Abstract
The type III secretion system is a dedicated machinery used by many pathogens to deliver toxins directly into the cytoplasm of a target cell. Expression and secretion of the type III effectors are triggered by cell contact. In Pseudomonas aeruginosa and Yersinia spp., expression can be triggered in vitro by removing calcium from the medium. The mechanism underlying either mode of regulation is unclear. Here we characterize a transposon insertion mutant of P. aeruginosa PAO1 that displays a marked defect in cytotoxicity. The insertion is located upstream of several genes involved in histidine utilization and impedes the ability of PAO1 to intoxicate eukaryotic cells effectively in a type III-dependent fashion. This inhibition depends on the presence of histidine in the medium and appears to depend on the excessive uptake and catabolism of histidine. The defect in cytotoxicity is mirrored by a decrease in exoS expression. Other parameters such as growth or piliation are unaffected. The cytotoxicity defect is partially complemented by an insertion mutation in cbrA that also causes overexpression of cbrB. The cbrAB two-component system has been implicated in sensing and responding to a carbon-nitrogen imbalance. Taken together, these results suggest that the metabolic state of the cell influences expression of the type III regulon.
Pseudomonas aeruginosa is an opportunistic pathogen that is a common cause of hospital-acquired infections. It generally infects immunocompromised patients, such as burn patients or cancer patients, in whom it causes wound infections, catheter infections, and ventilator-associated pneumonia (9, 30, 32). In cystic fibrosis patients, P. aeruginosa is the most common cause of severe, chronic lung infections, which ultimately lead to a decline in respiratory function and death (11).
P. aeruginosa possesses a large arsenal of virulence factors (11, 37). One virulence mechanism that has attracted significant attention in recent years is the ability to intoxicate eukaryotic cells by using a type III secretion system to inject toxins directly into the target cell (48). This mode of toxin delivery has also been termed contact-dependent secretion, since secretion of the effector molecules is triggered by cell contact (43). Four type III effector molecules (toxins) have been described in P. aeruginosa. Exoenzyme S (ExoS) and ExoT are highly homologous proteins that have a carboxy-terminal ADP-ribosylating motif and an amino-terminal GTPase-activating domain (10, 18, 20, 47). The GTPase-activating activity of these proteins targets small, Rho-like GTPases (19) and has been linked with cytoskeletal rearrangements and cell rounding in vitro (10, 18). The two other known effector molecules are ExoY and ExoU. ExoY is an adenylate cyclase (50), and ExoU is a necrotizing toxin with phospholipase activity (5, 6, 13, 35).
Type III secretion has been linked to more severe disease progression in ventilator-associated pneumonia (12, 15, 34), and type III effectors are also associated with increased virulence in animal models of infection (6, 28, 29).
The regulation of the type III effector genes and secretion machinery appears to be coordinated (45). ExoS expression is induced by cell contact but can also be turned on in vitro by removing calcium from the medium (7, 49). This induction of effector genes under low-calcium conditions depends on the presence of an intact type III secretion machinery, suggesting that the machinery itself is involved in sensing the calcium signal (7, 25, 45). This low-calcium regulation has also been observed in Yersinia sp., whose type III secretion machinery is highly homologous to that of P. aeruginosa (17, 27). The mechanism by which the extracellular calcium concentration is translated into a transcriptional signal remains enigmatic. However, it was recently reported that the ability of P. aeruginosa strain CHA to respond to low-calcium conditions was abolished by transposon insertions in the aceAB genes, which encode pyruvate dehydrogenase (4). It was suggested that pyruvate dehydrogenase may act directly as a regulator of gene expression, but another interpretation is that there is some form of metabolic control over the transcription of the type III secretion genes.
In this paper, we report the isolation and characterization of a transposon insertion mutation in P. aeruginosa strain PAO1 that results in overexpression of histidine utilization genes and simultaneously abolishes the ability of the bacterium to induce type III-mediated cytotoxicity. The cytotoxicity defect can be partially suppressed by an insertion in cbrA. This gene encodes the sensor kinase of a two-component system reported to be involved in sensing and responding to a carbon-nitrogen imbalance (31). These findings suggest that the metabolic state of the cell is factored into the decision to induce expression of type III effectors by P. aeruginosa.
MATERIALS AND METHODS
Bacterial strains and media.
All of the P. aeruginosa strains described in this report are listed in Table 1. Escherichia coli DH5α λpir and SM10 λpir were used, respectively, for cloning and conjugative transfer of plasmids into P. aeruginosa and were derived from laboratory stocks. Bacteria were grown in Luria-Bertani (LB) medium unless indicated otherwise. Exconjugants of P. aeruginosa were selected on Pseudomonas Isolation Agar (Becton Dickinson).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or description | Reference or source |
|---|---|---|
| Strains | ||
| PAO1 | Wild type | 16 |
| PAK | Wild type | 40 |
| I.11E | PAO1 with TnTGL3 insertion upstream of PA5099 (Fig. 2) | This study |
| PAO1 ΔpopB | ΔpopB | This study |
| PAO1 pilA | pilA::pP30Δ | This study |
| PAO1 ΔhutT | ΔhutT | This study |
| PAO1 ΔhutH | ΔhutH | This study |
| PAO1 ΔhutG | ΔhutG | This study |
| PAO1 ΔhutI | ΔhutI | This study |
| PAO1 cbrA::TnFAC | cbrA::TnFAC | This study |
| PAO1 exoS::GL3 | ΔexoS::(GFP lacZ) | This study |
| PAK exoS::GL3 | ΔexoS::(GFP lacZ) | This study |
| I.11E ΔhutT | TnTGL3(I.11E) ΔhutT | This study |
| I.11E ΔhutH | TnTGL3(I.11E) ΔhutH | This study |
| I.11E ΔhutI | TnTGL3(I.11E) ΔhutI | This study |
| I.11E ΔhutG | TnTGL3(I.11E) ΔhutG | This study |
| I.11E hutU | TnTGL3(I.11E) hutU::pP30Δ | This study |
| I.11E ΔcbrA | TnTGL3(I.11E) ΔcbrA | This study |
| Plasmids | ||
| pTGL3 | Suicide vector bearing TnTGL3 (Fig. 1) | This study |
| pPSV18 | Shuttle vector with pBR322 origin, PA origin, lac promoter, bla (ampicillin and carbenicillin resistance) | This study |
| pPSV32 | Shuttle vector with pBR322 origin, PA origin, lac promoter, lacIqaaC1 (gentamicin resistance) | This study |
| pP18-hutT-PA5099 | Chromosomal fragment carrying genes hutT, hutH, and PA5099 in pPSV18 | This study |
| pP18-hutT | hutT in pPSV18 | This study |
| pP18-PA5099 | PA5099 in pPSV18 | This study |
| pP32-cbrB | cbrB in pPSV32 | This study |
Plasmids and strain constructions.
