Abstract
Trichomonas vaginalis produces soluble factors that have been reported to have the ability to damage target cells in vitro, and it has been hypothesized that these factors may play a role in the pathogenesis of human trichomoniasis. A lytic factor (LF) was purified from T. vaginalis, and the molecular characteristics of LF were determined. T. vaginalis extract was subjected to hydrophobic chromatography with a 10 to 60% N-propanol gradient in 0.1 M ammonium acetate, resulting in the elution of LF from the column at 30% N-propanol. Cytotoxicity assays revealed that LF was cytotoxic to WEHI 164 cells and bovine red blood cells, and inactivation of LF by treatment with trypsin suggested that the active component of LF was a protein. Size exclusion chromatography of LF produced two fractions at 144 and 168 kDa, and analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis of LF under reducing conditions revealed two subunits of 57 and 60 kDa. Results of a fluorescence assay of LF on carboxyfluorescein-labeled liposomes composed of phosphatidylcholine-cholesterol showed that liposomes were hydrolyzed, suggesting that LF had phospholipase activity. Thin-layer chromatography analysis of BODIPY (4,4-difluoro-3a,4adiaza-s-indacene)-labeled phosphatidylcholine treated with LF demonstrated products that migrated identically to the products produced by treatment with phospholipase A2 (PLA2). These results suggest that LF is a PLA2 and may be an important virulence factor of T. vaginalis mediating the destruction of host cells and contributing to tissue damage and inflammation in trichomoniasis.
Human trichomoniasis, a sexually transmitted disease caused by Trichomonas vaginalis, is a disease ranging from a mild to a more severe infection causing vaginitis in women and urethritis in men (18). T. vaginalis is one of the most common causes of vaginitis in women and is estimated to account for 20 to 30% of all vaginitis cases worldwide (10). The mechanisms of pathogenesis of the trichomonad parasite in the urogenital tract and avoidance of the host immune system response are not well understood although T. vaginalis is known to cause cytopathic effects (CPE) on host cells which likely play a role in pathogenesis.
Since T. vaginalis is an extracellular, flagellate protozoan parasite, it has been proposed that the CPE are due to both contact-dependent and contact-independent mechanisms. There has been a description of four adhesion proteins that may mediate adherence to the vaginal epithelial cells in vivo (4), an event that leads to efficient CPE in vitro (1). Contact-dependent CPE has been well studied, and numerous potentially pathogenic molecules, such as cysteine proteinases (10), as well as hemolytic activity (17), that may be part of the molecular mechanism of contact-dependent CPE have also been reported. Two cysteine proteinases are known to be expressed on the surface of this parasite and to bind to host cells (2, 3, 23), and inhibition of cysteine proteases was shown to lower adhesion and cytotoxicity (2, 3). In contrast, how secreted proteases of T. vaginalis function in CPE or pathogenesis has not been elucidated so that the molecular mechanisms involved remain unclear.
Hogue first reported the CPE of T. vaginalis for human cells in vitro and hypothesized that the pathogenesis observed was directly due to secreted toxins from T. vaginalis (16). Subsequently CPE mediated by cell-free, parasite-derived filtrates on cultured cells and contact-dependent destruction of host cells have been reported (14, 16, 23, 26). Secreted soluble factors, termed cell-detaching factor (CDF) and T. vaginalis factor (TVF), cause a variety of morphological changes to certain mammalian cells in vitro (15, 21). Many of these morphological effects of parasite-derived soluble factors were reversible when the mammalian cells were subsequently cultured in media without CDF or TVF (15, 21), but no data on the specific biochemical alterations on the target cells were noted.
With the exception of cysteine proteinases, the parasite enzymatic activities that may be involved in the CPE on host cells, including target cell lysis, remain unknown. Because several microbial lytic molecules (e.g., bacterial hemolysins) have been identified as phospholipases or lipases, we hypothesized that a lipase or phospholipase could account for the target cell lysis and may be an important virulence factor of T. vaginalis.
Analysis of T. vaginalis extracts identified a lytic factor (LF) that hydrolyzes lipids and phospholipids, lyses nucleated mammalian cells and red blood cells, and hydrolyzes liposomes in vitro, suggesting that LF contains lipase or phospholipase activity. Further investigation revealed that the LF specifically hydrolyzed phosphatidylcholine to yield products similar to those produced by phospholipase A2 (PLA2), suggesting that the major enzymatic component of LF responsible for host cell destruction is a PLA2.
MATERIALS AND METHODS
Parasites.
T. vaginalis strain UAB 5-1, isolated in 2001 (provided by J. Swebke, University Alabama Medical Center, Birmingham), was grown in modified Diamond's medium, pH 6.0 (13), containing 10% heat-inactivated fetal bovine serum (Atlanta Biologicals, Atlanta, Ga.) at 37°C in 500-ml screw-cap serum bottles. Parasites were counted with a hemocytometer, collected by centrifugation (400 × g, 25°C, 5 min), and washed twice in phosphate-buffered saline (PBS; 10 mM sodium phosphate, pH 7.2, 150 mM NaCl).
Parasite extracts.
Whole-cell parasite extracts were prepared by adding 108 parasites/ml to extraction buffer, which contained 50 mM Tris (pH 8.0), 100 mM NaCl, 5 mM EDTA, 1% Triton X-100, 10 μ[/mu] leupeptin, and 10 μM N-trans-epoxysuccinyl-l-leucylamido-(4-guanidino) butane (E-64; Sigma, St. Louis, Mo.), followed by incubation on ice for 30 min with gentle mixing every 5 min. Cellular debris was pelleted (1,000 × g, 10°C, 30 min), and the supernatant was stored at −20°C. The bicinchoninic acid (BCA) protein assay (Pierce Chemicals, Rockford, Ill.) was used to determine protein yields.
