Abstract
Direct evidence that Escherichia coli Shiga toxin (Stx) acts against bovine leukemia virus (BLV)-expressing cells was obtained. The active A subunit of Stx type 1 (StxA1) targeted a selected population of permeable cells expressing BLV and inhibited BLV replication in a culture of bovine peripheral blood mononuclear cells. Cells were cultured with and without StxA1, and at various times cells expressing BLV were identified by being stained with MW1 monoclonal antibody specific for the BLV protein gp51. Before culture, permeable cells were tagged by uptake of one of the following: acetoxymethyl of 2′,7′-bis-(2-carboxyethyl)-5-(and 6)-carboxyfluorescein (BCECF), BCECF conjugated to 70-kDa dextran, or 70-kDa dextran conjugated to fluorescein. The tagged cells costaining with anti-gp51 were selectively eliminated in StxA1-treated cultures. Electron microscopy analysis of purified B lymphocytes showed sharply reduced numbers of BLV particles in StxA1-treated cultures.
Shiga toxin (Stx)-producing Escherichia coli (STEC) strains are prevalent in the gastrointestinal flora of healthy domesticated and wild ruminant species worldwide, with herd prevalence approaching 100% and very high incidences within herds (2, 3, 5, 13). In humans, certain serotypes of STEC are pathogenic and may cause hemorrhagic colitis and life-threatening sequelae (11). Stx type 1 (Stx1) and type 2 (Stx2), produced by STEC, belong to a large family of ribosome-inactivating proteins found in many plants and in some bacteria (16). These proteins exhibit antiviral activities against plant and animal viruses (1, 7, 10, 18). The virally infected cells sensitive to ribosome-inactivating proteins are thought to internalize the toxins through virus-induced permeable cell membranes (1, 4, 8, 19). Because such intoxication of virally infected cells is independent of endocytosis and does not involve expression of toxin receptors, antiviral effects may be exerted by enzymatic portions of toxins alone. We proposed that Stxs produced by bovine STEC, part of the normal flora of bovines, have antiviral activity in cattle and that this activity may reduce the severity of bovine viral infections, such as those with bovine leukemia virus (BLV), or delay the onset of an acute viral disease (7).
Previously, we showed that Stx1 holotoxin and the enzymatic subunit A of Stx1 (StxA1) were equally effective in suppressing BLV-induced spontaneous lymphocyte proliferation (SLP) when added within the first 12 h of culture of bovine peripheral blood mononuclear cells (PBMC) from BLV-positive cattle (7). The enzymatic activity of StxA1 was required for this effect (1). We also showed that the antiviral effect was completely independent of receptor-mediated endocytosis and hypothesized that StxA1 suppresses SLP by acting on rare, highly permeable cells expressing BLV proteins ex vivo, preventing these cells from producing sufficient amounts of BLV particles upon culture to induce SLP (1). Our previous attempt to detect the presence of StxA1 in the permeable cells by dual staining and by using radiolabeled toxin was unsuccessful, most likely because ex vivo very few PBMC from BLV-positive cattle are permeable to macromolecules, whereas while more cells become permeable upon expressing BLV in culture, fully enzymatically active StxA1 can kill intoxicated cells at extremely low intracellular concentrations, and cells may not accumulate detectable amounts of the toxin (1). The results presented here give direct evidence that the permeable cells expressing BLV ex vivo were eliminated from PBMC cultures treated with StxA1 and that BLV replication was inhibited in these cultures. We used electron microscopy (EM) to assess viral replication and flow cytometry to monitor the fate of BLV-expressing cells after toxin exposure.
BLV is an oncogenic retrovirus responsible for the enzootic form of bovine lymphosarcoma (9). In cattle, BLV infection may be asymptomatic for 1 to 7 years and then progress to persistent lymphocytosis (PL), which is characterized by a neoplasia of B lymphocytes (6). Although as many as 70% of the B lymphocytes in an infected animal can contain an integrated provirus, BLV expression is rare and detected in <2% of the PBMC (15). B lymphocytes from PL cattle are in three categories: BLV-negative cells, BLV-positive cells that contain provirus but do not express BLV proteins, and cells in which BLV is replicating. The last cells express the BLV protein gp51 (17), which is present in their cell membranes, and so these cells can be identified by staining with the monoclonal antibody MW1, specific for the D epitope of gp51 (12). The BLV genome is generally repressed in vivo, but removal of PBMC from immune plasma and placement in culture precipitate a derepression and conversion of cells containing provirus to cells expressing gp51. Once cells are in culture, a dynamic situation of cells transitioning from provirus-positive to virus-expressing status occurs. This continuous change in B-lymphocyte viral status prevented the methods in our previous work from demonstrating direct evidence for Stx antiviral activity by measuring changes in total gp51 expression. Here we used novel reagents to tag permeable cells ex vivo, before culture, and monitored the fate of tagged, virus-expressing cells over a 24-h period of culture with or without StxA1. We also used EM to look specifically at the effect of toxin on BLV.
