Abstract
Analysis of the rabbit retinal connectome RC1 reveals that the division between the ON and OFF inner plexiform layer (IPL) is not structurally absolute. ON cone bipolar cells make non-canonical axonal synapses onto specific targets and receive amacrine cell synapses in the nominal OFF layer, creating novel motifs, including inhibitory crossover networks. Automated transmission electron microscope (ATEM) imaging, molecular tagging, tracing, and rendering of ≈ 400 bipolar cells reveals axonal ribbons in 36% of ON cone bipolar cells, throughout the OFF IPL. The targets include GABA-positive amacrine cells (γACs), glycine-positive amacrine cells (GACs) and ganglion cells. Most ON cone bipolar cell axonal contacts target GACs driven by OFF cone bipolar cells, forming new architectures for generating ON-OFF amacrine cells. Many of these ON-OFF GACs target ON cone bipolar cell axons, ON γACs and/or ON-OFF ganglion cells, representing widespread mechanisms for OFF to ON crossover inhibition. Other targets include OFF γACs presynaptic to OFF bipolar cells, forming γAC-mediated crossover motifs. ON cone bipolar cell axonal ribbons drive bistratified ON-OFF ganglion cells in the OFF layer and provide ON drive to polarity-appropriate targets such as bistratified diving ganglion cells (bsdGCs). The targeting precision of ON cone bipolar cell axonal synapses shows that this drive incidence is necessarily a joint distribution of cone bipolar cell axonal frequency and target cell trajectories through a given volume of the OFF layer. Such joint distribution sampling is likely common when targets are sparser than sources and when sources are coupled, as are ON cone bipolar cells.
Keywords: retina, inner plexiform layer, connectomics, circuitry, neural network, bipolar cell, axonal ribbon, axonal cistern, amacrine cell, bistratified diving ganglion cell, intrinsically photosensitive ganglion cell, crossover inhibition, within channel inhibition, ON-OFF crosstalk, functional network, structure-function, network topology
Introduction
Structure-function relationships have been explored in the retina for over a century. Ramón y Cajal observed differential bipolar cell stratification in the inner plexiform layer (IPL) and suspected direct structure-function correlations (Ramón y Cajal, 1892). Indeed, it has long since been established that ON and OFF channels occupy distinct domains within the mammalian IPL, with OFF cells that depolarize to light decrements stratified in the distal 40% of the IPL and ON cells that depolarize to light increments stratified in the proximal 60% of the IPL.(Famiglietti et al., 1977; Famiglietti and Kolb, 1976; MacNeil et al., 2004; Wässle et al., 2009; Werblin and Dowling, 1969). Nevertheless, examples of nominal cone bipolar cells breaking the mammalian IPL stratification rules were recently reported (Anderson et al., 2011a; Dumitrescu et al., 2009; Hoshi et al., 2009). Type 6, and possibly type 7 or 8, ON cone bipolar cells in mouse, and calbindin-positive layer 4/5 stratifying ON cone bipolar cells in rabbit, have been demonstrated targeting tyrosine hydrdoxylase-positive cells (TH1s), M1-type intrinsically photosensitive retinal ganglion cells (ipRGCs) and bsdGCs in stratum one of the IPL (Dumitrescu et al., 2009; Hoshi et al., 2009), thus representing an accessory ON input to the OFF IPL layers. These ribbon contacts appear in two varieties: en passant, occurring inside the main bipolar cell descending axons, and branched, occurring from small processes that branch off the main descending axon (Figure 2). (Anderson et al., 2011a) demonstrated by ATEM that pre-synaptic ribbon and postsynaptic conventional synaptic ultrastructures existed at axonal ribbon locations, but characterization of their cognate networks was incomplete.
Figure 2. A subset of ON cone bipolar cells make en passant & branched axonal ribbons.
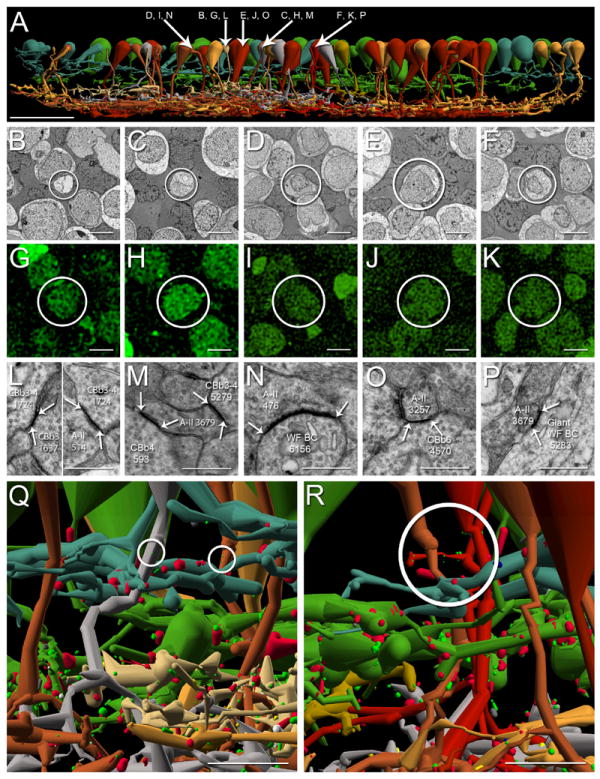
A. Vertically oriented renderings of 53 CBbs (neutral and warm colors) with axonal ribbons in the OFF IPL plotted against 48 CBas (cool colors). Cone bipolar cell color corresponds to depth of IPL stratification as follows: CBa1, sage; CBa2, green; CBb3, tan; CBb3-4, dark mustard; CBb4, silver; CBb5, mustard; CBb6, bright red; wide-field cone bipolar cell, deep red. Arrows, somas of CBbs referenced in B-P. Scale bar, 25 μm. B-K. CBbs indicated in A are confirmed as glycine-positive (B-F, TEM of CBb somas; G-K, glycine-positive labeling of corresponding somas in B-F). Scale bars, 5 μm. L-P. TEM of gap junctions between CBbs indicated in A and AII ACs. White arrows delineate gap junctions; A-II, AII amacrine cell; WF BC, wide field bipolar cell; scale bars, 0.5 μm. A, B, G, L. CBb3 1637 rendering (A), TEM of soma (B), corresponding glycine-positive signature (G), and indirect AII AC coupling via a gap junction with CBb3-4 1724 (L, left subpanel) which is couple to AII AC 514 (L, right subpanel). A, C, H, M. CBb4 593 rendering (A), TEM of soma (C), corresponding glycine-positive signature (H), and gap junction with AII AC 3679 (M). A, D, I, N. CBb5w 6156 rendering (A), TEM of soma (D), corresponding glycine-positive signature (I), and gap junction with AII AC 476 (N). A, E, J, O. CBb6 4570 rendering (A), TEM of soma (E), corresponding glycine-positive signature (J), and gap junction with AII AC 3257 (O). A, F, K, P. Wide-field cone bipolar cell 5283 rendering (A), TEM of soma (F), corresponding glycine-positive signature (K), and gap junction with AII AC 3679 (P). Q. CBb4 485 (silver) and CBb5w 180 (copper) form en passant axonal ribbon synapses (circles) among CBa1 and CBa2 arbors. Scale bar, 5 μm. R. Wide-field cone bipolar cell 16026 (red) forms branched axonal ribbon synapses (circle) among CBa1 and CBa2 arbors. Scale bar, 5 μm.
Indirect evidence exists to suspect that different ON cone bipolar cell types might communicate in the OFF IPL. First, in previous confocal imaging studies (Hoshi et al., 2009), only 23% of bsdGCs were apposed to calbindin positive bipolar cells, but most bsdGC spines were apposed to ribeye puncta. This indicates the remaining ribbons must be associated with other BC types. Also, many non-mammalian bipolar cell classes are multistratified, with axonal outputs in both the OFF and ON sublayers (Kolb, 1982; Pang et al., 2004; Ramon y Cajal, 1892; Scholes, 1975; Scholes and Morris, 1973; Sherry and Yazulla, 1993; Wong and Dowling, 2005) Moreover, infrequent reports of mammalian bistratified bipolar cells exist (Calkins et al., 1998; Famiglietti, 1981; Jeon and Masland, 1995; Kolb et al., 1990; Kolb et al., 1992; Linberg et al., 1996; Mariani, 1982; McGuire et al., 1984). These results impelled us to comprehensively classify ON cone bipolar cells that synapse in the OFF sublayer of the IPL.
In addition to the previously identified axonal ribbon targets, unknown targets with distinctive morphologies and ultrastructural elements were observed in retinal connectome RC1 (Anderson et al., 2011a). This strongly suggested additional cell types as targets. Axonal cisterns associated with post- synaptic densities were also discovered in the axons of ON cone bipolar cells (Anderson et al., 2011a), and are thus possible contributors to accessory ON networks. Sparse reports of rod bipolar cell axonal ribbons exist, implicating them as candidates for providing the ON input to ipRGCs, yet we demonstrate that rod bipolar cell axonal ribbons are not spatially coincident with ipRGCs and so cannot be responsible for ipRGC ON drive.
Electrophysiology with pharmacological blockade has revealed glycinergic crosstalk between ON and OFF channels at every synaptic tier in the retina, referred to as crossover inhibition (Chavez and Diamond, 2008; Chen et al., 2011; Liang and Freed, 2010; Manookin et al., 2008; Molnar et al., 2009; Roska et al., 2006; Werblin, 2010). Multi-stratified GACs are implicated as the source, yet the network topologies responsible remain speculative. Crossover inhibition has been posited to achieve a range of functions, including fidelity restoration of photic drive distorted by glutamate synapse nonlinearities, which would otherwise constrain OFF channels to negative contrast processing (Liang and Freed, 2010; Molnar et al., 2009; Werblin, 2010). Given that some of the targets of axonal ribbon synapses are GACs, ON-OFF crossover is one possible function of this accessory input. We show that crossover inhibition can definitely arise from accessory ON bipolar cell networks.
γACs mediate feedback, nested feedback, and feedforward networks throughout the retina, yet the reasons for the great diversity of types (wide-field, narrow-field, mono-, and multi-stratified) remain a mystery (Marc and Liu, 2000; Wagner and Wagner, 1988). We show examples of wide-field, OFF layer, monostratified γAC processes postsynaptic to ON cone bipolar cell axonal ribbons and presynaptic to both ON and OFF cone bipolar cells, arguing for the existence of γAC-mediated within- and cross channel inhibition in addition to GAC-mediated within- and cross channel inhibition. Many instances of GAC-mediatedand γAC-mediated crossover inhibition motifs have been identified in RC1 that do not involve axonal ribbons (data not shown), but that will be the subject of future papers.
In summary, ON cone bipolar cells participate in accessory ON input throughout the OFF sublayer of IPL, targeting not only the previously characterized ipRGCs and bsdGCs, but also newly identified targets. As yet unknown targets exist in RC1, some of which which may be sparse TH1 axonal cell dendrites reported by Dumitrescu et al. (2009) and Hoshi et al. (2009). Additionally, preliminary data reveal that sixty-eight of ninety-seven (70.1%) measured ON cone bipolar cells contain one or more post-synaptic densitites (PSDs) to amacrine cell input in the OFF IPL, and recently discovered axonal cisterns appear in 55 of 113 (48.7%) ON cone bipolar cell axons measured thus far. This specificity enhances the likelihood that accessory ON networks are evolved strategies rather than systemic oddities. Further, such networks are not readily predicted with physiological techniques. ON cone bipolar cell axonal ribbons inject both convergent and divergent ON input to several ganglion cell, GAC, and γAC networks, thus constructing ON-OFF amacrine cells and ganglion cells, and mediating within- and cross-channel inhibition. We show that both monad and dyad versions of axonal ribbons can involve single-ribbon or multi-ribbon forms. Some rod bipolar cells possess axonal ribbons, but they are very close to their initial axon terminal branches, only contact AI (A17) and AII ACs, and do not supply the rod signals discovered in ipRGCs. Ultimately, analysis of axonal ribbons yields a refactoring of the mammalian inner plexiform layer where the OFF layer contains precisely multiplexed ON cone bipolar cell inputs.
Materials & Methods
Tissue
Connectome volume RC1 was assembled from a light-adapted female Dutch Belted rabbit (Oregon Rabbitry, OR) after in vivo excitation mapping as described in Anderson et al. (2011a) in accord with Institutional Animal Care and Use protocols of the University of Utah, the ARVO Statement for the Use of Animals in Ophthalmic and Visual Research, and the Policies on the Use of Animals and Humans in Neuroscience Research of the Society for Neuroscience.
Computational Molecular Phenotyping (CMP)
Retinal neurons in RC1 were classified by CMP per Marc and Jones (2002) by using an array of small-molecule signatures (4-aminobutyrate [GABA], glycine, L-glutamate, L-glutamine, taurine, and the activity marker 1-amino-4-guanidobutane [AGB]). Briefly, the isolated rabbit eye was hemisected and immersion-fixed overnight in 1% paraformaldehyde, 2.5% glutaraldehyde, 3% sucrose, 0.01% CaCl2, in 0.1 M phosphate buffer, pH 7.4. Tissues were then dehydrated in graded methanols and acetone and embedded in epoxy resin. Tissues were then serial sectioned at 70-90 nm onto 12-spot Teflon-coated slides (Cel Line, Fisher Scientific, Waltham, MA). Antibody exposure and silver intensification is described below under antibody characterization. Incubation of all antibodies generated against small-molecular targets was performed overnight at room temperature, and visualization was with goat anti-rabbit secondary IgG coated with 1.4 nm gold (Amersham, Arlington Heights, IL) and silverintensified (Kalloniatis and Fletcher, 1993).
Small-Molecular Antibody Characterization
Anti-hapten IgGs from Signature Immunologics (Salt Lake City, UT; Table 1) have been extensively characterized in prior publications (Marc et al., 1995; Marc,1999a,b; Marc and Cameron, 2002; Marc and Jones, 2002). Each is an IgG isotype (determined by affinity chromatography and immunoblotting) produced in rabbit hosts immunized with glutaraldehyde-amino acid conjugates to bovine serum albumin (BSA) as described in Marc et al. (1995). Five analysis types were used to characterize the specificity and detectivity of each anti-hapten IgG: 1) dependence on target molecule trapping; 2) immunodot assays against cognate small molecule–protein conjugates; 3) competition assays against free and bis-conjugates of small molecules (Table 2); 4) binding curves on quantitative artificial antigen stacks; and 5) cluster analysis (Marc et al., 1995).
