Abstract
Monocytes are a heterogeneous population of bone marrow-derived cells that are recruited to sites of infection and inflammation in many models of human diseases, including those of the central nervous system (CNS). Ly6Chi/CCR2hi inflammatory monocytes have been identified as the circulating precursors of brain macrophages, dendritic cells and arguably microglia in experimental autoimmune encephalomyelitis; Alzheimer’s disease; stroke; and more recently in CNS infection caused by Herpes simplex virus, murine hepatitis virus, Theiler’s murine encephalomyelitis virus, Japanese encephalitis virus and West Nile virus. The precise differentiation pathways and functions of inflammatory monocyte-derived populations in the inflamed CNS remains a contentious issue, especially in regard to the existence of monocyte-derived microglia. Furthermore, the contributions of monocyte-derived subsets to viral clearance and immunopathology are not well-defined. Thus, understanding the pathways through which inflammatory monocytes migrate to the brain and their functional capacity within the CNS is critical to inform future therapeutic strategies. This review discusses some of the key aspects of inflammatory monocyte trafficking to the brain and addresses the role of these cells in viral encephalitis.
Keywords: Ly6Chi inflammatory monocytes, Viral encephalitis, Neurotrophic virus, CCL2, CCR2, VLA-4, LFA-1, Integrins
Background
Virus infection of the brain can cause severe and life-threatening disease. Despite this, few therapies beyond intensive supportive care are available to treat patients with encephalitis [1,2]. Anti-viral drugs have been developed for some viruses that can infect the brain, such as Herpes simplex virus (HSV)-1 and 2, and human immunodeficiency virus (HIV), but even with these treatments outcomes remain relatively poor [2-5]. Many patients succumb to disease, and survivors often suffer permanent neurological sequelae [6-9].
While the development and clinical implementation of novel anti-viral drugs may improve patient outcomes, it is becoming increasingly clear that therapies targeting pathogenic elements of the host immune response may be critical for successful intervention during infection [10-14]. Monocyte infiltration is a hallmark of central nervous system (CNS) inflammation, including viral infection. These cells migrate into the infected brain, where they differentiate into dendritic cell (DC), macrophage and, arguably, microglial populations. Once differentiated, these cells engage in a number of potent effector functions including antigen presentation and T cell stimulation, the production and secretion of numerous pro-inflammatory mediators as well as reactive oxygen species (ROS), all of which are focused on viral containment and clearance (Table 1). However, unbalanced and poorly controlled migration and effector functions of these cells may result in immune-mediated pathology, resulting in tissue damage and destruction during some infections (Table 1). Therefore, it is of high importance to understand the processes driving monocyte development, recruitment, differentiation and function, to aid in the development of novel therapeutics that inhibit immunopathological responses.
Table 1.
Evidence for macrophage-driven pathogenesis and control of viral encephalitis
| Macrophage-derived mediators | Pathogenic and anti-viral functions in the central nervous system | Pathogenic role in mouse models | Anti-viral role in mouse models | |
|---|---|---|---|---|
|
Pro-inflammatory cytokines |
IL-1β |
↑ pro-inflammatory cytokines |
IL-1β−/− mice resistant to fatal neurovirulent Sindbis virus encephalitis [15] |
IL-1β−/− mice exhibit increased mortality and virus loads in HSV-1 encephalitis [16] |
| ↑ leukocyte chemoattractants | ||||
|
IL-6 |
↑ adhesion molecules |
IL-6−/− mice exhibit reduced seizures in TMEV encephalitis [17] |
|
|
| ↑ NO/reactive oxygen species production | ||||
| ↑ neuronal misfiring/ seizures | ||||
| ↑ neuronal | ||||
| ↑ breakdown of BBB | ||||
| ↑ MMP | ||||
| Reviewed in [18-22] | ||||
|
IL-12 |
IL-12−/− mice show decreased clinical score during MHV encephalitis [23] |
Infusion of IL-12 reduces viral loads and improves survival during vesicular stomatitis virus encephalitis [24] |
||
|
TNF |
TNF-R−/− mice show improved survival in rabies virus encephalitis [25] |
TNF−/− mice exhibit increased mortality and virus loads in HSV-1 encephalitis [16] |
||
|
Free radicals |
NO/reactive oxygen species |
↑ neuronal misfiring/ seizures |
Inhibition of NOS2 prolonged survival in rabies virus encephalitis by delaying virus replication and inhibiting of apoptosis [26] |
NOS2−/− mice show increased susceptibility to CNS invasion and death in Murray Valley virus encephalitis [27] |
| ↑ neuronal damage/death | ||||
| ↑ formation of reactive oxygen species | ||||
| Inhibition of NOS2 reduces mortality during Junin virus encephalitis [28] and neurovirulent Sindbis virus encephalitis [29] | ||||
| Inhibition of NOS2 prolonged survival of WNV-infected animals [30] | ||||
| Reviewed in [31] | ||||
|
Proteases |
MMP |
↑ breakdown of the BBB |
MMP-9−/− mice show reduced viral loads and increased survival during WNV encephalitis [32] |
|
| ↑ neuronal damage/death | ||||
| ↑ demyelination | ||||
| ↑ pro-inflammatory cytokines | ||||
| Reviewed in [33,34] | ||||
| Neurotransmitters | Glutamate | ↑ neuronal misfiring/seizures |
Competitive and non-competitive glutamate receptor antagonists promote survival during neurovirulent Sindbis virus encephalitis [35,36] and improved outcomes during coronavirus encephalitis [37] | |
| ↑ neuronal damage/death | ||||
| ↑ production of NO/ROS | ||||
| Reviewed in [38] | ||||
BBB blood brain barrier; CNS central nervous system; HSV herpes simplex virus; MDP macrophage/dendritic cell precursor; MHV murine hepatitis virus; MMP matrix metalloproteinases; NO nitric oxide; NOS2 nitric oxide synthase-2; ROS reactive oxygen species; TMEV Theiler’s murine encephalomyelitis virus; WNV West Nile virus.
Monocytes are derived from hematopoietic precursors in the bone marrow
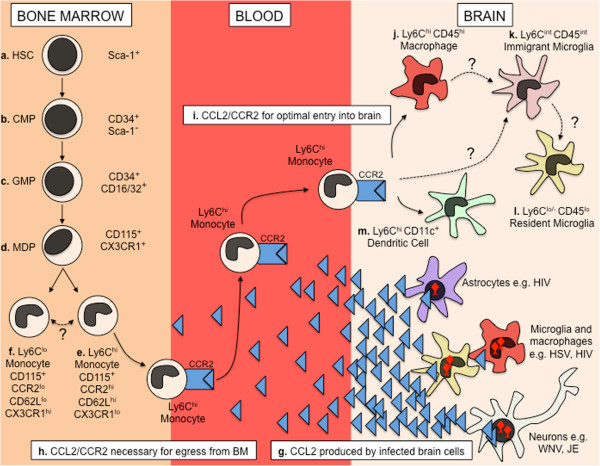
Monocytes are derived from hematopoietic stem cells (HSC) in the bone marrow (BM) (Figure 1). The earliest defined precursor is the common myeloid precursor (CMP), distinguished from HSC by the expression of CD34 but not SCA-1 [39-42] (Figure 1). These cells give rise to a pool of precursors called granulocyte/macrophage precursors (GMPs), which express CD16/32 [39]. Included within this subset is the recently defined macrophage/DC precursor (MDP), which specifically expresses high levels of the PU.1-controlled chemokine receptor CD115 (CSF-1R/M-CSFR), chemokine receptor CX3CR1 (fractalkine receptor), and Flt-3 (CD135/Flk2) [43-48] (Figure 1). The MDP gives rise to CD11b+, CD115+, F4/80+, CD11c-, Ly6G- monocytes, that can be isolated from the BM and blood [49-52] (Figure 1). The spleen has also been identified as an important reservoir of undifferentiated monocytes that are rapidly deployed to sites of inflammation, including the ischemic heart and brain [53-55]. Furthermore, a recent study has shown that cardiac infarction triggers a significant increase in numbers of MDPs in the spleen, which supply monocytes throughout the duration of acute inflammation [56]. Whether the spleen is a significant source of monocytes during CNS infection is yet to be determined, but presents a critical area of future investigation. It is likely that both the BM and spleen are critical for supplying monocytes to the infected CNS, particularly in cases of acute and severe infection, in which large numbers of these cells are rapidly deployed and recruited to the brain.
Figure 1.