PBMC from PL cattle were prepared as described before (7), by density centrifugation on Accu-Paque (1.086 g/ml; Accurate Chemical and Scientific Corp., Westbury, N.Y.), several washes in 4:1 phosphate-buffered saline (PBS)-acid citrate dextrose (ACD), and suspension in PBS with 0.5% (wt/vol) bovine serum albumin. The highly permeable cells were tagged by passive absorption of acetoxymethyl of 2′,7′-bis-(2-carboxyethyl)-5-(and 6)-carboxyfluorescein (BCECF), BCECF conjugated to 70-kDa dextran (BCECF-dextran), or 70-kDa dextran conjugated to fluorescein (70kDa-Fl) (Molecular Probes, Eugene, Oreg.). We have shown that BLV-expressing B cells are highly permeable to macromolecules up to 500 kDa and that 70-kDa molecules serve best to discriminate permeable cells (1) (data not shown). The 70-kDa dextran was selected for this study because of a molecular mass similar to that of Stx1 holotoxin (70,661 Da). PBMC were incubated on ice for 1 h in PBS with 0.5% bovine serum albumin containing one of the following compounds: acetoxymethyl BCECF (1.0 μM), BCECF-dextran (0.5 mg/ml), and 70kDa-Fl (0.5 mg/ml). After tagging, the cells were washed twice with PBS-ACD and resuspended in Dulbecco modified essential medium with 20% fetal bovine serum, at pH 7.4. Three milliliters of PBMC suspension (4.0 × 106 cells/ml) was placed in loosely capped, inclined 15-ml conical tubes and incubated in 6.5% CO2 at 37°C for 24 h without toxin or with 1.0 μg of StxA1/ml. The 24-h incubation provided the most consistent results compared to 6-, 12-, 48-, and 72-h incubations (data not shown). The cells were harvested by adding 12 ml of cold PBS-ACD containing 1 mM EDTA, centrifuging the mixture at 220 × g for 8 min, and washing the cells in PBS-ACD-EDTA. After harvest, cells expressing virus were identified by staining with MW1, a monoclonal antibody specific for BLV protein gp51 (WSU Monoclonal Antibody Center, Pullman, Wash.), followed by anti-mouse immunoglobulin G1 (IgG1) polyclonal antibody conjugated to Tri-Color (Caltag Laboratories, Burlingame, Calif.). Flow cytometry data were acquired for 100,000 cells per sample, by using a FACSCalibur flow cytometer equipped with a 488-nm argon laser (BD Biosciences, San Jose, Calif.).
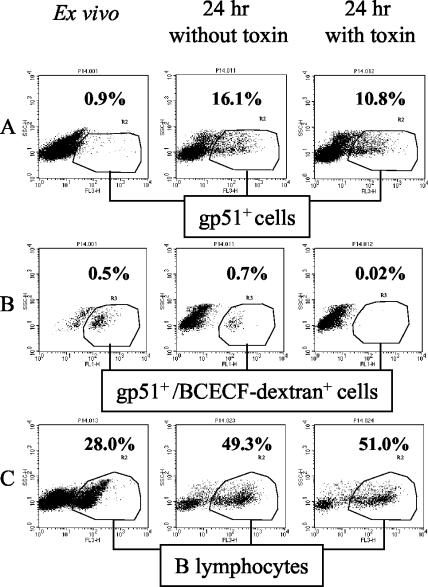
The percentage of cells expressing the BLV protein gp51 was low ex vivo and increased after 24 h of culture (Fig. 1A). This increase most likely resulted from a conversion of gp51-negative cells containing provirus to gp51-positive cells containing replicating virus. Measurements of the direct impact of StxA1 treatment on expression of gp51 gave highly variable results in experiments performed with the same or different donors. In some experiments there was no measurable reduction, although percentages of gp51-expressing cells were usually reduced, by 6 to 40% (data not shown). Occasionally, gp51 expression could not be quantitated because of a continuous (smeared) distribution of cells along the axis of red fluorescence. Thus, simple measurement of gp51 percentage expression could not be relied upon as a consistent assay of antiviral activity of StxA1. It is possible that the viral particles, released from the cells killed or damaged by toxin, were binding to BLV receptors on gp51-negative B cells (i.e., cells not expressing BLV), making these cells appear gp51 positive on flow cytometric dot plots. Accordingly, we assessed StxA1 impact on the rare permeable cells expressing gp51 ex vivo by measuring MW1 binding (red fluorescence) in conjunction with green fluorescence resulting from preloading the ex vivo cells prior to culture with one of the reagents passively absorbed by permeable cells. This method enabled us to determine the fate of the permeable cells independently of the overall changes in gp51 expression. In