Abstract
Some diseases are characterized by prevalence in the affected tissues of type 1 T lymphocytes, which secrete gamma interferon (IFN-γ) and other proinflammatory cytokines. For example, type 1 T cells predominate in the lesions of patients with Lyme disease, which is caused by the bacterium Borrelia burgdorferi. We used an in vitro model of the blood vessel wall to test the premise that the vascular endothelium actively recruits circulating type 1 T cells to such lesions. When T lymphocytes isolated from human peripheral blood were examined, the populations that traversed monolayers of resting human umbilical vein endothelial cells (HUVEC) or HUVEC stimulated by interleukin-1β or B. burgdorferi were markedly enriched for T cells that produced IFN-γ compared to the initially added population of T cells. No enrichment was seen for cells that produced interleukin-4, a marker for type 2 T lymphocytes. Very late antigen-4 and CD11/CD18 integrins mediated passage of the T cells across both resting and stimulated HUVEC, and the endothelium-derived chemokine CCL2 (monocyte chemoattractant protein 1) was responsible for the enhanced migration of T cells across stimulated HUVEC. These results suggest that the vascular endothelium may contribute to the selective accumulation of type 1 T cells in certain pathological lesions, including those of Lyme disease.
T lymphocytes can be classified functionally based on the cytokines that they secrete. Type 1 T lymphocytes are characterized by production of interleukin (IL)-2, gamma interferon (IFN-γ), and tumor necrosis factor beta, which help orchestrate cell-mediated immunity. In contrast, type 2 T lymphocytes secrete cytokines that promote humoral immunity, including IL-4 and IL-5 (22). Both CD4+ T helper (Th) cells and CD8+ T cytotoxic (Tc) cells contain type 1 and type 2 subsets (9). A number of diseases are typified by preferential accumulation of type 1 or type 2 T cells in affected tissues (11, 24). For instance, type 1 T cells are the predominant lymphocytic population in the extravascular lesions of patients with Lyme disease (16, 34, 35). In addition, the severity of Lyme arthritis is directly correlated with a greater ratio of Th1 to Th2 cells in the synovial fluids of the involved joints (16).
Extravascular accumulation of specific subpopulations of T lymphocytes may be due to their preferential migration across the endothelium, their local differentiation once they have emigrated, or their enhanced survival, proliferation, or retention within tissues. In support of the first of these possibilities, several in vitro studies indicate that endothelial adhesion molecules and/or chemoattractants may be responsible for the different migratory capacities of distinct subsets of T lymphocytes. Human Th2 cells, polarized and activated in vitro, express the chemokine receptor CCR2 more strongly and migrate across the endothelium to a greater degree than do similarly produced Th1 lymphocytes (5). Secretion of the chemoattractant cytokine CCL5 (formerly called RANTES) by rat aortic endothelial cells activated with IFN-γ enhances the transmigration of rat Th1-type cell lines but not Th2-type lines (21). Polarized murine Th1 cells produced in vitro bind to the endothelial adhesion molecules P- and E-selectin, whereas Th2 cells do not (1). However, subsequent reports suggest that the correlation between the Th1 phenotype and expression of specific chemokine receptors (25) or ligands for selectins (33) is much less distinct for T cells polarized in vivo rather than in vitro.
These studies support the notion that accumulation of specific subsets of T lymphocytes in tissues may be regulated at the level of extravasation, but all were limited to examination of type 1 and type 2 T cells that were generated in vitro. We previously used a well-characterized model of the blood vessel wall, consisting of human umbilical vein endothelial cells (HUVEC) cultured on connective tissue substrates prepared from human amnion, to study the transmigration of T lymphocytes freshly isolated from human peripheral blood. Prior activation of HUVEC with IL-1β or Borrelia burgdorferi, the causative organism of Lyme disease, increases the number of T cells that penetrate the endothelial monolayers by approximately twofold (15). The present study used the HUVEC-amnion model and freshly isolated T lymphocytes to examine the migratory behavior of type 1 and type 2 T cells. Our results demonstrate that the population of T lymphocytes that migrates across the endothelium is markedly enriched for IFN-γ-secreting, type 1 T cells.
MATERIALS AND METHODS
Culture of B. burgdorferi.
The HBD1 strain of B. burgdorferi, originally isolated from human blood (3), was used between passages 42 and 60. The spirochetes were grown at 33°C in Barbour-Stoenner-Kelly medium modified to minimize the content of lipopolysaccharide, as described previously (30). Bacteria were harvested in late-log-phase growth, centrifuged at 5,000 × g for 20 min at room temperature, and resuspended to the desired concentration in medium 199 (Invitrogen Corp., Grand Island, N.Y.) containing 20% heat-inactivated fetal bovine serum (HyClone Laboratories, Logan, Utah) for use in experiments. In some studies, an equal volume of uninoculated spirochetal growth medium was processed in parallel with the spirochetes, and HUVEC-amnion cultures were exposed to this sham preparation. The level of migration of T lymphocytes across sham-treated cultures was consistently similar to that across unstimulated endothelium, indicating that exogenous lipopolysaccharide was not introduced during processing of the bacteria.
Transendothelial migration of T lymphocytes.
HUVEC were isolated with collagenase as previously described (17) and cultured in medium 199 containing 20% fetal bovine serum, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 2 μg of amphotericin B per ml until confluent. HUVEC cultures from several cords were trypsinized, pooled, and plated at 1.5 × 105 cells/cm2 onto acellular connective tissue substrates derived from human amnion (14). In various experiments, amniotic substrates with a surface area of either 1 or 2 cm2 were used. HUVEC were grown on the amniotic tissue for 7 to 10 days, a time that allows transendothelial electrical resistance to develop (17). HUVEC-amnion cultures were then treated at 37°C for 24 h with culture medium lacking antibiotics as a negative control or the same medium containing 1 U of recombinant IL-1β (Collaborative Biomedical, Bedford, Mass.) per ml or 10 B. burgdorferi organisms per endothelial cell.
