Abstract
NG2 cells are an abundant glial cell type in the adult brain. They are distinct from astrocytes, mature oligodendrocytes, and microglia. NG2 cells generate oligodendrocytes and a subpopulation of protoplasmic astrocytes in the ventral forebrain during development. To determine whether NG2 cells generate reactive astrocytes in the lesioned brain, stab wound injury was created in adult NG2creBAC:ZEG double transgenic mice, in which enhanced green fluorescent protein (EGFP) is expressed in NG2 cells and their progeny, and the phenotype of the EGFP+ cells was analyzed at 10 and 30 days post lesion (dpl). The majority (>90%) of the reactive astrocytes surrounding the lesion that expressed glial fibrillary acidic protein (GFAP) lacked EGFP expression, and conversely the majority (>90%) of EGFP+ cells were GFAP-negative. However, 8% of EGFP+ cells co-expressed GFAP at 10 dpl. Most of these EGFP+GFAP+ cells were morphologically distinct from hypertrophic reactive astrocytes and exhibited weak GFAP expression. NG2 was detected in a fraction of the EGFP+GFAP+ cells found at 10 dpl. By 30 dpl the number of EGFP+GFAP+ cells had decreased more than four-fold from 10 dpl. A similar transient appearance of EGFP+GFAP+ cells with simple morphology was observed in NG2creER™:ZEG double transgenic mice in which EGFP expression had been induced in NG2 cells prior to injury. NG2 cell-specific deletion of the oligodendrocyte lineage transcription factor Olig2 using NG2creER™:Olig2fl/fl:ZEG triple transgenic mice did not increase the number of EGFP+ reactive astrocytes. These findings suggest that NG2 cells are not a major source of reactive astrocytes in the neocortex.
Keywords: NG2, reactive astrocyte, oligodendrocyte
INTRODUCTION
The central nervous system reacts to different types of lesions with reactive astrogliosis (Duchen, 1992; Pekny and Nilsson, 2005; Sofroniew, 2009), characterized by astrocyte hyperplasia, hypertrophy, and increased expression of the astrocyte-specific intermediate filament protein glial fibrillary acidic protein (GFAP). Although reactive astrogliosis is vital to limiting the extent of brain injury in the acute phase, it has been shown to have detrimental effects on neuronal regeneration in the chronic phase (Bush et al., 1999; McKeon et al., 1991; Wilhelmsson et al., 2004). While earlier ultrastructural studies suggested that reactive astrocytes arise from existing mature astrocytes (Cavanagh, 1970; Latov et al., 1979), more recent studies have raised the possibility that other cell types such as NG2 cells also contribute to astrogliosis (Alonso, 2005).
NG2 cells are identified by the expression of the NG2 chondroitin sulfate proteoglycan and multi-processed morphology. They exist ubiquitously throughout the mature central nervous system and are distinct from astrocytes, mature oligodendrocytes, and microglia (reviewed in Nishiyama, 2007; Nishiyama et al., 2009). We have demonstrated that during development NG2 cells in the dorsal forebrain generate predominantly oligodendrocytes but not astrocytes, while those in the embryonic ventral forebrain generate a subpopulation of protoplasmic astrocytes in the gray matter in addition to oligodendrocytes (Zhu et al., 2008, 2011).
NG2 cells continue to proliferate slowly in the mature brain where they represent the major proliferative cell population (Dawson et al., 2003). NG2 cells undergo enhanced proliferation in response to a variety of insults to the brain (Di Bello et al., 1999; Levine, 1994; McTigue et al., 2001). Recently, several studies have used pulse-chase labeling with 5-bromo-2′-deoxyuridine (BrdU) in various lesion paradigms and reported that the proliferated cells generate reactive astrocytes (Alonso, 2005; Cassiani-Ingoni et al., 2006, 2007; Magnus et al., 2007, 2008; Zhao et al., 2009). Since NG2 cells represent a significant proportion of the proliferating cells in such lesions, it was suggested in these studies that the proliferated NG2 cells contribute to reactive astrocytes. However, it is also possible that NG2-negative cells that proliferated in the lesions generated astrocytes.
In this study, we have used constitutively active and tamoxifen-inducible NG2-cre transgenic mouse lines to directly determine whether NG2 cells generate reactive astrocytes in response to a neocortical stab wound. Our results suggest that a low level of GFAP is transiently expressed in a small fraction of NG2 cell progeny, but the majority of reactive astrocytes are not derived from NG2 cells.
MATERIALS AND METHODS
Animals
All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Connecticut. The generation of NG2cre-BAC and NG2creER™BAC transgenic mice, which express constitutively active Cre and tamoxifen-inducible Cre, respectively, in NG2 cells, have been described (Zhu et al., 2008, 2011). NG2creBAC and NG2creER™BAC DNA were initially injected into C57Bl/6 oocytes, but the mice have been subsequently bred into the FvB strain. Z/EG mice (Novak et al., 2000) were obtained from Dr. Caiying Guo at the University of Connecticut Health Center (currently at Howard Hughes Medical Institute Janelia Farm). NG2creBAC and NG2creER™BAC mice were crossed to Z/EG mice to generate NG2creBAC:ZEG and NG2creER™BAC:ZEG double transgenic mice, respectively. Conditional Olig2 knockout mice were generated in Dr. Richard Lu’s laboratory (Yue et al., 2006) and crossed with NG2creER™BAC and Z/EG mice to generate NG2creER™BAC:ZEG:Olig2 fl/fl or NG2creER™BAC:ZEG:Olig2fl/+ triple transgenic mice. These mice have a mixed genetic background of 129, C57Bl/6, and FvB.
Adult mice between 6 and 12 weeks of age were used for all experiments. Stab wound lesions were created with a needle blade by making a sagittal cut through the neocortex and corpus callosum 3.94-mm lateral from the midline and extending from 2.35-mm anterior to 4.45-mm posterior from the bregma. Mice were sacrificed 10 and 30 days after lesioning (n = 6 or 7 for NG2creBAC:ZEG; n = 3 to 6 for NG2creER™BAC:ZEG; and n = 3 for NG2creER™BAC:ZEG:Olig2fl/fl mice for each time point). For experiments with NG2creER™BAC mice, Cre was induced by intraperitoneal injection of 1 mg of 4-hydroxytamoxifen (4OHT), dissolved in 0.1 mL of 1:19 mixture of ethanol and vegetable oil, twice a day for 5 consecutive days, and lesioning was performed two days after the last 4OHT injection.
To detect proliferating cells, 25 mg kg−1 of 5-ethynyl-2′-deoxyuridine (EDU; InVitrogen) was injected intraperitoneally at 3 dpl, and the mice were sacrificed 2 h later.
Tissue Processing
Mice were anesthetized and perfused as previously described (Zhu et al., 2008). The brains were cryoprotected and frozen as previously described (Watanabe et al., 2002), and coronal sections (20 µm) were cut on a cryostat (HM-500, Microm).
