Abstract
In cattle and other ruminants, infection with the intracellular pathogen Mycobacterium avium subsp. paratuberculosis results in a granulomatous enteritis (Johne's disease) that is often fatal. The key features of host immunity to M. avium subsp. paratuberculosis infection include an appropriate early proinflammatory and cytotoxic response (Th1-like) that eventually gives way to a predominant antibody-based response (Th2-like). Clinical disease symptoms often appear subsequent to waning of the Th1-like immune response. Understanding why this shift in the immune response occurs and the underlying molecular mechanisms involved is critical to future control measures and diagnosis. Previous studies have suggested that M. avium subsp. paratuberculosis may suppress gene expression in peripheral blood mononuclear cells (PBMCs) from infected cows, despite a continued inflammatory reaction at sites of infection. In the present study, we tested the hypothesis that exposure to M. avium subsp. paratuberculosis suppresses a proinflammatory gene expression pattern in PBMCs from infected cows. To do this, we examined expression of genes encoding interleukin-1α (IL-1α), IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12p35, IL-16, and IL-18, as well as genes encoding gamma interferon (IFN-γ), transforming growth factor β (TGF-β), and tumor necrosis factor alpha (TNF-α), in PBMCs, intestinal lesions, and mesenteric lymph nodes of cattle naturally infected with M. avium subsp. paratuberculosis. Cytokine gene expression in these cells and tissues was compared to expression in similar cells and tissues from control uninfected cattle. Our comprehensive results demonstrate that for most cytokine genes, including the genes encoding IFN-γ, TGF-β, TNF-α, IL-1α, IL-4, IL-6, IL-8, and IL-12p35, differential expression in PBMCs from infected and control cattle did not require stimulation with M. avium subsp. paratuberculosis. In fact, stimulation with M. avium subsp. paratuberculosis tended to reduce the differential expression observed in infected and uninfected cows for genes encoding IFN-γ, IL-1α, and IL-6. Only IL-10 gene expression was consistently enhanced by M. avium subsp. paratuberculosis stimulation of PBMCs from subclinically infected cattle. In ileal tissues from M. avium subsp. paratuberculosis-infected cattle, expression of the genes encoding IFN-γ, TGF-β, IL-5, and IL-8 was greater than the expression in comparable tissues from control uninfected cattle, while expression of the gene encoding IL-16 was lower in tissues from infected cattle than in control tissues. Mesenteric lymph nodes draining sites of M. avium subsp. paratuberculosis infection expressed higher levels of IL-1α, IL-8, IL-2, and IL-10 mRNA than similar tissues from control uninfected cattle expressed. In contrast, the genes encoding TGF-β and IL-16 were expressed at lower levels in lymph nodes from infected cattle than in tissues from uninfected cattle. Taken together, our results suggest that cells or other mechanisms capable of limiting proinflammatory responses to M. avium subsp. paratuberculosis develop in infected cattle and that a likely place for development and expansion of these cell populations is the mesenteric lymph nodes draining sites of infection.
Johne's disease is caused by the facultative intracellular bacterium Mycobacterium avium subsp. paratuberculosis. Although disease associated with M. avium subsp. paratuberculosis infection is largely restricted to ruminant species, this organism has been implicated as a casual or exacerbating agent in human Crohn's disease (9, 32) and can infect other monogastric species (3, 10).
In ruminants, infections with M. avium subsp. paratuberculosis can persist in a subclinical state for several years (24, 27, 30, 42). Although intestinal lesions develop during the subclinical period of infection, there are few outward signs of disease. Lesions associated with M. avium subsp. paratuberculosis infection are often diffuse and granulomatous and are typically restricted to the ileum and particularly to the ileocecal valve region of the small intestine (48). Pathogenesis associated with M. avium subsp. paratuberculosis infection is in large part due to a severe immune pathology and chronic inflammation (8, 37, 43). Lymph nodes draining sites of M. avium subsp. paratuberculosis infection often exhibit hyperplasia with enhanced numbers of T cells and infiltrating macrophages (8, 33). Large numbers of acid-fast bacilli can often be found in infected ileal tissues and associated lymph nodes, predominantly associated with macrophages.
Following the initial exposure to M. avium subsp. paratuberculosis, an appropriate T cell response, characterized by the release of proinflammatory cytokines, such as gamma interferon (IFN-γ), interleukin-1α (IL-1α), and IL-6, as well as by production of IL-2, develops (for reviews see references 4, 12, and 15). However, loss of this early proinflammatory and cytotoxic or Th1-like response and development of a Th2-like response, characterized by production of immunoglobulin G1 antibodies (for reviews see references 15, 18, and 39), are often associated with progression to clinical disease. The mechanisms responsible for the shift from Th1-like to Th2-like immune responses to M. avium subsp. paratuberculosis are unknown but may be related to unknown host genetic factors, to constant exposure of immune cells to antigen presented by infected macrophages, or to development of antigen-specific or general regulatory cell populations. Whatever the underlying cause, changes in the immune responses to M. avium subsp. paratuberculosis likely involve altered expression of various cytokines (39).
In several recent reports the authors described the patterns of expression of a limited number of cytokines (IFN-γ, IL-1α, and IL-6) in either peripheral blood mononuclear cells (PBMCs), lesions, or mesenteric lymph nodes of M. avium subsp. paratuberculosis-infected cattle and sheep (4, 7, 23, 25, 28, 35, 38, 40). Although important in providing a framework for future work, these studies generally did not provide a comprehensive overview of cytokine gene expression in M. avium subsp. paratuberculosis-infected animals. Our specific goal in the present study was to test the hypothesis that M. avium subsp. paratuberculosis can suppress proinflammatory cytokine gene expression in PBMCs from infected cows. In addition, we wished to provide a link between observations made with peripheral immune cells and sites of M. avium subsp. paratuberculosis infection and lymph nodes draining these sites. To do this, we developed and validated real-time quantitative reverse transcriptase PCR (Q-RT-PCR) assays for 14 bovine cytokine transcripts. These tools were used to study expression of all 14 cytokine genes in PBMCs, intestinal lesions, and mesenteric lymph nodes from cattle naturally infected with M. avium subsp. paratuberculosis.
MATERIALS AND METHODS
Experimental animals.
The subclinically infected and control cattle used in this study were Holstein cows that ranged in age from 24 to 48 months and were housed in the same commercial dairy operation. Clinically infected cows were obtained from a second commercial dairy operation in which Johne's disease-positive cows were isolated in a separate herd and kept in production until clinical symptoms appeared. All clinically infected cows exhibited chronic diarrhea and various degrees of emaciation. The immune status of all study animals with regard to M. avium subsp. paratuberculosis infection was confirmed by a serum enzyme-linked immunosorbent assay (ELISA) (IDEXX Herdcheck; IDEXX Laboratories, Inc., Westbrook, Maine) and by IFN-γ testing with a commercial assay (BioCor, Inc., Des Moines, Iowa) or by real-time Q-RT-PCR (P. Coussens, C. Colvin, J. M. G. Rosa, J. Perez-Laspuir, and M. Elftman, submitted for publication). Fecal culture testing by a U. S. Department of Agriculture-approved testing laboratory (Michigan State University Animal Health Diagnostic Laboratory, East Lansing) was conducted to confirm the infection status.
The control uninfected animals (n = 4) had shown negative responses in all tests for more than 3 years of periodic testing. Subclinically M. avium subsp. paratuberculosis-infected cows (n = 6) were strongly positive as determined by the serum ELISA (IDEXX value, >5) during the entire testing period, exhibited occasional diarrhea, and were all fecal culture positive (5 to 25 CFU/g of feces). Tests for IFN-γ in subclinically infected cows were strongly positive when results were analyzed as recommended by the manufacturer (BioCor, Inc.). Clinically infected cows (n = 4) shed >100 CFU of M. avium subsp. paratuberculosis per g of feces and were ELISA positive and IFN-γ negative. All animal housing, sampling, and euthanasia employed in this study were approved by the Michigan State University All University Animal Use and Care Committee.
Preparation of PBMCs and tissue collection.
Blood samples were obtained from all animals via the coccygeal (tail) vein by using 2.5-cm 21-gauge multiple-sample needles and a series of 10 8-ml Vacutainer tubes containing acid-citrate dextrose as an anticoagulant (BD Vacutainer, Rutherford, N.J.). PBMCs were prepared as previously described (14, 45, 50). PBMCs from each cow were split into two equal aliquots; one aliquot was used for stimulation with M. avium subsp. paratuberculosis, and the other aliquot was used for phosphate-buffered saline (PBS) (nil) treatment. Stimulation of PBMCs was performed by using 106 live M. avium subsp. paratuberculosis bacteria per ml of medium added in 200 μl of PBS. Nil stimulation was performed by adding 200 μl of PBS alone. All PBMCs were then incubated at 37°C for 16 to 18 h in complete RPMI 1640 (16) without antibiotics in a humidified atmosphere consisting of 95% air and 5% CO2. This protocol was selected because overnight stimulation with antigen is standard in IFN-γ testing and results in significant production of this cytokine (12). In a typical protocol, each treatment (nil and M. avium subsp. paratuberculosis stimulation) was applied to two or three 75-cm2 flasks per cow, and each flask contained approximately 2 × 107 PBMCs.
