Abstract
Background:
Ankle edema is a common adverse effect of amlodipine, an L-type calcium channel blocker (CCB). Cilnidipine is a newer L/N-type CCB, approved for treatment of essential hypertension.
Aim:
This study was designed to determine whether cilnidipine can produce resolution of amlodipine-induced edema while maintaining adequate control of hypertension.
Materials and Methods:
A prospective study was performed on 27 patients with essential hypertension with amlodipine-induced edema. Concomitant nephropathy, cardiac failure, hepatic cirrhosis, or other causes of edema, and secondary hypertension were excluded by appropriate tests. Amlodipine therapy was substituted in all the cases with an efficacy-equivalent dose of cilnidipine. Clinical assessment of ankle edema and measurement of bilateral ankle circumference, body weight, blood pressure, and pulse rate were performed at onset of the study and after 4 weeks of cilnidipine therapy.
Results:
At completion of the study, edema had resolved in all the patients. There was a significant decrease in bilateral ankle circumference and body weight (P < 0.001). There was no significant change in mean arterial blood pressure and pulse rate.
Conclusions:
Therapy with cilnidipine resulted in complete resolution of amlodipine-induced edema in all the cases without significant worsening of hypertension or tachycardia. Cilnidipine is an acceptable alternative antihypertensive for patients with amlodipine-induced edema.
Keywords: Ankle edema, Calcium channel blocker, Cilnidipine, Hypertension, L/N-type calcium channel
Introduction
Ankle edema is a common adverse effect of amlodipine, a widely used L-type calcium channel blocker (CCB), seen in up to 15% of patients receiving the drug.[1] Although it is a self-limited and relatively minor adverse effect in the most affected patients, amlodipine-induced edema can occasionally be severe, even progressing to anasarca.[2] Moreover, even mild edema can be perceived as disfiguring by some patients and lead to reduced drug compliance or complete discontinuation of therapy. This is unfortunate, as amlodipine is otherwise a highly effective antihypertensive.
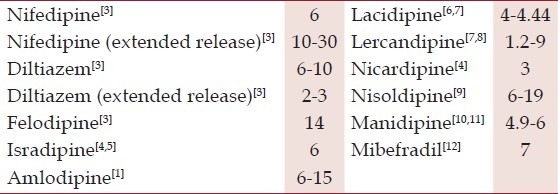
The usual approach to patients with amlodipine-induced edema involves cessation of amlodipine therapy and substitution with an alternative antihypertensive. Although the incidence of edema is relatively lower with other CCBs when compared to amlodipine [Table 1], replacement antihypertensives in these patients are typically drawn from a different class such as a thiazide diuretic or angiotensin converting enzyme inhibitor, in an attempt to avoid recurrence of edema. Although these alternative drug classes are effective at resolving edema, they possess a relatively weaker antihypertensive effect in Asian Indians.[13] Satisfactory resolution of edema is therefore gained at the cost of poorer control of hypertension. Substitution of amlodipine with another CCB could potentially provide similar control of blood pressure, and therefore, be a better strategy, provided there was adequate resolution of edema.
Table 1.
Incidence of ankle edema with various calcium channel blockers
Cilnidipine is a third generation L/N-type CCB[14] and is approved for the therapy of essential hypertension. A recent meta-analysis on the efficacy and safety of cilnidipine has demonstrated good tolerability and an antihypertensive efficacy equivalent to amlodipine.[15] There is however, no available data on the tolerability of cilnidipine in patients with amlodipine-induced edema. This study was, therefore, planned to determine whether cilnidipine therapy can produce resolution of amlodipine-induced edema while maintaining adequate control of blood pressure.
Materials and Methods
Institutional ethical committee clearance was obtained prior to initiation of the study. Informed consent for participation was taken from all the patients included in the study. Kasturba Hospital, Manipal, is a major tertiary care center in Southwestern India, catering to nearly 4.36 million individuals across the three surrounding districts of Udupi, Uttara Kannada, and Dakshina Kannada.
A prospective longitudinal study was planned to determine the effect of substitution of amlodipine with cilnidipine in hypertensive patients with amlodipine-induced ankle edema. Concomitant causes for edema including nephropathy, cardiac failure, hepatic cirrhosis, and hypoalbuminemia due to any other cause, were excluded by renal and liver function tests, thyroid profile, and baseline echocardiography. Varicose veins were ruled out by clinical examination.
Secondary causes for hypertension were evaluated beyond the aforementioned investigations only when the clinical suspicion was high or blood pressure was uncontrolled. The included patients typically maintained control of hypertension with less than three anti-hypertensive drugs. Ten patients were on monotherapy with amlodipine. Nine patients were receiving amlodipine and a RAS inhibitor. Four patients each were on a combination of amlodipine with a beta-blocker, and amlodipine with a thiazide diuretic.
