Abstract
Highly purified vitamin B2 (riboflavin 5′-sodium phosphate; purity > 97%) treatment by intravenous infusion at doses above those used clinically to treat vitamin B2 deficiency showed therapeutic effects in mice not only in cases of endotoxin- and exotoxin-induced shock but also in cases of gram-negative and gram-positive bacterial infection even after the toxemia had already begun.
Despite the advances in antibiotic therapy and intensive care medicine, sepsis still has a high mortality rate (1, 6, 8, 18). Considering that etiological pathogenesis is complicated (7, 14) and that patients are very heterogenous regarding underlying illness, site of infection, microbiological etiology, and physiological derangement (2), it is not surprising that strategies only block each detrimental factor and that treatment with drugs that were discovered by pretreatment in a single sepsis model has been shown to have disappointing outcomes (5, 9, 13, 16). Apart from these approaches it has been previously reported that vitamin B2 enhanced host resistance to infection (3), stimulated neutrophil functions (17), and boosted macrophage function (15). The purpose of this study was to evaluate (on the basis of a new immunotherapeutic concept) the therapeutic effects of highly purified vitamin B2 on levels of mouse mortality caused by toxin-induced shock and bacterial infection.
Highly purified vitamin B2 (riboflavin 5′-sodium phosphate; purity > 97%) was purchased from Sigma (St. Louis, Mo.) or was synthesized at Kashima Plant, Eisai Co., Ltd. Human activated protein C (Anact C [APC]) was purchased from Teijin (Tokyo, Japan). Endotoxin (Escherichia coli O111:B4 lipopolysaccharide [LPS]; Sigma), gram-positive bacteria (a clinical isolate of Staphylococcus aureus E311122), and gram-negative bacteria (a clinical isolate of E. coli E01292) were intravenously injected into male ICR mice (6 weeks of age), and exotoxin (S. aureus enterotoxin B [SEB]; Toxin Technology, Inc., Sarasota, Florida) was intraperitoneally injected into BALB/c mice (6 weeks of age). Saline (control), vitamin B2, and APC were intravenously given through a tail vein without anesthesia. Survival rates were determined at 7 days after LPS and SEB injection and E. coli infection. Survival rates were determined at 14 days after S. aureus infection. All experiments were approved by the Animal Care and Use Committee of Eisai.
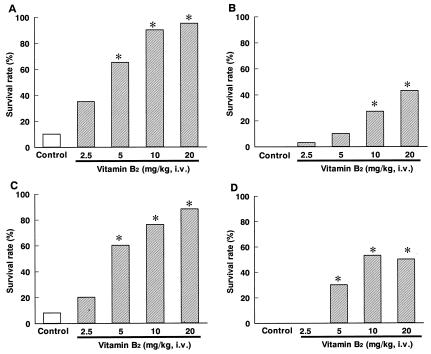
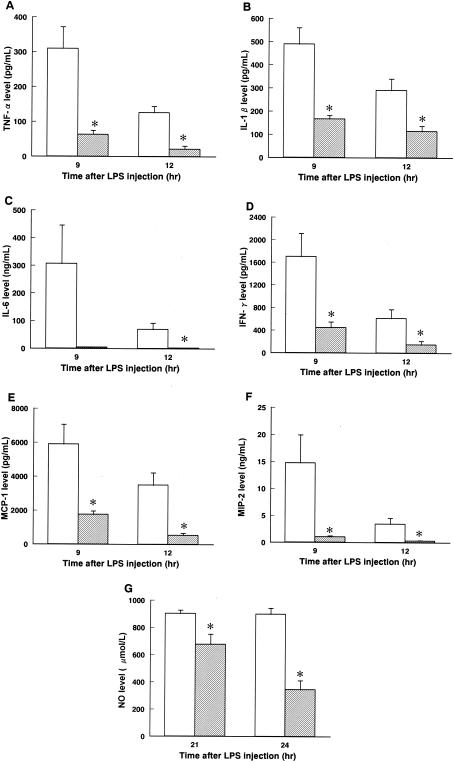
Vitamin B2 was administered with a bolus injection 6 h after LPS injection. The survival rate of the control group was 10% (2 of 20). At doses of vitamin B2 of 2.5, 5, 10, and 20 mg/kg of body weight, survival levels were 35% (7 of 20), 65% (13 of 20 [P < 0.05]), 90% (18 of 20 [P < 0.05]), and 95% (19 of 20 [P < 0.05]), respectively (Fig. 1A). Moreover, treatment with vitamin B2 at 20 mg/kg 6 h after LPS injection not only lowered levels of excessive plasma proinflammatory cytokines, including tumor necrosis factor alpha (Fig. 2A), interleukin-1 beta (IL-1β) (Fig. 2B), IL-6 (Fig. 2C), gamma interferon (Fig. 2D), monocyte chemotactic protein 1 (Fig. 2E), and macrophage inflammatory protein 2 (Fig. 2F), but also decreased NO levels (Fig. 2G).