Samples of ileum and mesenteric lymph nodes were obtained either at the time of slaughter in a commercial abattoir (control cows) or immediately following euthanasia (infected cows) with an overdose of sodium pentabarbitol (85 mg/kg). All infected animals (n = 4 to 6, depending upon the cytokine) exhibited thickening of the ileal mucosa, convolution of the interior of the ileum, and enlargement of adjacent draining lymph nodes. All control animals (n = 3) had a healthy ileum and mesenteric lymph nodes with no visible pathological signs of Johne's disease. Three individual sections (∼10 g each) of ileal tissue were harvested from different regions immediately adjacent (within 20 cm) to the ileal-cecal junction in each infected and control cow. For each infected cow, three separate lymph nodes draining sites of inflammation were collected. Individual tissues were divided in two for RNA extraction and future histological examination. All tissues used for RNA extraction were immediately placed into 50-ml conical tubes containing RNAlater (Ambion Corp, Austin, Tex.) and frozen in liquid nitrogen for storage at −80°C until they were used.
RNA extraction.
RNA was extracted from nil- and M. avium subsp. paratuberculosis-stimulated PBMCs by using Trizol reagent (Invitrogen Life Technologies Corp., Carlsbad, Calif.) as previously described (14, 50). Ileal tissues and lymph nodes were removed from storage at −80°C, and ∼2 g of each tissue sample from each infected and control cow was combined within cow and ground into a powder with a sterile RNase-free mortar and pestle in liquid nitrogen. RNA was extracted from the powdered tissues by using Trizol reagent (Invitrogen Life Technologies Corp.) essentially as recommended by the manufacturer. All RNA samples were treated with RNase-free DNase I (Promega Corp., Madison, Wis.). The quality and quantity of extracted total RNA were estimated by UV spectrophotometry and electrophoresis on 1.0% native agarose gels.
Real-time Q-RT-PCR.
Real-time Q-RT-PCRs were performed by using an Applied Biosystems 7000 DNA sequence detection system (Perkin-Elmer Corp., Foster City, Calif.). Total RNA extracted from M. avium subsp. paratuberculosis- and nil-stimulated PBMCs or tissue samples was converted into first-strand cDNA by adding 2 μg of total RNA to a 12-μl reaction mixture containing 10 mM Oligo(dT)15-18 primer. Following 5 min of incubation at 70°C, the reaction mixture was quick chilled to 20°C and modified by addition of 4 μl of 5× buffer supplied by the reverse transcriptase manufacturer (the final reagent concentrations were 50 mM Tris-HCl [pH 8.3], 75 mM KCl, and 3 mM MgCl2), each deoxynucleoside triphosphate at a concentration of 1 mM, 200 U of Superscript II RNase H− reverse transcriptase (Invitrogen Life Technologies Corp.), and dithiothreitol (final concentration, 10 mM); the total reaction mixture volume was 20 μl. The reverse transcription reaction was allowed to proceed at 42°C for 60 min, and then the mixture was heated to 70°C for 15 min and cooled to 37°C prior to addition of 2 U of DNase-free RNase H (Invitrogen Life Technologies Corp.). The mixture was then incubated at 37°C for 20 min in the presence of RNase H to remove the original RNA templates. The RNase H was subsequently inactivated by heating the mixture at 70°C for 10 min. First-strand cDNAs were purified by extraction with Quick-Clean resin (BD Biosciences, Inc., Alameda, Calif.) and precipitation in ethanol. Final cDNA pellets were suspended in 52 μl of RNase-free double-distilled H2O. The concentration of cDNA in each sample was determined by UV spectrophotometry and adjusted with RNase-free double-distilled H2O to a final working concentration of 10 ng per μl. All cDNA dilutions were stored at −80°C until they were used in Q-RT-PCRs.
Q-RT-PCR was performed by using SYBR Green PCR master mixture (Perkin-Elmer Corp.), 20 ng of template cDNA, and gene-specific primers. All primers were designed by using Primer Express software (Perkin-Elmer Corp.) and were synthesized by a commercial facility (Operon Technologies, Alameda, Calif.). Sequences used for primer design were obtained from public databases (GenBank, National Center for Biotechnology Information) as full-length cDNA sequences when available. When no bovine cDNA sequence was available, expressed sequence tag (EST) sequences derived from the Bos taurus gene index from The Institute for Genome Research and corresponding to the target cytokine (based in most cases on similarity to the corresponding human or mouse cytokine sequences) were used. The primer sequences are listed in Table 1 and posted at www.nbfgc.msu.edu along with additional information, including the expected melting temperatures of products and appropriate primer concentration ratios.
TABLE 1.
Primers used for Q-RT-PCR of bovine cytokines and for generation of standards
| Gene | Primera | Sequence | Primer for standard | Standard size (bp) |
|---|---|---|---|---|
| IFN-γ | F | TGGAGG ACTTCA AAAAGC TGATT | AGCCAA ATTGTC TCCTTC TACTTC | 261 |
| IFN-γ | R | TTTATG GCTTTG CGCTGG AT | CTGACT TCTCTT CCGCTT TCTG | |
| IL-1α | F | TTGGTG CACATG GCAAGT G | GAACGA CGCCCT CAATCA AA | 475 |
| IL-1α | R | GCACAG TCAAGG CTATTT TTCCA | GGGCTC GACTCC TTCAT | |
| IL-1β | F | GCCTTCAATAACTGTGGAACCAAT | NDb | ND |
| IL-1β | R | GTATATTTCAGGCTTGGTGAAAGGA | ND | |
| IL-2 | F | CCTCAA CTCCTG CCACAA TGTA | CCTCAA CTCCTG CCACAA TGTA | 376 |
| IL-2 | R | GTTTGC AACGAG TGCAAG AGTTA | CCCTGT AGTTCC AAAACG ATTCTC | |
| IL-4 | F | GCCACA CGTGCT TGAACA AA | CATTGT TAGCGT CTCCTG GTA | 403 |
| IL-4 | R | TGCTTG CCAAGC TGTTGA GA | GCTCGT CTTGGC TTCATT C | |
| IL-5 | F | TGGTGG CAGAGA CCTTGA CA | CTGGTG GCAGAG ACCTTG ACACT | 320 |
| IL-5 | R | GAATCA TCAAGT TCCCAT CACCTA | CAACTT TCCATT GTCCAC TCTGTG | |
| IL-6 | F | GGCTCC CATGAT TGTGGT AGTT | AGCGCA TGGTCG ACAAAA TCT | 523 |
| IL-6 | R | GCCCAG TGGACA GGTTTC TG | GCCCAG TGGACA GGTTTC TG | |
| IL-8 | F | GGAAAA GTGGGT GCAGAA GGT | CTAAAC CCCAAG GAAAAG TG | 443 |
| IL-8 | R | GGTGGT TTTTTC TTTTTC ATGGA | ACCAAG GCGCAG TTCAAC AG | |
| IL-10 | F | CTTGTC GGAAAT GATCCA GTTTT | ND | ND |
| IL-10 | R | TCAGGC CCGTGG TTCTCA | ND | |
| IL-12p35 | F | GCCACG GTATAG AGGATT TTCCT | CTTTCT TCAAAT GCAGCA TTGG | 271 |
| IL-12p35 | R | GGGTCT GGGTGA TACAAC GAA | GGGTCT GGGTGA TACAAC GAA | |
| IL-16 | F | GAGGGC GGTCCC AGAAGT | GGACCT CACGCG GTTTGA | 345 |
| IL-16 | R | CTCTCT AGATGC AGTCTG TCGTTT GT | CTCTCT AGATGC AGTCTG TCGTTT GT | |
| IL-18 | F | GAAAAT GATGAA GACCTG GAATCA | GAAAAT GATGAA GACCTG GAATCA | 352 |
| IL-18 | R | AACTTG GTCATT CAATT TCGTAT GA | GTCCTG GAACAC TTCTTT GA | |
| TGF-β1 | F | CTGAGC CAGAGG CGGACT AC | CTGAGC CAGAGG CGGACT AC | 262 |
| TGF-β1 | R | TGCCGT ATTCCA CCATTA GCA | TTGCTG AGGTAG CGCCAG GAATTG | |
| TNF-α | F | CGGTGG TGGGAC TCGTAT G | CAAGTA ACAAGC CGGTAG CC | 352 |
| TNF-α | R | CTGGTT GTCTTC CAGCTT CACA | TGGAAG ACTCCT CCCTGG TA |
F, forward; R, reverse.
ND, not done.
All reactions were performed in duplicate, and Q-RT-PCR data were analyzed by using the 2−(ΔΔCt) method as described previously (26). To assess the effect of stimulation (M. avium subsp. paratuberculosis stimulation versus nil stimulation) on cytokine expression in PBMCs within an animal, the β-actin gene was used as the control gene (for calculation of ΔCt) and nil-stimulated samples for each animal were used as the calibrator (for calculation of ΔΔCt). To assess differential gene expression between infection groups and between tissues, the β-actin gene was used as the control gene and the appropriate mean control cow value was used as the calibrator.