Unfractionated T lymphocytes, CD4+ T cells, and CD8+ T cells were isolated by negative selection with MACS immunomagnetic beads (Miltenyi Biotec, Auburn, Calif.) (15) and resuspended in culture medium. T cells (5 × 105 to 2 × 106/cm2) were added to the apical sides of the treated cultures at 37°C for 4 h. The ratio of T cells to HUVEC ranged from approximately 4:1 to 16:1. To determine the total number of T lymphocytes associated with each culture, the tissues were fixed in 10% formalin, stained with Wright stain, and counted en face by light microscopy (15). Portions of each tissue were embedded and cross-sectioned to distinguish T cells that were adherent to the apical side of the endothelium from those that had migrated beneath (27).
In some experiments, 25 to 30 μg of an immunoglobulin type IgG1 monoclonal antibody (MAb) to the chemokine CCL2 (formerly known as monocyte chemoattractant protein-1 [MCP-1]) (R&D Systems, Minneapolis, Minn.) or an isotype-matched control MAb (MOPC21; Sigma, St. Louis, Mo.) per ml was added to HUVEC-amnion cultures during the last 4 h of stimulation; cultures were elevated on silicone supports so that the MAb could be placed both above and beneath. T cells were then added in the presence of additional MAb. In other studies, T cells were incubated for 30 min with culture medium containing 10 μg of an IgG1 MAb to very late antigen-4 (VLA-4) (HP1/2; a gift of Roy R. Lobb, Biogen Inc., Cambridge, Mass.) and/or 40 μg of an IgG1 MAb to CD18 (TS1/18; provided by Richard T. Coughlin, Cambridge Biotech, Worcester, Mass.) per ml, prior to their addition to the HUVEC-amnion cultures. MOPC21 again served as an isotype-matched control MAb.
Intracellular staining for cytokines.
Following a transendothelial migration assay, adherent T cells were removed from the apical surface of the HUVEC by vigorous washing in buffer devoid of Ca2+ and Mg2+. The migrated populations of T cells were liberated from three or four HUVEC-amnion cultures for each set of experimental conditions by incubating for 20 to 30 min at 37°C in 0.5 mg of collagenase D (Roche Diagnostics Corp., Indianapolis, Ind.) per ml and 10 μg of hyaluronidase (Worthington Biochemical Corp., Lakewood, N.Y.) per ml. The digestion solution also contained soybean trypsin inhibitor (10 μg/ml), tosyl-l-phenylalanine chloromethyl ketone (37 μg/ml), and one Mini-Complete EDTA-free protease inhibitor cocktail tablet per 10 ml (all from Roche Applied Science, Indianapolis, Ind.). Harvested cells then were pooled, washed three times in Hanks' balanced salt solution (Invitrogen), and resuspended in 1 ml of RPMI 1640 (Sigma) containing 10% heat-inactivated fetal bovine serum (15). We previously demonstrated that this digestion protocol alters neither the viability of the T cells nor their expression of cell surface markers of interest, including CD4 and CD8 (15).
The migrated cells and an aliquot of the starting population of T cells were then activated with 25 ng of phorbol 12-myristate 13-acetate (PMA) per ml and 1 μg of ionomycin per ml in the presence of 10 μg of brefeldin A per ml (all from Sigma) for 4 h. The cells were subsequently incubated with fluorescently labeled MAb to CD4 or CD8, fixed, permeabilized, and then incubated with fluorescently labeled MAb to IFN-γ or IL-4 with the Fast Immune Cytokine System (BD Biosciences, San Jose, Calif.). Cells incubated with fluorescently labeled, isotype-matched MAbs to irrelevant antigens (Caltag Laboratories, Burlingame, Calif.) served as negative controls. Two-color flow cytometry was performed with a FACScan flow cytometer (BD Biosciences). Lymphocytes were gated according to their forward scatter versus side scatter to exclude debris, clumps, and endothelial cells (15). A total of 10,000 cells was analyzed for each sample. For each experimental group, an additional two cultures were fixed and analyzed microscopically, as described in the preceding section, to verify that the treated endothelia were indeed activated and that adherent lymphocytes were removed by the washing procedure.
Quantitation of chemokines.
HUVEC were seeded at 1.75 × 105 cells/well in 48-well tissue culture plates and grown to confluence. Cells were incubated for 24 h with either 0.5 ml of medium alone or medium containing 10 B. burgdorferi organisms per endothelial cell. The amounts of the chemokines CCL5 (RANTES) and CXCL8 (IL-8) in the cell-free conditioned media were measured with commercial enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems and Antigenix America, Inc., Huntington Station, N.Y.). CCL5 bound to HUVEC was measured with a whole-cell ELISA (30) and a MAb to CCL5 (21445.1; R&D Systems).
Statistics.
Data obtained for the transmigration assays were subjected to an ordinary one-way analysis of variance with the Tukey-Kramer multiple-comparison posttest (GraphPad Instat, GraphPad Software Inc., San Diego, Calif.). Data for the phenotypic analyses were subjected to a two-way analysis of variance of a randomized block design with the Tukey-Kramer multiple-comparison posttest (Jandel SigmaStat, SPSS Science, Chicago, Ill.).
