Abstract
Signal transducer and activator of transcription 3 (STAT3) is a key mediator of the inflammatory response by macrophages and other immune cell types. The naturally occurring polyphenol resveratrol is associated with anti-proliferative and anti-inflammatory properties via mechanisms implicating inhibition of STAT3 signaling. Here, we report that the small-molecule analogs of resveratrol, RSVA314 and RSVA405, are potent inhibitors of STAT3. RSVA314 and RSVA405 inhibited both constitutive and stimulated STAT3 in HEK293 cells and lipopolysaccharide (LPS)-activated RAW 264.7 macrophages, respectively. The small-molecule analogs inhibited STAT3 nearly 50 times more potently than did resveratrol (apparent IC50 ~ 0.5 μM). We further show that RSVA405 interfered with the inflammatory response by RAW 264.7 cells upon LPS stimulation by inhibiting IKK and IκBα phosphorylation and by decreasing the expression of several cytokines, including the NF-κB target genes, tumor necrosis factor-α and interleukin-6. Downstream activation of STAT1 upon LPS stimulation was also inhibited by RSVA405. Consequently, RSVA405 significantly interfered with the phagocytotic activity and proliferation of LPS-activated RAW 264.7 macrophages. Finally, we found that the effect of the two small-molecule analogs on STAT3 phosphorylation could be prevented by inhibitors of protein tyrosine phosphatases (PTPs), indicating that the small-molecules acted by promoting the dephosphorylation of STAT3 by PTPs.
Keywords: STAT3, Inflammation, RSVA314, RSVA405, Resveratrol
INTRODUCTION
Signal transducer and activator of transcription 3 (STAT3) is a transcription factor member of the STAT protein family [1]. STAT3 is activated during cytokine receptor signaling and is implicated in the immune response. STATs are activated by tyrosine phosphorylation by the JAK kinases and are inactivated by dephosphorylation by specific protein tyrosine phosphatases (PTPs), including SHP-1, SHP-2, or T-cell PTP [2,3]. The role of STAT3 in inflammation is complex. It has been proposed that its sustained activation has essential anti-inflammatory functions, whereas its transient activation promotes inflammation [2]. Upon activation, STAT3 translocates to the nucleus to activate the expression of several inflammatory cytokines, including interleukin (IL)-1β and IL-6, the receptors of which require STATs for intracellular signal transduction. Thus, STAT3 is critical for the activation and amplification of the inflammatory response.
Macrophages can be activated by the endotoxin lipopolysaccharide (LPS), a molecule found on the outer membrane of bacteria. LPS triggers stimulation of an array of signal transduction pathways, which include the nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB). These signal transduction pathways control the production of several cytokines, including tumor necrosis factor-α (TNF-α) and IL-6. LPS specifically binds Toll-like receptor 4 (TLR4) to promote signal transduction via the activation of intracellular pathways specific to two adaptor proteins, myeloid differentiation primary response gene 88 (MyD88) and Toll/IL-1R domain-containing adapter molecule 1 (TRIF) [4]. The MyD88-dependent pathway activates IκB kinase (IKK), which in turn inactivates IκBα and triggers NF-κB-dependent transcription [5–7]. The TRIF-dependent pathway activates the IKK-related kinase TBK1 (TNF receptor-associated factor family member-associated NF-κB activator-binding kinase-1) to engage another transcription factor, IRF3 [8,9].
Resveratrol (trans-3,4′,5-trihydroxystilbene) is a natural polyphenol found in red wine associated with several biological functions [10,11]. We recently showed that this polyphenol has anti-inflammatory properties in LPS-stimulated murine RAW 264.7 macrophages [12]. Resveratrol acted—at least in part—by interfering with TLR4 oligomerization upon receptor stimulation [12]. Recently, using a kinase screen analyzing 28 major phospho-proteins, we identified a series of structurally related small-molecule analogs of resveratrol that are potent activators of AMP-activated protein kinase (AMPK), a kinase critically involved in cellular energy homeostasis [13,14].This screen revealed that the small-molecule analogs of resveratrol not only activate AMPK but also inhibit STAT3 in our cell-based assay [13]. In this context, and because previous work indicates that resveratrol is associated with STAT3 inhibitory and anti-proliferative properties both in immune and cancer cells [15,16], we sought to determine whether the identified analogs of resveratrol retained STAT3 inhibitory activity and interfere with the inflammatory response in activated macrophages.