LF purification. (i) Hydrophobic chromatography.
LF was purified by hydrophobic chromatography (31) on octyl-Sepharose (Amersham-Pharmacia, Arlington Heights, Ill.). Briefly, octyl-Sepharose beads were washed twice with 2 volumes of 5% N-propanol in 0.1 M ammonium acetate, pH 7.0. Parasite extract (2 mg/ml of beads) or bovine serum albumin (BSA; 500 μg/ml of beads; negative control) was added, and the mixture was rotated end-over-end overnight at 4°C. The supernatant was decanted, and the beads were washed with 20 volumes of 5% N-propanol in 0.1 M ammonium acetate, pH 7.0. The column was eluted with a ladder gradient of 10 to 60% N-propanol (10% N-propanol at 30 ml/increment) in 0.1 M ammonium acetate. The presence of LF or BSA was monitored by periodic acid-Schiff (Sigma) staining of fractions spotted onto nitrocellulose paper or by BCA protein assay (Pierce Chemicals). Fractions containing carbohydrate material or protein (BSA) were concentrated under vacuum (Speedvac; Bio-Rad), resuspended in Hanks balanced salt solution (HBSS), pH 7.2, and pooled into groups of four fractions for further analysis.
(ii) Gel filtration.
Octyl-Sepharose LF preparations were further fractionated by size exclusion chromatography on Sephacryl 200 (Amersham Pharmacia). A column (1- by 25-cm bed volume) was packed in 0.1 M ammonium acetate, pH 7.0 (buffer), and the packed column was calibrated with a sample containing molecular weight standards (myosin, BSA, ovalbumin, and β-lactoglobulin), all at 1 mg/ml. For each chromatogram 500 μl of LF preparations was typically added to the column and 1-ml fractions were collected at a flow rate of 450 μl/min. The protein contents of fractions were determined by the BCA protein assay (Pierce Chemicals), and fractions were retained at 4°C for functional assays.
SDS-PAGE.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed using 10% T gels (19) that were either developed with silver staining (Novex; Invitrogen, Carlsbad, Calif.) or electroblotted (36) onto a nitrocellulose membrane and analyzed by Western blotting.
Periodate-sensitive epitopes.
Western blots were probed with antibodies or treated with periodate and then probed with antibodies. Briefly, strips were washed with 50 mM sodium acetate buffer, pH 7.0, for 5 min. Strips were then soaked either in 20 mM periodate in 50 mM sodium acetate buffer, pH 4.5, or in 50 mM sodium acetate, pH 4.5, in the dark for 1 h. After three washes (10 min each) in 50 mM sodium acetate, strips were soaked in 1% glycine in 150 mM NaCl for 30 min and washed in 5% BLOTTO (5% skim milk in PBS) for 30 min. Goat anti-T. vaginalis serum was added, and strips were incubated overnight at 4°C. Strips were washed in three changes of 5% BLOTTO followed by peroxidase-conjugated rabbit anti-goat immunoglobulin G (ICN/Cappel, Costa Mesa, Calif.) and incubated for 1 h. After three washes in PBS strips were developed with tetramethylbenzidine membrane peroxidase substrate (KPL, Gaithersburg, Md.).
Cytotoxicity assays.
The cytotoxicity of T. vaginalis parasites and purified LF was measured with the murine fibroblast cell line WEHI 164 (27) (American Type Culture Collection, Manassas, Va.). WEHI 164 cells were plated in 96- or 24-well plates at 2.0 × 105 or 2.0 × 106/well, respectively, depending on experimental design, in complete Dulbecco's modified Eagle medium (4 mM l-glutamine, 1 mM sodium pyruvate, 10% fetal bovine serum, 25 μg/ml gentamicin sulfate). Plates were incubated at 37°C in a 5% CO2, humidified atmosphere for 4 to 6 h to allow the WEHI 164 cells to adhere to culture plates. Serial dilutions of the parasite material (viable parasites, extract, LF, etc.) or LF treated with trypsin (20 μl, 130 μg/ml) or E-64 (20 μl, 100 mM) (Sigma) were then added to the WEHI 164 cells. Viable parasites were incubated directly on WEHI 164 cells or added to a Transwell filter (Corning Incorporated, Corning, N.Y.) inserted into the plate wells. A negative control was prepared with serial dilutions of BSA fractions eluted from the octyl-Sepharose column. A tumor necrosis factor alpha standard (National Institutes of Health) was used as a positive control for cytotoxicity assays. After incubation at 37°C overnight, medium was decanted and monolayers were washed with warm HBSS. HBSS was removed, and fixative (4% formalin) was added for 10 min, followed by crystal violet solution (25% ethanol, 0.55% crystal violet, 0.145 M NaCl) for 10 min. After three washes with HBSS, plates were allowed to dry overnight. Samples were solubilized with an acid-detergent solution (0.5 M acetic acid containing 0.5% SDS), and absorbance was measured at 550 nm on a spectrophotometer (Thermomax; Molecular Devices, Sunnyvale, Calif.). Percent cytotoxicity was calculated with the following equation: percent cytotoxicity = [(untreated WEHI OD550 − treated WEHI OD550)/untreated WEHI OD550] × 100, where OD550 is optical density at 550 nm.
Enzyme assays. (i) Lipase assay.
