Abstract
Enterotoxigenic Escherichia coli (ETEC) strains remain a formidable cause of diarrheal disease. To identify novel surface proteins of ETEC, we performed TnphoA mutagenesis of prototype ETEC strain H10407 and discovered a secreted protein not previously recognized in ETEC. DNA sequencing of the interrupted locus in mutant TnphoA.977 revealed a candidate 4,095-bp open reading frame without significant homology to commensal E. coli K-12 genomic DNA. Translation of this sequence revealed that it encoded a predicted peptide of 147.7 kDa that bears significant homology to members of the autotransporter family of bacterial virulence factors, particularly the serine protease autotransporters of the Enterobacteriaceae proteins. The gene identified in H10407, eatA (ETEC autotransporter A), encodes a potential serine protease motif (GDSGSP) in the secreted amino-terminal domain, and the predicted peptide shows more than 80% homology with SepA, a virulence protein secreted by Shigella flexneri. DNA hybridization and PCR demonstrated that eatA resides on the 92-kDa pCS1 virulence plasmid of H10407 and that it is present in multiple clinical ETEC strains. Immunoblots with antisera directed against a recombinant EatA passenger protein fragment identified a 110-kDa protein in supernatants purified from H10407 but not from the TnphoA.977 mutant or H10407-P, which lacks pCS1. EatA possesses serine protease activity that is abolished by mutations within a serine protease catalytic triad formed by residues H134, D162, and S267. Finally, interruption of the eatA gene retarded fluid accumulation in the rabbit ileal loop model, suggesting that this autotransporter contributes to the virulence of ETEC.
Enterotoxigenic Escherichia coli (ETEC) strains comprise a diverse group of pathogens that collectively are a leading cause of diarrheal disease and infant mortality in developing countries. These organisms are distinguished by the elaboration of plasmid-encoded heat-labile and heat-stable enterotoxins. Both of these toxins stimulate production of cyclic nucleotides (cyclic AMP and cyclic GMP, respectively) in intestinal epithelial cells, resulting in activation of the cystic fibrosis transmembrane regulator (CFTR) chloride channel and net secretion of fluid into the intestinal lumen (8, 20).
In addition to these known toxins, a second group of plasmid-encoded surface proteins, referred to as colonization factor antigens (CFAs), has been the subject of intensive investigation. These heterogeneous molecules are required for efficient colonization of the small intestine, where the organisms elaborate and deliver their enterotoxins. Although early studies demonstrated that these molecules could be used as targets to induce protective immunity (13), subsequent vaccine development efforts have been hindered by the molecular and antigenic heterogeneity of these molecules (38, 45).
The illnesses associated with these pathogens range from mild, self-limited diarrhea to severe, cholera-like disease associated with profound and rapid fluid loss (16, 32, 53). While the cellular actions of the toxins that define this group of pathogens have been investigated in some detail, other pathogen and/or host factors that may account for these apparent differences in virulence are poorly understood. One potential explanation is that some ETEC strains possess additional factors that modulate known virulence factors or that independently affect pathogenicity.
Recently, studies have indicated that these organisms, similar to other E. coli pathotypes, possess additional potential virulence genes in addition to the known toxins and colonization factor antigen molecules (19, 34). Included in these potential virulence genes is tibA (35), which encodes an adhesin that is a member of the autotransporter family of proteins. The autotransporters are a growing family of virulence proteins secreted by gram-negative bacteria that play a number of roles in pathogenesis (26), functioning diversely as adhesins, toxins, proteases, and mediators of intracellular motility. The majority of these proteins comprise three basic functional domains, including an amino-terminal leader sequence, a secreted “passenger” domain, and a carboxy-terminal β-core.
Two different mechanisms by which the passenger domains are processed have been proposed. In the first, the carboxy-terminal ends of these molecules form a monomeric hydrophilic β-barrel channel in the outer membrane, permitting export of an unfolded passenger domain (47). More recent data favor a model in which the carboxy-terminal domains assemble to form an oligomeric ring complex in the outer membrane that in turn serves as a central conduit for the export of the amino termini (60). In some autotransporter molecules, the passenger domain remains attached to the C terminus anchored in the outer membrane, whereas other passenger domains are released from the surface of the organism. A number of autotransporters also bear serine protease motifs within their passenger domains; this is a common feature of the molecules of this class produced by the Enterobacteriaceae, and hence they are referred to as SPATE (serine protease autotransporters of the Enterobacteriaceae) proteins (27).
In our efforts to identify additional surface-expressed molecules which might be exploited in vaccine development, we employed TnphoA mutagenesis, which provides powerful selection for potential virulence factors by identifying genes that encode exported proteins (37, 59). Here we report the identification of a second autotransporter protein of the prototypical ETEC strain H10407 that may function as a virulence determinant.
MATERIALS AND METHODS
Strains and plasmids.
The strains and plasmids used in the course of these studies are listed in Table 1. Clinical ETEC strains used in these studies were maintained as frozen stocks at −80°C and were initially obtained from collections maintained at Walter Reed Army Institute of Research. Strain ThroopD, originally isolated from a patient with severe cholera-like diarrheal illness (16), was kindly provided by Richard Finkelstein. The prototype ETEC strain H10407 used in these studies has recently been used in clinical challenge studies at the Walter Reed Army Institute of Research and was provided by Marcia Wolf, who also graciously provided the isolated 92-kDa, 64-kDa, and 6-kDa plasmids used in these studies.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant genotype or descriptiona | Reference(s) |
|---|---|---|
| Strains | ||
| H10407 | ETEC serotype O78:H11, LT+ ST+ | 14 |
| MM294 | endA hsdR pro supF | 59 |
| TnphoA.977 | eatA::TnphoA mutant obtained by random H10407 mutagenesis | This study |
| BL21 (DE3) | F−ompT hsdSB (rB− mB−) gal dcm(DE3) | Novagen |
| SM10λpir | thi thr leu tonA lacY supE recA::RP4-2-Tc::Mu | 29, 56 |
| DH5αpir | supE44 ΔlacU 169 (φ80lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | 57 |