RESULTS
To determine whether transendothelial migration promotes preferential accumulation of human type 1 or type 2 T cells, unfractionated T lymphocytes isolated from peripheral blood were added to unstimulated HUVEC-amnion cultures or cultures that had been stimulated with IL-1β or B. burgdorferi for 24 h. Cells that migrated were harvested, and their phenotypes were compared to those of the initially added population with respect to expression of CD4, CD8, IFN-γ, and IL-4. The populations of cells that underwent transendothelial migration were enriched for type 1, IFN-γ-secreting T lymphocytes regardless of the activation status of the endothelium. Moreover, this enrichment was noted in both CD4+ Th and CD8+ Tc subsets (Table 1). A comparable enrichment for Th1 cells in the populations that migrated was seen when purified CD4+ T cells were used in the assay (Table 2).
TABLE 1.
Populations of unfractionated T lymphocytes that traverse HUVEC are enriched for cells that secrete IFN-γ
| Donor | Surface marker | % of T lymphocytes secreting IFN-γ
|
|||
|---|---|---|---|---|---|
| Initial population | Migrated across un- stimulated HUVECa | Migrated across IL- 1β-treated HUVECab | Migrated across B. burg- dorferi-treated HUVECa,b | ||
| A | CD4 | 10 | 21 | 25 | 28 |
| CD8 | 13 | 37 | 28 | 37 | |
| B | CD4 | 17 | 44 | 44 | 39 |
| CD8 | 10 | 27 | 23 | 25 | |
| C | CD4 | 24 | 29 | 31 | 30 |
| CD8 | 29 | 40 | 43 | 43 | |
| D | CD4 | 13 | 36 | 28 | 33 |
| CD8 | 13 | 32 | 24 | 27 | |
For both CD4+ and CD8+ subsets, the migrated populations show significant enrichment for IFN-γ-secreting cells compared to the initial population (P < 0.05).
Total numbers of migrated cells were counted in two cultures per experimental group, as described in Materials and Methods, to ensure that treatment with IL-1β or B. burgdorferi indeed activated the HUVEC. On average, 2.3 ± 0.9-fold more T lymphocytes traversed stimulated HUVEC than unstimulated endothelium in these experiments.
TABLE 2.
Populations of purified CD4+ T lymphocytes that traverse HUVEC are enriched for cells that secrete IFN-γ
| Donor | % of CD4+ T lymphocytes secreting IFN-γ
|
|||
|---|---|---|---|---|
| Initial population | Migrated across unstimulated HUVECa | Migrated across IL-1β-treated HUVECab | Migrated across B. burgdorferi-treated HUVECa,b | |
| B | 30 | 62 | 53 | 62 |
| E | 26 | 51 | 38 | 49 |
| F | 12 | 39 | 29 | 34 |
All migrated populations showed significant enrichment for cells that secrete IFN-γ compared to the initial population (P < 0.05).
On average, 2.3 ± 0.8-fold more T lymphocytes traversed stimulated HUVEC than unstimulated endothelium in these experiments.
When purified CD8+ T cells were studied, however, the degree of enrichment for Tc1 cells in the migrated populations was less than that observed for unfractionated T cells in three of four experiments. Enrichment for CD8+ T cells that secrete IFN-γ was significant only for cells that traversed B. burgdorferi-stimulated endothelium (Table 3). The unfractionated T cells and the CD8+ subset were isolated from the same donors (although on different days), and the protocols for their purification differed only by the presence or absence of MAb to CD4 in the mixture of antibodies used for negative selection. A possible explanation for the discrepant results, then, is that the presence of CD4+ T cells may promote the migration of CD8+ type 1 T cells.
TABLE 3.
Populations of purified CD8+ T lymphocytes that traverse HUVEC are not markedly enriched for cells that secrete IFN-γ
| Donora | % of CD8+ T lymphocytes secreting IFN-γ
|
|||
|---|---|---|---|---|
| Initial population | Migrated across unstimulated HUVEC | Migrated across IL-1β-treated HUVECb | Migrated across B. burgdorferi-treated HUVECb,c | |
| A | 23 | 63 | 57 | 64 |
| B | 33 | 60 | 54 | 61 |
| C | 69 | 69 | 66 | 78 |
| D | 30 | 27 | 38 | 51 |
The donors correspond to those used for the experiments in Table 1, but the T cells were isolated on different days.
On average, 1.7 ± 0.2-fold more T lymphocytes traversed stimulated HUVEC than unstimulated endothelium in these experiments.
The population of CD8+ T lymphocytes that migrated across B. burgdorferi-activated endothelium showed significant enrichment for cells that secrete IFN-γ compared to the initial population (P < 0.05).
For both CD4+ and CD8+ subsets, the initial populations and all migrated populations contained similar proportions of IL-4-producing, type 2 T cells (Table 4). In seven separate experiments, the initially added populations contained 4.0% ± 2.4% type 2 T cells, whereas populations that traversed resting HUVEC or HUVEC exposed to IL-1β or B. burgdorferi contained 5.1% ± 2.9%, 4.3% ± 2.4%, and 3.5% ± 2.0% type 2 T cells, respectively.
TABLE 4.