Lipase activity was measured with polyoxyethylene sorbitan substrates (Tween 20 and Tween 80) as described previously (35). The reaction mixture, which consisted of 0.01 ml of 10% Tween in 50 mM Tris hydrochloride, pH 7.6 (Tris buffer), 0.01 ml of 1 M CaCl2 in Tris buffer, and 0.23 ml of 50 mM Tris-hydrochloride, pH 7.6, was mixed with 0.05 ml of fraction material or phospholipase D (PLD; Sigma). Reagent blanks were prepared with 0.05 ml of water. Duplicates of each mixture were prepared and incubated at 37°C for 2 h. The turbidity of each mixture was measured at 405 nm on a spectrophotometer (Thermomax; Molecular Devices).
(ii) Protease assay.
Protease activity was measured with azocoll as described earlier (8). Briefly, 11 mg of azocoll (Sigma)/ml in 0.1 M potassium phosphate buffer, pH 7.8, was preequilibrated at 42°C for 20 min in 1.0-ml aliquots. The reaction was initiated by the addition of 0.1 ml of enzyme to the azocoll solution. After mixing, 0.25 ml of the sample was removed at 5-min intervals and added to 1 ml of 5% of trichloroacetic acid to stop reaction, and absorbance was measured at 550 nm on a spectrophotometer (Thermomax).
Liposome preparation.
Liposomes were prepared essentially as described by Senior and Gregoriadis (29). Briefly, 0.25 ml of phosphatidylcholine (Sigma) in chloroform (100 mg/ml) was mixed with 0.63 ml of cholesterol (Sigma) in chloroform (20 mg/ml), and the lipid solution (1:1, molar) was dried by rotary evaporation in a 50-ml round-bottom flask. Two milliliters of aqueous 20 mM 5(6)-carboxyfluorescein (CF) (Molecular Probes, Eugene, Oreg.), warmed to 40°C, was added to the lipid film with shaking to dislodge lipid film. This was followed by sonication to reduce the multilamellar vesicles to small unilamellar vesicles (liposomes). Sonication was performed in a 40°C water bath with 1-min bursts at 60% output (50 Sonic Dismembrator; Fisher, Denver, Colo.) followed by 30-s cooling periods (10 min total). Large vesicles together with liberated probe tip fragments were pelleted (3,000 × g, 25°C, 7 min). Unincorporated CF was removed from the liposomes by gel filtration (Sepharose CL-4B) and centrifugation (1,000 × g,10°C, 30 min), and liposome fractions with CF incorporated were stored at 4°C. For assays liposomes were incubated with LF, BSA, or buffer (PBS) at 37°C for 2 h and treatment groups were then read on a fluorometer at an excitation wavelength of 435 nm and emission wavelength of 538 nm. The extent of lysis was expressed as the percent release, calculated with the following equation: percent CF release = [(control OD538 − unknown OD538)/control OD538] × 100.
Phospholipid hydrolysis.
Ten microliters of 1 mM BODIPY (4,4-difluoro-3a,4adiaza-s-indacene)-labeled phosphatidylcholine (BPC; Molecular Probes) was dried under a slow stream of nitrogen, dissolved in 50 μl of solution I (0.5 mM octylglucoside, 0.4 mM NaCl, 60 mM HEPES, pH 7.0), and briefly sonicated. Fifty microliters of solution II (5 mM EGTA, 2 mM EDTA, 1 mM dithiothreitol) or HBSS, pH 7.0, was added to the BPC solution, and 5 μl of LF, PLD, PLA2 (Sigma), or HBSS (blank) was added to 12.5 μl of BPC. Treatment groups were incubated for 2 h at 37°C. A 5-μl sample of each reaction mixture was then plated on a K6 silica gel 60 thin-layer chromatography plate (Whatman, Maidstone, United Kingdom) and allowed to dry. Samples were resolved in chloroform-methanol-acetic acid solvent (45:45:10), and hydrolysis was detected by inspection under far-UV (365-nm) illumination.
Statistical analysis.
Data were analyzed by a one-way analysis of variance, followed by Tukey's multiple-comparison test (Prism; GraphPad Software, San Diego, Calif.) to detect significant differences (P < 0.05) among treatment groups.
RESULTS
T. vaginalis CPE.
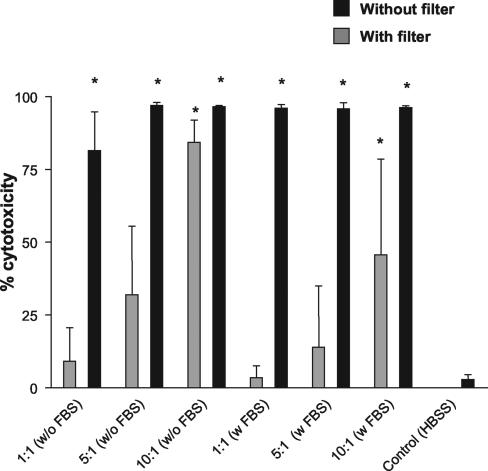
Previous reports have indicated that the CPE of T. vaginalis on mammalian cells in vitro may result from parasite-to-cell contact (3, 12, 14, 23) and soluble products released by the parasite (2, 21, 28). To address the question of whether contact is required for cytotoxicity, T. vaginalis was incubated with WEHI 164 cells in the presence or absence of Transwell filters. T. vaginalis added directly to the WEHI 164 cells, allowing parasite-to-cell contact, produced a high cytotoxic effect (>80%) compared to controls in the presence or absence of fetal bovine serum (FBS) at all parasite/cell ratios tested (10:1, 5:1, and 1:1) (Fig. 1).
FIG. 1.