RESULTS
RSVA314 and RSVA405 inhibit LPS-induced STAT3 activity, intracellular signaling, and cytokine response in activated RAW 264.7 macrophages
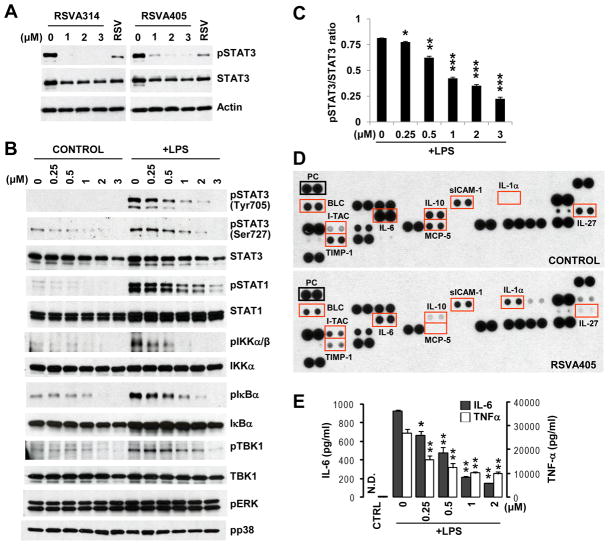
Previous studies from our laboratory indicated that 3 μM of the resveratrol analogs, RSVA314 or RSVA405, inhibited the activating phosphorylation of STAT3 at Tyr705 in HEK293 cells [13], suggesting that the two compounds inhibited constitutive STAT3. Western blot (WB) analyses confirmed the effect of RSVA314 and RSVA405 on phospho-Tyr705-STAT3 in HEK293 cells (Fig. 1A). We then assessed the effect of the most potent compound, RSVA405,on stimulated STAT3 in LPS-activated RAW 264.7 macrophages. The compound dose-dependently inhibited the LPS-induced increase of STAT3 phosphorylation at Tyr705 and Ser727, another phosphorylation site required for maximal activation of STAT3 [17]. Total STAT3 levels were also slightly affected by RSVA405 treatment (Fig. 1B). The decrease of total STAT3 is expected if the inhibitory effect on phospho-STAT3 is long enough to allow a transcriptional response, which is the case in this study (24 hrs). Indeed, STAT3 in several immune cells, including macrophages, can bind to its promoter to control its own transcription [18,19]. However, quantification of phospho-STAT3 and total STAT3 levels revealed a robust and significant decrease of the ratio pSTAT3/STAT3, demonstrating that the effect on phospho-STAT3 surpassed and probably preceded the effect on total STAT3 (Fig. 1C). In order to assess the anti-inflammatory properties of RSVA405, we analyzed its effect on the increase of several inflammatory markers in LPS-activated RAW 264.7 cells. RSVA405 inhibited the increase of phosphorylation of IKKα/β and IκBα, indicating that this compound interfered with the activation of the MyD88-dependent pathway by blocking NF-κB signaling in LPS-stimulated macrophages (Fig. 1B). RSVA405 also inhibited TBK1 phosphorylation, indicating that the TRIF-dependent pathway was also repressed (Fig. 1B).
Figure 1. RSVA314 and RSVA405 inhibit LPS-induced STAT3 activity, intracellular signaling, and cytokine response in activated RAW 264.7 macrophages.
(A) HEK293 cells were treated for 16 hrs with the indicated concentrations of RSVA314 and RSVA405, or with 40 μM resveratrol (RSV). Total protein extracts were then analyzed by WB using antibodies directed against phospho-Tyr705 STAT3 (pSTAT3), total STAT3, and actin. (B) RAW 264.7 cells pretreated for 16 hrs with the indicated concentrations of RSVA405 were stimulated with LPS or not (Control) for 8 hrs. Total protein extracts were then analyzed by WB using antibodies directed against the indicated proteins. (C) Densitometric analysis and quantification of the ratio pSTAT3/STAT3 obtained from three independent experiments performed as in (B). Histogram shows the mean ± S.D. (*, p<0.05; **, p<0.01; ***, p<0.001), as compared to the pSTAT3/STAT3 ratio in the control group. (D) Conditioned medium from RAW 264.7 cells pre-incubated for 16 hrs in the absence (Control) or presence of 2 μM RSVA405 and then stimulated with LPS for 8 hrs, was analyzed on cytokine arrays. PC, positive control corresponding to the reference spots on the arrays. (E) Conditioned medium from RAW 264.7 cells treated as in (D) was analyzed by ELISA for IL-6 and TNF-α secretion. Histograms show the mean ± S.D. (*, p<0.01; **, p<0.001; n=3). N.D., not detected; CTRL, non-treated control.