Development of monocytes in the bone marrow and recruitment to the virus-infected brain. Monocytes are generated from hematopoietic precursors in the bone marrow (BM). Sca-1+ Lin- HSC (a) give rise to CD34+, Sca-1- CMP (b). These cells in turn give rise to a pool of precursors known as granulocyte/macrophage precursors (GMPs), which express CD34 and CD16/32 (c). A fraction of these progenitors also express CD115 and CX3CR1 and are known as macrophage/dendritic cell precursor (MDP) (d). MDPs are the direct precursors of Ly6Chi inflammatory monocytes (e). MDPs also give rise to circulating Ly6Clo/- monocytes directly, or via a Ly6Chi monocyte intermediate (f). During viral encephalitis, large quantities of the chemokine CCL2 is produced by infected astrocytes, macrophages/microglia and/or neurons (g). CCL2 binds the chemokine receptor CCR2, expressed at high levels by Ly6Chi inflammatory monocytes, which promotes the egress of these cells from the BM (h) into the blood, and thus recruitment from the blood into the infected central nervous system (CNS) (i). Here, these cells can give rise to CD45hi Ly6Chi macrophages (j) and/or CD45int Ly6Cint immigrant microglia (k), although it is unclear whether Ly6Cint immigrant microglia are derived from a Ly6Chi macrophage intermediate or directly differentiate from Ly6Chi monocytes. Furthermore, it is unclear whether recruited macrophages and immigrant microglia give rise to CD45lo Ly6Clo/- resident microglia (l) if/when virus is cleared from the CNS. In some models of viral encephalitis, Ly6Chi inflammatory monocytes can also give rise to Ly6Chi/CD11c+ DC in the brain (m).
Monocytes are classified into two phenotypically and functionally distinct subsets
The MDPs give rise to two phenotypically and functionally distinct subsets of monocytes [50,57]. Ly6Chi monocytes are characterized by high expression of the chemokine receptor CCR2, adhesion molecule CD62L and low expression of the fractalkine receptor CX3CR1[48,51,58]. These cells have been termed ‘inflammatory’ because they are selectively recruited to sites of inflammation and infection in many models of disease, including atherosclerosis [59-62]; rheumatoid arthritis [63]; experimental colitis [64]; cardiac infarction [65]; and CNS infections including experimental autoimmune encephalomyelitis (EAE) [66,67], amyotrophic lateral sclerosis [68], and stroke [53]. Recent studies have shown that these cells are also recruited to the virus-infected brain in animal models of HSV, HIV, murine hepatitis virus (MHV), Theiler’s murine encephalomyelitis virus (TMEV) and a number of flaviviral encephalitides, where they give rise to macrophage, DC and, arguably, to microglial populations [11,13,14,69].
Conversely, Ly6Clo/- monocytes are smaller in size than their Ly6Chi counterparts and express low levels of CCR2 and CD62L and high levels of CX3CR1[48,51,58] (Figure 1). Several studies have shown that Ly6Chi monocytes can give rise to circulating Ly6Clo/- monocytes [58,70-72]. Interest in this subset has increased substantially in the past few years [72,73]. Recent studies have described the patrolling behavior of these cells in the vasculature [73], and have shown that in some models of disease they rapidly enter inflamed tissue and can contribute to early inflammatory responses before domination by Ly6Chi monocytes [73]. In the resolution phase of some diseases, Ly6Clo/- monocytes are critical for wound healing and angiogenesis [50]. While apparently important in the periphery, the role of Ly6Clo/- monocytes during CNS infection remains poorly defined, with little evidence supporting their migration into the brain during inflammation [74].
Monocyte egress from the bone marrow is controlled by chemokine/chemokine receptor interactions
The importance of monocyte-derived cells in the pathogenesis of brain infection highlights the importance of understanding the pathway(s) through which monocytes migrate from the periphery into the brain. It is apparent that this process is regulated by cytokine/chemokine and integrin/cellular adhesion molecule interactions that facilitate emigration from the BM into the blood and entry into the CNS. For example, the chemokine receptor CXCR4 and one of its ligands CXCL12 (SDF-1) directly enhance VLA-4-dependent adhesion and thereby aid in retaining immature cells in the BM. Deficiency in either molecule results in impaired myelopoiesis [75-80]. In addition to CXCR4, CCR2 and its ligands, CCL2 and CCL7 (MCP-3), are a critical requirement for Ly6Chi monocyte egress from the BM into the blood. CCL2/CCR2 deficiency or blockade with antibody results in monocyte accumulation in the BM in multiple disease models, including EAE, WNV and HSV encephalitides [11,61,67,81-87].
Monocyte recruitment into the infected brain is dependent on chemokine/chemokine receptor interactions
A number of chemokines and their receptors have been implicated in the recruitment of Ly6Chi monocytes from the blood and into the brain. CCR5 is expressed by Ly6Chi monocytes and is important for trafficking to sites of inflammation in some models of disease. In the brain, its ligand CCL5 (RANTES) expression is highly upregulated during infection/inflammation, including WNV, MHV, HSV and tick-borne encephalitis virus encephalitides [88-92]. Another chemokine of interest that controls the trafficking of monocytes into the brain parenchyma is SDF-1/CXCL12, in conjunction with its receptor CXCR4, expressed by monocytes [93]. In animal models of CNS inflammation including EAE [94], HIV [95] and WNV [96], there is significant upregulation of CXCL12. In EAE and WNV, CXCL12 has been shown to play an important role in retaining leukocytes in the perivascular space, thereby inhibiting infiltration into the parenchyma. Loss of this interaction resulted in the loss of perivascular cuffs and uncontrolled infiltration of CXCR4+ leukocytes, including monocytes, into the parenchyma. [94,96].
While it is clear that there are a multitude of soluble mediators that represent potential targets for future therapies aimed at blocking monocyte migration, the CCR2/CCL2 axis remains the most potent pathway based on the available literature. Ly6Chi /CCR2hi monocyte recruitment into the CNS in models of stroke [53], peripheral inflammation [97], Alzheimer’s disease (AD) [98,99] and EAE [67,74,100,101] are all dependent on CCR2/CCL2 signaling (Figure 1). In the context of viral encephalitis, the CCL2/CCR2 axis is also very important. The major producers of CCL2 appear to be different depending on the infectious agent, with microglia serving as important sources during HSV infection [16,102], neurons in the case of WNV infection [11] and astrocytes in HIV encephalitis [103]. No matter the source of CCL2, the inhibition of CCL2 can significantly reduce the infiltration of inflammatory monocyte-derived macrophages and microglia into the infected brain [11-13,69,88,102,104-108].
Monocyte recruitment into the infected brain is dependent on integrin/adhesion molecule interactions
The focus in the last decade has been heavily on the chemokines involved in monocyte trafficking, however, cellular adhesion molecules and their integrin ligands are obviously also important. In most models of viral infection, very late antigen-4 (VLA-4) and leukocyte function-associated antigen-1 (LFA-1) are expressed by Ly6Chi monocytes. In addition, their respective binding partner’s vascular cell adhesion molecule-1 (VCAM-1) and inter-cellular adhesion molecule-1 (ICAM-1) are usually upregulated on endothelium and other cell types in the inflamed brain [109-115].
The importance of VLA-4 and VCAM-1 and LFA-1 and ICAM-1 in the recruitment of Ly6Chi monocytes to sites of inflammation is evident in experiments using gene knockout animals or specific blockade of these molecules. VLA-4 and VCAM-1 interactions are critical for monocyte migration to the heart in models of atherosclerosis and arterial injury [116-118] and the inflamed peritoneum [119]. VLA-4 is also critical for Ly6Chi monocyte infiltration of the CNS in several models of inflammation, including EAE and spinal cord injury [97,109,120]. During viral infection of the brain, we have found that recruitment of monocytes to the CNS is also VLA-4-dependent. VLA-4 antibody neutralization significantly impairs the recruitment of Ly6Chi monocytes to the infected brain, in both WNV and JEV infection ([30], CvV et al., unpublished observations). LFA-1 and ICAM-1 interactions are also important for monocyte recruitment to atherosclerotic plaques [121,122] and to the CNS during EAE [110]. We have shown that LFA-1 is also important for recruitment of monocytes to the WNV-infected brain, however blockade resulted in a smaller reduction in monocytes infiltration compared to VLA-4 neutralization, which suggests the differential use of adhesion molecules by Ly6Chi monocyte subsets which enter the WNV-infected brain [30].