experiments performed with PBMC from four different cattle and using unconjugated BCECF or 70kDa-Fl dextran, StxA1 treatment consistently reduced the percentage of preloaded cells positive for gp51 (double fluorescing) by 32 to 73% (data not shown), showing that the ex vivo BLV-expressing and permeable cells were being eliminated by toxin treatment within 24 h of culture. The fact that some cells permeable to BCECF or to 70-kDa dextran survived exposure to StxA1 indicates that the cells permeable to these compounds were not uniformly sensitive to toxin. However, when 70-kDa dextran-BCECF conjugate (a more discriminating label) was used to tag the permeable cells, the double-fluorescing cells (permeable and gp51 positive) were completely eliminated from culture by toxin treatment. The overall percentage of gp51-positive cells, which includes the cells newly expressing gp51 in vitro that were not permeable ex vivo (not tagged), was reduced by 33%, showing that as cells transition from carrying provirus to expressing BLV they become sensitive to toxin (Fig. 1B). Taken together, these results indicate that the cells expressing gp51 and permeable ex vivo were selectively eliminated by StxA1 within 24 h of culture. The treatment with StxA1 did not affect the overall cell composition. Thus, the percentages of viable B lymphocytes (identified by forward and side scatter characteristics) were similar in cultures treated and not treated with StxA1 (Fig. 1C).
FIG. 1.
Impact of StxA1 on permeable cells expressing BLV. Permeable cells were tagged prior to culture with BCECF-dextran (green fluorescence, Fl-1). At 0 and 24 h after culture with and without StxA1 cells were labeled with monoclonal antibodies conjugated to Tri-Color (red fluorescence, Fl-3), and analyzed by flow cytometry. BLV-expressing cells were labeled with MW1 antibody specific for BLV protein gp51. B lymphocytes were labeled with GB25A antibody, specific for bovine CD21-like antigen. The cells considered positive for green or red fluorescence are circled. (A) Percentages of gp51-positive cells among PBMC (only 10 to 25% of total cells are shown for clarity). (B) BCECF-dextran-associated green fluorescence of these cells, gated as shown in panel A. (C) Percentages of B lymphocytes in total cells.
Because BLV primarily infects B lymphocytes (6), the impact of StxA1 treatment on BLV replication in cultures of PBMC was assessed by EM analysis of B lymphocytes. PBMC were cultured as described above, and at 0 and 24 h cells were centrifuged, washed with PBS-ACD-EDTA, resuspended in the same, and centrifuged in a density gradient by layering the cell suspension on Accu-Paque (1.086 g/liter) to remove the nonviable cells. B lymphocytes were purified by being stained with a mixture of murine monoclonal IgM antibodies BAS9A and BAQ44A specific for the bovine B-lymphocyte markers B-B1 and B-B2, respectively (WSU Monoclonal Antibody Center), and separated by positive selection on Midi-MACS Miltenyi columns with Miltenyi microbeads coated with polyclonal rat anti-mouse IgM (Miltenyi Biotec, Auburn, Calif.) in accordance with the manufacturer's instructions. Purified B lymphocytes were stained in succession with MW1 (an IgG1 antibody), polyclonal goat anti-mouse γ chain (Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.), and rabbit F(ab′) anti-goat IgG conjugated to 1.4-nm gold particles (Molecular Probes). The cell plugs were fixed overnight at 4°C in 2.5% glutaraldehyde solution with 2% paraformaldehyde in 0.1 M cacodylate buffer with 0.2 M sucrose, then treated with an LI Silver enhancement kit (Molecular Probes) per the manufacturer's instructions, and embedded in LR White resin by a routine method (14). Sections were analyzed using a JEOL-JEM 1200EX electron microscope (JEOL USA Inc., Peabody, Mass.).