We then assessed the effect of RSVA405 treatment on cytokine production in LPS-activated RAW 264.7 cells. Using cytokine arrays, we found that 2 μM RSVA405 led to a robust decrease of the levels of several cytokines and chemokines, including IL-6, IL-10, IL-27, MCP-5 (C-C motif chemokine 12), and TIMP-1 (metalloproteinase inhibitor 1)(Fig. 1D). A modest reduction in sICAM-1 (soluble intercellular adhesion molecule 1)and BLC (C-X-C motif chemokine 13) levels were also observed, whereas IL-1α and I-TAC (C-X-C motif chemokine 11) levels were increased in these conditions (Fig. 1D). Using ELISA, we confirmed the inhibitory effect of RSVA405 treatment on LPS-stimulated IL-6 secretion in RAW 264.7 cells(Fig. 1E). We further found that LPS-stimulated TNF-α secretion was also significantly inhibited by RSVA405 treatment in RAW 264.7 cells (Fig. 1E). In line with these results, we observed a strong inhibition by RSVA405 of the downstream effector STAT1 in LPS-activated macrophages (Fig. 1B). The effect of RSVA405, however, was relatively specific to the STAT pathway because its treatment did not affect the phosphorylation levels of extracellular signal-regulated kinase 1 and 2 (ERK1/2) or p38 mitogen-activated protein kinase (MAPK) (Fig. 1B), two MAPKs required for signal transduction in macrophages. Altogether these data show that RSVA405 inhibited LPS-induced intracellular signaling and cytokine response in activated macrophages with an apparent IC50 around 0.5–1μM(Figs. 1Cand1E).
RSVA405 inhibits the phagocytic activity of LPS-activated RAW 264.7cells
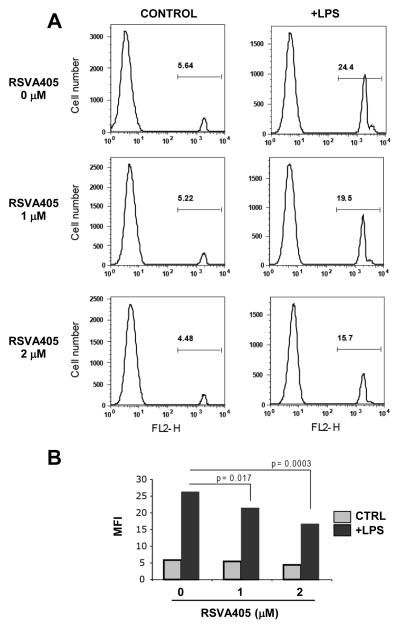
Using fluorescent red-labeled latex bead internalization and FACS analysis, we found that phagocytic activity of LPS-activated RAW 264.7 cells was significantly inhibited by pretreatment with RSVA405 (Figs. 2A and 2B).
Figure 2. RSVA405 inhibits the phagocytic activity of LPS-activated RAW 264.7 cells.
RAW 264.7 cells pretreated for 16 hrs with the indicated concentrations of RSVA405 were stimulated with LPS or not (Control) for 8 hrs. Internalization of fluorescent red-labeled latex beads were analyzed by flow cytometry. Shown are representative FACS analysis (A) and mean fluorescence intensity (MFI) quantification (B).
