Abstract
Background
Endothelin-1 (ET-1) is a proinflammatory mediator and elevated in the regions of several brain injury and inflammatory diseases. The deleterious effects of ET-1 on endothelial cells may aggravate brain inflammation mediated through the regulation of cyclooxygenase-2 (COX-2)/prostaglandin E2 (PGE2) system in various cell types. However, the signaling mechanisms underlying ET-1-induced COX-2 expression in brain microvascular endothelial cells remain unclear. Herein we investigated the effects of ET-1 in COX-2 regulation in mouse brain microvascular endothelial (bEnd.3) cells.
Results
The data obtained with Western blotting, RT-PCR, and immunofluorescent staining analyses showed that ET-1-induced COX-2 expression was mediated through an ETB-dependent transcriptional activation. Engagement of Gi- and Gq-protein-coupled ETB receptors by ET-1 led to phosphorylation of ERK1/2, p38 MAPK, and JNK1/2 and then activated transcription factor NF-κB. Moreover, the data of chromatin immunoprecipitation (ChIP) and promoter reporter assay demonstrated that the activated NF-κB was translocated into nucleus and bound to its corresponding binding sites in COX-2 promoter, thereby turning on COX-2 gene transcription. Finally, up-regulation of COX-2 by ET-1 promoted PGE2 release in these cells.
Conclusions
These results suggested that in mouse bEnd.3 cells, activation of NF-κB by ETB-dependent MAPK cascades is essential for ET-1-induced up-regulation of COX-2/PGE2 system. Understanding the mechanisms of COX-2 expression and PGE2 release regulated by ET-1/ETB system on brain microvascular endothelial cells may provide rationally therapeutic interventions for brain injury or inflammatory diseases.
Keywords: Endothelin-1, COX-2, MAPK, NF-κB, Brain microvascular endothelial cells
Background
Cerebral capillary and microvascular endothelial cells play an active role in maintaining cerebral blood flow, microvascular tone and blood-brain barrier (BBB) functions [1]. In the development of various vascular diseases, an early finding is dysfunction of the vascular endothelium that is closely related to clinical events in patients with atherosclerosis and hypertension [2,3]. The vasoactive mediators such as endothelin (ET) could be produced by endothelial cells to maintain hemodynamic responses. Production and release of ETs from cultured endothelial cells are regulated at transcription and translation levels by a variety of chemical and physical stimuli and the levels of ET, ET-1 especially, are elevated in shock, myocardial infarction, and kidney failure indicative of enhanced formation in these diseases [4]. Moreover, the bioactivity of ET-1 triggers vasoconstriction and pro-inflammatory action which have been implicated in the pathogenesis of hypertension and vascular diseases [3-6]. The effects of ET-1 are mediated through a G protein-dependent regulation, including two types of ET receptors: ET type A (ETA) and type B (ETB) [7]. ETA is involved in constriction and proliferation of vascular smooth muscle cells, whereas ETB on endothelial cells mediates the generation of nitric oxide, which acts as vasodilator and inhibits platelet aggregation [8]. Moreover, ET-1 also plays a substantial role in the normal development or in the central nervous system (CNS) diseases. In brain, endothelial cells [9] and astrocytes [10] are potential sources of ET-1 release in response to hypoxic/ischemic injury of the brain. A report has shown that the ETB receptors are located on brain endothelial and vascular smooth muscle cells, and modulate post-injury responses of these cells in the CNS [6]. Thus, there is an increasing interest in the regulatory role of endothelial cells in neurovascular coupling, which matches adequate supply of cerebral blood flow with the local metabolic demands that are imposed by neural activity [11]. As a fundamental component of the neurovascular unit, endothelial dysfunction has been shown to be implicated in neurodegenerative diseases [11,12]. Circumstantial evidence has further demonstrated that overexpression of ET-1 on endothelial cells has deleterious effects on ischemic brain [1]. It has been demonstrated that endothelial ET-1 induces cytokines or chemokines (e.g., interleukine-1 or interleukin-8) production and secretion by non-neuronal cells, including astrocytes and human brain-derived endothelial cells, which directly contributes to BBB breakdown during CNS inflammation [11,13]. These findings suggest that ET-1 might be involved in neuroinflammation. However, the detailed mechanisms responsible for ET-1 action are still limited.
Cyclooxygenase (COX), known as prostaglandin-endoperoxide synthase, is a rate-limiting key enzyme in the synthesis of prostaglandins (PGs). In this process, phospholipase A2 catalyzes the release of arachidonic acid (AA) from membrane phospholipids, while COX catalyzes the conversion of AA into PGs [14,15]. COX exists two isoforms: COX-1, which is constitutively expressed under normal conditions in most tissues, mediates regulating normal physiological responses and controls vascular homeostasis; COX-2, is not detectable in most normal tissues or cells, but its expression can be induced by a variety of stimuli such as cytokines, endotoxin, and growth factors to produce PGs during inflammatory responses in various cell types like vascular endothelial and smooth muscle cells [16,17]. Previous reports have shown that COX-2 immunoreactivity is a characteristic finding in the synovial macrophage and vascular cells of patients with arthritis and atherosclerosis, respectively. Moreover, several studies have indicated COX-2 as a major therapeutic target for the treatment of inflammatory disorders like arthritis [14]. The mice with homozygous deletion of the cox-2 gene lead to a striking reduction of endotoxin-induced inflammation [18]. Accordingly, COX-2 may play a crucial role in the development of various inflammatory responses including vascular inflammation. In the CNS, several studies have indicated that up-regulation of COX-2 leads to production of PGs which are potent inflammatory mediators in neurodegenerative disorders [19].
ET-1 is known to activate ET receptors (ETA or ETB), a heterotrimeric G protein-coupled receptor (GPCR), which stimulate multiple signaling pathways and regulate diverse cellular functions [7,20-22]. The principal mechanism underlying activation by ET-1 is mediated through ETB receptors coupling Gq proteins, resulting in activation of phospholipase C (PLC)-β, phosphoinositide (PI) hydrolysis, and formation of inositol trisphosphate (IP3) and diacylglycerol, leading to Ca2+ increase and protein kinase C (PKC) activation [22]. Activation of a Gi protein-coupled ETB receptor has been also shown to inhibit adenylyl cyclase activity [23]. Additionally, several studies have demonstrated that activation of Gq and Gi protein-coupled receptors via different signal pathways could activate diverse mitogen-activated protein kinases (MAPKs) [24]. It has been shown that ET-1-stimulated MAPKs activation to regulate various cellular responses including cell survival, growth, proliferation, and cellular hypertrophy in several cell types [21,25]. Several studies have suggested that up-regulation of COX-2 requires activation of MAPKs and related transcription factors in various cell types [22,26,27]. Our previous reports also demonstrate that several GPCR agonists (e.g. sphingosine 1-phosphate, thrombin, and bradykinin) stimulate MAPKs and NF-κB activation associated with COX-2 expression in rat VSMCs and astrocytes [28,29]. Although several pro-inflammatory mediators have been extensively confirmed to rapidly up-regulate NF-κB-dependent genes such as COX-2 and play a critical role in inflammation [29,30], the signaling mechanisms by which ET-1-induced MAPKs activation linked to COX-2 expression and PGE2 production are not completely defined in brain microvascular endothelial cells.
In this study, we investigated the molecular mechanisms underlying ET-1-induced COX-2 expression in mouse brain microvascular endothelial (bEnd.3) cells. These findings suggested that ET-1 induces COX-2 expression at the transcriptional and translational levels, which is mediated through the ETB receptor (coupling to Gi and Gq)-dependent activation of ERK1/2, p38 MAPK, JNK1/2, and NF-κB pathway, leading to PGE2 biosynthesis in mouse bEnd.3 cells. These results provide new insights into the mechanisms of ET-1 action which may be therapeutic value in brain inflammatory diseases.