T. vaginalis cytotoxicity for WEHI 164 cells is present with or without parasite-to-cell contact. Cytotoxicity of T. vaginalis cultured on WEHI 164 cells for 24 h was determined with or without Transwell filters and in the presence or absence of FBS. Data shown are means ± standard deviations from three individual experiments. *, significant difference (P < 0.05) between treatment group and control (WEHI 164 cells plus HBSS).
T. vaginalis added to filter inserts to prevent contact between the parasite and target produced lower cytotoxic levels than cultures without inserts. However, significant cytotoxicity was observed in the presence or absence of FBS at parasite-to-target ratios of 10:1 (Fig. 1). Although cytotoxicity levels at parasite-to-target ratios of 5:1 and 1:1 were not significant in the presence of filter barriers, a dose effect was evident as the ratio decreased. Thus contact between parasites and targets was not required for cytotoxicity for WEHI 164 cells.
Chromatographic purification of LF.
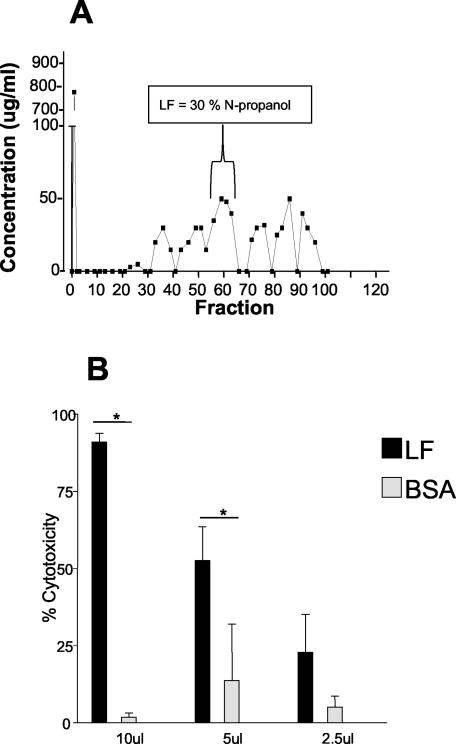
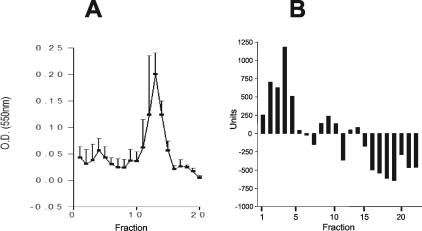
Since we observed cytotoxic activity of soluble parasite material on WEHI 164 cells and since it is well known that several microorganisms express lipolytic activity, which can play a role in their pathogenesis, including host cell membrane destruction, we examined the lipolytic activity present in T. vaginalis. Results of hydrophobic chromatography of T. vaginalis extract on octyl-Sepharose revealed that cytotoxic material, termed LF, eluted at 30% N-propanol, as detected by assay of WEHI 164 cells (Fig. 2). When LF was tested for cytotoxic activity on WEHI 164 cells, it exhibited a cytotoxic effect, with the kinetics of cell lysis ranging from 4 to 24 h depending on the concentration of LF in the preparation (data not shown). The addition of 10 μl of LF to 5.0 × 105 WEHI 164 cells had a cytotoxic effect of greater than 90% (Fig. 2). LF when added at 5 and 2.5 μl had cytotoxic effects on WEHI 164 cells of 50 and 25%, respectively, and LF lysis was also observed with bovine red blood cell targets (data not shown). When 10 μl of BSA material eluted from the octyl-Sepharose column (negative control) was added to WEHI 164 cells, there was no significant cytotoxic effect (Fig. 2). Parasite material which eluted off the column at 40% N-propanol in 0.1 M ammonium acetate also was not cytotoxic (data not shown). LF fractions contained carbohydrate material as detected by periodic acid-Schiff staining of fractions spotted onto nitrocellulose paper (data not shown).
FIG. 2.
Purified LF is cytotoxic to WEHI 164 cells. (A) Octyl-Sepharose chromatogram of T. vaginalis extract with LF elution at 30% N-propanol. (B) Cytotoxicity of LF and BSA, purified by octyl-Sepharose chromatography as described in Materials and Methods, lyophilized, and reconstituted in HBSS. Cytotoxicity was measured on WEHI 164 cells as described for Fig. 1. Data shown are means ± standard deviations from three individual experiments. *, significant difference (P < 0.05) between treatment groups.
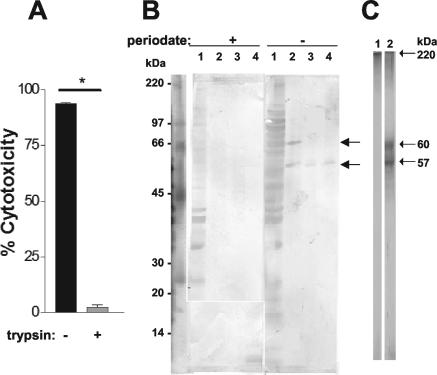
The effects of protease treatment on LF activity were examined by digestion with trypsin followed by a cytotoxicity assay. Treatment of LF (10 μl) with trypsin inhibited the cytotoxic activity on the WEHI 164 cells by 90% (Fig. 3A). As a control for the effects of trypsin on WEHI 164 cells, BSA eluted from the octyl-Sepharose column was treated with serial dilutions of trypsin, the dilutions that hydrolyzed BSA were added to BSA, and the mixture was assayed on WEHI 164 cells for lytic effects. Although 130 μg of trypsin/ml hydrolyzed BSA, it produced no detectable lysis of the WEHI 164 cells (data not shown).