RSVA405 inhibits RAW 264.7cell proliferation without affecting cell viability
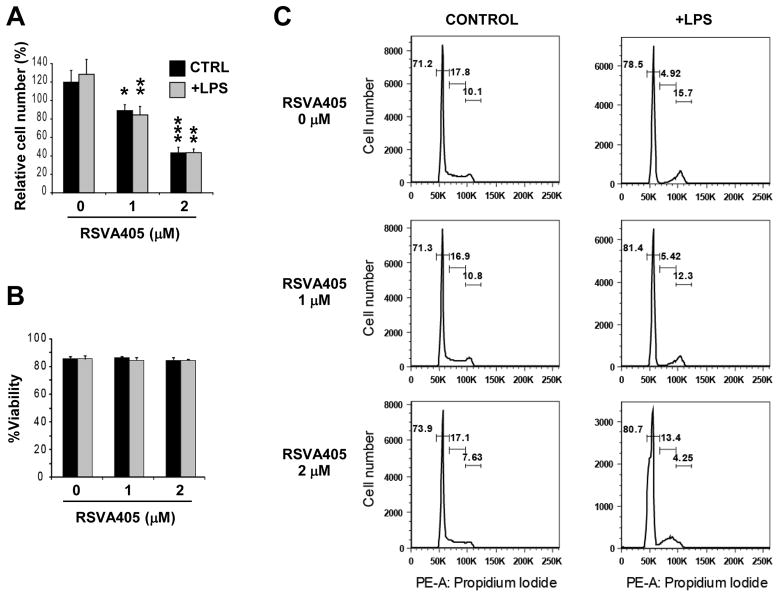
To further characterize the response of RAW 264.7 cells to RSVA405 treatment, we analyzed macrophage proliferation and cell viability. RSVA405 significantly lowered the proliferation of non-activated and LPS-activated RAW 264.7 cells (Fig. 3A). Trypan blue exclusion assay revealed no decrease in cell viability associated with RSVA405 treatment, at concentrations as high as 2 μM (Fig. 3B), indicating that the effect of this compound on activated macrophages is not due to cell toxicity. Flow cytometry analysis using propidium iodide staining further indicated that RSVA405 treatment did lead to the appearance of a subdiploid peak characteristic of the presence of an apoptotic cell population (Fig. 3C). Together these results show that RSVA405 interfered with macrophage proliferation without affecting cell viability.
Figure 3. RSVA405 inhibits RAW 264.7 cell proliferation without affecting cell viability.
RAW 264.7 cells pretreated for 16 hrs with the indicated concentrations of RSVA405 were stimulated with LPS or not (CTRL, Control)for 8 hrs. Cell counts (A) and cell viability (B) were determined by Trypan blue exclusion assay. Histograms show the mean ± S.D. (*, p<0.05; **, p<0.01; ***, p<0.001; n=4). Cell cycle progression analysis was performed by propidium iodide staining and flow cytometry (C).
RSVA314 and RSVA405 inhibit STAT3 activity by promoting the dephosphorylation of STAT3 by PTPs
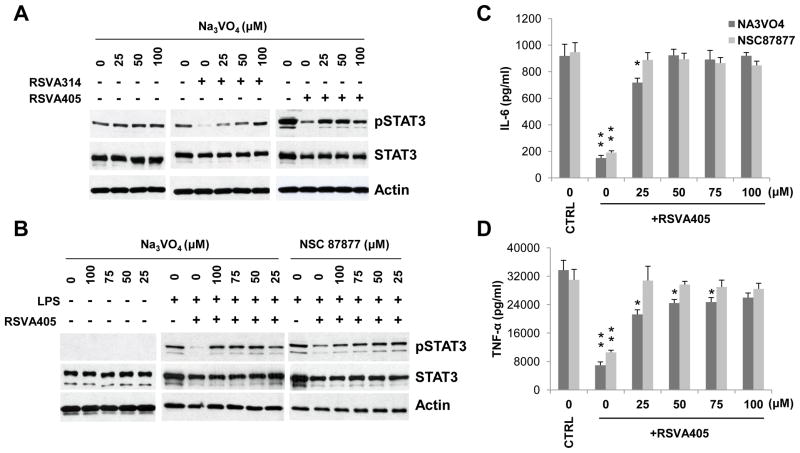
To gain insight into the mechanism by which RSVA314 and RSVA405 inhibit STAT3, we then asked whether these compounds promote STAT3 dephosphorylation. Strikingly, we observed that pretreatment with sodium orthovanadate, a general inhibitor of PTPs, completely prevented the inhibitory effect of RSVA314 and RSVA405 on STAT3 phosphorylation in HEK293 cells, whereas the PTP inhibitor had no effect on the constitutive levels of phospho-STAT3 (Fig. 4A). Sodium orthovanadate also prevented the effect of RSVA405 on phospho-STAT3 in LPS-activated RAW 264.7 macrophages (Fig. 4B). Pretreatment with NSC 87877, a specific inhibitor of SHP-1 and SHP-2[20], also prevented the effect of RSVA405 on phospho-STAT3 in LPS-activated macrophages (Fig. 4B). Importantly, both sodium orthovanadate and NSC 87877 pre-treatment prevented the inhibitory effect of RSVA405 on IL-6 and TNF-α secretion in LPS-activated RAW 264.7 macrophages (Figs. 4C and 4D). Thus, the small-molecules acted by promoting the inactivating dephosphorylation of STAT3 by PTPs.