Results
ET-1 induces COX-2 expression and PGE2 release in bEnd.3 cells
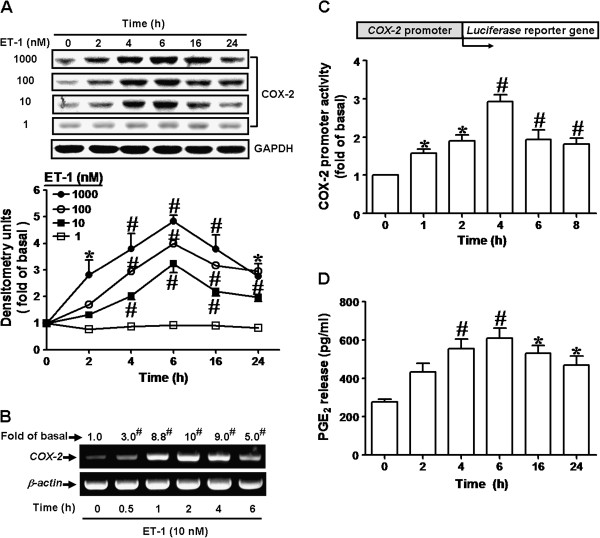
To investigate the effect of ET-1 on COX-2/PGE2 system, bEnd.3 cells were incubated with various concentrations of ET-1 for the indicated time intervals. The data showed that ET-1 induced COX-2 expression in a time- and concentration-dependent manner (Figure 1A). There was a significant increase within 2-4 h, reached a maximal response within 6 h, and declined within 24 h. ET-1 also time-dependently induced COX-2 mRNA expression in bEnd.3 cells, determined by RT-PCR (Figure 1B). There was a significant increase in COX-2 mRNA within 30 min, and reached a maximal response within 2 h. Moreover, to confirm whether ET-1 induces COX-2 expression via the transcription activity of COX-2 promoter, cells were transiently transfected with COX-2 promoter-luciferase reporter construct and then stimulated with ET-1 for the indicated time intervals. As shown in Figure 1C, ET-1 time-dependently induced COX-2 promoter-luciferase activity in bEnd.3 cells. A maximal response was obtained within 4 h. Our previous studies have shown that COX-2 expression induced by BK or sphingosine 1-phosphate is mainly responsible for prostanoid (PGE2) release in various cell types [28,29]. Thus, to determine whether ET-1 could induce PGE2 biosynthesis, we collected the conditioned media and determined PGE2 levels by using an EIA kit. The results showed that ET-1 time-dependently stimulated PGE2 release (Figure 1D) and a significant PGE2 production was observed within 4 h, reached a maximal response within 6 h and slightly declined within 24 h. These results suggested that ET-1 induces COX-2/PGE2 system via up-regulating COX-2 gene expression in bEnd.3 cells.
Figure 1.
ET-1 induced COX-2 expression and PGE2 release. (A) Time and concentration dependence of ET-1-induced COX-2 expression, cells were treated with various concentration ET-1 for the indicated time intervals. (B) Time dependence of ET-1-induced COX-2 mRNA expression, cells were treated with 10 nM ET-1 for the indicated time intervals. COX-2 mRNA was analyzed by RT-PCR. (C) Time dependence of ET-1-induced COX-2 promoter transcription activity, cells were transfected a COX-2 promoter–luciferase reporter gene and then incubated with ET-1 for the indicated times. The promoter reporter assay was performed as described in “Materials and Methods”. (D) PGE2 release induced by ET-1, the conditioned media were collected to assay PGE2 level by EIA as described in “Materials and Methods” (n=3 in each group; *P<0.05, #P<0.01 compared with vehicle).
ET-1 upregulates COX-2 expression via an ETB receptor
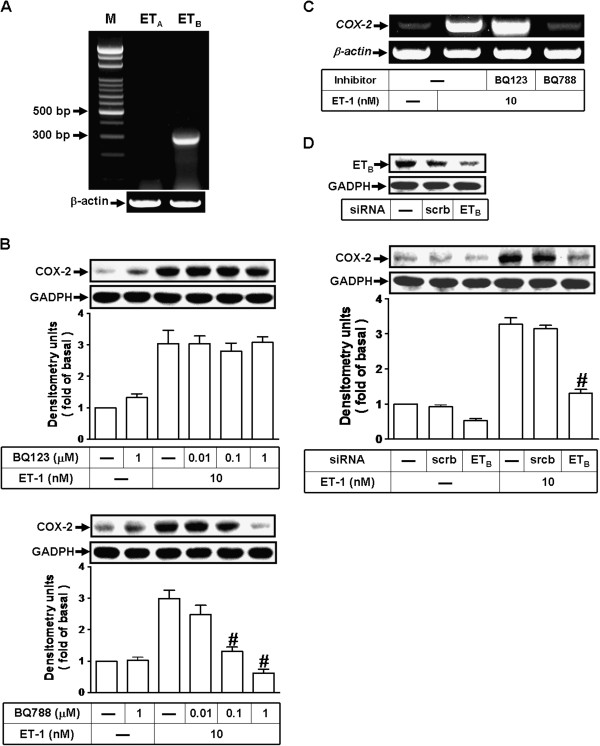
ET-1 exerts its biological effects via ET receptors, including ETA and ETB, which are members of GPCR superfamily [7]. First, we determined which subtypes of ET receptors are expressed on bEnd.3 cells by RT-PCR. The data showed that ETB but not ETA receptors are expressed on bEnd.3 cells (Figure 2A). Next, to identify the subtypes of ET receptors involved in ET-1-induced COX-2 expression, pretreatment with BQ-788 (an ETB antagonist), but not BQ-123 (an ETA antagonist), attenuated the ET-1-induced COX-2 protein (Figure 2B) and mRNA (Figure 2C) expression, suggesting that ETB receptor is predominantly involved in these responses. To further confirm this note, transfection of cells with ETB siRNA significantly down-regulated ETB protein expression and inhibited ET-1-induecd COX-2 expression (Figure 2D). These data suggested that ET-1-induced COX-2 expression is mediated through an ETB receptor-dependent manner in these cells.
Figure 2.
Involvement of ETB receptors in ET-1-induced COX-2 expression. (A) RT-PCR analysis of ET receptor expression in bEnd.3 cells. Lane 1: maker. Lane 2: ETA primers and bEnd.3 RNA. Lane 3: ETB primers and bEnd.3 RNA. (B, C) Cells were pretreated with BQ-123 or BQ-788 for 1 h and then incubated with ET-1 for (B) 6 h and (C) 1 h. (D) Cells were transfected with siRNA for ETB receptor for 24 h and then exposed to ET-1 for 6 h. The (B, D) COX-2 protein and (C) mRNA were analyzed by Western blot and RT-PCR, respectively as described in Figure 1. Data are expressed as mean ± SEM of three individual experiments (n=3 in each group, #P<0.01 as compared with cells stimulated by ET-1 alone).
Involvement of a Gi and Gq protein-coupled ETB receptor in ET-1-induecd COX-2 expression
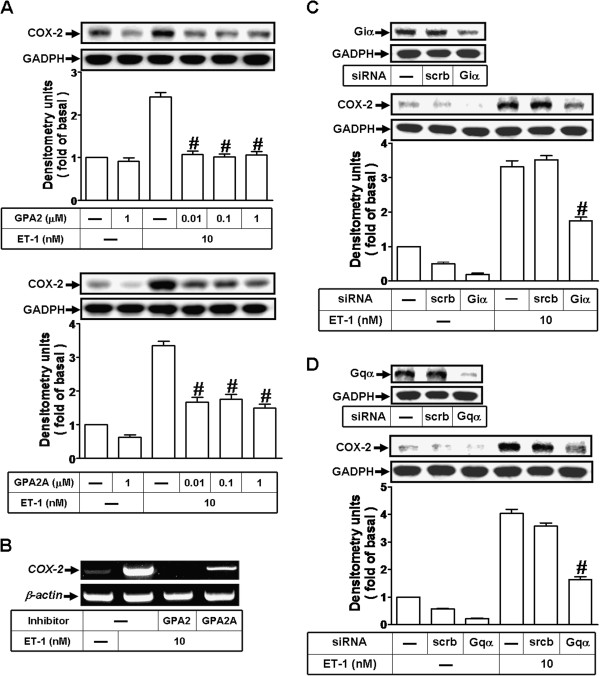
ET receptor has been shown to be a pleiotropic GPCR for ET-1 which is coupled to G proteins including Gi and Gq[7,22]. To further determine which of G proteins was involved in ET-1-induced COX-2 expression, pretreatment with either Gi protein antagonist GP antagonist-2 (GPA2) or Gq protein antagonist GP antagonist-2A (GPA2A) concentration-dependently attenuated ET-1-induced COX-2 protein (Figure 3A) and mRNA (Figure 3B) expression. Furthermore, to confirm these results, as shown in Figure 3C and D, transfection with either Giα or Gqα down-regulated Giα or Gqα protein, respectively, and attenuated ET-1-induced COX-2 expression. These data demonstrated that ET-1-induced COX-2 expression is mediated through either Gi or Gq protein-coupled ETB receptors in bEnd.3 cells.