Immunohistochemistry
Immunohistochemistry of fixed frozen section was performed as previously described (Watanabe et al., 2002). The following primary antibodies were used: mouse antibodies to Aldehyde dehydrogenase family 1 member L1 (Aldh1L1) (1:1,000 dilution, NeuroMab), GFAP (1:400, Sigma), nestin (1:200, Chemicon); NeuN (1:5,000, Chemicon); vimentin (1:200, Sigma); rat anti-CD11b antibody, (1:400, Serotec); rabbit antibodies to fibronectin (1:1,000, Sigma), GFAP (1:1,000, DAKO), glutathione S-transferase π(GST-π) (1:4,000, MBL), NG2 (1:500, Chemicon), Olig2 (1:20,000, kindly provided by Drs. Charles Stiles and John Alberta, Dana-Farber Cancer Institute, Boston, MA), and P0 (kindly proved by Dr. Peter Brophy, University of Edingburgh). Secondary species-specific and fluorophore-conjugated antibodies were from Jackson ImmunoResearch and Molecular Probes.
Immunolabeling with rabbit polyclonal anti-GFAP antibody was more robust than staining with mouse monoclonal anti-GFAP antibody. Mouse anti-GFAP immunolabeling was enhanced by including 0.3% Triton X-100 in the primary antibody solution. Antigen retrieval was performed for Olig2 immunolabeling by treating sections for 30 min at 65°C in 10 mM sodium citrate buffer, pH 6.0, prior to incubation with mouse anti-GFP and rabbit anti-Olig2 antibodies.
EDU was detected using Alexa 594-conjugated azide (Click-iT Assay System, InVitrogen).
Microscopy and Photomicrograph Production
Confocal images were obtained with a Leica TCS SP2 laser scanning confocal microscope. Serial z-stacks were analyzed to determine co-localization of EGFP fluorescence with cell type-specific markers. Figures were prepared by using Leica Confocal Software, Adobe Photoshop 9.0, and Adobe Illustrator CS3 (Adobe Systems, San Jose, CA). Image manipulations were limited to linear grayscale level adjustments.
Cellular Analysis and Quantification
To examine the fate of NG2 cells in the perilesional cortex, the total number of enhanced green fluorescence (EGFP)-positive cells and the number of cells that were double positive for EGFP and GFAP, Aldh1L1, nestin, vimentin, NG2, or GST-π were counted in the neocortex within 300 µm of the stab wound. Sections from anterior, middle, and posterior portions of the lesion were used for quantification. Between 200 and 500 EGFP+ cells were scored in NG2creBAC:ZEG mice, and 30 to 90 EGFP+ cells were scored in NG2creER™BAC:ZEG mice. EGFP+ cells that were identified as pericytes by virtue of their elongated morphology and location were not included in the counts.
RESULTS
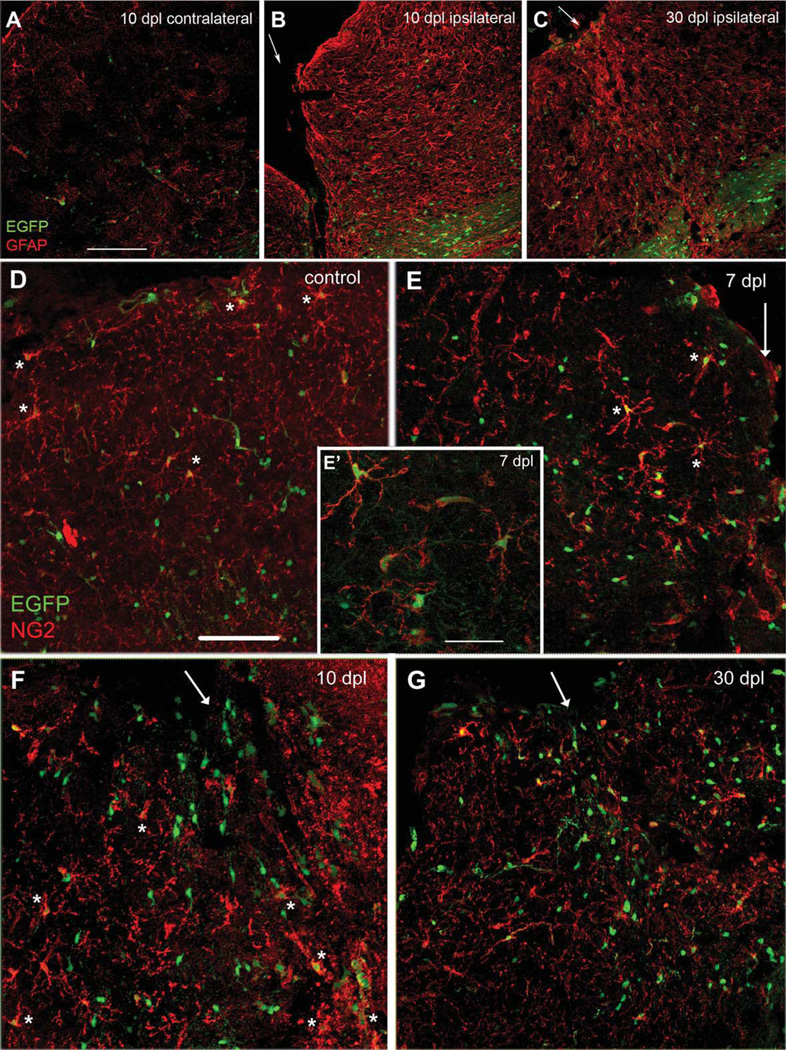
The cortical stab wound was easily identifiable 10 days after lesioning (10 dpl) as a gap in tissue with retracted edges (Fig. 1B). GFAP expression around the wound was markedly upregulated over a wide area spanning ~300 µm from the lesion edge, compared with the contralateral cortex (Fig. 1A) and non-lesioned cortex (not shown), and strongly GFAP+ cells with hypertrophic processes were abundant. At 30 dpl, the wound edges had fused in most cases, and the number of intensely GFAP+ hypertrophic reactive astrocytes around the wound had decreased, resulting in a thin band of increased GFAP immunoreactivity (Fig. 1C). At 3 dpl, there was increased cell proliferation around the lesion, and ~50% of the proliferating cells were NG2 cells (Supp. Info. Fig. 1).
Fig. 1.
GFAP and NG2 immunolabeling of neocortical stab wound in NG2creBAC:ZEG mice. (A–C) EGFP fluorescence (green) and immunolabeling with rabbit anti-GFAP antibody (red). A. Contralateral cortex at 10 dpl. B. Ipsilateral cortex at 10 dpl. C. Ipsilateral cortex at 30 dpl. Arrows indicate lesion center. Upper left is pial surface and lower right is corpus callosum. Intense GFAP immunoreactivity is seen over a wide area around the lesion at 10 dpl, which subsides by 30 dpl but remains elevated above the contralateral cortex. Most of the strongly GFAP+ hypertrophic astrocytes do not express EGFP, and most of the EGFP+ cells do not express GFAP. Scale bar in A, 150 µm. (D–G) EGFP fluorescence (green) and immunolabeling with rabbit anti-NG2 antibody (red). D. Contralateral cortex at 7 dpl. Scale bar, 100 µm. E. Ipsilateral cortex at 7 dpl. Inset (E’) is a higher magnification of perilesional cortex showing the presence of EGFP in NG2+ cells with activated morphology. Scale bar in E’, 50 µm. F. Ipsilateral cortex at 10 dpl. G. Ipsilateral cortex at 30 dpl. Arrows indicate lesion center. Top is pial surface. Asterisks indicate examples of NG2+EGFP+ cells. The majority of NG2+ cells express EGFP and exhibit reactive morphology, and increased NG2 immunoreactivity is seen around the lesion at 7 and 10 dpl. Increased number of EGFP+ cells is seen around the lesion from 7 dpl through 30 dpl.