| DH10B T1 | F−mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 deoR recA1 endA1 araD139 Δ(ara leu)7697 galU galK λ−rpsL nupG tonA | 28 |
| LMG194 | F− ΔlacX74 galE thi rpsL ΔphoA (Pvull) Δara-714 leu::Tn10 | Invitrogen |
| Plasmids | ||
| pRT291 | IncP1 TnphoA, Kmr Tcr | 59 |
| pPH1HI | IncP1, tra+ Spr Gmr | 50 |
| pCVD422 | pACYC184-based cloning plasmid with MluI linker inserted into EcoRV site | 10 |
| pRE107 | R6K ori-based suicide plasmid derived from pGP704, Ampr | 11 |
| pSG76-C | R6K ori-based suicide plasmid, Cmr | 48 |
| pPIR-K | pSC101ts-based pir helper plasmid, Kmr | 48 |
| pST76K-Sac | Temperature-sensitive sacB suicide plasmid, Kmr | 48 |
| pT7Blue-3 | PCR cloning vector, Ampr Kmr | Novagen |
| pET-30b(+) | Polyhistidine fusion protein expression vector, Kmr | Novagen |
| pGEM-7zf(+) | Cloning vector, Ampr | Promega |
| pJTH019 | 8,653-bp BspHI fragment containing the eatA-TnphoA junction from strain TnphoA.977 cloned into pCVD422 | This study |
| pJTH023 | 611-bp PCR amplicon internal to eatA gene cloned into EcoRV site of pT7Blue-3 | This study |
| pJTH024 | 647-bp EcoRI fragment from pJTH023 cloned into pRE107 (Ampr) | This study |
| pWY002 | Recombination of pJTH024 in H10407, excision with NotI and religation | This study |
| pJTH025 | 1,483-bp eatA gene fragment cloned into pT7Blue-3 | This study |
| pET30b(+).EatA | Six-histidine-rEatA fusion expression plasmid, Kmr | This study |
| pSP001 | 1,579-bp internal eatA fragment cloned into pT7Blue-3 | This study |
| pSP007 | 1,628-bp SalI-SphI pSP001 fragment cloned into pSG76-C | This study |
| pSP008 | Recombination of pSP007 with native H10407 plasmid, Cmr | This study |
| pSP009 | 4,267-bp amplicon cloned into EcoRV site of pSTBlue-1 | This study |
| pSP010 | 3,253-bp BstXI eatA fragment from pSP009 into pST76KSac | This study |
| PGPS2.1 | 4,490-bp Tn7 (cat) donor plasmid, R6K-g ori Cmr Tcr | NEBb |
| pSP011 | Transposition of Tn7 Cmr cassette into pSP010 eatA fragment | This study |
| pBAD-TOPO | 4,126-bp expression plasmid; arabinose promoter | Invitrogen, 23 |
| pSP014 | 4,185-bp eatA amplicon cloned into pBAD-TOPO | This study |
| pSP018 | pSP014 altered by SDM for Ser267 → Gly267 substitution of EatA | This study |
| pSP019 | pSP014 altered by SDM for His134 → Arg134 substitution of EatA | This study |
| PSP020 | pSP014 altered by SDM for Asp162 → Ala162 substitution of EatA | This study |
Ampr, Cmr, Kmr, Spr, and Tcr denote ampicillin, chloramphenicol, kanamycin, spectinomycin, and tetracycline resistance, respectively. SDM, site-directed mutagenesis.
NEB, New England Biolabs.
TnphoA mutagenesis.
The recipient strain for construction of TnphoA mutants, H10407.S, is a spontaneous streptomycin-resistant mutant of ETEC strain H10407. This isolate is fully virulent when grown on low concentrations of streptomycin and is equivalent to the parent in its ability to synthesize and release heat-labile toxin as measured by Gm1 ganglioside enzyme-linked immunosorbent assays. Plasmid pRT291 bearing TnphoA (59) was introduced into H10407.S by conjugation with SM10 (56). Transconjugants were then selected on Luria agar containing streptomycin (10 μg/ml) and kanamycin (45 μg/ml). Plasmid incompatibility was then used to select for authentic TnphoA transpositions into ETEC H10407 genomic DNA by mating streptomycin- and kanamycin-resistant colonies with colonies of MM294(pPH1JI) on Luria agar plates, followed by selection on plates containing streptomycin, kanamycin, spectinomycin (75 μg/ml), and 5-bromo-4-chloro-3-indolylphosphate (XP) indicator (40 μg/ml). Potential gene fusions with TnphoA were identified by selecting blue colonies from these plates. Blue colonies were isolated by streak purification on the same medium and saved for subsequent analysis.
DNA sequencing.
The DNA sequence was obtained with an Applied Biosystems 373 automated sequencer and edited with Sequencher 3.0 (GeneCodes, Ann Arbor, Mich.).
Cloning and expression of eatA.
To identify the location of the TnphoA insertion in strain TnphoA.977, genomic DNA was first digested with BspHI and ligated into the corresponding site of pCVD422 (10). DNA sequencing of the resulting plasmid, pJTH019, was initiated with primer TnphoA.179 (5′-CCA TCC CAT CGC CAA TCA-3′ ) oriented in the direction of the gene fusion junction. DNA sequence obtained in this fashion was in turn used to generate additional primers for sequencing by primer walking. Translation of the potential ′phoA fusion partner sequence was then used in BlastP searches, revealing a high degree of homology with the autotransporter protein SepA. Primer SPATE 5′0.1 (5′-CAA GGA GCT ATC TAT TTT-3′ ), based on sequence upstream from the candidate gene fusion partner, and a degenerate primer 5′-RTTNGCRTTDATNGCRTTRTC-3′ (where R is A or G; N is A, C, G, or T; and D is A, G, or T), based on conserved sequences at the 3′ end of SPATE-encoding genes, including sepA, were used to amplify an additional adjacent DNA sequence from ETEC H10407 genomic DNA. The resulting 3.6-kb PCR amplicon was then digested with Sau3AI and cloned into the BamHI site of pGEM-7Zf(+). DNA sequence was obtained from the resulting clones with M13 forward and reverse vector primers and assembled with Sequencher.
Sequence data obtained in this fashion were used to generate a 611-bp amplicon with primers jf032502.2 (5′-CAACATTTTGCCAGACAT-3′) and jf032502.3 (5′-CATCCTTAACTGACCAAA-3′). This fragment was cloned (pJTH023, Table 1) and ultimately used to construct suicide plasmid pJTH024, which was introduced into H10407-S by mating with SM10λpir for integration by allelic exchange with the eatA locus. The integrated plasmid and surrounding sequence were rescued by NotI digestion, religation, and transformation of Escherichia coli DH5α pir to yield pWY002. Finally, sequence generated from pWY002 was used to construct an additional suicide plasmid, pSP007, which was introduced into H10407(pPir-K) by electroporation, followed by selection at 42°C. Total plasmid DNA extracted from the resulting chloramphenicol-resistant, kanamycin-sensitive colonies was then used to transform DH5α pir to chloramphenicol resistance. The resulting plasmid, pSP008, was then used to sequence the 3′ end of eatA and the downstream flanking sequence. Primers SPATE 5′0.1 (5′-CAA GGA GCT ATC TAT TTT-3′ ) and jf100902.1 (5′-GTCGCTGTACTATCGTTA-3′) were used to amplify a 4,267-bp product which was cloned into the EcoRV site of pSTBlue-1 to produce pSP009.
For high-level eatA expression, the gene was first amplified by PCR with the downstream jf100902.1 primer with jf111302.2 (5′-GAGGAATAATAAATGAATAAAGTGTTCTCT-3′) containing a ribosome-binding site and translational stop codons (italic) preceding the ATG start site. The resulting product was cloned into pBAD-TOPO to yield pSP014. LMG194 was then used as the host expression strain for induction with arabinose.
Production of polyclonal antisera against recombinant EatA.