Monocytes differentiate into macrophages and dendritic cells in the infected brain
In models of CNS diseases, such as EAE and stroke, Ly6Chi monocytes have been shown to primarily differentiate into macrophage and DC populations exhibiting a M1 pro-inflammatory phenotype, which in-vitro effectively stimulates Th1 and Th17 responses in T cells [53,66,67,74]. Similarly, in models of viral encephalitis, Ly6Chi monocytes have been shown to give rise to M1 pro-inflammatory CD45hi macrophages and CD11c+ DC populations, which express high levels of nitric oxide (NO) and TNF during HSV, WNV, MHV, TMEV and JEV ([11-14,30,69], CvV et al., unpublished observations). We have shown that these CD45hi macrophages are highly effective at processing and presenting antigen and effectively stimulate T cell proliferation [30].
Resident microglia originate from a myeloid lineage distinct to that of infiltrating monocytes
Microglia are the resident macrophage population of the brain. Similar to other tissue resident cells such as Kupffer cells of the liver and Langerhans cells of the epidermis, microglia originate from the yolk sac during embryogenesis, from a myeloid lineage that is independent of BM HSC and therefore distinct from that of BM-derived monocytes [123-125]. Microglia can be distinguished from infiltrating monocyte-derived macrophages and DC by their low to intermediate expression of CD45 and lack of Ly6C expression [11,126]. In most infections, resident microglia play functionally distinct roles from that of monocyte-derived cells. For example, during acute WNV encephalitis, resident microglia express lower levels of pro-inflammatory mediators such as NO, express lower levels of MHC-II, and show a significantly reduced capacity to process antigen and stimulate T cell proliferation compared to the highly activated infiltrating macrophages [30]. In comparison, in acute TMEV infection, resident microglia and infiltrating macrophages express similar levels of pro-inflammatory cytokines and show similar antigen processing and presentation capacity; however, in chronic stages of disease, macrophages are more efficient at stimulating T cell responses [127].
Monocytes may serve as microglial precursors during brain infection
There is evidence to suggest that infiltrating monocytes have the capacity to give rise to microglial cells in some models of CNS inflammation, including AD, Parkinson’s disease, EAE, as well as in infectious models such as scrapie and bacterial meningitis [128-134]. These immigrant microglial cells appear to play distinct functional roles compared to their resident counterparts during disease. For example, immigrant microglia are more efficient at clearing amyloid plaques than resident microglia during AD [128,135]. However, a caveat of these studies has been in the use of irradiation to generate BM chimeras to distinguish resident microglial from BM-derived cells. There are currently no immunophenotypic markers that can definitively separate these two populations. As a result, the generation of chimeras can be used distinguish tissue resident and BM-derived populations. However, irradiation can disrupt the blood–brain barrier (BBB) and promote CCL2 production, resulting in the recruitment of monocytes to the CNS [136]. Therefore, it is difficult to conclude whether monocyte engraftment is a normal feature of disease in unperturbed animals or whether it is primarily the result of brain preconditioning by irradiation. A recent study using the parabiosis model in place of irradiated BM chimeras has shown that engraftment of monocyte-derived microglia during EAE is only a transient response [137]. The parabiosis models have also been employed to show that there is no significant engraftment of monocyte-derived microglia in facial nerve axonomy or amyotrophic lateral sclerosis [138]. Also, another recent study has compared the recruitment of monocyte-derived microglia into brain during AD, using chimeric mice generated with or without head protection during irradiation. They found that these cells do not engraft the brain of protected animals [99]. However, one major caveat of the head-protection model is the existence of BM in the skull that may be capable of reconstituting the animal. Further studies are required to definitively determine whether monocyte-derived cells can give rise to microglia and if these cells truly engraft the parenchyma and remain there if/when disease is resolved.
There are few studies that examine the recruitment of monocyte-derived microglia during viral infection of the CNS. We have shown that in WNV encephalitis, inflammatory monocytes not only give rise to CD45hi macrophages in the brain, but also to a CD45int subset, which is phenotypically analogous to activated resident microglia, apart from the expression of Ly6C [11,30]. Although chimeras were initially utilized to investigate this phenomenon, we further confirmed that the recruitment of these monocyte-derived cells was not the result of BBB breakdown, using methods that do not use any irradiation including bone marrow adoptive transfer studies and microparticle-based systems which track these cells with minimal perturbation of the disease system [11]. Furthermore, these cells were found to contribute to the immunopathogenesis of WNV encephalitis, as CCL2 blockade significantly reduced recruitment into the CNS and prolonged survival of lethally-infected animals [11]. Current studies in our laboratory aim to determine whether monocyte-derived microglia truly engraft the brain parenchyma during WNV encephalitis, the functional role of these cells throughout infection, and whether these cells remain in the CNS after disease is resolved.
Monocytes contribute to viral clearance or viral burden in different models of infection
Ly6Chi monocytes appear to play a paradoxical role in many disease models. For example, higher mortality rates and increased pathogen loads are seen in Toxoplasma [139,140], Listeria [83,141], Cryptococcus [142,143], Yersinia infections [144], HSV-2, [145] and coronavirus [146], as well as MHV [88] when these cells are depleted. On the other hand, Ly6Chi monocytes are direct targets for pathogens such as HIV, TMEV, Listeria and Toxoplasma [12,69,147-152]. Infected monocytes can be directly responsible for the dissemination of infection in a “Trojan horse” fashion into the CNS thereby potentiating disease and increasing potential mortality [153-156].
Monocytes significantly contribute to immunopathology during brain infection
An arguable role of monocytes during brain infection is their potential contribution to immune-mediated pathology. In several models of CNS disease, Ly6Chi inflammatory monocytes cause significant damage and destruction in the brain, directly contributing to morbidity and mortality. Ly6Chi monocytes contribute significantly to the pathogenesis of disease during stroke [53]. Mice with CCL2−/− and CCR2−/− deficiency show smaller infarcts and enhanced functional outcomes relative to wild-type controls following transient cerebral ischemia [157,158]. Similarly, in models of traumatic brain injury, CCL2−/− mice showed reductions in macrophage infiltration and lesion volume compared to wild-type mice, corresponding with improved functional recovery after injury [159]. In addition, CCR2−/− and CCL2−/− mice exhibit milder symptoms and, in some models, are completely resistant to the development of EAE [100,136,160,161]. Furthermore, a recent study has shown that Ly6Chi monocyte recruitment to the CNS is detrimental in amyotrophic lateral sclerosis [68]. In the case of encephalitic disease, studies in our laboratory using WNV as well as others using TMEV have shown that Ly6Chi monocytes are recruited into the infected brain where they contribute significantly to the immunopathogenesis of disease. Inhibition of inflammatory monocyte migration into the WNV or TMEV-infected brain can significantly reduce morbidity and mortality [11,12,69,108]. Furthermore, abrogation of monocyte migration into the CNS during MHV encephalitis results in the delayed onset of demyelinating disease [105]. The precise pathways through which inflammatory monovcytes contribute to pathology are still under intense investigation. However, it is clear that differentiation into effector cells such as macrophages and DC plays a substantial role. Once differentiated, these cells are significant producers of NO, matrix metalloproteinases (MMP) and other factors known to culminate in tissue destruction, breakdown of the BBB, as well as neuronal damage (Table 1). While in many organs such toxicity is not a major concern due to regenerative capabilities, the brain is largely comprised of many irreplaceable cellular subsets. As such not only is mortality a concern, in patients that survive serious CNS inflammatory insults will often suffer long-term sequelae and neurological imbalance [6-9].
Conclusions
Although Ly6Chi monocyte infiltration is a hallmark of viral encephalitis, the role of these cells in viral clearance and immunopathology is not well defined. While it is clear that these cells are critical for the control and clearance of some viruses, they are directly responsible for recruiting others into CNS, or cause significant immunopathology. Future studies which target monocyte development and migration to the CNS in a therapeutic manner will not only provide significant insight into pathways by which monocytes are recruited to the CNS, but will identify new targets for intervention during viral encephalitis.
Abbreviations
AD: Alzheimer’s disease; BBB: Blood–brain barrier; BM: Bone marrow; CNS: Central nervous system; CMP: Common myeloid precursor; DC: Dendritic cells; EAE: Experimental autoimmune encephalomyelitis; GMP: Granulocyte/macrophage precursor; HIV: Human immunodeficiency virus; HSC: Hematopoietic stem cells; HSV: Herpes simplex virus; ICAM-1: Inter-cellular adhesion molecule-1; JEV: Japanese encephalitis virus; LFA-1: Leukocyte function-associated antigen-1; MDP: Macrophage/DC precursor; MHV: Murine hepatitis virus; MMP: Matrix metalloproteinases; NO: Nitric oxide; NOS2: Nitric oxide synthase-2; ROS: Reactive oxygen species; TMEV: Theiler’s murine encephalomyelitis virus; VCAM-1: Vascular cell adhesion molecule-1; VLA-4: Very late antigen-4; WNV: West Nile virus.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
RLT drafted the manuscript. DRG, CD, CVV, ILC and NJCK contributed to the interpretation and critical evaluation of content and revision of the manuscript. All authors read and approved the final manuscript.