Most of the GFAP+ Reactive Astrocytes in NG2creBAC:ZEG Mice Lacked EGFP Expression
To determine whether NG2 cells in the neocortex of adult mice generate astrocytes in response to injury, the fate of NG2 cells was analyzed around the stab wound in NG2creBAC:ZEG mice, in which constitutively active Cre expressed in NG2 cells permanently activated EGFP expression. EGFP was detected in 92% of NG2 cells (Fig. 1D–G), consistent with our previous studies (Zhu et al., 2008). In the uninjured neocortex of adult NG2creBAC:ZEG mice, the majority of EGFP+ cells were NG2 cells or oligodendrocytes and did not express GFAP (Fig. 1A). By 7 dpl, there was an increased number of NG2 cells with reactive morphology characterized by increased NG2 immunoreactivity and thicker processes around the lesion, and the reactive NG2 cells expressed EGFP (Fig. 1E, asterisks, and Fig. 1E’). The number of EGFP+ cells remained elevated around the lesion through 30 dpl (Fig. 3G).
Fig. 3.
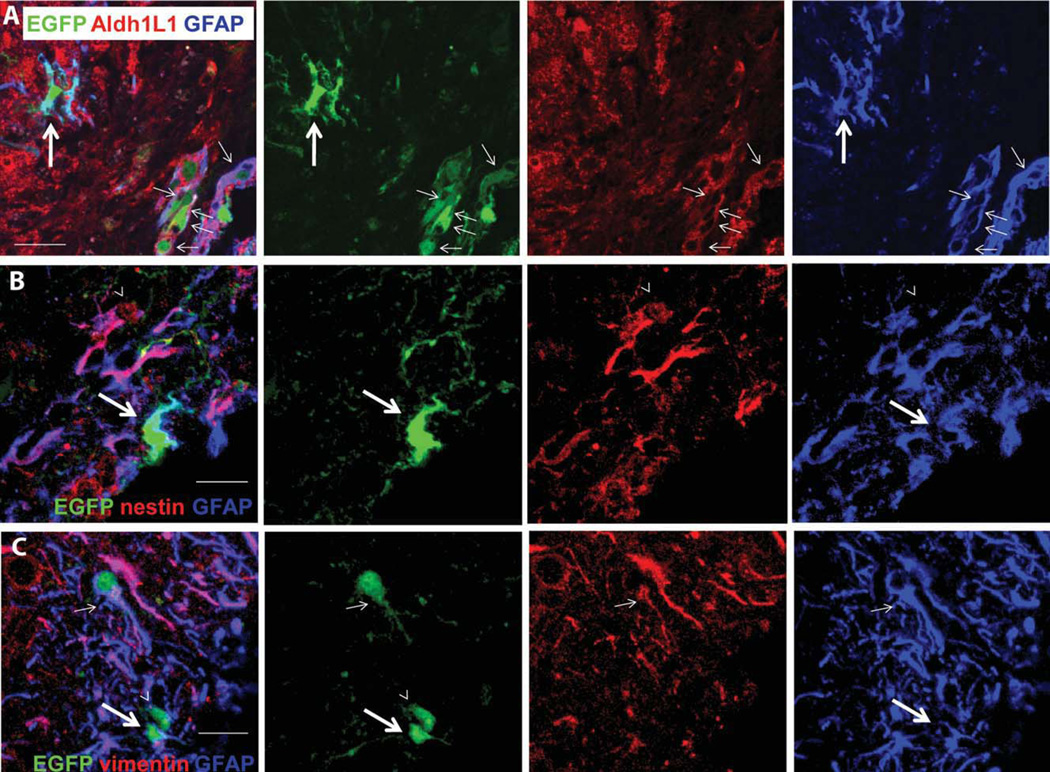
Labeling of EGFP+ cells in NG2creBAC:ZEG double transgenic mice for Aldh1L1 (A), nestin (B), and vimentin (C) at 10 dpl. (A) A cluster of EGFP+GFAP+ cells with simple morphologies (thin arrows) at the lesion border close to the pial surface expressing Aldh1L1 (red). An EGFP+GFAP+ cell with processes (thick arrows) lacks Aldh1L1 expression. (B) An EGFP+GFAP+ cell that lacks nestin expression (thick arrows) and an EGFP− hypertrophic GFAP+ cell that co-expresses nestin (arrowheads). (C) An EGFP+GFAP+ cell that has a vimentin+ process (thin arrows), an EGFP+GFAP+ cell that is vimentin-negative (thick arrows), and an EGFP+ cell that does not express GFAP or vimentin (arrowheads). Scale bars: A, 30 µm; B, 15 µm; C, 20 µm.
Among the GFAP+ cells that appeared around the stab wound, 91% ± 7% were EGFP-negative at 10 dpl (Fig. 2E, asterisks), and 98% ± 1% were EGFP-negative at 30 dpl. Thus, the majority of GFAP+ astrocytes surrounding the lesion in the neocortex had not originated from NG2 cells. Most of the NG2-negative EGFP+ cells were oligodendrocytes.
Fig. 2.