Baseline parameters including clinical evidence of ankle edema, pulse rate, blood pressure, bilateral ankle circumference, and body weight were recorded for all patients. Presence of edema was assessed clinically by applying sustained pressure with the ball of the thumb over the medial malleolus for 30 seconds. Pulse rate was measured by palpating the radial artery at the wrist, with the patient supine, after 15 min of rest. Blood pressure was measured by a mercury sphygmomanometer in the right arm, with the patient supine, after 15 min of rest. Ankle circumference was determined with a tape measure, 1 cm above the medial malleolus, with the patient standing. Weight (to the nearest 0.1 kg) was measured with an electronic weighing scale, while the subjects were fasting and wearing only their undergarments.
All patients were then initiated on an efficacy-equivalent dose of cilnidipine (5 mg of amlodipine is equivalent to 10 mg of cilnidipine). Amlodipine therapy was stopped on the day of initiating cilnidipine. The patients were followed-up for four weeks. Relevant parameters were then recorded again for all the patients.
Statistical analysis
Based on the assumption that there would be 80% reduction of amlodipine-induced ankle edema with cilnidipine, calculation showed that a sample size of 16 was required (power = 80%, α =0.05). Data analysis was done with Statistical Product and Service Solutions (SPSS) Statistics version 17.0 (Chicago IL, USA). Continuous variables were presented as median with interquartile range (IQR) or mean ± standard deviation. Wilcoxon signed-rank test was used to compare the means of variables before and after administration of cilnidipine. P <0.05 were considered indicative of statistical significance.
Results
Of the 27 patients included in the study, 15 (55.6%) were male. The median age was 60 years (IQR 54-66). Median duration of therapy with amlodipine at the time of inclusion in the study was 12 months (IQR 3-60). Twenty patients (74.1%) were receiving the median dose, i.e., 5 mg of amlodipine daily (Range 2.5-10). Baseline hemodynamic data, ankle circumferences, and body weight are detailed in Table 2.
Table 2.
Comparison of anthropometric and hemodynamic parameters at the start and end of the study
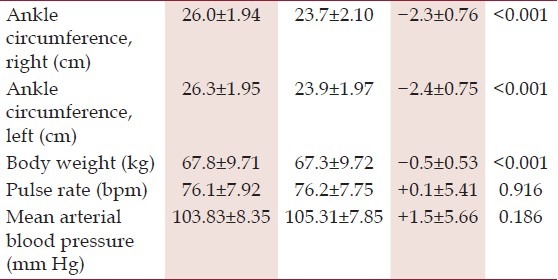
Reassessment after 1 month showed complete clinical resolution of ankle edema in all 27 patients. There was a significant decrease in ankle circumference and body weight. Comparison of hemodynamic parameters revealed a non-significant rise in mean arterial blood pressure, and no significant change in pulse rate. These findings are detailed in Table 2.
Discussion
A number of mechanisms have been postulated for CCB-induced edema. The principal mechanism involves interference of normal auto-regulatory postural vasoconstrictor reflexes.[16] In healthy individuals, reflex pre-capillary vasoconstriction in response to venous congestion protects the capillary bed from increased blood pressure, thereby restricting hydrostatic filtration of fluid into the interstitium. L-type CCBs like amlodipine directly inhibit pre-capillary vasoconstriction through arteriolar dilatation, thus promoting interstitial edema. Other contributory mechanisms include capillary hypertension and increased microvascular permeability. Preferential dilatation of pre-capillary resistance vessels by L-type CCBs with relative sparing of the post-capillary vascular tone results in significant capillary hypertension and promotes fluid hyperfiltration into the interstitium; this phenomenon has been validated with felodipine.[17] Increased microvascular permeability has also been demonstrated by interstitial extravasation of plasma protein-bound Evans-blue dye following administration of nifedipine and lacidipine.[18,19]
In contrast to amlodipine which acts primarily through blockade of L-type Ca2+ channels, cilnidipine acts through dual blockade of L-type and N-type Ca2+ channels.[20] Whereas L-type Ca2+ channel blockade produces vasodilation of peripheral resistance vessels akin to amlodipine, inhibition of neuronal N-type Ca2+ channels disrupts sympathetic nervous outflow, lowering plasma catecholamine levels, and thereby producing further vasodilatation. This unique mechanism of action results in vasodilation of both pre- and post-capillary resistance vessels reducing capillary hypertension and consequent hyperfiltration of fluid into the interstitium. The superior renoprotection of cilnidipine over other CCBs[21–25] through attenuation of glomerular hyperfiltration has been attributed to sympathetic blockade[26] and inhibition of N-type Ca2+ channels.[27] Reduction of capillary hyperfiltration in the peripheral systemic circulation would appear to be an extension of the same phenomenon.
