Abstract
Herein, we report a systematic study of the Larock indole annulation designed to explore the scope and define the generality of its use in macrocyclization reactions, its use in directly accessing the chloropeptin I versus II DEF ring system as well as key unnatural isomers, its utility for both peptide-derived and more conventional carbon-chain based macrocycles, and its extension to intramolecular cyclizations with formation of common ring sizes. The studies define a powerful method complementary to the Stille or Suzuki cross-coupling reactions for the synthesis of cyclic or macrocyclic ring systems containing an embedded indole, tolerating numerous functional groups and incorporating various (up to 28-membered) ring sizes. As a result of the efforts to expand the usefulness and scope of the reaction, we also disclose a catalytic variant of the reaction along with a powerful Pd2(dba)3 derived catalyst system, and an examination of the factors impacting reactivity and catalysis.
Introduction
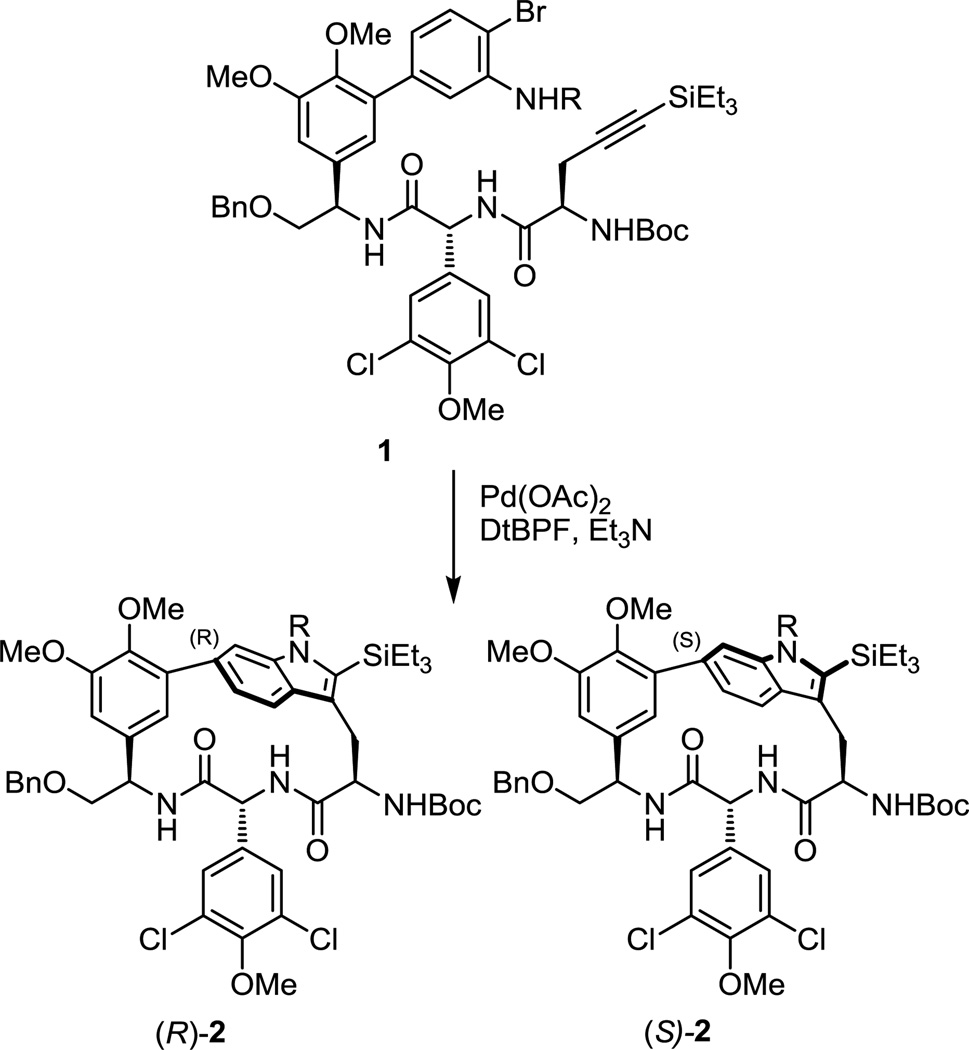
Embedded macrocyclic indoles and their oxidized variants are a recurring structural motif present in many natural products.1 To the best of our knowledge, no one had previously reported the use of a Pd(0)-mediated Larock-indole annulation reaction2 to furnish both the macrocycle and the indole in a single transformation. Confronted with the challenges posed by the natural products chloropeptin I and II,1b–j structures rivaling those of the glycopeptides antibiotics3 in complexity, our initial attempts to achieve cyclization of the macrocycle containing the tryptophan-derived moiety were unsuccessful using a range of cyclization strategies including free radical cyclization reactions4 and peptide coupling reactions.5 As a result, we examined modifications of a Larock annulation protocol permitting the use of aryl bromides originally developed by Farina6 and co-workers to achieve the key macrocyclization concurrent with introduction of the indole en route to a total synthesis of these natural products.5 This key reaction provided the fully functionalized DEF ring system of chloropeptin II in superb conversion (89%) and good atropdiastereoselectivity (4:1 R:S, R = Ac, Figure 1). Moreover, we found that increasing the size of the substrate aniline amide improved the modest atropdiastereoselectivity, providing the macrocycle 2 in good yield (70%) with complete control of the atropselectivity (>20:1 R:S, R = benzoyl, Figure 1). The use of further modified reaction conditions, enlisting a soluble tertiary amine base (vs NaHCO3) avoided competitive de-chlorination and epimerization. The use of nonpolar aprotic solvents increased both the rate and conversions of the macrocyclization, permitting the use of lower reaction temperatures (110 °C), and the incorporation of a large removable terminal alkyne substituent (–SiEt3) as first disclosed by Larock2,7 was used to control the indole cyclization regioselectivity. These studies represented the first reported examples of what we believe to be a powerful macrocyclization strategy rivaling the more conventional Stille8 and Suzuki9 cross-coupling reactions.