FIG. 1.
Effects of intravenous (i.v.) bolus injection of highly purified vitamin B2 on levels of mouse mortality caused by toxin-induced shock and bacterial infection. (A) LPS-induced shock; (B) SEB-induced shock; (C) E. coli infection; (D) S. aureus infection. *, P < 0.05 versus the results for the control group (Steel test).
FIG. 2.
Effect of highly purified vitamin B2 on plasma cytokine and NO levels in cases of LPS-induced shock. (A) Tumor necrosis factor alpha (TFN-α); (B) IL-1β; (C) IL-6; (D) gamma interferon (IFN-γ); (E) monocyte chemotactic protein 1 (MCP-1); (F) macrophage inflammatory protein 2 (MIP-2); (G) NO. Open columns and hatched columns indicate saline (control)- and vitamin B2-treated groups, respectively. Data are expressed as means ± standard errors of the means (n = 6). *, P < 0.05 versus the results for the control group (unpaired t test).
At 6 h after injection of SEB-d-galactosamine, vitamin B2 was administered with a bolus injection. The survival rate of the control group was 0% (0 of 30). The survival rates of the groups treated with vitamin B2 at 2.5, 5, 10, and 20 mg/kg were 3% (1 of 30), 10% (3 of 30), 27% (8 of 30 [P < 0.05]), and 43% (13 of 30 [P < 0.05]), respectively (Fig. 1B).
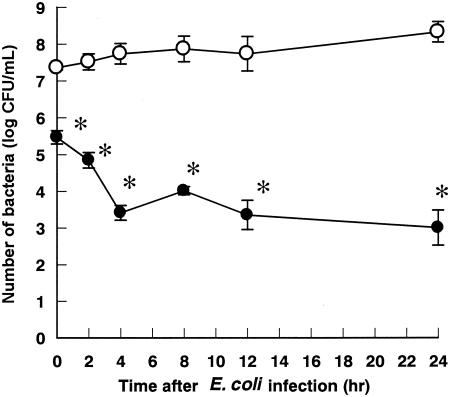
Vitamin B2 was given 24 h before E. coli inoculation (2.07 × 108 CFU). The survival rate of the control group was 8% (2 of 25). The survival rates of the groups treated with vitamin B2 at 2.5, 5, 10, and 20 mg/kg were 20% (5 of 25), 60% (15 of 25 [P < 0.05]), 76% (19 of 25 [P < 0.05]), and 88% (22 of 25 [P < 0.05]), respectively (Fig. 1C). In addition, vitamin B2 administered at 20 mg/kg 24 h before E. coli inoculation significantly reduced levels of the bacteria in the blood (Fig. 3).
FIG. 3.
Effect of highly purified vitamin B2 on the clearance of E. coli from blood in mice. Open and closed circles represent saline (control)- and vitamin B2-treated groups, respectively.. Each value represents the mean ± standard error of the mean (n = 5). *, P < 0.05 versus the results for the control group (unpaired t test).
Vitamin B2 was given with a bolus injection immediately and 1 and 2 days after S. aureus inoculation (1.69 × 108 CFU). The survival rate of the control group was 0% (0 of 30). The survival rates of the groups treated with vitamin B2 at 2.5, 5, 10, and 20 mg/kg were 0% (0 of 29), 30% (9 of 30 [P < 0.05]), 53% (16 of 30 [P < 0.05]), and 50% (15 of 30 [P < 0.05]), respectively (Fig. 1D).