Figure 4. RSVA314 and RSVA405 inhibit STAT3 activity by promoting the dephosphorylation of STAT3 by PTPs.
(A) HEK293 cells were preincubated in the absence (−) or presence (+) of 2 μM RSVA314 or RSVA405for 16 hrs and then were treated with the indicated concentrations of sodium orthovanadate (Na3VO4) for 4 hrs. Total protein extracts were analyzed by WB using antibodies directed against the indicated proteins. (B) RAW 264.7 cells were preincubated in the absence (−) or presence (+) of 2 μM RSVA405 for 16 hrs and then were treated or not with LPS for 8 hrs in the presence of the indicated concentrations of Na3VO4 (only during the last 4 hrs of treatment) or NSC 87877. WB analysis was then performed as in (A). (C and D) Conditioned medium from LPS-activated RAW 264.7 cells treated as in (B) was analyzed by ELISA for IL-6 and TNF-α secretion. Histograms show the mean ± S.D. (*, p<0.05; **, p<0.001, as compared to the respective control (CTRL) conditions; n=3).
DISCUSSION
In the present study, we show that the two analogs of resveratrol, RSVA314and RSVA405, inhibited both constitutive STAT3 in HEK293 cells and stimulated STAT3 in activated RAW 264.7 macrophages, with a potency 50 times higher than resveratrol (apparent IC50 ~ 0.5 μM). STAT3 has a critical role in cytokine-mediated inflammation and immunity. In this context, the identification of potent STAT3 inhibitors that can modulate the immune response is of interest. In the past few years, several direct and indirect STAT3 inhibitors with anti-proliferative and anti-inflammatory effects have been identified [21,22], including the natural compound resveratrol [1,16]. We found that RSVA405 interfered with the signaling response, cytokine release, proliferation, and phagocytotic activity of LPS-activated RAW 264.7 macrophages, indicating that this class of novel small-molecules may be of therapeutic value to control inflammation. It is important to note, however, that the small-molecules do not exclusively target STAT3. We previously reported that additional protein kinases, such as AMPK, are modulated by RSVA314 and RSVA405in neuronal cells and adipocytes [13,14]. It will be interesting to determine in future studies whether these other protein targets are also modulated by the RSVA compounds in immune cells.
Our results also showed that IL-1α is robustly up regulated upon treatment with RSVA405. IL-1α has been implicated in anti-proliferative properties in several cell systems [23,24], suggesting that RSVA405 might exert its anti-inflammatory effect in macrophages in part by up regulating IL-1α secretion.
Previous work from our laboratory indicated that RSVA314 and RSVA405 are not cytotoxic in different cell lines, including in HEK293 cells [13]. In the present study, we confirmed that RSVA405 did not affect viability of both quiescent and LPS-activated RAW 264.7 macrophages at concentrations efficiently inhibiting STAT3 phosphorylation. Furthermore, previous in vivo studies performed in our laboratory demonstrated that oral administration of RSVA405 at doses up to 100 mg/kg/day for 11 weeks is well tolerated in mice [14]. These results show that RSVA405 is not toxic in vitro and in vivo in mice and thus motivate future studies aimed at determining whether these compounds have beneficial effects in different pro-inflammatory mouse models.
In a recent study, we showed that RSVA405 has potent anti-adipogenic properties and can lower the body weight gain observed in mice fed a high-fat diet (HFD) [14]. HFD mice represent a model of obesity, a condition that leads to many symptoms of chronic low-grade inflammation [25]. Notably, adipose tissue is infiltrated by macrophages that produce elevated levels of various pro-inflammatory cytokines, such as IL-6 and TNF-α [26]. In the present study, we showed that RSVA405 could potently lower IL-6 and TNF-α secretion in response to LPS stimulation in macrophages. In subsequent studies, it will be interesting to determine whether RSVA405 can reduce inflammation in pro-inflammatory mouse models and whether STAT3 activity inhibition in adipose macrophages contributes to the beneficial effect of RSVA405 observed in the HFD mice.