Contributor Information
Rachael L Terry, Email: rachael.terry@northwestern.edu.
Daniel R Getts, Email: d-getts@gettsconsulting.com.
Celine Deffrasnes, Email: Celine.Deffrasnes@csiro.au.
Caryn van Vreden, Email: caryn.v.v@gmail.com.
Iain L Campbell, Email: icamp@usyd.edu.au.
Nicholas JC King, Email: nickk@pathology.usyd.edu.au.
Acknowledgements
The authors cited in this review were supported by the National Health and Medical Research Council grants 512413 and 1007757 to NJCK and ILC. RLT was supported by an Australian Postgraduate Award and a Wenkart Foundation Scholarship.
References
- Chaudhuri A, Kennedy P. Diagnosis and treatment of viral encephalitis. Postgrad Med J. 2002;78:575–583. doi: 10.1136/pmj.78.924.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley R, Gnann J. Viral encephalitis: familiar infections and emerging pathogens. Lancet. 2002;359:507–513. doi: 10.1016/S0140-6736(02)07681-X. [DOI] [PubMed] [Google Scholar]
- Raschilas F, Wolff M, Delatour F, Chaffaut C, De Broucker T, Chevret S, Lebon P, Canton P, Rozenberg F. Outcome of and prognostic factors for herpes simplex encephalitis in adult patients: results of a multicenter study. Clin Infect Dis. 2002;35:254–260. doi: 10.1086/341405. [DOI] [PubMed] [Google Scholar]
- Lawrence DM, Major EO. HIV-1 and the brain: connections between HIV-1-associated dementia, neuropathology and neuroimmunology. Microbes Infect. 2002;4:301–308. doi: 10.1016/S1286-4579(02)01542-3. [DOI] [PubMed] [Google Scholar]
- Domingues RB. Treatment of viral encephalitis. Cent Nerv Syst Agents Med Chem. 2009;9:56–62. doi: 10.2174/187152409787601905. [DOI] [PubMed] [Google Scholar]
- Utley TF, Ogden JA, Gibb A, McGrath N, Anderson NE. The long-term neuropsychological outcome of herpes simplex encephalitis in a series of unselected survivors. Neuropsychiatry Neuropsychol Behav Neurol. 1997;10:180–189. [PubMed] [Google Scholar]
- McGrath N, Anderson NE, Croxson MC, Powell KF. Herpes simplex encephalitis treated with acyclovir: diagnosis and long term outcome. J Neurol Neurosurg Psychiatry. 1997;63:321–326. doi: 10.1136/jnnp.63.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur JC. HIV dementia: an evolving disease. J Neuroimmunol. 2004;157:3–10. doi: 10.1016/j.jneuroim.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Ito Y, Kimura H, Yabuta Y, Ando Y, Murakami T, Shiomi M, Morishima T. Exacerbation of herpes simplex encephalitis after successful treatment with acyclovir. Clin Infect Dis. 2000;30:185–187. doi: 10.1086/313618. [DOI] [PubMed] [Google Scholar]
- Gras G, Kaul M. Molecular mechanisms of neuroinvasion by monocytes-macrophages in HIV-1 infection. Retrovirology. 2010;7:30. doi: 10.1186/1742-4690-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getts DR, Terry RL, Getts MT, Muller M, Rana S, Shrestha B, Radford J, Van Rooijen N, Campbell IL, King NJ. Ly6c+ "inflammatory monocytes" are microglial precursors recruited in a pathogenic manner in West Nile virus encephalitis. J Exp Med. 2008;205:2319–2337. doi: 10.1084/jem.20080421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophi GP, Massa PT. Central neuroinvasion and demyelination by inflammatory macrophages after peripheral virus infection is controlled by SHP-1. Viral Immunol. 2009;22:371–387. doi: 10.1089/vim.2009.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeton S, Kim T, O'Malley K, Perlman S. Maturation and localization of macrophages and microglia during infection with a neurotropic murine coronavirus. Brain Pathol. 2008;18:40–51. doi: 10.1111/j.1750-3639.2007.00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques CP, Cheeran MC, Palmquist JM, Hu S, Urban SL, Lokensgard JR. Prolonged microglial cell activation and lymphocyte infiltration following experimental herpes encephalitis. J Immunol. 2008;181:6417–6426. doi: 10.4049/jimmunol.181.9.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang XH, Goldman JE, Jiang HH, Levine B. Resistance of interleukin-1beta-deficient mice to fatal Sindbis virus encephalitis. J Virol. 1999;73:2563–2567. doi: 10.1128/jvi.73.3.2563-2567.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergerie Y, Rivest S, Boivin G. Tumor necrosis factor-alpha and interleukin-1 beta play a critical role in the resistance against lethal herpes simplex virus encephalitis. J Infect Dis. 2007;196:853–860. doi: 10.1086/520094. [DOI] [PubMed] [Google Scholar]
- Libbey JE, Kennett NJ, Wilcox KS, White HS, Fujinami RS. Interleukin-6, produced by resident cells of the central nervous system and infiltrating cells, contributes to the development of seizures following viral infection. J Virol. 2011;85:6913–6922. doi: 10.1128/JVI.00458-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaftel SS, Carlson TJ, Olschowka JA, Kyrkanides S, Matousek SB, O'Banion MK. Chronic interleukin-1beta expression in mouse brain leads to leukocyte infiltration and neutrophil-independent blood brain barrier permeability without overt neurodegeneration. J Neurosci. 2007;27:9301–9309. doi: 10.1523/JNEUROSCI.1418-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5:629–640. doi: 10.1038/nri1664. [DOI] [PubMed] [Google Scholar]
- Spooren A, Kolmus K, Laureys G, Clinckers R, De Keyser J, Haegeman G, Gerlo S. Interleukin-6, a mental cytokine. Brain Res Rev. 2011;67:157–183. doi: 10.1016/j.brainresrev.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Park KM, Bowers WJ. Tumor necrosis factor-alpha mediated signaling in neuronal homeostasis and dysfunction. Cell Signal. 2010;22:977–983. doi: 10.1016/j.cellsig.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation. 2008;5:45. doi: 10.1186/1742-2094-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapil P, Atkinson R, Ramakrishna C, Cua DJ, Bergmann CC, Stohlman SA. Interleukin-12 (IL-12), but not IL-23, deficiency ameliorates viral encephalitis without affecting viral control. J Virol. 2009;83:5978–5986. doi: 10.1128/JVI.00315-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu T, Barna M, Reiss CS. Interleukin-12 promotes recovery from viral encephalitis. Viral Immunol. 1997;10:35–47. doi: 10.1089/vim.1997.10.35. [DOI] [PubMed] [Google Scholar]
- Camelo S, Lafage M, Lafon M. Absence of the p55 Kd TNF-alpha receptor promotes survival in rabies virus acute encephalitis. J Neurovirol. 2000;6:507–518. doi: 10.3109/13550280009091951. [DOI] [PubMed] [Google Scholar]
- Ubol S, Sukwattanapan C, Maneerat Y. Inducible nitric oxide synthase inhibition delays death of rabies virus-infected mice. J Med Microbiol. 2001;50:238–242. doi: 10.1099/0022-1317-50-3-238. [DOI] [PubMed] [Google Scholar]
- Lobigs M, Mullbacher A, Wang Y, Pavy M, Lee E. Role of type I and type II interferon responses in recovery from infection with an encephalitic flavivirus. J Gen Virol. 2003;84:567–572. doi: 10.1099/vir.0.18654-0. [DOI] [PubMed] [Google Scholar]
- Gomez RM, Yep A, Schattner M, Berria MI. Junin virus-induced astrocytosis is impaired by iNOS inhibition. J Med Virol. 2003;69:145–149. doi: 10.1002/jmv.10254. [DOI] [PubMed] [Google Scholar]
- Tucker PC, Griffin DE, Choi S, Bui N, Wesselingh S. Inhibition of nitric oxide synthesis increases mortality in Sindbis virus encephalitis. J Virol. 1996;70:3972–3977. doi: 10.1128/jvi.70.6.3972-3977.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getts DR, Terry RL, Getts MT, Muller MA, Radford J, Rana S, Ashhurst T, Deffrasnes C, Hofer M, Thomas S, Campbell IL, King NJ. Targeted blockade in lethal West Nile virus encephalitis shows a critical role for VLA-4-dependant recruitment of nitric oxide-producing macrophages. J Neuroinflammation. 2012;9:246. doi: 10.1186/1742-2094-9-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese V, Mancuso C, Calvani M, Rizzarelli E, Butterfield DA, Stella AM. Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nat Rev Neurosci. 2007;8:766–775. doi: 10.1038/nrn2214. [DOI] [PubMed] [Google Scholar]