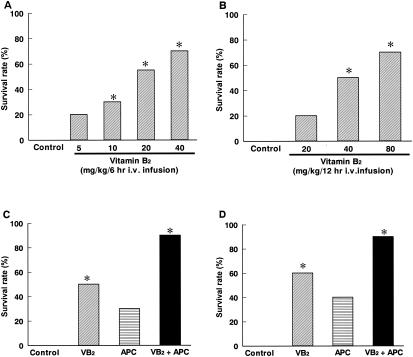
Furthermore, we investigated the therapeutic effect of vitamin B2 in long-term intravenous treatment by infusion using an infusion pump (Natsume, Tokyo, Japan). Infusion of vitamin B2 for 6 h (E. coli infection) or 12 h (S. aureus infection) was begun 1 h after inoculation with bacteria. In the E. coli infection experiments, the survival rate of the control group was 0% (0 of 30). Survival rates of the groups treated with vitamin B2 at 5, 10, 20, and 40 mg/kg/6 h were 20% (2 of 10), 30% (3 of 10 [P < 0.05]), 55% (11 of 20 [P < 0.05]), and 70% (7 of 10 [P < 0.05]), respectively (Fig. 4A). In the S. aureus infection experiments, the survival rate of the control group was 0% (0 of 10) and the survival rates of the groups treated with vitamin B2 at 20, 40, and 80 mg/kg/12 h were 20% (2 of 10), 50% (5 of 10 [P < 0.05]), and 70% (7 of 10 [P < 0.05]), respectively (Fig. 4B).
FIG. 4.
Therapeutic effect of intravenous (i.v.) infusion of highly purified vitamin B2 (VB2) on levels of mouse mortality caused by bacterial infection and toxin-induced shock. (A) E. coli infection; (B) S. aureus infection; (C) LPS-induced shock; (D) SEB-induced shock. *, P < 0.05 versus the results for the control group (Steel test).
Infusion of vitamin B2 with or without APC for 6 h was commenced 6 h after LPS or SEB injection. The survival rate of the control group was 0% (0 of 10) for LPS-treated mice. The survival rates of the groups treated with vitamin B2 (10 mg/kg/6 h), APC (75 units/kg/6 h), and vitamin B2 plus APC were 50% (5 of 10 [P < 0.05]), 30% (3 of 10), and 90% (9 of 10 [P < 0.05]), respectively (Fig. 4C). In SEB-treated mice, the survival rates of the control group and the groups treated with vitamin B2 (20 mg/kg/6 h), APC (300 units/kg/6 h), and vitamin B2 plus APC were 0% (0 of 10), 60% (6 of 10 [P < 0.05]), 40% (4 of 10), and 90% (9 of 10 [P < 0.05]), respectively (Fig. 4D).
Treatment with vitamin B2 lowered the levels of cytokines (4, 10, 11) and NO (12) that are implicated in the pathogenesis of sepsis and likely enhanced the eradication of bacteria. Thus, vitamin B2 may provide an approach for immunotherapy in the treatment of many inflammatory diseases (including systemic inflammatory response syndrome, cachexia, chronic rheumatism, and nephritis) without excessive suppression of the host defense system. Simultaneous administration of vitamin B2 with APC reduced the mortality of endotoxin- and exotoxin-induced shock, suggesting that therapy with vitamin B2 combined with agents having other properties could be an effective approach to treat sepsis.
In conclusion, treatment with highly purified vitamin B2 (riboflavin 5′-sodium phosphate; purity > 97%) reduced levels of mortality in mice with septic shock and may be useful for the treatment of patients with sepsis.
