Abstract
Background:
Microalbuminuria has been described as a marker of generalized vascular damage.
Aims:
The aim of the present study was to determine the prevalence of erectile dysfunction (ED) and microalbuminuria in adult male Nigerians with newly diagnosed hypertension. We also evaluated the relations between ED and microalbuminuria, electrocardiographic left ventricular hypertrophy, serum lipids, and cigarette smoking.
Materials and Methods:
A total of 81 male adult Nigerians with newly diagnosed hypertension were recruited into the study. There were also 75 age- and sex-matched healthy normotensive controls. ED was evaluated using a standardized questionnaire of the International Index of Erectile Function and microalbuminuria was determined using the Micra Test strips (Boehringer Manneheim GMBh, Mannheim, Germany).
Results:
Eighty-one hypertensive patients and 75 normotensive controls were studied. Mean age of the patients and the controls was 53.8 ± 5.6 and 51.2 ± 7.1 respectively. ED was found in 32.1% of the hypertensive patients and 16% of normotensive controls (P < 0.001). The prevalence of microalbuminuria was significantly higher in patients with ED than in those without it (65.4% vs. 23.6%, P < 0.0001).
Conclusion:
The study shows that ED and microalbuminuria are common in male adult Nigerians with hypertension. It also demonstrates that male ED is associated with an increased risk of cardiovascular disease.
Keywords: Erectile dysfunction, Hypertension, Left ventricular hypertrophy, Microalbuminuria
Introduction
Hypertension (HT) is a common cardiovascular disease globally and it causes significant morbidity and mortality. HT affects about one-fifth of adult Nigerians.[1–4] It is a risk factor for erectile dysfunction (ED) in men. Many studies have reported a higher prevalence of ED in hypertensive induviduals compared with normotensive individuals.[5–7] ED is defined as the persistent inability to achieve and/or maintained penile erection sufficient for sexual intercourse.[6] Male ED is associated with markers for increased risk of cardiovascular disease. Microalbuminuria (MA) is an important predictor of cardiovascular risk and complications in hypertensives.[8–10] MA has been described as a marker of generalized vascular damage. HT plays an important role in endothelial damage which may affect the nitric oxide production and/or release leading to ED.[11–13]
There are few Nigerian studies on male ED and HT. Our study focused on ED and MA in newly diagnosed adult male Nigerian hypertensives. The primary objective was to determine the prevalence of MA and ED in newly diagnosed hypertensive Nigerians. In addition, we also evaluated the relations between ED and MA and some other cardiovascular risk factors in essential HT.
Materials and Methods
The study was conducted at the cardiology clinic of a tertiary referral hospital in Nigeria. The study population consisted of 81 newly diagnosed adult male Nigerian hypertensive patients. The study was done in newly diagnosed hypertensive patients who were yet to commence antihypertensive drugs. There were 75 age-and sex-matched healthy normotensive controls. The study protocol was approved by the Ethics and Research Committee of the study center. All the participants gave informed consent and the procedures followed were consistent with institutional guidelines. Individuals with diabetes mellitus, renal disease, endocrine disorder, heart failure, liver disease, prostatic enlargement, psychiatric illness, obesity, and proteinuria were excluded from the study.