Table 1.
Primary Antibodies Used in This Study
| Antibody | Immunogen, Host Species | Source | Dilution Used |
|---|---|---|---|
| AGB | BSA-glutaraldehyde- (1-amino-4-guanidobutane) conjugate, rabbit | Signature Immunologics B100/rabbit-polyclonal | 1:4,000 |
| GABA | BSA-glutaraldehyde- (4-aminobutyrate) conjugate, rabbit | Signature Immunologics YY100/rabbit-polyclonal | 1:32,000 |
| Glycine | BSA-glutaraldehyde (glycine) conjugate, rabbit | Signature Immunologics G100/rabbit-polyclonal | 1:4,000 |
| L-glutamate | BSA-glutaraldehyde- (L-glutamate), conjugate, rabbit | Signature Immunologics E100/rabbit-polyclonal | 1:32,000 |
| L-glutamine | BSA-glutaraldehyde- (L-glutamine) conjugate, rabbit | Signature Immunologics Q100/rabbit-polyclonal | 1:4,000 |
| Taurine | BSA-glutaraldehyde- (taurine) conjugate, rabbit | Signature Immunologics TT100/rabbit-polyclonal | 1:16,000 |
Abbreviations. AGB 1-Amino-4-guanidobutane, GABA γ-Aminobutyric acid.
Table 2.
IgG Competitive Sensitivities Computed From Inhibition Assays
| Bis-conjugate | γ | G | E | Q | Τ |
|---|---|---|---|---|---|
| γ | 0 | 8 | 5 | 7 | 6 |
| G | 6 | 0 | 5 | 7 | 6 |
| E | 4 | 9 | 0 | 5 | 6 |
| Q | 6 | 9 | 5 | 0 | 6 |
| Τ | 5 | 10 | 5 | 7 | 0 |
Bis-conjugate Legend. IgG competitive sensitivities computed from inhibition assays and expressed as log differential inhibition: log [C]/[T], where [C] and [T] are the concentrations of any conjugate (C) or the cognate target conjugate (T) required for 100% binding block. γ GABA, G glycine, E glutamate, Q glutamine, τ taurine,.
RC1 Assembly, Analysis, and Sharing
Bipolar cell networks in the ultrastructural rabbit retinal connectome RC1 (Anderson et al. 2011a) were annotated with the Viking viewer (Anderson et al., 2011b), and explored via 3D rendering and graph visualization of connectivity (Anderson et al., 2011b). Small molecule signals embedded in RC1 for computational molecular phenotyping (CMP) include 4-aminobutyrate, glycine, L-glutamate, L-glutamine, taurine, and the activity marker 1-amino-4-guanidobutane (AGB). Combined with morphological reconstruction CMP permits robust bipolar cell classification (Anderson et al., 2011a). RC1 was acquired by ATEM at 2.18 nm resolution and assembled into a volume with the NCRToolset (Anderson et al., 2009). Molecular-ultrastructural registrations were generated with ir-tweak (Anderson et al., 2011a; Anderson et al., 2009; Anderson et al., 2011b)3D renderings are built from disk annotations in Vikingplot (Anderson et al., 2011b), allowing rendering of surfaces and characterization of areas and volumes. All cells rendered in this paper are publicly available as Google Collada *.dae files via the Connectome Viz application. These can be imported into 3D visualization tools such as Collada or Blender (www.blender.org). One defect in converting disk topologies to volumes for rendering of tapered processes sometimes led to somas or varicose neurites with vertically peaked shapes. These anomalies will be repaired in future code sets. Networks were visualized as directed multigraphs with Connectome Viz, and topologies explored with Structure Viz (Anderson et al., 2011b). The RC1 dataset and these associated analytical tools are publically available at connectomes.utah.edu. Quantitative features of connections (numbers of synapses, axon dimensions, etc.) can be queried within these various tools and with Microsoft SQL.
Identification of IPL layers
The ON-OFF border of the IPL is not absolute and we adopted a structural reference to define the transition between zones dominated by OFF and ON cone bipolar cells. In practice, the axial location of the ON-OFF border was set as most proximal surface of the AII AC lobule nearest a given bipolar cell. The OFF layer was defined as the region between the most distal GABA+ (γ+) processes and the ON-OFF border. Similarly, the ON layer was defined as the region between the most proximal γ+ processes and the ON-OFF border. For simplicity we refer to these regions as the ON and OFF layers, corresponding to the older but less descriptive sublamina a and sublamina b, respectively. As in previous work we define the amacrine cell layer - IPL border as level 0 and the ganglion cell layer - IPL border as level 100 (Marc, 1986).
Cell Classification
All cells were classified using three criteria: molecular signatures, synaptic connectivity, and morphology. Bipolar cells were further sub-classified according to their stratifications within the IPL, compared to the rabbit bipolar cell classification scheme outlined by MacNeil et al. (2004). An itemization of the rules required for cell identity follows.
Rules for Bipolar Cells
Virtually all bipolar cells possess ribbon synapses. Their somas reside in the inner nuclear layer (INL) and they are glutamate-positive. Glycine-positive (G+) bipolar cells coupled to AII AC arboreal dendrites via gap junctions and stratified in the proximal 60% of the IPL were classified as ON cone bipolar cells with their precise level of stratification used to further refine their class memberships (CBb3, CBb3n, CBb3-4, CBb4, CBb5, CBb6, wide-field cone bipolar cell, & rod bipolar cell). Anderson et al. (2011a) showed that quantitative G+ signatures are an absolute discriminator of bipolar cell ∷ AII AC coupling. Glycine-negative (G-) bipolar cells that stratified in the distal 40% of the IPL and were both presynaptic and postsynaptic to AII AC appendages were defined as OFF cone bipolar cells, with their precise level of stratification used to further refine their class (CBa1, CBa1w, CBa1-2, CBa1-2n). Bipolar cells with G-signatures stratified in most proximal IPL, presynaptic to AII AC arboreal dendrites, neither postsynaptic nor coupled to them, and presynaptic and postsynaptic to γ+ AI ACs were classified as rod bipolar cells. There are 104 rod bipolar cells in RC1. These independent classifiers are, collectively, errorless (Anderson et al., 2011a). There are instances where CBa and CBb terminals (never rod bipolar cells) make synaptic contacts lacking classical synaptic ribbons. We call these bipolar cell conventional synapses, and they occur in terminals with numerous ribbons at other sites. One glutamate-positive bipolar cell class (CBa1w) is presynaptic and postsynaptic to AII ACs but lacks ribbons and only makes bipolar cell conventional synapses. These cells are not discussed in this paper as they are not involved with the characterization of axonal synapses.
Rules for Amacrine cells
Amacrine cells possessed conventional synapses only(not ribbon synapses) with somas residing in the INL, except for ON starburst amacrine cells whose somas reside in the ganglion cell layer. G and γ signals further refined their classification as GACs and γACs. Cells with moderate glycine signals, presynaptic lobular appendages in the OFF IPL, and coupled and postsynaptic arboreal dendrites in the ON IPL were defined as AII ACs.
Rules for Ganglion Cells
Ganglion cells discussed in this paper were glutamate-positive, lacked presynaptic specializations, were never postsynaptic to rod bipolar cells and had somas placed in the GCL or processes that traversed the entire volume. Based on cone bipolar cell input patterns they were further classified as ON, OFF, or ON-OFF. Some classes were also γ+ to differing extents (Marc and Jones, 2002) due to amacrine cell coupling.
Axonal Ribbon Synapses
Axonal ribbon synapses were defined by presynaptic and postsynaptic form in all cases, with the presynaptic ribbon itself surrounded by a halo or cluster of synaptic vesicles, a dense pre-synaptic membrane, complete glial withdrawal from the contact site, an evenly spaced synaptic cleft, and an unambiguous postsynaptic density on the target process. Synaptic clefts of synapses sectioned at oblique angles were often obscured, but were recaptured via goniometric re-imaging at higher resolution when necessary. Axonal ribbon synapses were defined as residing distal to the first branch point of each bipolar cell’s primary axonal arborization. While this criterion is formally arbitrary, it distinguishes pure axonal ribbons from those in the thin branches between terminal swellings in the axonal arborization.
Image Preparation
As described in our prior papers on connectomics (Anderson et al., 2009), display TEM images in this paper were produced by remapping RC1 volume tiles to gamma 1.3. Optical and TEM overlays used the TEM greyscale brightness combined with the hue, and saturation from the optical image as described in Anderson et al. (2011a). 3D versions and network maps of annotated cells were generated in Vikingplot and Viz applications (Anderson et al., 2011b).
Results
The rabbit retinal connectome volume RC1 is a serial section, 2 nm resolution, 16.4 terabyte TEM image collection assembled into a cylindrical data volume ≈ 0.25 mm wide and ≈ 0.025 mm high spanning the mid-inner nuclear layer through the GC layer (Figure 1 A), augmented by molecular channels capping and intercalated every 30 sections through it (Anderson et al., 2011a; Anderson et al., 2009; Anderson et al., 2011b). The CMP channels include aspartate, glutamate, 4-aminobutyrate (GABA), glycine, glutamine, taurine, and AGB as a marker of light-driven activity. These channels permit robust classification of cells (Anderson et al., 2011a; Anderson et al., 2009; Anderson et al., 2011b; Marc and Jones, 2002; Marc et al., 1995) and form an analytic statistic independent of network motif measures. The 0.25 mm wide volume disc represents a mixture of sampling domains including complete, semi-complete and partial architectures (Figure 1 B). The complete architectures include ≈ 360 bipolar cells and ≈ 50 narrow field amacrine cells. The semi-complete architectures include ≈ 40 bipolar cells, ≈ 50 medium to wide-field amacrine cells, and 15 ganglion cells with somas in the volume and dendrites extending beyond it. The partial architectures include large numbers (hundreds) of traversing amacrine cell and ganglion cell dendrites and axonal amacrine cell fields arising from somas outside the volume. This in no way invalidates use of partial architectures. Many of these traversing elements are still identifiable from their molecular signatures and corresponding network motifs. The size of the volume is limited by storage and time. The 2 nm resolution essential for mapping small synapses and the gap junctions that provide diverse coupling topologies in retinal networks and serve as network identity signatures for specific neurons requires 16.5 terabytes (Tb) of raw data and ≈ 50 Tb total, and required 5 months to image. A volume containing complete wide-field amacrine cells would require many years of capture time to produce. Even so, the network motifs that emerge from deep analyses of partial elements such as crossing ganglion cell dendrites still accurately capture the native structure of the source cells, especially since no evidence exists for (and much against) network anisotropy in individual GC and amacrine cell dendrites. Finally, the connectivity map of any volume is a compromise between intrinsic connections arising from cells completely inside the volume and extrinsic connections arising from cells outside the volume. For example, cortical connectome volumes contain far more extrinsic than intrinsic elements (Briggman and Bock, 2011). For the purposes of this manuscript, we mined the axons of all bipolar cells for the presence of axonal ribbons and reconstructed the targets of these ribbons. Table 3 contains a legend for the color scheme used to represent synapse types in all 3D reconstructions displayed throughout this manuscript. All cell identification numbers used in this manuscript are identifiers that can be invoked in Viking, VikingPlot, and Viz tools (Anderson et al., 2011a) to validate all of the ultrastructural features, network motifs and statistics we report here. RC1 is an open-source, open-access, open-data resource.
Figure 1. RC1 overview.
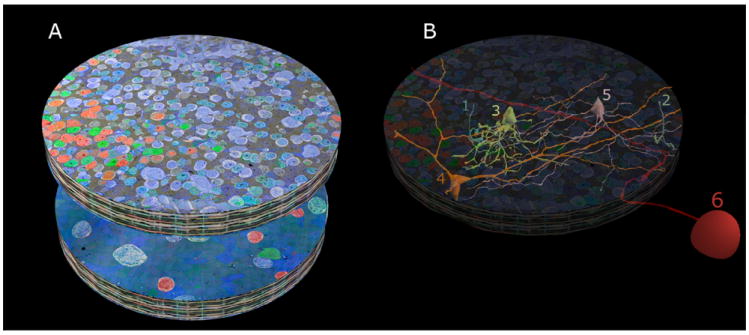
A. The RC1 volume with its top section (001) beginning in mid-INL and ending in the GCL at section 371, shown in a mirror image below. RC1 is a short cylinder ≈ 250 μm in diameter and ≈ 30 μm high containing 341 TEM sections and 11 intercalated CMP sections. The cylinder is capped at top and bottom with 10-section CMP series allowing molecular segmentation. TEM section 001 is a near-horizontal plane section through the INL visualized with GABA.glycine.glutamate → red.green.blue transparency mapping and a dark gold alpha channel (ANDed taurine + glutamine channels) described in Anderson et al. 2011a. Similarly TEM section 371 is a near-horizontal plane section through the GCL visualized with GABA.AGB.glutamate → red.green.blue transparency mapping. B. Representative cells contained in RC1 are rendered in 3D onto the volume. Many complete copies of small cells exist (tens to hundreds) such as rod bipolar cells (cells 1,2) and AII ACs (cell 3). A few semi-complete copies (5-10) of medium-diameter cell classes have their somas and much of their arbors within RC1, but extend outside it, such as interstitial γACs (cell 4) and AI amacrine cells (cell 5). Finally, RC1 contains many processes from partial cells: large cells such as wide-field amacrine cells or OFF α ganglion cells (cell 6) with somas outside the volume and often fully traversing it.
Table 3.
