Abstract
Sphingosine phosphate lyase (SPL) is an intracellular enzyme responsible for the irreversible catabolism of the lipid signaling molecule sphingosine-1-phosphate (S1P). SPL catalyzes the cleavage of S1P resulting in the formation of hexadecenal and ethanolamine phosphate. S1P functions as a ligand for a family of ubiquitously expressed G protein-coupled receptors that mediate autocrine and paracrine signals controlling cell migration, proliferation and programmed cell death pathways. S1P has also been implicated in developmental and pathological angiogenesis, cancer, inflammation, allergy, diabetes, lymphocyte trafficking and morphogenesis of the heart, kidney and brain as well as their response to ischemic injury.
As the final enzyme in the sphingolipid degradative pathway, SPL commands the only exit point for sphingolipid intermediates and their flow into phospholipid metabolism. So, in addition to regulating S1P levels, SPL is the gatekeeper of a critical node of lipid metabolic flow. The recent crystallization of a prokaryotic SPL has provided insight into the function and potential regulation and drug targeting of this enzyme. Considering the many physiological and pathological functions of S1P signaling, it seems likely that targeting SPL to modulate S1P signaling could be useful in a variety of clinical contexts.
In this review we discuss the recent highlights related to SPL-mediated biology, the structure of the SPL protein, the function of its products, new insights regarding the usefulness of SPL targeting in treating human diseases and the consequences of permanent SPL disruption in mice.
Keywords: sphingosine-1-phosphate, S1P lyase, apoptosis, signal transduction, sphingolipids, hexadecenal
Introduction
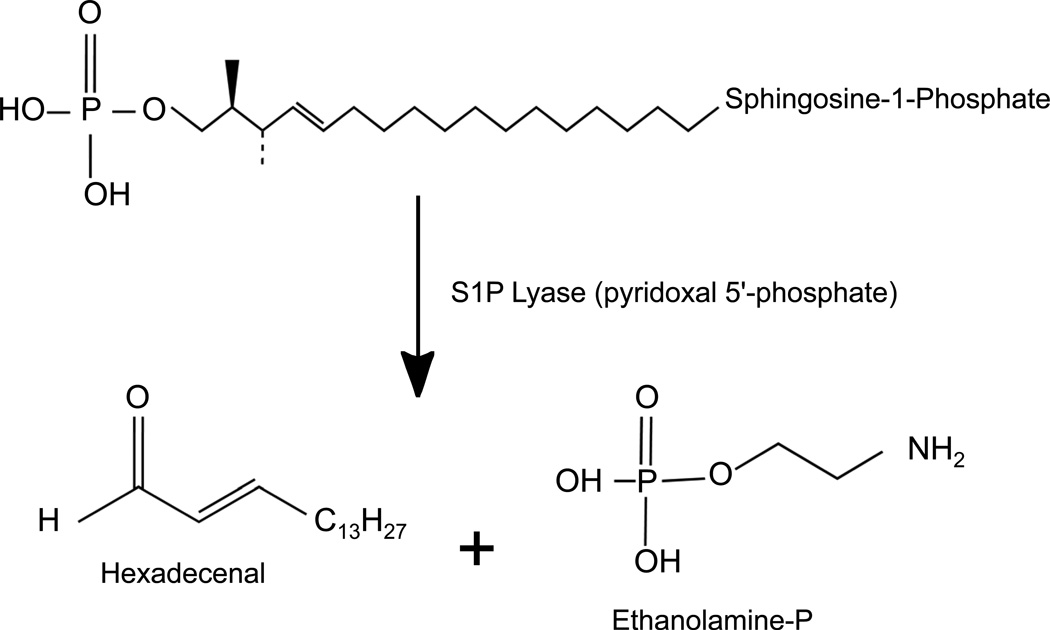
Sphingosine phosphate lyase (SPL) is an intracellular enzyme responsible for the irreversible catabolism of the lipid signaling molecule sphingosine-1-phosphate (S1P) (Figure 1) (Van Veldhoven, 2000). SPL catalyzes the cleavage of S1P at the C2-C3 carbon-carbon bond, resulting in formation of ethanolamine phosphate and the long chain aldehyde hexadecenal. SPL is a member of the superfamily of pyridoxal 5’-phosphate (PLP)-dependent enzymes. SPL activity was described in 1969 (Stoffel et al., 1969), and dpl1, the first SPL gene to be identified, was cloned in 1997 (Saba et al., 1997). This discovery was soon followed by the cloning of Sgpl1 genes encoding the murine and human SPL proteins (Van Veldhoven et al., 2000; Zhou et al., 1998). The substrate of the reaction, S1P, functions as a ligand for a family of ubiquitously expressed G protein-coupled receptors that mediate autocrine and paracrine signals controlling cell migration, proliferation and programmed cell death pathways (Hla et al., 2011). Thus, much of the attention SPL has gained in recent years relates to its ability to regulate S1P pools available for cell signaling.
Figure 1. SPL enzymatic reaction.
SPL is an integral membrane protein of the ER whose enzymatic activity catalyzes the cleavage of S1P at the C2-C3 carbon bond, resulting in depletion of S1P and formation of ethanolamine phosphate and hexadecenal. The enzyme requires pyridoxal 5’-phosphate as a cofactor.
An essential role for S1P signaling has been implicated in developmental angiogenesis and morphogenesis of the heart, kidney, brain and auditory system (Skoura et al., 2009). S1P-mediated events are also implicated in pathological angiogenesis, cancer, inflammation, allergy, diabetes, and the response to ischemic injury of the heart, kidney and brain. S1P’s role in regulating the trafficking of lymphocytes has brought it into the clinical arena as a pharmacological target for autoimmune diseases (Matloubian et al., 2004). The role of S1P receptor signaling in regulating the differentiation, migration and actions of other cells of hematopoietic origin is also gaining attention (Jenne et al., 2009; Keul et al., 2011; Liu et al., 2011). Additionally, recent studies have implicated intracellular S1P signaling in the regulation of NFκB activation, epigenetic control of gene transcription, mitochondrial function and calcium homeostasis (Spiegel et al., 2011a; Spiegel et al., 2011b).
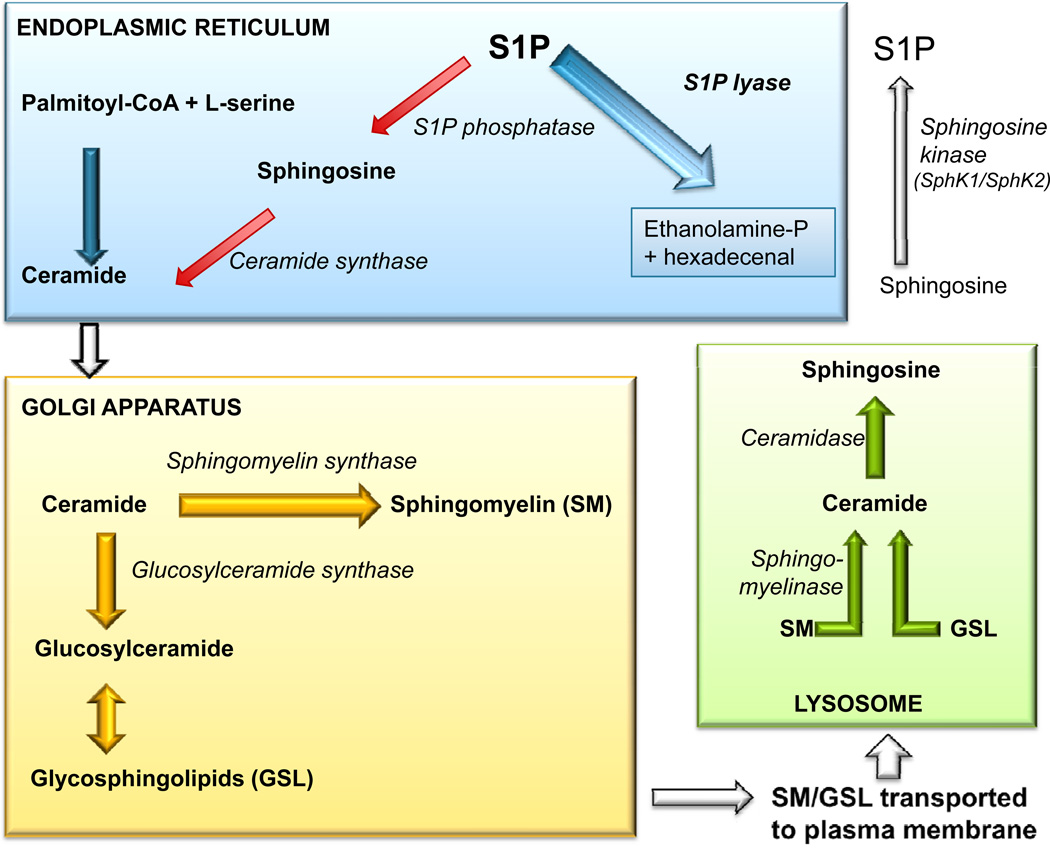
S1P is generated via phosphorylation of the sphingoid base sphingosine by the actions of two sphingosine kinases, SphK1 and SphK2 (Figure 2). These enzymes are partially redundant in function, although specific actions have been attributed to each (Pitson, 2011). For example, SphK1 is a downstream target of many growth factors, functions as an oncogene in model systems, is overexpressed in various types of cancer, and is a cofactor for the TRAF2 E3 ubiquitin ligase (Alvarez et al., 2010). In contrast, nuclear SphK2 generates S1P within a histone deacetylase complex, leading to inhibition of HDAC 1 and 2 activities, thereby contributing to the epigenetic regulation of gene transcription (Hait et al., 2009).