Editor: F. C. Fang
REFERENCES
- 1.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference. 1992. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit. Care Med. 20:864-874. [PubMed] [Google Scholar]
- 2.Angus, D. C., and R. S. Wax. 1991. Epidemiology of sepsis: an update. Crit. Care Med. 29:S109-S116. [DOI] [PubMed] [Google Scholar]
- 3.Araki, S., M. Suzuki, M. Fujimoto, and M. Kimura. 1995. Enhancement of resistance to bacterial infection in mice by vitamin B2. J. Vet. Med. Sci. 57:599-602. [DOI] [PubMed] [Google Scholar]
- 4.Barriere, S. L., and S. F. Lowry. 1995. An overview of mortality risk prediction in sepsis. Crit. Care Med. 23:376-393. [DOI] [PubMed] [Google Scholar]
- 5.Bernard, G. R., A. P. Wheeler, J. A. Russell, R. Schein, W. R. Summer, K. P. Steinberg, W. J. Fulkerson, P. E. Wright, B. W. Christman, W. D. Dupont, S. B. Higgins, B. B. Swindell, and The Ibuprofen in Sepsis Study Group. 1997. The effects of ibuprofen on the physiology and survival of patients with sepsis. N. Engl. J. Med. 336:912-918. [DOI] [PubMed] [Google Scholar]
- 6.Bone, R. C. 1991. Let's agree on terminology: definitions of sepsis. Crit. Care Med. 19:973-976. [DOI] [PubMed] [Google Scholar]
- 7.Bone, R. C. 1991. The pathogenesis of sepsis. Ann. Intern. Med. 115:457-469. [DOI] [PubMed] [Google Scholar]
- 8.Cohen, J. 2002. The immunopathogenesis of sepsis. Nature 420:885-891. [DOI] [PubMed] [Google Scholar]
- 9.Dhainaut, J. F., A. Tenaillon, M. Hemmer, P. Damas, P., Y. Le Tulzo, P. Radermacher, M. D. Schaller, J. P. Sollet, M. Wolff, L. Holzapfel, F. Zeni, J. M. Vedrinne, F. de Vathaire, M. L. Gourlay, P. Guinot, J. P. Mira, and the BN 52021 Sepsis Investigator Group. 1998. Confirmatory platelet-activating factor receptor antagonist trial in patients with severe gram-negative bacterial sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. Crit. Care Med. 26:1963-1971. [DOI] [PubMed] [Google Scholar]
- 10.Dinarello, C. A. 1997. Proinflammatory and anti-inflammatory cytokines as mediators in the pathogenesis of septic shock. Chest 112:321S-329S. [DOI] [PubMed]
- 11.Dinarello, C. A. 2000. Proinflammatory cytokines. Chest 118:503-508. [DOI] [PubMed] [Google Scholar]
- 12.Endo, S., K. Inada, H. Nakae, N. Arakawa, T. Takakuwa, Y. Yamada, T. Shimamura, T. Suzuki, S. Taniguchi, and M. Yoshida. 1996. Nitrite/nitrate oxide (NOx) and cytokine levels in patients with septic shock. Res. Commun. Mol. Pathol. Pharmacol. 91:347-356. [PubMed] [Google Scholar]
- 13.Fein, A. M., G. R. Bernard, G. J. Criner, E. C. Fletcher, J. T. Good, Jr., W. A. Knaus, H. Levy, G. M. Matuschak, H. M. Shanies, R. W. Taylor, T. C. Rodell, and the CP-0127 SIRS and Sepsis Study Group. 1997. Treatment of severe systemic inflammatory response syndrome and sepsis with a novel bradykinin antagonist, deltibant (CP-0127). Results of a randomized, double-blind, placebo-controlled trial. JAMA 277:482-487. [PubMed] [Google Scholar]
- 14.Glauser, M. P. 2000. Pathophysiologic basis of sepsis: considerations for future strategies of intervention. Crit. Care Med. 28:S4-S8. [DOI] [PubMed] [Google Scholar]
- 15.Kimura, M., M. Suzuki, and S. Araki. 1996. In vitro and in vivo effects of riboflavin sodium phosphate on the phagocytic activity of peritoneal macrophages in mice. Anim. Sci. Technol. 67:368-373. [Google Scholar]
- 16.Marshall, J. C. 2003. Such stuff as dreams are made on: mediator-directed therapy in sepsis. Nat. Rev. Drug Discov. 2:391-405. [DOI] [PubMed] [Google Scholar]
- 17.Osame, S., S. Araki, and M. Kimura. 1995. Effects of vitamin B2 on neutrophil functions in cattle. J. Vet. Med. Sci. 57:493-495. [DOI] [PubMed] [Google Scholar]
- 18.Parrillo, J. E. 1993. Pathogenetic mechanisms of septic shock. N. Engl. J. Med. 328:1471-1477. [DOI] [PubMed] [Google Scholar]