Abstract
To investigate the role of innate immunity in variable efficacy of Mycobacterium bovis BCG vaccination in Malawi and the United Kingdom, we examined 24-h tumor necrosis factor alpha, interleukin-1β (IL-1β), and IL-10 responses to mycobacterial purified protein derivatives (PPDs). The rank order in stimulatory potency for different PPDs was the same for all three cytokines. Before vaccination Malawians made higher pro- and anti-inflammatory responses than did United Kingdom subjects. Fewer than 5% of United Kingdom subjects made IL-10 in response to any PPD, compared to 19 to 57% responders among Malawians. Priming for regulatory IL-10 may contribute to the smaller increase in gamma interferon responses in Malawians compared to United Kingdom subjects following BCG vaccination.
The efficacy of vaccination against tuberculosis with Mycobacterium bovis bacillus Calmette-Guérin (BCG) varies geographically (reviewed in reference 10), one reason for which relates to differences in exposure to environmental mycobacteria (3). This is reflected in (i) higher prevaccination gamma interferon (IFN-γ) responses to environmental mycobacterial antigens in Malawi than in the United Kingdom (5), (ii) greater BCG-attributable increases in IFN-γ responses in the United Kingdom than in Malawi (5), and (iii) greater BCG-attributable increases in IFN-γ response to M. tuberculosis purified protein derivative (PPD) in Malawians with low initial responsiveness to PPDs of M. avium, M. intracellulare, and M. scrofulaceum (the MAIS complex) than in those with high initial responses (3). The higher prevalence of IFN-γ responsiveness to PPDs of the environmental MAIS complex than to PPDs of M. tuberculosis, M. bovis, or the more distantly related fast-growing species M. vaccae and M. fortuitum in Malawi (3) might relate to both relative exposure to these different species and the composition of cross-reacting T-cell peptide epitopes in the preparations. Alternatively, or in addition, they could reflect the ability of PPDs to induce proinflammatory (e.g., tumor necrosis factor alpha [TNF-α] and interleukin-1β [IL-1β]) or anti-inflammatory (e.g., IL-10) cytokine responses that regulate the adaptive immune response. To address this, we here examine 24-h TNF-α, IL-1β, and IL-10 responses in stimulated whole-blood cultures. This reveals differences in the magnitude of responsiveness to different mycobacterial PPDs as well as apparent in vivo priming for higher prevaccination responses in Malawian than in United Kingdom subjects.
Study design.
Recruitment and study design are described in detail elsewhere (4, 5, 23). In Malawi 633 young adults were recruited: of 562 eligible for BCG vaccination, 378 received BCG vaccine and 184 were given placebo. One year later, 329 of 378 vaccinees and 154 of 184 from the placebo group were retested for cytokine responses. In the United Kingdom (5, 23), 424 children were recruited: of those eligible for BCG vaccination, 282 received BCG vaccine and 115 controls received no injection. One year later 246 of 282 vaccinees and 104 of 115 controls were retested. Individuals acting as placebo group members or controls were vaccinated at the 1-year follow-up. Whole-blood assays were performed as described elsewhere (4, 5), and supernatants were collected 24 h poststimulation with seven PPDs or one “new tuberculin,” derived from six different species of mycobacteria. PPDs of M. tuberculosis (batch RT48, lot 191), M. avium (batch RS10/2, lots 37 and 39), M. intracellulare (batch RS23, lot 27), and M. scrofulaceum (batch RS95, lots 17 and 18) were prepared (15) at the Statens Serum Institut, Copenhagen, Denmark. M. avium CVL, a standard avian PPD (24) produced in 1954 at the Central Veterinary Laboratory, was obtained from the Veterinary Laboratories Agency. M. intracellulare PPD-B, prepared in 1970 according to a standard protocol (6), was obtained from the U.S. Public Health Service. M. bovis CVL PPD was from the National Institute of Biological Standards and Controls (South Mimms, United Kingdom), and M. vaccae new tuberculin (20) (batch R877R) was from J. Stanford (University College London). Endotoxin testing was carried out by the European Endotoxin Testing Service (Verviers, Belgium). Lipoarabinomannan (LAM) content was measured by enzyme-linked immunosorbent assay (ELISA) with antibody CS-35 (12), which recognizes arabinofuranosyl-containing motifs on all mycobacterial LAMs as the probe and BCG LAM as the standard. All preparations were used in whole-blood assays at a final concentration of 5 μg/ml. Lipopolysaccharide (LPS; Sigma; final concentration, 1 μg/ml) was used as a nonmycobacterial stimulus known to trigger innate immune responses. Quantitative TNF-α, IL-1β, and IL-10 ELISAs were carried out using commercially available antibody pairs (PharMingen, San Diego, Calif.; Biosource, Nivelles, Belgium). Recombinant cytokines (2,000 to 31 pg/ml; PharMingen or Biosource) were used for the standard curves; the lower limit of detection for all assays was 31 pg/ml. Plates were read and analyzed using Revelation 3.04 software (Dynex, Chantilly, Va.). Medium-alone negative-control values were subtracted from all results. Control supernatants were exchanged between the Malawi and United Kingdom labs to ensure comparability. Statistical analyses were carried out using STATA 7. Associations between pairs of variables were quantified using the Spearman rank correlation coefficient. Differences between the United Kingdom and Malawi were assessed using the Mann-Whitney test for median responses and the chi-square test for the percentage with a “positive” (i.e., >62-pg/ml) response. Linear regression was used for analysis of the change in TNF-α and IL-10 responses between recruitment and 1 year postvaccination. Values below the limit of detection of the assay were set to 15 pg/ml. A log transformation (base 2) was used for both the prevaccination and the postvaccination response. Analysis was based upon the difference between these two values.