In summary, we propose that the small-molecules RSVA314 and RSVA405 are novel potent inhibitors of STAT3 with anti-inflammatory properties in RAW 264.7 macrophages. Importantly, we found that effect of RSVA314 and RSVA405 on STAT3 phosphorylation could be prevented by inhibitors of PTPs and SHP-1/2, indicating that these compounds did not inhibit directly STAT3 but instead acted by promoting PTP-mediated dephosphorylation of STAT3. These results motivate further in vivo studies aimed at determining whether RSVA314 and RSVA405 have therapeutic value against conditions associated with deregulated STAT3 activation and abnormal immune responses.
EXPERIMENTAL PROCEDURES
Materials and Antibodies
Antibodies directed against total STAT3, phospho-Tyr705 STAT3 (pSTAT3), phospho-Ser727 STAT3(pSTAT3 (Ser)), total STAT1, phospho-Tyr701 STAT1 (pSTAT1), total IKKα, phospho-Ser176/180 IKKα/β (pIKKα/β), total IκBα, phospho-Ser32 IκBα (pIκBα), total TBK1, phospho-Thr202/Tyr204 ERK1/2 (pERK1/2), and phospho-Thr180/Tyr182 p38 (pp38) were obtained from Cell Signaling Technology (Danvers, MA). Antibody against phospho-Ser172 TBK1 (pTBK1) were from Epitomics (Burlingame, CA). Anti-actin antibody was from BD Transduction Laboratories (San Deigo, CA). Synthetic resveratrol (trans-resveratrol) and E. coli LPS were from Sigma-Aldrich (St. Louis, MO). Sodium orthovanadate (Na3VO4) and NSC 87877 were from Calbiochem-EMD Millipore (Billerica, MA). RSVA314 (N′-[4-(diethylamino)-2-hydroxybenzylidene]-2-hydroxybenzohydrazide) and RSVA405 (N′-[4-(diethylamino)-2-hydroxybenzylidene] isonicotinohydrazide) [13] were from Chembridge (Hit2Lead compounds # 5194489 and 5113025, respectively; San Diego, CA) and were dissolved in DMSO.
Trypan blue exclusion assay
Cells were incubated with 0.2% trypan blue for 5 min. Cells that did not take up the dye (viable cells) and cells that took up the dye (dead cells) were counted using a hemocytometer. For each sample four fields where counted and the numbers averaged. Each treatment was done in triplicate.
Cell culture and cell treatments
Murine macrophage cell line RAW 264.7 and HEK293 cells were maintained in DMEM plus 10% FBS, penicillin, streptomycin, at 37°C, 5% CO2. All cell lines were tested negative for mycoplasma contaminants [27]. HEK293 cells were treated at confluence for the indicated concentrations and incubation times. RAW 264.7 cells, at a density of 2 × 106 cells per 35-mm well, were treated with the different drugs and for the indicated concentrations and incubation times. RAW 264.7 cells were stimulated with 10 ng/ml LPS for the indicated incubation times.
Immunoblotting
Protein extracts were analyzed by WB using the antibodies listed above. Cell extracts obtained in Laemmli buffer were resolved on SDS-PAGE, followed by electrotransfer to nitrocellulose membranes. Following a blocking step in 5% milk in Tween-Tris-buffered saline, membranes were incubated with primary and secondary antibodies. Membranes were then developed and visualized with enhanced chemiluminescence from Thermo Scientific Pierce, (Rockford, IL).
ELISA and mouse cytokine array
Conditioned medium from RAW 264.7 cell cultures were collected and spun for 5 min at 14,000 × g at room temperature. Supernatants were analyzed for IL-6 and TNF-α levels by ELISA (R&D duo kits, R&D systems, Minneapolis, MN) and for the production of selected mouse cytokines and chemokines on membrane arrays (Proteome Profiler Mouse Cytokine Array Panel A, R&D Systems), as per the manufacturer’s instructions.
Phagocytosis assay
RAW 264.7 cells (1.0 × 106 cells/ml) were incubated with fluorescent red-labeled latex beads (10 μl of 2 μM beads in 2 ml DMEM, L3030, Sigma-Aldrich) for 2 hrs. Cells were then detached and washed 4–5 times with ice-cold phosphate-buffered saline (PBS). Cells were fixed with 3.7% formaldehyde and analyzed on BD FACSCalibur flow cytometer.