Synapse Color Scheme – 3D reconstructions
| Synapse Type | Color |
|---|---|
| Ribbon | Green |
| Conventional | Blue |
| Post-Synaptic Density | Red |
| Gap Junction | Yellow |
| Adherens Junction | White |
| Cistern Contact | Grey |
ON Cone Bipolar Cell Axonal Ribbons Throughout the OFF IPL form Accessory ON Pathways
ON cone bipolar cells make numerous axonal ribbon contacts throughout the OFF IPL: 175 of 398 (44%) bipolar cells in RC1 are ON cone bipolar cells. Thirty-four of these bipolar cells are semi-complete, with incomplete descending axons, thus we cannot determine the frequency of axonal ribbons in this subset. Fifty-four of the remaining complete 141 ON cone bipolar cells possess axonal ribbons (Figure 2). Thus 38% of the measurable ON cone bipolar cells make accessory ON axonal synapses. Three of these contain axonal ribbons only in the ON IPL, the remaining 51 of 141 bipolar cells (36%) contain one or more axonal ribbons in the OFF IPL. Importantly, most of these make multiple contacts through the OFF IPL and, on average, each ON cone bipolar cell that makes axonal synapses will do so at three different instances. For clarity we will use the MacNeil et al. (2004) rabbit bipolar cell morphological classification scheme to describe bipolar cells throughout this manuscript. Briefly, the MacNeil et al. (2004) scheme abbreviates “cone bipolar” as “CB”, OFF laminae of the IPL as “a”, ON laminae of the IPL as “b”, with numbers representing the specific IPL sublaminae within which bipolar cell axons primarily arborize. For instance, an OFF cone bipolar cell that primarily arborizes in sublamina 1 is referred to as “CBa1”, and an ON cone bipolar cell that primarily arborizes in sublamina 5 is referred to as “CBb5”, etc. Wide-field bipolar cells and rod bipolar cells are simply stated as such. Further cone bipolar cell subsets deemed as narrow and wide are additionally labeled with “n” or “w”, respectively, as in “CBb3n” or “CBa1w.” We introduce two newly discovered morphological bipolar cell classes, CBb5w and CBb6, which make axonal ribbons. Moreover, all major classes of ON cone bipolar cell (CBb3, CBb3n, CBb3-4, CBb4, CBb5, CBb5w, CBb6, Wide-field cone bipolar cell) make axonal ribbons, five of which are highlighted throughout this manuscript (Figure 3). CBb5w cells costratify with CBb5 cells, yet they possess axonal arbor field diameters ≈ 40-55 μm versus the 25-30 μm field diameters of most cone bipolar cells. CBb6’s are non-wide field bipolar cells that stratify alongside rod bipolar cells, more deeply than any other class of cone bipolar cell.
Figure 3. All major classes of ON cone bipolar cells possess axonal ribbons, stereogram.
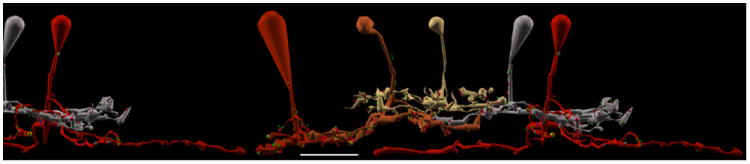
The five CBbs highlighted in figure 2 are displayed in isolation for clarity. Varied numbers of axonal ribbons across CBb classes span the IPL. Cone bipolar cell color corresponds to depth of IPL stratification. Specific cone bipolar cell colors as follows: CBb3, tan; CBb4, silver; CBb5w, copper; CBb6, bright red (left); wide-field cone bipolar cell, deep red (right). Note the class-specific arborization thickness, pattern of varicosities, and axonal arbor diameters. Spatial relationships are preserved. Scale bar,10 μm.
Previous studies indicated that the functional IPL stratification schemes require amendment to include an accessory ON layer at the most distal portion of IPL stratum one, and perhaps throughout the entire OFF IPL (Dumitrescu et al., 2009; Hoshi et al., 2009). Our data are consistent with mixed ON-OFF processing throughout levels 0-45% of the IPL, consistent with bipolar cell stratification patterns in non-mammalians.
Rod Bipolar Cell Axonal Ribbons Do Not Provide Accessory ON Drive
In contrast to CBbs, 61 of 105 (58%) rod BCs also make bona fide axonal ribbon synapses (synapses in the axon above the primary branch point), but these are virtually all within the upper part of the ON IPL with only a few breaking into the nominal OFF IPL (Figure 4). Further, virtually all of these (>90%) are contacts with identified AI or AII ACs. Every rabbit rod bipolar cell axon branches into 2 or 3 trunks as soon as it enters the ON IPL and immediately makes both pre- and postsynaptic specializations. The location of every axonal ribbon distal to the branch was mapped and we found that 89% were exclusively in sublamina b while 11% weakly breached the a/b border by an average of 600 nm. Over 90% of the traced targets of rod bipolar cell ribbons were verified as processes of AI or AII ACs. Indeed, all the AII AC processes were arboreal dendrites and never lobules.
Figure 4. CBb versus rod bipolar cell axonal ribbon depths.
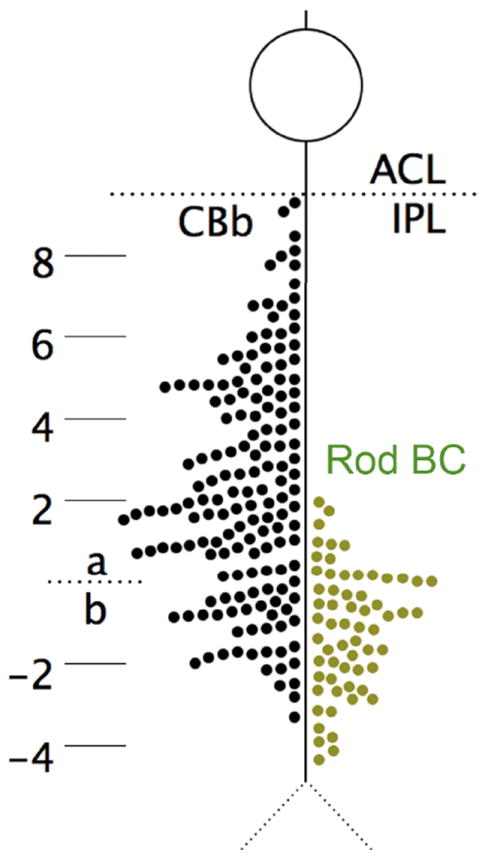
The distribution of 160 axonal ribbons in 54 CBbs and 63 ribbons in 63 of 104 rod bipolar cells in RC1. Ribbon positions are measured relative to the sublamina a/b border, defined as the proximal face of the nearest AII amacrine cell lobule. CBb axonal ribbons are distributed throughout sublamina a. Rod bipolar cell axonal ribbons are excluded from 80% of sublamina a. ACL, amacrine cell layer; Rod BC, rod bipolar cell.
CBb axonal ribbon frequency is approximately three times greater than rod axonal ribbons, and CBb axonal ribbon frequency (122 axonal ribbons) in sublamina a is approximately eight times greater than rod axonal ribbons (15 axonal ribbons), for fewer bipolar cells. Further, the IPL ON-OFF border is not distinct but is rather a blend of CBa and CBb terminals. The distribution of CBb axonal ribbons represents a unique accessory pathway in the OFF channel, whereas the distribution of rod bipolar cell axonal ribbons reflects the targeting of normal ON pathway amacrine cells near the a/b border.
The upper 80% of the OFF IPL displays no rod bipolar cell axonal ribbons. We posited that this might be due to the heavy layer of Müller cell processes that ensheath the rod bipolar cells. This may be partly correct, but clearly depends on the nature of the target. For example, arboreal dendrites of AII ACs readily induce desheathing of rod bipolar cell axons, but lobular processes never do, leading to an obvious bias for forming axonal ribbons in the ON IPL. But AI ACs, which are both presynaptic and postsynaptic to rod bipolar cells in the ON IPL effectively induce desheathing in the OFF IPL and were presynaptic to rod bipolar cell axons (this network will be the subject of other papers), but were never postsynaptic. Thus the formation of axonal ribbons is both site- and function-specific. The comparison of rod bipolar cell and ON cone bipolar cell ribbons shows that their roles are very different.
Finally, though ipRGCs receive rod signals (Aggelopoulos and Meissl, 2000; Dacey et al., 2005; Wong et al., 2007), the network pathway for this transmission remains unclear. The primary and secondary scotopic pathways, and rod bipolar cell axonal ribbon pathways have all been implicated, so we examined the relationship between rod bipolar cell axonal ribbons and M1 ipRGCs in the RC1 volume. We discovered that rod bipolar cell axonal ribbons are not cospatial with an M1 ipRGC dendrite present in the RC1 volume (Figure 5), hence this pathway cannot provide rod signals to M1 ipRGCs in the rabbit retina. Though ipRGC 12208’s identity cannot be absolutely confirmed because there was no melanopsin immunolabeling in RC1, it monostratifies at the IPL/INL border, sparsely branches, accepts axonal ribbon input from every ON cone bipolar cell it contacts (wide-field cone bipolar cell 6156 and wide-field cone bipolar cell 5283), and refuses input from two OFF cone bipolar cells (Figure 4. G-H). All of the above are consistent with M1-type ipRGCs (Dumitrescu et al., 2009; Graham et al., 2008; Hoshi et al., 2009). Henceforth, we shall simply refer to it as ipRGC 12208.
Figure 5. Rod bipolar cell axonal ribbons cannot drive M1 ipRGCs.
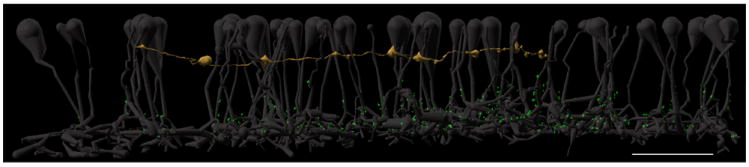
All 63 rod bipolar cells (ghosts) with axonal ribbons in RC1 are displayed against ipRGC 12208 (sand). Note that all ribbon synapses (bright green dots), including the axonal ribbons, are too proximal in the IPL to form synapses with the ipRGC. Scale bar, 20 μm.
Ganglion Cell Targets
We identified axonal ribbons from CBbs in the OFF IPL targeting bistratified diving ganglion cells (bsdGCs), multistratified ganglion cells, intrinsically photosensitive ganglion cells (ipRGCs), and other ON-OFF multistratified and OFF layer monostratified ganglion cell processes (Figure 6). Unexpectedly, a chain of coupled ON cone bipolar cells provides axonal ribbon input to the bsdGC. Furthermore, multiple ON cone bipolar cell classes synaptically converge to common targets, and individual ON cone bipolar cells diverge to multiple targets, via axonal ribbons.
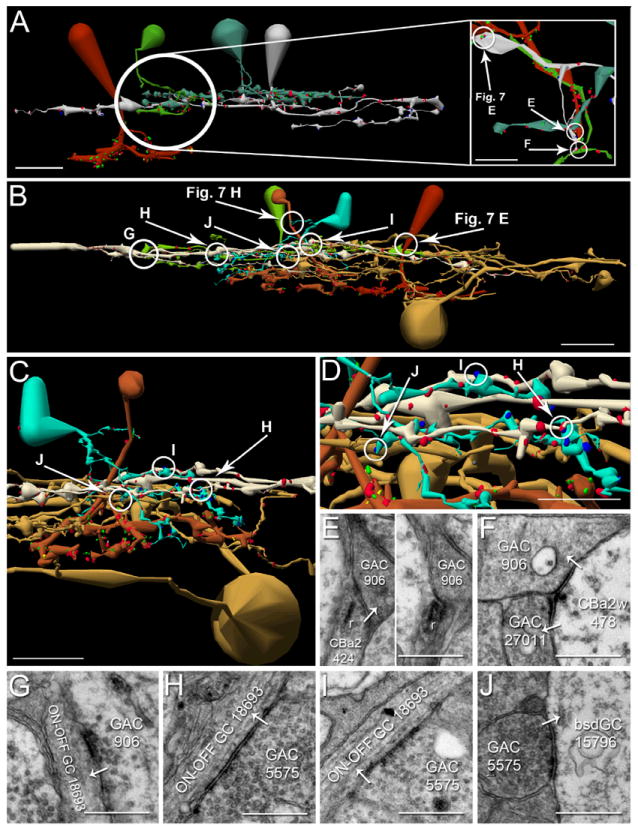
Figure 6. Ganglion cell axonal ribbon targets.
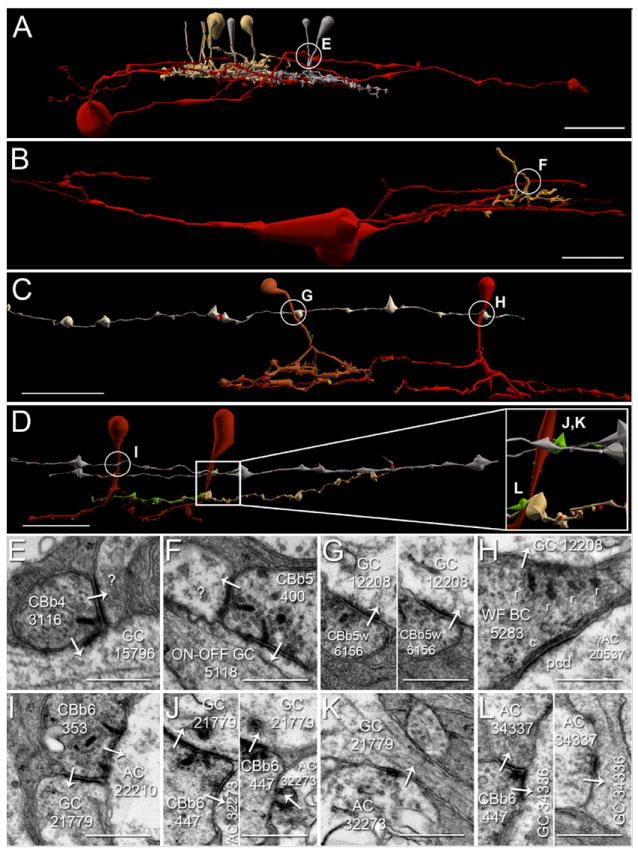
A-D. Renderings of five CBb classes forming axonal ribbons onto multiple ganglion cell classes, vertical orientation. Circles indicate location of synapses shown in E-L. Scale bars (A-B), 25 μm; scale bars (C-D), 20 μm. E-L. TEM of synapses indicated in A-D. White arrows indicate synapse directionality. GC, ganglion cell; WF BC, wide field bipolar cell; AC, amacrine cell; r, ribbons; c, cistern; pcd, post-citernal density; scale bars, 0.5 μm;. A,E. CBb4 3116 (left cell of the silver pair that intersect ganglion cell 15796 (red)) forms an axonal single-ribbon dyad with bsdGC 15796 and an unknown cell. CBb4 3116 participates in a chain of seven coupled CBb3s (tan) and CBb4s (silver). The bsdGC 15796 dendritic target of the axonal ribbon abruptly ascends to the OFF IPL where it receives the input before returning to the ON IPL distally (far right of panel A). B,F. CBb5 400 (mustard) forms an axonal multi-ribbon dyad with ON-OFF ganglion cell 5118 (red) and an unknown cell. C,G,H. CBb5w 6156 (copper) and wide-field cone bipolar cell 5283 (red) converge an axonal single-ribbon monad and axonal multi-ribbon monad, respectively, onto ipRGC 12208 (off white). Note the omega figure in the right subpanel of panel G. Wide-field cone bipolar cell 5283 forms an axonal cistern onto γAC 20537 (not shown in C, see Fig. 8, B-C) in the same plane of section as the four-ribbon axonal monad onto ipRGC 12208. D,I,J,K,L. CBb6 353 (red, left cell) and CBb6 447 (red, right cell) both form multi-ribbon axonal dyads (I,J) onto OFF-layer monostratified ganglion cell 21779 (silver) and another amacrine cell, amacrine cell 22210 (not shown inD for clarity,I) and amacrine cell 32273 (upper bright green cell in D, D inset,J), respectively. Amacrine cell 32273 creates both feedback (J, right subpanel) and feedforward (K) inhibition motifs via conventional synapses onto CBb6 447 and ganglion cell 21779, respectively. CBb6 447 also forms a single-ribbon axonal dyad in the ON IPL onto multistratified ganglion cell process 34336 (beige in D, D inset,L left subpanel) and amacrine cell 34337 (lower bright green cell in D, D inset,L left subpanel). Amacrine cell 34337 forms a conventional synapse onto ganglion cell 34336 (L right subpanel), thus completing a feedforward inhibition motif.