PPDs trigger higher prevaccination TNF-α, IL-1β, and IL-10 responses in Malawi than in the United Kingdom.
Prevaccination inflammatory cytokine responses are presented as median responses (picograms per milliliter) and percent responders (>62 pg/ml) in Fig. 1. Two clear patterns emerge: (i) the rank order of potency of the different mycobacterial preparations is essentially the same for TNF-α, IL-1β, and IL-10 responses, and (ii) Malawians make higher pro- and anti-inflammatory responses than do United Kingdom individuals. The latter is not due to differences in culture conditions, since both populations made similar TNF-α, IL-1β, and IL-10 responses to control LPS stimulation (≥90% responders; data not shown). Nor is the responsiveness to the mycobacterial preparations due to LPS contamination, since the level of endotoxin measured in all of the mycobacterial antigens used here was <0.05 endotoxin units/ml (<5 pg/ml). The rank order of potency of the PPD or new tuberculin preparations holds true across both populations for TNF-α and IL-1β, with the percentage of individuals responding to each of the antigens in Malawi strongly correlated with the percentage of individuals responding to the same antigens in the United Kingdom (TNF-α, r = 0.97, P < 0.001; IL-1β, r = 0.95, P < 0.001). In Malawi there were significant correlations (r = 0.52 to 0.68, P < 0.001) for individual TNF-α against IL-1β responses for all seven PPDs and for M. vaccae new tuberculin. TNF-α and IL-1β responses each also were correlated (r = 0.35 to 0.65, P < 0.001) with IL-10 responses. Among United Kingdom adolescents, for whom all median responses were generally lower (Fig. 1), the only moderately strong correlations were between TNF-α and IL-1β (r = 0.51, P < 0.001) for responses to M. avium CVL and between TNF-α and IL-10 (r = 0.60, P < 0.001) and IL-1β and IL-10 (r = 0.57, P < 0.001) for responses to M. vaccae new tuberculin. The prevalence of IL-10 responsiveness to PPDs was extremely low, with the exception of the response to M. vaccae new tuberculin. Interestingly, M. vaccae new tuberculin produced the highest TNF-α, IL-1β, and IL-10 responses in both populations, and M. tuberculosis PPD produced the lowest responses.
FIG. 1.
Median responses (picograms per milliliter) and percent responders (>62 pg/ml) for seven mycobacterial PPDs and M. vaccae new tuberculin. Data are presented in order of magnitude of TNF-α responses. Group sizes were as stated previously except for IL-1β responses in United Kingdom (UK) subjects, where n = 100. SSI, Statens Serum Institut; CVL, Central Veterinary Laboratory.
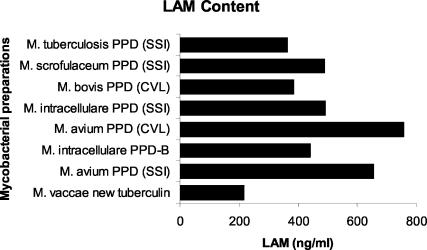
LAM content.