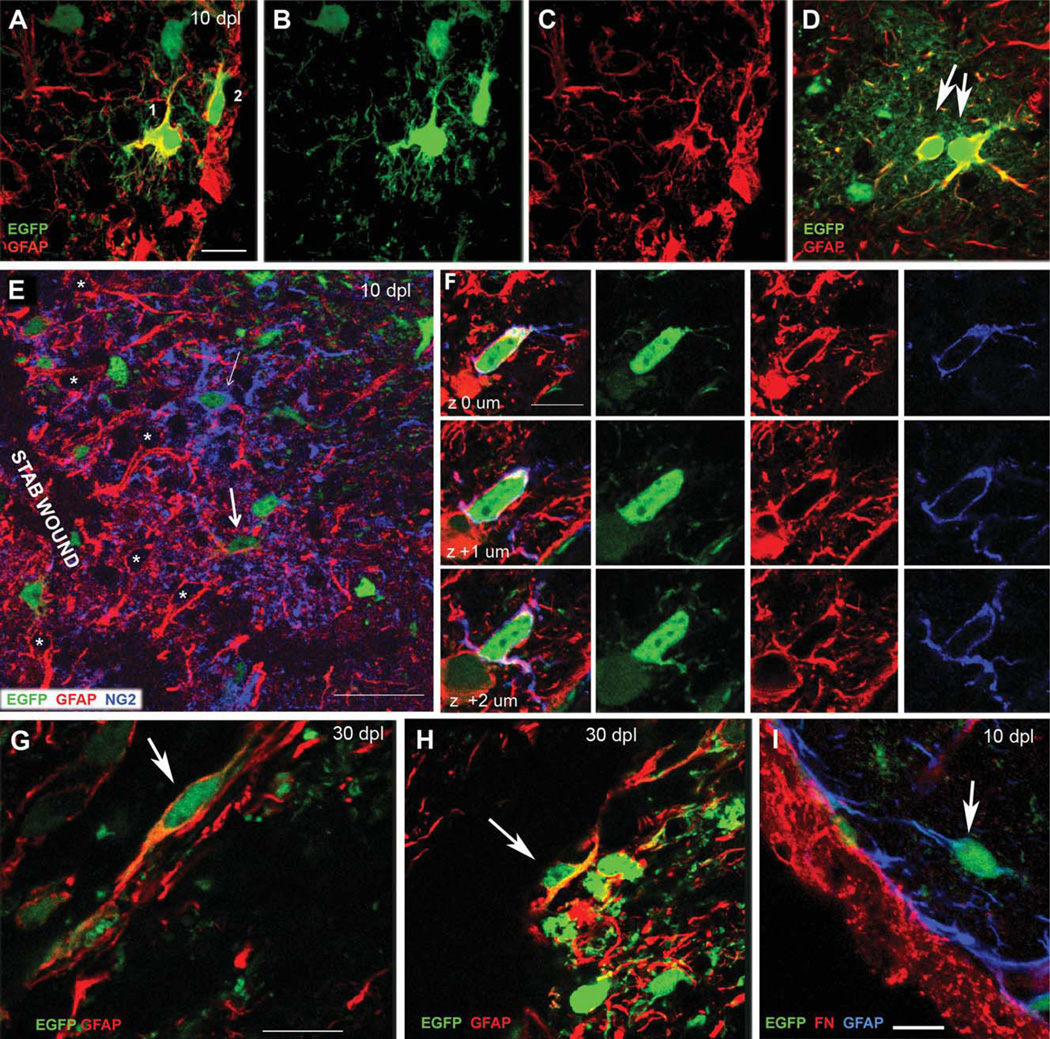
GFAP immunolabeling of EGFP+ cells around the lesion in NG2creBAC:ZEG mice. (A–C) EGFP fluorescence (green) and GFAP immunolabeling (red) of two EGFP+GFAP+ cells at the lesion edge (along the right edge of the images) at 10 dpl. A: merged. B: EGFP. C: GFAP. The cell further away from the lesion (Cell 1) exhibits NG2 cell-like morphology with several branched processes. The cell closer to the lesion (Cell 2) is aligned along the edge of the wound and has fewer processes. Neither resembles a normal protoplasmic astrocyte. (D) EGFP+GFAP+ astrocytes in the ventral forebrain showing the typical bushy protoplasmic astrocyte morphology (arrows) that arise from embryonic NG2 cells under normal conditions. (E, F) EGFP fluorescence and double immunolabeling for GFAP (red) and NG2 (blue) around the lesion at 10 dpl. E: A single z-section through the perilesional neocortex The majority of reactive astrocytes with strongly GFAP+ hypertrophic processes (*) do not express EGFP. Many EGFP+NG2+ cells are present but few are EGFP+GFAP+. Thick arrow indicates an EGFP+GFAP+NG2− cell with simple morphology. Thin arrow shows an EGFP+NG2+GFAP− cell with typical polydendrocyte morphology with multiple thin branched processes. (F) Consecutive z-sections through a rare EGFP+GFAP+NG2+ cell. (G and H) EGFP fluorescence (green) and GFAP immunolabeling (red) showing elongated EGFP+GFAP+ cells with simple morphology (arrows) along the lesion border at 30 dpl. (I) EGFP fluorescence (green) and double immunolabeling for GFAP (blue) and fibronectin (red) at 10 dpl, showing that the elongated EGFP+GFAP+ cells are distinct from fibronectin+ fibroblasts in the meninges. Scale bars in A–D, F, and G–I represent 10 µm. Scale bar in E = 30 µm.
GFAP Expression in a Small Subpopulation of EGFP+ Cells
A small number of EGFP+GFAP+ cells were found in the ipsilateral neocortex close to the lesion border (Fig. 2A–C,E (cell with thick arrow), and F). They comprised 9% ± 7.3% of the GFAP+ cells at 10 dpl, and the percentage declined to 2.2% ± 1% by 30 dpl (Table 1). No EGFP+GFAP+ cells were found in the neocortex away from the lesion or in the contralateral cortex. GFAP expression in the EGFP+GFAP+ cells was often weaker than that in the surrounding reactive astrocytes.
TABLE 1.
Antigenic Phenotype of EGFP+ Cells Around the Lesion in NG2creBAC:ZEG Mice
| Marker | 10 dpl | 30 dpl |
|---|---|---|
| GFAP+ | 7.9 ± 6.6 | 2.2 ± 1.1 |
| Aldh1L1+ | 1.1 ± 2.0 | 1.6 ± 2.3 |
| NG2 | 28.0 ± 14.0 | 16.0 ± 5.0 |
| GST-π | 52.0 ± 11.0 | 62.0 ± 9.0 |
Numbers represent percentages of EGFP+ cells expressing the antigens indicated in the left column. Data are shown as means ± standard deviations.
Perilesional EGFP+GFAP+ cells (Fig. 2A–C) lacked the typical bushy protoplasmic astrocytic morphology (Wilhelmsson et al., 2004) seen in the ventral forebrain where NG2 cells generate astrocytes during normal embryonic development (Zhu et al., 2008, 2011) (Fig. 2D). At 10 dpl, some EGFP+GFAP+ cells had several thin processes with a few branches and resembled NG2 cells (Fig. 2A, Cells 1 and 2). These cells were found mostly at 10 dpl and were rarely seen at 30 dpl. The second type of EGFP+GFAP+ cells could be found at both 10 and 30 dpl and had simple bipolar or elongated morphology with one or two unbranched processes or lacked processes altogether. These elongated EGFP+GFAP+ cells were found in clusters at the lesion border close to the pial surface (Fig. 2G,H, arrows).
A small fraction of EGFP+ cells co-expressed GFAP and NG2, and EGFP+GFAP+NG2+ cells represented 15% ± 11% of the EGFP+GFAP+ cells. The EGFP+GFAP+NG2+ cells had a few thin processes (Fig. 2F) and were only detected at 10 dpl. The other population of EGFP+GFAP+ cells that exhibited simple elongated morphology did not express NG2. The numerous NG2+ cells around the lesion that had the typical polydendrocyte morphology with multiple branched processes did not express GFAP (Fig. 2E, thin arrow).
Fewer than 2% of the EGFP+ cells around the lesion at 10 and 30 dpl expressed Aldh1L1, an antigen that is expressed by protoplasmic astrocytes in the normal neocortex (Cahoy et al., 2008) (Table 1). We never observed EGFP+ cells that were Aldh1L1+ but GFAP−. Most of the EGFP+GFAP+Aldh1L1+ cells had very simple morphology and were found clustered at the lesion border close to the pial surface (Fig. 3A, thin arrows). Some of the process-bearing EGFP+GFAP+ cells did not express Aldh1L1 (Fig. 3A, thick arrow).
We examined whether EGFP+GFAP+ cells expressed nestin and vimentin, which are intermediate filaments found in immature glia and are upregulated in reactive astrocytes (Sofroniew, 2009). Double-labeling for GFAP and nestin revealed that none of the EGFP+GFAP+ cells expressed nestin (Fig. 3B), while many of the hypertrophic GFAP+EGFP− cells were nestin+. Vimentin was detected in a small fraction of the EGFP+GFAP+ cells (Fig. 3C, small arrows). The majority of EGFP+GFAP+ cells were vimentin-negative, while the majority of the EGFP-negative hypertrophic GFAP+ cells expressed vimentin (Fig. 3C).