The dual mechanisms of cilnidipine can therefore explain both the low incidence of ankle edema and the excellent antihypertensive action that it possesses. Reduced inhibition of the local vasoconstrictor reflexes that normally prevent excessive fluid filtration in dependent regions could also contribute to the lack of edema with cilnidipine therapy; further studies are required to elucidate this possibility.
Conclusion
Cilnidipine is an effective and well-tolerated alternative antihypertensive in patients with amlodipine-induced edema. Further studies are required to elucidate the various pharmacodynamic properties of cilnidipine that are responsible for the absence of associated edema.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Osterloh I. The safety of amlodipine. Am Heart J. 1989;118:1114–9. doi: 10.1016/0002-8703(89)90838-7. [DOI] [PubMed] [Google Scholar]
- 2.Sener D, Halil M, Yavuz BB, Cankurtaran M, Arioğul S. Anasarca edema with amlodipine treatment. Ann Pharmacother. 2005;39:761–3. doi: 10.1345/aph.1E410. [DOI] [PubMed] [Google Scholar]
- 3.Opie LH, Gersh BJ. Drugs for the Heart. 6th ed. New York, NY: Elsevier Saunders; 2004. Calcium channel blockers (Calcium antagonists) pp. 50–79. [Google Scholar]
- 4.Kubota K, Pearce GL, Inman WH. Vasodilation-related adverse events in diltiazem and dihydropyridine calcium antagonists studied by prescription-event monitoring. Eur J Clin Pharmacol. 1995;48:1–7. doi: 10.1007/BF00202163. [DOI] [PubMed] [Google Scholar]
- 5.Chrysant SG, Cohen M. Sustained blood pressure control with controlled-release isradipine. Am J Hypertens. 1995;8:87–9. doi: 10.1016/0895-7061(94)00158-8. [DOI] [PubMed] [Google Scholar]
- 6.Lindholm LH, Tcherdakoff P, Zanchetti A. Safety profile of lacidipine: Update from a clinical trials database. Drugs. 1999;57:27–9. doi: 10.2165/00003495-199957001-00004. [DOI] [PubMed] [Google Scholar]
- 7.Leonetti G, Magnani B, Pessina AC, Rappelli A, Trimarco B, Zanchetti A, et al. Tolerability of long-term treatment with lercanidipine versus amlodipine and lacidipine in elderly hypertensives. Am J Hypertens. 2002;15:932–40. doi: 10.1016/s0895-7061(02)03000-5. [DOI] [PubMed] [Google Scholar]
- 8.Barrios V, Navarro A, Esteras A, Luque M, Romero J, Tamargo J, et al. Antihypertensive efficacy and tolerability of lercanidipine in daily clinical practice. The ELYPSE Study. Blood Press. 2002;11:95–100. doi: 10.1080/08037050211265. [DOI] [PubMed] [Google Scholar]
- 9.Opie LH, Müller FO, Myburgh DP, Rosendorff C, Sareli P, Seedat YK, et al. Efficacy and tolerability of nisoldipine coat-core formulation in the treatment of essential hypertension: The South African multicenter ANCHOR Study. Ambulatory Nisoldipine Coat-Core Hypertension Outpatient Response (ANCHOR) Investigators. Am J Hypertens. 1997;10:250–60. doi: 10.1016/s0895-7061(96)00384-6. [DOI] [PubMed] [Google Scholar]
- 10.Cheer SM, McClellan K. Manidipine: A review of its use in hypertension. Drugs. 2001;61:1777–99. doi: 10.2165/00003495-200161120-00010. [DOI] [PubMed] [Google Scholar]
- 11.Fogari R, Zoppi A, Lusardi P, Mugellini A. Efficacy and tolerability of manidipine hydrochloride in the long-term treatment of mild-moderate hypertension. Manidipine Efficacy in Long-Term Treatment Group. Blood Press Suppl. 1996;5:24–8. [PubMed] [Google Scholar]
- 12.Karch FE, Pordy R, Benz JR, Carr A, Lunde NM, Marbury T, et al. Comparative efficacy and tolerability of two long-acting calcium antagonists, mibefradil and amlodipine, in essential hypertension. Mibefradil Hypertension Study Group. Clin Ther. 1997;19:1368–78. doi: 10.1016/s0149-2918(97)80011-2. [DOI] [PubMed] [Google Scholar]
- 13.Gupta AK, Poulter NR, Dobson J, Eldridge S, Cappuccio FP, Caulfield M, et al. Ethnic differences in blood pressure response to first and second-line antihypertensive therapies in patients randomized in the ASCOT Trial. Am J Hypertens. 2010;23:1023–30. doi: 10.1038/ajh.2010.105. [DOI] [PubMed] [Google Scholar]