The similar rank order of stimulatory potency of PPDs suggests a common recognition and signaling pathway and/or some interdependence among these cytokines for their induction. Such coordinated regulation of all three cytokines has been observed previously in the response of human monocytes to bacterial LPS (11), where it was demonstrated that IL-10 synthesis is uniquely dependent on endogenous proinflammatory cytokines TNF-α and IL-1β. LPS triggers macrophages through the combined action of the adaptor molecule CD14 and the signal-transducing molecule Toll-like receptor 2 (TLR2) (1, 22). Mycobacteria also possess ligands that trigger both TLR2 and TLR4 (reviewed in reference 21). The mycobacterial ligands for TLR2 include LAM, a biosynthetic precursor of LAM phosphatidylinositolmannan, and the 19-kDa lipoprotein of M. tuberculosis, both of which could copurify with proteins and/or peptides in the PPD preparative process. LAM derived from ubiquitous, fast-growing, nonpathogenic mycobacteria (PILAM or AraLAM) binds CD14 and TLR2 (21) and elicits potent pro- and anti-inflammatory responses. The biochemically distinct (13) LAM from M. tuberculosis and M. bovis (ManLAM) fails to activate TLR2- or TLR4-transfected cells (21) and is a poor inducer of TNF-α, IL-1β, and IL-10 responses. Using the CS-35 antibody, we found that all of the mycobacterial PPDs contained LAM (Fig. 2) at concentrations (364 to 757 ng/ml) of the order of magnitude (1,000 ng/ml) of purified LAM used previously (16, 17) to induce macrophage pro- and anti-inflammatory responses. However, since the CS-35 antibody binds less avidly to ManLAM than to AraLAM (12) and mycobacteria differ in relative content of ManLAM and AraLAM (13), the absolute amount of LAM measured in the assay will not directly reflect its stimulatory potency. Hence, although there was no direct correlation between the magnitude of cytokine responses elicited by the different PPDs and LAM content as measured in our assay (data not shown), the rank order of potency of the preparations does reflect the relative content of qualitatively different LAMs. LAMs from different strains of M. tuberculosis (Erdman, H37Rv, and H37Ra) and attenuated M. bovis BCG are all mannose capped but vary in the extent of capping between 40 and 70% (13). This is consistent with our observation that PPDs from M. tuberculosis and M. bovis are at the bottom end of the rank order for potency as triggers in our assays (Fig. 1). In contrast, the more distantly related fast-growing M. vaccae is likely, like other fast-growing strains, to have the arabinan domain of LAM uncapped (AraLAM) or be capped with phosphoinositide motifs (PILAM) (13). This is consistent with its position as the most potent trigger for all three cytokines in our assays, despite the lower levels of LAM (217 ng/ml) detected by our assay. Members of the MAIS (M. avium, M. intracellulare, and M. scrofulaceum) complex are intermediate, both in terms of their potency as triggers for pro- and anti-inflammatory cytokines in our assays and in having LAMs capped mainly with single mannose residues rather than the di- and trimannopyranoside motifs found in M. tuberculosis and M. bovis-derived ManLAM (14). Overall our data are consistent with the hypothesis that qualitatively different LAMs could be the major contributors to the innate immune responses that we have observed to be elicited by PPDs in whole-blood assays.
FIG. 2.
LAM concentrations, as determined by ELISA with anti-LAM CS-35 antibody, which recognizes arabinofuranosyl-containing motifs common to all LAMs, in seven mycobacterial PPDs and M. vaccae new tuberculin. Data are presented in order of potency as inducers of pro- and anti-inflammatory responses. SSI, Statens Serum Institut; CVL, Central Veterinary Laboratory.
Effect of BCG vaccination on TNF-α, IL-1β, and IL-10 responses.
Higher prevaccination pro- and anti-inflammatory responses in Malawian than in United Kingdom adolescents suggested in vivo priming, possibly due to exposure to environmental mycobacteria. To determine whether M. bovis BCG vaccination itself could prime for enhanced inflammatory responses, we compared pre- and postvaccination responses. In order to determine whether there was a significant vaccine-related change in response between years 1 and 2, we compared (Table 1, vaccine/placebo ratio) how many times higher (or lower) the vaccine-induced change was than the placebo. For TNF-α responses, there were no significant vaccine-related changes in response to any antigens in Malawi or the United Kingdom (Table 1, ratio of change in vaccinated group to change in placebo group of close to 1). For IL-10 responses, there was a vaccine-related reduction in response to M. bovis and M. avium CVL PPDs (borderline significance; P = 0.058; P = 0.065), M. scrofulaceum PPD (P = 0.015), and M. vaccae new tuberculin (P = 0.02) in Malawi (Table 1, ratio of change in vaccinated group to change in placebo group of <1) but no vaccine-related change in response to any antigens in the United Kingdom. Hence, although we were measuring immediate (24-h) innate anti-inflammatory responses to mycobacterial antigen preparations in vitro, there were changes in responses over the year in Malawi that appear to be attributable to vaccine-induced in vivo effects that reduce IL-10 responses.