Figure 3.
ET-1 induces COX-2 expression via a Gi and Gq proteins-coupled ETB receptor. (A, B) Cells were pretreated with Gi antagonist (GPA2) or Gq antagonist (GPA2A) for 1 h and then exposed to ET-1 for (A) 6 h and (B) 1 h. (C, D) Cells were transfected with siRNA for (C) Giα or (D) Gqα protein for 24 h and then exposed to ET-1 for 6 h. The (A, C, D) COX-2 protein and (B) mRNA were analyzed by Western blot and RT-PCR, respectively as described in Figure 1. Data are expressed as mean ± SEM of three individual experiments (n=3 in each group, #P<0.01 as compared with ET-1 alone).
ET-1-induced COX-2 expression is mediated through MAPKs
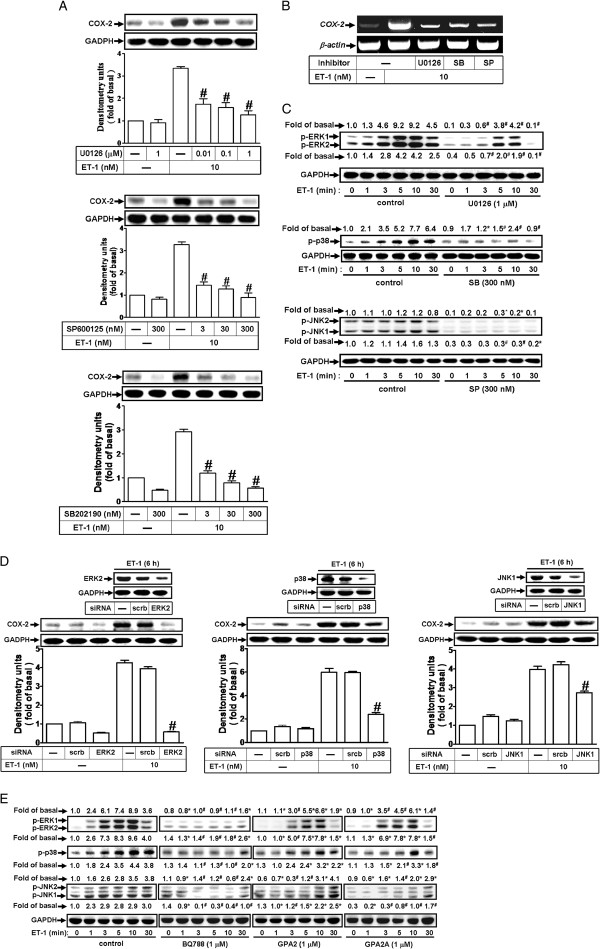
Activation of MAPKs by ET-1 could modulate cellular functions of endothelial cells [31]. To investigate the roles of ERK1/2, p38 MAPK, and JNK1/2 in ET-1-induced COX-2 expression, pretreatment with the inhibitor of MEK1/2 (U0126), p38 MAPK (SB202190), or JNK1/2 (SP600125) attenuated ET-1-induced COX-2 protein (Figure 4A) and mRNA (Figure 4B) expression in bEnd.3 cells, suggesting the involvement of ERK1/2, p38 MAPK, and JNK1/2 in ET-1-induced responses. To further determine whether ET-1-stimulated ERK1/2, p38 MAPK, and JNK1/2 phosphorylation is involved in COX-2 expression, as shown in Figure 4C, ET-1 time-dependently stimulated ERK1/2, p38 MAPK, and JNK1/2 phosphorylation which was attenuated by pretreatment with U0126, SB202190, or SP600125 during the period of observation. Moreover, to ensure the roles of MAPKs in ET-1-induced COX-2 expression, transfection with siRNA of ERK2, p38 MAPK, or JNK1 down-regulated the expression of total ERK2, p38 MAPK, or JNK1 protein and attenuated ET-1-induced COX-2 expression (Figure 4D). These data indicated that phosphorylation of ERK1/2, p38 MAPK, and JNK1/2 is involved in ET-1-induced COX-2 expression in bEnd.3 cells. To demonstrate whether ET-1 stimulates ERK1/2, p38 MAPK, and JNK1/2 phosphorylation via a G protein-coupled ETB receptor cascade, pretreatment with BQ-788, GPA2, or GPA2A attenuated ET-1-stimulated ERK1/2, p38 MAPK, and JNK1/2 phosphorylation during the period of observation (Figure 4E). These results demonstrated that G(i/q) protein-coupled ETB-dependent activation of ERK1/2, p38 MAPK, and JNK1/2 by ET-1 is, at least in part, required for COX-2 expression in bEnd.3 cells.
Figure 4.
ET-1-induced COX-2 expression is mediated through MAPKs phosphorylation. (A, B) Cells were treated with 10 nM ET-1 for (A) 6 h and (B) 1 h in the absence or presence of U0126, SB202190, or SP600125. The COX-2 protein and mRNA expression were determined by Western blot and RT-PCR. (C) Time dependence of ET-1-stimulated ERK1/2, p38 MAPK, and JNK1/2 phosphorylation, cells were incubated with 10 nM ET-1 for the indicated times in the absence or presence of U0126 (1 μM), SB202190 (300 nM), or SP600125 (300 nM). (D) Cells were transfected with siRNA of ERK2, p38 MAPK, or JNK1 and then exposed to ET-1 for 6 h. (E) Cells were pretreated with BQ-788 (1 μM), GPA2 (1 μM), or GPA2A (1 μM) for 1 h and then incubated with ET-1 (10 nM) for the indicated times. The cell lysates were collected and analyzed by Western blotting using an anti-COX-2, anti-phospho-ERK1/2, anti-phospho-p38 MAPK, anti-phospho-JNK1/2, anti-ERK2, anti-p38 MAPK, anti-JNK1, or anti-GAPDH (as an internal control) antibody. Data are expressed as mean ± SEM of at least three individual experiments (n=3 in each group; *P<0.05, #P<0.01 as compared with ET-1 alone).
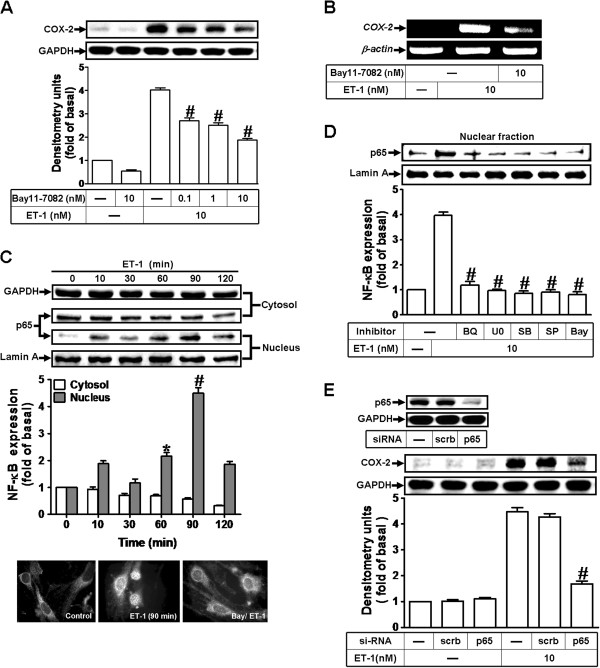
NF-κB is required for ET-1-induced COX-2 expression
ET-1 has been shown to modulate cellular functions through activation of NF-κB signaling in various cell types [32]. To examine whether activation of NF-κB is required for ET-1-induced COX-2 expression, as shown in Figure 5A and B, pretreatment with a selective NF-κB inhibitor Bay11-7082, which blocks activation of NF-κB signaling, attenuated ET-1-induced COX-2 protein and mRNA expression in bEnd.3 cells. To determine whether the involvement of NF-κB in ET-1-induced responses mediated through NF-κB (p65 subunit) translocation, as shown in Figure 5C, ET-1 time-dependently stimulated translocation of NF-κB p65 from cytosol into nucleus determined by Western blot. A maximal response was obtained within 90 min and sustained over 120 min (upper part). Moreover, we also confirmed the NF-κB p65 translocation by an immunofluorescence staining. The imaging data confirmed that ET-1 stimulated the p65 translocation at 90 min, which was inhibited by pretreatment with Bay11-7082 (Figure 5C, lower part). We further demonstrated that ET-1 stimulated translocation of NF-κB p65 was attenuated by pretreatment with the inhibitor of ETB receptor (BQ-788), MEK1/2 (U0126), p38 MAPK (SB202190), JNK1/2 (SP600125), or NF-κB (Bay11-7082) (Figure 5D). To further verify that NF-κB p65 is essential for ET-1-induced COX-2 expression, as shown in Figure 5E, transfection with p65 siRNA significantly reduced the p65 protein expression (upper panel) and the ET-1-induced COX-2 expression (lower panel). The results suggested that ET-1-stimulated NF-κB translocation mediated through ETB receptor, ERK1/2, p38 MAPK, and JNK1/2 is required for COX-2 induction in bEnd.3 cells.