Populations of T lymphocytes that traverse HUVEC are neither enriched for nor depleted of T cells that secrete IL-4
| Donor | Type of T cells addeda | % of T lymphocytes secreting IL-4
|
|||
|---|---|---|---|---|---|
| Initial population | Migrated across unstimulated HUVEC | Migrated across IL-1β-treated HUVECb | Migrated across B. burgdorferi-treated HUVECb | ||
| B | CD4+ | 9.0 | 8.5 | 6.6 | 5.7 |
| E | CD4+ | 4.7 | 6.9 | 6.2 | 6.3 |
| F | CD4+ | 3.1 | 8.3 | 4.5 | 4.4 |
| B | CD8+ | 3.3 | 5.1 | 5.8 | 3.3 |
| C | CD8+ | 3.9 | 0.7 | 0.8 | 1.3 |
| G | CD8+ | 2.3 | 3.4 | —c | 1.7 |
| D | Unfractionated (CD4+, CD8+) | 1.5 (1.2, 0.4) | 2.9 (1.0, 1.9) | 2.1 (1.5, 0.6) | 1.9 (1.4, 0.5) |
Assays were performed with unfractionated T lymphocytes or purified CD4+ or CD8+ T cells. For the study in which unfractionated T cells were added, the percentages of IL-4-secreting cells are reported for the total populations of T lymphocytes and for the CD4+ and CD8+ subsets.
On average, 2.2 ± 0.7-fold more T lymphocytes traversed stimulated HUVEC than unstimulated endothelial monolayers in these experiments.
The endothelium was not activated by IL-1β in this experiment, as assessed by comparing the numbers of T cells that migrated across untreated versus IL-1β-treated HUVEC.
In all experiments, unstimulated T cells were added to the endothelium; the cells were activated with PMA and ionomycin in the presence of brefeldin A to induce production and retention of cytokines only after the transmigration assay was completed. For both initially added and migrated populations, T cells that were incubated with brefeldin A but not PMA and ionomycin failed to express IFN-γ or IL-4 (data not shown), indicating that transmigration per se does not induce production of these cytokines.
With the HUVEC-amnion model, we previously observed that CD45RA+RO− (naïve) T cells migrate poorly regardless of the activation status of the endothelium. In contrast, CD45RA−RO+ (memory/effector) T cells are present in approximately equal proportions in the initially added and migrated populations, and CD45RA+RO+ (transitional) T cells are enriched two- to threefold in the populations that migrate (15). Although both CD45RA+RO+ and type 1 T cells preferentially accumulate in this model, the relationship between these two subsets is not clear. Indeed, purified CD45RA+RO+ T cells produce much less mRNA for IFN-γ when activated than do CD45RA−RO+ T cells (2). We attempted to address this issue directly by staining cells simultaneously for CD45RA, CD45RO, and IFN-γ. However, processing of the lymphocytes to detect intracellular IFN-γ markedly lowered their surface expression of the CD45 markers and thus artificially altered the classification of cells with respect to naïve, transitional, and memory/effector phenotypes. In a typical experiment, the mean fluorescence intensity of staining for CD45RA decreased from 333 to 61 after activation and permeabilization of the T cells, and that for CD45RO was lowered from 1,145 to 301.
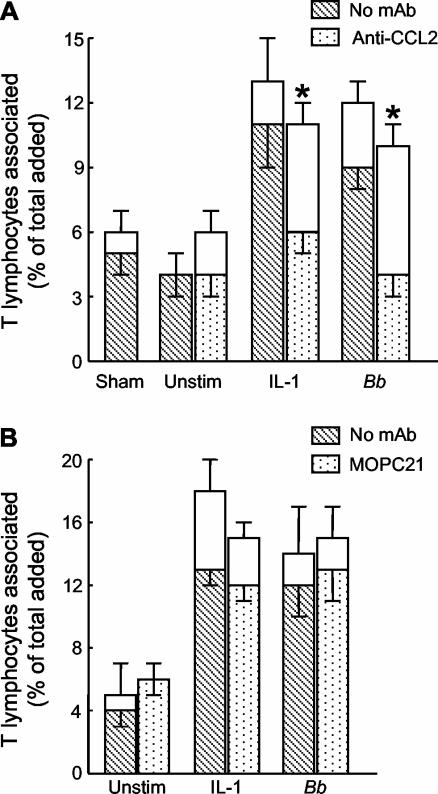
A MAb to the chemokine CCL2 (MCP-1), used at 25 μg/ml, completely abolished the enhanced migration of T lymphocytes across endothelium treated with IL-1β or B. burgdorferi, although it did not affect their traversal of unstimulated endothelium (Fig. 1A). In a control experiment, we observed that migration of T cells across endothelium treated with 5 U of IL-1β per ml was inhibited to the same degree by either 20 or 30 μg of MAb to CCL2 per ml (data not shown). In addition, endothelium stimulated with 5 U of IL-1β per ml produces substantially more CCL2 than does B. burgdorferi-activated endothelium (8). Therefore, any concentration of MAb to CCL2 above 20 μg/ml should be saturating when 1 U of IL-1β per ml or spirochetes are used as a stimulus, as in Fig. 1A. An isotype-matched control MAb to an irrelevant antigen had no effect on migration (Fig. 1B).
FIG. 1.
Enhanced migration of T lymphocytes across stimulated endothelium is dependent on CCL2 (MCP-1). HUVEC were left unstimulated (Unstim), treated with a sham preparation of bacteria, or stimulated with 1 U of IL-1β per ml or B. burgdorferi (Bb) at 10 spirochetes per endothelial cell for 24 h. During the last 4 h of stimulation, 25 μg of MAb to CCL2 per ml (A), 30 μg of the isotype-matched control MAb MOPC21 per ml (B), or an equal volume of phosphate-buffered saline (No MAb) was added. T lymphocytes (5 × 105 cells/cm2) were then added to the endothelium for 4 h in the presence of additional MAb. The total height of each bar represents the percentage of added T cells that became associated with the cultures. The lower (patterned) portion of each bar represents the percentage of cells that migrated beneath the endothelium; the upper (unfilled) portion illustrates the percentage that was adherent to the apical surface of the HUVEC. Bars represent the means ± standard deviations of three to four replicate samples. For A, data shown are representative of two separate experiments. Asterisks denote significant reductions in migration compared to control assays performed in the absence of MAb (P < 0.001).