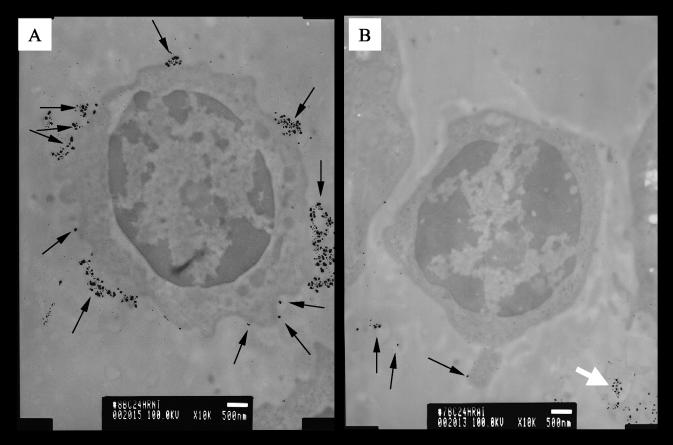
BLV particles present on cell surfaces were easily identified as large silver grains (Fig. 2). Only rare cells could be identified ex vivo as BLV positive (data not shown). However, 24 h postculture most of the B lymphocytes cultured without toxin had >100 BLV particles on the cell membrane (Fig. 2A), whereas B lymphocytes from StxA1-treated cultures had <10 BLV particles (Fig. 2B and Table 1). Rarely, large numbers of silver grains were observed among cells treated with toxin, but they were diffuse and appeared not to be associated with cells, so they may have represented the remnants of virus-expressing cells (Fig. 2B). These diffuse aggregates were not observed among cells cultured without toxin. Differential cell counts showed no B lymphocytes with high numbers of BLV particles in cultures with toxin (Table 1). In addition, the numbers of BLV particles directly observed by EM were reduced in cultures with toxin (data not shown). Importantly, the data presented in Table 1 show suppression of BLV replication in B cells that are still viable, as indicated by passage through density centrifugation and forward/side scatter ratios (data not shown). The numbers of disintegrated cells are similar in toxin-treated and untreated cultures because only the rare cells expressing BLV ex vivo appear to be eliminated within the first 24 h by StxA1 treatment.
FIG. 2.
Impact of StxA1 on B lymphocytes expressing BLV. BLV particles associated with purified B lymphocytes were identified by gold staining for the BLV protein gp51, followed by silver enhancement. Only external, cell-surface-associated gp51 was detected. Arrows indicate individual viral particles or aggregates of viral particles, identified by large silver grains. Bars, 0.5 μm. (A) Typical B lymphocyte after 24 h of culture without toxin, having copious aggregates of large silver grains. (B) Typical B lymphocyte after 24 h of culture with StxA1, having sparse silver grains. Among B cells incubated with toxin, diffused aggregates of silver grains were occasionally observed not associated with the cell surface, and this pattern is indicated by a white arrow at the lower right corner of panel B.
TABLE 1.
Inhibition of BLV replication in bovine PBMC culture by StxA1
| Treatment | No. of cellsa containing:
|
No. of disintegrated cells | ||
|---|---|---|---|---|
| >100 grainsb | 10-50 grains | <10 grains | ||
| No toxin | 68 | 14 | 2 | 16 |
| StxA1c,d | 0 | 1 | 85 | 14 |
Cells were harvested after 24 h of culture, and a total of 100 cells were counted in a sample of purified B lymphocytes.
Silver grains were >30 nm in diameter.
A 1.0-μg/ml amount of toxin was added at the start of culture.
Distribution of cell counts across categories was significantly different between two treatments (chi-square statistic = 158.6, df = 3, P < 0.001).
These findings give direct evidence that permeable cells expressing BLV ex vivo are selectively eliminated from cultures treated with StxA1 and that the toxin suppresses BLV replication in B cells. Experiments to test the antiviral effects of Stx in vivo in a BLV infection model are planned.
Acknowledgments
This work was supported, in part, by the Idaho Agriculture Experiment Station, U.S. Department of Agriculture NRICGP grant 99-35201-8539, and Public Health Service grants NO1-HD-0-3309 and P20-RR-15587 from the National Institutes of Health.
We thank C. Davitt for preparing sections for EM and Edward Wagner for help in obtaining blood samples from BLV-infected cows.