- 14.Ozawa Y, Hayashi K, Kobori H. New generation calcium channel blockers in hypertensive treatment. Curr Hypertens Rev. 2006;2:103–111. doi: 10.2174/157340206776877370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu G, Wu H, Du B, Qin L. The efficacy and safety of cilnidipine on mild to moderate essential hypertension: A systematic review and meta-analysis of randomized controlled trials in Chinese patients. Cardiovasc Hematol Disord Drug Targets. 2012;12:56–62. doi: 10.2174/187152912801823165. [DOI] [PubMed] [Google Scholar]
- 16.Pedrinelli R, Dell’Omo G, Mariani M. Calcium channel blockers, postural vasoconstriction and dependent oedema in essential hypertension. J Hum Hypertens. 2001;15:455–61. doi: 10.1038/sj.jhh.1001201. [DOI] [PubMed] [Google Scholar]
- 17.Gustafsson D, Länne T, Bjerkhoel P, Johansson P, Lundvall J. Microvascular effects and oedema formation of felodipine in man. J Hypertens Suppl. 1989;7:S161–7. [PubMed] [Google Scholar]
- 18.Valentin JP, Ribstein J, Halimi JM, Mimran A. Effect of different calcium antagonists on transcapillary fluid shift. Am J Hypertens. 1990;3:491–5. doi: 10.1093/ajh/3.6.491. [DOI] [PubMed] [Google Scholar]
- 19.Hulthén UL, Cao Z, Rumble JR, Cooper ME, Johnston CI. Vascular hypertrophy and albumin permeability in a rat model combining hypertension and diabetes mellitus. Effects of calcium antagonism, angiotensin converting enzyme inhibition, and angiotensin II-AT1-receptor blockade. Am J Hypertens. 1996;9:895–901. doi: 10.1016/s0895-7061(96)00177-x. [DOI] [PubMed] [Google Scholar]
- 20.Uneyama H, Uchida H, Konda T, Yoshimoto R. Cilnidipine: Preclinical profile and clinical evaluation. Cardiovasc Drug Rev. 1999;17:341–57. [Google Scholar]
- 21.Fujita T, Ando K, Nishimura H, Ideura T, Yasuda G, Isshiki M, et al. Antiproteinuric effect of the calcium channel blocker cilnidipine added to renin-angiotensin inhibition in hypertensive patients with chronic renal disease. Kidney Int. 2007;72:1543–9. doi: 10.1038/sj.ki.5002623. [DOI] [PubMed] [Google Scholar]
- 22.Katayama K, Nomura S, Ishikawa H, Murata T, Koyabu S, Nakano T. Comparison between valsartan and valsartan plus cilnidipine in type II diabetics with normo- and microalbuminuria. Kidney Int. 2006;70:151–6. doi: 10.1038/sj.ki.5000349. [DOI] [PubMed] [Google Scholar]
- 23.Morimoto S, Yano Y, Maki K, Iwasaka T. Renal and vascular protective effects of cilnidipine in patients with essential hypertension. J Hypertens. 2007;25:2178–83. doi: 10.1097/HJH.0b013e3282c2fa62. [DOI] [PubMed] [Google Scholar]
- 24.Kojima S, Shida M, Yokoyama H. Comparison between cilnidipine and amlodipine besilate with respect to proteinuria in hypertensive patients with renal diseases. Hypertens Res. 2004;27:379–85. doi: 10.1291/hypres.27.379. [DOI] [PubMed] [Google Scholar]
- 25.Miwa Y, Tsuchihashi T, Ohta Y, Tominaga M, Kawano Y, Sasaguri T, et al. Antiproteinuric effect of cilnidipine in hypertensive Japanese treated with renin-angiotensin-system inhibitors – A multicenter, open, randomized trial using 24-hour urine collection. Clin Exp Hypertens. 2010;32:400–5. doi: 10.3109/10641961003667914. [DOI] [PubMed] [Google Scholar]
- 26.Hatta T, Takeda K, Shiotsu Y, Sugishita C, Adachi T, Kimura T, et al. Switching to an L/N-type calcium channel blocker shows renoprotective effects in patients with chronic kidney disease: The Kyoto Cilnidipine Study. J Int Med Res. 2012;40:1417–28. doi: 10.1177/147323001204000420. [DOI] [PubMed] [Google Scholar]
- 27.Konno Y, Kimura K. Vasodilatory effect of cilnidipine, an L-type and N-type calcium channel blocker, on rat kidney glomerular arterioles. Int Heart J. 2008;49:723–32. doi: 10.1536/ihj.49.723. [DOI] [PubMed] [Google Scholar]