RESULTS
Development of Q-RT-PCR primers and standards for bovine cytokines.
For each cytokine monitored in this study, a set of primers compatible with Q-RT-PCR detection was constructed. An additional primer set was chosen from the Primer Express software output file, such that amplification with this primer set produced an amplification product that entirely contained sequences corresponding to the Q-RT-PCR products and that was large enough (250 to 600 bp) to isolate by agarose gel electrophoresis and to purify by standard methods (45). The larger fragments could then serve as standards in assessing the linearity of Q-RT-PCR detection for each cytokine. In addition, these standards could be used in cases where absolute quantitation (number of mRNA molecules) was required. All selected Q-RT-PCR primers, standard primers, and standard sizes are listed in Table 1. Standard products for most cytokines (except IL-10 and IL-1β) used in the present study were purified, and the resulting products were assessed by agarose gel electrophoresis (Fig. 1). Q-RT-PCR analysis performed by using amounts of standard cytokine products ranging from 0.025 to 50 pg demonstrated that these assays were linear over a broad range of potential cytokine transcript concentrations (Fig. 2).
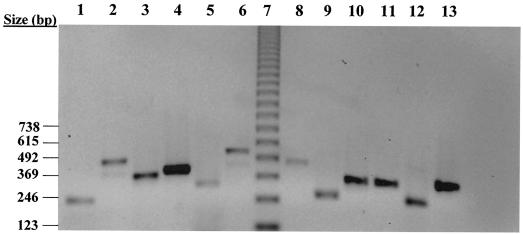
FIG. 1.
Agarose gel electrophoresis of cytokine Q-RT-PCR product standards. Standard products were amplified by Q-RT-PCR as described in Materials and Methods. Following purification with a Wizard PCR purification system (Promega Corp.), products were electrophoresed through a 1.5% agarose gel containing Tris-acetate-EDTA buffer and 10 μg of ethidium bromide per ml at 100 V for 60 min. Isolated products were visualized and photographed under UV illumination. The image is contrast inverted. Lane 1, IFN-γ; lane 2, IL-1α; lane 3, IL-2; lane 4, IL-4; lane 5, IL-5; lane 6, IL-6; lane 7, 2 μg of 123-bp ladder; lane 8, IL-8; lane 9, IL-12p35; lane 10, IL-16; lane 11, IL-18; lane 12, TGF-β1; lane 13, TNF-α.
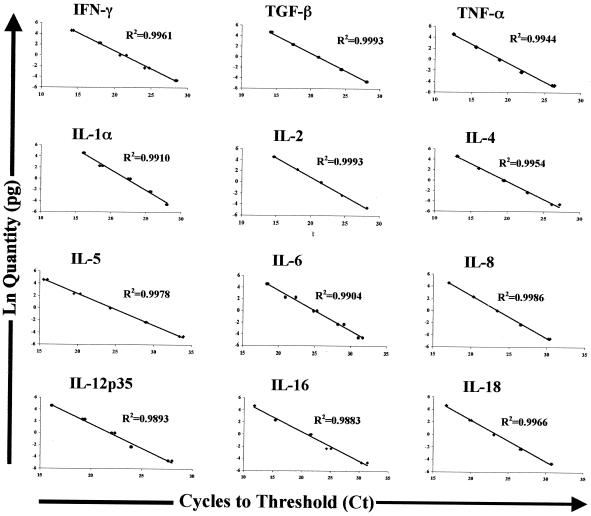
FIG. 2.
Linearity of Q-RT-PCR measurement of bovine cytokine cDNA. Cytokine standard products purified as described in the legend to Fig. 1 were used to assess the linearity of Q-RT-PCR by using SYBR Green for detection of various bovine cytokine transcripts. Purified products were added to Q-RT-PCR mixtures at various levels ranging from 0.025 to 50 pg, and Q-RT-PCR was performed as described in Materials and Methods. Q-RT-PCR data were analyzed by regressing ln(known quantity) on cycles to threshold (Ct) for each cytokine. The cytokines monitored and the R2 value for each resulting regression line are indicated.
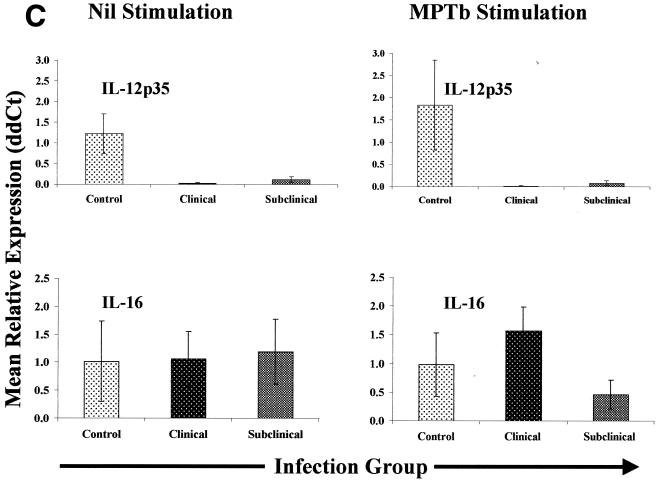
Expression of cytokine transcripts in nil- and M. avium subsp. paratuberculosis-stimulated PBMCs from Johne's disease-positive and control cows.
Q-RT-PCR monitoring of IFN-γ transcripts in PBMCs from infected cows revealed that for both clinically and subclinically infected cow PBMCs, the effect of 16 to 18 h of M. avium subsp. paratuberculosis stimulation on IFN-γ gene expression (effect of treatment) was relatively small compared to the effect of nil stimulation (less-than-twofold increase). The largest differences in IFN-γ gene expression were observed between nil-stimulated PBMCs from infected cows and similarly treated cells from controls (Fig. 3). In fact, when IFN-γ gene expression was compared between groups (infected cows versus control cows), PBMCs from all infected cows produced an overall mean of 147-fold more IFN-γ mRNA than PBMCs from the control group produced (no M. avium subsp. paratuberculosis stimulation). Nil-stimulated PBMCs from subclinically infected cows tended to produce more IFN-γ mRNA (the mean was 240-fold higher than the control mean) than PBMCs from clinically infected cows produced (the mean was 53-fold higher than the control mean) (Fig. 3). Although exposure to M. avium subsp. paratuberculosis tended to reduce differences in IFN-γ gene expression between infection groups, stimulated PBMCs from infected cows still produced an overall mean of 5.7-fold more IFN-γ mRNA than similarly treated cells from control cows produced. Taken together, results obtained with IFN-γ indicated that PBMCs from infected cows inherently produced significantly more IFN-γ mRNA than similar cells from control cows produced. Due to these novel observations with IFN-γ mRNA, we focused our attention regarding expression of other cytokines on comparisons within treatment groups (nil stimulation or M. avium subsp. paratuberculosis stimulation) and between infection groups (control versus subclinically and clinically infected).
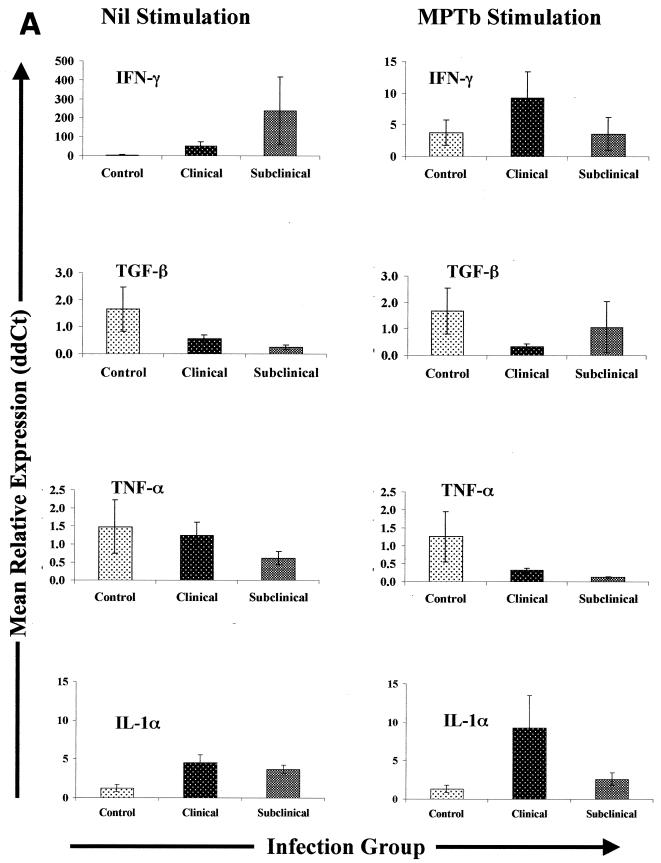
FIG. 3.