Figure 5.
NF-κB (p65) is essential for ET1-1-induced COX-2 expression. (A, B) Cells were treated with 10 nM ET-1 for 6 h in the absence or presence of Bay11-7082. The COX-2 protein and mRNA expression were determined by Western blot and RT-PCR as described in Figure 1. (C) Time dependence of ET-1-stimulated p65 NF-κB translocation by subcellular isolation, Western blot, and immunofluorescent stain. (D) Cells were pretreated with U0126 (1 μM), SB202190 (300 nM), SP600125 (300 nM), BQ-788 (1 μM), or Bay11-7082 (10 nM) for 1 h and then incubated with ET-1 (10 nM) for 90 min. The nuclear fraction was analyzed by Western blot. (E) Cells were transfected with p65 siRNA and then exposed to ET-1 for 6 h. The cell lysates were collected and analyzed by Western blotting using an anti-COX-2, anti-p65, anti-Lamin A, or anti-GAPDH (as an internal control) antibody. Data are expressed as mean ± SEM of at least three individual experiments (n=3 in each group; #P<0.01 as compared with ET-1 alone).
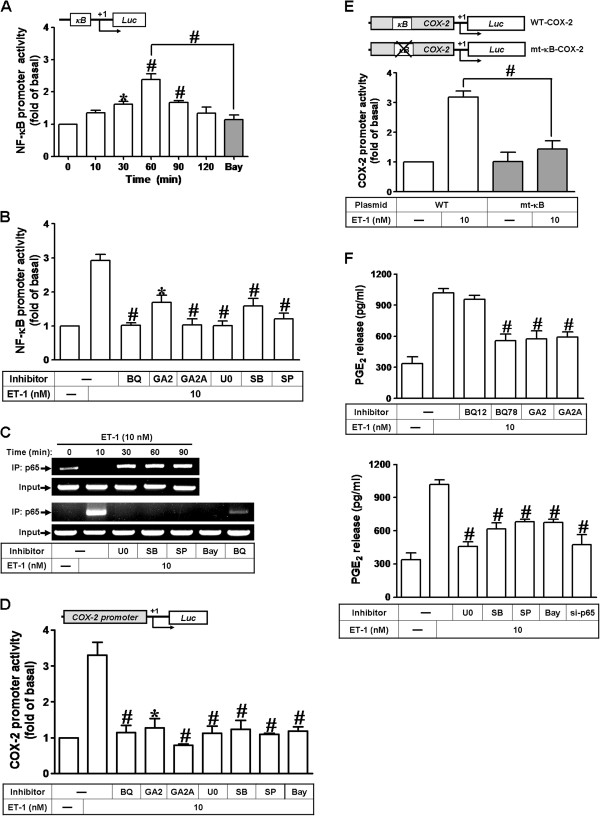
Involvement of NF-κB in COX-2 gene promoter activity stimulated by ET-1
We have found that ET-1 stimulates translocation of NF-κB p65 leading to COX-2 expression (Figure 5). Next, we examined whether activation of NF-κB is essential for ET-1-induced COX-2 gene up-regulation. The transcriptional activity of NF-κB was evaluated by a promoter (containing NF-κB binding sites)-luciferase activity assay. As shown in Figure 6A, ET-1 enhanced NF-κB transcriptional activity in a time-dependent manner with a maximal response within 60 min, which was significantly inhibited by pretreatment with an inhibitor of NF-κB (Bay11-7082, 10 nM). Moreover, pretreatment with BQ-788 (1 μM), GPA2 (1 μM), GPA2A (1 μM), U0126 (U0, 1 μM), SB202190 (SB, 300 nM), or SP600125 (SP, 300 nM) attenuated NF-κB transcriptional activity stimulated by ET-1 (Figure 6B), demonstrating that ET-1 enhances the NF-κB transcriptional activity through an ETB-dependent activation of MAPKs. Subsequently, we determined that ET-1-stimulates NF-κB p65 binding activity in a time-dependent manner by ChIP-PCR analysis (Figure 6C). ET-1-stimulated NF-κB p65 binding activity was inhibited by pretreatment with U0126, SB202190, SP600125, Bay11-7082, or BQ-788 (Figure 6C, lower part). In addition, we have demonstrated that ET-1 time-dependently induces COX-2 promoter activity (Figure 1C). We further demonstrated that ET-1-increased the COX-2 promoter activity was significantly inhibited by pretreatment with BQ-788, GPA2, GPA2A, U0126, SB202190, SP600125, or Bay11-7082 (Figure 6D), suggesting that ET-1 stimulates COX-2 promoter activity via the ETB-dependent activation of MAPKs and NF-κB in bEnd.3 cells. To further ensure that NF-κB indeed mediates ET-1-induced COX-2 promoter activity through binding to its regulatory element within the COX-2 promoter region, the wild-type (WT) and mutated by a single-point mutation of the NF-κB binding site (mt-κB) COX-2 promoters were constructed (as illustrated in Figure 6E, upper panel). ET-1-stimulated COX-2 promoter activity was significantly attenuated in bEnd.3 cells transfected with mt-κB-COX-2 (Figure 6D, lower panel), indicating that NF-κB element was essential for ET-1-induced COX-2 promoter activity. These results further confirmed that ET-1 induces COX-2 promoter activity via enhancing NF-κB binding to the κB binging site within COX-2 promoter region in bEnd.3 cells.
Figure 6.
ET-1-stimulated COX-2 promoter activity is mediated through NF-κB-dependent pathway. (A) Time dependence of ET-1-enhanced NF-κB transcription activity, cells were transfected with a NF-κB-luciferase reporter gene and then exposed to ET-1 for the indicated times. (B) After transfection, the cells were pretreated with BQ-788 (1 μM), GPA2 (1 μM), GPA2A (1 μM), U0126 (1 μM), SB202190 (300 nM), SP600125 (300 nM), or (A) Bay11-7082 (10 nM) for 1 h and then incubated with ET-1 (10 nM) for 60 min. (C) Cells were pretreated with BQ-788, U0126, SB202190, or SP600125 for 1 h and then incubated with ET-1. The p65 NF-κB binding activity was analyzed by ChIP-PCR as described in Methods. (D) For COX-2 promoter activity, cells were transfected with a COX-2-promoter-luciferase reporter gene and then exposed to ET-1. After transfection, the cells were pretreated with BQ-788, GPA2, GPA2A, U0126, SB202190, SP600125, or Bay11-7082 for 1 h and then incubated with ET-1 for 6 h. (E) Schematic representation of a 5′-promoter regions of the mouse different COX-2 promoter constructs, either wild-type (WT) or mutation of the κB-binding site (mt-κB) fused to the pGL-luciferase reporter gene, the translational start site (+1) of the luciferase reporter gene was indicated by an arrow. Cells were transfected with WT COX-2 promoter reporter gene (WT-COX-2) or NF-κB mutated COX-2 promoter reporter gene (mt-κB-COX-2) and then incubated with or without ET-1 for 6 h. The promoter reporter assay was performed as described in Methods. (F) Cells were pretreated with BQ-123, BQ-788, GPA2, GPA2A, U0126, SB202190, SP600125, Bay11-7082, or transfected with p65 siRNA and then incubated with ET-1 for 6 h. The PGE2 levels were analyzed by EIA. Data are expressed as mean ± SEM of at least three individual experiments (n=3 in each group; #P<0.01 as compared with ET-1 alone).