Since HUVEC can produce CCL5 (RANTES) in response to IFN-γ and tumor necrosis factor alpha (23), we considered the possibility that this chemokine might participate in emigration of T cells in our system. However, the amount of CCL5 secreted by unstimulated or B. burgdorferi-activated endothelium for 24 h was below the limit of detection (approximately 8 pg/ml) of a CCL5 ELISA. When the same conditioned media were assayed for the chemokine CXCL8 (IL-8), a significantly greater amount was detected in B. burgdorferi-treated samples than in untreated controls (10.0 ± 0.1 ng/ml versus 0.8 ± 0.1 ng/ml; P < 0.001). Thus, the HUVEC responded to B. burgdorferi by secreting CXCL8 despite their failure to produce measurable amounts of CCL5. Furthermore, we detected no CCL5 on the surface of endothelium exposed to B. burgdorferi for 24 h, although, again, conditioned media from these HUVEC contained significantly elevated levels of CXCL8 (35.4 ± 4.4 ng/ml compared to 0.2 ± 0.1 ng/ml secreted by unstimulated control cultures; P < 0.001).
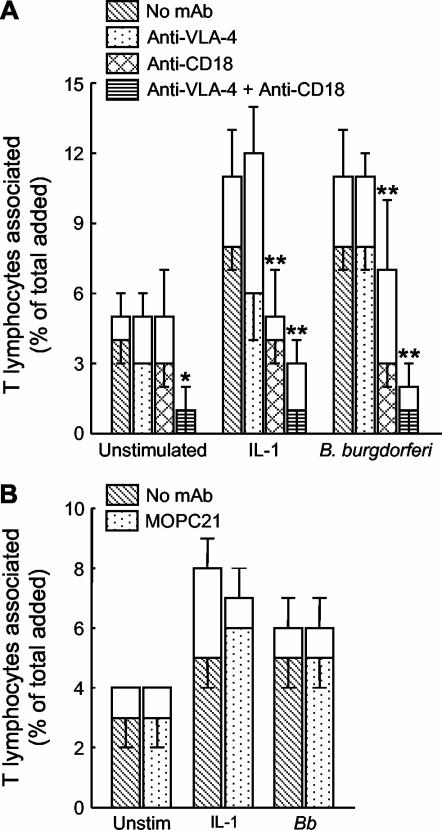
To determine the relative contributions of VLA-4 and CD11/CD18 to the migration of the T lymphocytes, assays were performed in the presence of MAbs to these integrins. The concentrations of MAbs used were saturating, as determined by flow cytometry. MAb to VLA-4 by itself had little effect on transmigration, whereas MAb to CD18 significantly reduced traversal of endothelium that was activated with IL-1β or B. burgdorferi (by 50% and 63%, respectively). When MAbs to VLA-4 and CD18 were used in combination, the migration of T lymphocytes across resting, IL-1β-stimulated, and B. burgdorferi-treated endothelium was almost completely abolished (Fig. 2A). In contrast, migration was not altered by an isotype-matched control MAb (Fig. 2B).
FIG. 2.
VLA-4 and CD11/CD18 integrins mediate the transendothelial migration of T lymphocytes. T lymphocytes (5 × 105 cells/cm2) were resuspended in medium containing 10 μg of MAb to VLA-4 per ml and/or 40 μg of MAb to CD18 per ml (A), 40 μg of the isotype-matched control MAb MOPC21 per ml (B), or an equivalent volume of phosphate-buffered saline (No MAb). The T lymphocytes were then added for 4 h to HUVEC that had either been left unstimulated (Unstim) or previously activated with IL-1β or B. burgdorferi (Bb) for 24 h. The total height of each bar represents the percentage of added T cells that became associated with the cultures. The lower (patterned) portion of each bar depicts the percentage of cells that migrated beneath the endothelium; the upper (unfilled) portion denotes the percentage that was adherent to the apical surface. Bars represent the means ± standard deviations of three to four replicate samples. For A, data shown are representative of two separate experiments. Asterisks denote significant reductions in migration compared to control assays performed in the absence of MAb: *, P < 0.01; **, P < 0.001.
DISCUSSION
In our in vitro model of extravasation, populations of T lymphocytes that migrated across unstimulated, IL-1β-treated, and B. burgdorferi-activated HUVEC were significantly enriched for cells that secreted IFN-γ upon activation (Table 1). To our knowledge, this is the first demonstration that the process of transendothelial migration contributes to the selective accumulation of human type 1 T cells. Regulation at the level of extravasation may explain, at least in part, why the lesions of patients with Lyme disease (16), rheumatoid arthritis (4), and lung cancer (19) contain a higher proportion of type 1 T cells than does their peripheral blood.
In contrast to T cells that produced IFN-γ, IL-4-secreting T lymphocytes were present in similar proportions in both the initially added and migrated populations (Table 4). The percentages of type 2 T cells in all populations were low compared to those of type 1 T cells. However, we have previously shown significant enrichment in migrated populations for CD69, an early marker of activation that is expressed by comparably small numbers of the cells (15). Thus, it is likely that we would have detected a consistent enrichment for type 2 T cells had it occurred. Under the conditions that we used, then, type 1 but not type 2 T lymphocytes selectively accumulated in the migrated populations. Consequently, enrichment of type 1 T lymphocytes in the migrated populations is unlikely to be merely secondary to the increased tendency of CD45RO-bearing cells to traverse the endothelium. If such were the case, then the migrated populations should have contained increased proportions of type 2 T cells as well.