Propidium iodide staining
RAW 264.7 cells (1.0 × 106 cells/ml) were fixed and permeabilized with ice-cold ethanol. Plates were washed twice and harvested by mechanical scraping with ice-cold PBS. Cells were spun down at 500 × g for 5 min at 4°C and cell pellets were resuspended in 200 μl PBS. Ice-cold 70% ethanol (1.8 ml) was then added drop by drop to the cell suspensions under vigorous stirring. Cells were stained with propidium iodide/RNase staining solution (0.1% Triton-X-100 in PBS, 100 μg/ml DNase-free RNase A, 40 μg/ml propidiumiodide) for 1 hr. For each sample, 10,000 cells were analyzed on BD FACSCalibur
Statistical analyses
All data sets were analyzed using unpaired Student’s t-test.
Acknowledgments
This work was supported in part by the National Institutes of Health grant PO1 AT004511 (National Center for Complementary and Alternative Medicine Project 2 to PM).
Abbreviations
- STAT
signal transducer and activator of transcription
- LPS
lipopolysaccharide
- PTP
protein tyrosine phosphatase
- IL
interleukin
- NF-κB
nuclear factor κ-light-chain-enhancer of activated B cells
- TNF
tumor necrosis factor
- TLR
Toll-like receptor
- MyD88
myeloid differentiation primary response gene 88
- TRIF
Toll/IL-1R domain-containing adapter molecule 1
- IKK
IκB kinase
- TBK1
TNF receptor-associated factor family member-associated NF-κB activator-binding kinase-1
Footnotes
The authors declare no conflict of interest.
References
- 1.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 2007;7:454–65. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- 3.Rawlings JS, Rosler KM, Harrison DA. The JAK/STAT signaling pathway. J Cell Sci. 2004;117:1281–3. doi: 10.1242/jcs.00963. [DOI] [PubMed] [Google Scholar]
- 4.Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 2007;13:460–9. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–51. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- 6.Sanjo H, Takeda K, Tsujimura T, Ninomiya-Tsuji J, Matsumoto K, Akira S. TAB2 is essential for prevention of apoptosis in fetal liver but not for interleukin-1 signaling. Mol Cell Biol. 2003;23:1231–8. doi: 10.1128/MCB.23.4.1231-1238.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shim JH, Xiao C, Paschal AE, Bailey ST, Rao P, Hayden MS, Lee KY, Bussey C, Steckel M, Tanaka N, Yamada G, Akira S, Matsumoto K, Ghosh S. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 2005;19:2668–81. doi: 10.1101/gad.1360605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fitzgerald KA, McWhirter SM, Faia KL, Rowe DC, Latz E, Golenbock DT, Coyle AJ, Liao SM, Maniatis T. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol. 2003;4:491–6. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- 9.Sharma S, tenOever BR, Grandvaux N, Zhou GP, Lin R, Hiscott J. Triggering the interferon antiviral response through an IKK-related pathway. Science. 2003;300:1148–51. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- 10.Fremont L. Biological effects of resveratrol. Life Sci. 2000;66:663–73. doi: 10.1016/s0024-3205(99)00410-5. [DOI] [PubMed] [Google Scholar]
- 11.Vingtdeux V, Dreses-Werringloer U, Zhao H, Davies P, Marambaud P. Therapeutic potential of resveratrol in Alzheimer’s disease. BMC Neurosci. 2008;9(Suppl 2):S6. doi: 10.1186/1471-2202-9-S2-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Capiralla H, Vingtdeux V, Zhao H, Sankowski R, Al-Abed Y, Davies P, Marambaud P. Resveratrol mitigates lipopolysaccharide-and Abeta-mediated microglial inflammation by inhibiting the TLR4/NF-kappaB/STAT signaling cascade. J Neurochem. 