We have found that ET-1 time-dependently induces PGE2 release (Figure 1D). Here, we further determined the involvement of these signaling components in ET-1-induced PGE2 release, as shown in Figure 6F, ET-1-induced PGE2 release was markedly attenuated by pretreatment with BQ-788, GPA2, GPA2A, U0126, SB202190, SP600125, Bay11-7082, or transfection with p65 siRNA. These results demonstrated that ETB-mediated activation of MAPKs (i.e., ERK, p38 MAPK, and JNK) and NF-κB by ET-1 is essential for COX-2 up-regulation and PGE2 release in bEnd.3 cells.
Discussion
Several lines of evidence have demonstrated that high levels of PGs, synthesized by inducible COX-2, are involved in inflammatory responses. The up-regulation of COX-2 has been shown to display a wide range of biological activities in different tissues, including development, proliferation, cancers, and inflammation [14,15]. Moreover, ET-1 is elevated in the regions of vascular injuries and inflammation [7,8]. Circumstantial evidence has further demonstrated that overexpression of ET-1 on endothelial cells has deleterious effects on ischemic brain [1,5,6]. Reid et al. (1995) suggest that the ET-1 model provides new insights into the mechanisms of cerebral ischemia and reperfusion injury, and evaluates the usefulness of novel strategies of neuroprotection [33]. ET-1 has been shown to up-regulate the expression of COX-2 through MAPKs in various cell types [26,27,34]. However, little is known about the effect of ET-1 on COX-2 expression in brain vascular endothelial cells. Here, we applied cultured models of mouse bEnd.3 cells coupled with Western blot analysis, selective pharmacological inhibitors, transfection with siRNAs, immunofluorescenct staining, and promoter assay to investigate the molecular mechanisms underlying ET-1-induced COX-2 expression and PGE2 release. Our results demonstrate that in bEnd.3 cells, activation of ETB receptor-dependent MAPKs (ERK1/2, p38, and JNK1/2) and NF-κB signaling cascade is essential for ET-1-induced COX-2 gene expression and PGE2 release.
ET-1 activates ET receptor subtypes (ETA and ETB) which are coupled to various G proteins such as Gq and Gi and then lead to multiple signaling pathways and regulate diverse cellular functions [7,20-22]. Thus, we first demonstrated a significant expression of ETB receptor in mouse bEnd.3 cells (Figure 2A). The involvement of ETB receptors in these responses is confirmed by that pretreatment with BQ-788 (an ETB receptor antagonist) reduced the ET-1-induced COX-2 protein and mRNA expression (Figure 2B and 2C), promoter activity (Figure 6D), and PGE2 release (Figure 6F), but not by an ETA receptor antagonist BQ-123. Subsequently, we confirmed these results by transfection with ETB siRNA (Figure 2D), suggesting that ETB receptor predominantly mediates ET-1-induced COX-2 expression and PGE2 release in bEnd.3 cells. Next, several subtypes of G proteins are potentially implicated in ET-1-induced COX-2 expression. We use GPA2 (a Gi protein antagonist) and GPA2A (a Gq protein antagonist) to interrupt G protein signaling and consequent COX-2 expression (Figure 3A). Moreover, the inhibitory effects of GPA2 and GPA2A on COX-2 induction by ET-1 were also observed in its mRNA (Figure 3B), promoter activity (Figure 6D), and PGE2 release (Figure 6F), indicating that ET-1-induced COX-2 expression and PGE2 release is mediated through a GPCR (i.e. ETB) coupling to either Gi or Gq protein in bEnd.3 cells, consistent with previous studies from esophageal smooth muscle cells [34] and rat brain astrocytes [22]. In contrast, previous reports have shown that ET-1 induces COX-2 expression via ETA receptors in peripheral lung microvascular smooth muscle cells [25] and ET-1 (ETA) receptors linked to phospholipase C and phospholipase A2 activation and prostanoid secretion (e.g. PGE2) in cultured human brain microvascular endothelial cells [35,36]. However, in respiratory and cardiovascular systems, both ET receptor subtypes, ETA in particular, are involved in progression of several diseases [37,38]. There differences may be due to cell type specific or different experimental conditions.
Abnormal MAPK regulation might be implicated in several models of CNS injury and inflammation [39]. Several lines of evidence demonstrate that MAPKs could be activated by GPCR agonists through different signaling pathways [24]. MAPKs activation by ET-1 has been shown to modulate various cellular responses in several cell types [22,25]. Activation of ERK1/2 (p44/p42 MAPK) might be implicated in the expression of inflammatory genes in several models of vascular injury and inflammation [17,28]. In this study, we demonstrated that ET-1 stimulated an ETB receptor-dependent cascade of sequential ERK1/2 phosphorylation (Figure 4E), which contributes to induction of COX-2 protein and mRNA levels (Figure 4A and 4B), promoter activity (Figure 6D), and PGE2 release (Figure 6F). The involvement of ERK1/2 in COX-2 expression and PGE2 release was furthe confirmed by transfection of cells with p42 siRNA (Figure 4D). These results are consistent with those of obtained with COX-2 expression induced by BK, thrombin, or ET-1 in various cell types [17,26,28]. Additionally, we found that expression of COX-2 and release of PGE2 induced by ET-1 were also attenuated by the inhibitor of p38 MAPK or JNK1/2. Pretreatment with SB202190 or SP600125 both markedly reduced ET-1-induced expression of COX-2 protein and mRNA (Figure 4A and 4B), promoter activity (Figure 6D), and PGE2 release (Figure 6F). Moreover, we also demonstrated that ET-1 stimulates phosphorylation of p38 MAPK and JNK via an ETB-dependent manner (Figure 4C and 4E). Similarly, we further confirmed these results by transfection with siRNA for p38 MAPK or JNK1 that attenuated ET-1-induced COX-2 expression (Figure 4D). These data clearly indicated that in bEnd.3 cells, three MAPK cascades (i.e. ERK1/2, p38 MAPK, and JNK1/2) are required for ET-1-induced COX-2 expression and PGE2 release. These results are consistent with those of obtained with up-regulation of COX-2 by ET-1 via p38 MAPK in glomerular mesangial cells or esophageal smooth muscle cells [27,34]. For the role of JNK1/2, we are the first presented that JNK1/2 plays a critical role in induction of COX-2 by ET-1 in endothelial (bEnd.3) cells.
It has been well established that inflammatory responses following exposure to extracellular stimuli are highly dependent on activation of NF-κB transcription factor, which plays an important role in regulation of several gene expression [40]. The 5’-flanking region of the COX-2 promoter has been shown to contain several binding sequences for various transcription factors including NF-κB [41]. Therefore, the regulation of COX-2 transcription may be mediated by aberrant activation of several distinct transcription factors dependent on agonists [29,42]. These reports suggest that NF-κB plays a critical role in the regulation of COX-2 expression in the development of the inflammatory responses. Our data showed that ET-1-induced COX-2 gene expression and PGE2 release was significantly abolished by a selective NF-κB inhibitor Bay11-7082 (Figures 5 and 6) or NF-κB p65 siRNA (Figures 5E and 6F), suggesting that NF-κB (p65) is involved in ET-1-induced COX-2 expression in bEnd.3 cells. Moreover, ET-1-stimulated NF-κB p65 translocation (Figure 5C), binding to COX-2 promoter region (Figure 6C and 6E), and NF-κB transcriptional activity (Figure 6A) was significantly inhibited by Bay11-7082 and the MAPK inhibitor U0126 (MEK1/2), SB202190 (p38 MAPK), or SP600125 (JNK1/2) (Figures 5 and 6). Our data further showed that ET-1-stimulated NF-κB transcriptional activity was significantly attenuated by blocking Gi and Gq protein-coupled ETB receptor-dependent pathways (Figure 6B), indicating that ET-1-induced activation of NF-κB is mediated through ETB receptor-dependent activation of three MAPKs cascades. These findings are consistent with recent studies indicating that COX-2 expression and prostacyclin release induced by thrombin were mediated through MAPKs and NF-κB activation in endothelial cells [16] and vascular smooth muscle cells [17] and COX-2 expression and PGE2 release induced by BK via ERK1/2 linking to NF-κB activation in astrocytes [29]. The involvement of NF-κB in ET-1-induced COX-2 expression is also consistent with previous reports indicating that ET-1-stimulated activation of NF-κB regulates expression of target genes involved in various CNS inflammatory processes [22]. Moreover, our recent data have also demonstrated that in bEnd.3 cells, c-Src-dependent transactivation of EGFR/PI3K/Akt and MAPKs linking to c-Jun/AP-1 cascade is essential for ET-1-induced COX-2/PGE2 upregulation [43]. We suggest that the findings of these two studies might have a crosstalk in MAPKs and lead to COX-2 expression induced by ET-1 in these cells. The interplay between these two pathways in the induction of COX-2 will be investigated in the future.