FIG. 3.
Proteolytic cleavage of LF eliminates cytotoxic activity. LF and BSA were treated with trypsin for 2 h, followed by an assay on WEHI 164 cells and analysis by SDS-PAGE. (A) WEHI 164 cell cytotoxicity assay of LF and trypsin-treated LF. Pooled data from two individual experiments are shown. *, significant difference (P < 0.05) between treatment groups. (B) LF samples were subjected to SDS-PAGE (10% T gel), electroblotted onto a nitrocellulose membrane, and probed with goat anti-T. vaginalis. Lanes 1, T. vaginalis extract; lanes 2, LF; lanes 3, LF at a 1:4 dilution treated with trypsin; lanes 4, LF at a 1:8 dilution treated with trypsin. The Western blot is representative of results of three individual experiments. (C) SDS-PAGE (10% T) of fractions of LF resolved by size exclusion chromatography (fractions 1 to 5). Lane 1, nonreducing conditions; lane 2, reducing conditions. Results shown are representative of three individual experiments.
To determine the molecular effects of protease on LF components, Western blots of trypsin-treated LF were probed with goat anti-T. vaginalis polyvalent serum. Untreated LF probed with goat anti-T. vaginalis polyvalent serum revealed two major bands (Fig. 3B) at approximately 60 and 57 kDa. When trypsin treated LF was probed with goat anti-T. vaginalis polyvalent serum, there was only one distinguishable band at 57 kDa. Interestingly, when LF was treated with periodate, the goat anti-T. vaginalis polyvalent serum was unable to recognize the epitopes of LF on the 60- and 57-kDa bands (Fig. 3B), indicating that immunogenic epitopes detected in the two bands are glycosylated.
Enzyme assays.
T. vaginalis has many proteases, notably cysteine proteases, and at least 23 cysteine proteases have been identified in T. vaginalis (9). Since proteases have been implicated in target cell damage, we examined LF cytotoxicity in the presence of the cysteine protease inhibitor E-64, and the results indicated that E-64 did not inhibit cytotoxicity on WEHI 164 cells. The BSA control, treated with the same levels of E-64, showed no signs of cytotoxicity on WEHI 164 cells (data not shown). An azocoll assay of LF revealed no protease activity compared to that in an equal amount of the positive control, trypsin, which hydrolyzed azocoll after as little as 25 min of treatment (data not shown). These results suggested that the cytotoxic effects of LF were not primarily due to protease activity.
LF hydrolyzes phospholipase substrates.
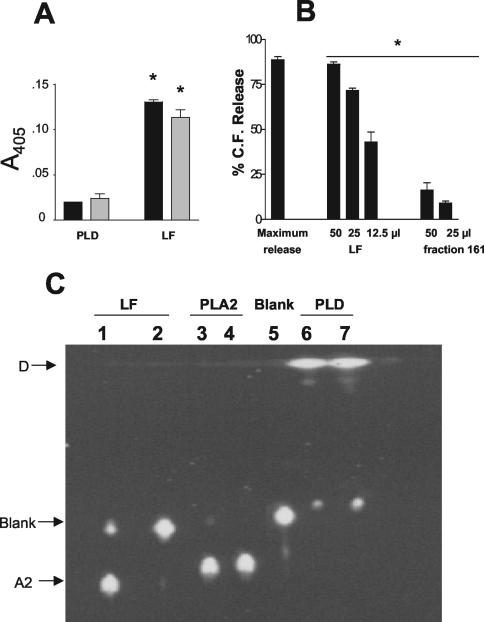
The lipase activity of LF was tested on substrates Tween 20 and Tween 80, which are hydrolyzed by lipases or phospholipases, producing an insoluble product detected by its turbidity measured at 405 nm. LF produced a turbidity 6.5-fold higher than PLD with Tween 20 and 4.7-fold higher with Tween 80 (Fig. 4A).
FIG. 4.
LF hydrolysis of phospholipase substrates. (A) LF and PLD (positive control) were incubated with Tween 20 or Tween 80 for 2 h at 37°C, and turbidity was recorded at 405 nm. Results are shown for data pooled from two individual experiments. *, significant difference (P < 0.05) between treatment groups. (B) LF, fraction 161 (negative control), and 1% Triton X-100 (maximum release) were incubated with CF-liposomes composed of phosphatidylcholine-cholesterol (1:1 ratio) for 2 h at 37°C, and CF release was detected by fluorimetry (excitation, 435 nm; emission, 538 nm). Data shown were pooled from three individual experiments. *, significant difference (P < 0.05) between treatment groups. (C) BPC substrate was incubated with LF, PLA2, blank (HBSS), or PLD for 2 h at 37°C, and products (D, blank, and A2) were resolved by thin-layer chromatography and detected by inspection under far UV (365 nm). Lane 1, 2× LF; lane 2, 1× LF; lane 3, 1× PLA2; lane 4, 2× PLA2; lane 5, blank (HBSS); lane 6, 2× PLD; lane 7, 1× PLD. Results shown are representative of three individual experiments.