We stained sections for GFAP and fibronectin to determine whether some of the EGFP+GFAP+ cells with simple morphology were meningeal fibroblasts. While, fibronectin immunoreactivity was strongly detected along the pial surface and around blood vessels in the cortex, it was not detected on EGFP+GFAP+ cells (Fig. 2I). The elongated EGFP+GFAP+ cells did not express the Schwann cell antigen P0 (not shown).
We further characterized the phenotype of EGFP+ cells around the lesion using antibodies to NG2 and the mature oligodendrocyte antigen GST-π. The majority of the remaining EGFP+ cells were NG2+ cells or GST-π+ oligodendrocytes, which together accounted for ~80% of the EGFP+ cells at both 10 and 30 dpl (Table 1). CD11b+ macrophages/microglia accounted for ~2% of the EGFP+ cells, but these cells were distinct from GFAP+ astrocytes.
Most of the GFAP+ Reactive Astrocytes in NG2creER™BAC:ZEG Mice Lacked EGFP Expression
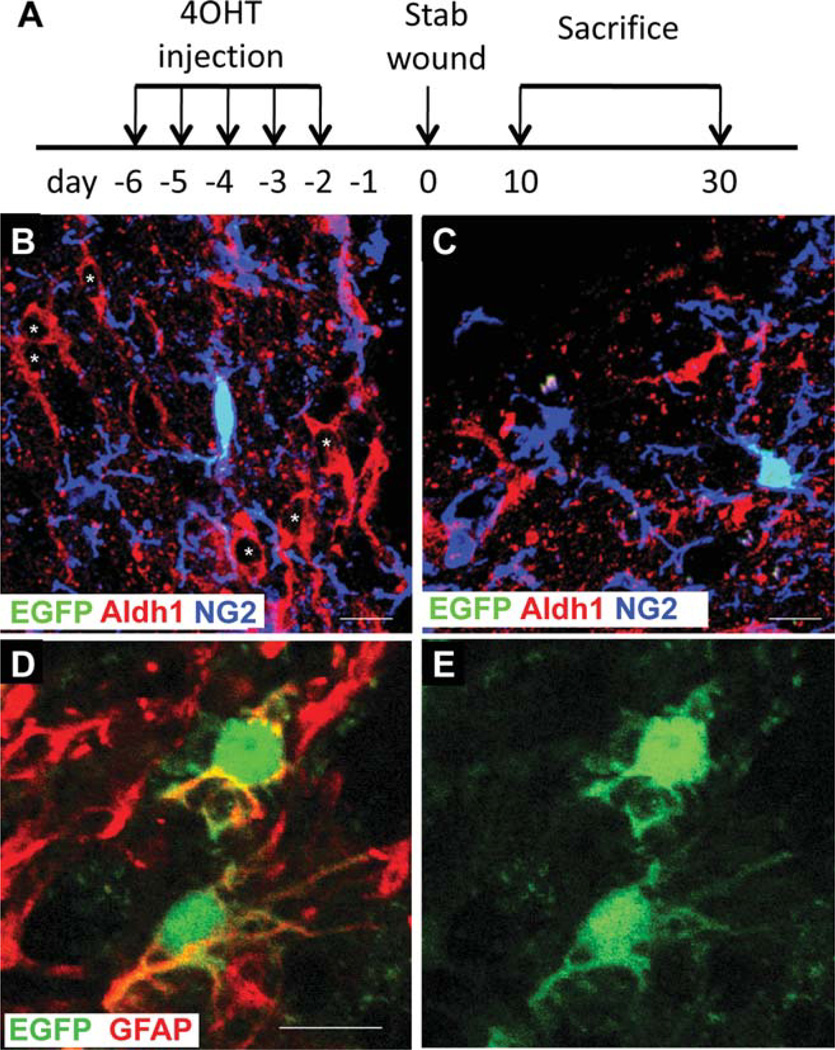
We previously reported that 1–2%of EGFP+ cells in the neocortex of NG2creBAC:ZEG double transgenic mice expressed S100β and had the morphological characteristics of protoplasmic astrocytes at P14 (Zhu et al., 2008). To determine whether the perilesional EGFP+GFAP+ cells described above had been generated de novo from NG2 cells, we created a similar stab wound in NG2creER™BAC:ZEG double transgenic mice (Zhu et al., 2011) after Cre activation by 4-hydroxytamoxifen (4OHT) (Fig. 4A). Cre was induced prior to stab wound injury in order to avoid Cre activation in a subpopulation of activated macrophages that express NG2 (Bu et al., 2001). Cre induction efficiency was between 1 and 2% in these mice when analyzed 1 day after the last day of 4OHT injection. To compensate for the low induction efficiency, we have examined a larger number of sections, so that 30–90 EGFP+ cells were analyzed for each animal. In unlesioned NG2creER™BAC:ZEG mice, no EGFP+GFAP+ cells were found one day after the last 4OHT injection into P60 mice (Zhu et al., 2011). The majority of the EGFP+ cells seen at 10 and 30 dpl expressed NG2 (84% ± 12% and 83% ± 5.3% of EGFP+ cells at 10 and 30 dpl, respectively) and exhibited the typical polydendrocyte morphology (Fig. 4B,C). Other EGFP+ cells seen at 30 dpl were oligodendrocytes that expressed GST-π (0% and 4.1% ± 0.8% of EGFP+ cells at 10 and 30 dpl, respectively).
Fig. 4.
Phenotype of EGFP+ cells around the lesion in NG2creER™BAC: ZEG mice. (A) Scheme of Cre induction, lesioning, and sampling of NG2creER™BAC:ZEG mice. (B,C) EGFP fluorescence (green) and double immunolabeling for NG2 (blue in B and C) and Aldh1L1 (red in B and C). The majority of reactive astrocytes that express GFAP (asterisks in B) or Aldh1L1 (C) do not express EGFP. Most EGFP+ cells are NG2+. (D,E) EGFP fluorescence (green) and immunolabeling for GFAP (red) showing two rare EGFP+ cells that are weakly labeled for GFAP and have thin processes distinct from those of protoplasmic or reactive astrocytes. D: merged image. E: EGFP fluorescence only. Scale bars = 10 µm.
At 10 dpl, 5.7% ± 5.2% of the EGFP+ cells around the lesion expressed GFAP, and the percentage declined to 0.4% ± 0.8% by 30 dpl. These cells lacked the typical astrocyte morphology and often exhibited weaker GFAP immunoreactivity than surrounding reactive astrocytes (Fig. 4D,E). Many of the EGFP+GFAP+ cells had a small number of thin, unbranched and tortuous processes. Only 10% of the EGFP+GFAP+ cells that were evaluated co-expressed NG2 at 10 dpl. No EGFP+GFAP+NG2+ triple labeled cells were detected at 30 dpl. None of the EGFP+ cells expressed Aldh1L1. These observations are consistent with the findings obtained from NG2creBAC:ZEG mice which express Cre constitutively in NG2 cells.
Olig2 Deletion Did Not Divert NG2 Cells to Astroglial Fate
Olig2 is a basic helix-loop-helix transcription factor that is required for oligodendrocyte specification and differentiation (Lu et al., 2002; Zhou et al., 2002). Deletion or cytoplasmic translocation of Olig2 in cultured neural stem cells promotes their astrocyte differentiation. To determine whether deletion of Olig2 could direct NG2 cells to become reactive astrocytes, the fate of NG2 cells was followed in mice that were triple transgenic for NG2creER™BAC, Z/EG, and Olig2fl/fl (Olig2 knockout) and compared with that in NG2creER™BAC:ZEG:Olig2fl/+ mice (Olig2 control). Cre was induced prior to stab wound injury as described above and shown in Fig. 4A.