To generate plasmid pPSV18, the β-lactamase gene (bla) and lac promoter-polylinker region of pUC18 were combined with a pBR322 origin and the Pseudomonas origin of replication (PAori) from pUC181.1 (8). The PstI fragment from plasmid pUC181.1, carrying the PAori, was first cloned into the BsmI site of a pBR322 derivative, pBR-mob, with a short linker to introduce SwaI and PacI sites (generated by annealing primers 5′-ATTAATTAATTTAAATTTACCG-3′) and 5′-GTAAATTTAAATTAATTAATTGCA-3′). The origin was then subcloned between the NarI/MscI sites of pBR322 as a NarI/SwaI fragment, generating plasmid pBR-PAori. pPSV18 was generated by combining the polylinker and most of the bla gene of pUC18 (pUC18 digested with SapI, blunted with T4 DNA polymerase, and subsequently digested with AhdI) with the SfoI/AhdI fragment of pBR-PAori, carrying the pBR322 origin and the PAori. Plasmid pPSV28 was derived from plasmid pPSV18 by replacing the bla gene with the aacC1 (gentamicin resistance) gene, derived originally from plasmid pBSL182 (1). aacC1 was amplified with primers gent5sm (5′-AAAAAAATATTACGCGTCAATTCTCGAATTGACATAA-3′) and gent3mma (5′-AAAAAGACTCCCCGTCCAATTGACGCGTCGGCCGGGAAGCCGATCTCG-3′) and cloned into pPSV18 as an SspI/AhdI fragment. Plasmid pPSV30 was generated by cloning the EcoRI fragment from plasmid pBSL237 (2), carrying the RP4 origin of transfer, into the MfeI site of pPSV28. Finally, plasmid pPSV32 was derived from plasmid pPSV30 by cloning the lacIq gene, amplified with primers lacIq5 (5′-AAAAAAATATTAATGGAGCAAAACCTTTCGCGGTAT-3′) and lacI3 (5′-AAAAAAATATTCTCACATTAATTGCGTTGCGCTCA-3′), as an SspI fragment into pPSV30.
Transposon TnTGL3 was constructed by first replacing the Kmr cassette on pSC123 (S. Chiang, unpublished data) with the MluI fragment carrying the tetracycline resistance gene from pBSL199 (1), thus generating pTnTet. In a second step, a cassette with the green fluorescent protein (GFP)-encoding gene and a translationally coupled copy of lacZ was generated by splicing together two PCR products (44). The GFP-encoding gene was amplified from pGFPmut3 (3) with primers G5PST (5′-AAAACTGCAGGAAGGAGATATACATATGAGTAAAG-3′) and DGL3 (5′-AATCATGGTCATTTGTATAGTTCATCCATGCC-3′). The lacZ gene was amplified from the chromosome of E. coli strain MG1655 with primers DLG5 (5′-GAACTATACAAATGACCATGATTACGGATTCACT-3′) and L3PST (5′-GCAGACATGGCCTGCAGGGTTATTATTATTTTTGAC-3′). These two PCR products were spliced together in a second PCR step and cloned into pTnTet as a PstI fragment, generating pTGL3 (harboring transposon TnTGL3). The sequences of the GFP-encoding and lacZ genes in pTGL3 were confirmed by sequencing.
Plasmid pP18-hutT-PA5099 was generated by amplifying the chromosomal region carrying genes PA5097 (hutT) to PA5099 with primers 5097 3′ (5′-AAAAAAAGCTTCTCAGGCCGCCGTCGGTTCCGTCACA-3′) and perm5 (5′-GGCGCGCGAATTCCAGCGGAGTACAGCA-3′) and cloning it into pPSV18 as an EcoRI/HindIII fragment. Plasmid pP18-hutT was derived from plasmid pP18-hutT-PA5099 by digestion with EcoRI and EcoRV, followed by end filling with T4 DNA polymerase and religation. Plasmid pP18-PA5099 was derived from plasmid pP18-hutT-5099 by digestion with BamHI and HindIII, followed by end filling with T4 DNA polymerase and religation. Plasmid pP32-cbrB was generated by amplifying the cbrB gene from the chromosome of PAO1 with primers cbrB5 (5′-AAAAAGAATTCAGAGAGCTGAATACATGGCACATATT-3′) and cbrB3 (5′-AAAAAAGCTTCCGCGGTAACAGGTTGCAGGGTGTTA-3′) and cloning the product as an EcoRI/HindIII fragment into plasmid pPSV32.
Deletion mutants of P. aeruginosa PAO1 were generated with a version of plasmid pEX18Gm (14) adapted for use with the Gateway recombinational cloning system (45). In-frame deletions were constructed in this vector by generating two PCR products that carried the flanking regions for the deletion site and splicing the two products together in a second PCR. The internal primers for the two PCR products defined the site of the deletion, as well as complementary 24-bp sequences (5′-TTCAGCATGCTTGCGGCTCGAGTT-3′ and 5′-AACTCGAGCCGCAAGCATGCTGAA-3′, respectively) that allowed the two initial PCR products to be spliced together to generate the in-frame deletion. The flanking primers contained the sequences necessary for subsequent recombinational cloning of the PCR product carrying the deletion (attB1 [5′-GGGGACAAGTTTGTACAAAAAAGCAGGCT-3′] and attB2 [5′-GGGGACCACTTTGTACAAGAAAGCTGGGT-3′]). The actual cloning was performed with the Gateway cloning kit (Invitrogen) in accordance with the manufacturer's instructions.
