Abstract
Objective:
To study the utility of Fournier's Gangrene Severity Index (FGSI) with mortality predictive value in our tertiary institutes in North India.
Materials and Methods:
A retrospective study of 95 cases of Fournier's Gangrene (FG), admitted from 2009 to 2011, was carried out. We analysed clinical and laboratory findings, various prognostic factors, surgical treatments and their outcomes in FG patients. FGSI was used as individual variable to estimate the severity of FG; the effects of these factors on mortality were also evaluated.
Results:
The overall mean age was 46.5 ± 15.6 (range 24-82) years. Anorectal and urological regions were the main sites of the infection. The most common site of infection origin was scrotum in 81.3% in group A and 41.2% in group B. One or more predisposing factors such as diabetes mellitus (DM; 55%) malignancies (4.6%), chronic renal failure (4.5%) and previous surgery (9.2%) were detected. We observed mortality in 26.5% cases (17/65). The FGSI calculated averaged 5.95 ± 365 in group A and 9.44 ± 2.56 in group B, at the time of admission (P > 0.05).
Conclusion:
In FG, an early diagnosis and early surgical debridement are essential. The FGSI seems to be an excellent tool for the outcome prediction.
KEYWORDS: Fournier's gangrene, Fournier's gangrene severity index, sepsis
INTRODUCTION
Fournier's Gangrene (FG) is a fulminant infection, including necrotising fasciitis of the genital, perineal and/or perianal regions. It was initially described by Baurinne in 1764 and is named after Jean Alfred Fournier, a French dermatologist who in 1883 described it.[1,2]
This condition is potentially fatal, affects any age and gender, has been reported even in neonates,[2,3] is characterised by rapid progression of infection in soft tissue, caused by the synergistic action of several agencies that extend along fascial planes, causing necrosis of these tissues and destruction.[2,4]
This necrosis is secondary to thrombosis of small vessels, which is due to endarteritis obliterans caused by the spread of microorganisms into the subcutaneous space (platelet aggregation stimulated by heparinase produced by aerobic and anaerobic bacteria),[2,5] which in addition to generating local oedema, hypoxia, difficulty in local blood supply, favours the development of anaerobic bacteria. These microorganisms produce hydrogen and nitrogen that accumulate in tissues, causing crepitation.[4]
The most frequent concomitant diseases are diabetes mellitus (DM; present between 32% and 66% of cases),[4] alcoholism and cancer, among other immunosuppressive diseases.
Mortality has been reported in different series to range from 16 to 40%.[5,6]
Its clinical presentation is variable, but often presents with oedema, erythema, pain, fever and increased volume; crepitus is present in 50-62% of cases.[5] The time interval from onset of symptoms specific to the process until the request for medical care is from 2 to 7 days, on average. This time determines the extent of the necrotic area and a critical influence on the prognosis.[7] Of the imaging studies, X-rays are useful in demonstrating the presence of gas in soft tissues; ultrasound is more useful as it can demonstrate the presence of diffuse oedema, thickness of the scrotal wall and possibly the penis, and the presence of scrotal gas.[5]
It is a situation that warrants urgent radical surgical treatment (debridement), in addition to the use of antibiotics.[8]
The management ranges from emergency surgery (debridement), managing topically (sodium hypochlorite, hydrogen superoxide and even honey), and administering antibiotics, to hyperbaric oxygen.[9,10]
Loar et al. have described gangrene severity index of Fournier (FGSI)[4,11,12] [Table 1], which is useful for evaluating the prognosis and to stratify risks in these patients.
Table 1.