TABLE 1.
Vaccination with M. bovis BCG and changes in proinflammatory (TNF-α) or anti-inflammatory (IL-10) responses to mycobacterial antigens as indicated by comparison of vaccine and placebo groupsa
| Antigen | Response by cytokine and country
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TNF-α
|
IL-10
|
|||||||||||
| Malawi
|
UK
|
Malawi
|
UK
|
|||||||||
| Vaccine/placebo ratio | 95% CI | P | Vaccine/placebo ratio | 95% CI | P | Vaccine/placebo ratio | 95% CI | P | Vaccine/placebo ratio | 95% CI | P | |
| M. tuberculosis PPD (SSI) | 1.17 | 0.86-1.59 | 0.315 | 1.14 | 0.88-1.48 | 0.318 | 0.97 | 0.82-1.15 | 0.741 | 0.97 | 0.86-1.09 | 0.562 |
| M. bovis PPD (CVL) | 1.04 | 0.71-1.53 | 0.843 | 1.31 | 1.03-1.67 | 0.071 | 0.84 | 0.70-1.01 | 0.058 | 0.87 | 0.84-1.12 | 0.664 |
| M. avium PPD (CVL) | 1.00 | 0.68-1.48 | 0.989 | 1.30 | 0.96-1.78 | 0.094 | 0.84 | 0.70-1.01 | 0.065 | 1.03 | 0.85-1.25 | 0.785 |
| M. avium PPD (SSI) | 0.97 | 0.76-1.24 | 0.799 | 1.21 | 0.91-1.62 | 0.188 | 0.97 | 0.80-1.18 | 0.740 | 1.10 | 0.79-1.53 | 0.556 |
| M. intracellulare PPD (SSI) | 0.83 | 0.63-1.09 | 0.178 | 1.26 | 0.93-1.71 | 0.128 | 0.90 | 0.76-1.05 | 0.185 | 1.05 | 0.83-1.34 | 0.660 |
| M. intracellulare PPD-B | 1.11 | 0.77-1.61 | 0.571 | 1.06 | 0.77-1.44 | 0.735 | 0.89 | 0.66-1.22 | 0.473 | 1.15 | 0.81-1.62 | 0.440 |
| M. scrofulaceum PPD (SSI) | 1.02 | 0.76-1.35 | 0.917 | 0.96 | 0.77-1.21 | 0.743 | 0.83 | 0.71-0.96 | 0.015 | 0.99 | 0.86-1.13 | 0.838 |
| M. vaccae new tuberculin | 0.88 | 0.67-1.15 | 0.346 | 1.19 | 0.79-1.78 | 0.406 | 0.79 | 0.64-0.96 | 0.020 | 0.92 | 0.57-1.50 | 0.741 |
The vaccine/placebo ratio takes into account a comparison of year 1 and year 2 data for the same individual. Ratios of >1 indicate that vaccination is associated with an enhanced response; ratios of <1 indicate that vaccination is associated with a reduced response. Significant (P < 0.05) and borderline significant (0.05 < P ≤ 0.065) responses are shown by boldface. Abbreviations: SSI, Statens Serum Institut; CVL, Central Veterinary Laboratory; UK, United Kingdom; CI, confidence interval.
Conclusions.
Recent research has focused on mechanisms that allow innate immunity to drive and modulate the adaptive immune response (2, 7-9, 18, 19), particularly in relation to M. tuberculosis infection (21) and to vaccination (7). Innate immunity involves rapid recognition of pathogen-associated molecular patterns via pattern recognition receptors, leading to the induction of inflammatory cytokines by macrophages and expression of costimulatory molecules on dendritic cells. We have shown that, in addition to providing protein antigens for recall of adaptive T-cell IFN-γ responses (3, 4, 23), mycobacterial PPDs also elicit potent early-innate TNF-α, IL-1β, and IL-10 responses in whole-blood assays. While we cannot discount some role for dendritic cells and T cells in production of 24-h inflammatory cytokine responses, it seems likely that monocytes dominate the response. This stimulation of innate responses by mycobacterial PPDs may influence detection of antigen-specific T-cell responses in whole-blood cultures.