Conclusions
In this study, we reported here that ET-1/ET (ETB) receptor system exerts its effects on COX-2 gene expression and PGE2 release in mouse bEnd.3 cells. The Gi and Gq protein-coupled ETB receptor, ERK1/2, p38 MAPK, JNK1/2, and NF-κB cascades cooperatively mediated these effects of ET-1. These findings concerning ET-1-induced COX-2/PGE2 system imply that ET-1 might play a critical role in brain injury, vascular inflammation, and CNS diseases, mediated through MAPK-dependent activation of NF-κB pathway in bEnd.3 cells. Pharmacological approaches suggest that targeting COX-2/PGE2 system and their upstream signaling components should yield useful therapeutic targets for brain injury and inflammatory diseases.
Methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM)/F-12 medium, fetal bovine serum (FBS), and TRIzol were from Invitrogen (Carlsbad, CA). Hybond C membrane and enhanced chemiluminescence (ECL) Western blot detection system were from GE Healthcare Biosciences (Buckinghamshire, UK). Anti-COX-2 monoclonal antibody was from BD Transduction Laboratories (San Diego, CA). Phospho-ERK1/2, phospho-p38, phospho-JNK1/2 antibody kits were from Cell Signaling (Danver, MA). p65, p42 (ERK2), p38, and JNK1 antibodies were from Santa Cruz (Santa Cruz, CA). Anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody was from Biogenesis (Boumemouth, UK). BQ-123, BQ-788, GP antagonist-2, GP antagonist-2A, U0126, SB202190, SP600125, and Bay11-7082 were from Biomol (Plymouth Meeting, PA). Bicinchoninic acid (BCA) protein assay reagent was from Pierce (Rockford, IL). ET-1, enzymes, and other chemicals were from Sigma (St. Louis, MO).
Mouse brain microvascular endothelial cell culture
Mouse brain microvascular endothelial cells (bEnd.3) were purchased from Bioresource Collection and Research Centre (BCRC, Hsinchu, Taiwan) and grew in DMEM/F-12 containing 10% FBS and antibiotics (100 U/ml penicillin G, 100 μg/ml streptomycin and 250 ng/ml fungizone) at 37°C in a humidified 5% CO2 atmosphere. The cell line is acquired from mouse BALB/c strain brain cerebral cortex endothelial polyoma middle T antigen transformed, which was performed STR-PCR profile at BCRC. All the experiments were performed using this cell line and approved by the ethic approval of Chang Gung University. Confluencent cells were released with 0.05% (w/v) trypsin/0.53 mM EDTA for 5 min at 37°C. The cell suspension (2 × 105 cells/ml) was plated onto 6-well culture plates (2 ml/well) or 10-cm culture dishes (10 ml/dish) for the measurement of protein or RNA expression, respectively. Culture medium was changed after 24 h and then every 3 days. Experiments were performed with cells from passages 5 to 13.
Preparation of cell extracts and Western blot analysis
Growth-arrested cells were incubated with ET-1 at 37°C for various time intervals. The cells were washed with ice-cold phosphate-buffered saline (PBS), scraped, and collected by centrifugation at 45,000 × g for 1 h at 4°C to yield the whole cell extract, as previously described [17]. Samples were analyzed by Western blot, transferred to nitrocellulose membrane, and then incubated overnight using an anti-COX-2, phospho-ERK1/2, phospho-p38 MAPK, phospho-JNK1/2, p42, p38, JNK1, p65, or GAPDH antibody. Membranes were washed with TTBS four times for 5 min each, incubated with a 1:2000 dilution of anti-rabbit horseradish peroxidase antibody for 1 h. The immunoreactive bands were detected by ECL reagents.
Total RNA extraction and gene expression
For reverse transcription PCR (RT-PCR) analysis, total RNA was extracted from mouse brain endothelial cells stimulated by ET-1, as previously described [17]. The cDNA obtained from 0.5 μg total RNA was used as a template for PCR amplification. Oligonucleotide primers were designed based on Genbank entries for mouse COX-2 and β-actin. The following primers were used for amplification reaction: for COX-2: 5′-(AAAACCGTGGGGAATGTATGAGC)-3′ (sense), 5′-(GATGGGTGAAGTGCTGGGGAAAG)-3′ (anti-sense); ETA: 5′-(GGCGCAATCGCTGACAATGCTGAG)-3′ (sense), 5′-(CCACGTAGATAAGGTCTCCAAGGG)-3′ (anti-sense); TB: 5′-(CGTGTTCGTGCTAGGCATCATCGG)-3′ (sense), 5′-(CGACTCCAAGAAGCAACAGCTCGA)-3′ (anti-sense); for β-actin: 5′-(GAACCCTAAGGCCACCGTG)-3′ (sense), 5′-(TGGCATAGAGGTCTTTACGG)-3′ (anti-sense). PCR mixes contained 10 μl of 5X PCR buffer, 1.25 mM of each dNTP, 100 pmol of each forward and reverse primer, and 2.5 units of Taq polymerase (Takara, Shiga, Japan). The final reaction volume was 50 μl. Amplification was performed in 25 cycles at 94°C, 20 s; 60°C, 40 s; 72°C, 40 s [44]. After the last cycle, all samples were incubated for an additional 10 min at 72°C. PCR fragments were analyzed on 2% agarose 1X TAE gel containing ethidium bromide and their size was compared to a molecular weight marker. Amplification of β-actin, a relatively invariant internal reference RNA, was performed in parallel, and cDNA amounts were standardized to equivalent β-actin mRNA levels. These primer sets specifically recognized only the genes of interest as indicated by amplification of a single band of the expected size (500 bp for COX-2, 343 bp for ETA; 293 bp for ETB, and 514 bp for β-actin) and direct sequence analysis of the PCR products.
Immunofluorescence staining
Cells were plated on 6-well culture plates with coverslips. Cells were shifted to a serum-free DMEM/F-12 for 24 h and treated with 10 nM ET-1. After washing twice with ice-cold PBS, the cells were fixed with 4% (w/v) paraformaldehyde in PBS for 30 min, and then permeabilized with 0.3% Triton X-100 in PBS for 15 min. The staining was performed by incubating with 10% normal goat serum in PBS for 30 min followed by incubating with a primary anti-p65 NF-κB polyclonal antibody (1:200 dilution) for 1 h in PBS with 1% BSA, washing thrice with PBS, incubating for 1 h with fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit antibody (1:200 dilution) in PBS with 1% BSA, washing thrice with PBS, and finally mounting with aqueous mounting medium. The images observed under a fluorescence microscope (ZEISS, Axiovert 200M).
Chromatin immunoprecipitation assay
To detect the in vivo association of nuclear proteins with mouse COX-2 promoter, chromatin immunoprecipitation (ChIP) analysis was conducted as previously described [45]. Briefly, the bEnd.3 cells were cross-linked with 1% formaldehyde for 10 min at 37°C and washed thrice with ice-cold PBS containing 1 mM phenylmethylsulfonyl fluoride (PMSF) and 1% aprotinin. Soluble chromatin was prepared using a ChIP assay kit (Upstate) according to the manufacturer’s recommendations and immunoprecipitated without (control) or with anti-p65 NF-κB antibody and normal goat immunoglobulin G (IgG). Following washes and elution, precipitates were heated overnight at 65°C to reverse cross-linking of DNA and protein. DNA fragments were purified by phenol-chloroform extraction and ethanol precipitation. The purified DNA was subjected to PCR amplification using the primers specific for the region (-371 to +70) containing the NF-κB binding site present in the COX-2 promoter region, sense primer: 5′-GGGGGAGGGAAGCTGTGACACTCTTGAGCTTT-3′; antisense primer: 5′-GACAGTGCTGAGATTCTTCGTGAGCAGAGTCC-3′. PCR fragments were analyzed on 2% agarose in 1X TAE gel containing ethidium bromide and the size (441 bp) was compared to a molecular weight marker.