The primers used to make deletion constructs were ΔhutT (5′-flanking PCR product, 5097/5-1 [5′-TTCAGCATGCTTGCGGCTCGAGTTCGTCATCCCTCGGCTCTCCTCTT-3′] and 5097/5-2 [5′-GGGGACCACTTTGTACAAGAAAGCTGGGTCCATGCTCGGTTCGCGGGCGCCGTTCGAT-3′]; 3′-flanking PCR product, 5097/3-1 [5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTCCAGCTCCGGCGCCTGGTCGTGGAAGGT-3′] and 5097/3-2 [5′-AACTCGAGCCGCAAGCATGCTGAAGCCCAGGGAGGGCTGTGACGGAACCGA-3′]), ΔhutH (5′-flanking PCR product, 5098/5-1 [5′-TTCAGCATGCTTGCGGCTCGAGTTGCTCATCGAAGGCTCCTTGTTCGGTT-3′] and 5098/5-2 [5′-GGGGACCACTTTGTACAAGAAAGCTGGGTTTCAGCCGCCTGATGCTGCTCAGCGACAT-3′]; 3′-flanking PCR product, 5098/3-1 [5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTGATCACCACGACATTGAGGA-3′] and n5098/3-2 [5′-AACTCGAGCCGCAAGCATGCTGAACGGCTGTTGCCGAGTCTTTGATA-3′]), and ΔhutG (5′-flanking PCR product, hutG5-1 [5′-TTCAGCATGCTTGCGGCTCGAGTTACTCAGGACTTCATCCACGGTATACCT-3′] and hutG5-2 [5′-GGGGACCACTTTGTACAAGAAAGCTGGGTGCGAGGCCAGCGAGGAAGAATTGCT-3′]; 3′-flanking PCR product, hutG3-1 [5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTGGTGAAGCGGCCGGGATAGTCGTAGT-3′] and hutG3-2 [5′-AACTCGAGCCGCAAGCATGCTGAAATCCGCGAACTGCTGGAAAGCCTCCTCGCCT-3′]). A deletion in exoS was constructed as described previously (39), with primers ExoS 5′ (5′-TACAAAAAAGCAGGCTGGCTCTGGTTCGGCTACTGGATCC-3′) and ExoS SOE 3′ (5′-ATCCTGTTCCTGGACCTCGCCTGAGTCGACTGGGAATTCCAAACGCCCACTGGCGGCCTGGTG-3′) for the 5′-flanking region and ExoS 3′ (5′-TACAAGAAAGCTGGGTGAGCGCTTCGTCATCCTCAATCCG-3′) and ExoS SOE 5′ (5′-CACCAGGCCGCCAGTGGGCGTTTGGAATTCCCAGTCGACTCAGGCGAGGTCCAGGAACAGGAT-3′) for the 3′-flanking region.
The exoS reporter was constructed by cloning the PstI fragment carrying the GFP-encoding and lacZ genes (blunted with T4 DNA polymerase) from pTGL3 into the EcoRI site of pDONRΔexoS (again made blunt with T4 DNA polymerase). The resulting plasmid was then recombined into deletion vector pEXGmGW with the Gateway recombination system (Invitrogen), resulting in plasmid pEXΔexoS::GL3.
Mutations in pilA and hutU were generated by insertional mutagenesis with pP30Δ. pP30Δ was derived from pPSV30 by digesting pPSV30 sequentially with MscI and SmaI and religation, thus removing the lac promoter and PAori of pPSV30. Internal fragments of hutU and pilA were cloned into this vector (primers hutUint5 [5′-AAAAACTGCAGAACCCCAGGGAACTGGTGGTTTA-3′] and hutUint3 [5′-AAAAACTGCAGGCCTTCGGCGGTGTAGCGCTGGAT-3′], as well as pilAint5 [5′-AAAAACTGCAGAGGCTTTACCTTGATCGAACTGA-3′] and pilAint3-1 [5′-AAAAACTGCAGGAACATCGGATCCTGGGTAGATT-3′], respectively [the underlined sequences are restriction sites for cloning]), and the resulting plasmids, pP30ΔhutUint and pP30ΔpilA1int, were moved into SM10Δλpir and subsequently mated into P. aeruginosa PAO1 to generate the null mutants.
Fluorescence-activated cell sorting.
The pool of transposon insertion mutants was grown in tryptic soy broth (Becton Dickinson) supplemented with 100 mM sodium glutamate and 1% glycerol. Low-calcium conditions were achieved by addition of 1 mM EGTA, and calcium-replete conditions were achieved by adding 1 mM CaCl2. Fluorescent or nonfluorescent cells, depending on the stage in the enrichment cycle, were sorted with a FACScalibur flow cytometer (Becton Dickinson).
Phage transduction.
Transduction was performed with a clear derivative of phage DMS3 (J. Budzik and G. A. O'Toole, unpublished data).
Cell lines and tissue culture conditions.
A549 cells (CCL-185; ATCC), a human epithelial cell line, and RAW264.7 cells (IB-71; ATCC), a mouse monocyte-derived cell line, were grown in RPMI 1640 tissue culture medium (CellGro) supplemented with 2 mM glutamine, 10 mM HEPES, and 10% fetal bovine serum (FBS) (RP10 medium). In experiments in which tissue culture medium without histidine was required (see Fig. 5 and 6), RPMI medium was generated in accordance with the published formulation (CellGro) but omitting histidine (RPMI-His). In those experiments, the data points of strains grown in the presence of histidine reflect results obtained with the same batch of RPMI-His to which histidine was added back to the concentration found in complete RPMI medium.
FIG. 5.
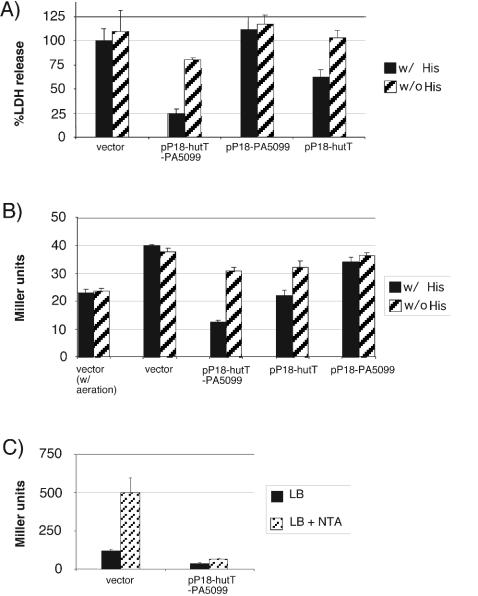
Effect of overexpression of hutT and hutH on cytotoxicity and exoS expression. hutT (pP18-hutT), PA5099 (pP18-PA5099), or the chromosomal region spanning hutT, hutH, and PA5099 (pP18-hutT-PA5099) was expressed in multicopy in either wild-type PAO1 (A) or PAO1 ΔexoS::GL3 (B). In panel A, cytotoxicity was assayed by monitoring LDH release by infected RAW264.7 macrophages. In panel B, exoS expression was monitored by β-galactosidase assay in cells grown with aeration (first two columns [w/aeration]) or under tissue culture conditions (no aeration, 5% CO2). Both experiments were performed with RPMI medium supplemented with 10% FBS and glutamine and containing histidine (w/His) or lacking histidine (w/o His). In panel C, exoS expression was monitored in strain PAK ΔexoS::GL3 carrying either plasmid pPSV18 (vector control) or pP18-hutT-PA5099. The bacteria were grown aerobically in LB medium to an optical density at 600 nm of approximately 0.3, at which point bacteria were diluted 1:1 into either fresh LB medium or LB medium containing the calcium chelator nitrilotriacetic acid (NTA; 10 mM final concentration) and incubated for another 3 h. Following this, the cultures were placed on ice and the β-galactosidase activity was determined.
FIG. 6.