Editor: A. D. O'Brien
REFERENCES
- 1.Basu, I., W. A. Ferens, D. M. Stone, and C. J. Hovde. 2003. Antiviral activity of Shiga toxin requires enzymatic activity and is associated with increased permeability of the target cells. Infect. Immun. 71:327-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertin, Y., K. Boukhors, N. Pradel, V. Livrelli, and C. Martin. 2001. Stx2 subtyping of Shiga toxin-producing Escherichia coli isolated from cattle in France: detection of a new Stx2 subtype and correlation with additional virulence factors. J. Clin. Microbiol. 39:3060-3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beutin, L., D. Geier, H. Steinruck, S. Zimmermann, and F. Scheutz. 1993. Prevalence and some properties of verotoxin (Shiga-like toxin)-producing Escherichia coli in seven different species of healthy domestic animals. J. Clin. Microbiol. 31:2483-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carrasco, L. 1995. Modification of membrane permeability by animal viruses. Adv. Virus Res. 45:61-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cobbold, R., and P. Desmarchelier. 2000. A longitudinal study of Shiga-toxigenic Escherichia coli (STEC) prevalence in three Australian dairy herds. Vet. Microbiol. 71:125-137. [DOI] [PubMed] [Google Scholar]
- 6.Esteban, E. N., R. M. Thorn, and J. F. Ferrer. 1985. Characterization of the blood lymphocyte population in cattle infected with the bovine leukemia virus. Cancer Res. 45:3225-3230. [PubMed] [Google Scholar]
- 7.Ferens, W. A., and C. J. Hovde. 2000. Antiviral activity of Shiga toxin 1: suppression of bovine leukemia virus-related spontaneous lymphocyte proliferation. Infect. Immun. 68:4462-4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez-Puentes, C. 1984. Permeability to inhibitors of protein synthesis in virus infected cells. Mol. Biol. Rep. 10:65-68. [DOI] [PubMed] [Google Scholar]
- 9.Ferrer, J. F. 1980. Bovine lymphosarcoma. Adv. Vet. Sci. Comp. Med. 24:1-68. [PubMed] [Google Scholar]
- 10.Girbes, T., J. M. Ferreras, R. Iglesias, L. Citores, C. De Torre, M. L. Carbajales, P. Jimenez, F. M. De Benito, and R. Munoz. 1996. Recent advances in the uses and applications of ribosome-inactivating proteins from plants. Cell. Mol. Biol. (Noisy-Le-Grand) 42:461-471. [PubMed] [Google Scholar]
- 11.Griffin, P. M., and R. V. Tauxe. 1991. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol. Rev. 13:60-98. [DOI] [PubMed] [Google Scholar]
- 12.Jensen, W. A., S. E. Sheehy, M. H. Fox, W. C. Davis, and G. L. Cockerell. 1990. In vitro expression of bovine leukemia virus in isolated B-lymphocytes of cattle and sheep. Vet. Immunol. Immunopathol. 26:333-342. [DOI] [PubMed] [Google Scholar]
- 13.Khan, A., S. Yamasaki, T. Sato, T. Ramamurthy, A. Pal, S. Datta, N. R. Chowdhury, S. C. Das, A. Sikdar, T. Tsukamoto, S. K. Bhattacharya, Y. Takeda, and G. B. Nair. 2002. Prevalence and genetic profiling of virulence determinants of non-O157 Shiga toxin-producing Escherichia coli isolated from cattle, beef, and humans, Calcutta, India. Emerg. Infect. Dis. 8:54-62. [PubMed] [Google Scholar]
- 14.Lah, J. J., D. M. Hayes, and R. W. Burry. 1990. A neutral pH silver development method for the visualization of 1-nanometer gold particles in pre-embedding electron microscopic immunocytochemistry. J. Histochem. Cytochem. 38:503-508. [DOI] [PubMed] [Google Scholar]
- 15.Mirsky, M. L., C. A. Olmstead, Y. Da, and H. A. Lewin. 1996. The prevalence of proviral bovine leukemia virus in peripheral blood mononuclear cells at two subclinical stages of infection. J. Virol. 70:2178-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stirpe, F., L. Barbieri, M. G. Battelli, M. Soria, and D. A. Lappi. 1992. Ribosome-inactivating proteins from plants: present status and future prospects. Bio/Technology 10:405-412. [DOI] [PubMed] [Google Scholar]
- 17.Stone, D. M., L. K. Norton, and W. C. Davis. 2000. Spontaneously proliferating lymphocytes from bovine leukaemia virus-infected, lymphocytotic cattle are not the virus-expressing lymphocytes, as these cells are delayed in G0/G1 of the cell cycle and are spared from apoptosis. J. Gen. Virol. 81:971-981. [DOI] [PubMed] [Google Scholar]
- 18.Wang, P., and N. E. Tumer. 2000. Virus resistance mediated by ribosome inactivating proteins. Adv. Virus Res. 55:325-355. [DOI] [PubMed] [Google Scholar]
- 19.Yamaizumi, M., T. Uchida, and Y. Okada. 1979. Macromolecules can penetrate the host cell membrane during the early period of incubation with HVJ (Sendai virus). Virology 95:218-221. [DOI] [PubMed] [Google Scholar]