Fournier's gangrene severity index score system
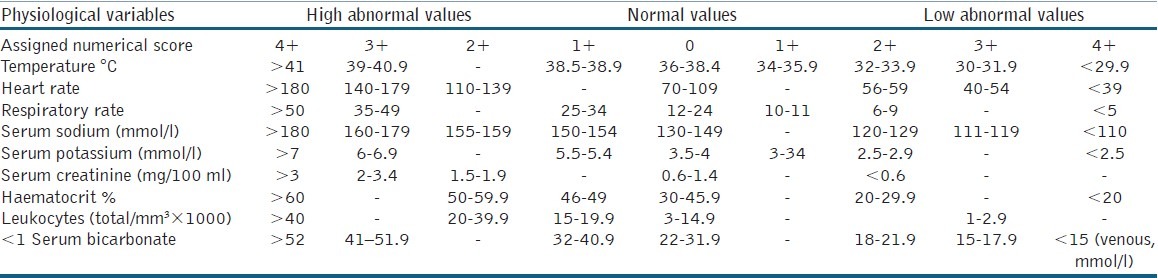
FGSI is a numerical score obtained from a combination of physiological hospital admission parameters that include temperature, heart rate, respiration rate, sodium, potassium, creatinine, leukocytes, haematocrit and bicarbonate. They stabilised that an FGSI score above 9 is sensitive and specific as a mortality predictor in FG patients.
In this study, we identify various predicting factors for the mortality in FG patients, with special reference to FGSI score system.
MATERIALS AND METHODS
It is a retrospective study of 95 patients identified as having FG, admitted from August 2009 to August 2011, i.e., in a period of 2 years.
The data were collected from the Department of Surgery of GSVM Medical College, Kanpur, UP; MRA Medical College, Ambedkar Nagar, UP; and Government Medical College, Haldwani, Uttarakhand.
FG diagnosis was established on clinical basis. Patient's age, gender, infection source, predisposing factors, clinical findings, various surgical procedures, and laboratory results were analysed.
FGSI was calculated by evaluating nine hospital admission parameters: temperature, respiratory rate, hear rate, sodium, potassium, creatinine, serum bicarbonate, leukocyte count and haemocrit. Evaluation criteria were gauged from 0 to +4 as described by Loar et al.[4] We excluded patients with periurethral and scrotal abscesses if there was no evidence of extension to soft-tissue or necrosis.
In this study, we divided all FG patients into two categories: survivors group A (n=69) and nonsurvivors group B (n=26) [Table 1].
Univariate analyses (Chi-square and Student's t-test) were used for comparisons. A P value of <0.05 was considered statistically significant.
RESULTS
We analysed 95 cases from three tertiary institutes of North India over a period of 2 years. The overall mean age was 46.5 ± 15.6 (range 24–82) years. The patients of group B (nonsurvivors) were slightly older (although not significant). 80% patients (76/95) were males.
The most common site of infection origin was scrotum in 81.3% in group A and 41.2% in group B. The perianal origin was found in 18.7% in group A and 58.8% in group B (P < 0.05) [Table 2].
Table 2.
Comparison of the results between the two groups
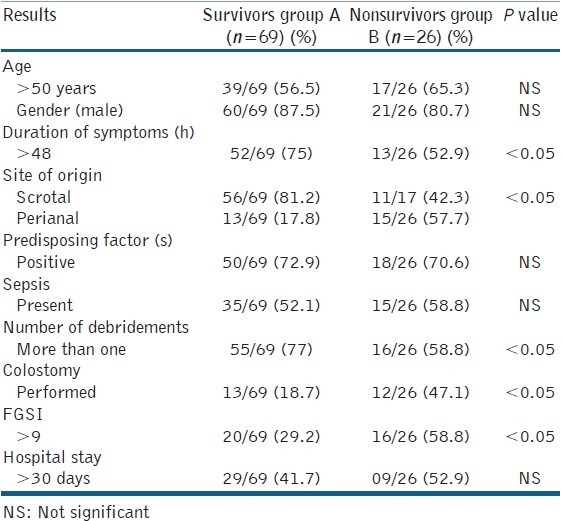
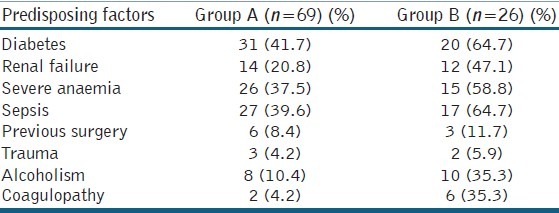
One or more predisposing factors were present in 72.6% cases totally (69/95). DM was present in 54.7% (52/95) cases. But the number of DM patients was more in group B (20/26) as compared to group A (31/69) [Table 3]. Seven patients of group B had coagulopathy. Eleven out of 26 patients in group B had shock. Severe anaemia was present in 45% of all the patients. Two patients were found to be HIV positive in group B.