At 10 dpl, 99 ± 1.6% of EGFP+ cells in Olig2 control mice expressed Olig2. In Olig2 knockout mice 92.4% ± 1.9% of EGFP+ cells lacked Olig2 immunoreactivity at 10 dpl (not shown). At 30 dpl, 97% ± 3.3% of EGFP+ cells in Olig2 control mice expressed Olig2, whereas in Olig2 knockout mice 90% ± 1.2% of EGFP+ lacked Olig2 immunoreactivity (not shown). This indicated successful deletion of the Olig2 gene in the majority of EGFP+ cells.
In brains in which Olig2 had been deleted in NG2 cells, there was no significant difference in the extent of reactive gliosis following stab wound. The majority of the hypertrophic, strongly GFAP+ astrocytes that were found around the lesion at 10 and 30 dpl in Olig2 knockout mice did not express EGFP, and the majority of EGFP+ cells did not express GFAP at 10 or 30 dpl (Fig. 5A–C). A small fraction of EGFP+ cells expressed GFAP (2.8% ± 3.9% at 10 dpl and 0.4 ± 0.7% at 30 dpl). The percentage of EGFP+ cells that also expressed GFAP in Olig2 knockout lesions was not significantly different from that in wild type lesions. The morphology of the EGFP+GFAP+ cells in Olig2 knockout mice was not typical of reactive astrocytes (Fig. 5D–F) and was similar to the EGFP+GFAP+ cells found in Olig2 heterozygous or wild type lesions. The majority of the EGFP+ cells in Olig2 knockout lesions at 10 and 30 dpl were NG2+. Since introduction of the Olig2-VP16 mutation in the adult brain has been shown to induce generation of neurons after stab wound injury (Buffo et al., 2005), we immunolabeled Olig2 knockout sections for NeuN but did not find EGFP+ cells that expressed NeuN or displayed neuronal morphology. These observations indicate that Olig2 deletion in NG2 cells does not promote astroglial differentiation from NG2 cells around a stab wound.
Fig. 5.
Phenotype of EGFP+ cells around the lesion in Olig2 knockout mice. EGFP fluorescence (green) and immunolabeling for GFAP (red) at 10 dpl. (A–C) Example of a typical field where the EGFP+ cell does not express GFAP. (D–F) Example of a rare EGFP+ cell that also expresses GFAP. A and D, merged images; B and E, EGFP channel only; C and F, GFAP channel only. Scale bars: A, 10 µm; D, 20 µm.
DISCUSSION
In vivo fate mapping of NG2 cells in response to a neocortical stab wound in NG2creBAC:ZEG and NG2creER™BAC:ZEG mice revealed that the majority of GFAP+ reactive astrocytes were not derived from NG2 cells, and that the majority of EGFP+ progeny of NG2 cells did not exhibit the morphological and antigenic characteristics of reactive astrocytes, even in the absence of Olig2. NG2 cells remained mainly as NG2 cells or differentiated into oligodendrocytes around the lesion. GFAP was detected in fewer than 10% of the EGFP+ cells derived from NG2 cells at 10 dpl, and a small fraction of these cells also expressed NG2. Most of the EGFP+GFAP+ cells had thin processes and morphologically did not resemble reactive astrocytes. The majority of the EGFP+GFAP+ cells did not co-express other astrocytic markers such as Aldh1L1, nestin, or vimentin. The number of EGFP+GFAP+ cells decreased to background levels by 30 dpl. We previously showed that in the neocortex of NG2creBAC:ZEG double transgenic mice, fewer than 1.8% of EGFP+ cells were protoplasmic astrocytes, in contrast to a much higher percentage of NG2 cell-derived astrocytes found in the ventral forebrain (Zhu et al., 2008). Thus, in NG2cre-BAC:ZEG mice, the 1–2% of EGFP+ cells in the neocortex that expressed astrocyte markers at both 10 and 30 dpl are likely to be normal resident astrocytes that had been generated from NG2 cells during embryonic development (Zhu et al., 2011).
The increased number EGFP+GFAP+ cells that appeared at 10 dpl (increased to 8% above a background of 1–2%) may represent NG2 cells in the oligodendrocyte lineage that had transiently upregulated GFAP expression. The EGFP+GFAP+ cells appeared at 4 dpl, were most prevalent at 10 dpl, and declined thereafter. These cells could represent NG2 cells that had initiated a reprogramming event to become astrocytes, but somehow the pathway may have been aborted prior to their full differentiation into astrocytes. Transient expression of a low level of GFAP might have resulted from translation of GFAP mRNA, which has been detected in some NG2 cells (Matthias et al., 2003; Zhou et al., 2000). The decline in the number of EGFP+GFAP+ cells after 10 dpl may have been caused by loss of GFAP expression in NG2 cell progeny that remained within the oligodendrocyte lineage and/or by cell death. Similar transient coexpression of GFAP and NG2 in a small subpopulation of cells around a cortical lesion has been reported (Hampton et al., 2007; Zhao et al., 2009). The intracellular signaling pathways necessary for astrocyte differentiation in these EGFP+GFAP+ progeny of NG2 cells may have been repressed by the high level of Noggin present in the extracellular environment around the lesion (Hampton et al., 2007). The identity of EGFP+GFAP+ cells with elongated morphology remains unclear. It is possible that transcription of NG2 had occurred transiently in reactive astrocytes or that they had been generated from the minority of EGFP1 astrocytes found in the neocortex of NG2creBAC:ZEG mice (Zhu et al., 2008). Although it was recently shown that NG2 cells can generate Schwann cells during remyelination (Zawadzka et al., 2010), the elongated EGFP+GFAP+ cells did not express the Schwann cell antigen P0.
Our findings differ from several recently published reports in which it was proposed that reactive astrocytes originate from NG2 cells. One study using BrdU pulse chase labeling showed a decrease in the percentage of the BrdU+ cells that were NG2+ and a concomitant increase in the percentage of BrdU+ cells that were GFAP+ between 2 and 6 days after stab wound injury (Alonso, 2005). On the basis of these observations, the author concluded that proliferated NG2 cells had differentiated into GFAP+ reactive astrocytes Using a similar approach, other studies have also made similar conclusions in various lesion paradigms (Horky et al., 2006; Magnus et al., 2007, 2008; Zhao et al., 2009). However, only a subpopulation of the proliferating cells expressed NG2 (33% in Alonso, 2005; up to 50% in Horky et al., 2006; 22% in Magnus et al., 2007; 55% in Magnus et al., 2008). Therefore, it is highly likely that the BrdU+GFAP+ cells that appeared in the lesion in the previously reported studies had originated from astrocyte precursor cells that expressed neither NG2 nor GFAP. This is consistent with the findings that quiescent GFAP-negative astrocyte precursors that express mRNA encoding fibroblast growth factor receptor 3 (FGFR3) and glutamate aspartate transporter (GLAST) proliferate in response to injury and generate GFAP+ reactive astrocytes (Buffo et al., 2008; Bush et al., 1999; Zawadzka et al., 2010). These findings suggest that resident astrocytes that are GFAP−, GFAP mRNA+, and GLAST mRNA+, rather than NG2 cells, proliferate around a lesion and generate reactive astrocytes.