Figure 2. Sphingolipid metabolic pathway.
The de novo biosynthesis of sphingolipids initiates in the endoplasmic reticulum, where L-serine and palmitoyl-CoA are condensed to form the initial sphingoid base, which subsequently undergoes reduction, N-acylation and desaturation to form ceramide. The newly synthesized ceramide is then transported to the golgi apparatus where it is converted to higher order sphingolipids such as sphingomyelin (SM) and glycosphingolipids (GSL). These are transported to the plasma membrane where they are used as structural components. SM and GSL can be degraded in the plasma membrane or lysosome to form ceramide which can be further deacylated back to sphingosine. Sphingosine can be phosphorylated to form sphingosine-1-phosphate (S1P). S1P can be irreversibly degraded by S1P lyase or be dephosphorylated by phosphatases and utilized for the salvage pathway (red arrows).
S1P can be eliminated by dephosphorylation reactions catalyzed by the actions of S1P-specific phosphatases, as well as by enzymes of the nonspecific lipid phosphate phosphatase family (LPPs) (Pyne et al., 2005). Each of these enzymes regenerates sphingosine which can be rapidly rephosphorylated, thereby restoring S1P levels. Lipid phosphatases may be important in controlling local S1P levels within specific tissue niches, as demonstrated by a recent study implicating LPP3 as an essential regulator of lymphocyte egress from the thymus (Breart et al., 2011). In contrast to the phosphatases, SPL catalyzes an irreversible cleavage reaction that provides global control over circulating S1P levels and tissue S1P pools available for signaling, thereby commanding a pivotal position in which to influence S1P-dependent biological and pathological processes (Bektas et al., 2010). As the final enzyme in the sphingolipid degradative pathway, SPL controls the only exit point for sphingolipid intermediates and their flow into phospholipid metabolism. Thus, in addition to regulating S1P levels, SPL is the gatekeeper of a critical node of lipid metabolic flow.
In 2005, a study published by Jason Cyster’s research group established that pharmacological inhibition of SPL in mice produces lymphopenia, revealing the potential utility of targeting this enzyme as a strategy for achieving immunosuppression in autoimmune diseases (Schwab et al., 2005). Subsequent to this discovery, interest in SPL-mediated biology and pharmacological modulation of the enzyme has increased. In addition, the recent crystallization of a prokaryotic SPL has provided interesting insights into the function, regulation and drug targeting of this enzyme (Bourquin et al., 2010).
The utility of targeting SPL to modulate lymphocyte trafficking in autoimmune diseases is now an area of active clinical research (Bagdanoff et al., 2010), whereas the potential for targeting SPL in disease states other than autoimmune diseases has been largely unexamined. Considering the many physiological and pathological functions of S1P signaling, it seems likely that targeting SPL to modulate S1P signaling could be useful in a variety of clinical contexts. Several recent reports have explored SPL-suppression strategies in preclinical models of various disease states, including ischemia/reperfusion (I/R) injury, radiation injury and lung infection (Bandhuvula et al., 2011; Kumar et al., 2011b; Zhao et al., 2011). The potential for delivering SPL as an S1P-depleting therapy has also been explored (Huwiler et al., 2011).
However, disruption of the murine Sgpl1 gene to generate SPL knockout mice has revealed significant metabolic and immunological phenotypes associated with the SPL null state. These findings indicate that chronic and complete SPL suppression may have untoward consequences (Allende et al., 2010; Bektas et al., 2010). New insights regarding the biological functions of the SPL products are also beginning to emerge, raising questions about the potential effect of chronic SPL suppression on product depletion (Kumar et al., 2011a).
This review will focus on recent insights related to SPL-mediated cell biology, the structure of prokaryotic and eukaryotic SPL proteins as revealed by crystallization, the function of SPL reaction products, the consequences of permanent SPL disruption in mice, and SPL targeting in several preclinical models of human diseases. For more information on the biochemical characteristics of SPL, its tissue distribution, transcriptional and post-transcriptional regulation, developmental functions, other aspects of SPL-mediated biology in human cells and metazoan model systems, and its important role in immune regulation, the reader is referred to several recent reviews (Alexander et al., 2011; Bourquin et al., 2011; Gräler, 2010; Kumar et al., 2009; Serra et al., 2010; Van Veldhoven, 2000).
SPL and lipid homeostasis
In 2007, Soriano and colleagues published the first characterization of an SPL knockout mouse generated by a gene trap insertion mutagenesis screen (Schmahl et al., 2007). The important features of mice homozygous for the Sgpl1 null allele included runting, death at the time of weaning, anemia, lymphopenia, infrequent anomalies of kidney, cartilage and bone, and defective fibroblast cell migration. These phenotypes were consistent with the group’s identification of Sgpl1 in a screen for downstream targets of platelet-derived growth factor (PDGF) signaling (Chen et al., 2004). The null heterozygotes of this line have been particularly useful in establishing potential benefits of targeting SPL for therapeutic purposes (as described below). Further characterization of the SPL knockout mouse by others have confirmed and expanded our understanding of the immunological functions of SPL, which degrades tissue S1P and thereby contributes to forming the S1P chemical gradient between thymus and circulation along which mature lymphocytes migrate as they egress from the thymus (Vogel et al., 2009; Weber et al., 2009).
By regulating flow of sphingolipid biochemical intermediates into the phospholipid pathway, SPL represents the only exit point for sphingolipid degradation. Accumulation of sphingolipid intermediates contributes to the characteristic phenotypes of metazoan SPL mutants, including defects of muscle homeostasis and reproduction (Herr et al., 2003; Li et al., 2001; Mendel et al., 2003). Not surprisingly, SPL knockout mice were found to have high levels of S1P and other sphingolipids including ceramides and sphingomyelins (SM) in serum (Bektas et al., 2010). However, they also exhibited high levels of total and free cholesterol, cholesterol esters, triglycerides and phospholipids in serum. In particular, VLDL-associated triacylglycerols (TAG), cholesterol associated with LDL and HDL, and phospholipids associated with HDL were all elevated in the SPL knockout mouse compared to heterozygote and wild type control mice. Sphingolipid intermediates including sphingosine, dihydrosphingosine, ceramides, sphingomyelins, dihydrosphingosine-1-phosphate and S1P were elevated in knockout mouse liver, whereas phosphatidylethanolamine, phosphatidylserine and cardiolipin were reduced. Other histological features indicate that these sphingolipids are likely generated through the recycling of S1P via the salvage pathway of sphingolipid metabolism, which regenerates ceramide from sphingosine. Although free cholesterol levels were not affected, cholesterol esters, diacylglycerol (DAG) and triacylglycerol (TAG) were all appreciably increased in the mutant liver.
The mechanism of increased cholesterol in SPL mutants is not known but could result from interactions between SM and cholesterol in the membrane, the known effects of ceramide on cholesterol efflux, or indirect inflammatory changes resulting from sphingolipid accumulation. It is likely that DAG and TAG are generated when excess ceramide is reincorporated into SM in a reaction catalyzed by SM synthase, which utilizes ceramide and phosphatidylcholine, generates SM and releases DAG, the latter which can then be synthesized into TAG.