To identify the mature EatA protein in concentrated supernatants of ETEC, polyclonal antisera directed against a recombinant EatA fragment were raised in rabbits. First, a 1,483-bp region of the eatA gene encoding a portion of the passenger domain was amplified by PCR with primers jf020602.1 (5′-TTTTTGGATCCCTACGATAAGAATGGAGT-3′) and jf020602.2 (5′-TTTGGCTCGAGCGACCGTACGCCTTTGATT-3′) (italics represent the locations of BamHI and XhoI restriction sites, respectively). The resulting amplicon was cloned into pT7Blue-3 to yield pJTH025. A BamHI/XhoI fragment from pJTH025 was then cloned into the corresponding sites of pET-30b(+) to construct an expression plasmid, pET-30b(+).EatA, encoding a six-histidine tag-EatA fusion protein (r6H.EatA88-581). DNA sequencing of pET-30b(+).EatA was performed with T7 and SP6 vector primers to ensure that the insert encoding amino acids 88 to 581 of EatA was in-frame with the polyhistidine tag. Following expression of the protein in E. coli BL21(DE3) and purification by Ni affinity chromatography, r6H.EatA88-581 was used to immunize two New Zealand White rabbits as previously described (17). Polyclonal rabbit antisera were then absorbed on an E. coli lysate column (Pierce Biotechnology) to remove cross-reacting antibodies.
Detection of the secreted EatA passenger domain in culture supernatants.
Immunoblotting was used to identify the putative passenger domain in culture supernatants of ETEC strain H10407 and mutants. Briefly, cultures of each strain were grown overnight in LB with or without appropriate antibiotics. Cultures were centrifuged at 6,000 × g, and the remaining bacteria were removed from resulting supernatants by passage through a 0.22-μm sterile filtration flask (Millipore). Supernatant proteins were then concentrated 10-fold with a 76-mm, 30,000-molecular-weight-cutoff filter (Millipore YM30) to a final volume of approximately 10 ml. Supernatants were further concentrated to a final volume of 0.5 to 1.0 ml (100- to 200-fold concentrate) with centrifugal filtration devices (Ultrafree 15, Millipore; 50,000 molecular weight cutoff).
After separation of supernatant proteins by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (30) and transfer to nitrocellulose, immunoblotting was performed with purified rabbit polyclonal anti-r6H.EatA88-581 serum (1:1,000), and goat anti-rabbit immunoglobulin G(Fc)-horseradish peroxidase (1:60,000) (Pierce). All blocking and incubation steps were carried out in 5% skim milk-Tris-buffered saline (pH 7.2)-0.05% Tween 20. Detection was carried out with luminol-based chemiluminescent substrate (SuperSignal; Pierce).
DNA hybridization and PCR studies.
Primers SPATE 5′0.1 (5′-CAAGGAGCTATCTATTTT-3′) and jf112001.2 (5′-GTGGCGCTACTAAAATCA-3′) were used to produce the 423-bp probe 1. Primers jf061402.1 (5′-GATAAACTGAGGCTTGAT-3′) and jf061402.3 (5′-GCTCCAGCATACAAAGAA-3′) were used to generate probe 2, encompassing a 1,580-bp sequence internal to the eatA gene. Probes were labeled by random primer labeling of PCR products with digoxigenin.
In colony hybridization studies, isolated colonies were grown overnight at 37°C on a positively charged nylon filter on the surface of a Luria agar plate. Liberation of DNA from bacterial colonies and UV cross-linking were carried out as previously described (55). Both colony and Southern blotting hybridizations were carried out under stringent conditions without formamide. PCR to identify eatA in clinical ETEC strains employed primers jf061402.1 and jf061402.3.
Oligopeptide cleavage.
p-Nitroanilide substrates (Sigma, Calbiochem, and Bachem) were prepared as 10 mM stock solutions in dimethyl sulfoxide. Concentrated supernatants from LMG194(pSP014) were dialyzed overnight against 0.1 M MOPS (morpholinepropanesulfonic acid) (pH 7.3)-0.2 M NaCl-0.01 M ZnCl2. Reactions were performed in a total volume of 300 μl at 37°C, and absorbance readings (SpectraMax Plus; Molecular Devices) at 405 nm were obtained at 5-min intervals over the course of 12 to 16 h and plotted kinetically (SoftMax Pro v4.3). Values were expressed as mean Vmax in milliunits per minute for comparison between substrates and to examine the effect of inhibitors or mutations within the putative serine protease motif. Phenylmethylsulfonyl fluoride was added at a final concentration of 0.01 to 1 mM.
Insertional and site-directed mutagenesis of eatA.
To confirm the phenotype of the eatA mutant generated by random TnphoA mutagenesis, we constructed a directed mutation in eatA by introduction of a chloramphenicol resistance cassette. First, a 3,253-bp BstXI fragment from pSP009 containing a portion of eatA was cloned into the suicide plasmid pST76K-Sac to create pSP010. A chloramphenicol resistance cassette was then introduced into the insert region of pSP010 by Tn7-based (9) in vitro transposition (46) from pGPS2.1, yielding pSP011. Finally, the pSP011 suicide plasmid was used to replace the wild-type eatA sequence by double homologous recombination (5). After selection for chloramphenicol-resistant, kanamycin-sensitive colonies, the presence of the mutation was confirmed by PCR.
Site-directed mutagenesis was performed with either pS132P009 or pSP014 as the template with QuikChange XL reagents (Stratagene) and primer pairs jf121902.1 (5′-CAATATGTTGTCACAGCAAAGCGTGTTAATGGATCAGATATAATG-3′) and jf121902.2; jf121902.5 (5′-GAGAACAACCATAATAGCCTTGCTATTAAAATACGGCGTTTAAAT-3′) and jf121902.6; and jf121902.3 (5′-CTATCTAAATAAAGGTGATGGTGGCTCTCCTTTATTTGCGT-3′) and jf121902.4, where the second primer in each series is the reverse complement of the first. These primers were designed to confer single nucleotide changes resulting in amino acid substitutions of His134 to Arg134, Asp162 to Ala162, and Ser267 to Gly267, respectively.
Cyclic AMP assays.
Bacteria were grown overnight, diluted 1:100, and grown for an additional 90 min prior to inoculating triplicate wells of a 96-well plate containing confluent HCT-8 cell monolayers at a multiplicity of infection of approximately 100:1. After 2, 4, or 6 h of incubation, the supernatant was removed, and the monolayers were washed four times with RPMI. Intracellular cyclic AMP concentrations in the target monolayers were then determined by enzyme-linked immunosorbent assay (Amersham Pharmacia Biotech).
Rabbit ileal loop assays.
Rabbit ileal loop assays were performed as previously described with 2-kg male New Zealand White rabbits (19). Bacteria were grown overnight in 2-ml cultures in Luria broth containing appropriate antibiotics, diluted 1:100 on the morning of the procedure, and grown to an optical density at 600 nm of approximately 0.1. Approximately 108 bacteria were resuspended in 1 ml of phosphate-buffered saline (pH 7.4) and placed on ice prior to instillation in rabbit ileal loops. A fraction of each preparation was diluted in sterile 0.9% NaCl and grown overnight on LB plates to precisely determine the inoculum. Loops were harvested after approximately 16 h, and the accumulated fluid in each loop was determined by harvesting the fluid in preweighed tubes. Alternatively, 109 bacteria were instilled in each loop, and the incubation time was shortened from 16 to 7 h. Immediately after recovery of the accumulated fluid, the center of each loop was excised and preserved in 10% buffered formalin for subsequent histopathologic analysis. In all of the experiments, control loops contained 1 ml of phosphate-buffered saline without bacteria.