A liposome assay was performed next, since this method produces less variation than, and has superior sensitivity to, Tween assays and since liposomes with a precise lipid composition (phospholipase target molecules such as phosphatidylcholine) could be used. Liposomes that consisted of phosphatidylcholine-cholesterol (1:1 ratio) were prepared to determine if LF would hydrolyze phospholipids that are substrates for all four subclasses of phospholipases (PLA, PLB, PLC, and PLD). CF-labeled liposomes incubated at 37°C with LF for 2 h released up to 90% of the maximum releasable CF (Fig. 4B), determined by treatment with 1% Triton X-100. Decreasing concentrations of LF added to the liposomes resulted in decreasing release of CF, demonstrating that lysis due to LF occurred in a dose-dependent manner. A control fraction that eluted from the octyl-Sepharose column at 60% N-propanol (fraction 161), distinct from the fractions containing LF, was also tested for CF release after lyophilization and reconstitution in the same manner as those of LF. When 50 and 25 μl of fraction 161 were incubated with liposomes only 25 and 12% CF releases, respectively, were observed (Fig. 4B).
To examine the products of phospholipid degradation by LF, the products of LF activity on phosphatidylcholine were examined by thin-layer chromatography. BPC was hydrolyzed in the presence of LF, and the products of treatment with LF and PLA2 had similar migration patterns when separated by thin-layer chromatography (Fig. 4C). This migration pattern of the hydrolysis products of LF on phosphatidylcholine also clearly differed from those for nonhydrolyzed BPC (lane 5) and PLD-hydrolyzed BPC (Fig. 4C, lanes 6 and 7).
Size exclusion chromatography.
To determine the sizes of LF components, size exclusion chromatography was used to fractionate LF material from the octyl-Sepharose column. Material in the LF preparation eluted off the Sephacryl 200 column in two major regions of the chromatogram: one in fractions 1 to 5 and the other in fractions 11 to 15 (Fig. 5). Fractions 1 to 5 and 11 to 15 correlated to 167 and 144 kDa, respectively, based on the calibration of the gel filtration column (data not shown). The first 22 fractions were tested for the ability to hydrolyze liposomes, and a detectable level of CF release was observed with fractions 1 to 5 (Fig. 5). SDS-PAGE analysis of fractions 1 to 5 under reducing conditions revealed 60- and 57-kDa components (Fig. 3C, lane 2), while nonreducing conditions produced a single band >200 kDa (Fig. 3C, lane 1).
FIG. 5.
Size exclusion chromatography of LF. (A) LF from octyl-Sepharose was loaded onto a Sephacryl 200 column and eluted with 0.1 M ammonium acetate buffer, pH 7.0, and protein was detected with the BCA assay (550 nm). (B) Lytic activity of fractions was determined by incubation with CF-liposomes composed of phosphatidylcholine-cholesterol (1:1 ratio) for 2 h at 37°C, and CF release was detected by fluorimetry (excitation, 435 nm; emission, 538 nm). Results shown are representative of three individual experiments.
Octyl-Sepharose chromatography yielded LF with an activity of 67 U/μg of protein in the CF assay, and further purification by size exclusion chromatography on Sephacryl 200 produced LF with an activity of 245 U/μg, a 3.6-fold increase in specific activity.
DISCUSSION
T. vaginalis has been shown to cause CPE upon contact with host cells (16, 18, 26) and soluble parasite products (15, 21), and enzymes such as cysteine proteases (9, 23) have been implicated in this process (2, 3, 23). However, the molecular mechanism of T. vaginalis CPE for host cells may involve several parasite products and host responses and remains to be well defined. This prompted us to investigate the cytotoxicity of a recent clinical isolate of T. vaginalis, and the results confirmed that this parasite produced potent cytotoxic effects on mammalian cells through parasite-host cell contact, as expected, and produced soluble products that mediated cytotoxicity across a barrier membrane (Fig. 1).
In the present study a hydrophobic lytic product, LF, was purified from extracts of T. vaginalis that hydrolyzed the phospholipid phosphatidylcholine (Fig. 4B), a substrate of phospholipases (PLA, PLB, PLC, and PLD), to products characteristic of PLA2 (Fig. 4C). Further analysis of LF demonstrated two distinct bands around 60 and 57 kDa when it was electrophoresed under reducing conditions (Fig. 3B and C); these products are similar in size to a PLA2 purified from sheep platelets (20). Although secretion of cysteine proteases from T. vaginalis has been reported (28) and may play a role in cytotoxicity (2), additional experiments failed to demonstrate detectable protease activity in the purified LF although it retained the lytic effect on red blood cells (data not shown) and WEHI 164 target cells (Fig. 2).
Previously McGregor et al. (22) reported increased levels of PLA2 activity in vaginal secretions of pregnant women with various sexually transmitted infections including T. vaginalis infection and suggested that this may contribute to the pathogenesis of preterm labor and birth which was previously correlated with T. vaginalis infection (10). In addition to the phospholipases of bacteria such as Staphylococcus aureus and Clostridium perfringens, which are well known to mediate nucleated host cell lysis and hemolysis (24, 32, 33), phospholipases such as PLA have recently been shown to be produced by several parasitic protozoa (5, 7, 30, 38), and the capacity for host tissue damage either through active release or release after parasite destruction by host responses has been suggested (38). Recently subcellular fractions of T. vaginalis were shown to produce hemolysis of human and rat red blood cells which was inhibited by the Rosenthal inhibitor (37), suggesting that this activity was dependent on a phospholipase and extending earlier reports of hemolysis by intact parasites (17, 12). The results presented here confirm these earlier observations and strongly suggest that the principal enzyme activity in LF is a PLA2.