Consistent with the accumulation of phospholipids in the liver was the finding of increased lipid deposits and lipid droplets in liver parenchyma of knockout mice. Despite the high levels of these lipids in the liver and circulation, SPL knockout mice were lean, harboring reduced stores of adipose tissue compared to controls. Microarray analysis revealed a marked upregulation of genes involved in lipid metabolism in the knockout liver, including PPAR gamma, an important nuclear receptor that regulates lipid storage. The expression of genes involved in S1P metabolism was not altered in the SPL knockout liver, whereas downregulation of one subunit of serine palmitoyltransferase was observed, suggesting internal feedback between the final step in sphingolipid degradation and the initial, rate-limiting step of sphingolipid biosynthesis.
The features of high circulating and stored lipids in the presence of low body weight and lean body mass, which are reminiscent of the human lipodystrophies, suggest that SPL may be a critical regulator of body metabolism and could potentially play a role in obesity, diabetes, atherosclerosis and metabolic syndrome. Further, these findings suggest that complete SPL inhibition may have significant effects on lipid homeostasis, due to the absolute requirement for this enzyme in sphingolipid catabolism and widespread effects caused by the accumulation of ceramide, S1P and other sphingolipid intermediates.
SPL deficiency in innate and adaptive immunity
S1P is produced by erythrocytes, secreted by mast cells, platelets and endothelial cells, and is highly enriched in blood and lymph. S1P signaling plays a critical role in lymphocyte trafficking by serving as a stimulus for mature, S1P receptor 1 (S1PR1)-expressing T cells to egress from the thymus and enter the circulation (Hla et al., 2011). SPL knockout mice are severely lymphopenic, exhibit T and B cell deficiency in the blood and spleen and impairment of T cell egress from the thymus, leading to accumulation of “single positive” mature T cells in the thymic parenchyma (Schwab et al., 2005). This was thought to be due to the role of thymic SPL in establishing and maintaining an S1P chemical gradient between the thymus and the circulation. However, recent findings suggest that the story is not this simple, and that other S1P degrading enzymes contribute to the regulation of local S1P levels in thymic niches (Breart et al., 2011). Thus, thymic emigration may be perturbed only when the effects of global and complete SPL deficiency override the normal mechanisms of local S1P regulation. Defects in the development of T and B cells, the thymic settling of bone marrow-derived T cell precursors, and effects on lymphocyte viability in response to thymic retention may also contribute to the lymphopenia of SPL knockout mice (Gräler, 2010).
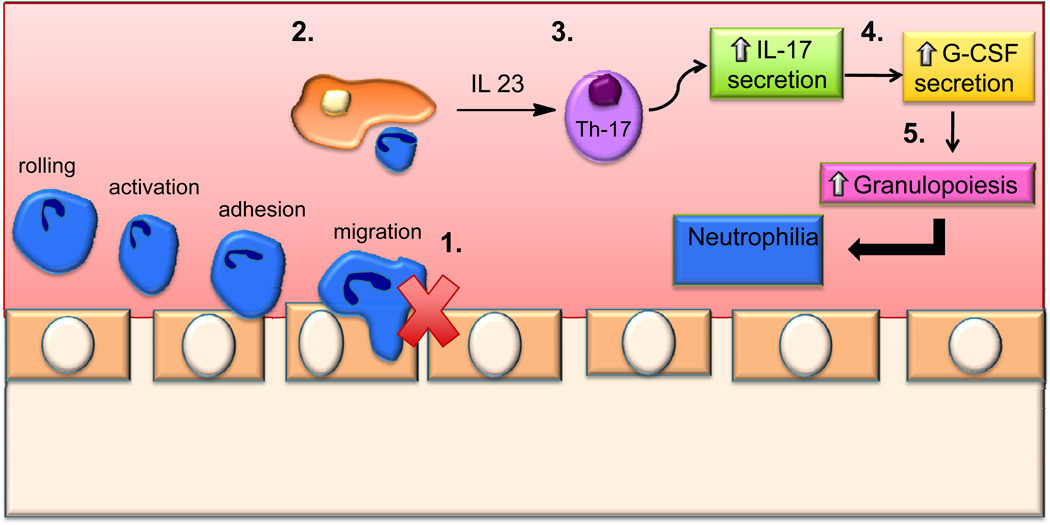
In contrast to lymphopenia, SPL knockout mice exhibit peripheral neutrophilia and monocytosis (Figure 3) (Allende et al., 2010). The neutrophils of SPL-deficient mice have impaired migration into inflamed tissues and fail to respond properly to a chemotactic stimulus. This defect results in peripheral neutrophilia, elevated splenic neutrophils and monocytes, and an abnormal neutrophil homeostatic regulatory loop marked by increased granulopoeisis in the bone marrow, without evidence of defective myeloid cell differentiation. Acute phase reactants are highly elevated in SPL knockout mice. Increased expression of Vcam-1 in the liver and high levels of pro-inflammatory cytokines in the serum provide evidence of a systemic inflammatory response. Additional studies established that this inflammation is not due to opportunistic infection.
Figure 3. SPL deficiency in innate and adaptive immunity.
1) SPL inhibition impairs neutrophil migration. 2) Phagocytosis of apoptotic neutrophils by macrophages and dendritic cells. 3) Increased IL-23 expression leads to increased numbers of Th-17 cells. 4) Increase in Th17 cells stimulates IL-17 secretion, which in turns increases G-CSF production. 5) G-CSF stimulates granulopoiesis, consequently resulting in neutrophilia.
The triad of neutrophilia, increased granulopoiesis and impaired entry of neutrophils into tissues are reminiscent of the findings observed in humans afflicted with leukocyte adhesion deficiency. Consistent with this comparison, neutrophil cell surface expression of adhesion molecules including L-selectin, Mac-1, integrins αL, β2 and α2 is decreased in SPL knockout mice. In addition, the IL-23/IL-17/GCSF cytokine axis that controls granulopoeisis was found to be activated. SPL/S1PR4 double knockout mice exhibited a lower neutrophil count and serum pro-inflammatory cytokines than SPL single knockout mice, suggesting involvement of S1PR4. However, SPL knockout mouse survival and lymphopenia were unaffected by S1PR4 status. Based on these findings, SPL activity and S1PR4-dependent signaling pathways represent new potential targets for manipulating innate immunity and inflammation.
Role of SphK1 and SPL in human lung endothelial cell motility
S1P is a potent angiogenic and vascular maturation factor regulating endothelial cell proliferation, migration and remodeling (Kono et al., 2004; Lucke et al., 2010). Extracellular S1P signals through S1PR1 to induce migration of human lung endothelial cells. Endothelial cells release relatively little S1P under basal conditions, but laminar shear stress leads to an increase in S1P release, suggesting that under certain circumstances vascular endothelium can serve as important source of circulating S1P (Venkataraman et al., 2008).
A recent study found that human pulmonary artery endothelial cells (HPAECs) express SphK1 and SphK2, but that only SphK1 regulates S1P-mediated endothelial cell migration (Berdyshev et al., 2011). Downregulation of SPL expression or chemical inhibition of SPL activity augmented intracellular S1P levels in HPAECs as well as extracellular S1P levels, and SPL suppression also stimulated wound healing. S1P-induced HPAEC migration is mediated through a pathway involving the Rho GTPase Rac1 and the Ras GTPase activating-like protein IQGAP1. Both of these proteins are important and interacting regulators of the organization of the cytoskeleton (White et al., 2009). SphK1 and SPL exerted opposite effects on Rac1 activation and IQGAP1 redistribution to the periphery in a pertussis toxin-sensitive manner, suggesting involvement of Gi-linked S1PRs.
S1P links glycosphingolipid metabolism to neurodegeneration
In most cell types, S1P and ceramide have an antagonistic effect on cell survival, with S1P serving as a pro-survival stimulus and ceramide activating cellular stress responses that lead to cell death (Cuvillier et al., 1996). However, S1P responses are cell-type specific. For example, S1P induces proliferation in neuronal progenitor cells, whereas S1P accumulation was found to induce apoptosis in an S1PR-independent fashion in the hippocampal neurons of SPL knockout mice (Hagen et al., 2009). A recent study was conducted to determine the molecular mechanism underlying the enhanced apoptosis observed in SPL knockout mouse neurons (Hagen et al., 2011).