All the participants underwent a detailed history and a thorough physical examination. History of alcohol ingestion and cigarette smoking was included. Blood pressure was measured using mercury column sphygmomanometer and cuff of appropriate size. A standardized protocol was followed, in which systolic blood pressure (SBP) and diastolic blood pressures (DBP) were measured on the left arm after the participants had been seated for at least 5 min. Three measurements were done at least 5 min apart and the mean value was used for the study. HT was defined as SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg, or use of antihypertensive drugs.[1] General as well as specific examination of the external genitals for local abnormalities and digital rectal examination for prostatic enlargement were conducted. Venous blood samples were collected and analyzed for fasting plasma glucose (FPG), serum total cholesterol (TC), and serum triglyceride (TG). Resting 12-lead electrocardiogram (ECG) of all the patients and controls were recorded using Schiller AT-1 machine at a sensitivity of 10 mm/mV and a paper speed of 25 mm/sec. ECG left ventricular hypertrophy (LVH) was diagnosed based on standard ECG criteria.[14–16]
ED was evaluated using a standardized questionnaire of the International Index of Erectile Function (IIEF).[17–19,5] We used an “inform-then-probe” approach, whereby the participants were generously assured that their ED (if present) was not an uncommon condition. The questionnaire was administered by the researchers. The IIEF has been proved to be a reliable and widely used test to define sexual function.[17,20,21] The IIEF consists of 14 questions on several aspects of sexual function. ED is graded as severe (6-10 points), moderate (11-16 points), mild (17-25 points), and none (26-30 points).[17]
MA was determined using the Micra Test strips (Boehringer Mannheim, Mannheim, Germany). This dipstick has been found to be a fast, accurate, and relatively, the cheapest way to screen patients for the presence of MA.[22,23] There are four color blocks on the test strip corresponding to negative (or 0), 20, 50, and 100 mg/l of albumin. The test was done on three consecutive first morning-voided urine samples collected at 3 weeks’ intervals. MA was considered to be present when two of the three urine samples tested produce a reaction color corresponding to 20 mg/l or more. The mean value of the MA was also recorded for each participant.
Statistical study
The data entry and analysis was done in SPSS software version 16.0. Data were reported as means and standard deviation (SD) and percentages. Statistical analyses were done using the Student's t-test and Chi?square to compare means and proportions respectively. P < 0.05 was considered as significant.
Results
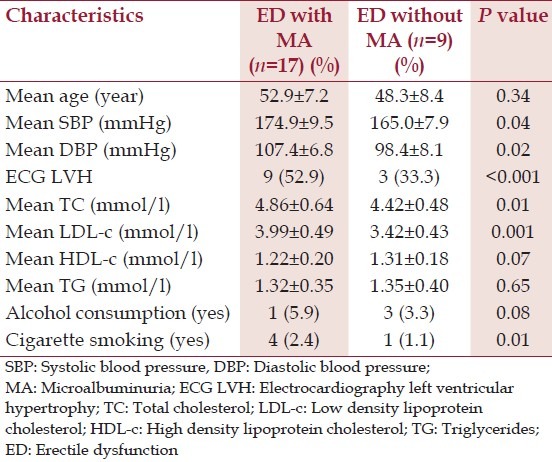
Eighty-one newly diagnosed hypertensive patients and 75 normotensive controls were studied. The mean age of the patients and the controls was 53.8 ± 5.6 and 51.2 ± 7.1 respectively. The other characteristics of the patients and the controls are described in Table 1. ED was found in 32.1% of hypertensive patients and 16% of normotensive controls and the difference was statistically significant (<0.001). Among the hypertensive patients, there was a significant difference between the mean age of those with ED and those without ED (54.2 ± 8.5 vs. 49.2 ± 6.8, P = 0.02). The prevalence of MA was significantly higher in patients with ED (65.4%) than in those without ED (23.6%) (P < 0.001). Hypertensive patients with ED were significantly more likely to have ECG LVH than their counterparts without ED (P < 0.001). The other characteristics and features of hypertensive patients with ED compared with hypertensive patients without ED are shown in Table 2. When hypertensive patients with ED are divided into two subsets based on the presence or otherwise of MA, and then compared, the results obtained are shown in Table 3: The means of SBP (P = 0.04), DBP (P = 0.02), ECG LVH (P < 0.001), serum TC (P = 0.1), serum LDL-c (P = 0.001) were significantly higher in those with MA than their counterparts without MA. Multiple regression analysis showed that ED was independently associated with MA, ECG LVH, age, DBP, and cigarette smoking.
Table 1.