2012;120:461–72. doi: 10.1111/j.1471-4159.2011.07594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vingtdeux V, Chandakkar P, Zhao H, d’Abramo C, Davies P, Marambaud P. Novel synthetic small-molecule activators of AMPK as enhancers of autophagy and amyloid-beta peptide degradation. FASEB J. 2011;25:219–31. doi: 10.1096/fj.10-167361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vingtdeux V, Chandakkar P, Zhao H, Davies P, Marambaud P. Small-molecule activators of AMP-activated protein kinase (AMPK), RSVA314 and RSVA405, inhibit adipogenesis. Mol Med. 2011;17:1022–30. doi: 10.2119/molmed.2011.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wung BS, Hsu MC, Wu CC, Hsieh CW. Resveratrol suppresses IL-6-induced ICAM-1 gene expression in endothelial cells: effects on the inhibition of STAT3 phosphorylation. Life Sci. 2005;78:389–97. doi: 10.1016/j.lfs.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 16.Mankan AK, Greten FR. Inhibiting signal transducer and activator of transcription 3: rationality and rationale design of inhibitors. Expert Opin Investig Drugs. 2011;20:1263–75. doi: 10.1517/13543784.2011.601739. [DOI] [PubMed] [Google Scholar]
- 17.Wen Z, Zhong Z, Darnell JE., Jr Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241–50. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- 18.Quintana FJ, Jin H, Burns EJ, Nadeau M, Yeste A, Kumar D, Rangachari M, Zhu C, Xiao S, Seavitt J, Georgopoulos K, Kuchroo VK. Aiolos promotes T(H)17 differentiation by directly silencing Il2 expression. Nat Immunol. 2012 doi: 10.1038/ni.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hutchins AP, Poulain S, Miranda-Saavedra D. Genome-wide analysis of STAT3 binding in vivo predicts effectors of the anti-inflammatory response in macrophages. Blood. 2012;119:e110–9. doi: 10.1182/blood-2011-09-381483. [DOI] [PubMed] [Google Scholar]
- 20.Chen L, Sung SS, Yip ML, Lawrence HR, Ren Y, Guida WC, Sebti SM, Lawrence NJ, Wu J. Discovery of a novel shp2 protein tyrosine phosphatase inhibitor. Mol Pharmacol. 2006;70:562–70. doi: 10.1124/mol.106.025536. [DOI] [PubMed] [Google Scholar]
- 21.Yu PJ, Jin H, Zhang JY, Wang GF, Li JR, Zhu ZG, Tian YX, Wu SY, Xu W, Zhang JJ, Wu SG. Pyranocoumarins Isolated from Peucedanum praeruptorum Dunn Suppress Lipopolysaccharide-Induced Inflammatory Response in Murine Macrophages Through Inhibition of NF-kappaB and STAT3 Activation. Inflammation. 2012;35:967–77. doi: 10.1007/s10753-011-9400-y. [DOI] [PubMed] [Google Scholar]
- 22.Park SY, Baik YH, Cho JH, Kim S, Lee KS, Han JS. Inhibition of lipopolysaccharide-induced nitric oxide synthesis by nicotine through S6K1-p42/44 MAPK pathway and STAT3 (Ser 727) phosphorylation in Raw 264.7 cells. Cytokine. 2008;44:126–34. doi: 10.1016/j.cyto.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Maund SL, Barclay WW, Hover LD, Axanova LS, Sui G, Hipp JD, Fleet JC, Thorburn A, Cramer SD. Interleukin-1alpha mediates the antiproliferative effects of 1,25-dihydroxyvitamin D3 in prostate progenitor/stem cells. Cancer Res. 2011;71:5276–86. doi: 10.1158/0008-5472.CAN-10-2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gariboldi M, Monti E. Antiproliferative effects of interleukin-1 alpha and nitric oxide release in a human ovarian carcinoma cell line. Int J Oncol. 1996;9:499–503. doi: 10.3892/ijo.9.3.499. [DOI] [PubMed] [Google Scholar]
- 25.Das UN. Is obesity an inflammatory condition? Nutrition. 2001;17:953–66. doi: 10.1016/s0899-9007(01)00672-4. [DOI] [PubMed] [Google Scholar]
- 26.Surmi BK, Hasty AH. Macrophage infiltration intoadipose tissue: initiation, propagation and remodeling. Future Lipidol. 2008;3:545–56. doi: 10.2217/17460875.3.5.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao H, Dreses-Werringloer U, Davies P, Marambaud P. Amyloid-beta peptide degradation in cell cultures by mycoplasma contaminants. BMC Res Notes. 2008;1:38. doi: 10.1186/1756-0500-1-38. [DOI] [PMC free article] [PubMed] [Google Scholar]