An interesting feature of our study was the dramatic difference in pro- and anti-inflammatory responses between Malawi and the United Kingdom. This could reflect a difference in the relative numbers of circulating monocytes and/or expression of higher levels of pattern recognition receptors on circulating monocytes in Malawian than in United Kingdom adolescents. Of particular note was the failure of United Kingdom subjects to make IL-10 in response to PPDs compared to Malawians. In contrast, the apparent priming for immunoregulatory IL-10 responses in Malawians might contribute to smaller BCG vaccine-induced IFN-γ responses to M. tuberculosis PPD (3-5). As our data suggest that BCG vaccination reduced the IL-10 response to some PPDs in Malawians, this may be further evidence of a role for in vivo priming and boosting of the innate immune response.
In conclusion, our results demonstrate that mycobacterial PPD preparations do contain molecules that act as ligands to stimulate innate immune responses. Importantly, major differences detected in Malawi compared to the United Kingdom indicate that in vivo priming for innate immune responses occurs and may contribute to the mechanisms that determine differences in levels of protection against M. tuberculosis following BCG vaccination in these two populations.
Acknowledgments
Studies were approved by the National Health Sciences Research Committee of Malawi, the Ethics Committee of the London School of Hygiene and Tropical Medicine, and the Local Research Ethics Committee of Redbridge and Waltham Forest Health Authority.
We thank K. Haslov, G. Hewinson, J. Stanford, and the Central Veterinary Laboratory, Weybridge, United Kingdom, for providing the mycobacterial antigen preparations; Evans Medical for donating the M. bovis BCG vaccine and placebo; and P. Brennan for making available the CS-35 monoclonal antibody as part of NIH, NIAID contract NO1 AI-25469 (http://www.cvmbs.colostate.edu/mip/leprosy/materialsavailable.html). We thank the people of Karonga for participating in these studies and the National Health Sciences Research Committee of Malawi for permission to publish this paper. We thank the staff and students of the United Kingdom schools who participated in the study and the staff of the Redbridge and Waltham Forest Health Authority for their help with the United Kingdom school study.
The work in Malawi was supported by The Wellcome Trust and that in the United Kingdom was supported by the British Leprosy Relief Association (LEPRA).
Editor: A. D. O'Brien
REFERENCES
- 1.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675-680. [DOI] [PubMed] [Google Scholar]
- 2.Bendelac, A., and D. T. Fearon. 1997. Innate pathways that control acquired immunity. Curr. Opin. Immunol. 9:1-3. [DOI] [PubMed] [Google Scholar]
- 3.Black, G. F., H. M. Dockrell, A. C. Crampin, S. Floyd, R. E. Weir, L. Bliss, L. Sichali, L. Mwaungulu, H. Kanyongoloka, B. Ngwira, D. K. Warndorff, and P. E. Fine. 2001. Patterns and implications of naturally acquired immune responses to environmental and tuberculous mycobacterial antigens in northern Malawi. J. Infect. Dis. 184:322-329. [DOI] [PubMed] [Google Scholar]
- 4.Black, G. F., P. E. M. Fine, D. K. Warndorff, S. Floyd, R. E. Weir, J. M. Blackwell, L. Bliss, L. Sichali, L. Mwaungulu, S. Chaguluka, E. Jarman, B. Ngwira, and H. M. Dockrell. 2001. Relationship between IFN-gamma and skin test responsiveness to Mycobacterium tuberculosis PPD in healthy, non-BCG-vaccinated young adults in northern Malawi. Int. J. Tuberc. Lung Dis. 5:664-672. [PubMed] [Google Scholar]
- 5.Black, G. F., R. E. Weir, S. Floyd, L. Bliss, D. K. Warndorff, A. C. Crampin, B. Ngwira, L. Sichali, B. Nazareth, J. M. Blackwell, K. Branson, S. D. Chaguluka, L. Donovan, E. Jarman, E. King, P. E. Fine, and H. M. Dockrell. 2002. BCG-induced increase in interferon-gamma response to mycobacterial antigens and efficacy of BCG vaccination in Malawi and the UK: two randomised controlled studies. Lancet 359:1393-1401. [DOI] [PubMed] [Google Scholar]