Since treatment of pregnant women with T. vaginalis infection was recently shown to significantly increase their risk of preterm birth (6), it is tempting to speculate that destruction of parasites by drug therapy could release parasite enzymes such as PLA2 and proteases that may mediate cell destruction and contribute to the unfavorable pregnancy outcome. This is suggested by the major symptoms of trichomoniasis, which seem largely due to the inflammatory response T. vaginalis causes in the host. Because PLA2 releases the fatty acid arachidonic acid, which is then converted to prostanoids and leukotrienes by cyclooxygenases (COX) and lipoxygenases (11, 25, 34), the PLA2 of T. vaginalis may be of particular importance to this inflammation process. In other work we have shown that Tritrichomonas foetus, which is similar to T. vaginalis, stimulates a macrophage cell line (J774) to produce COX-2 (J. M. Voyich, T. McCormick, and D. E. Burgess, unpublished data), which could act synergistically with arachidonic acid released by PLA2. Thus, participation in the arachidonic acid pathway and host cell destruction by released PLA2 (34) leading to inflammation may be convergent effects of this parasite-derived enzyme leading to enhanced inflammation in trichomoniasis.
In conclusion, our results show that T. vaginalis produces soluble LF that lyses nucleated target cells and that its principal constituent is PLA2 enzymatic activity. These findings demonstrate that T. vaginalis can produce CPE against mammalian target cells without requiring parasite contact with the target cell and that the molecular mechanism is largely due to PLA2 activity.
Acknowledgments
This work was supported in part by NIH R15AI45526-01A1 and USDA 00-35008-9241 to Donald Burgess.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Alderete, J. F., and G. E. Garza. 1988. Identification and properties of Trichomonas vaginalis proteins involved in cytadherence. Infect. Immun. 56:28-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez-Sanchez, M. E., L. Avila-Gonzalez, C. Becerril-Garcia, L. V. Fattel-Facenda, J. Ortega-Lopez, and R. Arroyo. 2000. A novel cysteine proteinase (CP65) of Trichomonas vaginalis involved in cytotoxicity. Microb. Pathog. 28:193-202. [DOI] [PubMed] [Google Scholar]
- 3.Arroyo, R., and J. F. Alderete. 1995. Two Trichomonas vaginalis surface proteinases bind to host epithelial cells and are related to levels of cytoadherence and cytotoxicity. Arch. Med. Res. 26:279-285. [PubMed] [Google Scholar]
- 4.Arroyo, R., J. Engbring, and J. F. Alderete. 1992. Molecular basis of host epithelial cell recognition by Trichomonas vaginalis. Mol. Microbiol. 6:853-862. [DOI] [PubMed] [Google Scholar]
- 5.Barbour, S. E., and F. Marciano-Cabral. 2001. Naegleria fowleri amoebae express a membrane-associated calcium-independent phospholipase A(2). Biochim. Biophys. Acta 1530:123-133. [DOI] [PubMed] [Google Scholar]
- 6.Carey, J. C., M. A. Klebanoff, and the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. 2003. What have we learned about vaginal infections and preterm birth? Semin. Perinatol. 27:212-216. [DOI] [PubMed] [Google Scholar]
- 7.Cassaing, S., J. Fauvel, M. H. Bessieres, S. Guy, J. P. Seguela, and H. Chap. 2000. Toxoplasma gondii secretes a calcium-independent phospholipase A(2). Int. J. Parasitol. 30:1137-1142. [DOI] [PubMed] [Google Scholar]
- 8.Chavira, R., Jr., T. J. Burnett, and J. H. Hageman. 1984. Assaying proteinases with azocoll. Anal. Biochem. 136:446-450. [DOI] [PubMed] [Google Scholar]
- 9.Coombs, G. H., and M. J. North. 1983. An analysis of the proteinases of Trichomonas vaginalis by polyacrylamide gel electrophoresis. Parasitology 86(Pt. 1):1-6. [DOI] [PubMed] [Google Scholar]
- 10.Cotch, M. F., J. G. Pastorek II, R. P. Nugent, D. E. Yerg, D. H. Martin, and D. A. Eschenbach. 1991. Demographic and behavioral predictors of Trichomonas vaginalis infection among pregnant women. Obstet. Gynecol. 78:1087-1092. [PubMed] [Google Scholar]
- 11.Cummings, B. S., J. McHowat, and R. G. Schnellmann. 2000. Phospholipase A(2)s in cell injury and death. J. Pharmacol. Exp. Ther. 294:793-799. [PubMed] [Google Scholar]
- 12.Dailey, D. C., T. H. Chang, and J. F. Alderete. 1990. Characterization of Trichomonas vaginalis haemolysis. Parasitology 101:171-175. [DOI] [PubMed] [Google Scholar]
- 13.Diamond, L. S. 1957. The establishment of various trichomonads of animals and man axenic cultures. J. Parasitol. 43:488-490. [PubMed] [Google Scholar]
- 14.Fiori, P. L., P. Rappelli, M. F. Addis, F. Mannu, and P. Cappuccinelli. 1997. Contact-dependent disruption of the host cell membrane skeleton induced by Trichomonas vaginalis. Infect. Immun. 65:5142-5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garber, G. E., L. T. Lemchuk-Favel, and W. R. Bowie. 1989. Isolation of a cell-detaching factor of Trichomonas vaginalis. J. Clin. Microbiol. 27:1548-1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hogue, M. J. 1943. The effect of Trichomonas vaginalis on tissue-culture cells. Am. J. Hyg. 37:142-152. [Google Scholar]