Table 3.
Predisposing factors of Fournier's gangrene
The most common symptoms at the time of admission in the hospital were fever (85%), increased scrotal volume (84%) [Figure 1], and perineal or genital pain (71%). The average time of the symptoms prior to referral to the treatment was 6.4 days (range 2–15 days) in group A and 9.5 days in group B (range 1–19 days) [Table 2].
Figure 1.
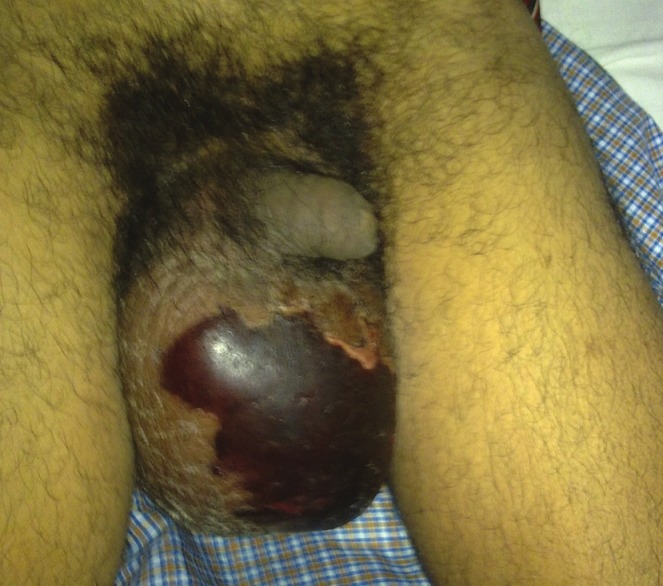
Photograph of an Fournier's Gangrene patient with scrotal origin
The surgical procedure preformed was debridement in all the patients. Overall, cystostomy was done in 28.8%. Twenty percent patients had colostomy and 8% patients had bilateral orchidectomy. As far as extensive debridement was concerned, 86.7% was done in the first 8 h of admission to the hospitals. 65.2% patients had undergone debridement twice or thrice overall [Table 2].
The most frequent bacteria were Escherichia coli in 51.8%, followed by Enterococcus faecalis in 28%. Polymicrobial infection was found in 62.5% in group A and 72.5% in group B (P < 0.05).
Overall, thirty-five patients required intensive care unit stay for an average of 7 days. The overall stay in the hospital was 22.5 days (range 10 hours-77 days). Death occurred in 26/95 patients (27.5%).
The FGSI index calculated ranged from 0 to 14, with average 5.95 ± 365 in group A and 9.44 ± 2.56 in group B, at the time of admission (P < 0.05) [Table 2].
DISCUSSION
FG, first described as a rapidly progressing idiopathic infection, includes any necrotising infection of the external genitals and perineum in both men and women.[7] It is usually a polymicrobial infection whose probable physiopathology is due to endarteritis obliterans of the small and superficial veins, resulting in gangrene.[2] Despite aggressive wide-spectrum antibiotic treatment, aggressive surgical debridement, intensive care and anaesthesia, the mortality rates are as high as 43% in some series.[3]
Anaerobic and aerobic organisms that have been isolated from the most common wounds are: E. coli, Bacteroides spp., Streptococcus spp., Enterococcus spp. and Staphylococcus.[1,3] In the present series, E. coli was the predominant bacterium. There is no consensus on clinical variables for predicting FG results. Lower limb and abdominal wall involvement are associated with high mortality rate. Studies have shown that aggressive therapy, age, comorbidities and time of presentation do not affect prognosis.