Plasmid construction, transient transfection and luciferase assays
The mouse COX-2 promoter was constructed as described previously [46] with some modifications. The upstream region (-907 to +70) of the mouse COX-2 promoter was cloned to the pGL3-basic vector containing the luciferase reporter system. Introduction of a double-point mutation into the NF-κB-binding site (TGGGGA to TGGGAC) to generate pGL-COX2-mκB was performed using the following (forward) primer: 5′-GGGGTACCGCAAATAATTTTTTATCAAACACTGTTTCTG-3′ (corresponding to a region from -907 to +70). The underlined nucleotides indicate the positions of substituted bases. The mutant construct was cloned into the pGL3-basic vector containing the luciferase reporter system. All plasmids were prepared by using QIAGEN plasmid DNA preparation kits. The siRNAs for p42, p38, JNK1, p65, and scrambled control were from Dharmacon Research Inc (Lafayette, CO), and NF-κB or COX-2 promoter constructs were transfected into cells using the Lipofetamine-2000 transfection reagent according to the instructions of manufacture (Invitrogen, Carlsbad, CA). The transfection efficiency (~60%) was determined by transfection with enhanced EGFP. To assess promoter activity, cells were collected and disrupted by sonication in lysis buffer (25 mM Tris-phosphate, pH 7.8, 2 mM EDTA, 1% Triton X-100, and 10% glycerol). After centrifugation, aliquots of the supernatants were tested for luciferase activity using a luciferase assay system. Firefly luciferase activities were standardized to β-galactosidase activity.
Measurement of PGE2 release
The cells were seeded in 12-well plates and grown to confluence. Cells were shifted to serum-free DMEM/F-12 medium for 24 h, and then treated with ET-1 for various time intervals. The culture supernatants were collected to measure PGE2 levels using an EIA kit as specified by the manufacturer (Cayman Chemical).
Statistical analysis of data
All data were estimated using GraphPad Prism Program (GraphPad, San Diego, CA). Quantitative data were analyzed by one-way ANOVA followed by Tukey’s honestly significant difference tests between individual groups. Data were expressed as mean±SEM. A value of P<0.05 was considered significant.
Abbreviations
CNS: Central nervous system; BBB: Blood-brain barrier; ET-1: Endothelin-1; COX-2: Cyclooxygenase-2; PGE2: Prostaglandin E2; bEnd.3: Brain microvascular endothelial cells; DMEM/F-12: Dulbecco’s modified Eagle’s medium/Ham’s nutrient mixture F-12; FBS: Fetal bovine serum; ECL: Enhanced chemiluminescence; BCA: Bicinchoninic acid; PBS: Phosphate-buffered saline; GPCR: G protein-coupled receptor; siRNA: Small interfering RNA; RT-PCR: Reverse transcription-polymerase chain reaction; ChIP: Chromatin immunoprecipitation.
Competing interests
The authors have no competing interests to declare.
Authors’ contributions
CCL, RHS, PLC, and SEC designed and performed experiments, acquisition and analysis of data, and drafted the manuscript. HLH and RHS helped to perform experiments and prepare the manuscript. HLH and CMY have conceived of the study, participated in its design and coordination, CMY has been involved in drafting the manuscript and revising it critically for important intellectual content and has given final approval of the version to be published. All authors read and approved the final manuscript.
Contributor Information
Chih-Chung Lin, Email: chihchung@adm.cgmh.org.tw.
Hsi-Lung Hsieh, Email: hlhsieh@mail.cgust.edu.tw.
Ruey-Horng Shih, Email: r.h.shih51@gmail.com.
Pei-Ling Chi, Email: chi542738@yahoo.com.tw.
Shin-Ei Cheng, Email: cindy0137rachel@yahoo.com.tw.
Chuen-Mao Yang, Email: chuenmao@mail.cgu.edu.tw.
Acknowledgements
This work was supported by the Ministry of Education, Taiwan, Grant number: EMRPD1B0311 and EMRPD1B0321; National Science Council, Taiwan, Grant number: NSC101-2321-B-182-013, NSC101-2320-B-182-039-MY3, NSC99-2321-B182-003, NSC98-2320-B-182-004-MY3, NSC98-2314-B-182-021-MY3, NSC99-2321-B182-003, and NSC98-2320-B-255-001-MY3; Chang Gung Medical Research Foundation, Grant number: CMRPD180373, CMRPD1B0381, CMRPG391033, CMRPG3B1091, and CMRPF1A0062. The authors also thank Ms. C.Y. Lee and Mr. L.D. Hsiao for their technical assistance in preparation of this manuscript.
References
- McCarron RM, Chen Y, Tomori T, Strasser A, Mechoulam R, Shohami E, Spatz M. Endothelial-mediated regulation of cerebral microcirculation. J Physiol Pharmacol. 2006;57:133–144. [PubMed] [Google Scholar]
- Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation. 2005;111:363–368. doi: 10.1161/01.CIR.0000153339.27064.14. [DOI] [PubMed] [Google Scholar]
- Böhm F, Pernow J. The importance of endothelin-1 for vascular dysfunction in cardiovascular disease. Cardiovasc Res. 2007;76:8–18. doi: 10.1016/j.cardiores.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Anggård EE, Botting RM, Vane JR. Endothelins. Blood Vessels. 1990;27:269–281. [PubMed] [Google Scholar]
- Levin ER. Endothelins. N Engl J Med. 1995;333:356–363. doi: 10.1056/NEJM199508103330607. [DOI] [PubMed] [Google Scholar]
- Kawanabe Y, Nauli SM. Endothelin. Cell Mol Life Sci. 2011;68:195–203. doi: 10.1007/s00018-010-0518-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubanyi GM, Polokoff MA. Endothelins: molecular biology, biochemistry, pharmacology, physiology, and pathophysiology. Pharmacol Rev. 1994;46:325–415. [PubMed] [Google Scholar]
- D’Uscio LV, Barton M, Shaw S, Lüscher TF. Endothelin in atherosclerosis: importance of risk factors and therapeutic implications. J Cardiovasc Pharmacol. 2000;35:S55–S59. doi: 10.1097/00005344-200000002-00013. [DOI] [PubMed] [Google Scholar]
- Chen P, Shibata M, Zidovetzki R, Fisher M, Zlokovic BV, Hofman FM. Endothelin-1 and monocyte chemoattractant protein-1 modulation in ischemia and human brain-derived endothelial cell cultures. J Neuroimmunol. 2001;116:62–73. doi: 10.1016/S0165-5728(01)00280-6. [DOI] [PubMed] [Google Scholar]
- Hasselblatt M, Lewczuk P, Löffler BM, Kamrowski-Kruck H, von Ahsen N, Sirén AL, Ehrenreich H. Role of the astrocytic ET(B) receptor in the regulation of extracellular endothelin-1 during hypoxia. Glia. 2001;34:18–26. doi: 10.1002/glia.1036. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Guo S, Lo EH. Dysfunctional cell-cell signaling in the neurovascular unit as a paradigm for central nervous system disease. Stroke. 2009;40:S4–S7. doi: 10.1161/STROKEAHA.108.534388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zidovetzki R, Chen P, Chen M, Hofman FM. Endothelin-1-induced interleukin-8 production in human brain-derived endothelial cells is mediated by the protein kinase C and protein tyrosine kinase pathways. Blood. 1999;94:1291–1299. [PubMed] [Google Scholar]
- Williams CS, Mann M, DuBois RN. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene. 1999;18:7908–7916. doi: 10.1038/sj.onc.1203286. [DOI] [PubMed] [Google Scholar]
- Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- Syeda F, Grosjean J, Houliston RA, Keogh RJ, Carter TD, Paleolog E, Wheeler-Jones CP. Cyclooxygenase-2 induction and prostacyclin release by protease-activated receptors in endothelial cells require cooperation between mitogen-activated protein kinase and NF-κB pathways. J Biol Chem. 2006;281:11792–11804. doi: 10.1074/jbc.M509292200. [DOI] [PubMed] [Google Scholar]