Nucleotide sequence accession number.
The eatA gene has been assigned GenBank accession number AY163491.
RESULTS
eatA gene is encoded on a virulence plasmid of ETEC H10407 and distributed widely among ETEC strains.
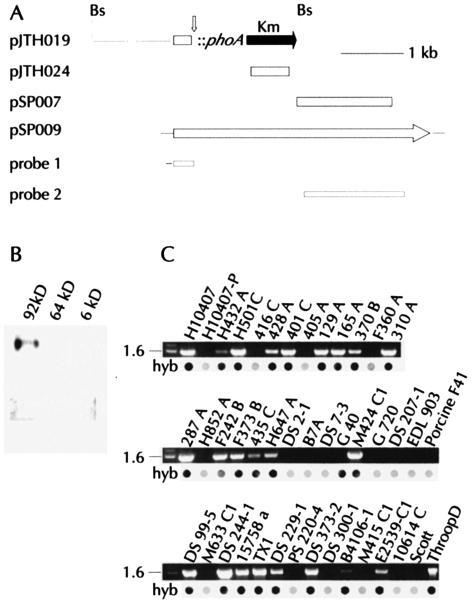
Analysis of DNA sequence upstream from the TnphoA insertion on plasmid pJTH019 (Fig. 1A) by BlastN searches (2) identified regions of significant homology with pWR501 (61), derived from the pWR100 virulence plasmid of Shigella flexneri 2a. To determine whether this locus might reside on one of the known virulence plasmids of ETEC H10407, we performed Southern blotting experiments with individual plasmids previously isolated from this strain. As shown in Fig. 1B, a labeled probe constructed from the 5′ end of the locus hybridized to a BamHI fragment from the 92-kDa plasmid, but gave no detectable signal with either the 64-kDa or 6-kDa plasmid. When plasmid DNA, genomic DNA, or DNA from colony lysates from H10407-P, which lacks the 92-kDa plasmid, served as templates in PCRs with primers used to construct probes 1 and 2, no amplicon was produced. Similarly, colony hybridization experiments with probe 2 and H10407-P were negative (Fig. 1C).
FIG. 1.
(A) Map of pJTH019 demonstrating the location of the TnphoA insertion within the 5′ end of eatA. BspHI sites (Bs) flank the insert. Maps of plasmids bearing full-length eatA (pSP009) and eatA subclones (pJTH024, pSP007). Relative locations of eatA locus DNA probes 1 and 2 used in DNA hybridization studies are depicted at the bottom of the map. (B) Hybridization of eatA gene probe 1 in Southern blot of BamHI-digested DNA from isolated ETEC H10407 virulence plasmids of 92, 64, and 6 kDa. (C) PCR and colony hybridization (hyb) data (probe 2) from H10407, H10407-P, as well as other clinical ETEC strains from diverse geographic origins. Size markers of 1.6 and 2.0 kb are shown at the left of each gel image.
Analysis of DNA sequence downstream from eatA demonstrated significant homology with pWR100, the major virulence plasmid of Shigella flexneri, as well as the EAF plasmid from enteropathogenic E. coli. Furthermore, a search of DNA sequence contigs from the 92-kDa plasmid also identified the eatA gene (V. Burland, personal communication). Analysis of DNA from additional human clinical ETEC isolates showed that 25 (61%) of 41 hybridized with probe 2, and 24 of these were also positive by PCR. These findings suggest that eatA or similar homologous genes are widely distributed among clinical human ETEC strains.
EatA is a member of the SPATE family of autotransporter molecules.
DNA sequencing of the candidate open reading frame revealed little or no homology to known virulence genes or to genes found in E. coli K-12. However, BlastP comparisons demonstrated considerable homology with the autotransporter family of virulence proteins. The predicted 147.7-kDa peptide encoded by eatA is most closely related to the Shigella flexneri protein SepA (3), to which it is more than 70% identical (Fig. 2).
FIG. 2.
ClustalW alignment (http://www.ch.embnet.org/index.html) of the predicted peptide sequences of EatA and its closest homologue, the Shigella flexneri protein SepA. Regions shaded black indicate identity. The two proteins have several features in common with other SPATE proteins, including an extended signal peptide and a putative serine protease site within the proposed passenger domain. Amino acids which form part of the serine protease catalytic triad are indicated (*).
Analysis of the predicted peptide sequence of EatA revealed several features common to the SPATE family of virulence molecules. First, the EatA precursor begins with an unusually long signal peptide with a cleavage site between amino acids 56 and 57, as predicted by SignalP (43). Within this long signal peptide are several features common to other SPATE proteins, including initiation in an M1, N, R/K followed by Y5/F, X, I/LV, X, Y/W, and finally a highly conserved I17AVSELAR motif of unknown function (24). The passenger domain bears a signature serine protease motif formed by a potential catalytic triad comprised of His134, Asp162, and the Ser267 residue within the GDSGS autotransporter consensus sequence (15) (Fig. 2 and 3). As in prior comparisons of the autotransporters (22), we found that EatA shows a high degree of homology to many of these molecules within the highly conserved carboxy-terminal β-barrel transport region (Table 2), whereas only the passenger domain of SepA shows marked similarity with the corresponding portion of EatA.
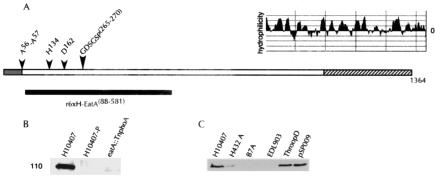
FIG. 3.
(A) Proposed structure of EatA molecule. Predicted signal peptide is shaded grey. Cleavage site between amino acids 56 and 57 was predicted by Signal P (http://www.cbs.dtu.dk/services/SignalP/) (43). The passenger domain is unshaded and extends from A57 to approximately amino acid 1098, based on prior sequence alignments of SepA and other autotransporter proteins (3). The carboxy-terminal β-barrel transport region is hatched, with predicted Kyte-Doolittle (30) hydrophilicity plot (MacVector). The location of amino acids H134, D162, and S267 within the conserved SPATE GDSGSP motif (amino acids 265 to 270), proposed to form the serine protease catalytic triad, are shown above the passenger domain. The black bar depicts the region of EatA (amino acids 88 to 581) represented in the recombinant protein used to raise polyclonal rabbit antisera. (B) Anti-EatA immunoblot of supernatants from H10407 (wild type), H10407-P (cured of the 92-kDa virulence plasmid), and TnphoA.977, bearing the eatA::TnphoA mutation. (C) Anti-EatA immunoblot of concentrated supernatants from clinical ETEC strains and a recombinant E. coli strain expressing the cloned eatA gene on pSP009.
TABLE 2.
Homologues of EatA among members of the SPATE family of autotransporters
| Homologue | % Identical (% similar)a
|
Putative function | Reference(s) | ||
|---|---|---|---|---|---|
| Complete peptide | Passenger domain | Autotransporter domain | |||
| SepA | 74 (85) | 74 (84) | 81 (91) | Cytotoxin | 3, 4 |
| Pic | 52 (68) | 47 (64) | 71 (85) | Mucinase | 25 |
| Hbp | 45 (63) | 42 (59) | 58 (76) | Hemoglobin protease | 44 |
| Tsh | 45 (63) | 42 (59) | 58 (76) | Hemagglutination | 49 |
| SigA | 37 (53) | 26 (40) | 71 (86) | Cytopathic protease | 1 |
| EspC | 31 (46) | 38 (51) | 83 (92) | Enterotoxin | 39, 58 |
| Sat | 30 (46) | 32 (48) | 72 (86) | Cytotoxin | 22 |
| Pet | 29 (46) | 28 (45) | 73 (86) | Enterotoxin | 42 |
BlastP comparisons.