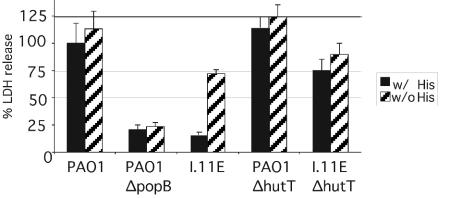
Cytotoxicity of the I.11E insertion mutant in the presence (w/His) or absence (w/o His) of histidine. LDH release by RAW264.7 macrophages infected with P. aeruginosa PAO1, a popB deletion mutant, and the transposon insertion mutant I.11E, as well as mutants of PAO1 and I.11E lacking hutT, was measured. The infections were carried out with RPMI tissue culture medium supplemented with glutamine and 10% FBS and containing (w/His) or lacking (w/o His) histidine.
Cytotoxicity assays.
Two types of assays were used to detect cytotoxicity. The first measures the ability of P. aeruginosa to cause rounding of epithelial cells. A549 cells were seeded in 24-well plates at approximately 8 × 104/well on the day prior to the experiment. On the day of the experiment, cells were infected at a multiplicity of infection of 50 (assuming ∼105 A549 cells/well) for 4 h. The infection was stopped by removing the medium and incubating the cells in a phosphate-buffered 4% paraformaldehyde solution. The extent of cell rounding was assessed by phase microscopy.
The second cytotoxicity assay exploits the ability of P. aeruginosa to cause necrosis in cultured RAW264.7 macrophages. The extent of necrosis was assayed by monitoring the release of the cytoplasmic enzyme lactate dehydrogenase (LDH). On the day of the experiment, RAW264.7 cells were scraped up, washed once with fresh RP10 medium, resuspended in fresh RP10 medium without the phenol red indicator dye (Invitrogen), and then dispensed into a 96-well plate (Corning) at 104/well. The cells were infected with P. aeruginosa at an multiplicity of infection of 50. After 300 to 330 min of infection, the extent of LDH release was assayed with the Cytotox96 kit in accordance with the manufacturer's (Promega) instructions.
β-Galactosidase assays.
Cells were permeabilized with chloroform-sodium dodecyl sulfate, and β-galactosidase activity was assayed as described previously (26).
DNA microarray analysis.
The expression profiles of mutant and wild-type PAO1 were determined with Affymetrix GeneChip microarrays. The wild-type and mutant were grown statically in a 5% CO2 atmosphere in 100 ml of RP10 medium. After 3 h of incubation, the cells were spun down, resuspended in Trizol reagent (GIBCO), and processed as described previously (45). The experiment was only performed once, and the results were not verified beyond the experiments indicated in this study (exoS expression and the effect of overexpression of the histidine degradation genes).
RESULTS
Isolation of a transposon insertion mutant that affects type III secretion-mediated cytotoxicity in P. aeruginosa PAO1.
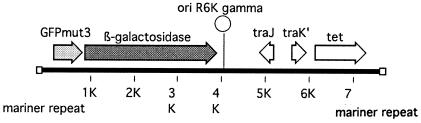
GFP has been used to identify differentially regulated genes (23, 24, 36, 41, 42). We used a modified version of this usually plasmid-based approach to identify differentially regulated genes in P. aeruginosa. Specifically, we designed a transposon (TnTGL3) with a promoterless GFP-encoding gene and a translationally coupled lacZ gene for this purpose (Fig. 1).
FIG. 1.
Organization of promoter trap transposon TnTGL3. The transposon carries a promoterless version of GFPmut3, as well as a translationally coupled lacZ gene (indicated by the light and dark grey arrows, respectively).
At least one set of known virulence genes, encoding the type III secretion machinery and effectors, is regulated by low calcium. We therefore decided to use our system to identify additional genes that are induced under low-calcium conditions. A pool of approximately 58,000 transposon insertion mutants was grown in LB medium under low-calcium conditions, and fluorescent bacteria were isolated by fluorescence-activated cell sorting. This pool of insertions was then grown in the presence of Ca2+ and sorted for nonfluorescent bacteria. After a second round of growth under low-calcium conditions and sorting for fluorescent bacteria, the output pool was plated for single colonies. Of 190 colonies picked from this output pool, 77 (37%) responded to low calcium when tested individually. Sequencing of 36 mutants revealed 20 unique insertions, defining 11 genes or intergenic regions. This set of genes included an insertion four nucleotides upstream of pcrH, which encodes a proposed chaperone of the type III needle complex protein PopD. With the exception of two insertions (one in PA0388 and one just upstream of PA5099), this set matched a list of calcium-regulated genes previously identified by DNA microarray analysis (45).
P. aeruginosa causes rounding in epithelial cells and necrosis of macrophages in vitro. These activities depend on the presence of a functional type III secretion system. Since the type III effectors and secretion machinery genes are subject to induction under low-calcium conditions and we identified an insertion upstream of pcrH, we decided to subject the pool of low-calcium-regulated insertions to a secondary screen for cytotoxicity. One of these 77 mutants, I.11E, failed to induce rounding in A549 cells (data not shown) and displayed a significant defect in lysing RAW264.7 macrophages (Fig. 3).
FIG. 3.
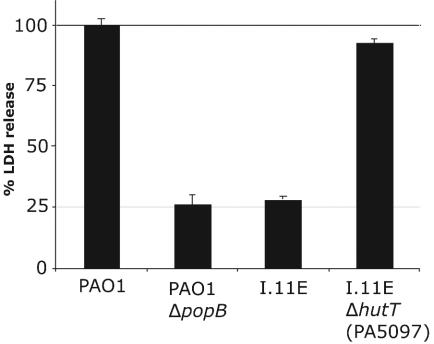
Suppression of the I.11E phenotype by deletion of PA5097 (hutT). LDH release by RAW264.7 macrophages infected with P. aeruginosa PAO1, a mutant with an in-frame deletion of popB, the transposon insertion mutant I.11E, as well as a mutant of PAO1 combining the I.11E insertion with an in-frame deletion of PA5097 (hutT). The infections were carried out in complete RPMI tissue culture medium (which contains histidine) supplemented with glutamine and 10% FBS.
A transposon insertion resulting in overexpression of the histidine utilization genes results in loss of cytotoxicity.
The transposon insertion in mutant I.11E is located immediately upstream of gene PA5099, which encodes a putative transporter (Fig. 2). The mutant phenotype was not due to a secondary mutation, since crossing out the transposon insertion restored cytotoxicity to wild-type levels (data not shown). We also determined growth curves in LB medium and under tissue culture conditions and performed twitching motility assays to rule out a growth defect or loss of piliation as a cause for the loss of cytotoxicity. Neither growth nor piliation was affected in the mutant (data not shown).