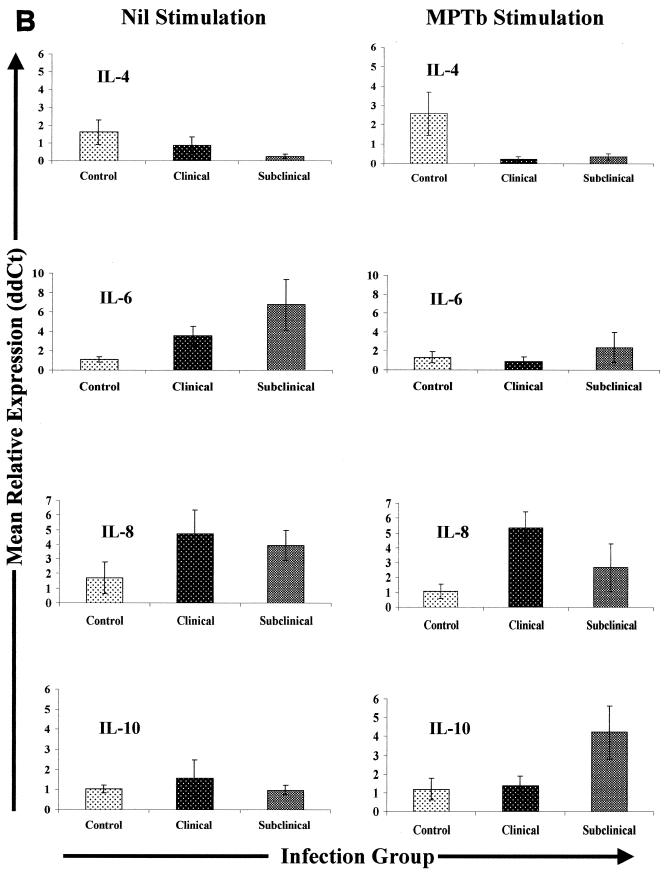
Cytokine gene expression in PBMCs from Johne's disease-positive and control cows. PBMCs were isolated and stimulated with either PBS (Nil) or M. avium subsp. paratuberculosis (MPTb) as described in Materials and Methods. RNA isolation and Q-RT-PCR were performedas described in Materials and Methods. Q-RT-PCR data were analyzed by using the 2−(ΔΔCt) method, with β-actin gene expression serving as a control for calculation of ΔCt values for each gene and the mean control value for each stimulation serving as the calibrator for final calculation of mean relative expression values (ddCt). Because the largest differences were observed between groups, data are presented so that they highlight between-group gene expression differences for each type of stimulation (nil or M. avium subsp. paratuberculosis). Likewise, data for each group of infected cows (subclinically infected and clinically infected) are displayed separately due to the significant differences (P > 0.05) in expression of some cytokine genes.
Transforming growth factor β (TGF-β) and tumor necrosis factor alpha (TNF-α) transcript levels were significantly lower (P < 0.05) in nil-stimulated PBMCs from clinically infected cows and subclinically infected cows (TGF-β only) than in similarly treated cells from controls. PBMCs from subclinically infected cows expressed significantly less TGF-β mRNA than cells from either controls or clinically infected cows expressed, and the difference between subclinically infected PBMCs and control cow PBMCs was highly significant (6.7-fold; P < 0.05). Stimulation with M. avium subsp. paratuberculosis had little or no effect on expression of TGF-β mRNA in PBMCs from control or clinically infected cows but had highly variable effects on TGF-β mRNA abundance in PBMCs from subclinically infected cows (Fig. 3). Stimulation of PBMCs from all infected cows with M. avium subsp. paratuberculosis significantly decreased TNF-α mRNA expression (P < 0.05) but had little effect on TNF-α gene expression in PBMCs from control cows (Fig. 3). Although curious, the failure to activate TNF-α gene expression suggests that the M. avium subsp. paratuberculosis preparations utilized in this study were not contaminated with endotoxin, as enhanced expression of TNF-α is a hallmark of immune cell stimulation by lipopolysaccharide or endotoxin and also occurs when cells from M. avium subsp. paratuberculosis-infected cattle are stimulated with lipopolysaccharide or endotoxin (P. M. Coussens, S. K. Chiang, and B. C. Tooker, unpublished observations).
Nil-stimulated PBMCs from both groups of infected cows expressed significantly more IL-1α mRNA than nil-stimulated cells from control cows expressed (P < 0.01). The mean relative levels of expression of IL-1α mRNA in nil-stimulated PBMCs from clinically and subclinically infected cows were 4.5- and 3.5-fold higher, respectively, than the levels of expression in similarly treated cells from controls (Fig. 3). The effect of M. avium subsp. paratuberculosis stimulation compared to nil stimulation was dependent upon the infection group. Stimulation with M. avium subsp. paratuberculosis had little or no effect on IL-1α mRNA expression in PBMCs from control cows but tended to increase IL-1α mRNA expression in PBMCs from clinically infected cows and to decrease IL-1α mRNA expression in PBMCs from subclinically infected cows. Thus, the difference between the levels of IL-1α mRNA expression in PBMCs from clinically and subclinically infected cows exposed to M. avium subsp. paratuberculosis approached statistical significance (P = 0.09).
Expression of IL-4 mRNA was markedly different for different infection groups. IL-4 mRNA expression in nil-treated PBMCs from subclinically infected cows was significantly lower (P < 0.05) than IL-4 mRNA expression in PBMCs from either control or clinically infected cows (Fig. 3). Stimulation with M. avium subsp. paratuberculosis resulted in significantly lower expression of IL-4 mRNA in PBMCs from all infected cows than in similarly treated cells from controls (P < 0.05) and in reduced differences between PBMCs from clinically and subclinically infected cows.
IL-6 mRNA expression was significantly greater (P < 0.05) in nil-stimulated PBMCs from both clinically and subclinically infected cows than in similarly treated cells from controls. The mean level of expression of transcripts encoding IL-6 was 3.5-fold higher in nil-stimulated clinically infected cow PBMCs than in nil-stimulated cells from controls, while the mean level of IL-6 gene expression was more than 6.5-fold higher in nil-stimulated cells from subclinically infected cows than in similarly treated cells from controls. The difference in IL-6 mRNA expression between nil-stimulated PBMCs from clinically and subclinically infected cows was also statistically significant (P < 0.05). Stimulation with M. avium subsp. paratuberculosis tended to reduce differences in IL-6 mRNA expression, such that there were no statistically significant differences in IL-6 mRNA expression between the infection groups following stimulation with M. avium subsp. paratuberculosis (Fig. 3).
Expression of transcripts encoding IL-8 was significantly greater (mean, ∼4.7-fold; P < 0.05) in nil-stimulated PBMCs from clinically infected cows than in cells from controls. IL-8 gene expression was also greater (mean, ∼4-fold; P < 0.1) in nil-stimulated PBMCs from subclinically infected cows than in nil-stimulated cells from controls. Stimulation of PBMCs with M. avium subsp. paratuberculosis did not change this pattern of expression of IL-8 mRNA, although there was a slight increase in IL-8 expression in PBMCs from clinically infected cows and a slight decrease in PBMCs from subclinically infected cows when M. avium subsp. paratuberculosis-stimulated and nil-stimulated samples were compared. Overall, PBMCs from infected cows expressed approximately fourfold more IL-8 mRNA than cells from control cows expressed (P < 0.05), and this trend was not significantly affected by M. avium subsp. paratuberculosis stimulation.
Expression of IL-10 mRNA was not significantly different in nil-stimulated PBMCs from infected and control cows. However, following stimulation with M. avium subsp. paratuberculosis, the mean expression of IL-10 mRNA was more than fourfold greater in PBMCs from subclinically infected cows than in similarly treated cells from controls (P < 0.1). In contrast, stimulation of PBMCs from clinically infected cows had little or no effect on expression of IL-10 mRNA (Fig. 3). The difference between the effect of M. avium subsp. paratuberculosis stimulation on IL-10 mRNA expression in PBMCs from subclinically infected cows and the effect of nil stimulation was highly significant (P < 0.01).
Overall, PBMCs from infected cows expressed 13- and 20-fold less IL-12p35 mRNA than cells from control cows with nil stimulation and M. avium subsp. paratuberculosis stimulation expressed, respectively. The most significant difference was in PBMCs from clinically infected cows, in which the expression of IL-12p35 mRNA was 30-fold less than the expression in cells from control cows with nil stimulation and more than 54-fold less than the expression in cells from control cows with M. avium subsp. paratuberculosis stimulation (P < 0.05). A similar, although more subtle, effect was observed in PBMCs from subclinically infected cows, whose nil-stimulated cells expressed significantly less (8.6-fold less; P < 0.05) IL-12p35 mRNA than cells from controls expressed. Stimulation with M. avium subsp. paratuberculosis further enhanced this difference, and cells from subclinically infected cows expressed 12-fold less IL-12p35 mRNA than similarly treated cells from controls expressed (P < 0.05).
Expression of IL-16 was not statistically different for any of the infection groups when PBMCs were nil stimulated. However, following M. avium subsp. paratuberculosis stimulation, there was an increase in expression of IL-16 mRNA in PBMCs from clinically infected cows compared to the expression in cells from controls and there was a decrease in expression in PBMCs from subclinically infected cows compared to the expression in cells from controls. The combination of these effects made the difference in IL-16 expression between PBMCs from clinically and subclinically infected cows highly significant (P < 0.01) (Fig. 3).