Secretion of the EatA passenger domain.
The passenger domains of the autotransporters are processed by several different mechanisms (27). The amino-terminal passenger domain may be deployed via the carboxy-terminal β-barrel for presentation at the cell surface but remain covalently attached to the rest of the molecule (35). Alternatively, the passenger domain may be proteolytically cleaved from the precursor protein at the bacterial surface, where it either retains contact via noncovalent interactions (40) or is freely secreted into the surrounding environment (15).
To determine the fate of the amino-terminal passenger domain of EatA, we performed immunoblots of concentrated supernatants with antibodies raised against a recombinant protein fragment representing amino acid residues 88 to 581 (Fig. 3A). These antibodies recognized a protein of 110 kDa in concentrated supernatants from cultures of ETEC H01407 (Fig. 3B). This finding is consistent with the size reported for the secreted passenger domain of SepA (3, 4). This molecule was not detected in supernatants of either the TnphoA.977 strain that bears an insertion in eatA or H10407-P, further supporting the localization of the eatA gene to the 92-kDa pCS1 plasmid.
In addition, we analyzed concentrated culture supernatants from a subset of clinical ETEC strains. As shown in Fig. 3C, two additional clinical ETEC strains, H432A and ThroopD, which possess the eatA gene as demonstrated by PCR and DNA hybridization studies, also secrete a molecule of identical molecular weight. Conversely, two eatA-negative clinical ETEC isolates, B7A and EDL903, did not secrete a 110-kDa protein recognized by anti-recombinant EatA antibodies. Finally, E. coli DH10BT1 bearing the eatA gene cloned on a recombinant plasmid, pSP009, produced a 110-kDa protein identical in size to that produced by all of the eatA-positive clinical strains. Together, these findings suggest that eatA, like sepA of Shigella flexneri, is located on a virulence plasmid and that it encodes an autotransporter molecule that freely releases the passenger domain into external milieu.
EatA is a serine protease.
Because of the significant homology of EatA to members of the SPATE family, we performed assays to detect serine protease activity within the EatA passenger domain. We tested the ability of concentrated supernatants from recombinant E. coli expressing eatA to cleave synthetic oligopeptides labeled with paranitroanilide. The EatA passenger domain, similar to that of SepA (4), cleaved substrates that are classically identified as substrates for cathepsin G (41), an antibacterial serine protease from human neutrophils (Fig. 4A). Similar to cathepsin G, the EatA passenger was most highly active in cleavage of methoxysuccinyl (MeOSuc)-Ala-Ala-Pro-Met-p-nitroanilide (pNA) (AAPM).
FIG. 4.
(A) Cleavage of synthetic p-nitroanilide oligopeptide substrates by recombinant EatA protein. (AAPM = MeOSuc-Ala-Ala-Pro-Met-pNA, AAPL = Suc-Ala-Ala-Pro-Leu-pNA, AAPF = Suc-Ala-Ala-Pro-Phe-pNA, AAPV = MeSuc-Ala-Ala-Pro-Val-pNA, MeOH, GGF = Suc-Gly-Gly-Phe-pNA, VPF = Suc-Val-Pro-Phe-pNA, FLF = Suc-Phe-Leu-Phe-pNA). Hydrolysis of each substrate was assessed by monitoring the increase in absorption over time at 405 nm. Activity was then expressed as Vmax in milliunits min−1. Values for each peptide represent the average of the Vmax data from three independent experiments relative to the values obtained for cleavage of AAPM substrate under the same conditions. (B) Effect of mutations within the putative serine protease catalytic triad or addition of phenylmethylsulfonyl fluoride on EatA enzymatic activity. Values represent activity of each mutant protein on the indicated substrate relative to the parent protein. (C) Mutations in the serine protease catalytic triad have no effect on the secretion of the EatA passenger domain. Immunoblots of concentrated supernatants from LMG194 bearing pBAD-TOPO-based plasmids expressing either the wild-type eatA gene (pSP014) or serine protease mutants (pSP018, pSP020, and pSP019, respectively). Concentrated supernatants from the ΔeatA deletion strain (eatA::Cm) and the deletion strain complemented with either the pBAD-TOPO vector containing a lacZ control insert (pSP013) or pSP014 are shown for comparison.
Previously, Fink et al. used ClustalW alignments of the amino acid sequences of different autotransporters, including SepA, to identify a candidate serine protease motif for the Haemophilus influenzae autotransporter Hap (15). Based on these data and a ClustalW alignment of EatA and SepA (Fig. 2), we predicted that residues H134, D162, and S267 could constitute the serine protease catalytic triad located within the EatA passenger domain. These data were also supported by predictions from the MEROPS protease database (http://merops.sanger.ac.uk/) which identified the corresponding SepA residues as comprising the potential active site of this molecule. Site-directed mutagenesis of these residues within the candidate triad, similar to the addition of the serine protease inhibitor phenylmethylsulfonyl fluoride, totally abolished the ability of the passenger domain to cleave pNA-labeled AAPM or AAPL (ALa-Ala-Pro-Leu) substrates (Fig. 4B).
Some members of the autotransporter family, such as Hap, rely on autoproteolysis for processing and release of the passenger domain from the outer membrane (15). However, immunoblot analysis of concentrated supernatants from recombinant E. coli expressing either wild-type eatA or versions bearing mutations within the serine protease catalytic triad demonstrated that the passenger domain is normally released from the bacterial surface via a process that does not involve this particular motif (Fig. 4C).
EatA leads to accelerated virulence in the rabbit ileal loop model of infection.
To assess the contribution of eatA to the virulence of the H10407 prototype ETEC strain, we tested the wild-type and ΔeatA strains in the rabbit ileal loop model. In these experiments, we saw no difference in the degree of loop fluid accumulation when the wild type was compared to the ΔeatA mutant after 16 h. Similarly, we were not able to demonstrate any difference between the mutant and wild type with respect to growth, secretion of heat-labile toxin, or the ability of these strains to stimulate a cyclic AMP response in target epithelial cells (data not shown). However, at 7 h following infection of the loops, we observed considerably more fluid in the loops containing the wild-type compared to those containing the mutant strain (Fig. 5A). We were not able to detect any difference in the number of organisms surviving in the loops at the end of 7 h, suggesting that the differences in fluid accumulation are not directly related to altered survival of the pathogen.
FIG. 5.
(A) EatA leads to accelerated virulence in a rabbit model of ETEC infections. The graph represents data obtained from six separate rabbit experiments. In each experiment, ETEC H10407 (wild type) was tested against the eatA deletion strain (▵) and uninfected (control) loops. (B) Histopathology of rabbit ileal loops 7 h after inoculation with bacteria. Images (×10 magnification) represent separate loops obtained from the same rabbit.