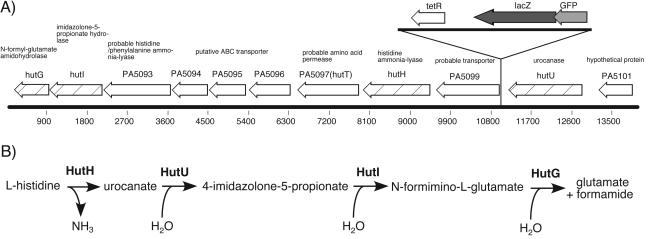
FIG. 2.
Chromosomal location of the I.11E insertion mutant. (A) The transposon insertion in the cytotoxicity mutant I.11E is located 9 bp upstream of gene PA5099. Histidine utilization genes are indicated by the hatched arrows. (B) The histidine utilization pathway (http://biocyc.org/). The gene product involved in each individual step is in boldface.
DNA microarray analysis demonstrated that nine genes downstream of the transposon insertion were at least 15-fold overexpressed (data not shown). To determine if overexpression of one of the adjacent genes was responsible for the observed phenotype, deletions in adjacent genes were combined with the transposon insertion mutation, and cytotoxicity of the resultant strains was determined with the macrophage lysis assay. The ability of the bacteria to kill RAW264.7 macrophages was restored by deleting PA5097, a putative amino acid transporter gene (Fig. 3). Deletion of PA5097 in wild-type PAO1 had no effect on cytotoxicity (data not shown).
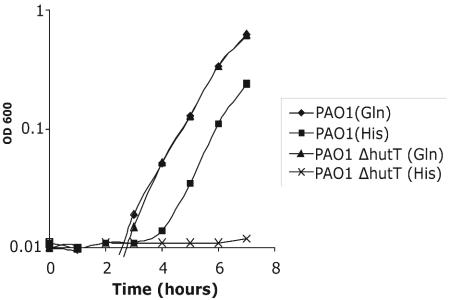
Since PA5097 is annotated as encoding a putative amino acid transporter and is situated among the histidine catabolism genes, we assayed the ability of a PA5097 deletion mutant to grow on each of the 20 amino acids as a sole carbon source. Of the amino acids that P. aeruginosa is able to utilize as a sole carbon source, the PA5097 deletion mutant only displayed a defect in utilizing histidine (Fig. 4). PA5097 therefore appears to encode the histidine transporter, and we have designated it hutT, in keeping with the nomenclature of the annotated histidine utilization genes. The unlinked ABC transporter hisJQMP is also annotated as a histidine transporter, on the basis of homology to the E. coli histidine transporter (38). However, since insertional inactivation of hutT, but not hisM, leads to histidine auxotrophy, the annotation of hisJQMP is likely to be incorrect (data not shown).
FIG. 4.
Growth of PAO1 and PAO1 ΔhutT on glutamine or histidine as the sole carbon source. Either strain was grown overnight in MOPS minimal medium with glucose as the sole carbon source. On the following day, the bacteria were diluted into MOPS minimal medium with either 10 mM glutamine or 10 mM histidine as the sole carbon source and grown at 37°C with aeration. Growth was monitored by measuring the absorbance of the culture at 600 nm. OD, optical density.
We next determined if overexpression of the histidine transporter by itself could replicate the mutant phenotype. To this end, a chromosomal fragment carrying genes PA5097 (hutT) to PA5099 was expressed from a multicopy plasmid in wild-type PAO1. Overexpression of this chromosomal fragment resulted in loss of cytotoxicity (Fig. 5A). Deletions of the plasmid that leave either PA5099 or hutT intact demonstrated that this defect depends on overexpression of hutT, not PA5099 (Fig. 5A). It is notable that the plasmid carrying only hutT did not have as severe an impact on cytotoxicity as the full-length construct. This difference is likely due to the effect of HutH, the histidine-ammonia lyase, which also contributes to the mutant phenotype (see below). The cytotoxicity defect was mirrored by a decrease in exoS transcription as measured by a GFP-lacZ reporter construct inserted into the chromosome at the exoS locus (Fig. 5B).
hutT overexpression represses exoS transcription in strain PAK.
In the course of this study, we discovered that strain PAO1 does not induce expression of the type III effector genes under low-calcium conditions in vitro, and its overall level of type III effector expression is low compared to that of other P. aeruginosa strains. We therefore decided to determine if the effect of overexpressing the histidine catabolic genes could be extended to other strains of P. aeruginosa. Strain PAK induces expression of exoS under low-calcium conditions (45). We therefore introduced our chromosomal exoS reporter into PAK, as well as the plasmid bearing the hutT-PA5099 chromosomal fragment. Overexpression of the hutT-PA5099 fragment in PAK resulted in repression of the exoS reporter (Fig. 5C) and loss of cytotoxicity (data not shown).
The defect in cytotoxicity is dependent on the presence of histidine in the medium and on an intact histidine degradation pathway.
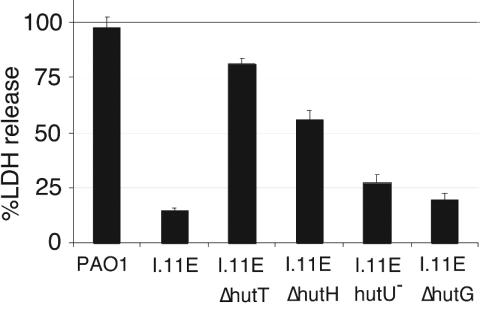
To determine if the cytotoxicity defect in the I.11E insertion mutant was a nonspecific side effect of overexpressing the histidine transporter or actually related to its function, we examined the effect of exogenous histidine on the mutant's cytotoxicity. The mutant phenotype was readily reversed by removing histidine from the tissue culture medium (Fig. 6), indicating that excessive uptake of histidine, rather than mere overexpression of the transporter, was responsible for the mutant phenotype. In fact, deletions in the histidine catabolic genes hutH, hutU, and hutG suppressed the mutant phenotype to various degrees, indicating that uptake and degradation of histidine are required for decreased cytotoxicity (Fig. 7). The poor suppression of the mutant phenotype by the mutations in later steps of the histidine utilization pathway (hutU, hutI, and hutG) may be due partly to the fact that combining these mutations with the I.11E insertion was deleterious for growth. This was especially true for hutI, which was not included in Fig. 7, and, to a lesser degree, hutG (data not shown). Deletion of hutH, hutI, or hutG in the wild-type background had no effect on cytotoxicity (data not shown).
FIG. 7.
Suppression of the I.11E phenotype by mutations in the histidine utilization pathway. LDH release by RAW264.7 macrophages infected with P. aeruginosa PAO1, the transposon insertion mutant I.11E, and mutants of PAO1 combining the I.11E insertion with mutations hutT, hutH, hutU, and hutG, was measured. The infections were carried out with RPMI tissue culture medium (complete) supplemented with glutamine and 10% FBS.
The histidine dependence of the mutant phenotype correlated with a histidine-dependent decrease in exoS expression in a strain overexpressing either hutT and hutH or just hutT (Fig. 5B).