Characteristics of hypertensive patients and the controls
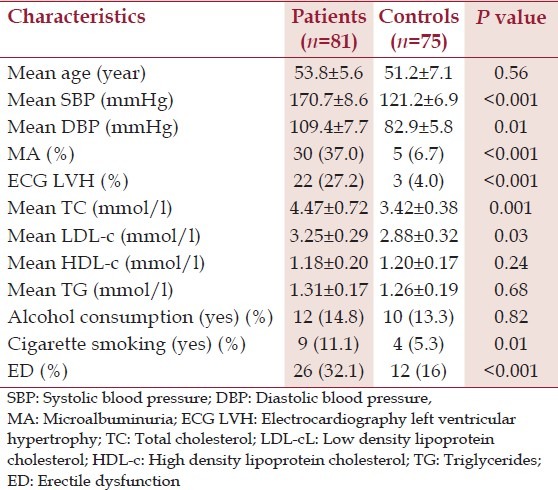
Table 2.
Characteristics of hypertensive patients with and without erectile dysfunction
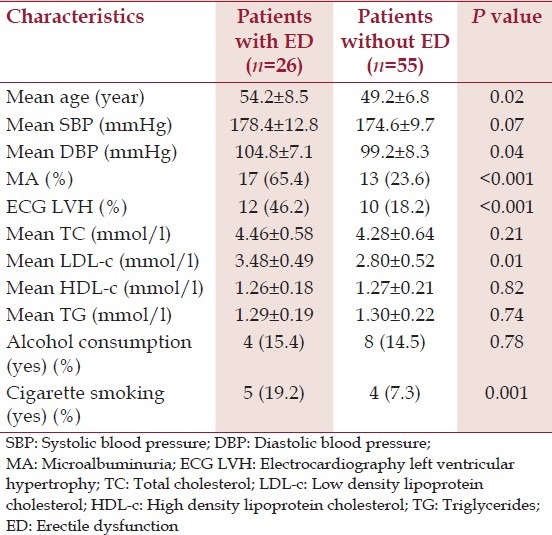
Table 3.
Characteristics of hypertensive patients with erectile dysfunction with and without microalbuminuria
Discussion
Our study demonstrated a higher prevalence of ED in hypertensive patients than in normotensive individuals (32.1% vs. 16.0%). This finding is consistent with reports from previous studies in Nigeria.[5,24] However, the prevalence of ED is lower than findings in some Caucasian studies.[6,7,21] For instance, Aranda et al.[6] reported a prevalence of ED among hypertensive patients of 46% in Spain where patients with diabetes mellitus were included in the study. In this study, diabetes mellitus was an exclusion criterion and only newly diagnosed hypertensive patients were used. Thus, the difference in prevalence may be due to study methodology including the selection of patients.
Hypertensive patients with ED have significantly higher cardiovascular risk factors when compared to their counterparts without ED and to the normotensive controls. This is consistent with findings in previous studies.[5,21] These cardiovascular factors have been implicated in the pathogenesis of ED in HBP.[25,26] MA was found in 37% of the hypertensive patients as against 6.7% in the controls. This finding is higher than that of Akinsola et al. who reported 17.4% prevalence of MA in HT. The difference may be attributed to patient selection as we recruited hypertensive patients who had never been treated previously for the study. Treatment of HBP with antihypertensive medications, particularly angiotensin-converting-enzyme (ACE) inhibitors, has been found to improve MA.[27] In our study, MA, a cardiovascular risk factor and marker of endothelial damage, is also significantly more common in hypertensives with ED than in hypertensives without ED. That is, patients with ED are more likely to have endothelial damage and dysfunction than their counterparts without ED. The study also revealed that ED may also be related to cardiac damage as significantly more hypertensive patients with ED have ECG LVH.