First, CBb4 3116 forms an axonal ribbon dyad onto bsdGC 15796 and a currently unidentified target (Figure 6 A,E). bsdGCs were identified in rabbit with dendrites that rise through the ON layer to stratify in the OFF IPL, where they receive CBb axonal ribbon input before re-entering the ON IPL (Hoshi et al., 2009). Our bsdGCs may be the same as the G9 ganglion cell identified by (Roska and Werblin, 2003), with depolarizing responses to light blocked by L-APB and enhanced by glycine and GABA receptor antagonists, and thus appear to be directly excited by ON cone bipolar cell input despite multistratification in both the ON and OFF IPL. Note the ganglion cell target process ascends to the OFF sublaminae where it receives the axonal ribbon input, then more distally returns to approximately the same IPL depth as the primary axonal arborization of the CBb4 that provides the axonal input. No OFF cone bipolar cell input to this ganglion cell has been found, despite abundant contact opportunities. Interestingly, CBb4 3116 participates in a chain of coupled CBbs across classes (CBb3 and CBb4). Moreover, none of these other CBbs, except CBb4 3116, have been discovered to synapse onto bsdGC 15796 despite costratification of their primary axonal arbors with it. CBb4 3116 only provides input to bsdGC 15796 at the axonal ribbon location in the OFF IPL. Furthermore, the descending axon of CBb4 4569, one of the chain of coupled CBbs, passes within 0.25 μm of the axonal ribbon input to bsdGC 15796 by CBb4 3116 and does not form an axonal ribbon. These results are consistent with and extend those of Hoshi et al. (2009) by validating the selective input from CBb cells in the OFF layer.
Second, an axonal ribbon contact from CBb5 400 drives multistratified ganglion cell 5118. We cannot currently verify whether this ganglion cell is a bsdGC or ON-OFF ganglion cell, as its OFF layer-stratifying processes exit the volume without descending to ON layers and no OFF inputs have been discovered as of yet. That said, ganglion cell 5118 appears morphologically distinct from bsdGC 15796, thus is likely a different ganglion cell class.
Third, CBb5w 6156 and wide-field cone bipolar cell 5283 convergently drive M1 ipRGC 12208 with axonal ribbons, a single-ribbon monad and four-ribbon monad, respectively (Figure 6 C,G,H). This convergent input from two CBb classes presumably indicates fusion of different CBb response profiles to extend the functional range of the ipRGC. This is concrete evidence for convergent axonal ribbon input from multiple bipolar cell classes onto ganglion cells.
Fourth, CBb6 447 and CBb6 353 converge axonal ribbon synapses onto OFF layer mono-stratified ganglion cell process 21779, and CBb6 447 diverges its output across the OFF and ON IPL via another axonal ribbon synapse in the ON layer to multistratified ganglion cell process 34336 (Figure 6 D,J,K,L). Both ganglion cell processes branch sparsely or not at all as they traverse nearly the entire width of the RC1 volume (257 μm) with no evidence of somata, indicating dendritic arbor radii of ≥ 250 μm and, thus, diameters ≥ 500 μm. Therefore, ganglion cell 21779 could belong to one of several classes of OFF layer-stratifying ganglion cells, but is unlikely to be an M1 ipRGC for two reasons. First, it monostratifies closer to the primary branch points of CBb3s than expected for an ipRGC. Second, it receives ribbon input from a partial trace of an OFF cone bipolar cell axonal arbor (data not shown), further inconsistent with M1 ipRGC electrophysiology. Ganglion cell 34336 could belong to any number of multistratified ganglion cell classes. This constitutes the first evidence that axonal ribbons in a single ON cone bipolar cell divergently drive targets in both the ON and OFF IPL. Importantly, all three of the axonal ribbons (across both CBb6s) form dyads onto a ganglion cell and amacrine cell targets, and both the amacrine cell targets of CBb6 447 conventionally synapse onto the ganglion cell target, thus forming CBb > amacrine cell ≥ ON-OFF ganglion cell feed forward motifs (Figure 6 I,J,K,L). Furthermore, amacrine cell 32273 provides feedback onto a finger-like projection from CBb 447 in addition to the feedforward to ganglion cell 21779, thus regulating both pre-synaptic bipolar cell release and post-synaptic ganglion cell membrane potential (Figure 6 J, right subpanel).
Combined, these results demonstrate that axonal ribbons from multiple CBb classes convergently and divergently drive multiple classes of ganglion cells across OFF and ON sublayers, and inject both ON excitation and ON inhibition to ON-OFF ganglion cells.
GAC Targets
Axonal ribbons from at least two CBb classes target both mono- and multi-stratified GACs (Figure 7). The first demonstrated reciprocal synapse at an axonal ribbon location appears between CBb6 4570 and monostratified GAC 906 (Figure 7 A,E), revealing axonal ribbons as sites of potential input as well as output. GAC 906 receives both ON and OFF inputs via monostratification in the overlapping region of ON-OFF processing in the mid-IPL described above. ON-OFF cells in the IPL are generally believed to be multistratified, yet this GAC, and ganglion cell 18693 described below, highlight ON-OFF comingling in the IPL as fundamental topology. This reinforces the fact that bipolar cells can multistratify to facilitate cross-channel communication; that they do not constrain their synaptic communication to discrete ON-OFF territories. GAC 5507 is currently a partial trace, so it is possibly multi-, rather than mono-stratified (Figure 7 C,G). Multistratified GAC 5575 is particularly interesting, as it extends a dendrite off its main trunk directly toward the descending axon of CBb5w 6156, where it receives axonal ribbon input (Figure 7 D,H). GAC 5575 divergently drives both ON cone bipolar cell ≥ ON ganglion cell (bsdGC) and ON cone bipolar cell ≥ ON-OFF ganglion cell inhibition, described in the text below. The combination of mono- and multi-stratified GAC targets suggests differential sign-inverting distribution of the CBb glutamatergic drive, but that will be explored in separate manuscripts.
Figure 7. GAC axonal ribbon targets.
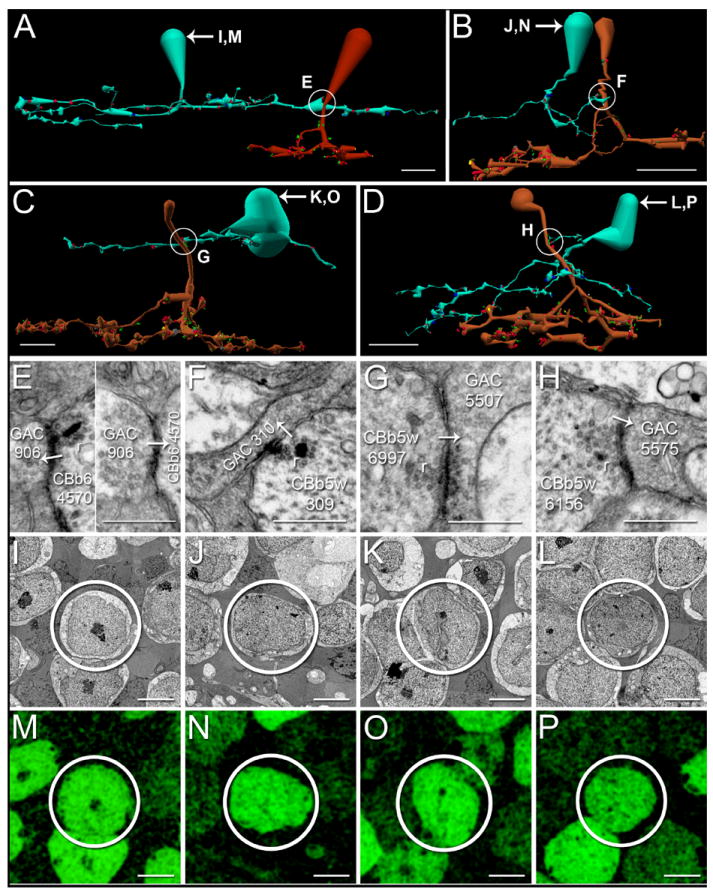
A-D. Renderings of CBbs targeting both mono- and multi-stratified GACs with axonal ribbons, vertical orientation. Circles indicate locations of synapses shown in E-H. Scale bars, 10 μm. E-H. TEM of axonal synapses at locations indicated A-D. White arrows indicate synapse directionality. r, ribbons; scale bars, 0.5 μm. I-L. TEM of GAC somas. Scale bars, 5 μm. M-P. Glycine-positive signatures of the corresponding GAC somas in I-L. Scale bars, 5 μm. A, E. CBb6 4570 (red) forms a single-ribbon monadic reciprocal synapse with GAC 906 (patina). B,F. CBb5w 309 (copper) forms a single-ribbon monadic synapse onto GAC 310 (patina). C,G. CBb5w 6997 (copper) forms a single-ribbon axonal monad with GAC 5507. The ribbon is very light, but possesses the characteristic halo of clear vesicles, and both pre- and post-synaptic densities are visible. D,H. CBb5w 6156 (copper) forms a single-ribbon axonal monad with GAC 5575 (patina).
γAC Targets Mediate Within- and Cross-Channel Inhibition
Three classes of ON cone bipolar cell were discovered to form γAC-mediated within- (Figure 8 A) and cross-channel (Figure 8 B-C) inhibitory motifs with axonal ribbons. First, CBb5 5562 drives multistratified γAC 5294 with an axonal ribbon (Figure 8 A,D,F). γAC 5294 forms a conventional synapse onto the primary telodendria of CBb5 5645 (Figure 8 A inset,G), completing a within channel inhibition motif. This within-channel inhibition is consistent with formation of the inhibitory surround of a center surround receptive field for CBb5 5645, yet this is the first report of such surround inhibition arising from axonal ribbon drive. Second, CBb6 5536 divergently drives a pair of amacrine cells, one of which is γ+ (Figure 8 E), at a branched axonal ribbon dyad site (Figure 8 B-C,H). Target amacrine cell 20537 is the γAC dendrite, and it spans most of the width of the RC1 volume without attachment to its soma, indicating a dendritic arbor radius ≥ 250 um, and therefore a dendritic arbor diameter ≥ 500 um. Thus γAC 20537 is a wide-field γAC. Target amacrine cell 19571 does not cross an immunolabeled section of the RC1 volume, and cannot be confirmed as γ+, but it is glycine negative (data not shown), and possesses the characteristic light cytoplasm (clear varicosities) of γACs. Furthermore, the two amacrine cell targets form a nested feedback architecture onto CBb6 5536 (Figure 8 H, right subpanel), a γAC network motif previously demonstrated in teleosts (Marc and Liu, 2000). Wide-field γAC 20537 also receives branched axonal ribbons from wide-field cone bipolar cell 16026 (Figure 8 B,C left inset,I), which combined with input from CBb6 5536 forms a CBb > γAC convergent motif. The second amacrine cell target of the divergence from the CBb6 5536 branched axonal ribbon creates a CBb > γAC ≥ CBa crossover inhibition motif (Figure 8 B,C right inset,J).
Figure 8. γAC axonal ribbon targets & axonal cisterns.