The mutant phenotype is suppressed by a transposon insertion in cbrA.
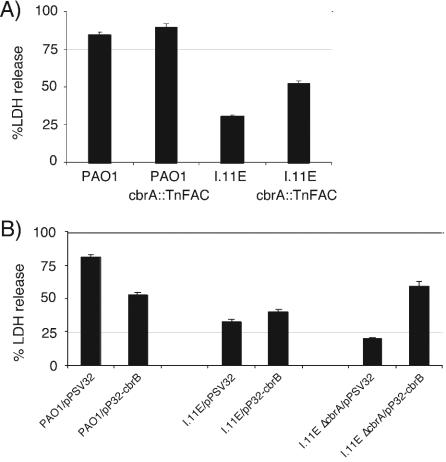
While constructing the hut deletion mutants in the I.11E background, we discovered that the original insertion mutant had a severe defect in growth on histidine as the sole carbon source and we used this phenotype to isolate suppressor mutants. The I.11E insertion mutant was subjected to a second round of transposon mutagenesis, this time with the mariner-based minitransposon TnFAC (46). From a pool of about 3,000 mutants, 3 were isolated that restored the ability of the I.11E mutant to grow efficiently on histidine as the sole carbon source. Of these, one partially restored cytotoxicity (Fig. 8A). The insertion in this mutant was located in cbrA, which encodes the sensor kinase of a two-component regulatory system (cbrAB) that has been implicated in sensing and responding to a carbon-nitrogen imbalance (31). The suppression phenotype is recapitulated by overexpressing cbrB in a mutant with an in-frame deletion in cbrA (Fig. 8B). Neither overexpression of cbrB alone nor deletion of cbrA alone affected cytotoxicity (Fig. 8B). A transductant in which the suppressing TnFAC insertion in cbrA was moved into wild-type PAO1 did not display significantly enhanced cytotoxicity. This suggests that cbrB is not simply an activator of type III secretion gene expression, but rather that the unique combination of deleting cbrA and overexpressing cbrB helps to, at least in part, alleviate the metabolic imbalance that caused the original insertion mutant to be noncytotoxic.
FIG. 8.
Suppression of the I.11E phenotype by overexpression of cbrB. (A) LDH release by RAW264.7 macrophages infected with P. aeruginosa PAO1, transposon insertion mutant I.11E, or either strain harboring the cbrA::TnFAC insertion. (B) LDH release by RAW264.7 macrophages infected with PAO1, the I.11E insertion mutant, and the I.11E insertion mutant combined with a deletion in cbrA harboring either the vector (pPSV32) or a plasmid expressing cbrB (pP32-cbrB). The infections were carried out with RPMI tissue culture medium (complete) supplemented with glutamine and 10% FBS. In panel B, the medium was supplemented with 10 mM isopropyl-β-d-thiogalactopyranoside.
DISCUSSION
Here we describe the isolation of a transposon insertion mutant of P. aeruginosa that is unable to intoxicate eukaryotic cells in a type III secretion system-dependent manner. The insertion mutation results in overexpression of genes required for histidine utilization, and the concomitant cytotoxicity defect can be alleviated by removing histidine from the tissue culture medium. This suggests that the cytotoxicity defect results from excessive uptake of histidine, and not, for example, from aberrant overexpression of a variety of membrane proteins. Consistent with this observation is the fact that the defect in cytotoxicity can also be alleviated by combining the original insertion mutation with deletions in histidine degradation pathway genes, most notably hutT (the histidine transporter) and hutH (the histidine-ammonia lyase, which catalyzes the first step in histidine degradation). The latter result indicates that both uptake and degradation of histidine are required for the loss of cytotoxicity.
Transcriptional profiling of the mutant indicated that, compared to that in the wild type, expression of the type III effectors and secretion machinery was reduced under the conditions of the cytotoxicity assays. We used a chromosomal exoS-lacZ reporter system to confirm this result. The defect in exoS expression is observed during overexpression of the histidine transporter (hutT) in a strain carrying a lacZ reporter gene in the exoS locus. This defect in exoS expression also depends on the presence of histidine in the medium.
The I.11E mutation causes PAO1 to grow extremely poorly on histidine as a sole carbon source. The reason for this appears somewhat puzzling, since none of the histidine catabolic genes are disrupted. If anything, they are overexpressed. One possible explanation is that overexpression of all of the histidine utilization genes except hutU (the gene for urocanase, which is located upstream of the insertion site [Fig. 2]) leads to a situation in which the relatively low level of urocanase creates a bottleneck in the catabolic pathway. As a result, it may be that ammonia levels rise because of the initial deamination reaction, while the assimilation of the carbon backbone proceeds more slowly, resulting in a metabolic imbalance that is deleterious for growth. This explanation fits conceptually with the results of the suppressor mutant screen. The defects in growth and cytotoxicity were partially suppressed by an insertion in cbrA, which also results in overexpression of cbrB. The cbrAB two-component regulatory system was originally described as being involved in controlling a number of catabolic operons. Strains mutant for cbrA or cbrB are incapable of growing on a variety of amino acids as the sole carbon source. This two-component regulatory system was postulated to sense and respond to a carbon-nitrogen imbalance because of the intriguing observation that a mutant is unable to grow on proline as the sole carbon and nitrogen source but can be complemented by addition of succinate to the medium. This complementation is abolished, however, by the further addition of ammonia to the medium (31). Since the cbrA::TnFAC insertion does not increase the cytotoxicity of the wild-type strain, it seems that this mutation helps counteract the metabolic imbalance incurred by the excessive uptake and catabolism of histidine.
A recent search for mutations that render P. aeruginosa strain CHA unable to induce its type III secretion machinery under low-calcium conditions identified a large number of insertions in the aceAB operon, which encodes the subunits of pyruvate dehydrogenase (4). The authors proposed that pyruvate dehydrogenase may act directly as a transcriptional activator. In view of our results, however, an alternative explanation is that these mutations affect the metabolic state of the cell, which, in turn, affects the expression of type III secretion genes.
Infection of a host represents a significant change in environment, and sensing its own metabolic state may be an expedient way to sense this transition and regulate virulence factor expression accordingly. Since several metabolic pathways funnel into and branch off of the tricarboxylic acid (TCA) cycle, its intermediates should be ideally suited to conveying the overall metabolic state of the cell. We propose that the I.11E and aceAB mutations affect the type III secretion system indirectly by modulating levels of TCA intermediates. Histidine is degraded to glutamate, which in turn is one step away from the TCA cycle intermediate α-ketoglutarate. Pyruvate dehydrogenase generates acetyl coenzyme A, which combines with oxaloacetate to form citrate, another TCA cycle intermediate. Current experiments are aimed at addressing this hypothesis directly, but there is clearly some supporting evidence from studies of other pathogenic bacteria. Glutamate is involved in the regulation of type III secretion genes in Yersinia sp. (33), and mutations in fatty acid biosynthesis genes in Salmonella sp. have also suggested that a change in metabolism may be factored into the expression of the type III secretion machinery on Salmonella pathogenicity island 1 (21, 22).
