Abstract
To understand the role of neutrophils in the development of rat tuberculosis in vivo, we utilized lipopolysaccharide (LPS)-induced neutrophilia in the lungs. LPS (50 μg/ml) was administered intratracheally to male Fischer rats. Rats were then infected with Mycobacterium tuberculosis by an airborne route. Intratracheal injection of LPS significantly blocked the development of pulmonary granulomas and significantly reduced pulmonary CFU (P < 0.01). LPS treatment with amphotericin B (an LPS inhibitor) or neutralizing anti-rat neutrophil antibody reversed the development of pulmonary lesions. LPS-induced transient neutrophilia prevented early mycobacterial infection. The timing of LPS administration was important. When given intratracheally at least 10 days after aerial infection, LPS did not prevent development of tuberculosis. Neutrophils obtained by bronchoalveolar lavage killed M. tuberculosis cells. These results indicate clearly that neutrophils participate actively in defense against early-phase tuberculosis.
When tubercle bacilli are introduced into the lung, neutrophils migrate and accumulate in the infected pulmonary lesions during the early stage of tuberculosis. This rapid neutrophil response controls fast-replicating intracellular bacteria but not slow-replicating Mycobacterium tuberculosis (5). However, it has been reported that murine neutrophils play a protective nonphagocytic role in systemic M. tuberculosis infection in mice (3). Neutrophils from M. avium-infected mice produce tumor necrosis factor alpha, interleukin-12 (IL-12), and IL-1β (4). Thus, little is known about immunologic function in the lungs (although neutrophils have been identified as sources of inflammatory cytokines and chemokines) (2). In circumstances in which neutrophils are not activated with granulocyte colony-stimulating factor, severe mycobacterial infection results (7). However, it remains unclear whether neutrophils play a protective role in defense against mycobacterial infection. In vivo studies of neutrophils in the lungs are challenging, because it is difficult to induce neutrophilia. We therefore modified the lipopolysaccharide (LPS)-induced neutrophilia system in rats so that we could utilize it to study rat tuberculosis (1).
Permission to experiment on animals was given by the Animal Experiment Committee of The Research Institute of Tuberculosis. We injected LPS (E. coli 0111:B4 LPS) purchased from Sigma (St. Louis, Mo.) (50 μg/0.5 ml) intratracheally into anesthetized 6-week-old female Fischer rats (three rats/group). On the day after the injection, the rats were infected with the M. tuberculosis Kurono strain (ATCC 25618) by an airborne route by placing them in the exposure chamber of an airborne infection apparatus (model 099CA4241; Gals-Col, Inc., Terre Haute, Ind.). The concentration was calculated to result in the uptake of around 200 viable bacilli by the rat lungs after inhalation exposure for 90 min under the experimental conditions of this study (8). For the pulmonary CFU assay, 7 weeks later the lungs were removed, homogenized, diluted with physiological saline, placed on Ogawa slant agar, and incubated at 37°C for 4 weeks. Lung tissue sections from paraffin blocks were stained with hematoxylin and eosin or stained using the Ziehl-Neelsen method for acid-fast bacilli. Similar infection experiments were performed three times. Bronchoalveolar lavage (BAL) was carried out to obtain neutrophils 24 h after the intratracheal introduction of LPS. Under these conditions, >107 rat neutrophils were obtained by BAL. The cell differential counts showed that more than 99% were neutrophils and less than 1% were alveolar macrophages; no eosinophils were found. This LPS-induced neutrophilia in the rat lung disappeared 4 weeks later. We changed the dosage of LPS administered, and an inoculation of 50 μg of LPS/rat was sufficient to induce optimal neutrophilia. No side effects were recognized with the use of LPS.