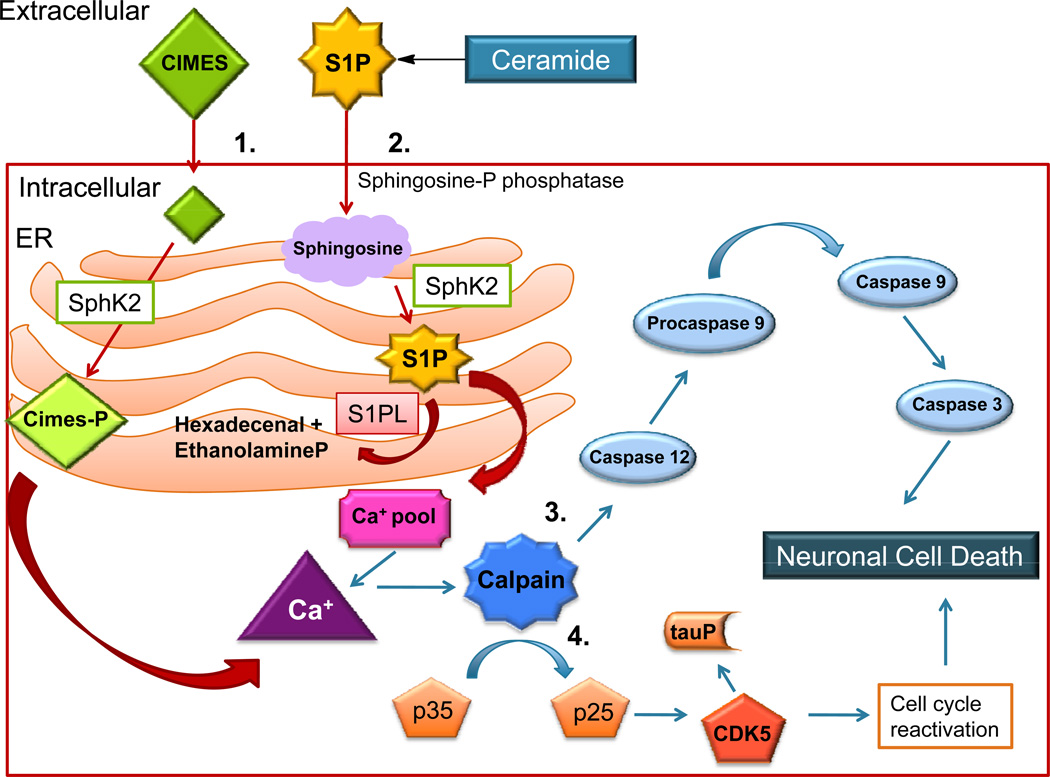
Cimes is a synthetic sphingosine analog which is phosphorylated by SphK2 in terminally differentiated neurons, where it accumulates and acts as an S1P analog. Many effects attributed to S1P intracellular functions were found to be induced by treatment of neurons with cimes, including the release of inositol 1,4,5-triphosphate sensitive intracellular calcium pools and neurotoxicity (Hagen et al., 2011). Using SPL-deficient neurons and cimes as complementary approaches to augment intracellular S1P signaling, it was shown that S1P accumulation induces mitochondrial-independent apoptosis in neurons through a mechanism involving calpain activation, induction of the ER stress response, upregulation of CDK5/p25, phosphorylation of Rb and inappropriate cell cycle re-entry (Figure 4).
Figure 4. Sphingoid base phosphates induce neuronal apoptosis.
1) CIMES (cis-4-methylsphingosine) is taken up by the cell and phosphorylated by SphK2. 2) S1P generated in the ER activates the release of calcium from inositol 1,4,5-triphosphate-sensitive calcium pools. 3) Calpain activity is triggered by increased calcium levels and activates stress-specific caspases, causing neuronal cell death. 4) CDK5 activation and tau hyperphosphorylation found in association with sphingoid base phosphate accumulation may contribute to neuronal cell death.
Calpain was also found to mediate cimes-induced tau hyperphosphorylation, the latter which is known to form neurofibrillary tangles in neurological disorders including “tauopathies” and Alzheimer’s disease. Interestingly, analysis of the cerebellar cortex of SPL-deficient mice reveals the presence of scattered groups of degenerating Purkinje cells. Neuroanatomical studies show that neurons with abundant SPL expression are those that degenerate first in SPL-deficient mice. This finding suggests that S1P accumulation and intracellular signaling may be an important link between impaired lipid metabolism and neurological disorders.
SPL as a target for cardioprotection from I/R injury
S1P signaling plays an important role in the development and functioning of the cardiovascular system. S1P signaling contributes to cardiac morphogenesis, angiogenesis, anti-atherogenic effects mediated by high-density lipoprotein, as well as the regulation of vascular tone and permeability. Recent studies have also implicated S1P signaling in protection from I/R injury of the heart and other tissues (Karliner et al., 2009). S1P signaling becomes activated in response to pre- and post-conditioning cardioprotective regimens. Reduced S1P synthesis and signaling contribute to I/R injury, whereas increasing S1P signaling through specific S1PRs may provide therapeutic benefit in the setting of I/R. Unfortunately, delivery of S1P is challenging due to binding by carrier proteins, ubiquitously expressed receptors and rapid metabolism. S1PR agonists such as FTY720 enhance functional recovery after I/R. However, FTY720’s clinical utility may be limited by bradycardia stemming from lack of receptor specificity. Thus, development of alternative methods for modulating S1P signaling for therapeutic benefit is warranted.
Using a murine ex vivo model of I/R injury to the heart, we explored how SPL activity responds to ischemic conditions and whether SPL inhibition might afford cardioprotection from I/R injury (Bandhuvula et al., 2011). SPL activity in ischemic tissues increased three-fold over baseline and returned to near baseline levels during reperfusion. In contrast to the dynamic response of SPL activity, SPL protein expression remained constant, suggesting a post-translational mechanism of SPL activation. Homozygous SPL null mice do not survive the post-natal period (Schmahl et al., 2007). Thus, to assess the effect of SPL inhibition on I/R injury to the heart, we compared the effects of I/R in heterozygous SPL null mice and littermate controls. We also employed a pharmacological SPL inhibitor in wild type mice and assessed the impact on I/R injury to the heart.
S1P levels decreased in both heterozygote and wild type hearts in response to I/R, consistent with SPL activation. Importantly, compared to control mice, the heterozygote null mice exhibited increased cardiac S1P levels, reduced infarct size and increased hemodynamic recovery from I/R injury, as measured by left ventricular developed pressure (LVDP). Similarly, pre-treatment of wild type mice with tetrahydroxybutylimidazole (THI), an imidazole compound found in caramel food coloring that serves as an SPL inhibitor, reduced tissue SPL activity, raised cardiac and plasma S1P levels, reduced the size of infarction by approximately 50% and improved functional recovery by the same degree compared to vehicle-treated controls.
THI-mediated cardioprotection was attenuated by pre-treatment with VPC23019, a small molecule S1PR1/3 antagonist, suggesting that THI acts in part through an S1PR-dependent mechanism. THI-mediated cardioprotection correlated with an S1PR-dependent effect on the protein translation machinery. However, other mechanisms also likely contribute to the effect of SPL inhibition on cardioprotection. Since individuals present to the medical system with a myocardial infarction in progress or an acute coronary syndrome, the potential of SPL inhibition as a useful medical intervention will depend upon the ability to achieve cardioprotection when the SPL inhibitor is administered after I/R. Thus, establishing the potential utility of targeting SPL as an effective cardioprotectant strategy will require preclinical studies wherein critical endpoints can be assessed, including cardiac function in a living animal, survival, and physiological responses to I/R hours or longer after injury. Further, the utility of targeting SPL for protection of other tissues from I/R or for cardioprotection from other insults, such as the potentially lethal side effects of chemotherapy and radiation in cancer patients, remain to be determined.
SPL as a target for protection of acute lung injury
S1P signaling promotes barrier functions, vascular integrity and inflammatory processes that are important in mediating the response to acute lung injury induced by trauma, aspiration, endotoxin exposure or infection (McVerry et al., 2005). Vascular permeability is a hallmark feature of acute lung injury, leading to flooding of alveoli and contributing to hypoxia and the high mortality associated with this condition. Intravenous delivery of S1P or the S1PR1 agonists have been shown to reduce lung edema in murine models of acute lung injury, raising the possibility that targeting SPL may be useful as a therapeutic strategy (Peng et al., 2004).