The enrichment for IFN-γ-secreting T cells that we observed might arise from either selective recruitment of existing type 1 T cells by endothelial adhesion molecules and/or chemoattractants or a skewing of T cells toward the type 1 phenotype during the migration process itself. A determination of whether enrichment of type 1 T cells in the migrated populations is accompanied by a corresponding depletion in populations that do not migrate would distinguish between these two possibilities. However, the small percentage of cells that migrated in our system precludes such an approach. Previously, we showed that fewer than 7.5% of T cells that migrate across resting or stimulated HUVEC express the early activation marker CD69 (15). Moreover, the migrated populations in the present study did not produce IFN-γ unless they were treated with PMA and ionomycin. These observations indicate that the process of transmigration does not overtly activate the T lymphocytes. However, we cannot discount the possibility that transendothelial migration predisposes them to secrete IFN-γ when subsequently activated.
The enhanced migration of T lymphocytes across HUVEC stimulated by IL-1β or B. burgdorferi was largely dependent on endothelium-derived CCL2 (MCP-1) (Fig. 1A). In contrast, MAb to CCL2 had no effect on the traversal of unstimulated endothelium by the T lymphocytes, although the population that migrated across resting HUVEC was also enriched for type 1 T cells. This result strongly suggests that CCL2 is not the only endothelium-derived signal that drives accumulation of type 1 T lymphocytes. It also implies that the enhanced migration of T cells across activated endothelium is not due simply to increased expression of the same signals that are produced by resting endothelium; rather, a qualitative change in mediators appears to be involved.
Migration across the endothelium requires the participation of not only chemoattractants, but also adhesion molecules. T lymphocytes express the integrins VLA-4 and CD11a/CD18, which bind to counterreceptors on endothelium, including vascular cell adhesion molecule 1 (VCAM-1), intercellular adhesion molecule 1 (ICAM-1), and junctional adhesion molecule 1 (JAM-1) (26, 31). In our system, traversal of either unstimulated or activated endothelium by T lymphocytes was inhibited only partially or not at all by individual blockade of VLA-4 and CD11/CD18 integrins. In contrast, migration was almost abolished when both adhesion pathways were blocked simultaneously (Fig. 2A). Our results are consistent with studies of cutaneous delayed-type hypersensitivity lesions in rats, in which accumulation of T cells is more strongly reduced when both VLA-4 and CD11a/CD18 are blocked than when the integrins are inhibited singly (18). Based on this observation and our data, it appears that VLA-4 and CD11a/CD18 mediate two alternative pathways that T cells can employ for extravasation, since migration is not substantially inhibited unless the functions of both integrins are eliminated. Our results further suggest that all T lymphocytes, including the type 1 subset, use VLA-4 and CD11a/CD18 to interact with endothelium. This conclusion is supported by the observation that adoptively transferred, alloreactive Th1 cell lines employ CD11a/CD18 and ICAM-1 to localize to the lung in mice (13).
We cannot be certain that enrichment for cells that secrete IFN-γ in our in vitro model results from selective recruitment of committed type 1 T cells. However, there is evidence to suggest that type 1 and type 2 T cells use different mechanisms to extravasate. Human polarized Th1 and Th2 cells share certain chemokine receptors, including that for CCL2 (MCP-1), but the expression of others differs (6). Consequently, Th1 and Th2 lymphocytes are attracted by overlapping but not identical spectra of chemokines (29). Moreover, activation of T cells induces changes in the levels of expression of particular chemokine receptors (29), raising the possibility that we might have obtained different results had we stimulated our T-cell preparations before adding them to the endothelium.
Variations between Th1 and Th2 cells in their expression of adhesion molecules have also been observed. Polarized human Th1 cells have greater amounts of CD18 than do Th2 cells (10), but whether this quantitative difference increases their ability to interact with endothelium is not known. Murine T cells polarized in vitro toward Th1 or Th2 phenotypes express P-selectin glycoprotein ligand 1 (PSGL-1). However, only PSGL-1 on Th1 cells is competent to bind to P-selectin on endothelium (7). A greater ability to bind to both E- and P-selectin accounts for the selective homing of these Th1 cells, as opposed to Th2 lymphocytes, to sites of inflammation when injected intravenously into syngeneic mice (1).
It is uncertain, however, whether Th1 and Th2 cells that arise from differentiation in vivo differ as distinctly in their ability to bind selectins (33) or the chemokine receptors that they express (25) as those generated in vitro. Differences between T cells polarized in vitro and in vivo may explain the discrepancy between our results and those of Biernacki et al. (5), who observed that human Th2 cells generated in vitro migrate across human brain endothelial cells and HUVEC to a greater extent than do their Th1 counterparts.
The potential importance of both chemoattractants and adhesion molecules in recruitment of specific subsets of T lymphocytes is underscored by a recent report from Katakai et al. (20). In this study, CD4+ T cells were isolated from spleens of mice with autoimmune gastritis and allowed to migrate across layers of the F-2 line of transformed murine endothelial cells. In agreement with our results, populations that migrated were enriched for IFN-γ-secreting cells but not IL-4-secreting cells. Migration of Th1 cells was only partially suppressed by pertussis toxin, which should block responses of the lymphocytes to chemokines and other chemoattractants that signal through Gαi-dependent pathways. The authors therefore suggest that interactions between leukocytic and endothelial adhesion molecules might provide additional signals that specifically stimulate the transendothelial migration of the type 1 T lymphocytes.
The enrichment of specific subpopulations of T lymphocytes in involved tissues is critical in the pathogenesis of particular diseases due to the effector functions that are elicited (12, 28, 32). The results presented here suggest that accumulation of type 1 T lymphocytes in the perivascular tissues may be controlled, at least in part, at the level of extravasation. Understanding the mechanisms involved in this regulation may contribute to the development of new therapies to treat diseases by altering the balance between type 1 and type 2 T-lymphocyte responses (32).