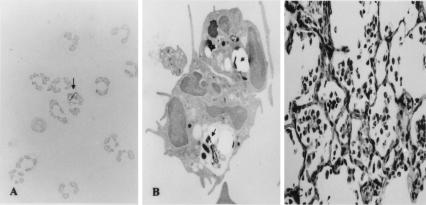
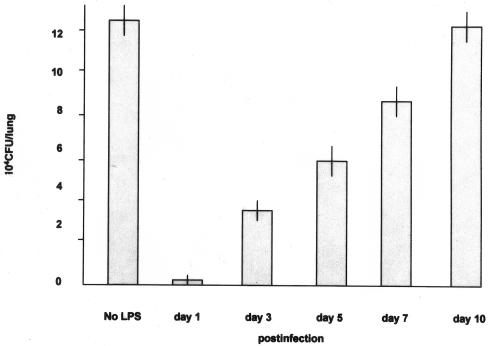
We grew cultures of neutrophils with M. tuberculosis overnight (multiplicity of infection, 10:1); then, the collected neutrophils were grown on Ogawa slant medium for 4 weeks. No CFU were found on the media. It is suggested that neutrophils possess direct or indirect killing activity for tubercle bacilli. As shown in Fig. 1A, less than 10% of the neutrophils ingested tubercle bacilli. Fig. 1B shows tubercle bacilli in phagosomes of neutrophils (9). Fig. 1C shows alveoli filled with neutrophils. No exudate was found in the alveoli. We performed time course infection experiments to examine the mechanism of rat neutrophil-mediated reduction of mycobacterial growth in vivo. The rats were infected by an airborne route at days 1, 3, 5, 7, and 10 after LPS (50 μg/0.5 ml) was injected intratracheally. The lung homogenates were grown on Ogawa slant culture for 4 weeks, and colonies were counted thereafter. As shown in Fig. 2, there was marked reduction of tubercle bacilli at day 1 after LPS injection but the pulmonary CFU level became gradually higher with the passage of time. These results show that rat neutrophils prevented early mycobacterial infection without ingesting tubercle bacilli. In the lungs treated intratracheally with LPS and infected with M. tuberculosis the following day, 200 ± 10 CFU were found; in the lungs infected with M. tuberculosis without LPS treatment, 1.3 × 105 ± 1 × 104 CFU were found (P < 0.01) (Fig. 3). At 7 weeks after aerosol infection, the lung tissues were removed for CFU assays and the lung homogenates were grown on Ogawa slant cultures for 4 weeks (Fig. 3). Pulmonary histopathology also showed that the size of granulomas was reduced markedly (Fig. 4B).
FIG. 1.
(A) Neutrophil-ingesting tubercle bacilli. The results of Ziehl-Neelsen staining are shown at a magnification of ×600. Arrow, neutrophil ingesting tubercle bacilli. (B) Electron micrograph of neutrophil-ingesting tubercle bacilli. Several tubercle bacilli (arrow) are evident in the neutrophil phagosomes. The image is shown at a magnification of ×5,000. (C) Alveoli filled with neutrophils. No exudate was found in the alveoli. The results of hematoxylin and eosin staining are shown at a magnification of ×200.
FIG. 2.
Bacterial load in a time course study starting at day 1 postinfection. The rats were infected by an airborne route at days 1, 3, 5, 7, and 10 after intratracheal injection of LPS (50 μg/0.5 ml). The lung homogenates were grown on Ogawa slant medium at 37°C for 4 weeks, and CFU were counted thereafter.
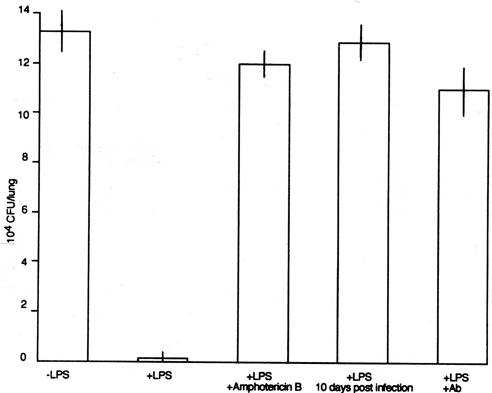
FIG. 3.