Expression of cytokine transcripts in ileal tissues of Johne's disease-positive and control cows.
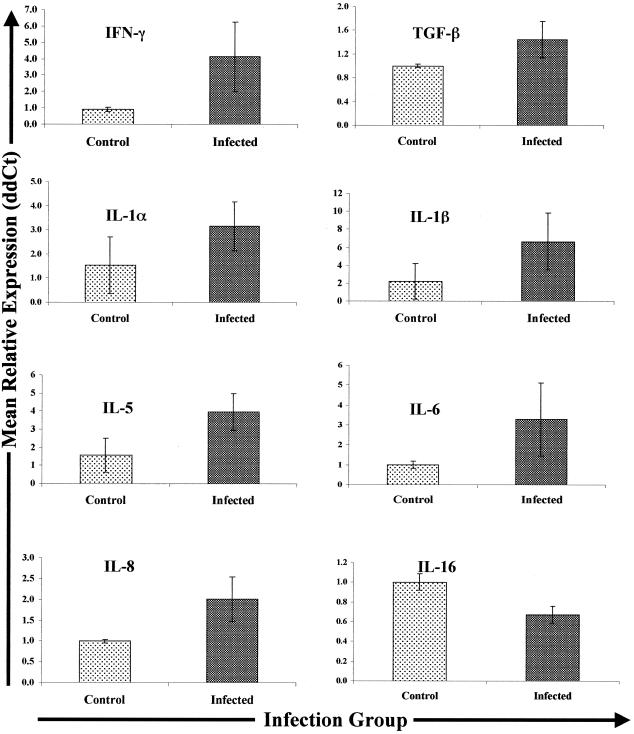
Expression of TNF-α, IL-2, IL-4, IL-10, and IL-18 transcripts was not significantly different in tissues from infected and control cows (data not shown). Despite variability within the infected group, ileal tissues from all infected cows showed enhanced expression of IFN-γ mRNA (expression was 1.7- to 14.6-fold higher than expression in control cow tissues). In fact, the relative IFN-γ expression in tissues from four of five individual infected cows was significantly greater (P < 0.05) than the mean relative IFN-γ expression in tissues from control cows. Overall, the mean expression of IFN-γ mRNA was fourfold greater in tissues from infected cows (n = 5) than in similar tissues from controls (Fig. 4).
FIG. 4.
Cytokine gene expression in ileal tissues from Johne's disease-positive and control cows. Tissues were collected, RNA was extracted, and Q-RT-PCR was performed as described in Materials and Methods. Q-RT-PCR data were analyzed by using the 2−(ΔΔCt) method, with β-actin gene expression serving as the control for calculation of ΔCt values for each gene within animal and the mean control value for each gene serving as the calibrator for final calculation of the mean relative expression values (ddCt). Only data for the genes exhibiting differential expression in the different infection groups (infected and control) are shown.
The relative expression of TGF-β mRNA was variable in ileal tissues from infected cows (n = 6). TGF-β mRNA was significantly more abundant (P < 0.05) in tissues from three of six individual infected cows than in tissues from controls (range, 1.3- to 2.9-fold more abundant). There was a modest increase in expression of TGF-β mRNA (1.3-fold increase) compared to expression in control tissues from a fourth cow, and two tissue samples from infected cows contained TGF-β mRNA levels similar to those in control cow tissues. Thus, while the mean expression of TGF-β mRNA was 1.5-fold greater in tissues from infected cows than in tissues from control cows (Fig. 4), the overall result was not statistically significant.
Similar to expression of IFN-γ mRNA, tissues from all infected cows exhibited enhanced expression of IL-1α mRNA compared to the expression in control cow tissues. The mean expression of IL-1α mRNA in tissues from infected cows was 3.2-fold greater than the expression in control cow tissues (P = 0.016). However, the expression of IL-1α was highly variable in tissues from infected cows and ranged from 1.2- to 8.0-fold greater than the mean expression in control cow tissues. A similar situation was observed for IL-1β transcripts; expression in all five infected cow tissue samples was greater than expression in control cow tissues (range, 1.3 to 22.2-fold greater; P = 0.031) (Fig. 4). Likewise, the mean expression of IL-5 transcripts was 3.9-fold greater in tissues from infected cows than in tissues from controls, and despite variability in tissues from the infected group, the results approached statistical significance (P = 0.107).
Three of five infected cow tissue samples showed significantly enhanced expression of IL-6 mRNA compared to expression in control cow tissues (range, 2.2- to 12.2-fold greater; P < 0.05). However, this was not consistent across all cows as tissue samples from one infected cow expressed levels of IL-6 mRNA similar to those in control cow tissues and tissues from another infected cow expressed almost twofold less IL-6 mRNA than control cow tissues expressed. Thus, although the mean relative expression of IL-6 transcripts in tissues from infected cows was more than threefold greater than the mean relative expression in control cow tissues, animal-to-animal variation reduced the statistical significance of these results (Fig. 4).
The mean relative expression of IL-8 mRNA was significantly greater (>2-fold greater; P < 0.05) in ileal tissues from all infected cows tested (n = 5) than in control cow tissues (range, 1.6- to 4.0-fold greater) (Fig. 4). In contrast, the mean relative levels of expression of both IL-12p35 mRNA (∼2-fold lower; P = 0.101) and IL-16 mRNA (∼1.5-fold lower; P < 0.05) were lower in ileal tissues from infected cows than in tissues from control cows (Fig. 4).
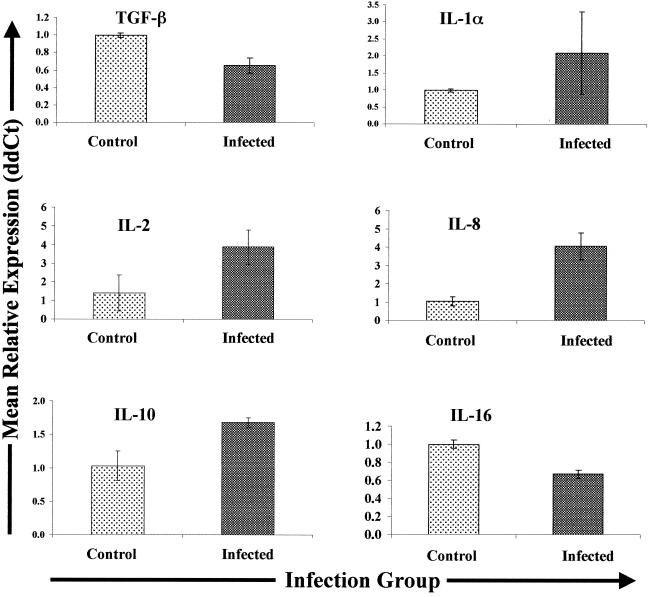
Expression of cytokine transcripts in mesenteric lymph nodes of Johne's disease-positive and control cows.
Mesenteric lymph nodes draining sites of inflammation in Johne's disease-positive cows and lymph nodes from uninfected control cows expressed similar amounts of IFN-γ, TNF-α, IL-1β, IL-4, IL-10, and IL-18. In contrast, the levels of TGF-β mRNA expression were lower (1.5-fold lower; P = 0.08) in lymph nodes from infected cows than in lymph nodes from control uninfected cows (Fig. 5). Expression of IL-1α mRNA was greater in lymph nodes of all infected cows relative to that in controls (mean, ∼2-fold greater); however, relative expression varied among individuals, and the level of IL-1α mRNA in lymph nodes from one infected cow was more than fivefold more than the mean levels found in lymph nodes from control cows.
FIG. 5.
Cytokine gene expression in mesenteric lymph nodes from Johne's disease-positive and control cows. Tissues were collected, RNA was extracted, and Q-RT-PCR was performed as described in Materials and Methods. Q-RT-PCR data were analyzed by using the 2−(ΔΔCt) method, with β-actin gene expression serving as a control for calculation of ΔCt values for each gene within animal and the mean control value for each gene serving as the calibrator for final calculation of mean relative expression values (ddCt). Only data for the genes exhibiting differential expression in the different infection groups (infected and control) are shown.
IL-2 mRNA was also more abundant (mean, 3.9-fold) in lymph nodes from infected cows than in similar tissues from controls (Fig. 5). Expression of IL-5, IL-6, and IL-12p35 mRNAs also tended to be greater (means, 3.4-, 1.6-, and 2.7-fold greater, respectively) in lymph nodes from infected cows. However, expression of these cytokine genes was highly variable in tissues from infected cows, and thus the differences were not statistically significant (data not shown). In contrast, IL-8 gene expression was significantly upregulated (mean, fourfold; P < 0.05) in lymph nodes from infected cows compared to expression in lymph nodes from the control cows (Fig. 5). Finally, the level of expression of IL-16 was significantly lower (1.5-fold lower; P < 0.05) in lymph nodes from infected cows than in similar tissues from control cows (Fig. 5).