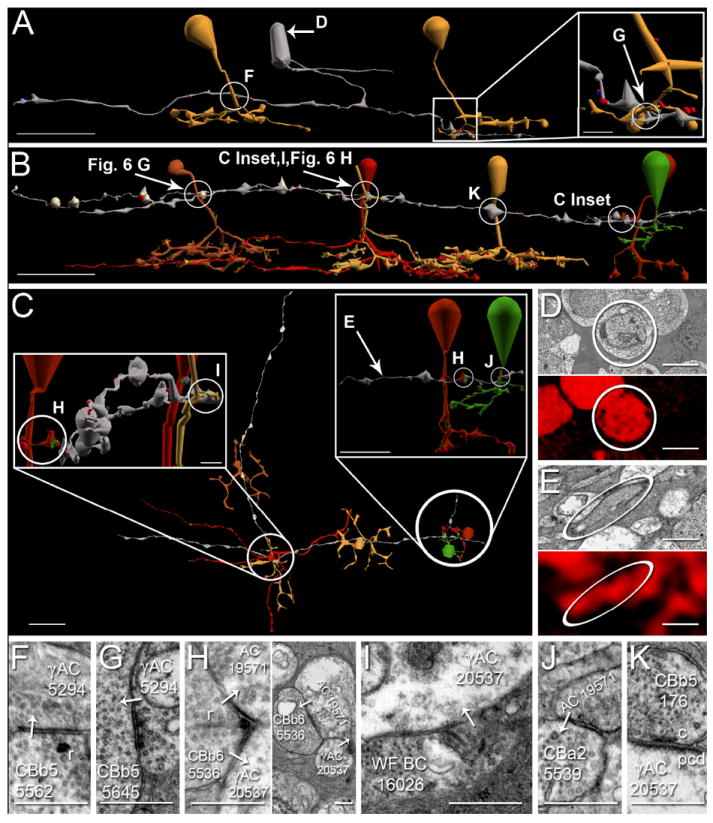
A-C. Renderings of axonal-ribbon driven γACs mediating within- and cross-channel, divergent and convergent, inhibitory networks, vertical orientation (A-B), horizontal orientation (C). Arrows, locations of γ+ signatures shown in D-E; circles, locations of synapses shown in F-K; scale bars, 20 μm. D-E. TEM of γACs in A-C with corresponding γ+ signatures. Scale bar (D), 5 μm; scale bar (E), 0.5 μm. F-K. TEM of synapses indicated in A-C. White arrows indicate synapse directionality. AC, amacrine cell; WF BC, wide field bipolar cell; r, ribbons; c, cistern; pcd, post-cisternal density; scale bars, 0.5 μm. A. CBb5 5562 (mustard, left) forms an axonal single ribbon monad onto multistratified γAC 5294 (silver, F). γAC 5294 forms a conventional synapse (A inset, G) onto CBb5 5645 (mustard, right), thus completing an axonal ribbon-mediated within channel inhibition motif. 5294’s soma is γ+ (D). B. A chain of five CBbs converge and diverge axonal ribbon and cistern contacts onto common γAC and ganglion cell targets, vertical orientation. CBb6 5536 (red, right) provides divergent input to amacrine cell 19571process (silver) and wide-field γAC 20537(silver) with an axonal ribbon dyad (H) at locations indicated in C insets. Wide-field γAC 20537 is γ+ (E). Amacrine cell 19571 cannot be confirmed as γ+, but is glycine negative, and participates in nested feedback with γAC 20537 (H, right subpanel). CBb5 176 (mustard) and wide-field cone bipolar cell 5283 (deep red, center) converge axonal cistern contacts onto γAC 20537 (K, Fig. 6 H, respectively). In the same plane of section wide-field cone bipolar cell 5283 drives ipRGC 12208 with a four-ribbon axonal monad (Fig. 6 H). This ipRGC receives convergent axonal ribbon input from CBb5w 6156 (copper, Fig. 5 G). C. Horizontal view of B. Scale bar, 20 μm. (Left inset): Rotated and zoomed-in vertical view of the circled area in the main panel (some cells removed for clarity). CBb6 5536 (red) and wide-field cone bipolar cell 16026 (sand) provide convergent, branched axonal ribbon input to γAC 20537 (H left subpanel & I, respectively). This view looks down the length of γAC 20537 (silver) between wide-field cone bipolar cell 16026 in the right foreground and CBb6 5536 in the left background. Wide-field cone bipolar cell 5283 (red, right) can be seen close to wide-field cone bipolar cell 16026. Scale bar, 2.5 μm. (Right inset): Rotated and zoomed-in vertical view of CBb > γAC ≥ CBa crossover inhibition. CBb6 5536 (red) provides a branched axonal ribbon dyad onto amacrine cell 19571 (H, left subpanel). amacrine cell 19571 forms a conventional synapse (J) onto CBa2 5539 (green) nearby, thus completing the crossover inhibition motif. Scale bar, 5 μm.
Axonal Cisterns Appear in Accessory ON Networks
Axonal cisterns, reported by (Anderson et al., 2011a), are characterized by a cistern adjacent to the plasma membrane of the nominal presynaptic cell, desheathed glia, an evenly spaced cleft similar to a synaptic cleft, and a definitive post-cisternal density (PCD) indistinguishable from classic postsynaptic densities. As an example, some targets collect from multiple cisterns. In addition to its axonal ribbon input, γAC 20537 contacts axonal cisterns from CBb5 176 and wide-field cone bipolar cell 5283 (Figure 8 B,K,Figure 5 H). The convergent axonal ribbon input to ipRGC 12208 described previously is linked to this γAC axonal ribbon network via the axonal cistern and axonal ribbons in the same plane of section by wide-field cone bipolar cell 5283. Taken together, this partial network of axonal ribbons and cisterns illuminate the complexity of axonal communication. The simultaneous divergence and convergence illustrated by the branched axonal ribbon dyad and monad from CBb6 5536 and wide-field cone bipolar cell 16026, respectively, spotlights the efficient design inherent in these networks.
Divergent ON-OFF GAC Inhibition to CBbs and ON-OFF Ganglion cells
We explored identified GAC axonal ribbon targets as possible crossover candidates. Axonal ribbon-driven GACs can distribute ON-OFF inhibition to both CBbs and ON-OFF ganglion cells (Figure 9). Specifically the following network motifs exist: CBa > ON-OFF GAC ≥ CBb, CBa > ON-OFF GAC ≥ ON-OFF ganglion cell, and CBb > ON-OFF GAC ≥ ON-OFF ganglion cell, all three of which constitute ON-OFF cross-inhibition.
Figure 9. Novel network topologies construct an ON-OFF GAC & underlie glycine-mediated within- and crosschannel inhibition.
A-D. Vertically orientated enderings of ON-OFF GAC construction and CBa > GAC ≥ CBb crossover inhibition (A), and CBb > GAC ≥ ganglion cell within- and crosschannel (crossover) inhibition motifs (B-D). Circles, location of synapses shown in E-J; scale bar (A), 10 μm; scale bar (A inset), 5 μm; scale bar (B-C), 20 μm; scale bar (D), 10 μm. E-J. TEM of synapses indicated by circles in A-D. White arrows indicate synapse directionality; GC,ganglion cell; scale bars, 0.5 μm. A,E-F. Axonal ribbon topologies employed for construction of a mono-stratified, ON-OFF GAC and CBa > GAC ≥ CBb crossover inhibition motifs. (A Inset): Rotated and zoomed in horizontal view of CBa2 424 (green), CBa2 478 (sage), GAC 906 (silver), and CBb6 4570 (red). CBa2 424 and CBa2 478 converge a single-ribbon monad and single ribbon dyad onto GAC 906 (E & F, respectively). GAC 906 forms a conventional synapse onto CBb6 4570 (red), reciprocal to an axonal ribbon (Fig. 6 E). B,G-J. Parallel CBb > GAC ≥ ON-OFF ganglion cell crosschannel inhibition, and divergent within- (CBb > GAC ≥ bsdGC) and crosschannel (CBb > GAC ≥ ON-OFF ganglion cell) inhibition. CBb6 4570 (red) drives GAC 906 (green) at the axonal synapse described in A. GAC 906 forms a conventional synapse onto mono-stratified ON-OFF ganglion cell 18693 (off-white, G). CBb5w 6156 (copper) drives narrow-field multi-stratified GAC 5575 (patina) with an axonal ribbon (Fig. 6 H). GAC 5575 forms conventional synapses onto mono-stratified ON-OFF ganglion cell 18693 at two locations (H,I). The above two synaptic chains thus form parallel CBb > GAC ≥ ON-OFF ganglion cell motifs that converge onto the same ganglion cell target. GAC 5575 also forms a conventional synapse onto bsdGC 15796 (sand, J), thereby creating divergent inhibitory motifs from CBb5w 6156 to two distinct classes of ganglion cell. C,H-J. Rotated zoom-in and isolation of divergent inhibition shown in B. Multi-stratified, narrow-field GAC 5575 (patina) receives axonal ribbon input from CBb5w 6156 (copper) at an OFF-layer branch (Fig. 7 H, not circled for anatomical clarity), and forms conventional synapses with ganglion cell 18693 (off-white,H-I) and bsdGC 15796 (sand, J). D,H-J. Zoom-in of GAC 5575 divergent inhibition in B-C for anatomical clarity and detail, better appreciation of network topologies, and synapse locations.
First, CBa2 424 and CBa2w 478 (a new CBa class discovered in RC1) drive mono-stratified GAC 906 with ribbon synapses (Figure 9 A,E,F). GAC 906 forms a conventional synapse onto CBb6 4570, reciprocal to an axonal ribbon (Figure 9 A,Figure 6 E), thus bestowing ON-OFF properties to GAC 906, and constructing a CBa > monostratified ON-OFF GAC ≥ CBb crossover inhibition motif. ON-OFF GAC 906 also synaptically diverges this ON-OFF inhibition to monostratified ON-OFF ganglion cell 18693. This is the first example of one GAC divergently distributing ON-OFF inhibition to both CBb and ON-OFF ganglion cell targets.
Next, CBb5w 6156 forms axonal ribbon synapses onto multi-stratified GAC 5575, (Figure 9 B,Figure 7 H), and GAC 906 and GAC 5575 cross inhibit each other (data not shown). GAC 906 therefore injects its ON-OFF properties to GAC 5575. Some ON-OFF amacrine cells are known to receive ON-OFF inhibition (Chen et al., 2011), and we add that the excitatory drive for this can arise from axonal ribbons. Each of the above ON-OFF GACs makes conventional synapses onto mid-IPL monostratified ON-OFF ganglion cell 18693 (Figure 9 B,G,H,I), forming parallel CBb > ON-OFF GAC ≥ ON-OFF ganglion cell motifs via two morphologically distinct GAC classes, thus blurring classical ideas of structure-function relationships. Clearly the relationships are complex. The OFF input to GAC 906 from the above two CBas further constructs a CBa > ON-OFF GAC ≥ ON-OFF ganglion cell motif. Examples of GAC-mediated crossover inhibition motifs via axonal ribbons from ON to pure OFF targets remains to be discovered in RC1.
Divergent ON-OFF GAC Inhibition to ON-OFF Ganglion cells and bsdGCs
bsdGCs obtain ON polarity response properties via direct synaptic drive from CBbs, some of which arises from axonal ribbons (Hoshi et al., 2009; Roska and Werblin, 2003). Here we report that axonal ribbons also drive ON-OFF inhibition to bsdGCs via one branch of a divergent inhibitory pathway. ON-OFF GAC 5575, introduced above, not only mediates CBb > ON-OFF GAC ≥ ON-OFF ganglion cell inhibition, it also synaptically diverges its signals to bsdGC 15796 (Figure 9 B,C,D,H,I,J). This constitutes the first reported evidence that a single narrow field multistratified GAC can disperse sign-inverted axonal ribbon excitatory signals to both ON-OFF ganglion cells and ON ganglion cells (bsdGCs), and emphasizes the inherently multiplexed nature of GACs.
ON Cone Bipolar Cell Axon Tangency Without Axonal Ribbon Synapses
Thirty-eight percent of CBbs in RC1 make axonal ribbons, which raises the question of why the other sixty percent do not. This requires some new terminology. Most neurites in the retina are directly apposed to those of other neurons without forming any specialization such as a synapse, gap junction or adherens junction (Anderson et al., 2011a). We refer to such neurite pairs as tangent processes. In some cases, a single descending axon simply bypasses a cell to which it is tangent without forming an axonal ribbon (Figure 10 A,C). More intriguing, two ON cone bipolar cell axons may be tangent to the same cell, with differential connectivity to it. For example, CBb4 3116 forms an axonal ribbon dyad onto a bsdGC 15796 and an unknown target, and CBb4 4569 is tangent to the same unknown process, without forming an axonal ribbon synapse (Figure 10 B,D). In the first case, the potential but unconsummated target is an OFF layer monostratified ganglion cell that may be a pure OFF ganglion cell, as we have identified only OFF cone bipolar cell input to this ganglion cell. Thus it may not be an appropriate target. In the second case, three interesting points arise: 1) the CBb without axonal ribbons in the figure does not make any axonal ribbons, 2) the CBbs are of the same class (CBb4), and 3) the CBbs are coupled by gap junctions and therefore share signaling attributes. One possibility for the differential connectivity is that ON cone bipolar cell coupling obviates the need for axonal ribbon input from both CBbs. That said, coupled CBbs do drive common targets from their telodendria, but never at the same locus. This topic will be addressed in future papers.
Figure 10. CBb axon tangency to potential targets without axonal ribbon synapses.
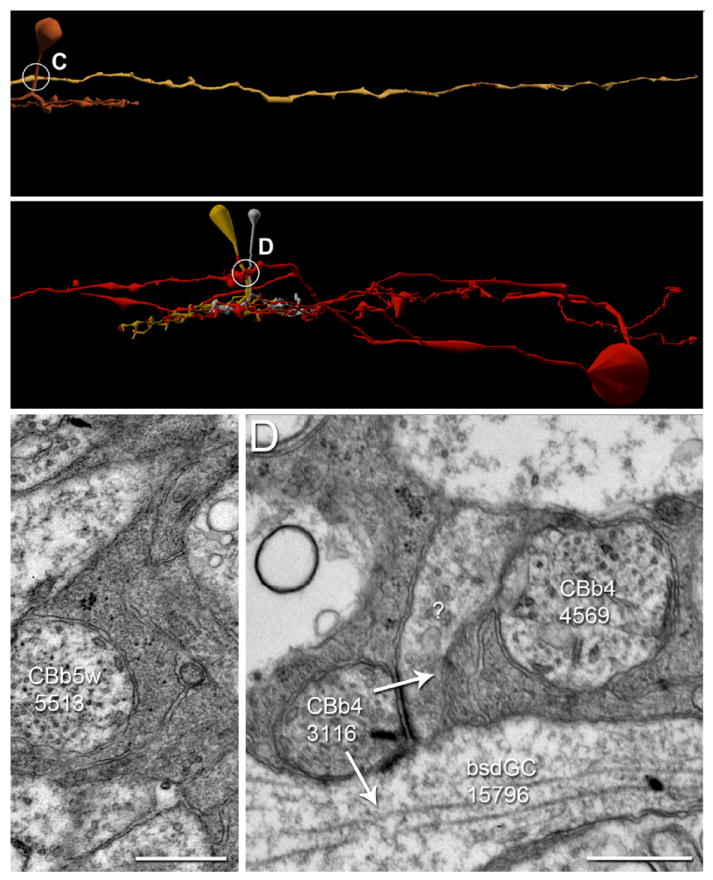
A-B. Renderings of CBbs with contact, but not synapses onto ganglion cells, vertical orientation. Circles, locations of synapses shown in C-D; scale bars, 20 μm. C-D. TEM of synapses indicated by circles in A-B. White arrows indicate synapse directionality; GC, ganglion cell; scale bars, 0.5 μm. A,C. CBb3 5513’s (copper) axon is tangent (adjacent with no intervening muller glia) to OFF ganglion cell 13858 (sand), yet does not form a synapse. B,D.CBb4 3116 (silver) forms an axonal ribbon dyad onto bsdGC 15796 (D, Fig. 6 A & E) and an unknown target (D), whereas CBb4 4569 (dark mustard) does not form an axonal ribbon onto the same unknown target despite being tangent to it. Incidently, CBb4 3116 and CBb4 4569 are gap junctionally coupled (data not shown).