Employing SPL heterozygous null mice and THI administration to wild type mice, SPL suppression was shown to raise S1P levels in lung tissue and bronchoalveolar lavage fluid (Zhao et al., 2011). An LPS-induced mouse model of acute lung injury was employed in which LPS is instilled intratracheally, followed by 24 hours of recovery and broncheoalveolar lavage (BAL) to obtain BAL fluid. Using this method, it was shown that SPL inhibition by either genetic or pharmacological approaches attenuated markers of disease including BAL total protein and interleukin-6 (IL-6) levels, as well as histological evidence of inflammatory cell infiltrate and signs of injury in lung parenchyma.
Lung SPL gene and protein levels were found to increase in response to LPS exposure, concomitant with a fall in S1P levels. Similarly, upregulation of SPL gene, protein and activity levels was observed in human lung microvascular endothelial cells (HLMVEC) exposed to LPS. LPS treatment of HLMVECs led to release of IL-6 into the media in a TLR4-dependent fashion that also required the TLR4 adapter protein MyD88. IL-6 secretion was enhanced by SPL overexpression and reduced by SPL knockdown. LPS exposure induced p38, ERK1/2 and IκB phosphorylation in HLMVECs, but only p38 and IκB activation were associated with IL-6 secretion. Importantly, knockdown of SPL attenuated LPS-induced p38 phosphorylation, IκB and IL-6 secretion and endothelial barrier disruption as measured by transendothelial resistance. These effects were mediated through redistribution to the cell periphery of Rac1, a downstream target of S1PR1 signaling that promotes cytoskeletal reorganization and thereby enhances vascular integrity. Results obtained from modulation of LPPs favor a mechanism by which intracellular S1P accumulated in response to SPL inhibition is transported outside of the cell, where it acts upon S1PR1 to mediate Rac1 redistribution.
In contrast to the effects on LPS-induced inflammation, SPL downregulation was not capable of influencing TNFα-induced changes in p38 and IκB, indicating that SPL plays a specific role in the response to LPS. Further, the SPL products hexadecenal and ethanolamine phosphate had no effect on p38 and IκB, indicating that the effects of SPL are mediated specifically through regulation of it substrate, S1P. It remains to be determined which cells are the source of increased SPL in response to LPS-induced lung injury, as well as the specific transporter involved in export of S1P to the extracellular space. However, these findings suggest the possibility that SPL inhibition strategies may be useful for preventing vascular leak in the treatment of acute lung injury.
Using exogenous SPL for therapeutic purposes
SPL inhibition has been proposed as a strategy for treatment of several disorders including autoimmune diseases, inflammation, I/R injury and lung infection. These applications involve strategies to raise S1P and thereby make use of protection from cell death or inhibition of lymphocyte trafficking. Alternatively, activation of endogenous SPL or delivery of an active form of SPL could potentially reduce S1P in contexts wherein S1P signaling is contributing to the disease state, such as in cancer, fibrosis and pathological angiogenesis. There is increasing evidence that inhibiting S1P signaling through the use of S1P-specific monoclonal antibodies has clinical utility in cancer and vascular disease. Thus, it seems likely that SPL delivery or activation could be useful as well.
Toward that end, Huwiler and colleagues recently developed a method for delivering a recently cloned bacterial SPL in active form in vitro and in vivo (Bourquin et al., 2010; Huwiler et al., 2011). They showed the efficacy of exogenous SPL in interrupting S1PR-dependent signaling through MAPKs, reducing connective tissue growth factor expression in mesangial cells, and inhibiting the proliferation and migration of endothelial cells and tumor cells. Interestingly, bacterial SPL delivery also reduced circulating S1P levels by 70% for several hours and inhibited tumor-cell induced angiogenesis in vivo. Although it will be important to establish whether bacterial SPL is immunogenic in humans and/or whether human SPL can be generated and delivered in a stable and active form, these exciting results suggest that delivery of SPL enzyme therapy as an S1P-reducing strategy may be a viable pharmacological approach in certain clinical contexts.
The SPL product 2-trans-hexadecenal
SPL may mediate profound effects on lipid metabolism, viability and immunity through its control over S1P and upstream sphingolipid intermediates. However, studies in F9 murine embryonic carcinoma cells and the human parasite Leishmania have provided evidence that the products of the SPL reaction may also harbor biological activity (Kariya et al., 2005; Zhang et al., 2007). These observations have raised the possibility that some of the biological impact of SPL may be mediated through product regulation.
To explore this possibility further, we recently investigated the cellular activities and signaling functions of the long chain aldehyde produced by SPL-mediated cleavage of S1P. Treatment of human and murine cell lines with exogenous 2-trans-hexadecenal induced cytoskeletal reorganization, cell detachment and apoptosis (Kumar et al., 2011a). These effects were mediated via a signaling pathway involving mixed lineage kinase 3 and the respective phosphorylation of MKK4/7 and c-Jun terminal kinase (JNK). Activation of this signaling pathway resulted in c-Jun phosphorylation, cytochrome c release, and modulation of Bcl-2 family member proteins, including Bax activation, Bid cleavage and increased translocation of Bim into mitochondria.
Our findings provide a novel paradigm of sphingolipid signaling whereby cleavage of the cytoprotective molecule S1P results in formation of a bioactive product that induces cytoskeletal reorganization and apoptosis through a stress-activated MAPK signaling pathway. JNK is induced in response to anti-cancer agents, irradiation, osmotic shock, heat shock and other stressful conditions in which SPL is activated or induced. This suggests the possibility that the aldehyde may contribute to JNK-mediated effects under these conditions.
The direct molecular mechanism by which hexadecenal activates this signaling pathway remains to be determined. Considering that fatty aldehydes are highly reactive molecules that can interact with amino groups present on proteins, as well as carbohydrates and nucleic acids, hexadecenal generation by SPL could impact many cellular components. In that regard, an important unanswered question is whether hexadecenal is capable of forming an adduct with DNA and thereby contributing to genomic instability, similar to other long chain aldehydes generated during fatty acid oxidation.
The intracellular levels of hexadecenal generated by S1P catabolism are low and, thus, the impact of these molecules in physiological settings will depend upon whether local concentrations are sufficient to activate neighboring targets. However, there are conditions in which hexadecenal may accumulate to substantial levels. For example, patients with Sjögren–Larsson syndrome lack the microsomal fatty aldehyde dehydrogenase FALDH required for long chain aldehyde breakdown (Rizzo, 2007). These patients exhibit neurological and cognitive defects and ichthiosis caused by accumulation of toxic fatty aldehydes and alcohols. Whether SPL contributes to the etiology of Sjögren–Larsson syndrome and, importantly, whether SPL inhibition could reduce symptomatology of affected patients yet remains to be determined.
SPL and the DNA damage response
DNA damage responses activated by single or double stranded DNA breaks serve to maintain the genomic integrity of eukaryotic cells. The main mechanisms by which cells protect the DNA involve DNA repair processes coupled with cell cycle checkpoints that allow the repair machinery sufficient time to act upon damaged DNA molecules before cell cycle progression into mitosis. These processes are critical for the protection of healthy cells in response to radiation, and their disruption can lead to a propensity to develop cancer. In contrast, these pathways are targeted for therapeutic purposes to treat cancer.
The G2/M checkpoint initiated by the cdk1-cyclin B complex specifically prevents entry into mitosis in the presence of DNA damage, although if damage is severe, this arrest may be prolonged, triggering apoptosis. Sphingolipids are recognized to play a significant role in the response of eukaryotic cells to ionizing radiation (Corre et al., 2010). Activation of sphingomyelinases in response to radiation exposure results in sphingomyelin hydrolysis and ceramide generation. Ceramide disrupts membrane rafts and induces apoptosis in many cell types. In contrast, S1P formed from the metabolism of ceramide acts as a radioprotectant of gonads, gut endothelium and other tissues (Bonnaud et al., 2010; Kim et al., 2003; Morita et al., 2000; Otala et al., 2004).
We recently explored whether SPL exerts an influence on the DNA damage response pathway. Upregulation of SPL protein expression was observed in HEK293 cells and NIH3T3 fibroblasts after radiation exposure (Kumar et al., 2011b). Overexpression of SPL enhanced radiation-induced apoptosis via a mechanism involving the mitochondrial permeability transition, whereas SPL knockdown reduced apoptosis. Cells overexpressing SPL initiated a G2/M cell cycle arrest similar to control cells but exhibited an abbreviated G2/M checkpoint. This effect occurred in a cdk1-cyclin-B-dependent fashion, leading to premature entry into mitosis and mitotic cell death.