- Wang P, Dai J, Bai F, Kong KF, Wong SJ, Montgomery RR, Madri JA, Fikrig E. Matrix metalloproteinase 9 facilitates West Nile virus entry into the brain. J Virol. 2008;82:8978–8985. doi: 10.1128/JVI.00314-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong VW. Metalloproteinases: mediators of pathology and regeneration in the CNS. Nat Rev Neurosci. 2005;6:931–944. doi: 10.1038/nrn1807. [DOI] [PubMed] [Google Scholar]
- Yong VW, Power C, Forsyth P, Edwards DR. Metalloproteinases in biology and pathology of the nervous system. Nat Rev Neurosci. 2001;2:502–511. doi: 10.1038/35081571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene IP, Lee EY, Prow N, Ngwang B, Griffin DE. Protection from fatal viral encephalomyelitis: AMPA receptor antagonists have a direct effect on the inflammatory response to infection. Proc Natl Acad Sci USA. 2008;105:3575–3580. doi: 10.1073/pnas.0712390105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nargi-Aizenman JL, Havert MB, Zhang M, Irani DN, Rothstein JD, Griffin DE. Glutamate receptor antagonists protect from virus-induced neural degeneration. Ann Neurol. 2004;55:541–549. doi: 10.1002/ana.20033. [DOI] [PubMed] [Google Scholar]
- Brison E, Jacomy H, Desforges M, Talbot PJ. Glutamate excitotoxicity is involved in the induction of paralysis in mice after infection by a human coronavirus with a single point mutation in its spike protein. J Virol. 2011;85:12464–12473. doi: 10.1128/JVI.05576-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks JJ, Teunissen CE, de Vries HE, Dijkstra CD. Macrophages and neurodegeneration. Brain Res Brain Res Rev. 2005;48:185–195. doi: 10.1016/j.brainresrev.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/S0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Weissman IL. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity. 1994;1:661–673. doi: 10.1016/1074-7613(94)90037-X. [DOI] [PubMed] [Google Scholar]
- Reddy MA, Yang BS, Yue X, Barnett CJ, Ross IL, Sweet MJ, Hume DA, Ostrowski MC. Opposing actions of c-ets/PU.1 and c-myb protooncogene products in regulating the macrophage-specific promoters of the human and mouse colony-stimulating factor-1 receptor (c-fms) genes. J Exp Med. 1994;180:2309–2319. doi: 10.1084/jem.180.6.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krysinska H, Hoogenkamp M, Ingram R, Wilson N, Tagoh H, Laslo P, Singh H, Bonifer C. A two-step, PU.1-dependent mechanism for developmentally regulated chromatin remodeling and transcription of the c-fms gene. Mol Cell Biol. 2007;27:878–887. doi: 10.1128/MCB.01915-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKoter RP, Walsh JC, Singh H. PU.1 regulates both cytokine-dependent proliferation and differentiation of granulocyte/macrophage progenitors. EMBO J. 1998;17:4456–4468. doi: 10.1093/emboj/17.15.4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagoh H, Himes R, Clarke D, Leenen PJ, Riggs AD, Hume D, Bonifer C. Transcription factor complex formation and chromatin fine structure alterations at the murine c-fms (CSF-1 receptor) locus during maturation of myeloid precursor cells. Genes Dev. 2002;16:1721–1737. doi: 10.1101/gad.222002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, Cumano A, Geissmann F. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311:83–87. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- Auffray C, Fogg D, Narni-Mancinelli E, Senechal B, Trouillet C, Saederup N, Leemput J, Bigot K, Campisi L, Abitbol M, Molina T, Charo I, Hume DA, Cumano A, Lauvau G, Geissmann F. CX3CR1+ CD115+ CD135+ common macrophage/DC precursors and the role of CX3CR1 in their response to inflammation. J Exp Med. 2009;206:595–606. doi: 10.1084/jem.20081385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogg D, Sibon C, Miled C, Jung S, Aucouturier P, Littman D, Cumano A, Geissmann F. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311:83–87. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Jung S, Littman D. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/S1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- Strauss-Ayali A, Conrad SM, Mosser D. Monocyte subpopulations and their differentiation patterns during infection. JLB. 2007;82:244–252. doi: 10.1189/jlb.0307191. [DOI] [PubMed] [Google Scholar]
- Bao Y, Kim E, Bhosle S, Mehta H, Cho S. A role for spleen monocytes in post-ischemic brain inflammation and injury. J Neuroinflammation. 2010;7:92. doi: 10.1186/1742-2094-7-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuschner F, Panizzi P, Chico-Calero I, Lee WW, Ueno T, Cortez-Retamozo V, Waterman P, Gorbatov R, Marinelli B, Iwamoto Y, Chudnovskiy A, Figueiredo JL, Sosnovik DE, Pittet MJ, Swirski FK, Weissleder R, Nahrendorf M. Angiotensin-converting enzyme inhibition prevents the release of monocytes from their splenic reservoir in mice with myocardial infarction. Circ Res. 2010;107:1364–1373. doi: 10.1161/CIRCRESAHA.110.227454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuschner F, Rauch PJ, Ueno T, Gorbatov R, Marinelli B, Lee WW, Dutta P, Wei Y, Robbins C, Iwamoto Y, Sena B, Chudnovskiy A, Panizzi P, Keliher E, Higgins JM, Libby P, Moskowitz MA, Pittet MJ, Swirski FK, Weissleder R, Nahrendorf M. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J Exp Med. 2012;209:123–137. doi: 10.1084/jem.20111009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderkötter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, Leenen PJM. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol. 2010;7:77–86. doi: 10.1038/nrcardio.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swirski FK, Weissleder R, Pittet MJ. Heterogeneous in vivo behavior of monocyte subsets in atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1424–1432. doi: 10.1161/ATVBAHA.108.180521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacke F, Alvarez D, Kaplan T, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swirski F, Libby P, Aikawa E, Alcaide P, Luscinskas F, Weissleder R, Pittet M. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruhl H, Cihak J, Plachy J, Kunz-Schughart L, Niedermeier M, Denzel A, Rodriguez Gomez M, Talke Y, Luckow B, Stangassinger M, Mack M. Targeting of Gr-1+,CCR2+ monocytes in collagen-induced arthritis. Arthritis Rheum. 2007;56:2975–2985. doi: 10.1002/art.22854. [DOI] [PubMed] [Google Scholar]
- Waddell A, Ahrens R, Steinbrecher K, Donovan B, Rothenberg ME, Munitz A, Hogan SP. Colonic eosinophilic inflammation in experimental colitis is mediated by Ly6C(high) CCR2(+) inflammatory monocyte/macrophage-derived CCL11. J Immunol. 2011;186:5993–6003. doi: 10.4049/jimmunol.1003844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahrendorf M, Swirski F, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo J, Libby P, Weissleder R, Pittet M. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King IL, Dickendesher TL, Segal BM. Circulating Ly-6C+ myeloid precursors migrate to the CNS and play a pathogenic role during autoimmune demyelinating disease. Blood. 2009;113:3190–3197. doi: 10.1182/blood-2008-07-168575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mildner A, Mack M, Schmidt H, Bruck W, Djukic M, Zabel MD, Hille A, Priller J, Prinz M. CCR2+Ly-6Chi monocytes are crucial for the effector phase of autoimmunity in the central nervous system. Brain. 2009;132:2487–2500. doi: 10.1093/brain/awp144. [DOI] [PubMed] [Google Scholar]