ED is often a sentinel manifestation of damage to the vascular endothelium.[28–30] The main link between ED and cardiovascular disease is the vascular endothelium, which has a fundamental role in the regulation of circulation. The formation of nitric oxide is a fundamental link between the endothelium and ED.[25] Nitric oxide is produced in the endothelial cells by nitric oxide synthase. Its release stimulates the relaxation of smooth muscle cells of the corpus carvernosum. Thus, once the ability to generate nitric oxide has been compromised, ED may ensue.[25] The study shows that MAL and ED might represent different variables with a common pathological process such as vascular damage. This hypothesis is consistent with the finding of Pedrinelli et al.[31] who reported that MA was strongly associated with C-reactive protein (CRP) which is an evidence of subclinical inflammation. CRP is also a cardiovascular risk factor and predicts cardiovascular prognosis independent of conventional risk factors.[32] Thus, vascular damage with possible atherosclerosis and the resultant inadequate blood flow to the erectile tissue might cause ED.
There are conflicting reports on the role of cigarette smoking in the etio-pathogenesis of ED. Moreira et al.[33] showed a 2.5-fold increased risk of ED among smokers in contrast to the study by Doumas et al.[21] and the Massachusetts Male Aging Study[34] where smoking was found not to be associated with ED. This notwithstanding, long-term cigarette smoking is a major risk factor for vasculogenic ED because of its effects on the vascular endothelium.[34] Cigarette smoking decreases the nitric oxide synthase activity in the penis. It also causes nicotine-induced vasoconstriction of the cavernous smooth muscle.[34] In our study, patients with ED were significantly more likely to be cigarette smokers than those without ED. This is consistent with the report of a Nigerian study by Opadijo.[5] The effect of alcohol on penile erection depends on the volume and the duration of consumption. Although small quantities of alcohol may improve erection and enhance libido, large amounts result in central sedation, depression of libido, and ED.[35] Polyneuropathy from chronic alcoholic intake may also cause or worsen ED.[35,36] In our study, we found no association between alcohol ingestion and ED. However, one of the limitations of the study is that the alcohol ingestion was not quantified.
Conclusion
The study reveals that both ED and MA are common in adult male Nigerians with HT. It also points out that male ED in hypertensive patients is associated with MA and the subsets with either or both have increased risk of cardiovascular disease. Cigarette smoking, a major risk factor for atherosclerosis and cardiovascular disease, is also associated with ED. With a somewhat common pathological process of vascular damage between ED and MA, it could be expected that antihypertensive drugs, particularly ACE-inhibitors and angiotensin-receptor blockers, that improve MA might have some impact on ED. This hypothesis is yet to be proven and may become a focus of research in the future.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 Report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 2.Akinkugbe OO. Current epidemiology of hypertension in Nigeria. Arch Ibadan Med. 2002;1:3–5. [Google Scholar]
- 3.The National Expert Committee on Non-communicable Diseases. Non-communicable diseases in Nigeria. Final Report of the National Survey. Federal Ministry of Health and Social Services. 1997 [Google Scholar]