Discussion
The analysis of RC1 and non-canonical ON cone bipolar cell axonal ribbon synapses in the OFF layer exposes new organizational concepts in retina and leads to a refactoring of the IPL. We first address the existence of mixed signaling strata and new network access schemas; the distinction between simple tangency and functional contact; and the importance of joint distributions for interpreting synaptic statistics. Then we will review key signaling features of specific targets of axonal ribbons. Since bipolar cell nomenclatures differ across species and we will now be discussing many of them, and since all cone bipolar cell classes in rabbit make axonal ribbons, we periodically depart from the McNeil et al. (2004) rabbit scheme for the discussion and simply refer to cone bipolar cells as ON cone bipolar cells and OFF cone bipolar cells.
First, why do ON cone bipolar cells target the OFF layer of IPL at all? The answer is partly evolutionary: the OFF layer of the IPL has been a mixed ON-OFF stratum throughout vertebrate descent. Every non-mammalian vertebrate class harbors multistratified ON bipolar cells, (Kolb, 1982; Pang et al., 2004; Ramon y Cajal, 1892; Scholes, 1975; Scholes and Morris, 1973; Sherry and Yazulla, 1993; Wong and Dowling, 2005) and their discovery in the mammalian retina demonstrates that no evolutionary mechanism has ever “purified” the OFF layer. But more concretely, mixed strata reflect important network access properties. Axonal ribbons provide ON inputs to unique monostratified cells such as TH1 axonal cells and M1 ipRGCs (Dumitrescu et al., 2009; Hoshi et al., 2009) that send their dendrites to the most distal layer of the IPL. That is an incomplete explanation since the very same ON cone bipolar cells also have outputs in the ON layer. The question should be reframed in future work: why do the target ON cells invade the OFF layer at all. We have preliminary data to show that, in addition to ON inputs, these cells seekinputs from CBa1-driven OFF γACs accessible only in the OFF layer. Ultimately, there is no unique distal ON stratum in the IPL. Indeed, the entire OFF layer is a stack of mixed ON-OFF strata with cone bipolar cell axonal ribbons distributed throughout (Figure 2, 3, 4). We propose that ON signals in the OFF layer provide unique network opportunities for crossover signaling and loci for mixing ON excitation with polarity-matched OFF inhibition.
Analysis of axonal ribbon sites reveals that specific rules control their incidence, though we clearly have a poor idea of the molecular mechanisms. ON cone bipolar cell axons are sheathed by three facing Müller cells throughout their transit of the OFF layer except at sites of potential target contact, where the Müller cells are parted by unknown mechanisms. As described by Anderson et al. (2011a) many neural processes are apposed without intervening glia but never make synapses, gap junctions or even adherens junctions. We, noted above, refer to such lack of functional contact as tangency. Many processes somehow induce unsheathing of Müller cells around CBb cells in the OFF layer yet remain simply tangent. Another important point is that ribbon synapses, whether in the axon or axon terminal, never appear at the membrane without an associated postsynaptic density. This suggests that complete synaptic contacts are induced by the target or source-target interactions, but that unsheathing to expose the source seems to be under the control of the target.
Finally, not all ON cone bipolar cells in a given class form axonal synapses, but members of all classes do form OFF layer axonal synapses. Using a very strict criterion, 36% of all identified ON cone bipolar cells in RC1 engage the OFF IPL with axonal ribbon synapses. Our analysis of sources and targets for these and other synaptic pairings suggests that the retina routinely invokes such partial motifs. Such sampling schemes conflict with our traditional expectations and methods of tabulating synaptic contacts (e.g. counting the percent of outputs onto a target). That approach to network analysis would lead us to ask: If most ON cone bipolar cells do not form axonal synapses, how can we argue that they are functional and not some statistical anomaly? We can approach this problem via graph theory, with cells represented as vertices and synaptic connections represented as edges. Every vertex in a directed graph represents a point of signal transfer between a source and target. In a multidigraph like the retina (Marc et al., 2012), each vertex represents the source or target for multiple edges. And given that the copy numbers for each class of vertices (i.e. each ultimate cell class, Marc and Jones (2002)) varies, as do their coverages and Hausdorff dimensions, one cannot optimize a complex biological system to give smooth statistics or provide 100% source contacts for all cells. Figure 11 provides a geometric proof of this. The white dots in Figure 11 represent the projection of 15 ON cone bipolar cell axons through a sampling plane of the IPL. In Figure 11 A, a set of cells from a single class (with individual cells in different colors) with a high coverage contacts every cone bipolar cell axon. Indeed the overlap of individual cells leads to multiple edges. The outflow efficiency appears to be 100%, with a mean contact number of 2.67 ± 0.7 (standard deviation). But it is important to grasp that these are meaningless metrics, especially the variance. The only metric that matters is the efficiency of target sampling, which is also 100%. This becomes clearer in Figure 11 B, where two different, sparse cell classes send dendrites through the axonal field. Only 6 of 15 axons are hit for an output efficiency of 40%. Indeed the output efficiency is even lower for each class. Yet, from the perspective of the targets, the two cells make synapses with 100% of the axons they encounter. This is critical for cells with low coverages such as ganglion cells. Their target sampling is perfect. Not all axons are hit because there is an oversupply of sources. The target doesn’t “know” that there are excess source axons since they are not needed. Thus the partial incidence of axonal synapses in ON cone bipolar cell axons reflects the spatial needs of the targets, not the sources. It does not represent any imprecision: QED. The key descriptor for such networks is the joint density distribution of source and target, expressed as a metric of signal transfer sites per unit area or volume of neural space.
Figure 11. Explanation of the interaction between sparse network topologies and joint distributions.
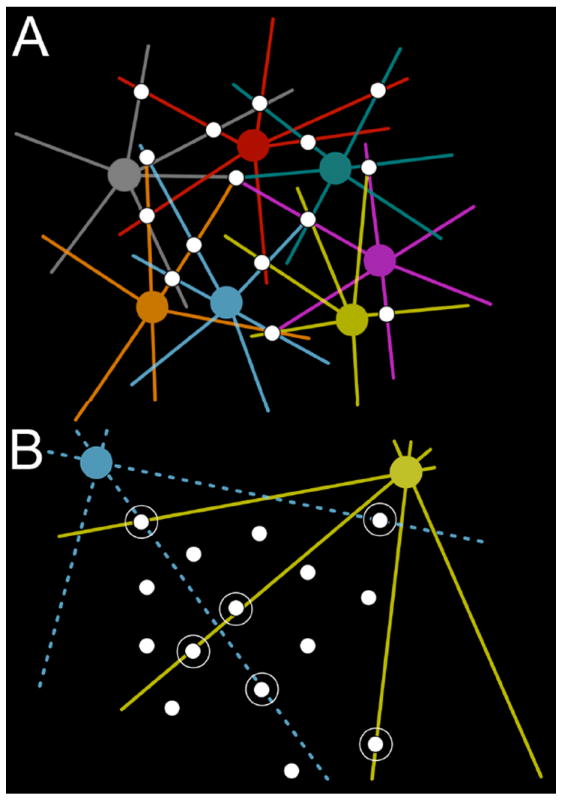
A. An array of bipolar cell axons (white) traverses the image plane of the retina. In the top field, a cell class with high coverage is shown in different colors for every instance of the class. Each bipolar cell axon is contacted several times for an average contact of 2.4. B. Two different classes of ganglion cells (yellow, blue) form part of their tiling by sampling from the bipolar cell array. Most bipolar cells are missed, for an average outflow contact of 0.375, which is meaningless. Six circled bipolar cells are contacted by the ganglion cells (none twice), and the ganglion cells are errorless in contacting encountered bipolar cells. As ganglion cells are not space filling cells, further inputs would be superfluous.
Two ON Cone Bipolar Cell Classes Converge Onto ipRGCs
The putative ipRGC identified in RC1 receives axonal ribbon input from every ON cone bipolar cell it encounters, wide-field cone bipolar cell 5283 and CBb5w 6156. It further refuses input from two OFF cone bipolar cells to which it is tangent (data not shown). Neural structure-function correspondence is widely agreed upon, and every tested class of bipolar cell identified based on unique morphology has thus far proven to possess unique physiological response properties (Masland, 2001). Thus wide-field cone bipolar cell 5283 and CBb5w 6156 contribute their presumably differential responses properties via sign conserving synapses to the ipRGC, thereby increasing the complexity or range of the ipRGC responses. This could represent convergence of different spectral sensitivities and/or flux range fractionation.
Coupled Bipolar Cell Input to bsdGCs
bsdGC 15796 is one target of an axonal ribbon dyad from CBb4 3116 (Figure 6 A), which belongs to a cluster of seven coupled ON cone bipolar cells that likely represent a patch in a larger sheet of coupled cone bipolar cells, similar to the coupled clusters of ON cone bipolar cells discovered in teleosts (Umino et al., 1994). It is striking that none of the other members of the coupled chain provide input to the bsdGC, despite a second axon from CBb4 4569 very close to the axonal ribbon input by CBb4 3116 (Figure 6 A; Figure 10 B-C), and costratification of ON cone bipolar cell primary axonal arbors with ON layer bsdGC arbors. Again, this reflects the concept of joint distributions where a limited bsdGC target architecture samples inputs from an array of excess sources. This would be especially true when sampling from coupled arrays since a single sampled input would provide some weighted mean output from a patch. Teleost coupled bipolar cells appear to receive variable input from cones, which introduces noise into the system, and modeling coupled bipolar cells as hexagonal arrays of isopotential units indicates that coupling increases the input signal to noise ratio without significantly sacrificing resolution (Umino et al., 1994). bsdGCs receive most of their ribbon input in the ON layer (Hoshi et al., 2009), and the need for axonal ribbon input remains a mystery. As noted above, it is likely that the primary function of OFF stratification in nominal ON cells is accessing OFF amacrine cell inputs.
γAC Targets, GAC Targets, and Crossover Inhibition
Physiological analyses show that ON and OFF channels cross-inhibit each other via glycinergic synapses at every tier of the IPL (Chen et al., 2011; Molnar et al., 2009; Roska and Werblin, 2003; Werblin, 2010). Functional reasons for this include possible restoration of linearity to rectified currents driven by AMPA and NMDA receptors, expanding photopic dynamic range into the scotopic domain, luminance-contrast distinction, better impedence matching in postsynaptic neurons, OFF cone bipolar cell gain and high frequency response increase, and limitation of OFF channels to negative contrast processing (Liang and Freed, 2010; Molnar et al., 2009; Werblin, 2010). We add evidence that ON cone bipolar cell axonal ribbons mediate crossover inhibition via synapses with both γACs and GACs, revealing network topologies not predicted from electrophysiology.
The GAC and γAC targets are both mono- and multistratified (Figures 7 & 8). Both GAC and γAC targets form feedback and feed forward motifs, and γAC targets also form nested feedback to axonal ribbons.
Given the extensive γAC networks at bipolar cell axon terminals, it is not surprising they engage axonal ribbons as well. γAC feedback and nested feedback onto bipolar cells fine-tunes bipolar cell presynaptic release (Marc and Liu, 2000), and is implicated in axonal ribbon release as well (Figures 7 & 9).
γAC-mediated crossover inhibition via axonal ribbons (Figure 8 B,C right inset) extends the functional repertoire of γACs, demanding dissection of the potentially differential functional role of glycinergic and GABAergic crossover inhibition. Two non-exclusive functional implications arise. First, glycine receptor (glyR)-mediated, GABA receptor (GABAR)-mediated inhibition of bipolar cells may manifest different kinetics that combine with amacrine cell presynaptic release, such that GABAAR- and glyR-mediated inhibition predominantly control the magnitude of bipolar cell glutamate release, whereas GABAC-mediated inhibition controls the timing of bipolar cell glutamate release by increasing its transiency (Eggers and Lukasiewicz, 2011; Eggers and Lukasiewicz, 2006a; 2006b; 2010; Eggers et al., 2007). Crossover inhibition networks may appropriate these kinetic differences to increase the range and complexity of bipolar cell and ganglion cell responses. Second, dual transmitters may optimize crossover inhibition by preventing synaptic occlusion, which occurs when two or more adjacent pre-synaptic terminals release the same neurotransmitter onto a shared postsynaptic target (Fatima-Shad and Barry, 1992; Gold and Martin, 1984). Since the postsynaptic cell detects these multiple GABAergic synaptic inputs via the same type of GABAARs, for example, adjacent GABAergic inputs cross-desensitize. Introduction of multiple neurotransmitters at these locations discretizes the signals, which may be necessary to properly effect crossover inhibition.
We now consider the functional role of dual transmitter-mediated crossover inhibition for the CBb > γAC ≥ CBa motif (Figure 8 B,C right inset). Most OFF cone bipolar cells receive ON inhibition (Molnar and Werblin, 2007). Further, OFF cone bipolar cells are dominated by glyR-mediated inhibition, though they also receive some GABAAR-mediated inhibition, but little GABACR-mediated inhibition (Eggers and Lukasiewicz, 2011). This is quantitatively inconsistent with the dominance of γAC inputs to CBa cells, but qualitatively matches observed higher GAC convergence on CBa as opposed to CBb cells. Given the similarites between glyR- and GABAAR-mediated OFF cone bipolar cell response kinetics in response to natural stimuli, there is no obvious kinetic advantage to the utilization of both to cross-inhibit OFF cone bipolar cells. Thus, dual γAC-mediated and GAC-mediated bipolar cell > amacrine cell ≥ bipolar cell crossover inhibition networks may reduce synaptic occlusion, rather than control OFF cone bipolar cell peak release. That said, examples of axonal ribbon-involved adjacent γAC and GAC processes sharing postsynaptic targets remain to be found. Though axonal ribbon-mediated OFF ≥ ON GABAergic crossover inhibition has not been discovered in the OFF layer, it has been found in the ON layer between OFF cone bipolar cell telodendria and ON cone bipolar cells, and is the topic of future papers.