CFU assay of infected lung tissues left untreated (−LPS), treated with LPS (50 μg/0.5 ml) (+LPS), treated with LPS (50 μg/0.5 ml) and amphotericin B (50 μg/0.5 ml) (+LPS +Amphotericin B), treated with LPS (50 μg/0.5 ml) 10 days after aerial infection (+LPS 10 days post infection), and treated with LPS and anti-rat neutralizing neutrophil Ab (500 μg/rat) (+LPS +Ab).
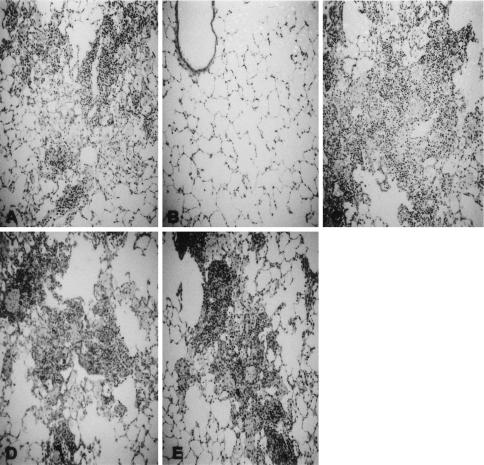
FIG. 4.
Representative histopathology of untreated infected lung tissues (A) and infected lung tissues treated with LPS (50 μg/0.5 ml) 1 day before aerial infection (B), treated with LPS 10 days after aerial infection (C), treated with LPS and amphotericin B (50 μg/0.5 ml) 1 day before aerial infection (D), and treated with LPS and anti-rat neutrophil Ab (500 μg/rat) 1 day before aerial infection (E). The results of hematoxylin and eosin staining are shown at a magnification of ×100.
Next, amphotericin B (Sigma), an LPS inhibitor, was used to demonstrate the direct effect of LPS on the rapid induction of rat neutrophils. LPS (50 μg/0.5 ml of pyrogen-free saline) and amphotericin B (50 μg) were mixed for 30 min; the mixture was then given to rats intratracheally. The following day, the rats were infected with M. tuberculosis aerially. At 7 weeks later, the CFU assay and pulmonary histopathology were performed. The pulmonary CFU level was 1.2 × 105 ± 1.0 × 104, and the difference between the results seen with LPS-treated rats and those seen with rats treated with LPS plus amphotericin B was statistically significant (P < 0.01). This marked increase of pulmonary CFU was confirmed by lung histopathology, which showed that large pulmonary granulomas were present (Fig. 4D).
To examine the effects of the presence of neutrophils induced by LPS on the growth of M. tuberculosis in vivo, neutralizing anti-rat neutrophil antibody (Ab) (catalog no. 22840D; PharMingen International) was used (6). LPS was inoculated intratracheally into the lungs of Fischer rats. The following day, 0.5 mg of Ab/0.5 ml of pyrogen-free saline was given intratracheally. One hour after Ab introduction into the rat lungs, the rats were infected with M. tuberculosis aerially. At 7 weeks later, pulmonary CFU were counted and histopathology was undertaken. As shown in Fig. 3 and 4E, pulmonary CFU levels increased and the granulomas became large because the rat neutrophils were killed with Ab and, thus, did not kill the tubercle bacilli.
Lastly, we examined the effect of the timing of the intratracheal inoculation of LPS on the development of tuberculosis. The rats were infected with the M. tuberculosis Kurono strain aerially. At 10 days later, LPS was administered intratracheally to the infected rats. We already know that the initial small pulmonary granulomas are formed 10 days after aerial infection. At 7 weeks after aerial infection with M. tuberculosis, pulmonary CFU and histopathology were examined again. Pulmonary CFU levels were not reduced significantly (Fig. 3), and the size of granulomas was not reduced substantially (Fig. 4C). Once the pulmonary granulomas were formed, rat neutrophils were ineffective for both the reduction of granuloma size and the elimination of tubercle bacilli.