- Butovsky O, Siddiqui S, Gabriely G, Lanser AJ, Dake B, Murugaiyan G, Doykan CE, Wu PM, Gali RR, Iyer LK, Lawson R, Berry J, Krichevsky AM, Cudkowicz ME, Weiner HL. Modulating inflammatory monocytes with a unique microRNA gene signature ameliorates murine ALS. J Clin Invest. 2012;122:3063–3087. doi: 10.1172/JCI62636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophi GP, Hudson CA, Panos M, Gruber RC, Massa PT. Modulation of macrophage infiltration and inflammatory activity by the phosphatase SHP-1 in virus-induced demyelinating disease. J Virol. 2009;83:522–539. doi: 10.1128/JVI.01210-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacke F, Randolph G. Migratory fate and differentiation of blood monocyte subsets. Immunobiology. 2006;211:609–618. doi: 10.1016/j.imbio.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Varol C, Landsman L, Fogg D, Greenshtein L, Gildor B, Margalit R, Kalchenko V, Geissmann F, Jung S. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J Exp Med. 2007;204:171–180. doi: 10.1084/jem.20061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison AM, King NJ. Accelerated dendritic cell differentiation from migrating Ly6C(lo) bone marrow monocytes in early dermal West Nile virus infection. J Immunol. 2011;186:2382–2396. doi: 10.4049/jimmunol.1002682. [DOI] [PubMed] [Google Scholar]
- Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- Saederup N, Cardona A, Croft K, Mizutani M, Cotleur A, Tsou C, Ransohoff R, Charo I. Selective chemokine receptor usage by central nervous system myeloid cells in CCR2-red fluorescent protein knock-in mice. PLoS One. 2010;5:e13693. doi: 10.1371/journal.pone.0013693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- Hidalgo A, Sanz-Rodriguez F, Rodriguez-Fernandez JL, Albella B, Blaya C, Wright N, Cabanas C, Prosper F, Gutierrez-Ramos JC, Teixido J. Chemokine stromal cell-derived factor-1alpha modulates VLA-4 integrin-dependent adhesion to fibronectin and VCAM-1 on bone marrow hematopoietic progenitor cells. Exp Hematol. 2001;29:345–355. doi: 10.1016/S0301-472X(00)00668-8. [DOI] [PubMed] [Google Scholar]
- Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1) J Exp Med. 1996;184:1101–1109. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama Y, Hidalgo A, Peired A, Frenette PS. Integrin alpha4beta7 and its counterreceptor MAdCAM-1 contribute to hematopoietic progenitor recruitment into bone marrow following transplantation. Blood. 2004;104:2020–2026. doi: 10.1182/blood-2003-12-4157. [DOI] [PubMed] [Google Scholar]
- Sanz-Rodriguez F, Hidalgo A, Teixido J. Chemokine stromal cell-derived factor-1alpha modulates VLA-4 integrin-mediated multiple myeloma cell adhesion to CS-1/fibronectin and VCAM-1. Blood. 2001;97:346–351. doi: 10.1182/blood.V97.2.346. [DOI] [PubMed] [Google Scholar]
- Serbina N, Pamer E. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- Shi C, Velazquez P, Hohl T, Leiner I, Dustin M, Pamer E. Monocyte trafficking to hepatic sites of bacterial infection is chemokine independent and directed by focal intercellular adhesion molecule-1 expression. J Immunol. 2010;184:6266–6274. doi: 10.4049/jimmunol.0904160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia T, Serbina NV, Brandl K, Zhong MX, Leiner IM, Charo IF, Pamer EG. Additive roles for MCP-1 and MCP-3 in CCR2-mediated recruitment of inflammatory monocytes during Listeria monocytogenes infection. J Immunol. 2008;180:6846–6853. doi: 10.4049/jimmunol.180.10.6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel D, Maurer J, Tittel A, Weisheit C, Cavlar T, Schumak B, Limmer A, van Rooijen N, Trautwein C, Tacke F, Kurts C. CCR2 mediates homeostatic and inflammatory release of Gr1(high) monocytes from the bone marrow, but is dispensable for bladder infiltration in bacterial urinary tract infection. J Immunol. 2008;181:5579–5586. doi: 10.4049/jimmunol.181.8.5579. [DOI] [PubMed] [Google Scholar]
- Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, Charo IF. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117:902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Cui L, Gonsiorek W, Min SH, Anilkumar G, Rosenblum S, Kozlowski J, Lundell D, Fine JS, Grant EP. CCR2 and CXCR4 regulate peripheral blood monocyte pharmacodynamics and link to efficacy in experimental autoimmune encephalomyelitis. J Inflamm (Lond) 2009;6:32. doi: 10.1186/1476-9255-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin N, Menasria R, Gosselin D, Rivest S, Boivin G. Impact of deficiency in CCR2 and CX3CR1 receptors on monocytes trafficking in herpes simplex virus encephalitis. J Gen Virol. 2012;93:1294–1304. doi: 10.1099/vir.0.041046-0. [DOI] [PubMed] [Google Scholar]
- Chen BP, Kuziel WA, Lane TE. Lack of CCR2 results in increased mortality and impaired leukocyte activation and trafficking following infection of the central nervous system with a neurotropic coronavirus. J Immunol. 2001;167:4585–4592. doi: 10.4049/jimmunol.167.8.4585. [DOI] [PubMed] [Google Scholar]
- Ruzek D, Salat J, Singh SK, Kopecky J. Breakdown of the blood–brain barrier during tick-borne encephalitis in mice is not dependent on CD8+ T-cells. PLoS One. 2011;6:e20472. doi: 10.1371/journal.pone.0020472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass WG, Lim JK, Cholera R, Pletnev AG, Gao JL, Murphy PM. Chemokine receptor CCR5 promotes leukocyte trafficking to the brain and survival in West Nile virus infection. J Exp Med. 2005;202:1087–1098. doi: 10.1084/jem.20042530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass WG, Liu MT, Kuziel WA, Lane TE. Reduced macrophage infiltration and demyelination in mice lacking the chemokine receptor CCR5 following infection with a neurotropic coronavirus. Virology. 2001;288:8–17. doi: 10.1006/viro.2001.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilela MC, Mansur DS, Lacerda-Queiroz N, Rodrigues DH, Lima GK, Arantes RM, Kroon EG, Da Silva Campos MA, Teixeira MM, Teixeira AL. The chemokine CCL5 is essential for leukocyte recruitment in a model of severe Herpes simplex encephalitis. Ann N Y Acad Sci. 2009;1153:256–263. doi: 10.1111/j.1749-6632.2008.03959.x. [DOI] [PubMed] [Google Scholar]
- Malik M, Chen YY, Kienzle MF, Tomkowicz BE, Collman RG, Ptasznik A. Monocyte migration and LFA-1-mediated attachment to brain microvascular endothelia is regulated by SDF-1 alpha through Lyn kinase. J Immunol. 2008;181:4632–4637. doi: 10.4049/jimmunol.181.7.4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCandless EE, Wang Q, Woerner BM, Harper JM, Klein RS. CXCL12 limits inflammation by localizing mononuclear infiltrates to the perivascular space during experimental autoimmune encephalomyelitis. J Immunol. 2006;177:8053–8064. doi: 10.4049/jimmunol.177.11.8053. [DOI] [PubMed] [Google Scholar]
- Peng H, Erdmann N, Whitney N, Dou H, Gorantla S, Gendelman HE, Ghorpade A, Zheng J. HIV-1-infected and/or immune activated macrophages regulate astrocyte SDF-1 production through IL-1beta. Glia. 2006;54:619–629. doi: 10.1002/glia.20409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCandless EE, Zhang B, Diamond MS, Klein RS. CXCR4 antagonism increases T cell trafficking in the central nervous system and improves survival from West Nile virus encephalitis. Proc Natl Acad Sci USA. 2008;105:11270–11275. doi: 10.1073/pnas.0800898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Mello C, Le T, Swain M. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. J Neurosci. 2009;29:2089–2102. doi: 10.1523/JNEUROSCI.3567-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman S, El Khoury J. Mechanisms of mononuclear phagocyte recruitment in Alzheimer's disease. CNS Neurol Disord Drug Targets. 2010;9:168–173. doi: 10.2174/187152710791011982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mildner A, Schlevogt B, Kierdorf K, Bottcher C, Erny D, Kummer MP, Quinn M, Bruck W, Bechmann I, Heneka MT, Priller J, Prinz M. Distinct and Non-Redundant Roles of Microglia and Myeloid Subsets in Mouse Models of Alzheimer's Disease. J Neurosci. 2011;31:11159–11171. doi: 10.1523/JNEUROSCI.6209-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izikson L, Klein RS, Charo IF, Weiner HL, Luster AD. Resistance to experimental autoimmune encephalomyelitis in mice lacking the CC chemokine receptor (CCR)2. J Exp Med. 2000;192:1075–1080. doi: 10.1084/jem.192.7.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogan RN, Elhofy A, Karpus WJ. Production of CCL2 by central nervous system cells regulates development of murine experimental autoimmune encephalomyelitis through the recruitment of TNF- and iNOS-expressing macrophages and myeloid dendritic cells. J Immunol. 2008;180:7376–7384. doi: 10.4049/jimmunol.180.11.7376. [DOI] [PubMed] [Google Scholar]
