogenes infection. J. Immunol. 170:2053-2063. [DOI] [PubMed] [Google Scholar]
- 30.Stenger, S., and R. L. Modlin. 2002. Control of Mycobacterium tuberculosis through Toll-like receptors. Curr. Opin. Immunol. 14:452-457. [DOI] [PubMed] [Google Scholar]
- 31.Sun, J. C., and M. J. Bevan. 2003. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science 300:339-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang, B., C. C. Norbury, R. Greenwood, J. R. Bennink, J. W. Yewdell, and J. A. Frelinger. 2001. Multiple paths for activation of naïve CD8 T cells: CD4-independent help. J. Immunol. 167:1283-1289. [DOI] [PubMed] [Google Scholar]
- 33.Weltman, A., and D. Rose. 1993. The safety of Bacillus Calmette-Guerin vaccination in HIV infection and AIDS. AIDS 7:149-157. [DOI] [PubMed] [Google Scholar]
- 34.Withrich, M., H. J. Filutowicz, T. Warner, G. S. Deepe, Jr., and B. S. Klein. 2003. Vaccine immunity to pathogenesis overcomes the requirement for CD4 help in exogenous antigen presentation to CD8 T cells: implications for vaccine development in immune-deficient hosts. J. Exp. Med. 197:1405-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu, Y., and Y. Liu. 1994. Viral induction of co-stimulatory activity on antigen-presenting cells bypasses the need for CD4+ T cell help in CD8+ T cell responses. Curr. Biol. 4:499-505. [DOI] [PubMed] [Google Scholar]
- 36.Xia, D., A. Sanders, M. Shah, A. Bickerstaff, and C. Orosz. 2001. Real-time polymerase chain reaction analysis reveals an evolution of cytokine mRNA production in allograft acceptor mice. Transplantation 72:907-914. [DOI] [PubMed] [Google Scholar]