- 17.Krieger, J. N., M. A. Poisson, and M. F. Rein. 1983. Beta-hemolytic activity of Trichomonas vaginalis correlates with virulence. Infect. Immun. 41:1291-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krieger, J. N., C. Jenny, M. Verdon, N. Siegel, R. Springwater, C. W. Critchlow, and K. K. Holmes. 1993. Clinical manifestations of trichomoniasis in men. Ann. Intern. Med. 118:844-849. [DOI] [PubMed] [Google Scholar]
- 19.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 20.Loeb, L. A., and R. W. Gross. 1986. Identification and purification of sheep platelet phospholipase A2 isoforms. Activation by physiologic concentrations of calcium ion. J. Biol. Chem. 261:10467-10470. [PubMed] [Google Scholar]
- 21.Lushbaugh, W. B., A. C. Turner, G. A. Gentry, and P. C. Klykken. 1989. Characterization of a secreted cytoactive factor from Trichomonas vaginalis. Am. J. Trop. Med. Hyg. 41:18-28. [PubMed] [Google Scholar]
- 22.McGregor, J. A., J. I. French, W. Jones, R. Parker, E. Patterson, and D. Draper. 1992. Association of cervicovaginal infections with increased vaginal fluid phospholipase A2 activity. Am. J. Obstet. Gynecol. 167:1588-1594. [DOI] [PubMed] [Google Scholar]
- 23.Mendoza-Lopez, M. R., C. Becerril-Garcia, L. V. Fattel-Facenda, L. Avila-Gonzalez, M. E. Ruiz-Tachiquin, J. Ortega-Lopez, and R. Arroyo. 2000. CP30, a cysteine proteinase involved in Trichomonas vaginalis cytoadherence. Infect. Immun. 68:4907-4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menestrina, G., C. L. Bashford, and C. A. Pasternak. 1990. Pore-forming toxins: experiments with S. aureus alpha-toxin, C. perfringens theta-toxin and E. coli haemolysin in lipid bilayers, liposomes and intact cells. Toxicon 28:477-491. [DOI] [PubMed] [Google Scholar]
- 25.Michiels, C., P. Renard, N. Bouaziz, N. Heck, F. Eliaers, N. Ninane, R. Quarck, P. Holvoet, and M. Raes. 2002. Identification of the phospholipase A(2) isoforms that contribute to arachidonic acid release in hypoxic endothelial cells: limits of phospholipase A(2) inhibitors. Biochem. Pharmacol. 63:321-332. [DOI] [PubMed] [Google Scholar]
- 26.Pindak, F. F., M. Mora de Pindak, and W. A. Gardner, Jr. 1993. Contact-independent cytotoxicity of Trichomonas vaginalis. Genitourin. Med. 69:35-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruddle, N. H., C. M. Bergman, K. M. Mcgrath, E. G. Lingenheld, M. L. Grunnet, S. J. Padula, and R. B. Clark. 1990. An antibody to lymphotoxin and tumor necrosis factor prevents transfer of experimental allergic encephalomyelitis. J. Exp. Med. 172:1193-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott, D. A., M. J. North, and G. H. Coombs. 1995. The pathway of secretion of proteinases in Trichomonas vaginalis. Int. J. Parasitol. 25:657-666. [DOI] [PubMed] [Google Scholar]
- 29.Senior, J., and G. Gregoriadis. 1982. Stability of small unilamellar liposomes in serum and clearance from the circulation: the effect of the phospholipid and cholesterol components. Life Sci. 30:2123-2136. [DOI] [PubMed] [Google Scholar]
- 30.Shuaibu, M., N. H. Kanbara, T. Yanagi, D. A. Ameh, J. J. Bonire, and A. J. Nok. 2001. Phospholipase A2 from Trypanosoma brucei gambiense and Trypanosoma brucei brucei: inhibition by organotins. J. Enzyme Inhib. 16:433-441. [DOI] [PubMed] [Google Scholar]
- 31.Singh, B. N. 1993. Lipophosphoglycan-like glycoconjugate of Tritrichomonas foetus and Trichomonas vaginalis. Mol. Biochem. Parasitol. 57:281-294. [DOI] [PubMed] [Google Scholar]
- 32.Snijder, H. J., and B. W. Dijkstra. 2000. Bacterial phospholipase A: structure and function of an integral membrane phospholipase. Biochim. Biophys. Acta 1488:91-101. [DOI] [PubMed] [Google Scholar]
- 33.Songer, J. G. 1997. Bacterial phospholipases and their role in virulence. Trends Microbiol. 5:156-161. [DOI] [PubMed] [Google Scholar]
- 34.Taketo, M. M., and M. Sonoshita. 2002. Phospholipase A2 and apoptosis. Biochim. Biophys. Acta 1585:72-76. [DOI] [PubMed] [Google Scholar]
- 35.Tirunarayanan, M. O., and H. Lundbeck. 1968. Investigations on the enzymes and toxins of staphylococci. Assay of lipase using Tween as the substrate. Acta Pathol. Microbiol. Scand. 72:263-276. [DOI] [PubMed] [Google Scholar]
- 36.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vargas-Villarreal, J., B. D. Mata-Cardenas, F. Gonzalez-Salazar, H. G. Lozano-Garza, E. I. Cortes-Gutierrez, R. Palaclos-Corona, H. G. Martinez-Rodriguez, E. Ramirez-Bon, and S. Said-Fernandez. 2003. Trichomonas vaginalis: identification of a phospholipase A-dependent hemolytic activity in a vesicular subcellular fraction. J. Parasitol. 89:105-112. [DOI] [PubMed] [Google Scholar]
- 38.Wainszelbaum, M., E. Isola, S. Wilkowsky, J. J. Cannata, J. Florin-Christensen, and M. Florin-Christensen. 2001. Lysosomal phospholipase A1 in Trypanosoma cruzi: an enzyme with a possible role in the pathogenesis of Chagas' disease. Biochem. J. 355:765-770. [DOI] [PMC free article] [PubMed] [Google Scholar]