Acknowledgments
This work was supported by Public Health Service grant AI47313 from the National Institute of Allergy and Infectious Diseases and grants from the National Office and Long Island chapter of the Arthritis Foundation.
We thank Roy Lobb and Richard Coughlin for generous gifts of reagents, James Rohlf and Raymond Mugno for expert help with statistics, Christopher Pullis and Corinne Leombruno for performing flow cytometry, and Jorge Benach, Howard Fleit, Richard Kew, and Amitabha Mazumder for helpful advice and critical review of the manuscript.
Editor: D. L. Burns
REFERENCES
- 1.Austrup, F., D. Vestweber, E. Borges, M. Löhning, R. Bräuer, U. Herz, H. Renz, R. Hallmann, A. Scheffold, A. Radbruch, and A. Hamann. 1997. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflamed tissues. Nature 385:81-83. [DOI] [PubMed] [Google Scholar]
- 2.Beckman, I., K. Shepherd, K. Dimopoulos, M. Ahern, F. Firgaira, and J. Bradley. 1994. Differential expression and regulation of cytokine mRNAs in normal human CD45R T cell subsets. Cytokine 6:116-123. [DOI] [PubMed] [Google Scholar]
- 3.Benach, J. L., E. M. Bosler, J. P. Hanrahan, J. L. Coleman, G. S. Habicht, T. F. Bast, D. J. Cameron, J. L. Ziegler, A. G. Barbour, W. Burgdorfer, R. Edelman, and R. A. Kaslow. 1983. Spirochetes isolated from the blood of two patients with Lyme disease. N. Engl. J. Med. 308:740-742. [DOI] [PubMed] [Google Scholar]
- 4.Berner, B., D. Akca, T. Jung, G. A. Muller, and M. A. Reuss-Borst. 2000. Analysis of Th1 and Th2 cytokines expressing CD4+ and CD8+ T cells in rheumatoid arthritis by flow cytometry. J. Rheumatol. 27:1128-1135. [PubMed] [Google Scholar]
- 5.Biernacki, K., A. Prat, M. Blain, and J. P. Antel. 2001. Regulation of Th1 and Th2 lymphocyte migration by human adult brain endothelial cells. J. Neuropathol. Exp. Neurol. 60:1127-1136. [DOI] [PubMed] [Google Scholar]
- 6.Bonecchi, R., G. Bianchi, P. P. Bordignon, D. D'Ambrosio, R. Lang, A. Borsatti, S. Sozzani, P. Allavena, P. A. Gray, A. Mantovani, and F. Sinigaglia. 1998. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J. Exp. Med. 187:129-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borges, E., W. Tietz, M. Steegmaier, T. Moll, R. Hallmann, A. Hamann, and D. Vestweber. 1997. P-selectin glycoprotein ligand-1 (PSGL-1) on T helper 1 but not on T helper 2 cells binds to P-selectin and supports migration into inflamed skin. J. Exp. Med. 185:573-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burns, M. J., and M. B. Furie. 1998. Borrelia burgdorferi and interleukin-1 promote the transendothelial migration of monocytes in vitro by different mechanisms. Infect. Immun. 66:4875-4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carter, L. L., and R. W. Dutton. 1996. Type 1 and type 2: a fundamental dichotomy for all T-cell subsets. Curr. Opin. Immunol. 8:336-342. [DOI] [PubMed] [Google Scholar]
- 10.Clissi, B., D. D'Ambrosio, J. Geginat, L. Colantonio, A. Morrot, N. W. Freshney, J. Downward, F. Sinigaglia, and R. Pardi. 2000. Chemokines fail to up-regulate β1 integrin-dependent adhesion in human Th2 T lymphocytes. J. Immunol. 164:3292-3300. [DOI] [PubMed] [Google Scholar]
- 11.D'Ambrosio, D., A. Iellem, L. Colantonio, B. Clissi, R. Pardi, and F. Sinigaglia. 2000. Localization of Th-cell subsets in inflammation: differential thresholds for extravasation of Th1 and Th2 cells. Immunol. Today 21:183-186. [DOI] [PubMed] [Google Scholar]
- 12.D'Elios, M., and G. Del Prete. 1998. Th1/Th2 balance in human disease. Transplant. Proc. 30:2373-2377. [DOI] [PubMed] [Google Scholar]
- 13.Dixon, A. E., J. B. Mandac, P. J. Martin, R. C. Hackman, D. K. Madtes, and J. G. Clark. 2000. Adherence of adoptively transferred alloreactive Th1 cells in lung: partial dependence on LFA-1 and ICAM-1. Am. J. Physiol. Lung Cell Mol. Physiol. 279:L583-L591. [DOI] [PubMed] [Google Scholar]
- 14.Furie, M. B., E. B. Cramer, B. L. Naprstek, and S. C. Silverstein. 1984. Cultured endothelial cell monolayers that restrict the transendothelial passage of macromolecules and electrical current. J. Cell Biol. 98:1033-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gergel, E. I., and M. B. Furie. 2001. Activation of endothelium by Borrelia burgdorferi in vitro enhances transmigration of specific subsets of T lymphocytes. Infect. Immun. 69:2190-2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gross, D. M., A. C. Steere, and B. T. Huber. 1998. T helper 1 response is dominant and localized to the synovial fluid in patients with Lyme arthritis. J. Immunol. 160:1022-1028. [PubMed] [Google Scholar]