Many prognostic factors such as advanced age,[1,13] primary anorectal infections,[1] DM,[9] delayed treatment, synergistic sepsis on admission,[8] anaemia,[14] and high FGSI score[4] have been reported in literature for FG. Other predisposing factors include local trauma, paraphimosis, periurethral extravasation of the urine, perirectal or anal infections, and surgeries such as circumcision or herniorrhaphy.[15]
In our study, we found that higher mortality was seen with increasing age in patients more than 50 years of age.[1] Overall mortality was 26.6%. This was consistent with other series.[12,14,16,17] In this study, it was observed that if the time interval between the first symptom and surgical intervention is increased, the mortality is increased, which is consistent with other studies.[14,16]
Although the majority of the patients presented in this series had DM (54.5%), other predisposing factors including previous surgeries (9.2%), trauma (4.6%) and alcoholism (16.9%) were also present. There is still controversy as to whether the coexistence of DM influences prognosis.[18] But in our study, which is consistent with the report of Korkut et al., DM was significant in the mortality group.
We found the presence of sepsis on admission also to be a prognostic factor for FG and its mortality, as reported by Unalp et al.[19]
Treatment for FG must be started as early as possible. Early and aggressive debridement and use of wide-spectrum antibiotics are the gold standard for decreasing the mortality and morbidity.[19]
Debridement must be repeated with the same aggressive approach when necessary.[19] We preformed repeated debridements in 65.2% of the FG patients in our study.
Some published series have emphasised that hyperbaric oxygen therapy can be helpful for the management of FG. Limitations in the availability and transfer of the patients to units offering this service restrict its application for the patients with FG.[20,21] Consequently, we did not utilise hyperbaric oxygen therapy for our patients.
FGSI, which was developed by Loar et al., is a good prognostic tool for assessing the FG patients.[4] Mean FGSI in our study was 5.8, 4.6 and 11.1 in all patients, in surviving patients and in patients who died, respectively. We also find that FGSI score system is a good tool for predicting severity of the disease and mortality risk of the patients.[19,22]
FG is an infectious process that can lead to death in up to 40% of patients. Early diagnosis and aggressive surgical interventions, and intensive postoperative care have undoubtedly controlled the mortality rates. Understanding the physiopathology and predisposing factors is essential for early diagnosis and treatment. There is currently no level I evidence for the use of indices for predicting mortality.
In our multicentre study, we have found that older age, DM, anaemia, sepsis, delay in initial treatment and FGSI core ≥9 are the important predicting severity factors.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Rodríguez Alonso A, Pérez García MD, Núñez López A, Ojea Calvo A, Alonso Rodrigo A, Rodríguez Iglesias B, et al. Fournier's gangrene: anatomo-clinical features in adults and children. Therapy update. Actas Urol Esp. 2000;24:294–306. doi: 10.1016/s0210-4806(00)72452-1. [DOI] [PubMed] [Google Scholar]
- 2.Alejandro GM, Juan Atianio AL, Jesus DG, Rafael MM, Laura SG. Fournier's gangrene: Our experience in 5 years, bibliographic review and assessment of the Fournier's gangrene severity index. Arch EspUrol. 2009;62:532–40. [PubMed] [Google Scholar]
- 3.Spirnak JP, Resnick MI, Hampel N, Persky L. Fournier's gangrene: Re-port of 20 patients. J Urol. 1984;131:289–91. doi: 10.1016/s0022-5347(17)50351-1. [DOI] [PubMed] [Google Scholar]