DISCUSSION
Immune responses to viral, bacterial, or parasitic challenges result from a complex interaction of various immune effector cells and signals. Cytokines make up a considerable portion of this signaling cascade, and the balance of various cytokines has a profound effect on the outcome of infectious challenges. The importance of various cytokines has been aptly proven by using transgenic knockout mice in which one or more cytokine genes have been disrupted (2, 13, 29). Similarly, the use of cytokine-specific monoclonal antibodies to prevent cytokine activity has also provided much insight (34, 44, 49).
While a simple dichotomy has been developed to explain the direction of immune responses in the mouse (the Th1-Th2 paradigm) (31), this restricted view of the immune response, based on cytokine profiles from immune cell subsets, is beginning to wear thin (21). In cattle and humans, it is clear that the Th1-Th2 dichotomy fails to explain many immune cell reactions to pathogenic challenges (5). The immune responses in these species are often a balance between the two extremes supported by the Th1-Th2 paradigm, with many immune cells falling into a Th0 classification characterized by production of both Th1 and Th2 cytokines.
In long-term chronic infections, such as M. avium subsp. paratuberculosis infections, immune responses also tend to be dynamic, changing from a predominately Th1-like pattern to a Th2-like pattern over the long course of infection. Clinical manifestations of Johne's disease in cattle and other ruminants are often associated with a shift in the immune response from a primarily proinflammatory and cytotoxic response (Th1-like) to an antibody-based response (Th2-like) (39). It is, however, unlikely that there is a sharp transition; rather, there is a slow progression along the classical Th1-Th2 line. Importantly, the same shift in immune responses is associated with development of clinical disease in human tuberculosis and other mycobacterial diseases.
Q-RT-PCR results presented in this paper support the novel conclusion that PBMCs from Johne's disease-positive cows inherently exhibit a different cytokine gene expression profile than PBMCs from control uninfected cows exhibit. Differential expression of IFN-γ, TGF-β, IL-1α, IL-4, IL-6, IL-8, and IL-12 p35 by PBMCs from control cows and Johne's disease-positive cows was readily observed without M. avium subsp. paratuberculosis stimulation. Overall, the pattern of cytokine gene expression in nil-stimulated PBMCs from subclinically and clinically infected cows was consistent with a proinflammatory Th1-like response, with genes encoding IFN-γ, IL-1α, IL-6, and IL-8 all expressed at levels significantly above the control levels. Consistent with this interpretation, genes encoding IL-4 and TGF-β were expressed at lower levels in nil-stimulated PBMCs from infected cows than in similarly treated cells from control cows.
The dramatically different levels of expression of IFN-γ mRNA observed in nil-stimulated PBMCs from infected and control cows were, in large part, obfuscated by overnight stimulation with M. avium subsp. paratuberculosis. Following exposure to M. avium subsp. paratuberculosis, the expression of transcripts encoding TNF-α, IL-4 (in PBMCs from clinically infected cows only), and IL-6 was also reduced in PBMCs from infected cows compared to expression in nil-stimulated cells from the same cows. It is interesting that three cytokine genes (the IFN-γ, TNF-α, and IL-6 genes) showing muted or repressed expression changes in PBMCs from infected cows exposed for 16 to 18 h to M. avium subsp. paratuberculosis are representative of the classic Th1 immune response class. In addition, another classic Th1 cytokine gene, the gene encoding IL-12p35, was inherently expressed at significantly lower levels in PBMCs from infected cows than in PBMCs from controls (regardless of stimulation). Based on these data, we concluded that exposure of PBMCs from infected cows to M. avium subsp. paratuberculosis results in suppression of the Th1-like or proinflammatory response. This conclusion is also consistent with recent data demonstrating that general suppression of immune cell gene expression is caused by overnight stimulation of PBMCs with M. avium subsp. paratuberculosis (16; Coussens et al., submitted). The mechanisms responsible for this suppression remain to be determined. Given the potent immune modulating effects of IL-10 in cattle (5, 6), results presented in this paper may have significant biological implications and may also help explain suppression of the proinflammatory response in subclinically infected cow PBMCs following exposure to M. avium subsp. paratuberculosis. However, the same potential mechanism cannot be used to explain similar reductions in IFN-γ, TNF-α, and IL-6 mRNA expression in PBMCs from the clinically infected cows used in this study, since PBMCs from these cows did not show enhanced expression of the IL-10 gene in response to M. avium subsp. paratuberculosis.
Ileal tissue from Johne's disease-positive cows exhibited enhanced expression of several cytokines (IFN-γ, TGF-β, IL-1α, IL-1β, IL-5, IL-6, and IL-8) compared to expression in similar tissues from control uninfected cows. In contrast, expression of IL-16 was significantly greater in ileal tissues from infected cows than in tissues from control cows. The enhanced expression of IL-1α and TGF-β mRNAs observed in this study is consistent with previous observations (1, 25). The possible consequences of enhanced expression of these two cytokines in ileal tissues from M. avium subsp. paratuberculosis-infected cows have been discussed previously (1, 25).
The pattern of enhanced cytokine gene expression in ileal tissues from infected cows is a dichotomy of classical Th1 (IFN-γ, IL-1α, and IL-6) and Th2 (IL-5) cytokine gene expression. Enhanced expression of mRNA encoding the chemotactic (CXC) cytokine IL-8 is consistent with the large numbers of macrophages found at sites of active M. avium subsp. paratuberculosis infection (a potential source of IL-8) but not with the reduced presence of neutrophils at sites of M. avium subsp. paratuberculosis infection (25). One possible explanation for this apparent discrepancy is that while neutrophils may indeed be recruited to sites of active infection via IL-8 signaling, they rapidly phagocytose M. avium subsp. paratuberculosis released from infected macrophages and are thus rapidly destroyed by apoptosis or necrosis following oxidative burst. Another possibility is that dramatic changes in lumen structure that typically accompany M. avium subsp. paratuberculosis infection reduce the ability of neutrophils to migrate into these areas, despite abundant expression of IL-8. The role of neutrophils in lesions associated with Johne's disease has not been investigated previously, but these cells are a major factor in mouse and human inflammatory gastrointestinal disorders (20). Likewise, enhanced expression of IL-5 suggests that eosinophils may be actively recruited to sites of M. avium subsp. paratuberculosis infection and activated. Like the role of neutrophils, the potential role of eosinophils in pathogenesis associated with M. avium subsp. paratuberculosis infection has not been extensively investigated, although eosinophils are also a major factor in several mouse models of inflammatory gastrointestinal disorders (22).
Perhaps just as interesting as the genes found to be differentially expressed in ileal tissues from Johne's disease-positive and control cows are the genes that are not differentially expressed. TNF-α gene expression in ileal tissues did not appear to be affected by M. avium subsp. paratuberculosis infection. This result is consistent with previous observations (25) and may help explain the diffuse nature of granulomas at sites of M. avium subsp. paratuberculosis infection, since TNF-α is required for proper formation of well-defined granulomas (19, 36). Similarly, IL-4 was not differentially expressed in tissues from infected and control cows, suggesting that the immune response at sites of M. avium subsp. paratuberculosis infection is a largely proinflammatory or Th1-like response.
Like expression in intestinal tissues, expression of transcripts encoding IL-8 was significantly greater (mean, ∼4-fold greater) in lymph nodes from infected cows draining sites of M. avium subsp. paratuberculosis infection than in similar lymph nodes from control cows. Although the difference was not as pronounced as the difference in ileal tissues, lymph nodes from Johne's disease-positive cows and other ruminants often contain more macrophages than similar lymph nodes from uninfected cows, although the number of neutrophils is typically small. Thus, we are again presented with a situation similar to the situation in infected ileal tissue, where cytokine gene expression profiles (enhanced expression of IL-8 mRNA) and cellular infiltrates (low neutrophil numbers) do not seem to correspond.
Lymph nodes from Johne's disease-positive cows were the only tissue tested that displayed significantly elevated levels of IL-2 (∼4-fold higher than the levels in control cow lymph nodes). Although the level of IL-2 expression tended to be elevated in PBMCs from subclinically infected cows (mean, 10-fold higher) and clinically infected cows (mean, 7-fold higher) following stimulation with M. avium subsp. paratuberculosis (compared to nil-stimulated cells), the results were highly variable and thus not statistically significant.
Previous studies have shown that the IL-2 receptor (CD25) is expressed at elevated levels in lymph nodes draining sites of M. avium subsp. paratuberculosis infection and vaccination (46). Taken together, overexpression of IL-2 and overexpression of CD25 could help explain the hyperplasia often observed in lymph nodes draining sites of M. avium subsp. paratuberculosis infection. In addition, these results suggest that T cells within these nodes are continuously stimulated to divide. As T cells from these reservoirs circulate throughout the animal, the specific T cells undergoing autocrine or paracrine growth in lymph nodes draining sites of M. avium subsp. paratuberculosis infection may have a profound influence on cells obtained from peripheral sites (41, 46, 47) and may help explain observed differences in gene expression profiles between PBMCs from Johne's disease-positive and uninfected control cows (Coussens et al., submitted). An interesting complement to this hypothesis is the reduced expression of IL-16 in lymph nodes from infected cows compared to the expression in lymph nodes from uninfected cows. IL-16 is a potent chemotactic factor for CD4+ T cells, interacting with the CD4 receptor (17). Taken in context, our results suggest a hypothesis in which entry of new T cells into lymph nodes draining sites of M. avium subsp. paratuberculosis infection is reduced but the cells already in the lymph nodes proliferate due to enhanced IL-2-CD25 signaling. If true, this hypothesis further suggests that over the long course of infection with M. avium subsp. paratuberculosis, T cell populations within lymph nodes draining sites of infection would change in favor of cells capable of responding to M. avium subsp. paratuberculosis antigens. As the infection progressed, this effect could also alter circulating T cell populations. Several lines of experimental evidence support such a hypothesis and further suggest that the balance of T cell populations is altered in favor of a regulatory type of cell (11, 46).