The effects of SPL on cell cycle progression and radiosensitivity were mediated in part by S1P depletion. However, a feedback mechanism that amplified ceramide generation in response to radiation also contributed to the effect. Knockdown of SPL improved the efficiency of DNA repair, whereas SPL overexpression delayed the kinetics of DNA repair. Further, inhibition of SPL by oral administration of the compound THI prolonged the survival of mice exposed to a lethal dose of radiation. These cumulative findings reveal SPL to be a regulator of the DNA damage response and a potential target for radioprotection. Our findings indicate the complex and sometimes unexpected interactions that characterize the sphingolipid biochemical pathway.
Solving the crystal structures of two SPL proteins
SPL is localized to the endoplasmic reticulum (ER), where it resides in the ER membrane by virtue of an N-terminal hydrophobic domain, with the active face of the enzyme facing the cytosol, providing access to S1P generated in the cytosol by sphingosine kinases. During the past year, the crystal structures and structure-function analyses of two SPL proteins were published in a report that provides, for the first time, direct insight into the three-dimensional topology, quaternary structure and reaction mechanism of the SPL enzyme from two species (Bourquin et al., 2010). The authors additionally propose a model of human SPL based on homology to the two solved crystal structures. One of these, the Saccharomyces cerevisiae Dpl1p, was the first SPL to be cloned, and the second is the first identified prokaryotic homologue from the commensal thermophilic bacterium Symbiobacterium thermophilum.
The crystal structure of the Symbiobacterium thermophilum SPL (StSPL) was found to contain dimmers exhibiting asymmetry with respect to cofactor binding. The StSPL protein contained an N-terminal disordered region and a core region exhibiting a fold characteristic of PLP-dependent enzymes and harboring the cofactor binding site. The Dpl1p structure (lacking the first 102 N-terminal residues) was found to be very similar to StSPL, including conservation at the cofactor binding mode and active site regions. Asymmetry in cofactor binding site occupancy was also observed in the Dpl1p crystal structure. StSPL was active in vitro, but Dpl1p was not. This finding suggested that the N-terminal flexible region of eukaryotic (and possibly bacterial) SPL may be necessary for accommodation of the substrate embedded in the membrane and for its presentation to the active site. A portion of the protein including the disordered regions mentioned above contribute to formation of a hydrophobic pocket that accommodates the substrate before its entrance into the active site. The N- and C-termini of SPL appear to surround the entry channel to the active site and may shield the substrate from solvent, thereby enhancing catalysis.
Site-directed mutagenesis studies combined with an in vivo enzyme assay established the functional relevance of several asymmetries and key residues surrounding the active site of StSPL. A reliable structure of human SPL was deduced based on the 45% conservation between human and yeast SPL amino acid sequences. Residue Y174 of Dpl1p was found to be conserved in human SPL (Y150) and may be important in its regulation. The crystal structure of SPL should facilitate crystallization and structural analysis of human SPL, which will be crucial for strategic design of inhibitors for use as immune modulators and in other clinical contexts.
Summary
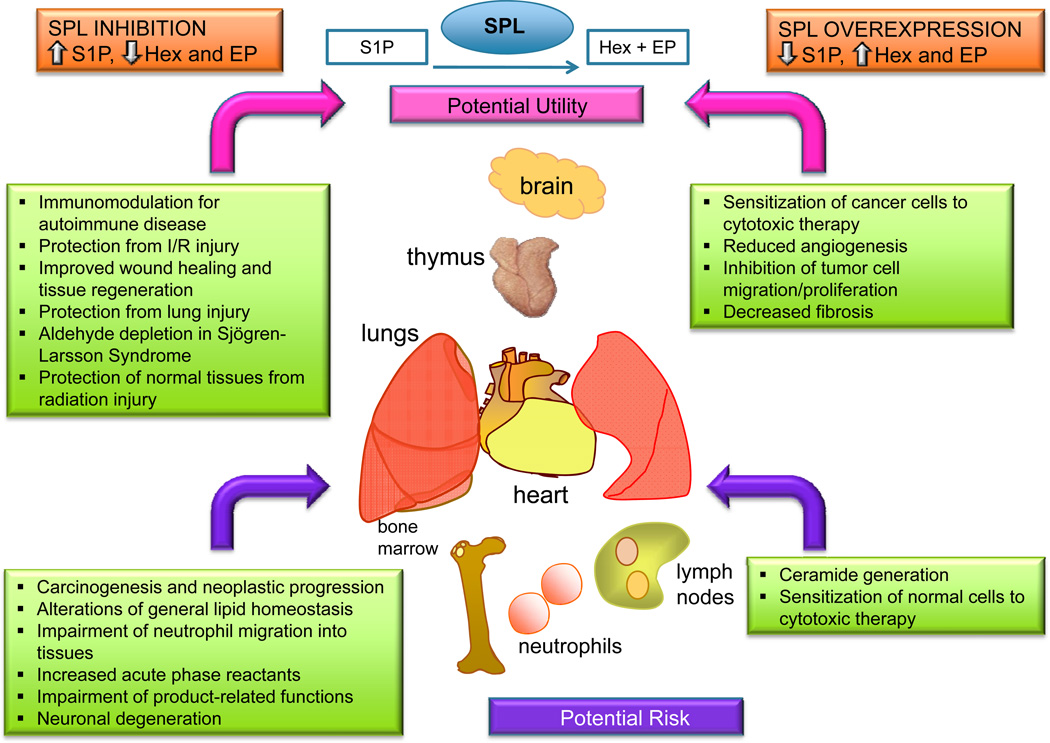
The potential utility of targeting SPL to treat diseases in which amplification of S1P signaling may be advantageous has recently been explored in a number of different contexts and disease model systems, as well as in human clinical trials. SPL suppression inhibits lymphocyte trafficking and induces lymphopenia, which may be useful in the treatment of autoimmune diseases, and clinical trials using the Lexicon drug LX2931 appear promising in this context (Bagdanoff et al., 2010). Studies utilizing preclinical models of acute lung injury, radiation injury and I/R injury of the heart have now provided evidence that temporary or incomplete SPL inhibition may be a useful strategy for reducing cell death and tissue injury in certain clinical contexts. We extrapolate from these findings that SPL inhibition could amplify S1P signals that promote cell survival and tissue homeostasis in response to various kinds of injury, such as hypoxia, metabolic insult, trauma and genetic defects. Alternatively, delivery of SPL as a strategy for lowering S1P levels may be useful in diseases of pathological angiogenesis, cancer or fibrosis (Figure 5).
Figure 5. Potential benefits and risks of modulating SPL for therapeutic purposes.
SPL inhibition is proposed as a strategy for raising S1P levels to inhibit lymphocyte trafficking and to promote cell survival in conditions causing tissue injury. Aldehyde depletion could be of potential benefit in Sjögren–Larsson syndrome. Potential risks include promotion of carcinogenesis, inhibition of innate immune functions, perturbation of lipid homeostasis, increased acute phase reactants. SPL delivery or upregulation is proposed as a strategy for depleting S1P in conditions such as cancer, fibrosis and pathologic angiogenesis, wherein monoclonal antibodies to S1P and sphingosine kinase inhibition have shown utility. Potential risks include sensitizing non-malignant cells to cytotoxic therapy and SPL product accumulation. Key determinants of safety and efficacy will likely include the degree of SPL inhibition or activation, the timing and duration of the intervention, and tissue specificity (as determined by the bioavailability and biodistribution of SPL modulating agents).
Much still remains to be learned about the biological functions of SPL and the ramifications of long-term SPL suppression. For example, the mechanism responsible for early death in SPL knockout mice remains unknown. Subtle effects of SPL modulation on lipid metabolism, vascular biology, innate immunity and SPL product depletion or enhancement in humans treated with SPL inhibitors or SPL delivery systems have not been examined over time. Further, the observed downregulation of SPL in some cancers, the potential effects of hexadecenal on DNA stability, and the influence of S1P signaling on inflammatory pathways suggest the possibility that chronic SPL inhibition or activation may have undesirable consequences. The degree, timing and tissue-specificity of SPL modulation may be critical parameters in determining safety and efficacy of these approaches. Despite these caveats, it is clear that SPL has promise as a drug target with potential uses in the treatment of several classes of disease and that its ability to modulate S1P signaling for therapeutic benefit is worthy of further investigation.