Histopathology of rabbit ileal loops obtained 7 h after inoculation with the wild-type strain demonstrated focal areas of mucosal destruction with infiltration of leukocytes in the mucosa and submucosa (Fig. 5B). The remainder of the mucosa appeared normal, as did specimens from loops infected with the negative control and the ΔeatA mutant. Together, these data support a model in which EatA is not absolutely required for virulence but may serve to accelerate the virulence of ETEC through a mechanism independent of the known virulence factors.
DISCUSSION
The clinical presentation of disease caused by ETEC varies widely, ranging from mild diarrhea to more severe cholera-like illness with accompanying rapid fluid loss and dehydration. Indeed, ETEC strains, including H10407, were originally isolated from patients with diarrheal illness clinically indistinguishable from cholera (52, 53). However, the properties that distinguish strains associated with severe disease from those associated with milder infections are as yet poorly understood. While ETEC strains are defined by the production of heat-labile toxin and/or heat-stable toxin, additional traits that may contribute to the virulence of these pathogens remain largely unexplored. Data from these and other studies support the concept that E. coli strains bearing genes for production of these toxins are otherwise genetically diverse.
Two of the eatA+ strains used in this study, H10407 and ThroopD, have been associated with severe clinical illness. ETEC H10407 was originally isolated from a patient with severe cholera-like diarrhea in Bangladesh (14). Similarly, strain ThroopD was originally isolated from a patient with over 50 liters of stool output over 4 days (16). Recently, in studies of ETEC in a mouse challenge model, Byrd et al. demonstrated that H10407 (O78:H11:CFA/I:LT+:ST+) was consistently more virulent than strain B7A (O148:H28:CS6:LT+:ST+) (7), which we demonstrate in these studies to lack the eatA gene. Human challenge studies with these ETEC strains have also suggested that H10407 is more virulent. In studies by Levine et al., H10407 required 20-fold fewer organisms (5 × 108) than B7A (1 × 1010) to induce diarrhea in orally challenged human volunteers. Likewise, the former strain caused diarrhea in 100% of volunteers, while B7A produced diarrhea in only 67% despite the higher dose (33).
In the classic paradigm for diarrheal disease caused by ETEC, the organisms elaborate heat-labile toxin or heat-stable toxin in the small intestine, eliciting fluid secretion without significant inflammation (21). However, a number of recent clinical studies suggest that the pathogenesis of human ETEC infections may be considerably more complex than previously appreciated. Bouckenooghe et al. demonstrated that among travelers with diarrhea in which ETEC was the only pathogen isolated, more than 25% had fecal leukocytes or elevated lactoferrin values (6). Similarly, in travelers with bacterial diarrhea due to ETEC, fecal cytokines, including interleukin-8 and interleukin-1 receptor alpha, were elevated to a greater extent than in cases of Salmonella infection (21). One potential implication of these studies is that there may be additional virulence determinants of ETEC that have not yet been described. While the role of the host inflammatory response in the pathogenesis of human ETEC infections has yet to be investigated, prior studies suggest that neutrophil migration itself may stimulate fluid secretion via a number of different mechanisms (18, 36).
While the precise function of the EatA protein described here remains to be defined, the results of the rabbit ileal loop experiments are similar to those attributed to SepA in Shigella flexneri (3). Benjelloun-Touimi et al. demonstrated that a sepA deletion mutation resulted in decreased fluid accumulation and inflammation in rabbit ileal loops relative to the wild-type S. flexneri strain. Similarly, we found that deletion of eatA led to attenuation of virulence in this model. Our inability to distinguish the wild type from the eatA deletion mutant in the loop model on prolonged incubation may be explained by the fact that the ΔeatA strain retained the capacity to effectively secrete and deliver heat-labile toxin.
Herein we demonstrate that EatA, like many other autotransporters produced by the Enterobacteriaceae, is a serine protease. While some molecules, such as Hap produced by H. influenzae, undergo autoproteolysis through an intramolecular mechanism that requires serine protease activity (15), processing of EatA occurred independently of the catalytic triad. EatA, like SepA (4), cleaved peptides that have previously been defined as substrates for cathepsin G, a serine protease produced by polymorphonuclear leukocytes which modulates or cleaves a diverse array of extracellular products, including proteoglycans (51), as well as cell surface protease-activated receptors (54). Although the protease activity of some of the autotransporters appears to be required for virulence (42), the relationship between the enzymatic and potential virulence functions of EatA is uncertain.
The initial findings described here suggest that eatA, which encodes a previously uncharacterized autotransporter of ETEC, contributes to the virulence of H10407. The finding that H10407 and another clinical isolate previously associated with severe disease produce EatA is intriguing; theoretically, the presence of the autotransporter might also lead to accelerated virulence in the course of human infections. Elucidation of a defined role for EatA in the pathogenesis of ETEC infections in humans could have potential implications for vaccine development, as it represents a surface-expressed target that is present in more than half of the clinical strains tested to date. Likewise, the ability to selectively protect against organisms that have the capacity to cause accelerated infections would be particularly important in reducing the number of deaths that ultimately result from rapid dehydration.
Acknowledgments
We thank Marcia Wolf for providing the isolated virulence plasmids from H10407 and Steven Savarino and Richard Finklestein for supplying the laboratory with ETEC strains H10407-P and ThroopD, respectively. We also thank Michael Donnenberg for supplying the TnphoA donor construct employed here and Alan Mast for advice regarding the oligopeptide cleavage assays. We are indebted to Stanley D. Kosanke for expeditious processing of the histopathology specimens. We thank Harry Courtney, James Dale, David Hasty and Dennis Kopecko for critical reviews of the manuscript.
This study was supported by grants from the Department of Veterans Affairs (to J.M.F.) and the National Institutes of Health (to J.M.F.) and by the NIH Medical Student Research Fellowship Program (to J.D.).