- 4.Kadiri S, Walker O, Salako BL, Akinkugbe O. Blood pressure, hypertension and correlates in urbanised workers in Ibadan, Nigeria: A revisit. J Hum Hypertens. 1999;13:23–7. doi: 10.1038/sj.jhh.1000722. [DOI] [PubMed] [Google Scholar]
- 5.Opadijo OG. Male erectile dysfunction in adults with hypertension: The role of vascular and cardiac damage. Cardiol Trop. 2003;29:3–7. [Google Scholar]
- 6.Aranda P, Ruilope LM, Calvo C, Luque M, Coca A, Gil de Miguel A. Erectile dysfunction in essential arterial hypertension and effects of sildenafil: Results of a Spanish national study. Am J Hypertens. 2004;17:139–45. doi: 10.1016/j.amjhyper.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Moreira ED, Jr, Lbo CF, Diament A, Nicolosi A, Glasser DB. Incidence of erectile dysfunction in men 40 to 69 years old: Results from a population-based cohort study in Brazil. Urology. 2003;61:431–6. doi: 10.1016/s0090-4295(02)02158-1. [DOI] [PubMed] [Google Scholar]
- 8.de Zeeuw D, Parving HH, Henning RH. Microalbuminuria as an early marker for cardiovascular disease. J Am Soc Nephrol. 2006;17:2100–5. doi: 10.1681/ASN.2006050517. [DOI] [PubMed] [Google Scholar]
- 9.Stehouwer CD, Smulders YM. Microalbuminuria and risk for cardiovascular disease: Analysis of potential mechanisms. J Am Soc Nephrol. 2006;17:2106–11. doi: 10.1681/ASN.2005121288. [DOI] [PubMed] [Google Scholar]
- 10.Karalliedde J, Viberti G. Microalbuminuria and cardiovascular risk. Am J Hypertens. 2004;17:986–93. doi: 10.1016/j.amjhyper.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia. 1989;32:219–26. doi: 10.1007/BF00285287. [DOI] [PubMed] [Google Scholar]
- 12.Jensen JS, Borch-Johnsen K, Jensen G, Feldt-Rasmussen B. Microalbuminuria reflects a generalized transvascular albumin leakiness in clinically healthy subjects. Clin Sci (Colch) 1995;88:629–33. doi: 10.1042/cs0880629. [DOI] [PubMed] [Google Scholar]
- 13.Sullivan ME, Thompson CS, Dashwood MR, Khan MA, Jeremy JY, Morgan RJ, et al. Nitric oxide and penile erection: Is erectile dysfunction another manifestation of vascular disease? Cardiovasc Res. 1999;43:658–65. doi: 10.1016/s0008-6363(99)00135-2. [DOI] [PubMed] [Google Scholar]
- 14.Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J. 1949;37:161–86. doi: 10.1016/0002-8703(49)90562-1. [DOI] [PubMed] [Google Scholar]
- 15.Morris JJ, Jr, Estes EH, Jr, Whalen RE, Thompson HK, Jr, Mcintosh HD. P-wave analysis in valvular heart disease. Circulation. 1964;29:242–52. doi: 10.1161/01.cir.29.2.242. [DOI] [PubMed] [Google Scholar]
- 16.Romhilt DW, Estes EH., Jr A point-score system for the ECG diagnosis of left ventricular hypertrophy. Am Heart J. 1968;75:752–8. doi: 10.1016/0002-8703(68)90035-5. [DOI] [PubMed] [Google Scholar]
- 17.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): A multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–30. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 18.Giuliano F, Chevret-Measson M, Tsatsaris A, Reitz C, Murino M, Thonneau P. Prevalence of erectile dysfunction in France: Results of an epidemiological survey of a representative sample of 1004 men. Eur Urol. 2002;42:382–9. doi: 10.1016/s0302-2838(02)00323-8. [DOI] [PubMed] [Google Scholar]
- 19.Korenman SG. Sexual function and dysfunction. In: Wilson JD, editor. Williams Textbook of Endocrinology. 9th ed. Philadelphia: WB Saunders; 1998. pp. 927–38. [Google Scholar]
- 20.Martin-Morales A, Sanchez-Cruz JJ, Saenz de Tejada I, Rodriguez-Vela L, Jimenez-Cruz JF, Burgos-Rodriguez R. Prevalence and independent risk factors for erectile dysfunction in Spain: Results of the Epidemiologia de la Disfuncion Erectil Masculina Study. J Urol. 2001;166:569–74. doi: 10.1016/s0022-5347(05)65986-1. [DOI] [PubMed] [Google Scholar]