- Wuest TR, Carr DJ. The role of chemokines during herpes simplex virus-1 infection. Front Biosci. 2008;13:4862–4872. doi: 10.2741/3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persidsky Y, Ghorpade A, Rasmussen J, Limoges J, Liu XJ, Stins M, Fiala M, Way D, Kim KS, Witte MH, Weinand M, Carhart L, Gendelman HE. Microglial and astrocyte chemokines regulate monocyte migration through the blood–brain barrier in human immunodeficiency virus-1 encephalitis. Am J Pathol. 1999;155:1599–1611. doi: 10.1016/S0002-9440(10)65476-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques CP, Hu S, Sheng W, Lokensgard JR. Microglial cells initiate vigorous yet non-protective immune responses during HSV-1 brain infection. Virus Res. 2006;121:1–10. doi: 10.1016/j.virusres.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Savarin C, Stohlman SA, Atkinson R, Ransohoff RM, Bergmann CC. Monocytes regulate T cell migration through the glia limitans during acute viral encephalitis. J Virol. 2010;84:4878–4888. doi: 10.1128/JVI.00051-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held KS, Chen BP, Kuziel WA, Rollins BJ, Lane TE. Differential roles of CCL2 and CCR2 in host defense to coronavirus infection. Virology. 2004;329:251–260. doi: 10.1016/j.virol.2004.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane TE, Hosking MP. The pathogenesis of murine coronavirus infection of the central nervous system. Crit Rev Immunol. 2010;30:119–130. doi: 10.1615/CritRevImmunol.v30.i2.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett JL, Elhofy A, Charo I, Miller SD, Dal Canto MC, Karpus WJ. CCR2 regulates development of Theiler's murine encephalomyelitis virus-induced demyelinating disease. Viral Immunol. 2007;20:19–33. doi: 10.1089/vim.2006.0068. [DOI] [PubMed] [Google Scholar]
- Yednock TA, Cannon C, Fritz LC, Sanchez-Madrid F, Steinman L, Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992;356:63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- Gordon EJ, Myers KJ, Dougherty JP, Rosen H, Ron Y. Both anti-CD11a (LFA-1) and anti-CD11b (MAC-1) therapy delay the onset and diminish the severity of experimental autoimmune encephalomyelitis. J Neuroimmunol. 1995;62:153–160. doi: 10.1016/0165-5728(95)00120-2. [DOI] [PubMed] [Google Scholar]
- Ceulemans AG, Zgavc T, Kooijman R, Hachimi-Idrissi S, Sarre S, Michotte Y. The dual role of the neuroinflammatory response after ischemic stroke: modulatory effects of hypothermia. J Neuroinflammation. 2010;7:74. doi: 10.1186/1742-2094-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persidsky Y, Buttini M, Limoges J, Bock P, Gendelman HE. An analysis of HIV-1-associated inflammatory products in brain tissue of humans and SCID mice with HIV-1 encephalitis. J Neurovirol. 1997;3:401–416. doi: 10.3109/13550289709031186. [DOI] [PubMed] [Google Scholar]
- Lewandowski G, Hobbs MV. Evidence for deficiencies in intracerebral cytokine production, adhesion molecule induction, and T cell recruitment in herpes simplex virus type-2 infected mice. J Neuroimmunol. 1998;81:58–65. doi: 10.1016/S0165-5728(97)00159-8. [DOI] [PubMed] [Google Scholar]
- Sharma A, Bhomia M, Honnold SP, Maheshwari RK. Role of adhesion molecules and inflammation in Venezuelan equine encephalitis virus infected mouse brain. Virol J. 2011;8:197. doi: 10.1186/1743-422X-8-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra MK, Dutta K, Saheb SK, Basu A. Understanding the molecular mechanism of blood–brain barrier damage in an experimental model of Japanese encephalitis: correlation with minocycline administration as a therapeutic agent. Neurochem Int. 2009;55:717–723. doi: 10.1016/j.neuint.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Cybulsky MI, Iiyama K, Li H, Zhu S, Chen M, Iiyama M, Davis V, Gutierrez-Ramos JC, Connelly PW, Milstone DS. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J Clin Invest. 2001;107:1255–1262. doi: 10.1172/JCI11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barringhaus KG, Phillips JW, Thatte JS, Sanders JM, Czarnik AC, Bennett DK, Ley KF, Sarembock IJ. Alpha4beta1 integrin (VLA-4) blockade attenuates both early and late leukocyte recruitment and neointimal growth following carotid injury in apolipoprotein E (−/−) mice. J Vasc Res. 2004;41:252–260. doi: 10.1159/000078646. [DOI] [PubMed] [Google Scholar]
- Huo Y, Hafezi-Moghadam A, Ley K. Role of vascular cell adhesion molecule-1 and fibronectin connecting segment-1 in monocyte rolling and adhesion on early atherosclerotic lesions. Circ Res. 2000;87:153–159. doi: 10.1161/01.RES.87.2.153. [DOI] [PubMed] [Google Scholar]
- Henderson RB, Hobbs JA, Mathies M, Hogg N. Rapid recruitment of inflammatory monocytes is independent of neutrophil migration. Blood. 2003;102:328–335. doi: 10.1182/blood-2002-10-3228. [DOI] [PubMed] [Google Scholar]
- Fleming JC, Bao F, Chen Y, Hamilton EF, Gonzalez-Lara LE, Foster PJ, Weaver LC. Timing and duration of anti-alpha4beta1 integrin treatment after spinal cord injury: effect on therapeutic efficacy. J Neurosurg Spine. 2009;11:575–587. doi: 10.3171/2009.6.SPINE08915. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Fan J. Atherosclerosis and inflammation mononuclear cell recruitment and adhesion molecules with reference to the implication of ICAM-1/LFA-1 pathway in atherogenesis. Int J Cardiol. 1998;66(1):S45–S53. doi: 10.1016/S0167-5273(98)00128-4. discussion S55. [DOI] [PubMed] [Google Scholar]
- Patel SS, Thiagarajan R, Willerson JT, Yeh ET. Inhibition of alpha4 integrin and ICAM-1 markedly attenuate macrophage homing to atherosclerotic plaques in ApoE-deficient mice. Circulation. 1998;97:75–81. doi: 10.1161/01.CIR.97.1.75. [DOI] [PubMed] [Google Scholar]
- Alliot F, Godin I, Pessac B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res Dev Brain Res. 1999;117:145–152. doi: 10.1016/S0165-3806(99)00113-3. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Naito M, Takeya M. Development and heterogeneity of macrophages and their related cells through their differentiation pathways. Pathol Int. 1996;46:473–485. doi: 10.1111/j.1440-1827.1996.tb03641.x. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick JD, Schwender S, Imrich H, Dorries R, Butcher GW, ter Meulen V. Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proc Natl Acad Sci USA. 1991;88:7438–7442. doi: 10.1073/pnas.88.16.7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack CL, Vanderlugt-Castaneda CL, Neville KL, Miller SD. Microglia are activated to become competent antigen presenting and effector cells in the inflammatory environment of the Theiler's virus model of multiple sclerosis. J Neuroimmunol. 2003;144:68–79. doi: 10.1016/j.jneuroim.2003.08.032. [DOI] [PubMed] [Google Scholar]
- Simard AR, Soulet D, Gowing G, Julien JP, Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer's disease. Neuron. 2006;49:489–502. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Malm TM, Koistinaho M, Parepalo M, Vatanen T, Ooka A, Karlsson S, Koistinaho J. Bone-marrow-derived cells contribute to the recruitment of microglial cells in response to beta-amyloid deposition in APP/PS1 double transgenic Alzheimer mice. Neurobiol Dis. 2005;18:134–142. doi: 10.1016/j.nbd.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Heppner FL, Greter M, Marino D, Falsig J, Raivich G, Hovelmeyer N, Waisman A, Rulicke T, Prinz M, Priller J, Becher B, Aguzzi A. Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat Med. 2005;11:146–152. doi: 10.1038/nm1177. [DOI] [PubMed] [Google Scholar]