Editor: B. B. Finlay
REFERENCES
- 1.Al-Hasani, K., I. R. Henderson, H. Sakellaris, K. Rajakumar, T. Grant, J. P. Nataro, R. Robins-Browne, and B. Adler. 2000. The sigA gene which is borne on the she pathogenicity island of Shigella flexneri 2a encodes an exported cytopathic protease involved in intestinal fluid accumulation. Infect. Immun. 68:2457-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 3.Benjelloun-Touimi, Z., P. J. Sansonetti, and C. Parsot. 1995. SepA, the major extracellular protein of Shigella flexneri: autonomous secretion and involvement in tissue invasion. Mol. Microbiol. 17:123-135. [DOI] [PubMed] [Google Scholar]
- 4.Benjelloun-Touimi, Z., M. S. Tahar, C. Montecucco, P. J. Sansonetti, and C. Parsot. 1998. SepA, the 110-kDa protein secreted by Shigella flexneri: two-domain structure and proteolytic activity. Microbiology 144:1815-1822. [DOI] [PubMed] [Google Scholar]
- 5.Blomfield, I. C., V. Vaughn, R. F. Rest, and B. I. Eisenstein. 1991. Allelic exchange in Escherichia coli with the Bacillus subtilis sacB gene and a temperature-sensitive pSC101 replicon. Mol. Microbiol. 5:1447-1457. [DOI] [PubMed] [Google Scholar]
- 6.Bouckenooghe, A. R., H. L. Dupont, Z. D. Jiang, J. Adachi, J. J. Mathewson, M. P. Verenkar, S. Rodrigues, and R. Steffen. 2000. Markers of enteric inflammation in enteroaggregative Escherichia coli diarrhea in travelers. Am. J. Trop. Med. Hyg. 62:711-713. [DOI] [PubMed] [Google Scholar]
- 7.Byrd, W., S. R. Mog, and F. J. Cassels. 2003. Pathogenicity and immune response measured in mice following intranasal challenge with enterotoxigenic Escherichia coli strains H10407 and B7A. Infect. Immun. 71:13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chao, A. C., F. J. de Sauvage, Y. J. Dong, J. A. Wagner, D. V. Goeddel, and P. Gardner. 1994. Activation of intestinal CFTR Cl-channel by heat-stable enterotoxin and guanylin via cyclic AMP-dependent protein kinase. EMBO J. 13:1065-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Craig, N. L. 1996. Transposon Tn7. Curr. Top. Microbiol. Immunol. 204:27-48. [DOI] [PubMed] [Google Scholar]
- 10.Donnenberg, M. S., S. B. Calderwood, A. Donohue-Rolfe, G. T. Keusch, and J. B. Kaper. 1990. Construction and analysis of TnphoA mutants of enteropathogenic Escherichia coli unable to invade HEp-2 cells. Infect. Immun. 58:1565-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edwards, R. A., L. H. Keller, and D. M. Schifferli. 1998. Improved allelic exchange vectors and their use to analyze 987P fimbria gene expression. Gene 207:149-157. [DOI] [PubMed] [Google Scholar]
- 12.Evans, D. G., D. J. Evans, Jr., and H. L. DuPont. 1977. Virulence factors of enterotoxigenic Escherichia coli. J. Infect. Dis. 136(Suppl.):S118-S123. [DOI] [PubMed] [Google Scholar]
- 13.Evans, D. G., D. Y. Graham, and D. J. Evans, Jr. 1984. Administration of purified colonization factor antigens (CFA/I, CFA/II) of enterotoxigenic Escherichia coli to volunteers. Response to challenge with virulent enterotoxigenic Escherichia coli. Gastroenterology 87:934-940. [PubMed] [Google Scholar]
- 14.Evans, D. G., R. P. Silver, D. J. Evans, Jr., D. G. Chase, and S. L. Gorbach. 1975. Plasmid-controlled colonization factor associated with virulence in Escherichia coli enterotoxigenic for humans. Infect. Immun. 12:656-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fink, D. L., L. D. Cope, E. J. Hansen, and J. W. Geme III. 2001. The Hemophilus influenzae Hap autotransporter is a chymotrypsin clan serine protease and undergoes autoproteolysis via an intermolecular mechanism. J. Biol. Chem. 276:39492-39500. [DOI] [PubMed] [Google Scholar]
- 16.Finkelstein, R. A., M. L. Vasil, J. R. Jones, R. A. Anderson, and T. Barnard. 1976. Clinical cholera caused by enterotoxigenic Escherichia coli. J. Clin. Microbiol. 3:382-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fleckenstein, J. M., J. T. Holland, and D. L. Hasty. 2002. Interaction of an outer membrane protein of enterotoxigenic Escherichia coli with cell surface heparan sulfate proteoglycans. Infect. Immun. 70:1530-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fleckenstein, J. M., and D. J. Kopecko. 2001. Breaching the mucosal barrier by stealth: an emerging pathogenic mechanism for enteroadherent bacterial pathogens. J. Clin. Investig. 107:27-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleckenstein, J. M., L. E. Lindler, E. A. Elsinghorst, and J. B. Dale. 2000. Identification of a gene within a pathogenicity island of enterotoxigenic Escherichia coli H10407 required for maximal secretion of the heat-labile enterotoxin. Infect. Immun. 68:2766-2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gabriel, S. E., K. N. Brigman, B. H. Koller, R. C. Boucher, and M. J. Stutts. 1994. Cystic fibrosis heterozygote resistance to cholera toxin in the cystic fibrosis mouse model. Science 266:107-109. [DOI] [PubMed] [Google Scholar]
- 21.Greenberg, D. E., Z. D. Jiang, R. Steffen, M. P. Verenker, and H. L. DuPont. 2002. Markers of inflammation in bacterial diarrhea among travelers, with a focus on enteroaggregative Escherichia coli pathogenicity. J. Infect. Dis. 185:944-949. [DOI] [PubMed] [Google Scholar]
- 22.Guyer, D. M., I. R. Henderson, J. P. Nataro, and H. L. Mobley. 2000. Identification of sat, an autotransporter toxin produced by uropathogenic Escherichia coli. Mol. Microbiol. 38:53-66. [DOI] [PubMed] [Google Scholar]
- 23.Guzman, L. M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henderson, I. R., R. Cappello, and J. P. Nataro. 2000. Autotransporter proteins, evolution and redefining protein secretion. Trends Microbiol. 8:529-532. [DOI] [PubMed] [Google Scholar]
- 25.Henderson, I. R., J. Czeczulin, C. Eslava, F. Noriega, and J. P. Nataro. 1999. Characterization of Pic, a secreted protease of Shigella flexneri and enteroaggregative Escherichia coli. Infect. Immun. 67:5587-5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henderson, I. R., and J. P. Nataro. 2001. Virulence functions of autotransporter proteins. Infect. Immun. 69:1231-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henderson, I. R., F. Navarro-Garcia, and J. P. Nataro. 1998. The great escape: structure and function of the autotransporter proteins. Trends Microbiol. 6:370-378. [DOI] [PubMed] [Google Scholar]
- 28.Killmann, H., R. Benz, and V. Braun. 1996. Properties of the FhuA channel in the Escherichia coli outer membrane after deletion of FhuA portions within and outside the predicted gating loop. J. Bacteriol. 178:6913-6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolter, R., M. Inuzuka, and D. R. Helinski. 1978. Trans-complementation-dependent replication of a low molecular weight origin fragment from plasmid R6K. Cell 15:1199-1208. [DOI] [PubMed] [Google Scholar]
- 30.Kyte, J., and R. F. Doolittle. 1982. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157:105-132. [DOI] [PubMed] [Google Scholar]
- 31.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 32.Lederer, W., and P. Echeverria. 1989. Enterotoxigenic Escherichia coli associated diarrhea: the clinical pattern in Khmer children. Acta Leiden. 58:141-150. [PubMed] [Google Scholar]