Control over a local inflammatory response seems to be of paramount importance for the long-term survival of the host. Enhanced expression of IL-10 in lymph nodes draining sites of M. avium subsp. paratuberculosis infection could help control local inflammatory responses. Given that IL-10 is produced by T cells, enhanced expression of IL-10 in lymph nodes from infected cows could be a consequence of enhanced proliferation of such a regulatory cell population in lymph nodes from M. avium subsp. paratuberculosis-infected cows. If these cells represent an antigen-specific suppressor population, this would also explain the dramatic increase in IL-10 gene expression in PBMCs from subclinically infected cows following exposure to M. avium subsp. paratuberculosis in vitro.
In summary, our comprehensive results suggest that PBMCs from Johne's disease-positive cows inherently exhibit a different cytokine gene expression profile than their counterparts from uninfected control cows exhibit. This unique expression profile appears to be a classic Th1-like proinflammatory signature with enhanced expression of genes encoding IFN-γ, IL-6, and IL-1α. This conclusion is further supported by reduced expression of TGF-β mRNA in PBMCs from infected cows, as well as normal levels of IL-10 and IL-4 (not different from the levels in controls). Curiously, the level of IL-12p35 gene expression is significantly lower in PBMCs from infected cows, raising the question of what is driving the significantly enhanced amounts of IFN-γ mRNA if IL-12 is not doing this.
Exposure of PBMCs from infected cows to M. avium subsp. paratuberculosis for 16 to 18 h results in a muted or repressed proinflammatory cytokine gene expression profile, suggesting a specific shutdown of this response. This effect is more apparent in PBMCs from subclinically infected cows than in cells from clinically infected cows, perhaps due to enhanced expression of IL-10 in PBMCs from subclinically infected cows following exposure to M. avium subsp. paratuberculosis. The effects observed in peripheral immune cells likely reflect what is happening at both sites of infection and lymph nodes draining these sites. One model to explain our results is that a proinflammatory cell population develops within lymph nodes draining sites of infection and another population, perhaps expressing IL-10 in an antigen-specific manner, also develops to control local tissue damage and immune responses. Since both of these cell populations would circulate, the peripheral response observed would depend upon the relative amounts of the two cell populations in the periphery at any given time.
Acknowledgments
We acknowledge the outstanding technical support of Sue Sipkovsky and Xiaoning Ren. We also thank George W. Smith for a critical review of the manuscript and Jeanne Burton for many helpful discussions.
We acknowledge the generous financial support of the College of Agriculture and Natural Science, the Michigan State University Agricultural Experiment Station, and the Office of the Vice President for Research and Graduate Studies at Michigan State University. Additional support for this project was provided by the Michigan Animal Industry Coalition, USDA IFAFS grant 2001-52100-11211, and grant 03-9100-0794-GR from the USDA Animal and Plant Health Inspection Service, Division of Veterinary Services.
Editor: B. B. Finlay
REFERENCES
- 1.Aho, A. D., A. M. McNulty, and P. M. Coussens. 2003. Enhanced expression of interleukin-1α and tumor necrosis factor receptor-associated protein 1 in ileal tissues of cattle infected with Mycobacterium avium subsp. paratuberculosis. Infect. Immun. 71:6479-6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appelberg, R., A. G. Castro, J. Pedrosa, and P. Minoprio. 1994. Role of interleukin-6 in the induction of protective T cells during mycobacterial infections in mice. Immunology 82:361-364. [PMC free article] [PubMed] [Google Scholar]
- 3.Beard, P. M., M. J. Daniels, D. Henderson, A. Pirie, K. Rudge, D. Buxton, S. Rhind, A. Greig, M. R. Hutchings, I. McKendrick, K. Stevenson, and J. M. Sharp. 2001. Paratuberculosis infection of nonruminant wildlife in Scotland. J. Clin. Microbiol. 39:1517-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Billman-Jacobe, H., M. Carrigan, F. Cockram, L. A. Corner, I. J. Gill, J. F. Hill, T. Jessep, A. R. Milner, and P. R. Wood. 1992. A comparison of the interferon gamma assay with the absorbed ELISA for the diagnosis of Johne's disease in cattle. Aust. Vet. J. 69:25-28. [DOI] [PubMed] [Google Scholar]
- 5.Brown, W. C., and D. M. Estes. 1997. Type 1 and type 2 responses in cattle and their regulation, p. 15-33. In V. E. C. J. Schijns and M. C. Horzinek (ed.), Cytokines in veterinary medicine, vol. 1. CAB International, New York, N.Y. [Google Scholar]
- 6.Brown, W. C., V. M. Woods, C. G. Chitko-McKown, S. M. Hash, and A. C. Rice-Ficht. 1994. Interleukin-10 is expressed by bovine type 1 helper, type 2 helper, and unrestricted parasite-specific T-cell clones and inhibits proliferation of all three subsets in an accessory-cell-dependent manner. Infect. Immun. 62:4697-4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burrells, C., C. J. Clarke, A. Colston, J. M. Kay, J. Porter, D. Little, and J. M. Sharp. 1999. Interferon-gamma and interleukin-2 release by lymphocytes derived from the blood, mesenteric lymph nodes and intestines of normal sheep and those affected with paratuberculosis (Johne's disease). Vet. Immunol. Immunopathol. 68:139-148. [DOI] [PubMed] [Google Scholar]
- 8.Burrells, C., C. J. Clarke, A. Colston, J. M. Kay, J. Porter, D. Little, and J. M. Sharp. 1998. A study of immunological responses of sheep clinically-affected with paratuberculosis (Johne's disease). The relationship of blood, mesenteric lymph node and intestinal lymphocyte responses to gross and microscopic pathology. Vet. Immunol. Immunopathol. 66:343-358. [DOI] [PubMed] [Google Scholar]
- 9.Chamberlin, W., D. Y. Graham, K. Hulten, H. M. El-Zimaity, M. R. Schwartz, S. Naser, I. Shafran, and F. A. El-Zaatari. 2001. Review article: Mycobacterium avium subsp. paratuberculosis as one cause of Crohn's disease. Aliment. Pharmacol. Ther. 15:337-346. [DOI] [PubMed] [Google Scholar]
- 10.Chiodini, R. J., and C. D. Buergelt. 1993. Susceptibility of Balb/c, C57/B6 and C57/B10 mice to infection with Mycobacterium paratuberculosis. J. Comp. Pathol. 109:309-319. [DOI] [PubMed] [Google Scholar]
- 11.Chiodini, R. J., and W. C. Davis. 1993. The cellular immunology of bovine paratuberculosis: immunity may be regulated by CD4+ helper and CD8+ immunoregulatory T lymphocytes which down-regulate gamma/delta+ T-cell cytotoxicity. Microb. Pathog. 14:355-367. [DOI] [PubMed] [Google Scholar]
- 12.Collins, M. T., D. C. Sockett, S. Ridge, and J. C. Cox. 1991. Evaluation of a commercial enzyme-linked immunosorbent assay for Johne's disease. J. Clin. Microbiol. 29:272-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper, A. M., A. D. Roberts, E. R. Rhoades, J. E. Callahan, D. M. Getzy, and I. M. Orme. 1995. The role of interleukin-12 in acquired immunity to Mycobacterium tuberculosis infection. Immunology 84:423-432. [PMC free article] [PubMed] [Google Scholar]
- 14.Coussens, P., C. Colvin, A. Abouzied, K. Wiersma, and S. Sipkovsky. 2002. Gene expression profiling of peripheral blood mononuclear cells from cattle infected with Mycobacteriumparatuberculosis. Infect. Immun. 70:5494-5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coussens, P. M. 2001. Interactions between Mycobacterium paratuberculosis and the bovine immune system. Anim. Health Res. Rev. 2:141-161. [PubMed] [Google Scholar]