Finally, since we were able to recapitulate the mutant phenotype in strain PAK with a plasmid that overexpressed the histidine transporter and histidine-ammonia lyase, it appears that metabolic regulation of the type III secretion system is a general phenomenon. If so, it may be possible to target specific metabolic pathways to prevent induction of the type III secretion machinery and thus improve clinical outcomes.
Acknowledgments
We thank George O'Toole for providing phage DMS3 prior to publication, as well as Stephen Lory for assistance with the microarray analysis.
A.R. was supported by a Helen Hay Whitney Foundation postdoctoral fellowship. M.C.W. was supported by a Cystic Fibrosis Foundation postdoctoral fellowship. This work was supported in part by grant AI26289 from the National Institutes of Health to J.J.M.
Editor: V. J. DiRita
REFERENCES
- 1.Alexeyev, M. F., and I. N. Shokolenko. 1995. Mini-Tn10 transposon derivatives for insertion mutagenesis and gene delivery into the chromosome of gram-negative bacteria. Gene 160:59-62. [DOI] [PubMed] [Google Scholar]
- 2.Alexeyev, M. F., and I. N. Shokolenko. 1995. RP4 oriT and RP4 oriT-R6K oriV DNA cassettes for construction of specialized vectors. BioTechniques 19:22-24, 26. [PubMed] [Google Scholar]
- 3.Cormack, B. P., R. H. Valdivia, and S. Falkow. 1996. FACS-optimized mutants of the green fluorescent protein (GFP). Gene 173:33-38. [DOI] [PubMed] [Google Scholar]
- 4.Dacheux, D., O. Epaulard, A. de Groot, B. Guery, R. Leberre, I. Attree, B. Polack, and B. Toussaint. 2002. Activation of the Pseudomonas aeruginosa type III secretion system requires an intact pyruvate dehydrogenase aceAB operon. Infect. Immun. 70:3973-3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finck-Barbancon, V., and D. W. Frank. 2001. Multiple domains are required for the toxic activity of Pseudomonas aeruginosa ExoU. J. Bacteriol. 183:4330-4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finck-Barbancon, V., J. Goranson, L. Zhu, T. Sawa, J. P. Wiener-Kronish, S. M. Fleiszig, C. Wu, L. Mende-Mueller, and D. W. Frank. 1997. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol. Microbiol. 25:547-557. [DOI] [PubMed] [Google Scholar]
- 7.Frank, D. W. 1997. The exoenzyme S regulon of Pseudomonas aeruginosa. Mol. Microbiol. 26:621-629. [DOI] [PubMed] [Google Scholar]
- 8.Frank, D. W., D. G. Storey, M. S. Hindahl, and B. H. Iglewski. 1989. Differential regulation by iron of regA and toxA transcript accumulation in Pseudomonas aeruginosa. J. Bacteriol. 171:5304-5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garau, J., and L. Gomez. 2003. Pseudomonas aeruginosa pneumonia. Curr. Opin. Infect. Dis. 16:135-143. [DOI] [PubMed] [Google Scholar]
- 10.Goehring, U. M., G. Schmidt, K. J. Pederson, K. Aktories, and J. T. Barbieri. 1999. The N-terminal domain of Pseudomonas aeruginosa exoenzyme S is a GTPase-activating protein for Rho GTPases. J. Biol. Chem. 274:36369-336372. [DOI] [PubMed] [Google Scholar]
- 11.Govan, J. R., and V. Deretic. 1996. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol. Rev. 60:539-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hauser, A. R., E. Cobb, M. Bodi, D. Mariscal, J. Valles, J. N. Engel, and J. Rello. 2002. Type III protein secretion is associated with poor clinical outcomes in patients with ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Crit. Care Med. 30:521-528. [DOI] [PubMed] [Google Scholar]
- 13.Hauser, A. R., P. J. Kang, and J. N. Engel. 1998. PepA, a secreted protein of Pseudomonas aeruginosa, is necessary for cytotoxicity and virulence. Mol. Microbiol. 27:807-818. [DOI] [PubMed] [Google Scholar]
- 14.Hoang, T. T., R. R. Karkhoff-Schweizer, A. J. Kutchma, and H. P. Schweizer. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77-86. [DOI] [PubMed] [Google Scholar]
- 15.Holder, I. A., A. N. Neely, and D. W. Frank. 2001. Type III secretion/intoxication system important in virulence of Pseudomonas aeruginosa infections in burns. Burns 27:129-130. [DOI] [PubMed] [Google Scholar]
- 16.Holloway, B. W. 1955. Genetic recombination in Pseudomonas aeruginosa. J. Gen. Microbiol. 13:572-581. [DOI] [PubMed] [Google Scholar]
- 17.Hueck, C. J. 1998. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62:379-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krall, R., G. Schmidt, K. Aktories, and J. T. Barbieri. 2000. Pseudomonas aeruginosa ExoT is a Rho GTPase-activating protein. Infect. Immun. 68:6066-6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krall, R., J. Sun, K. J. Pederson, and J. T. Barbieri. 2002. In vivo Rho GTPase-activating protein activity of Pseudomonas aeruginosa cytotoxin ExoS. Infect. Immun. 70:360-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu, S., T. L. Yahr, D. W. Frank, and J. T. Barbieri. 1997. Biochemical relationships between the 53-kilodalton (Exo53) and 49-kilodalton (ExoS) forms of exoenzyme S of Pseudomonas aeruginosa. J. Bacteriol. 179:1609-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lucas, R. L., and C. A. Lee. 2000. Unravelling the mysteries of virulence gene regulation in Salmonella typhimurium. Mol. Microbiol. 36:1024-1033. [DOI] [PubMed] [Google Scholar]
- 22.Lucas, R. L., C. P. Lostroh, C. C. DiRusso, M. P. Spector, B. L. Wanner, and C. A. Lee. 2000. Multiple factors independently regulate hilA and invasion gene expression in Salmonella enterica serovar Typhimurium. J. Bacteriol. 182:1872-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marra, A., J. Asundi, M. Bartilson, S. Lawson, F. Fang, J. Christine, C. Wiesner, D. Brigham, W. P. Schneider, and A. E. Hromockyj. 2002. Differential fluorescence induction analysis of Streptococcus pneumoniae identifies genes involved in pathogenesis. Infect. Immun. 70:1422-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mason, K. M., R. S. Munson, Jr., and L. O. Bakaletz. 2003. Nontypeable Haemophilus influenzae gene expression induced in vivo in a chinchilla model of otitis media. Infect. Immun. 71:3454-3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCaw, M. L., G. L. Lykken, P. K. Singh, and T. L. Yahr. 2002. ExsD is a negative regulator of the Pseudomonas aeruginosa type III secretion regulon. Mol. Microbiol. 46:1123-1133. [DOI] [PubMed] [Google Scholar]
- 26.Miller, J. H. 1992. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor Laboratory Press, Plainview, N.Y.