In cultures of neural stem cells, Olig2 inhibits astrocyte differentiation (Fukuda et al., 2004), and export of Olig2 from the nucleus to the cytoplasm induces astrocytic differentiation (Setoguchi and Kondo, 2004). In vivo, cytoplasmic translocation of Olig2 in NG2 cells has been correlated with the appearance of GFAP expression in NG2 cells after injury, and detection of cytoplasmic Olig2 in NG2 cells around a lesion has been used in these studies to support the notion that NG2 cells translocate Olig2 to the cytoplasm prior to differentiating into reactive astrocytes (Hampton et al., 2007; Magnus et al., 2007; Tatsumi et al., 2008; Zhao et al., 2009). In our study, Olig2 was found exclusively in the nucleus, and we did not observe cytoplasmic localization of Olig2, which is in agreement with the localization of Olig2 described by Chen et al. (2008). Therefore, although a possible gain-of-function of cytoplasmically translocated Olig2 could not be ruled out in the knockout mice, we think it is unlikely that Olig2 plays a major role in astrocyte differentiation from NG2 cells in the neocortex of adult mice.
Olig2 is expressed in astrocytes and is upregulated during their peak proliferation in response to injury (Chen et al., 2008; Sofroniew, 2009). Thus the presence of Olig2+GFAP+ cells around a lesion can be explained by upregulation of Olig2 in GFAP+ reactive astrocytes that arose from resident GFAP− astrocytes rather than conversion of NG2 cells into reactive astrocytes. Detection of Cre reporter+ cells after neocortical injury in Olig2-creER:reporter double transgenic mice (Tatsumi et al., 2008) is likely to have been caused by the expression of Olig2 in proliferating reactive astrocytes and their precursors (Chen et al., 2008).
NG2 cells have been shown to respond to a wide variety of insults to the brain by enhanced proliferation and morphological changes (Levine, 1994; Levine et al., 1998; Nishiyama et al., 1997; Watanabe et al., 2002). Our observations support the view that reactive NG2 cells and reactive astrocytes represent two distinct glial cell lineages, both of which respond to injury. The functional role of each reactive glial cell population in the lesion has yet to be established. Reactive astrocytes have been shown to produce chondroitin sulfate proteoglycans particularly of the lectican family that are highly inhibitory to axonal growth and regeneration (McKeon et al., 1991; Silver and Miller, 2004). By contrast, NG2 cells may counter the inhibitory effect of astrocytes by providing a more permissive environment for axonal growth (Busch et al., 2010; Yang et al., 2006). Further studies are needed to define the relative functional contribution of reactive NG2 cells and reactive astrocytes.
ACKNOWLEDGMENTS
The authors thank Drs. Charles Stiles and John Alberta (Dana-Farber Cancer Institute, Boston MA) who kindly provided the Olig2 antibody and Dr. Peter Brophy for his kind gift of the rabbit anti-P0 antibody.
Grant sponsors: NIH; Swedish Brain Foundation (Hjärnfonden); Swedish Medical Association.
Footnotes
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- Alonso G. NG2 proteoglycan-expressing cells of the adult rat brain: Possible involvement in the formation of glial scar astrocytes following stab wound. Glia. 2005;49:318–338. doi: 10.1002/glia.20121. [DOI] [PubMed] [Google Scholar]
- Bu J, Akhtar N, Nishiyama A. Transient expression of the NG2 proteoglycan by a subpopulation of activated macrophages in an excitotoxic hippocampal lesion. Glia. 2001;34:296–310. doi: 10.1002/glia.1063. [DOI] [PubMed] [Google Scholar]
- Buffo A, Vosko MR, Erturk D, Hamann GF, Jucker M, Rowitch D, Gotz M. Expression pattern of the transcription factor Olig2 in response to brain injuries: Implications for neuronal repair. Proc Natl Acad Sci USA. 2005;102:18183–18188. doi: 10.1073/pnas.0506535102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffo A, Rite I, Tripathi P, Lepier A, Colak D, Horn AP, Mori T, Gotz M. Origin and progeny of reactive gliosis: A source of multipotent cells in the injured brain. Proc Natl Acad Sci USA. 2008;105:3581–3586. doi: 10.1073/pnas.0709002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch SA, Horn KP, Cuascut FX, Hawthorne AL, Bai L, Miller RH, Silver J. Adult NG2+ cells are permissive to neurite outgrowth and stabilize sensory axons during macrophage-induced axonal dieback after spinal cord injury. J Neurosci. 2010;30:255–265. doi: 10.1523/JNEUROSCI.3705-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T, Svendsen CN, Mucke L, Johnson MH, Sofroniew MV. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23:297–308. doi: 10.1016/s0896-6273(00)80781-3. [DOI] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: A new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassiani-Ingoni R, Coksaygan T, Xue H, Reichert-Scrivner SA, Wiendl H, Rao MS, Magnus T. Cytoplasmic translocation of Olig2 in adult glial progenitors marks the generation of reactive astrocytes following autoimmune inflammation. Exp Neurol. 2006;201:349–358. doi: 10.1016/j.expneurol.2006.04.030. [DOI] [PubMed] [Google Scholar]
- Cassiani-Ingoni R, Muraro PA, Magnus T, Reichert-Scrivner S, Schmidt J, Huh J, Quandt JA, Bratincsak A, Shahar T, Eusebi F, et al. Disease progression after bone marrow transplantation in a model of multiple sclerosis is associated with chronic microglial and glial progenitor response. J Neuropathol Exp Neurol. 2007;66:637–649. doi: 10.1097/nen.0b013e318093f3ef. [DOI] [PubMed] [Google Scholar]
- Cavanagh JB. The proliferation of astrocytes around a needle wound in the rat brain. J Anat. 1970;106(Part 3):471–487. [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Miles DK, Hoang T, Shi J, Hurlock E, Kernie SG, Lu QR. The basic helix-loop-helix transcription factor olig2 is critical for reactive astrocyte proliferation after cortical injury. J Neurosci. 2008;28:10983–10989. doi: 10.1523/JNEUROSCI.3545-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson MR, Polito A, Levine JM, Reynolds R. NG2-expressing glial progenitor cells: An abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci. 2003;24:476–488. doi: 10.1016/s1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- Di Bello CI, Dawson MR, Levine JM, Reynolds R. Generation of oligodendroglial progenitors in acute inflammatory demyelinating lesions of the rat brain stem is associated with demyelination rather than inflammation. J Neurocytol. 1999;28:365–381. doi: 10.1023/a:1007069815302. [DOI] [PubMed] [Google Scholar]
- Duchen LW. General pathology of neurons and neuroglia. In: Adams JH, Duchen LW, editors. Greenfield’s neuropathology. 5th ed. New York: Oxford University Press; 1992. pp. 1–68. [Google Scholar]
- Fukuda S, Kondo T, Takebayashi H, Taga T. Negative regulatory effect of an oligodendrocytic bHLH factor OLIG2 on the astrocytic differentiation pathway. Cell Death Differ. 2004;11:196–202. doi: 10.1038/sj.cdd.4401332. [DOI] [PubMed] [Google Scholar]