- 16.Coussens, P. M., A. Jeffers, and C. Colvin. 2003. Rapid and transient activation of gene expression in peripheral blood mononuclear cells from Johne's disease positive cows exposed to Mycobacterium paratuberculosis in vitro. Microb. Pathog. 36:93-108. [DOI] [PubMed]
- 17.Cruikshank, W. W., H. Kornfeld, and D. M. Center. 2000. Interleukin-16. J. Leukoc. Biol. 67:757-766. [DOI] [PubMed] [Google Scholar]
- 18.Dargatz, D. A., B. A. Byrum, L. K. Barber, R. W. Sweeney, R. H. Whitlock, W. P. Shulaw, R. H. Jacobson, and J. R. Stabel. 2001. Evaluation of a commercial ELISA for diagnosis of paratuberculosis in cattle. J. Am. Vet. Med. Assoc. 218:1163-1166. [DOI] [PubMed] [Google Scholar]
- 19.Ehlers, S., S. Kutsch, E. M. Ehlers, J. Benini, and K. Pfeffer. 2000. Lethal granuloma disintegration in mycobacteria-infected TNFRp55−/− mice is dependent on T cells and IL-12. J. Immunol. 165:483-492. [DOI] [PubMed] [Google Scholar]
- 20.Elliott, S. N., and J. L. Wallace. 1998. Neutrophil-mediated gastrointestinal injury. Can. J. Gastroenterol. 12:559-568. [DOI] [PubMed] [Google Scholar]
- 21.Gor, D. O., N. R. Rose, and N. S. Greenspan. 2003. Th1-Th2: a Procrustean paradigm. Nat. Immunol. 4:503-505. [DOI] [PubMed] [Google Scholar]
- 22.Hogan, S. P., P. S. Foster, and M. E. Rothenberg. 2002. Experimental analysis of eosinophil-associated gastrointestinal diseases. Curr. Opin. Allergy Clin. Immunol. 2:239-248. [DOI] [PubMed] [Google Scholar]
- 23.Ishikawa, H., T. Shirahata, and K. Hasegawa. 1994. Interferon-gamma production of mitogen stimulated peripheral lymphocytes in perinatal cows. J. Vet. Med. Sci. 56:735-738. [DOI] [PubMed] [Google Scholar]
- 24.Kennedy, D. J., and G. Benedictus. 2001. Control of Mycobacterium avium subsp. paratuberculosis infection in agricultural species. Rev. Sci. Tech. O.I.E. (Off. Int. Epizoot.) 20:151-179. [DOI] [PubMed] [Google Scholar]
- 25.Lee, H., J. R. Stabel, and M. E. Kehrli, Jr. 2001. Cytokine gene expression in ileal tissues of cattle infected with Mycobacterium paratuberculosis. Vet. Immunol. Immunopathol. 82:73-85. [DOI] [PubMed] [Google Scholar]
- 26.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(t)) method. Methods 4:402-408. [DOI] [PubMed] [Google Scholar]
- 27.Manning, E. J., and M. T. Collins. 2001. Mycobacterium avium subsp. paratuberculosis: pathogen, pathogenesis and diagnosis. Rev. Sci. Tech. O.I.E. (Off. Int. Epizoot.) 20:133-150. [DOI] [PubMed] [Google Scholar]
- 28.McDonald, W. L., S. E. Ridge, A. F. Hope, and R. J. Condron. 1999. Evaluation of diagnostic tests for Johne's disease in young cattle. Aust. Vet. J. 77:113-119. [DOI] [PubMed] [Google Scholar]
- 29.Mendez-Samperio, P., E. Garcia-Martinez, M. Hernandez-Garay, and M. Solis-Cardona. 1997. Depletion of endogenous interleukin-10 augments interleukin-1 beta secretion by Mycobacterium bovis BCG-reactive human cells. Clin. Diagn. Lab. Immunol. 4:138-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merkal, R. S., A. B. Larsen, and G. D. Booth. 1975. Analysis of the effect of inapparent bovine paratuberculosis. Am. J. Vet. Res. 36:837-838. [PubMed] [Google Scholar]
- 31.Mosmann, T. R., H. Cherwinski, M. W. Bond, M. A. Giedlin, and R. L. Coffman. 1986. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 136:2348-2357. [PubMed] [Google Scholar]
- 32.Naser, S. A., I. Shafran, D. Schwartz, F. El-Zaatari, and J. Biggerstaff. 2002. In situ identification of mycobacteria in Crohn's disease patient tissue using confocal scanning laser microscopy. Mol. Cell Probes 16:41-48. [DOI] [PubMed] [Google Scholar]
- 33.Navarro, J. A., G. Ramis, J. Seva, F. J. Pallares, and J. Sanchez. 1998. Changes in lymphocyte subsets in the intestine and mesenteric lymph nodes in caprine paratuberculosis. J. Comp. Pathol. 118:109-121. [DOI] [PubMed] [Google Scholar]
- 34.North, R. J. 1998. Mice incapable of making IL-4 or IL-10 display normal resistance to infection with Mycobacterium tuberculosis. Clin. Exp. Immunol. 113:55-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perez, V., J. Tellechea, J. M. Corpa, M. Gutierrez, and J. F. Garcia Marin. 1999. Relation between pathologic findings and cellular immune responses in sheep with naturally acquired paratuberculosis. Am. J. Vet. Res. 60:123-127. [PubMed] [Google Scholar]
- 36.Roach, D. R., A. G. Bean, C. Demangel, M. P. France, H. Briscoe, and W. J. Britton. 2002. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J. Immunol. 168:4620-4627. [DOI] [PubMed] [Google Scholar]
- 37.Rook, G. A., R. A. Attiyah, and N. Foley. 1989. The role of cytokines in the immunopathology of tuberculosis, and the regulation of agalactosyl IgG. Lymphokine Res. 8:323-328. [PubMed] [Google Scholar]
- 38.Stabel, J. R. 2000. Cytokine secretion by peripheral blood mononuclear cells from cows infected with Mycobacterium paratuberculosis. Am. J. Vet Res. 61:754-760. [DOI] [PubMed] [Google Scholar]
- 39.Stabel, J. R. 2000. Transitions in immune responses to Mycobacterium paratuberculosis. Vet. Microbiol. 77:465-473. [DOI] [PubMed] [Google Scholar]
- 40.Stabel, J. R., and R. H. Whitlock. 2001. An evaluation of a modified interferon-gamma assay for the detection of paratuberculosis in dairy herds. Vet. Immunol. Immunopathol. 79:69-81. [DOI] [PubMed] [Google Scholar]
- 41.Storset, A. K., G. Berntsen, and H. J. Larsen. 2000. Kinetics of IL-2 receptor expression on lymphocyte subsets from goats infected with Mycobacterium avium subsp. paratuberculosis after specific in vitro stimulation. Vet. Immunol. Immunopathol. 77:43-54. [DOI] [PubMed] [Google Scholar]
- 42.Storset, A. K., H. J. Hasvold, M. Valheim, H. Brun-Hansen, G. Berntsen, S. K. Whist, B. Djonne, C. M. Press, G. Holstad, and H. J. Larsen. 2001. Subclinical paratuberculosis in goats following experimental infection. An immunological and microbiological study. Vet. Immunol. Immunopathol. 80:271-287. [DOI] [PubMed] [Google Scholar]
- 43.Strohmeier, G. R., and M. J. Fenton. 1999. Roles of lipoarabinomannan in the pathogenesis of tuberculosis. Microbes Infect. 1:709-717. [DOI] [PubMed] [Google Scholar]
- 44.Sugawara, I., H. Yamada, H. Kaneko, S. Mizuno, K. Takeda, and S. Akira. 1999. Role of interleukin-18 (IL-18) in mycobacterial infection in IL-18-gene-disrupted mice. Infect. Immun. 67:2585-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tooker, B. R., J. L. Burton, and P. M. Coussens. 2002. Survival tactics of M. paratuberculosis in bovine macrophage cells. Vet. Immunol. Immunopathol. 87:443-451. [DOI] [PubMed] [Google Scholar]
- 46.Valheim, M., H. J. Hasvold, A. K. Storset, H. J. Larsen, and C. M. Press. 2002. Localisation of CD25+ cells and MHCII+ cells in lymph nodes draining Mycobacterium avium subsp. paratuberculosis vaccination granuloma and the presence of a systemic immune response. Res. Vet. Sci. 73:77-85. [DOI] [PubMed] [Google Scholar]
- 47.Whist, S. K., A. K. Storset, and H. J. Larsen. 2000. The use of interleukin-2 receptor expression as a marker of cell-mediated immunity in goats experimentally infected with Mycobacterium avium ssp. paratuberculosis. Vet. Immunol. Immunopathol. 73:207-218. [DOI] [PubMed] [Google Scholar]
- 48.Whitlock, R. H., and C. Buergelt. 1996. Preclinical and clinical manifestations of paratuberculosis (including pathology). Vet. Clin. North Am. Food Anim. Pract. 12:345-356. [DOI] [PubMed] [Google Scholar]
- 49.Yamada, H., S. Mizumo, R. Horai, Y. Iwakura, and I. Sugawara. 2000. Protective role of interleukin-1 in mycobacterial infection in IL-1 alpha/beta double-knockout mice. Lab. Invest. 80:759-767. [DOI] [PubMed] [Google Scholar]
- 50.Yao, J., J. L. Burton, P. Saama, S. Sipkovsky, and P. M. Coussens. 2001. Generation of EST and cDNA microarray resources for the study of bovine immunolobiology. Acta Vet. Scand. 42:391-406. [PubMed] [Google Scholar]