- 4.Laor E, Palmer LS, Tolia BM, Reid RE, Winter HI. Outcome prediction in patients with Fournier's gangrene. J Urol. 1995;154:89–92. [PubMed] [Google Scholar]
- 5.Eke N. Fournier's gangrene: A review of 1726 cases. Br J Surg. 2000;87:718–28. doi: 10.1046/j.1365-2168.2000.01497.x. [DOI] [PubMed] [Google Scholar]
- 6.Ersay A, Yilmaz G, Akgun Y, Celik Y. Factors affecting mortility of Fournier's gangrene: Review of 70 patients. ANZ J Surg. 2007;77:43. doi: 10.1111/j.1445-2197.2006.03975.x. [DOI] [PubMed] [Google Scholar]
- 7.Fournier JA. Overwhelming Fournier's Gangrene. Semin Med. 1883;3:345. [Google Scholar]
- 8.Korkut M, Icoz G, Dayangac M, Akgun E, Yeniyay L, Erdogan O, et al. Outcome analysis in patients with Fournier's gangrene: Report of 45 cases. Dis Colon Rectum. 2003;46:649–52. doi: 10.1007/s10350-004-6626-x. [DOI] [PubMed] [Google Scholar]
- 9.Schaeffer AJ. Campbell's Urology. 8th ed. Argentina: Editorial Panamericana; 2007. Urinary tract infections: Fournier's gangrene; pp. 641–3. [Google Scholar]
- 10.Clayton MD, Fowler JE, Jr, Sharifi R, Pearl RK. Causes, presentation and survival of fifty-seven patients with necrotizing fasciitis of the male genitalia. Surg Gynecol Obstet. 1990;170:49–55. [PubMed] [Google Scholar]
- 11.Yeniyol CO, Suelozgen T, Arslan M, Ayder AR. Fournier's gangrene: Experience with 25 patients and use of Fournier's gangrene severity index score. Urology. 2004;64:218–22. doi: 10.1016/j.urology.2004.03.049. [DOI] [PubMed] [Google Scholar]
- 12.Tuncel A, Aydin O, Tekdogan U, Nalcacioglu V, Capar Y, Atan A. Fournier's gangrene: Three years of experience with 20 patients and validity of the Fournier's gangrene severity index score. Eur Urol. 2006;50:838–43. doi: 10.1016/j.eururo.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 13.Benizri E, Fabiani P, Migliori G, Chevallier D, Peyrottes A, Raucoules M, et al. Gangrene of the perineum. Urology. 1996;47:935–9. doi: 10.1016/S0090-4295(96)00058-1. [DOI] [PubMed] [Google Scholar]
- 14.Moorthy K, Rao PP, Supe AN. Necrotizing perianal infection: A fatal outcome of Fournier's gangrene. J R Coll Surg Edinb. 2000;27:1038–43. [PubMed] [Google Scholar]
- 15.Miller JD. The importance of early diagnosis and surgical treatment of necrotizing fasciitis. Surg Gynecol Obstet. 1983;157:197–200. [PubMed] [Google Scholar]
- 16.Norton KS, Johnson LW, Perry T, Perry KH, Sehon JK, Zibari GB. Management of Fournier's gangrene: An eleven year retrospective analysis of early recognition, diagnosis, and treatment. Am Surg. 2002;68:709–13. [PubMed] [Google Scholar]
- 17.Corman JM, Moody JA, Aronson WJ. Fournier's gangrene in a modern surgical setting: Improved survival with aggressive management. BJU Int. 1999;84:85–8. doi: 10.1046/j.1464-410x.1999.00140.x. [DOI] [PubMed] [Google Scholar]
- 18.Morpurgo E, Galandiuk S. Fournier's gangrene. Surg Clin North Am. 2002;82:1213–24. doi: 10.1016/s0039-6109(02)00058-0. [DOI] [PubMed] [Google Scholar]
- 19.Unalp HR, Kamer E, Derici H, Atahan K, Balci U, Demirdiven C, et al. Fournier's gangrene: Evaluation of 68 patients and analysis of prognostic variables. J Postgrad Med. 2008;54:102–5. doi: 10.4103/0022-3859.40775. [DOI] [PubMed] [Google Scholar]
- 20.Quatan N, Kirby RS. Improving outcomes in Fournier's gangrene. BJU Int. 2004;93:691–2. doi: 10.1111/j.1464-410X.2003.04753.x. [DOI] [PubMed] [Google Scholar]
- 21.Hejase MJ, Simonin JE, Bihrle R, Coogan CL. Genital Fournier's gangrene: Experience with 38 patients. Urology. 1996;47:734–9. doi: 10.1016/s0090-4295(96)80017-3. [DOI] [PubMed] [Google Scholar]
- 22.Lin E, Yang S, Chiu AW, Chow YC, Chen M, Lin WC, et al. Is Fournier's gangrene severity index useful for predicting outcome of Fournier's gangrene? Urol Int. 2005;75:119–22. doi: 10.1159/000087164. [DOI] [PubMed] [Google Scholar]