- 21.Doumas M, Tsakiris A, Doumas M, Grigorakis A, Papadopoulos A, Hounta A, et al. Factors affecting the increased prevalence of erectile dysfunction in Greek hypertensive compared with normotensive subjects. J Androl. 2006;27:469–77. doi: 10.2164/jandrol.04191. [DOI] [PubMed] [Google Scholar]
- 22.Tiu SC, Lee SS, Cheng MW. Comparison of six commercial techniques in the measurement of microalbuminuria in diabetic patients. Diabetes Care. 1993;16:616–20. doi: 10.2337/diacare.16.4.616. [DOI] [PubMed] [Google Scholar]
- 23.Comper WD, Jerums G, Osicka TM. Differences in urinary albumin detected by four immunoassays and high-performance liquid chromatography. Clin Biochem. 2004;37:105–11. doi: 10.1016/j.clinbiochem.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Nwofor AM, Yeboa ED, Dogunro AS. Sildenafil (Viagra™) in the treatment of male erectile dysfunction: a 6-week flexible dose of evaluation and safety. Niger J Health Biomed Sci. 2002;1:45–8. [Google Scholar]
- 25.Lue TF. Erectile dysfunction. N Engl J Med. 2000;342:1802–13. doi: 10.1056/NEJM200006153422407. [DOI] [PubMed] [Google Scholar]
- 26.Andersen KE, Hedlund P. New directions for erectile dysfunction therapies. Int Angiol. 2001;20:195–9. [Google Scholar]
- 27.Kirby M, Jackson G, Betteridge J, Friedli K. Is erectile dysfunction a marker for cardiovascular disease? Int J Clin Pract. 2001;55:614–8. [PubMed] [Google Scholar]
- 28.Sullivan ME, Keoghane SR, Miller MA. Vascular risk factors and erectile dysfunction. BJU Int. 2001;87:838–45. doi: 10.1046/j.1464-410x.2001.02211.x. [DOI] [PubMed] [Google Scholar]
- 29.Sasayama S, Ishii N, Ishikura F, Kamijima G, Ogawa S, Kanmatsuse K, et al. Men's Health Study: Epidemiology of erectile dysfunction and cardiovascular disease. Circ J. 2003;67:656–9. doi: 10.1253/circj.67.656. [DOI] [PubMed] [Google Scholar]
- 30.Mimran A, Ribstein J. Microalbuminuria in essential hypertension. J Hum Hypertens. 1996;10:657–61. [PubMed] [Google Scholar]
- 31.Pedrinelli R, Dell’Omo G, Di Bello V, Pellegrini G, Pucci L, Del Prato S, et al. Low-grade inflammation and microalbuminuria in hypertension. Arterioscler Thromb Vasc Biol. 2004;24:2414–9. doi: 10.1161/01.ATV.0000147415.40692.7f. [DOI] [PubMed] [Google Scholar]
- 32.Hackam DG, Anand SS. Emerging risk factors for atherosclerotic vascular disease: A critical review of the evidence. J Am Med Assoc. 2003;290:932–40. doi: 10.1001/jama.290.7.932. [DOI] [PubMed] [Google Scholar]
- 33.Moreira ED, Jr, Lbo CF, Diament A, Nicolosi A, Glasser DB. Incidence of erectile dysfunction in men 40 to 69 years old: Results from a population-based cohort study in Brazil. Urology. 2003;61:431–6. doi: 10.1016/s0090-4295(02)02158-1. [DOI] [PubMed] [Google Scholar]
- 34.Johannes CB, Araujo AB, Feldman HA, Derby CA, Kleinman KP, McKinlay JB. Incidence of erectile dysfunction in men 40 to 69 years old: Longitudinal results from the Massachusetts male aging study. J Urol. 2000;163:460–3. [PubMed] [Google Scholar]
- 35.Rimm EB, Bacon CG, Giovanucci EL, Kawachi I. Body weight, physical activity and alcohol consumption in relation to erectile dysfunction among US male health professionals free of major chronic diseases. In: Proceedings of the American Urological Association–95th Annual Meeting, Atlanta, April 29th-May 4th, 2000. J Urol. 2000;163:15–7. [Google Scholar]
- 36.Derby CA, Mohr BA, Goldstein I, Feldman HA, Johannes CB, McKinlay JB. Modifiable risk factors and erectile dysfunction: Can lifestyle changes modify risk? Urology. 2000;56:302–6. doi: 10.1016/s0090-4295(00)00614-2. [DOI] [PubMed] [Google Scholar]