- 17.Huang, A. J., M. B. Furie, S. C. Nicholson, J. Fischbarg, L. S. Liebovitch, and S. C. Silverstein. 1988. Effects of human neutrophil chemotaxis across human endothelial cell monolayers on the permeability of these monolayers to ions and macromolecules. J. Cell Physiol. 135:355-366. [DOI] [PubMed] [Google Scholar]
- 18.Issekutz, T. B. 1993. Dual inhibition of VLA-4 and LFA-1 maximally inhibits cutaneous delayed-type hypersensitivity-induced inflammation. Am. J. Pathol. 143:1286-1293. [PMC free article] [PubMed] [Google Scholar]
- 19.Ito, N., H. Nakamura, Y. Tanaka, and S. Ohgi. 1999. Lung carcinoma: analysis of T helper type 1 and 2 cells and T cytotoxic type 1 and 2 cells by intracellular cytokine detection with flow cytometry. Cancer 85:2359-2367. [PubMed] [Google Scholar]
- 20.Katakai, T., T. Hara, M. Sugai, H. Gonda, Y. Nambu, E. Matsuda, Y. Agata, and A. Shimizu. 2002. Chemokine-independent preference for T-helper-1 cells in transendothelial migration. J. Biol. Chem. 277:50948-50958. [DOI] [PubMed] [Google Scholar]
- 21.Kawai, T., M. Seki, K. Hiromatsu, J. W. Eastcott, G. F. Watts, M. Sugai, D. J. Smith, S. A. Porcelli, and M. A. Taubman. 1999. Selective diapedesis of Th1 cells induced by endothelial cell RANTES. J. Immunol. 163:3269-3278. [PubMed] [Google Scholar]
- 22.Lichtman, A. H., and A. K. Abbas. 1997. T-cell subsets: recruiting the right kind of help. Curr. Biol. 7:R242-R244. [DOI] [PubMed] [Google Scholar]
- 23.Marfaing-Koka, A., O. Devergne, G. Gorgone, A. Portier, T. J. Schall, P. Galanaud, and D. Emilie. 1995. Regulation of the production of the RANTES chemokine by endothelial cells. Synergistic induction by IFN-γ plus TNF-α and inhibition by IL-4 and IL-13. J. Immunol. 154:1870-1878. [PubMed] [Google Scholar]
- 24.Mosmann, T. R., and S. Sad. 1996. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol. Today 17:138-146. [DOI] [PubMed] [Google Scholar]
- 25.Nanki, T., and P. E. Lipsky. 2000. Lack of correlation between chemokine receptor and Th1/Th2 cytokine expression by individual memory T cells. Int. Immunol. 12:1659-1667. [DOI] [PubMed] [Google Scholar]
- 26.Ostermann, G., K. S. Weber, A. Zernecke, A. Schröder, and C. Weber. 2002. JAM-1 is a ligand of the β2 integrin LFA-1 involved in transendothelial migration of leukocytes. Nat. Immunol. 3:151-158. [DOI] [PubMed] [Google Scholar]
- 27.Randolph, G. J., and M. B. Furie. 1995. A soluble gradient of endogenous monocyte chemoattractant protein-1 promotes the transendothelial migration of monocytes in vitro. J. Immunol. 155:3610-3618. [PubMed] [Google Scholar]
- 28.Romagnani, S., P. Parronchi, M. M. D'Elios, P. Romagnani, F. Annunziato, M. P. Piccinni, R. Manetti, S. Sampognaro, C. Mavilia, M. De Carli, E. Maggi, and G. F. Del Prete. 1997. An update on human Th1 and Th2 cells. Int. Arch. Allergy Immunol. 113:153-156. [DOI] [PubMed] [Google Scholar]
- 29.Sallusto, F., and A. Lanzavecchia. 2000. Understanding dendritic cell and T-lymphocyte traffic through the analysis of chemokine receptor expression. Immunol. Rev. 177:134-140. [DOI] [PubMed] [Google Scholar]
- 30.Sellati, T. J., M. J. Burns, M. A. Ficazzola, and M. B. Furie. 1995. Borrelia burgdorferi upregulates expression of adhesion molecules on endothelial cells and promotes transendothelial migration of neutrophils in vitro. Infect. Immun. 63:4439-4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimizu, Y., W. Newman, Y. Tanaka, and S. Shaw. 1992. Lymphocyte interactions with endothelial cells. Immunol. Today 13:106-112. [DOI] [PubMed] [Google Scholar]
- 32.Spellberg, B., and J. E. Edwards, Jr. 2001. Type 1/Type 2 immunity in infectious diseases. Clin. Infect. Dis. 32:76-102. [DOI] [PubMed] [Google Scholar]
- 33.Tietz, W., Y. Allemand, E. Borges, D. von Laer, R. Hallmann, D. Vestweber, and A. Hamann. 1998. CD4+ T cells migrate into inflamed skin only if they express ligands for E- and P-selectin. J. Immunol. 161:963-970. [PubMed] [Google Scholar]
- 34.Yin, Z., J. Braun, L. Neure, P. Wu, U. Eggens, A. Krause, T. Kamradt, and J. Sieper. 1997. T cell cytokine pattern in the joints of patients with Lyme arthritis and its regulation by cytokines and anticytokines. Arthritis Rheum. 40:69-79. [DOI] [PubMed] [Google Scholar]
- 35.Yssel, H., M. C. Shanafelt, C. Soderberg, P. V. Schneider, J. Anzola, and G. Peltz. 1991. Borrelia burgdorferi activates a T helper type 1-like T cell subset in Lyme arthritis. J. Exp. Med. 174:593-601. [DOI] [PMC free article] [PubMed] [Google Scholar]