- 33.Levine, M. M., R. E. Black, C. C. Brinton, Jr., M. L. Clements, P. Fusco, T. P. Hughes, S. O'Donnell, R. Robins-Browne, S. Wood, and C. R. Young. 1982. Reactogenicity, immunogenicity and efficacy studies of Escherichia coli type 1 somatic pili parenteral vaccine in man. Scand. J. Infect. Dis. Suppl. 33:83-95. [PubMed] [Google Scholar]
- 34.Lindenthal, C., and E. A. Elsinghorst. 2001. Enterotoxigenic Escherichia coli TibA glycoprotein adheres to human intestine epithelial cells. Infect. Immun. 69:52-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindenthal, C., and E. A. Elsinghorst. 1999. Identification of a glycoprotein produced by enterotoxigenic Escherichia coli. Infect. Immun. 67:4084-4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madara, J., T. Patapoff, B. Gillece-Castro, S. Colgan, C. Parkos, C. Delp, and R. Mrsny. 1993. 5′-adenosine monophosphate is the neutraphil-derived paracrine factor that elicits chloride secretion from T84 intestinal epithelial cell monolayers. J. Clin. Investig. 91:2320-2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manoil, C., and J. Beckwith. 1985. TnphoA: a transposon probe for protein export signals. Proc. Natl. Acad. Sci. USA 82:8129-8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McConnell, M. M., M. L. Hibberd, M. E. Penny, S. M. Scotland, T. Cheasty, and B. Rowe. 1991. Surveys of human enterotoxigenic Escherichia coli from three different geographical areas for possible colonization factors. Epidemiol. Infect. 106:477-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mellies, J. L., F. Navarro-Garcia, I. Okeke, J. Frederickson, J. P. Nataro, and J. B. Kaper. 2001. espC pathogenicity island of enteropathogenic Escherichia coli encodes an enterotoxin. Infect. Immun. 69:315-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moormann, C., I. Benz, and M. A. Schmidt. 2002. Functional substitution of the TibC protein of enterotoxigenic Escherichia coli strains for the autotransporter adhesin heptosyltransferase of the AIDA system. Infect. Immun. 70:2264-2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakajima, K., J. C. Powers, B. M. Ashe, and M. Zimmerman. 1979. Mapping the extended substrate binding site of cathepsin G and human leukocyte elastase. Studies with peptide substrates related to the alpha 1-protease inhibitor reactive site. J. Biol. Chem. 254:4027-4032. [PubMed] [Google Scholar]
- 42.Navarro-Garcia, F., C. Sears, C. Eslava, A. Cravioto, and J. P. Nataro. 1999. Cytoskeletal effects induced by Pet, the serine protease enterotoxin of enteroaggregative Escherichia coli. Infect. Immun. 67:2184-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nielsen, H., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10:1-6. [DOI] [PubMed] [Google Scholar]
- 44.Otto, B. R., S. J. van Dooren, J. H. Nuijens, J. Luirink, and B. Oudega. 1998. Characterization of a hemoglobin protease secreted by the pathogenic Escherichia coli strain EB1. J. Exp. Med. 188:1091-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peruski, L. F., Jr., B. A. Kay, R. A. El-Yazeed, S. H. El-Etr, A. Cravioto, T. F. Wierzba, M. Rao, N. El-Ghorab, H. Shaheen, S. B. Khalil, K. Kamal, M. O. Wasfy, A. M. Svennerholm, J. D. Clemens, and S. J. Savarino. 1999. Phenotypic diversity of enterotoxigenic Escherichia coli strains from a community-based study of pediatric diarrhea in periurban Egypt. J. Clin. Microbiol. 37:2974-2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peters, J. E., and N. L. Craig. 2001. Tn7: smarter than we thought. Nat. Rev. Mol. Cell. Biol. 2:806-814. [DOI] [PubMed] [Google Scholar]
- 47.Pohlner, J., R. Halter, K. Beyreuther, and T. F. Meyer. 1987. Gene structure and extracellular secretion of Neisseria gonorrhoeae IgA protease. Nature 325:458-462. [DOI] [PubMed] [Google Scholar]
- 48.Posfai, G., M. D. Koob, H. A. Kirkpatrick, and F. R. Blattner. 1997. Versatile insertion plasmids for targeted genome manipulations in bacteria: isolation, deletion, and rescue of the pathogenicity island LEE of the Escherichia coli O157:H7 genome. J. Bacteriol. 179:4426-4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Provence, D. L., and R. Curtiss III. 1994. Isolation and characterization of a gene involved in hemagglutination by an avian pathogenic Escherichia coli strain. Infect. Immun. 62:1369-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruvkun, G. B., and F. M. Ausubel. 1981. A general method for site-directed mutagenesis in prokaryotes. Nature 289:85-88. [DOI] [PubMed] [Google Scholar]
- 51.Sabri, A., S. G. Alcott, H. Elouardighi, E. Pak, C. Derian, P. Andrade-Gordon, K. Kinnally, and S. F. Steinberg. 2003. Neutrophil cathepsin G promotes detachment-induced cardiomyocyte apoptosis via a protease-activated receptor-independent mechanism. J. Biol. Chem. 278:23944-23954. [DOI] [PubMed] [Google Scholar]
- 52.Sack, D. A., J. C. McLaughlin, R. B. Sack, F. Orskov, and I. Orskov. 1977. Enterotoxigenic Escherichia coli isolated from patients at a hospital in Dacca. J. Infect. Dis. 135:275-280. [DOI] [PubMed] [Google Scholar]
- 53.Sack, R. B., S. L. Gorbach, J. G. Banwell, B. Jacobs, B. D. Chatterjee, and R. C. Mitra. 1971. Enterotoxigenic Escherichia coli isolated from patients with severe cholera-like disease. J. Infect. Dis. 123:378-385. [DOI] [PubMed] [Google Scholar]
- 54.Sambrano, G. R., W. Huang, T. Faruqi, S. Mahrus, C. Craik, and S. R. Coughlin. 2000. Cathepsin G activates protease-activated receptor-4 in human platelets. J. Biol. Chem. 275:6819-6823. [DOI] [PubMed] [Google Scholar]
- 55.Sambrook, J., and D. W. Russell. 2001. Lysing colonies and binding of DNA on filters, p. 1.135-1.137. In N. Irwin (ed.), Molecular cloning: a laboratory manual, vol. 1. Cold Spring Harbor Press, Cold Spring Harbor, N.Y. [Google Scholar]
- 56.Simon, R., U. Priefer, and A. Puhler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 57.Stalker, D. M., R. Kolter, and D. R. Helinski. 1982. Plasmid R6K DNA replication. I. Complete nucleotide sequence of an autonomously replicating segment. J. Mol. Biol. 161:33-43. [DOI] [PubMed] [Google Scholar]
- 58.Stein, M., B. Kenny, M. A. Stein, and B. B. Finlay. 1996. Characterization of EspC, a 110-kilodalton protein secreted by enteropathogenic Escherichia coli which is homologous to members of the immunoglobulin A protease-like family of secreted proteins. J. Bacteriol. 178:6546-6554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taylor, R. K., C. Manoil, and J. J. Mekalanos. 1989. Broad-host-range vectors for delivery of TnphoA: use in genetic analysis of secreted virulence determinants of Vibrio cholerae. J. Bacteriol. 171:1870-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Veiga, E., E. Sugawara, H. Nikaido, V. de Lorenzo, and L. A. Fernandez. 2002. Export of autotransported proteins proceeds through an oligomeric ring shaped by C-terminal domains. EMBO J. 21:2122-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Venkatesan, M. M., M. B. Goldberg, D. J. Rose, E. J. Grotbeck, V. Burland, and F. R. Blattner. 2001. Complete DNA sequence and analysis of the large virulence plasmid of Shigella flexneri. Infect. Immun. 69:3271-3285. [DOI] [PMC free article] [PubMed] [Google Scholar]