- Hampton DW, Asher RA, Kondo T, Steeves JD, Ramer MS, Fawcett JW. A potential role for bone morphogenetic protein signaling in glial cell fate determination following adult central nervous system injury in vivo. Eur J Neurosci. 2007;26:3024–3035. doi: 10.1111/j.1460-9568.2007.05940.x. [DOI] [PubMed] [Google Scholar]
- Horky LL, Galimi F, Gage FH, Horner PJ. Fate of endogenous stem/progenitor cells following spinal cord injury. J Comp Neurol. 2006;498:525–538. doi: 10.1002/cne.21065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latov N, Nilaver G, Zimmerman EA, Johnson WG, Silverman AJ, Defendini R, Cote L. Fibrillary astrocytes proliferate in response to brain injury: A study combining immunoperoxidase technique for glial fibrillary acidic protein and radioautography of tritiated thymidine. Dev Biol. 1979;72:381–384. doi: 10.1016/0012-1606(79)90127-1. [DOI] [PubMed] [Google Scholar]
- Levine JM. Increased expression of the NG2 chondroitin-sulfate proteoglycan after brain injury. J Neurosci. 1994;14:4716–4730. doi: 10.1523/JNEUROSCI.14-08-04716.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JM, Enquist LW, Card JP. Reactions of oligodendrocyte precursor cells to alpha herpesvirus infection of the central nervous system. Glia. 1998;23:316–328. [PubMed] [Google Scholar]
- Lu QR, Sun T, Zhu Z, Ma N, Garcia M, Stiles CD, Rowitch DH. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell. 2002;109:75–86. doi: 10.1016/s0092-8674(02)00678-5. [DOI] [PubMed] [Google Scholar]
- Magnus T, Carmen J, Deleon J, Xue H, Pardo AC, Lepore AC, Mattson MP, Rao MS, Maragakis NJ. Adult glial precursor proliferation in mutant SOD1G93A mice. Glia. 2008;56:200–208. doi: 10.1002/glia.20604. [DOI] [PubMed] [Google Scholar]
- Magnus T, Coksaygan T, Korn T, Xue H, Arumugam TV, Mughal MR, Eckley DM, Tang SC, Detolla L, Rao MS, et al. Evidence that nucleocytoplasmic Olig2 translocation mediates brain-injury-induced differentiation of glial precursors to astrocytes. J Neurosci Res. 2007;85:2126–2137. doi: 10.1002/jnr.21368. [DOI] [PubMed] [Google Scholar]
- Matthias K, Kirchhoff F, Seifert G, Huttmann K, Matyash M, Kettenmann H, Steinhauser C. Segregated expression of AMPA-type glutamate receptors and glutamate transporters defines distinct astrocyte populations in the mouse hippocampus. J Neurosci. 2003;23:1750–1758. doi: 10.1523/JNEUROSCI.23-05-01750.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeon RJ, Schreiber RC, Rudge JS, Silver J. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J Neurosci. 1991;11:3398–3411. doi: 10.1523/JNEUROSCI.11-11-03398.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTigue DM, Wei P, Stokes BT. Proliferation of NG2-positive cells and altered oligodendrocyte numbers in the contused rat spinal cord. J Neurosci. 2001;21:3392–3400. doi: 10.1523/JNEUROSCI.21-10-03392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A. Polydendrocytes: NG2 cells with many roles in development and repair of the CNS. Neuroscientist. 2007;13:62–76. doi: 10.1177/1073858406295586. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): Multifunctional cells with lineage plasticity. Nat Rev Neurosci. 2009;10:9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Yu M, Drazba JA, Tuohy VK. Normal and reactive NG2+ glial cells are distinct from resting and activated microglia. J Neurosci Res. 1997;48:299–312. doi: 10.1002/(sici)1097-4547(19970515)48:4<299::aid-jnr2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Novak A, Guo C, Yang W, Nagy A, Lobe CG. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28:147–155. [PubMed] [Google Scholar]
- Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- Setoguchi T, Kondo T. Nuclear export of OLIG2 in neural stem cells is essential for ciliary neurotrophic factor-induced astrocyte differentiation. J Cell Biol. 2004;166:963–968. doi: 10.1083/jcb.200404104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi K, Takebayashi H, Manabe T, Tanaka KF, Makinodan M, Yamauchi T, Makinodan E, Matsuyoshi H, Okuda H, Ikenaka K, et al. Genetic fate mapping of Olig2 progenitors in the injured adult cerebral cortex reveals preferential differentiation into astrocytes. J Neurosci Res. 2008;86:3494–3502. doi: 10.1002/jnr.21862. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Toyama Y, Nishiyama A. Differentiation of proliferated NG2-positive glial progenitor cells in a remyelinating lesion. J Neurosci Res. 2002;69:826–836. doi: 10.1002/jnr.10338. [DOI] [PubMed] [Google Scholar]
- Wilhelmsson U, Li L, Pekna M, Berthold CH, Blom S, Eliasson C, Renner O, Bushong E, Ellisman M, Morgan TE, et al. Absence of glial fibrillary acidic protein and vimentin prevents hypertrophy of astrocytic processes and improves post-traumatic regeneration. J Neurosci. 2004;24:5016–5021. doi: 10.1523/JNEUROSCI.0820-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Suzuki R, Daniels SB, Brunquell CB, Sala CJ, Nishiyama A, Yang Z. NG2 glial cells provide a favorable substrate for growing axons. J Neurosci. 2006;26:3829–3839. doi: 10.1523/JNEUROSCI.4247-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue T, Xian K, Hurlock E, Xin M, Kernie SG, Parada LF, Lu QR. A critical role for dorsal progenitors in cortical myelination. J Neurosci. 2006;26:1275–1280. doi: 10.1523/JNEUROSCI.4717-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawadzka M, Rivers LE, Fancy SP, Zhao C, Tripathi R, Jamen F, Young K, Goncharevich A, Pohl H, Rizzi M, Rowitch DH, Kessaris N, Suter U, Richardson WD, Franklin RJ. CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell. 2010;6:578–590. doi: 10.1016/j.stem.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JW, Raha-Chowdhury R, Fawcett JW, Watts C. Astrocytes and oligodendrocytes can be generated from NG2+ progenitors after acute brain injury: Intracellular localization of oligodendrocyte transcription factor 2 is associated with their fate choice. Eur J Neurosci. 2009;29:1853–1869. doi: 10.1111/j.1460-9568.2009.06736.x. [DOI] [PubMed] [Google Scholar]
- Zhou M, Schools GP, Kimelberg HK. GFAP mRNA positive glia acutely isolated from rat hippocampus predominantly show complex current patterns. Brain Res Mol Brain Res. 2000;76:121–131. doi: 10.1016/s0169-328x(99)00341-1. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Anderson DJ. The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell. 2002;109:61–73. doi: 10.1016/s0092-8674(02)00677-3. [DOI] [PubMed] [Google Scholar]
- Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008;135:145–157. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]
- Zhu X, Hill RA, Dietrich D, Komitova M, Suzuki R, Nishiyama A. Age-dependent fate and lineage restriction of single NG2 cells. Development. 2011;138:745–753. doi: 10.1242/dev.047951. [DOI] [PMC free article] [PubMed] [Google Scholar]