Predicting the function of OFF ≥ ON, dual transmitter crossover inhibition is less clear, due to some slight discrepancies in the literature. Eggers and Lukasiewicz (2011) report that murine ON cone bipolar cells possess similar levels of GABAAR- and GABACR-mediated inhibition, and little to no glyR-mediated inhibition, whereas others report glycine-mediated crossover inhibition of ON cone bipolar cells (Molnar et al., 2009; Werblin, 2010). Presuming that glyR-, GABAAR-, and GABACR-mediated inhibition all occur in rabbit ON cone bipolar cells, which is consistent with amacrine cell networks in RC1, dual glycine- and GABA-mediated crossover inhibition would afford control of both the peak amplitude and the degree of prolonged release in ON cone bipolar cells. Synaptic occlusion reduction could be an additional benefit of dual-transmitter crossover inhibition in these cells as well, but more analysis is needed to determine the frequency of adjacent γAC and GAC inputs to common targets.
GAC- versus γAC-Mediated Cross-Channel Feedback and Feed Forward Inhibition
Many networks describe in this manuscript constitute axonal ribbon-mediated cross-channel feedback inhibition (CBb > γAC ≥ CBa and CBa > GAC ≥ CBb motifs), and cross-channel feed forward inhibition (CBb > GAC ≥ ganglion cell and CBa > GAC ≥ ganglion cell motifs). These motifs could also subserve kinetically appropriate ON-OFF response properties in polarity-opposite targets. Axonal ribbon reciprocal synapses can inject OFF components into ON channels, inject ON components into OFF channels, and construct ON-OFF target cells. GAC and γAC feedforward motifs discovered thus far are different. γACs feedforward to targets also directly driven by axonal ribbons by the CBb, whereas GACs feedforward to targets not directly driven by those axonal ribbons. We refer to these as in-class and cross-class feedforward motifs, respectively. One common form of glycinergic ON ≥ OFF crossover is provided by AII AC lobular dendrite synapses onto OFF cone bipolar cells and extensive input to OFF α and δ ganglion cells. Importantly, neither AII ACs nor OFF α / δ ganglion cells are targeted by ON cone bipolar cell axonal synapses, despite abundant opportunities.
The diversity of inputs to ON-OFF amacrine cells aligns with the complexity of amacrine cell/ganglion cell response properties. We show that an anatomical framework exists to support glycine- and GABA-mediated control of ON cone bipolar cell release at axonal ribbon locations, which may subserve both crossover inhibition and ON-OFF GAC regulation of ON cone bipolar cell axonal ribbon synapse release kinetics.
Rod Bipolar Cell Axonal Ribbons Are Distinct from ON Cone Bipolar Cell Axonal Ribbons
Despite the fact that multiple laboratories have reported very few, if any, axonal ribbons in rod bipolar cells (Chun et al., 1993; Ghosh et al., 2001; Tsukamoto et al., 2001), our results are more consistent with those of Strettoi et al. (1990), in which they reported occasional instances of output synapses along the descending axons of rod bipolar cells. Nonetheless, the rod bipolar cell axonal ribbons all occur en passant, with no evident branching, and are concentrated in the ON IPL (Figure 4). Those that breach the ON-OFF boundary do so marginally; they comprise ON drive to polarity-matched targets, distinct from ON-OFF crosstalk achieved by ON cone bipolar cell axonal ribbons in the rabbit retina. The absence of rod bipolar cell axonal ribbons in the distal OFF layer is significant since M1 ipRGCs exhibit rod responses (Aggelopoulos and Meissl, 2000; Dacey et al., 2005; Wong et al., 2007). Possible sources include the primary AII-mediated scotopic pathway, the secondary rod∷cone coupling scotopic pathway, or direct rod bipolar cell axonal synapses with M1 ipRGCs as suggested by Ostergaard et al. (2007). Our data demonstrate that rod input to M1 cells absolutely does not arise from rod bipolar cell axonal ribbons. Moreover, we have found no evidence of rod bipolar cell synapses onto ganglion cells of any type, and the rod bipolar cell axonal ribbons discovered thus far target only AI and AII ACs, both typical ON layer targets of rod bipolar cell ribbons. AI AC rod bipolar cell axonal ribbon targets are further consistent with previous work demonstrating that AI AC dendrites sometimes immediately appose GABA receptors on descending rod bipolar cell axons in the ON IPL sublaminae, expected for reciprocal synapses observed between AI ACs and rod bipolar cell ribbons (Wässle et al., 1991; Zhang et al., 2002).
Multiple Axonal Synaptic and Network Topologies Distribute Functionality
Axonal ribbons routinely construct convergent and divergent synaptic motifs. The synaptic topologies vary across these examples, including all combinations of single-versus multi-ribbon, and monadic versus dyadic synapses (Figures 6,7,8,9). Axonal ribbons also tend to be smaller than ribbons in the primary ON cone bipolar cell arbors. Distinct synaptic topologies are considered here.
First, wide-field cone bipolar cell 6156 forms single-ribbon, monadic axonal synapses to drive an ipRGC and a narrow-field, diffusely stratified GAC employed for divergent within- and cross-channel inhibition motifs (Figure 6 C,G; Figure 7 D,H). Second, wide-field cone bipolar cell 5283 drives the ipRGC targeted by CBb5w 6156 with a multi-ribbon, monadic axonal synapse, demonstrating different synaptic topological input to a common target, albeit from two classes of ON cone bipolar cell. Third, CBb6 5536 displays a single-ribbon, branched axonal synapse dyad to drive a pair of OFF layer, mono-stratified amacrine cell processes, which provide nested feedback to the CBb6, and one of which mediates CBb > γAC ≥ CBa crossover inhibition (Figure 8 C right inset,H). Finally, CBb5 400 forms a multi-ribbon, dyadic axonal synapse onto ganglion cell 5118 and a currently unidentified process (Figure 6 B,F).
No clear pattern emerges as to the rules governing axonal ribbon synaptic topologies, but we can eliminate two possibilities. First, the target cell does not govern axonal ribbon count, as evidenced by the ipRGC recipient to convergent input from two axonal ribbon monads with different numbers of ribbons. Second, cone bipolar cell class does not govern axonal ribbon synaptic topology, given that ON cone bipolar cells of the same class can instantiate different axonal synaptic topologies, and ON cone bipolar cells of different classes can share synaptic topologies. More source-target analysis is needed on this topic.
Monostratification Achieves ON-OFF Crosstalk via Axonal Ribbons
It is generally thought that ganglion cells acquire ON-OFF responses via bistratification across the ON and OFF IPL, yet in WT mice 11% of ganglion cells establish ON-OFF properties by P33 via mono-stratification of one thick band of dendrites in the middle IPL (Tian, 2008). Ganglion cell process 18693, targeted in CBb > ON-OFF GAC ≥ ON-OFF ganglion cell crossover inhibition (Figure 9 B,C,D), is one such mono-stratified ganglion cell. Unfortunately, this ganglion cell process exits RC1, so we cannot verify that it lacks another stratum of arborization. However, its annotated processes costratifiy in the mid-IPL with GAC 906 from which it receives crossover inhibition, driven by an axonal ribbon (Figure 9 B). This example of co-monostratification of a GAC and ganglion cell in the same crossover inhibition network demonstrates that multistratified bipolar cells can mediate ON-OFF crosstalk. Moreover, since it is now established that the entire OFF layer of the IPL contains mixed ON-OFF signal processing, it follows that almost any monostratified cell could develop ON-OFF responses.
Axonal Cisterns
Though the function of axonal cisterns is unknown, they are not randomly distributed and appear as well-ordered accessory ON network elements to common target cells (Figure 8 B,K; Figure 6 H). They are often in close proximity to axonal ribbon synapses (Figure 6 H), converge onto common targets (Figure 8 B), and have been observed reciprocal to conventional synapses (data not shown), suggesting that they are real structural or communicative elements of accessory ON networks. Indeed, preliminary analyses reveal that 55 of 113 (48.7%) measured cone bipolar cells contain one or more axonal cisterns. More complete analyses will be conducted in future manuscripts.
Axonal Ribbons are Routine Network Elements Throughout the IPL
Ten axonal ribbon-mediated network motifs have been discovered in RC1 thus far spanning all IPL sublaminae (Figure 12), emphasizing their routinity in cone bipolar cell signaling. It is highly likely that additional motifs exist, as many axonal ribbon targets and networks remain to be identified. The excitatory motifs provide direct axonal ribbon drive to an array of ganglion cell classes. The inhibitory motifs comprise both feedback and feedforward as they target GACs and γACs, which in turn form synapses onto CBas, CBbs, and several classes of ganglion cell.
Figure 12. Axonal ribbon motifs summary semi-schematic.
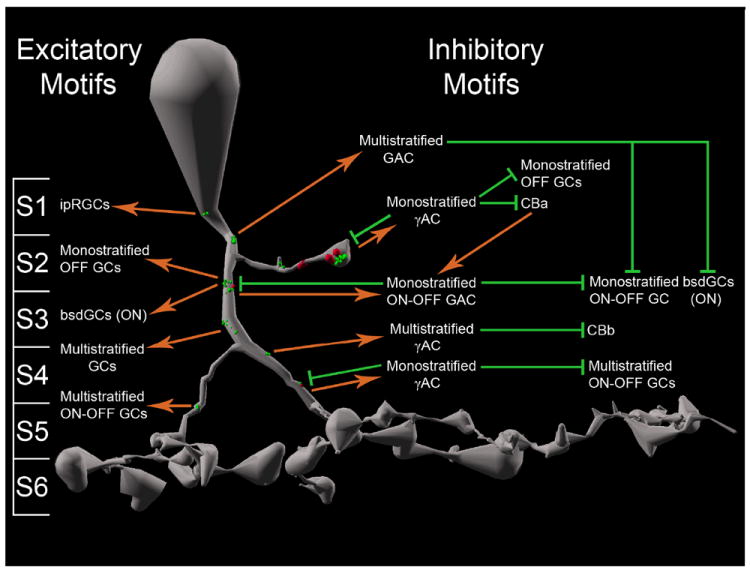
Wiring diagram for axonal ribbon motifs discovered across all cone bipolar cell classes in RC1 collapsed onto one representative cell. Spatial distributions of axonal ribbons have been preserved as best as possible to represent actual axonal ribbon locations. The axonal branch in sublamina 2 and the bifurcated descending axon are included for completeness, though both occur in a minority of cone bipolar cells. Note that in addition to abundant axonal ribbon output, cone bipolar descending axons are frequently postsynaptic to amacrine cell inputs. S1-S6, IPL sublaminae 1-6; orange arrows, excitatory ribbon synapses; green flathead arrows, inhibitory GAC- or γAC-mediated synapses; GC, ganglion cell.
Figure 12 collapses the network motifs reported in this manuscript onto one representative cone bipolar cell for clarity. As such two features that occur in a minority of cells are included, branched axonal ribbons in the canonical OFF IPL and bifurcated descending axons. It is important to distinguish between a bifurcated axon and the primary branch point of the telodendria. ON cone bipolar cell descending axon bifurcations occur in sublaminae 3-5, distinctly distal to the primary arborization of the cell. In such cases, the descending axon typically bifurcates into major (Figure 12, right branch) and minor (Figure 12, left branch) axons before each primarily arborizes. The major branch diameter remains comparable to the descending axon diameter distal to the bifurcation, while the minor branch point adopts a smaller diameter. Each branch retains axonal features such as predominant microtubule bundles and a scarcity of vescicles, except for vesicle clouds concentrated near axonal ribbons. In cases of clearly bifurcated descending axons distal to the primary arborization of the cell, such as that shown in figure 12, ribbon synapses in both the major and minor axons were still classified as “axonal”.
Note that in addition to abundant axonal ribbon output, cone bipolar descending axons are frequently postsynaptic to amacrine cell inputs, both reciprocal and non-reciprocal to axonal ribbons.
Table 4.
Abbreviations
| INL | Inner Nuclear Layer |
| IPL | Inner Plexiform Layer |
| GCL | Ganglion Cell Layer |
| GAC | Glycine-positive Amacrine Cell |
| γAC | GABA-positive Amacrine Cell |
| AI AC | AI Amacrine Cell = A17 Amacrine Cell |
| AII AC | AII Amacrine Cell |
| ipRGC | Intrinsically Photosensitive Retinal Ganglion Cell |
| bsdGC | Bistratified Diving Ganglion Cell |
| > | Sign-Conserving Synapse |
| ≥ | Sign-Inverting Synapse |
| GABAAR | GABAA Receptor |
| GABACR | GABAC Receptor |
| glyR | Glycine Receptor |
Acknowledgments
We thank James Tucker for assistance with imaging and discussions, and Hope Morrison for annotation of cells in the RC1 volume. The RC1 data set is freely available to be transferred to user media or viewed with Viking application upon request.
Support: NIH EY02576 (RM), NIH EY015128 (RM), NSF 0941717 (RM), NIH EY014800 Vision Core (RM), RPB unrestricted grant to Moran Eye Center, RPB Career Development Award (BWJ), Thome Foundation grant for AMD Research (BWJ)
Role of Authors
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: JSL, REM. Acquisition of data: JSL, JVH, REM. Analysis and interpretation of data: JSL, REM. Drafting of the manuscript: JSL, REM. Critical revision of the manuscript for important intellectual content: JSL, REM. Statistical analysis: JSL, REM. Obtained funding: REM. Administrative, technical, and material support: JRA, BWJ, CBW, SM. Study supervision: REM.
Footnotes
Conflict of Interest Statement
Robert E. Marc is a principal of Signature Immunologics, Inc., manufacturer of some reagents used in this work.