Acknowledgments
This work was supported by the Swim Across America Foundation and National Institutes of Health Grants RC1AI078516, R01CA77528, R01CA129438 and R01GM66954 (JDS).
Abbreviations
- BAL
bronchioalveolar lavage
- (DAG)
diacylglycerol
- ER
endoplasmic reticulum
- JNK
c-Jun n-terminal kinase
- LPP
lipid phosphate phosphohydrolase
- HLMVEC
human lung mesenchymal vascular endothelial cell
- HPAEC
human pulmonary artery endothelial cell
- IL-6
interleukin-6
- I/R
ischemia/reperfusion
- LVDP
left ventricular developed pressure
- PDGF
platelet derived growth factor
- PLP
pyridoxal 5’-phosphate
- S1P
sphingosine-1-phosphate
- S1PR1
S1P receptor 1
- SPL
sphingosine phosphate lyase
- StSPL
Symbiobacterium thermophilum SPL
- THI
tetrahydroxybutylimidazole
- TAG
triacylglycerol
References
- Alexander S, Alexander H. Lead genetic studies in Dictyostelium discoideum and translational studies in human cells demonstrate that sphingolipids are key regulators of sensitivity to cisplatin and other anticancer drugs. Semin Cell Dev Biol. 2011;22:97–104. doi: 10.1016/j.semcdb.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Allende M, Bektas M, Lee B, Bonifacino E, Kang J, Tuymetova G, et al. Sphingosine-1-phosphate lyase deficiency produces a pro-inflammatory response while impairing neutrophil trafficking. J Biol Chem. 2010;286:7348–7358. doi: 10.1074/jbc.M110.171819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez SE, Harikumar KB, Hait NC, Allegood J, Strub GM, Kim EY, et al. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature. 2010;465:1084–1088. doi: 10.1038/nature09128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdanoff J, Donoviel M, Nouraldeen A, Carlsen M, Jessop T, Tarver J, et al. Inhibition of sphingosine 1-Phosphate lyase for the treatment of rheumatoid arthritis: discovery of (E)-1-(4-((1R,2S,3R)-1,2,3,4-tetrahydroxybutyl)-1H-imidazol-2-yl)ethanone Oxime (LX2931) and (1R,2S,3R)-1-(2-(Isoxazol-3-yl)-1H-imidazol-4-yl)butane-1,2,3,4-tetraol (LX2932) J Med Chem. 2010;53:8650–8652. doi: 10.1021/jm101183p. [DOI] [PubMed] [Google Scholar]
- Bandhuvula P, Honbo N, Wang GY, Jin ZQ, Fyrst H, Zhang M, et al. S1P lyase: a novel therapeutic target for ischemia/reperfusion injury of the heart. Am J Physiol Heart Circ Physiol. 2011 doi: 10.1152/ajpheart.00946.2010. H1753–H61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bektas M, Allende ML, Lee BG, Chen W, Amar MJ, Remaley AT, et al. Sphingosine 1-phosphate lyase deficiency disrupts lipid homeostasis in liver. J Biol Chem. 2010;285:10880–10889. doi: 10.1074/jbc.M109.081489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdyshev EV, Gorshkova I, Usatyuk P, Kalari S, Zhao Y, Pyne NJ, et al. Intracellular S1P generation is essential for S1P-induced motility of human lung endothelial cells: role of sphingosine kinase 1 and S1P lyase. PLoS One. 2011;6:e16571. doi: 10.1371/journal.pone.0016571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnaud S, Niaudet C, Legoux F, Corre I, Delpon G, Saulquin X, et al. Sphingosine-1-phosphate activates the AKT pathway to protect small intestines from radiation-induced endothelial apoptosis. Cancer Res. 2010;70:9905–9915. doi: 10.1158/0008-5472.CAN-10-2043. [DOI] [PubMed] [Google Scholar]
- Bourquin F, Capitani G, Grutter MG. PLP-dependent enzymes as entry and exit gates of sphingolipid metabolism. Protein Sci. 2011;20:1492–1508. doi: 10.1002/pro.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourquin F, Riezman H, Capitani G, Grutter MG. Structure and function of sphingosine-1-phosphate lyase, a key enzyme of sphingolipid metabolism. Structure. 2010;18:1054–1065. doi: 10.1016/j.str.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Breart B, Ramos-Perez WD, Mendoza A, Salous AK, Gobert M, Huang Y, et al. Lipid phosphate phosphatase 3 enables efficient thymic egress. J Exp Med. 2011;208:1267–1278. doi: 10.1084/jem.20102551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Delrow J, Corrin P, Frazier J, Soriano P. Identification and validation of PDGF transcriptional targets by microarray-coupled gene-trap mutagenesis. Nat Genet. 2004;36:304–312. doi: 10.1038/ng1306. [DOI] [PubMed] [Google Scholar]
- Corre I, Niaudet C, Paris F. Plasma membrane signaling induced by ionizing radiation. Mutat Res. 2010;704:61–67. doi: 10.1016/j.mrrev.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind S, et al. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381:800–803. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- Gräler M. Targeting sphingosine 1-phosphate (S1P) levels and S1P receptor functions for therapeutic immune interventions. Cell Physiol Biochem. 2010;26:79–86. doi: 10.1159/000315108. [DOI] [PubMed] [Google Scholar]
- Hagen N, Hans M, Hartmann D, Swandulla D, van Echten-Deckert G. Sphingosine-1-phosphate links glycosphingolipid metabolism to neurodegeneration via a calpain-mediated mechanism. Cell Death Differ. 2011;18:1356–1365. doi: 10.1038/cdd.2011.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen N, Van Veldhoven PP, Proia RL, Park H, Merrill AH, Jr, Van Echten-Deckert G. Subcellular origin of sphingosine-1-phosphate is essential for its toxic effect in lyase deficient neurons. J Biol Chem. 2009;284:11346–11353. doi: 10.1074/jbc.M807336200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325:1254–1257. doi: 10.1126/science.1176709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr DR, Fyrst H, Phan V, Heinecke K, Georges R, Harris GL, et al. Sply regulation of sphingolipid signaling molecules is essential for Drosophila development. Development. 2003;130:2443–2453. doi: 10.1242/dev.00456. [DOI] [PubMed] [Google Scholar]
- Hla T, Brinkmann V. Sphingosine 1-phosphate (S1P): Physiology and the effects of S1P receptor modulation. Neurology. 2011;76:S3–S8. doi: 10.1212/WNL.0b013e31820d5ec1. [DOI] [PubMed] [Google Scholar]
- Huwiler A, Bourquin F, Kotelevets N, Pastukhov O, Capitani G, Grutter MG, et al. A Prokaryotic S1P Lyase Degrades Extracellular S1P In Vitro and In Vivo: Implication for Treating Hyperproliferative Disorders. PLoS One. 2011;6:e22436. doi: 10.1371/journal.pone.0022436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenne CN, Enders A, Rivera R, Watson SR, Bankovich AJ, Pereira JP, et al. T-bet-dependent S1P5 expression in NK cells promotes egress from lymph nodes and bone marrow. J Exp Med. 2009;206:2469–2481. doi: 10.1084/jem.20090525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariya Y, Kihara A, Ikeda M, Kikuchi F, Nakamura S, Hashimoto S, et al. Products by the sphingosine kinase/sphingosine 1-phosphate (S1P) lyase pathway but not S1P stimulate mitogenesis. Genes Cells. 2005;10:605–615. doi: 10.1111/j.1365-2443.2005.00862.x. [DOI] [PubMed] [Google Scholar]
- Karliner JS, Brown JH. Lipid signalling in cardiovascular pathophysiology. Cardiovasc Res. 2009;82:171–174. doi: 10.1093/cvr/cvp096. [DOI] [PubMed] [Google Scholar]
- Keul P, Lucke S, von Wnuck Lipinski K, Bode C, Graler M, Heusch G, et al. Sphingosine-1-phosphate receptor 3 promotes recruitment of monocyte/macrophages in inflammation and atherosclerosis. Circ Res. 2011;108:314–323. doi: 10.1161/CIRCRESAHA.110.235028. [DOI] [PubMed] [Google Scholar]
- Kim DS, Kim SY, Lee JE, Kwon SB, Joo YH, Youn SW, et al. Sphingosine-1-phosphate-induced ERK activation protects human melanocytes from UVB-induced apoptosis. Arch Pharm Res. 2003;26:739–746. doi: 10.1007/BF02976685. [DOI] [PubMed] [Google Scholar]