It is concluded that the presence of LPS-induced neutrophilia does not affect the reduction of established pulmonary granulomas. We carried out reverse transcription-PCR using neutrophils obtained by BAL from M. tuberculosis-infected rats. We observed significant expression of tumor necrosis factor alpha, IL-1 β, and IL-12 mRNA in M. tuberculosis-infected rat neutrophils (data not shown) (4, 10).
In summary, neutrophils play a protective role in the host defense mechanism against M. tuberculosis (early-phase tuberculosis). LPS-induced transient neutrophilia prevents early mycobacterial infection, and rat neutrophils possess some phagocytic activity. LPS-induced neutrophilia in the lung is useful for examining neutrophil function in the development of tuberculosis.
Editor: J. T. Barbieri
REFERENCES
- 1.Cardona, P. J., R. Llatjos, S. Gordillo, J. Diaz, B. Vinado, A. Ariza, and V. Ausina. 2001. Towards a human-like model of tuberculosis: intranasal inoculation of LPS induces intragranulomatous lung necrosis in mice infected aerogenically with Mycobacterium tuberculosis. Scand. J. Immunol. 53:65-71. [DOI] [PubMed] [Google Scholar]
- 2.Fulton, S. A., S. M. Reba, T. D. Martin, and W. H. Boom. 2002. Neutrophil-mediated mycobactericidal immunity in the lung during Mycobacterium bovis BCG infection in C57BL/6 mice. Infect. Immun. 70:5322-5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pedrosa, J., B. M. Saunders, R. Appelberg, I. M. Orme, M. T. Silva, and A. M. Cooper. 2000. Neutrophils play a protective nonphagocytic role in systemic Mycobacterium tuberculosis infection of mice. Infect. Immun. 68:577-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrofsky, M., and L. E. Bermudez. 1999. Neutrophils from Mycobacterium avium-infected mice produce TNF-alpha, IL-12, and IL-1 beta and have a putative role in early host response. Clin. Immunol. 91:354-358. [DOI] [PubMed] [Google Scholar]
- 5.Seiler, P., P. Aichele, B. Raupach, B. Odermatt, U. Steinhoff, and S. I. Kaufmann. 2000. Rapid neutrophil response controls fast-replicating intracellular bacteria but not slow-replicating Mycobacterium tuberculosis. J. Infect. Dis. 181:671-680. [DOI] [PubMed] [Google Scholar]
- 6.Sekiya, S., S. Gotoh, T. Yamashita, T. Watanabe, S. Saitoh, and F. Sendo. 1989. Selective depletion of rat neutrophils by in vivo administration of a monoclonal antibody. J. Leukoc. Biol. 46:96-102. [DOI] [PubMed] [Google Scholar]
- 7.Sugawara, I., S. Mizuno, H. Yamada, M. Matsumoto, and S. Akira. 2001. Disruption of nuclear factor-IL-6, a transcription factor, results in severe mycobacterial infection. Am. J. Pathol. 158:361-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sugawara, I., H. Yamada, and S. Mizuno. 2003. Relative importance of STAT4 in murine tuberculosis. J. Med. Microbiol. 52:29-34. [DOI] [PubMed] [Google Scholar]
- 9.Yamada, H., S. Mizuno, M. Reza-Gholizadeh, and I. Sugawara. 2001. Relative importance of NF-κB p50 in mycobacterial infection. Infect. Immun. 69:7100-7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamada, H., S. Mizuno, R. Horai, Y. Iwakura, and I. Sugawara. 2000. Protective role of IL-1 in mycobacterial infection in IL-1 α/β double-knockout mice. Lab. Investig. 80:759-767. [DOI] [PubMed] [Google Scholar]