- 6.Edwards, L. B., and C. E. Palmer. 1958. Epidemiologic studies of tuberculin sensitivity. 1. Preliminary results with purified protein derivatives prepared from atypical acid-fast organisms. Am. J. Hyg. 68:213-231. [PubMed] [Google Scholar]
- 7.Fearon, D. T. 2000. Innate immunity—beginning to fulfill its promise? Nat. Immunol. 1:102-103. [DOI] [PubMed] [Google Scholar]
- 8.Fearon, D. T. 1997. Seeking wisdom in innate immunity. Nature 388:323-324. [DOI] [PubMed] [Google Scholar]
- 9.Fearon, D. T., and R. M. Locksley. 1996. The instructive role of innate immunity in the acquired immune response. Science 272:50-53. [DOI] [PubMed] [Google Scholar]
- 10.Fine, P. E. 1995. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 346:1339-1345. [DOI] [PubMed] [Google Scholar]
- 11.Foey, A. D., S. L. Parry, L. M. Williams, M. Feldmann, B. M. Foxwell, and F. M. Brennan. 1998. Regulation of monocyte IL-10 synthesis by endogenous IL-1 and TNF-alpha: role of the p38 and p42/44 mitogen-activated protein kinases. J. Immunol. 160:920-928. [PubMed] [Google Scholar]
- 12.Kaur, D., T. L. Lowary, V. D. Vissa, D. C. Crick, and P. J. Brennan. 2002. Characterization of the epitope of anti-lipoarabinomannan antibodies as the terminal hexaarabinofuranosyl motif of mycobacterial arabinans. Microbiology 148:3049-3057. [DOI] [PubMed] [Google Scholar]
- 13.Khoo, K. H., A. Dell, H. R. Morris, P. J. Brennan, and D. Chatterjee. 1995. Inositol phosphate capping of the nonreducing termini of lipoarabinomannan from rapidly growing strains of Mycobacterium. J. Biol. Chem. 270:12380-12389. [DOI] [PubMed] [Google Scholar]
- 14.Khoo, K. H., J. B. Tang, and D. Chatterjee. 2001. Variation in mannose-capped terminal arabinan motifs of lipoarabinomannans from clinical isolates of Mycobacterium tuberculosis and Mycobacterium avium complex. J. Biol. Chem. 276:3863-3871. [DOI] [PubMed] [Google Scholar]
- 15.Magnusson, M., and M. W. Bentzon. 1958. Preparation of purified tuberculin RT23. Bull. W. H. O. 19:829-843. [PMC free article] [PubMed] [Google Scholar]
- 16.Means, T. K., E. Lien, A. Yoshimura, S. Wang, D. T. Golenbock, and M. J. Fenton. 1999. The CD14 ligands lipoarabinomannan and lipopolysaccharide differ in their requirement for Toll-like receptors. J. Immunol. 163:6748-6755. [PubMed] [Google Scholar]
- 17.Means, T. K., S. Wang, E. Lien, A. Yoshimura, D. T. Golenbock, and M. J. Fenton. 1999. Human toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J. Immunol. 163:3920-3927. [PubMed] [Google Scholar]
- 18.Medzhitov, R., and C. A. Janeway, Jr. 1997. Innate immunity: impact on the adaptive immune response. Curr. Opin. Immunol. 9:4-9. [DOI] [PubMed] [Google Scholar]
- 19.Medzhitov, R., and C. A. Janeway, Jr. 1997. Innate immunity: the virtues of a nonclonal system of recognition. Cell 91:295-298. [DOI] [PubMed] [Google Scholar]
- 20.Stanford, J. L., G. A. Rook, N. Samuel, F. Madlener, A. A. Khamenei, T. Nemati, F. Modabber, and R. J. Rees. 1980. Preliminary immunological studies in search of correlates of protective immunity carried out on some Iranian leprosy patients and their families. Lepr. Rev. 51:303-314. [DOI] [PubMed] [Google Scholar]
- 21.Stenger, S., and R. L. Modlin. 2002. Control of Mycobacterium tuberculosis through mammalian Toll-like receptors. Curr. Opin. Immunol. 14:452-457. [DOI] [PubMed] [Google Scholar]
- 22.Takeda, K., T. Kaisho, and S. Akira. 2003. Toll-like receptors. Annu. Rev. Immunol. 21:335-376. [DOI] [PubMed] [Google Scholar]
- 23.Weir, R. E., P. E. M. Fine, B. Nazareth, S. Floyd, G. F. Black, E. King, C. Stanley, L. Bliss, K. Branson, and H. M. Dockrell. 2003. Interferon-gamma and skin test responses of schoolchildren in southeast England to purified protein derivatives from Mycobacterium tuberculosis and other species of mycobacteria. Clin. Exp. Immunol. 134:285-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. 1955. International standard for purified protein derivative of avian tuberculin. WHO Tech. Rep. Ser. 96:11. [Google Scholar]