- Hsieh HL, Sun CC, Wang TS, Yang CM. PKC-δ/c-Src-mediated EGF receptor transactivation regulates thrombin-induced COX-2 expression and PGE2 production in rat vascular smooth muscle cells. Biochim Biophys Acta. 2008;1783:1563–1575. doi: 10.1016/j.bbamcr.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Ejima K, Layne MD, Carvajal IM, Kritek PA, Baron RM, Chen YH. Vom Saal J, Levy BD, Yet SF, Perrella MA: Cyclooxygenase-2-deficient mice are resistant to endotoxin-induced inflammation and death. FASEB J. 2003;17:1325–1327. doi: 10.1096/fj.02-1078fje. [DOI] [PubMed] [Google Scholar]
- Minghetti L. Cyclooxygenase-2 (COX-2) in inflammatory and degenerative brain diseases. J Neuropathol Exp Neurol. 2004;63:901–910. doi: 10.1093/jnen/63.9.901. [DOI] [PubMed] [Google Scholar]
- Arai H, Hori S, Aramori I, Ohkubo H, Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990;348:730–732. doi: 10.1038/348730a0. [DOI] [PubMed] [Google Scholar]
- Bouallegue A, Daou GB, Srivastava AK. Endothelin-1-induced signaling pathways in vascular smooth muscle cells. Curr Vasc Pharmacol. 2007;5:45–52. doi: 10.2174/157016107779317161. [DOI] [PubMed] [Google Scholar]
- Wang HH, Hsieh HL, Wu CY, Yang CM. Endothelin-1 enhances cell migration via matrix metalloproteinase-9 up-regulation in brain astrocytes. J Neurochem. 2010;113:1133–1149. doi: 10.1111/j.1471-4159.2010.06680.x. [DOI] [PubMed] [Google Scholar]
- Aramori I, Nakanishi S. Coupling of two endothelin receptor subtypes to differing signal transduction in transfected Chinese hamster ovary cells. J Biol Chem. 1992;267:12468–12474. [PubMed] [Google Scholar]
- Marshall CJ. MAP kinase kinase kinase, MAP kinase kinase and MAP kinase. Curr Opin Genet Dev. 1994;4:82–89. doi: 10.1016/0959-437X(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Marasciulo FL, Montagnani M, Potenza MA. Endothelin-1: the yin and yang on vascular function. Curr Med Chem. 2006;13:1655–1665. doi: 10.2174/092986706777441968. [DOI] [PubMed] [Google Scholar]
- Chen D, Balyakina EV, Lawrence M, Christman BW, Meyrick B. Cyclooxygenase is regulated by ET-1 and MAPKs in peripheral lung microvascular smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2003;284:L614–621. doi: 10.1152/ajplung.00215.2002. [DOI] [PubMed] [Google Scholar]
- Pratt PF, Bokemeyer D, Foschi M, Sorokin A, Dunn MJ. Alterations in subcellular localization of p38 MAPK potentiates endothelin-stimulated COX-2 expression in glomerular mesangial cells. J Biol Chem. 2003;278:51928–51936. doi: 10.1074/jbc.M309256200. [DOI] [PubMed] [Google Scholar]
- Hsieh HL, Wu CB, Sun CC, Liao CH, Lau YT, Yang CM. Sphingosine-1-phosphate induces COX-2 expression via PI3K/Akt and p42/p44 MAPK pathways in rat vascular smooth muscle cells. J Cell Physiol. 2006;207:757–766. doi: 10.1002/jcp.20621. [DOI] [PubMed] [Google Scholar]
- Hsieh HL, Wang HH, Wu CY, Jou MJ, Yen MH, Parker P, Yang CM. BK-induced COX-2 expression via PKC-δ-dependent activation of p42/p44 MAPK and NF-κB in astrocytes. Cell Signal. 2007;19:330–340. doi: 10.1016/j.cellsig.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Lin CC, Hsiao LD, Chien CS, Lee CW, Hsieh JT, Yang CM. Tumor necrosis factor-α-induced cyclooxygenase-2 expression in human tracheal smooth muscle cells: involvement of p42/p44 and p38 mitogen-activated protein kinases and nuclear factor-κB. Cell Signal. 2004;16:597–607. doi: 10.1016/j.cellsig.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Gogg S, Smith U, Jansson PA. Increased MAPK activation and impaired insulin signaling in subcutaneous microvascular endothelial cells in type 2 diabetes: the role of endothelin-1. Diabetes. 2009;58:2238–2245. doi: 10.2337/db08-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorlemmer A, Matter ML, Shohet RV. Cardioprotective signaling by endothelin. Trends Cardiovasc Med. 2008;18:233–239. doi: 10.1016/j.tcm.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid JL, Dawson D, Macrae IM. Endothelin, cerebral ischaemia and infarction. Clin Exp Hypertens. 1995;17:399–407. doi: 10.3109/10641969509087080. [DOI] [PubMed] [Google Scholar]
- Song HJ, Min YS, Shin CY, Jeong JH, Sohn UD. Activation of p38 MAPK is involved in endothelin-1-stimulated COX-2 expression in cultured Feline esophageal smooth muscle cells. Mol Cells. 2006;22:44–50. [PubMed] [Google Scholar]
- Stanimirovic DB, Bacic F, Uematsu S, Spatz M. Profile of prostaglandins induced by endothelin-1 in human brain capillary endothelium. Neurochem Int. 1993;23:385–393. doi: 10.1016/0197-0186(93)90082-G. [DOI] [PubMed] [Google Scholar]
- Stanimirovic DB, Yamamoto T, Uematsu S, Spatz M. Endothelin-1 receptor binding and cellular signal transduction in cultured human brain endothelial cells. J Neurochem. 1994;62:592–601. doi: 10.1046/j.1471-4159.1994.62020592.x. [DOI] [PubMed] [Google Scholar]
- De Lagausie P, de Buys-Roessingh A, Ferkdadji L, Saada J, Aisenfisz S, Martinez-Vinson C, Fund X, Cayuela JM, Peuchmaur M, Mercier JC, Berrebi D. Endothelin receptor expression in human lungs of newborns with congenital diaphragmatic hernia. J Pathol. 2005;205:112–118. doi: 10.1002/path.1677. [DOI] [PubMed] [Google Scholar]
- Lund AK, Lucero J, Lucas S, Madden MC, McDonald JD, Seagrave JC, Knuckles TL, Campen MJ. Vehicular emissions induce vascular MMP-9 expression and activity associated with endothelin-1-mediated pathways. Arterioscler Thromb Vasc Biol. 2009;29:511–517. doi: 10.1161/ATVBAHA.108.176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR. Peripheral and central mechanisms of inflammatory pain, with emphasis on MAP kinases. Curr Drug Targets Inflamm Allergy. 2004;3:299–303. doi: 10.2174/1568010043343804. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Karin M. Nuclear factor-κB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- Tanabe T, Tohnai N. Cyclooxygenase isozymes and their gene structures and expression. Prostaglandins Other Lipid Mediat. 2002;68-69:95–114. doi: 10.1016/s0090-6980(02)00024-2. [DOI] [PubMed] [Google Scholar]
- Kim Y, Fischer SM. Transcriptional regulation of cyclooxygenase-2 in mouse skin carcinoma cells. Regulatory role of CCAAT/enhancer-binding proteins in the differential expression of cyclooxygenase-2 in normal and neoplastic tissues. J Biol Chem. 1998;273:27686–27694. doi: 10.1074/jbc.273.42.27686. [DOI] [PubMed] [Google Scholar]
- Hsieh HL, Lin CC, Chan HJ, Yang CM. c-Src-dependent EGF receptor transactivation contributes to ET-1-induced COX-2 expression in brain microvascular endothelial cells. J Neuroinflammation. 2012;9:152. doi: 10.1186/1742-2094-9-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunot S, Vila M, Teismann P, Davis RJ, Hirsch EC, Przedborski S, Rakic P, Flavell RA. JNK-mediated induction of cyclooxygenase 2 is required for neurodegeneration in a mouse model of Parkinson’s disease. Proc Natl Acad Sci USA. 2004;101:665–670. doi: 10.1073/pnas.0307453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh HL, Wu CY, Yang CM. Bradykinin induces matrix metalloproteinase-9 expression and cell migration through a PKC-δ-dependent ERK/Elk-1 pathway in astrocytes. Glia. 2008;56:619–632. doi: 10.1002/glia.20637. [DOI] [PubMed] [Google Scholar]
- Yang F, Bleich D. Transcriptional regulation of cyclooxygenase-2 gene in pancreatic beta-cells. J Biol Chem. 2004;279:35403–35411. doi: 10.1074/jbc.M404055200. [DOI] [PubMed] [Google Scholar]