- 27.Miller, V. L. 2002. Connections between transcriptional regulation and type III secretion? Curr. Opin. Microbiol. 5:211-215. [DOI] [PubMed] [Google Scholar]
- 28.Nicas, T. I., J. Bradley, J. E. Lochner, and B. H. Iglewski. 1985. The role of exoenzyme S in infections with Pseudomonas aeruginosa. J. Infect. Dis. 152:716-721. [DOI] [PubMed] [Google Scholar]
- 29.Nicas, T. I., D. W. Frank, P. Stenzel, J. D. Lile, and B. H. Iglewski. 1985. Role of exoenzyme S in chronic Pseudomonas aeruginosa lung infections. Eur. J. Clin. Microbiol. 4:175-179. [DOI] [PubMed] [Google Scholar]
- 30.Nicolle, L. E. 2002. Resistant pathogens in urinary tract infections. J. Am. Geriatr. Soc. 50:S230-S235. [DOI] [PubMed] [Google Scholar]
- 31.Nishijyo, T., D. Haas, and Y. Itoh. 2001. The CbrA-CbrB two-component regulatory system controls the utilization of multiple carbon and nitrogen sources in Pseudomonas aeruginosa. Mol. Microbiol. 40:917-931. [DOI] [PubMed] [Google Scholar]
- 32.Pirnay, J. P., D. De Vos, C. Cochez, F. Bilocq, J. Pirson, M. Struelens, L. Duinslaeger, P. Cornelis, M. Zizi, and A. Vanderkelen. 2003. Molecular epidemiology of Pseudomonas aeruginosa colonization in a burn unit: persistence of a multidrug-resistant clone and a silver sulfadiazine-resistant clone. J. Clin. Microbiol. 41:1192-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramamurthi, K. S., and O. Schneewind. 2002. Type iii protein secretion in yersinia species. Annu. Rev. Cell Dev. Biol. 18:107-133. [DOI] [PubMed] [Google Scholar]
- 34.Roy-Burman, A., R. H. Savel, S. Racine, B. L. Swanson, N. S. Revadigar, J. Fujimoto, T. Sawa, D. W. Frank, and J. P. Wiener-Kronish. 2001. Type III protein secretion is associated with death in lower respiratory and systemic Pseudomonas aeruginosa infections. J. Infect. Dis. 183:1767-1774. [DOI] [PubMed] [Google Scholar]
- 35.Sato, H., D. W. Frank, C. J. Hillard, J. B. Feix, R. R. Pankhaniya, K. Moriyama, V. Finck-Barbancon, A. Buchaklian, M. Lei, R. M. Long, J. Wiener-Kronish, and T. Sawa. 2003. The mechanism of action of the Pseudomonas aeruginosa-encoded type III cytotoxin, ExoU. EMBO J. 22:2959-2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schneider, W. P., S. K. Ho, J. Christine, M. Yao, A. Marra, and A. E. Hromockyj. 2002. Virulence gene identification by differential fluorescence induction analysis of Staphylococcus aureus gene expression during infection-simulating culture. Infect. Immun. 70:1326-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith, R. S., and B. H. Iglewski. 2003. P. aeruginosa quorum-sensing systems and virulence. Curr. Opin. Microbiol. 6:56-60. [DOI] [PubMed] [Google Scholar]
- 38.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, I. T. Paulsen, J. Reizer, M. H. Saier, R. E. Hancock, S. Lory, and M. V. Olson. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 39.Sundin, C., M. C. Wolfgang, S. Lory, A. Forsberg, and E. Frithz-Lindsten. 2002. Type IV pili are not specifically required for contact dependent translocation of exoenzymes by Pseudomonas aeruginosa. Microb. Pathog. 33:265-277. [DOI] [PubMed] [Google Scholar]
- 40.Takeya, K., and K. Amako. 1966. A rod-shaped Pseudomonas phage. Virology 28:163-165. [DOI] [PubMed] [Google Scholar]
- 41.Valdivia, R. H., and S. Falkow. 1996. Bacterial genetics by flow cytometry: rapid isolation of Salmonella typhimurium acid-inducible promoters by differential fluorescence induction. Mol. Microbiol. 22:367-378. [DOI] [PubMed] [Google Scholar]
- 42.Valdivia, R. H., and S. Falkow. 1997. Fluorescence-based isolation of bacterial genes expressed within host cells. Science 277:2007-2011. [DOI] [PubMed] [Google Scholar]
- 43.Vallis, A. J., T. L. Yahr, J. T. Barbieri, and D. W. Frank. 1999. Regulation of ExoS production and secretion by Pseudomonas aeruginosa in response to tissue culture conditions. Infect. Immun. 67:914-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Warrens, A. N., M. D. Jones, and R. I. Lechler. 1997. Splicing by overlap extension by PCR using asymmetric amplification: an improved technique for the generation of hybrid proteins of immunological interest. Gene 186:29-35. [DOI] [PubMed] [Google Scholar]
- 45.Wolfgang, M. C., V. T. Lee, M. E. Gilmore, and S. Lory. 2003. Coordinate regulation of bacterial virulence genes by a novel adenylate cyclase-dependent signaling pathway. Dev. Cell 4:253-263. [DOI] [PubMed] [Google Scholar]
- 46.Wong, S. M., and J. J. Mekalanos. 2000. Genetic footprinting with mariner-based transposition in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 97:10191-10196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yahr, T. L., J. T. Barbieri, and D. W. Frank. 1996. Genetic relationship between the 53- and 49-kilodalton forms of exoenzyme S from Pseudomonas aeruginosa. J. Bacteriol. 178:1412-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yahr, T. L., J. Goranson, and D. W. Frank. 1996. Exoenzyme S of Pseudomonas aeruginosa is secreted by a type III pathway. Mol. Microbiol. 22:991-1003. [DOI] [PubMed] [Google Scholar]
- 49.Yahr, T. L., L. M. Mende-Mueller, M. B. Friese, and D. W. Frank. 1997. Identification of type III secreted products of the Pseudomonas aeruginosa exoenzyme S regulon. J. Bacteriol. 179:7165-7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yahr, T. L., A. J. Vallis, M. K. Hancock, J. T. Barbieri, and D. W. Frank. 1998. ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc. Natl. Acad. Sci. USA 95:13899-13904. [DOI] [PMC free article] [PubMed] [Google Scholar]