- Kokovay E, Cunningham LA. Bone marrow-derived microglia contribute to the neuroinflammatory response and express iNOS in the MPTP mouse model of Parkinson's disease. Neurobiol Dis. 2005;19:471–478. doi: 10.1016/j.nbd.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Priller J, Prinz M, Heikenwalder M, Zeller N, Schwarz P, Heppner FL, Aguzzi A. Early and rapid engraftment of bone marrow-derived microglia in scrapie. J Neurosci. 2006;26:11753–11762. doi: 10.1523/JNEUROSCI.2275-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djukic M, Mildner A, Schmidt H, Czesnik D, Bruck W, Priller J, Nau R, Prinz M. Circulating monocytes engraft in the brain, differentiate into microglia and contribute to the pathology following meningitis in mice. Brain. 2006;129:2394–2403. doi: 10.1093/brain/awl206. [DOI] [PubMed] [Google Scholar]
- Stalder AK, Ermini F, Bondolfi L, Krenger W, Burbach GJ, Deller T, Coomaraswamy J, Staufenbiel M, Landmann R, Jucker M. Invasion of hematopoietic cells into the brain of amyloid precursor protein transgenic mice. J Neurosci. 2005;25:11125–11132. doi: 10.1523/JNEUROSCI.2545-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Khoury J, Toft M, Hickman SE, Means TK, Terada K, Geula C, Luster AD. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med. 2007;13:432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- Mildner A, Schmidt H, Nitsche M, Merkler D, Hanisch UK, Mack M, Heikenwalder M, Bruck W, Priller J, Prinz M. Microglia in the adult brain arise from Ly-6C(hi)CCR2(+) monocytes only under defined host conditions. Nat Neurosci. 2007;10:1544–1553. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci. 2011;14:1142–1149. doi: 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- Robben PM, LaRegina M, Kuziel WA, Sibley LD. Recruitment of Gr-1+ monocytes is essential for control of acute toxoplasmosis. J Exp Med. 2005;201:1761–1769. doi: 10.1084/jem.20050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunay IR, Fuchs A, Sibley LD. Inflammatory monocytes but not neutrophils are necessary to control infection with Toxoplasma gondii in mice. Infect Immun. 2010;78:1564–1570. doi: 10.1128/IAI.00472-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59–70. doi: 10.1016/S1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- Osterholzer JJ, Curtis JL, Polak T, Ames T, Chen GH, McDonald R, Huffnagle GB, Toews GB. CCR2 mediates conventional dendritic cell recruitment and the formation of bronchovascular mononuclear cell infiltrates in the lungs of mice infected with Cryptococcus neoformans. J Immunol. 2008;181:610–620. doi: 10.4049/jimmunol.181.1.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynor TR, Kuziel WA, Toews GB, Huffnagle GB. CCR2 expression determines T1 versus T2 polarization during pulmonary Cryptococcus neoformans infection. J Immunol. 2000;164:2021–2027. doi: 10.4049/jimmunol.164.4.2021. [DOI] [PubMed] [Google Scholar]
- Ye Z, Uittenbogaard AM, Cohen DA, Kaplan AM, Ambati J, Straley SC. Distinct CCR2(+) Gr1(+) cells control growth of the Yersinia pestis DeltayopM mutant in liver and spleen during systemic plague. Infect Immun. 2011;79:674–687. doi: 10.1128/IAI.00808-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima N, Mattei LM, Iwasaki A. Recruited inflammatory monocytes stimulate antiviral Th1 immunity in infected tissue. Proc Natl Acad Sci USA. 2011;108:284–289. doi: 10.1073/pnas.1005201108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan T, Morrison TE, Funkhouser W, Uematsu S, Akira S, Baric RS, Heise MT. MyD88 is required for protection from lethal infection with a mouse-adapted SARS-CoV. PLoS Pathog. 2008;4:e1000240. doi: 10.1371/journal.ppat.1000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinat C, Mena I, Brahic M. Theiler's virus infection of primary cultures of bone marrow-derived monocytes/macrophages. J Virol. 2002;76:12823–12833. doi: 10.1128/JVI.76.24.12823-12833.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton HL, Twaddle G, Jelachich ML. The predominant virus antigen burden is present in macrophages in Theiler's murine encephalomyelitis virus-induced demyelinating disease. J Virol. 1995;69:2525–2533. doi: 10.1128/jvi.69.4.2525-2533.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clatch RJ, Miller SD, Metzner R, Dal Canto MC, Lipton HL. Monocytes/macrophages isolated from the mouse central nervous system contain infectious Theiler's murine encephalomyelitis virus (TMEV) Virology. 1990;176:244–254. doi: 10.1016/0042-6822(90)90249-Q. [DOI] [PubMed] [Google Scholar]
- Blakemore WF, Welsh CJ, Tonks P, Nash AA. Observations on demyelinating lesions induced by Theiler's virus in CBA mice. Acta Neuropathol. 1988;76:581–589. doi: 10.1007/BF00689596. [DOI] [PubMed] [Google Scholar]
- Aubert C, Chamorro M, Brahic M. Identification of Theiler's virus infected cells in the central nervous system of the mouse during demyelinating disease. Microb Pathog. 1987;3:319–326. doi: 10.1016/0882-4010(87)90002-7. [DOI] [PubMed] [Google Scholar]
- Wang H, Sun J, Goldstein H. Human immunodeficiency virus type 1 infection increases the in vivo capacity of peripheral monocytes to cross the blood–brain barrier into the brain and the in vivo sensitivity of the blood–brain barrier to disruption by lipopolysaccharide. J Virol. 2008;82:7591–7600. doi: 10.1128/JVI.00768-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets DA, Dillon MJ, Schawang JS, Van Rooijen N, Ehrchen J, Sunderkotter C, Leenen PJ. The Ly-6Chigh monocyte subpopulation transports Listeria monocytogenes into the brain during systemic infection of mice. J Immunol. 2004;172:4418–4424. doi: 10.4049/jimmunol.172.7.4418. [DOI] [PubMed] [Google Scholar]
- Drevets D, Dillon M, Schawang J, Stoner J, Leenen P. IFN-gamma triggers CCR2-independent monocyte entry into the brain during systemic infection by virulent Listeria monocytogenes. Brain Behav Immun. 2010;24:919–929. doi: 10.1016/j.bbi.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Charlier C, Nielsen K, Daou S, Brigitte M, Chretien F, Dromer F. Evidence of a role for monocytes in dissemination and brain invasion by Cryptococcus neoformans. Infect Immun. 2009;77:120–127. doi: 10.1128/IAI.01065-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courret N, Darche S, Sonigo P, Milon G, Buzoni-Gatel D, Tardieux I. CD11c- and CD11b-expressing mouse leukocytes transport single Toxoplasma gondii tachyzoites to the brain. Blood. 2006;107:309–316. doi: 10.1182/blood-2005-02-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling M, Strecker JK, Ringelstein EB, Schabitz WR, Kiefer R. The role of CC chemokine receptor 2 on microglia activation and blood-borne cell recruitment after transient focal cerebral ischemia in mice. Brain Res. 2009;1289:79–84. doi: 10.1016/j.brainres.2009.06.054. [DOI] [PubMed] [Google Scholar]
- Schilling M, Strecker JK, Schabitz WR, Ringelstein EB, Kiefer R. Effects of monocyte chemoattractant protein 1 on blood-borne cell recruitment after transient focal cerebral ischemia in mice. Neuroscience. 2009;161:806–812. doi: 10.1016/j.neuroscience.2009.04.025. [DOI] [PubMed] [Google Scholar]
- Semple BD, Bye N, Rancan M, Ziebell JM, Morganti-Kossmann MC. Role of CCL2 (MCP-1) in traumatic brain injury (TBI): evidence from severe TBI patients and CCL2−/− mice. J Cereb Blood Flow Metab. 2010;30:769–782. doi: 10.1038/jcbfm.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fife BT, Huffnagle GB, Kuziel WA, Karpus WJ. CC chemokine receptor 2 is critical for induction of experimental autoimmune encephalomyelitis. J Exp Med. 2000;192:899–905. doi: 10.1084/jem.192.6.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KJ, Strieter RM, Kunkel SL, Lukacs NW, Karpus WJ. Acute and relapsing experimental autoimmune encephalomyelitis are regulated by differential expression of the CC chemokines macrophage inflammatory protein-1alpha and monocyte chemotactic protein-1. J Neuroimmunol. 1998;92:98–108. doi: 10.1016/S0165-5728(98)00187-8. [DOI] [PubMed] [Google Scholar]