Author responsibilities: Lauritzen: 1° Author, network graph representation, mapping, and analysis, 3-dimensional reconstruction of functional networks, structural analysis, Anderson: 2° Author, CMP, TEM, imaging, network graph representation, RC1 construction and annotation software development, Jones: 2° Author, CMP, TEM, imaging, Watt: 3° Author, CMP, TEM, imaging, Mohammed: 3° Author, network graph software development, Hoang: 3° Author, network graph mapping, Marc: 1° Author, CMP, TEM, imaging, network graph software development, RC1 construction and annotation software development, network graph representation and analysis, 3-dimensional reconstruction of functional networks.
Definitions:
1° Author: Generated primary manuscript
2° Author: Generated manuscript sections and contributed substantial revisions
3° Author: Generated area-specific manuscript revisions
Literature Cited
- Aggelopoulos NC, Meissl H. Responses of neurones of the rat suprachiasmatic nucleus to retinal illumination under photopic and scotopic conditions. J Physiol. 2000;623:211–222. doi: 10.1111/j.1469-7793.2000.t01-1-00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JR, et al. Exploring the retinal connectome. Mol Vision. 2011a;17:355–379. [PMC free article] [PubMed] [Google Scholar]
- Anderson JR, et al. A computational framework for ultrastructural mapping of neural circuitry. PLoS Biol. 2009;7(3):e1000074. doi: 10.1371/journal.pbio.1000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JR, et al. The Viking viewer for connectomics: scalable multi-user annotation and summarization of large volume data sets. J Microscopy. 2011b;241:13–28. doi: 10.1111/j.1365-2818.2010.03402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggman KL, Bock DD. Volume electron microscopy for neuronal circuit reconstruction. Curr Opin Neurobiol. 2011;22:1–8. doi: 10.1016/j.conb.2011.10.022. [DOI] [PubMed] [Google Scholar]
- Calkins DJ, et al. Microcircuitry and mosaic of a blue-yellow ganlion cell in the primate retina. J Neurosci. 1998;18(9):3373–3385. doi: 10.1523/JNEUROSCI.18-09-03373.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez AE, Diamond JS. Diverse mechanisms underlie glycinergic feedback transmission onto rod bipolar cells in the rat retina. J Neurosci. 2008;28:7919–7928. doi: 10.1523/JNEUROSCI.0784-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, et al. Amacrine-to-acacrine cell inhibition: Spatiotemporal properties of GABA and glycine pathways. Vis Neurosci. 2011;28:193–204. doi: 10.1017/S0952523811000137. [DOI] [PubMed] [Google Scholar]
- Chun MH, et al. Electron microscopic analysis of the rod pathway of the rat retina. J Comp Neurol. 1993;332:421–432. doi: 10.1002/cne.903320404. [DOI] [PubMed] [Google Scholar]
- Dacey DM, et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- Dumitrescu ON, et al. Ectopic retinal ON bipolar cell synapses in the OFF inner plexiform layer: contacts with dopaminergic amacrine cells and melanopsin ganglion cells. J Comp Neurol. 2009;517:226–244. doi: 10.1002/cne.22158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers E, Lukasiewicz P. Multiple pathways of inhibition shape bipolar cell responses in the retina. Vis neurosci. 2011;28:95–108. doi: 10.1017/S0952523810000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz PD. GABA(A), GABA(C), and glycine receptor-mediated inhibition differentially affects light-evoked signaling from mouse retinal rod bipolar cells. J Physiol. 2006a;572:215–225. doi: 10.1113/jphysiol.2005.103648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz PD. Receptor and transmitter release properties set the time course of retinal inhibition. J Neurosci. 2006b;26:9413–9425. doi: 10.1523/JNEUROSCI.2591-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz PD. Interneuron circuits tune inhibition in retinal bipolar cells. J Neurophysiol. 2010;103:25–37. doi: 10.1152/jn.00458.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, et al. Presynaptic inhibition differentially shapes transmission in distinct circuits in the mouse retina. J Physiol. 2007;582:569–582. doi: 10.1113/jphysiol.2007.131763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famiglietti EV. Functional architecture of cone bipolar cells in mammalian retina. Vision Res. 1981;21:1559–1563. doi: 10.1016/0042-6989(81)90032-8. [DOI] [PubMed] [Google Scholar]
- Famiglietti EV, et al. Neuronal Architecture of on and off pathways to ganglion cells in carp retina. Science. 1977;198(4323):1267–1269. doi: 10.1126/science.73223. [DOI] [PubMed] [Google Scholar]
- Famiglietti EV, Kolb H. Structural basis for ON- and OFF-center responses in retinal ganglion cells. Science. 1976;194(4261):193–195. doi: 10.1126/science.959847. [DOI] [PubMed] [Google Scholar]
- Fatima-Shad K, Barry PH. A patch-clamp study of GABA- and strychnine-sensitive glycine-activated currents in post-natal tissue-cultured hippocampal neurons. Proc Biol Sci. 1992;250(1328):99–105. doi: 10.1098/rspb.1992.0136. [DOI] [PubMed] [Google Scholar]
- Ghosh KK, et al. Glutamate receptors in the rod pathway of the mammalian retina. J Neurosci. 2001;21:8636–8647. doi: 10.1523/JNEUROSCI.21-21-08636.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MR, Martin AR. Gamma-Aminobutyric acid and glycine activate Cl- channels having different characteristics in CNS neurones. Nature. 1984;308(5960):639–641. doi: 10.1038/308639a0. [DOI] [PubMed] [Google Scholar]
- Graham D, et al. Webvision: The Organization of the Retina and Visual System [Internet] Salt Lake City (UT): University of Utah Health Sciences Center; 1995-2008 Aug 01; 2008. Melanopsin Ganglion Cells: A bit of fly in the mammalian eye. [PubMed] [Google Scholar]
- Hoshi H, et al. ON inputs to the OFF layer: bipolar cells that break the stratification rules of the retina. J Neurosci. 2009;29(28):8875–8883. doi: 10.1523/JNEUROSCI.0912-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon CJ, Masland RH. A population of wide-field bipolar cells in the rabbit retina. J Comp Neurol. 1995;360:403–412. doi: 10.1002/cne.903600304. [DOI] [PubMed] [Google Scholar]
- Kolb H. The morphology of the bipolar cells, amacrine cells and ganglion cells in the retina of the turtle Pseudemys scripta elegans. Philos Trans R Soc Lond B Biol Sci. 1982;298(1092):355–393. doi: 10.1098/rstb.1982.0087. [DOI] [PubMed] [Google Scholar]
- Kolb H, et al. The synaptic organization of the dopaminergic amacrine cell in the cat retina. J Neurocytol. 1990;19(3):343–366. doi: 10.1007/BF01188404. [DOI] [PubMed] [Google Scholar]
- Kolb H, et al. Neurons of the human retina: a Golgi study. J Comp Neurol. 1992;318(2):147–187. doi: 10.1002/cne.903180204. [DOI] [PubMed] [Google Scholar]
- Liang Z, Freed M. The ON pathway rectifies the OFF pathway of the mammalian retina. J Neurosci. 2010;30(16):5533–5543. doi: 10.1523/JNEUROSCI.4733-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linberg KA, et al. Retinal neurons of the California ground squirrel, Spermophilus beecheyi: a Golgi study. J Comp Neurol. 1996;365:173–216. doi: 10.1002/(SICI)1096-9861(19960205)365:2<173::AID-CNE1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- MacNeil M, et al. The population of bipolar cells in the rabbit retina. J Comp Neurol. 2004;472:73–86. doi: 10.1002/cne.20063. [DOI] [PubMed] [Google Scholar]
- Manookin MB, et al. Disinhibition combines with excitation to extend the operating range of the OFF visual pathway in daylight. J Neurosci. 2008;28:4136–4150. doi: 10.1523/JNEUROSCI.4274-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc RE. Neurochemical stratification in the inner plexiform layer of the vertebrate retina. Vision Res. 1986;26(2):223–238. doi: 10.1016/0042-6989(86)90017-9. [DOI] [PubMed] [Google Scholar]
- Marc RE, Jones BW. Mjolecular phenotyping of retinal ganglion cells. J Neurosci. 2002;22:413–427. doi: 10.1523/JNEUROSCI.22-02-00413.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc RE, et al. Building Retinal Connectomes. Current Opinion in Neurobiology. 2012 doi: 10.1016/j.conb.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc RE, Liu WL. Fundamental GABAergic amacrine cell circuitries in the retina: nested Feedback, concatenated inhibition, and axosomatic synapses. J Comp Neurol. 2000;425:560–582. doi: 10.1002/1096-9861(20001002)425:4<560::aid-cne7>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Marc RE, et al. Pattern recognition of amino acid signatures in retinal neurons. J Neurosci. 1995;15:5106–5129. doi: 10.1523/JNEUROSCI.15-07-05106.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani AP. Biplexiform cells: ganglion cells of the primate retina that contact photoreceptors. Science. 1982;216:1134–1136. doi: 10.1126/science.6177044. [DOI] [PubMed] [Google Scholar]
- Masland RH. Neuronal diversity in the retina. Curr Opin Neurobiol. 2001;11(4):431–436. doi: 10.1016/s0959-4388(00)00230-0. [DOI] [PubMed] [Google Scholar]
- McGuire B, et al. Microcircuitry of bipolar cells in cat retina. J Neurosci. 1984;4(12):2920–2938. doi: 10.1523/JNEUROSCI.04-12-02920.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar A, et al. Crossover inhibition in the retina: circuitry that compensates for nonlinear rectifying synaptic transmission. J Comput Neurosci. 2009;27:569–590. doi: 10.1007/s10827-009-0170-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar A, Werblin F. Inhibitory feedback shapes bipolar cell responses in the rabbit retina. J Neurophysiol. 2007;98(6):3423–3435. doi: 10.1152/jn.00838.2007. [DOI] [PubMed] [Google Scholar]
- Ostergaard J, et al. Synaptic contact between melanopsin-containing retinal ganglion cells and rod bipolar cells. Invest Ophthalmol Vis Sci. 2007;48:3812–3820. doi: 10.1167/iovs.06-1322. [DOI] [PubMed] [Google Scholar]
- Pang JJ, et al. Stratum-by-stratum projection of light response attributes by retinal bipolar cells of Ambystoma. J Physiol. 2004;558:249–262. doi: 10.1113/jphysiol.2004.063503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon y, Cajal SR. La rétine des vertébrés. Cellule. 1892;9:121–225. [Google Scholar]
- Roska B, et al. Parallel processing in retinal ganglion cells: how integration of space-time patterns of excitation and inhibition form the spiking output. J neurophysio. 2006;95:3810–3822. doi: 10.1152/jn.00113.2006. [DOI] [PubMed] [Google Scholar]
- Roska B, Werblin F. Rapid global shifts in natural scenes block spiking in specific ganglion cell types. Nature Neurosci. 2003;6:600–608. doi: 10.1038/nn1061. [DOI] [PubMed] [Google Scholar]
- Scholes J. Colour receptors, and their synaptic connexions, in the retina of a cyprinid fish. Philos Trans R Soc Lond B Biol Sci. 1975;270(902):61–118. doi: 10.1098/rstb.1975.0004. [DOI] [PubMed] [Google Scholar]
- Scholes J, Morris J. Receptor—bipolar connectivity patterns in fish retina. Nature. 1973;241(5384):52–54. doi: 10.1038/241052a0. [DOI] [PubMed] [Google Scholar]
- Sherry DM, Yazulla S. Goldfish bipolar cells and axon terminal patterns: a Golgi study. J Comp Neurol. 1993;329(2):188–200. doi: 10.1002/cne.903290204. [DOI] [PubMed] [Google Scholar]
- Strettoi E, et al. Synaptic connections of rod bipolar cells in the inner plexiform layer of the rabbit retina. J Comp Neurol. 1990;295(3):449–466. doi: 10.1002/cne.902950309. [DOI] [PubMed] [Google Scholar]
- Tian N. Synaptic activity, visual experience and the maturation of retinal synaptic circuitry. J Physiol. 2008;586(18):4347–4355. doi: 10.1113/jphysiol.2008.159202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto Y, et al. Microcircuits for night vision in mouse retina. J Neurosci. 2001;21:8616–8623. doi: 10.1523/JNEUROSCI.21-21-08616.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umino O, et al. The network properties of bipolar-bipolar cell coupling in te retina of teleost fishes. Vis Neurosci. 1994;11(3):533–548. doi: 10.1017/s0952523800002443. [DOI] [PubMed] [Google Scholar]
- Wagner HJ, Wagner E. Amacrine cells in the retina of a teleost fish, the roach (Rutilus rutilus): a Gogi study on diferentiation and layering. Philos Trans R Soc Lond B Biol Sci. 1988;321(1206):263–324. doi: 10.1098/rstb.1988.0094. [DOI] [PubMed] [Google Scholar]
- Wässle H, et al. Cone contacts, mosaics, and territories of bipolar cells in the mouse retina. J Neurosci. 2009;29(1):106–117. doi: 10.1523/JNEUROSCI.4442-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wässle H, et al. The rod bipolar cell of the mammalian retina. Visual Neuroscience. 1991;7(1-2):99–112. doi: 10.1017/s095252380001097x. [DOI] [PubMed] [Google Scholar]
- Werblin F. Six different roles for crossover inhibition in the retina: Correcting the nonlinearities of synaptic transmission. Vis Neurosci. 2010;27:1–8. doi: 10.1017/S0952523810000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin F, Dowling JE. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969;32(3):339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]
- Wong KY, Dowling JE. Retinal bipolar cell input mechanisms in giant danio. III. ON-OFF bipolar cells and their color-opponent mechanisms. J Neurophysiol. 2005;94:265–272. doi: 10.1152/jn.00271.2004. [DOI] [PubMed] [Google Scholar]
- Wong KY, et al. Synaptic influences on rat ganglion-cell photoreceptors. J Physiol. 2007;582:279–296. doi: 10.1113/jphysiol.2007.133751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, et al. Confocal analysis of reciprocal feedback at rod bipolar terminals in the rabbit retina. J Neurosci. 2002;22(24):10871–10882. doi: 10.1523/JNEUROSCI.22-24-10871.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