- Kono M, Mi Y, Liu Y, Sasaki T, Allende ML, Wu YP, et al. The sphingosine-1-phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. J Biol Chem. 2004;279:29367–29373. doi: 10.1074/jbc.M403937200. [DOI] [PubMed] [Google Scholar]
- Kumar A, Byun HS, Bittman R, Saba JD. The sphingolipid degradation product trans-2-hexadecenal induces cytoskeletal reorganization and apoptosis in a JNK-dependent manner. Cell Signal. 2011a;23:11346–11353. doi: 10.1016/j.cellsig.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Oskouian B, Fyrst H, Zhang M, Paris F, Saba JD. S1P lyase regulates DNA damage responses through a novel sphingolipid feedback mechanism. Cell Death Dis. 2011b;2:e119. doi: 10.1038/cddis.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Saba JD. Lyase to live by: sphingosine phosphate lyase as a therapeutic target. Expert Opin Ther Targets. 2009;13:1013–1025. doi: 10.1517/14728220903039722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Foote C, Alexander S, Alexander H. Sphingosine-1-phosphate lyase has a central role in the development of Dictyostelium discoideum. Development. 2001;128:3473–3483. doi: 10.1242/dev.128.18.3473. [DOI] [PubMed] [Google Scholar]
- Liu J, Hsu A, Lee JF, Cramer DE, Lee MJ. To Stay or to Leave: Stem Cells and Progenitor Cells Navigating the S1P Gradient. World J Biol Chem. 2011;2:1–13. doi: 10.4331/wjbc.v2.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucke S, Levkau B. Endothelial functions of sphingosine-1-phosphate. Cell Physiol Biochem. 2010;26:87–96. doi: 10.1159/000315109. [DOI] [PubMed] [Google Scholar]
- Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- McVerry B, Garcia J. In vitro and in vivo modulation of vascular barrier integrity by sphingosine 1-phosphate: mechanistic insights. Cell Signal. 2005;17:131–139. doi: 10.1016/j.cellsig.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Mendel J, Heinecke K, Fyrst H, Saba JD. Sphingosine phosphate lyase expression is essential for normal development in Caenorhabditis elegans. J Biol Chem. 2003;278:22341–22349. doi: 10.1074/jbc.M302857200. [DOI] [PubMed] [Google Scholar]
- Morita Y, Perez GI, Paris F, Miranda SR, Ehleiter D, Haimovitz-Friedman A, et al. Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1-phosphate therapy. Nat Med. 2000;6:1109–1114. doi: 10.1038/80442. [DOI] [PubMed] [Google Scholar]
- Otala M, Suomalainen L, Pentikainen M, Kovanen P, Tenhunen M, Erkkila K, et al. Protection from radiation-induced male germ cell loss by sphingosine-1-phosphate. Biol Reprod. 2004;70:759–767. doi: 10.1095/biolreprod.103.021840. [DOI] [PubMed] [Google Scholar]
- Peng X, Hassoun PM, Sammani S, McVerry BJ, Burne MJ, Rabb H, et al. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med. 2004;169:1245–1251. doi: 10.1164/rccm.200309-1258OC. [DOI] [PubMed] [Google Scholar]
- Pitson SM. Regulation of sphingosine kinase and sphingolipid signaling. Trends Biochem Sci. 2011;36:97–107. doi: 10.1016/j.tibs.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Pyne S, Long J, Ktistakis N, Pyne N. Lipid phosphate phosphatases and lipid phosphate signalling. Biochem Soc Trans. 2005;33:1370–1374. doi: 10.1042/BST0331370. [DOI] [PubMed] [Google Scholar]
- Rizzo W. Sjögren-Larsson syndrome: molecular genetics and biochemical pathogenesis of fatty aldehyde dehydrogenase deficiency. Mol Genet Metab. 2007;90:1–9. doi: 10.1016/j.ymgme.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saba JD, Nara F, Bielawska A, Garrett S, Hannun YA. The BST1 gene of Saccharomyces cerevisiae is the sphingosine-1-phosphate lyase. J Biol Chem. 1997;272:26087–26090. doi: 10.1074/jbc.272.42.26087. [DOI] [PubMed] [Google Scholar]
- Schmahl J, Raymond CS, Soriano P. PDGF signaling specificity is mediated through multiple immediate early genes. Nat Genet. 2007;39:52–60. doi: 10.1038/ng1922. [DOI] [PubMed] [Google Scholar]
- Schwab S, Pereira J, Matloubian M, Xu Y, Huang Y, Cyster J. Lymphocyte sequestration through S1P lyase inhibition an disruption of S1P gradients. Science. 2005;309:1735–1739. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- Serra M, Saba JD. Sphingosine 1-phosphate lyase, a key regulator of sphingosine 1-phosphate signaling and function. Adv Enzyme Regul. 2010;50:349–362. doi: 10.1016/j.advenzreg.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoura A, Hla T. Lysophospholipid receptors in vertebrate development, physiology, and pathology. J Lipid Res. 2009;50(Suppl):S293–S298. doi: 10.1194/jlr.R800047-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat Rev Immunol. 2011a;11:403–415. doi: 10.1038/nri2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel S, Milstien S, Grant S. Endogenous modulators and pharmacological inhibitors of histone deacetylases in cancer therapy. Oncogene. 2011b doi: 10.1038/onc.2011.267. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffel W, LeKim D, Sticht G. Distribution and properties of dihydrosphingosine-1-phosphate aldolase (sphinganine-1-phosphate alkanal-lyase) Hoppe Seylers Z Physiol Chem. 1969;350:1233–1241. doi: 10.1515/bchm2.1969.350.2.1233. [DOI] [PubMed] [Google Scholar]
- Van Veldhoven PP. Sphingosine 1-phosphate lyase. In: Merrill AH Jr., Hannun YA, editors. Sphingolipid Metabolism and Cell Signaling Part A. Vol 311. New York: Academic Press; 2000. pp. 244–254. [Google Scholar]
- Van Veldhoven PP, Gijsbers S, Mannaerts GP, Vermeesch JR, Brys V. Human sphingosine-1-phosphate lyase: cDNA cloning, functional expression studies and mapping to chromosome 10q22. Biochim Biophys Acta. 2000;1487:128–134. doi: 10.1016/s1388-1981(00)00079-2. [DOI] [PubMed] [Google Scholar]
- Venkataraman K, Lee YM, Michaud J, Thangada S, Ai Y, Bonkovsky HL, et al. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ Res. 2008;102:669–676. doi: 10.1161/CIRCRESAHA.107.165845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel P, Donoviel M, Read R, Hansen G, Hazlewood J, Anderson S, et al. Incomplete inhibition of sphingosine 1-phosphate lyase modulates immune system function yet prevents early lethality and non-lymphoid lesions. PLoS ONE. 2009;4:e4112. doi: 10.1371/journal.pone.0004112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber C, Krueger A, Münk A, Bode C, Van Veldhoven PP, Gräler MH. Discontinued postnatal thymocyte development in sphingosine 1-phosphate-lyase-deficient mice. J Immunol. 2009;183:4292–4301. doi: 10.4049/jimmunol.0901724. [DOI] [PubMed] [Google Scholar]
- White CD, Brown MD, Sacks DB. IQGAPs in cancer: a family of scaffold proteins underlying tumorigenesis. FEBS Lett. 2009;583:1817–1824. doi: 10.1016/j.febslet.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Pompey J, Hsu F, Turk J, Bandhuvula P, Saba J, et al. Redirection of sphingolipid metabolism towards de novo synthesis of ethanolamine in Leishmania. EMBO J. 2007;26:1094–1104. doi: 10.1038/sj.emboj.7601565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Gorshkova IA, Berdyshev E, He D, Fu P, Ma W, et al. Protection of LPS-induced murine acute lung injury by sphingosine-1-phosphate lyase suppression. Am J Respir Cell Mol Biol. 2011;45:426–435. doi: 10.1165/rcmb.2010-0422OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Saba J. Identification of the first mammalian sphingosine phosphate lyase gene and its functional expression in yeast. Biochem Biophys Res Commun. 1998;242:502–507. doi: 10.1006/bbrc.1997.7993. [DOI] [PubMed] [Google Scholar]