Figure 1.
First generation Pd(0)-mediated macrocyclization.
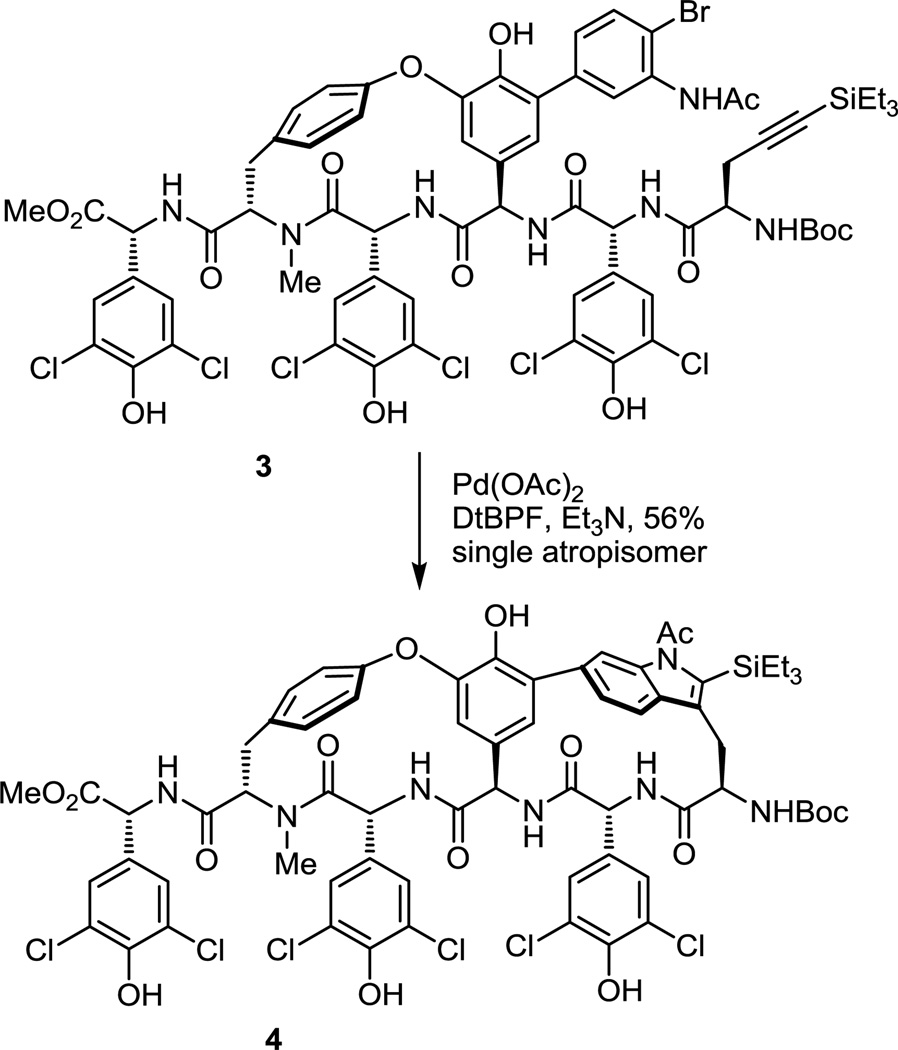
In a second generation approach to the chloropeptins in which the western macrocycle was already in place, the key macrocyclization was conducted with substrate 3, containing four phenols, five secondary amides, a carbamate, and six labile aryl chlorides, and provided the product 4 (56%) exclusively as a single atropisomer (>20:1, detection limits) possessing the natural (R)-configuration (Figure 2).5a In this instance, the increased complexity of the substrate and the reverse macrocyclization order did not diminish the atropdiastereoselectivity. Rather, it provided an improvement over the 4:1 selectivity that was observed with the analogous N-acetyl substrate 2 and the reaction exhibited superb functional group tolerance. Deliberate in the approach and by virtue of a single-step acid-catalyzed conversion of chloropeptin II to chloropeptin I,10 only cyclization to provide the more strained and challenging indole C6 versus C7-linked biaryl DEF ring system found in chloropeptin II was examined. Herein, we report a systematic study designed to explore the scope and define the generality of this new macrocyclization reaction, its use in also directly accessing the chloropeptin I versus II DEF ring system as well as key isomers, its utility for both peptide-derived and more conventional carbon chain-based macrocycles, its extension to intramolecular cyclizations with formation of more common ring sizes, and the factors impacting reactivity and catalysis.
Figure 2.
Second generation macrocyclization.
Results and Discussion
Macrocyclic Peptides
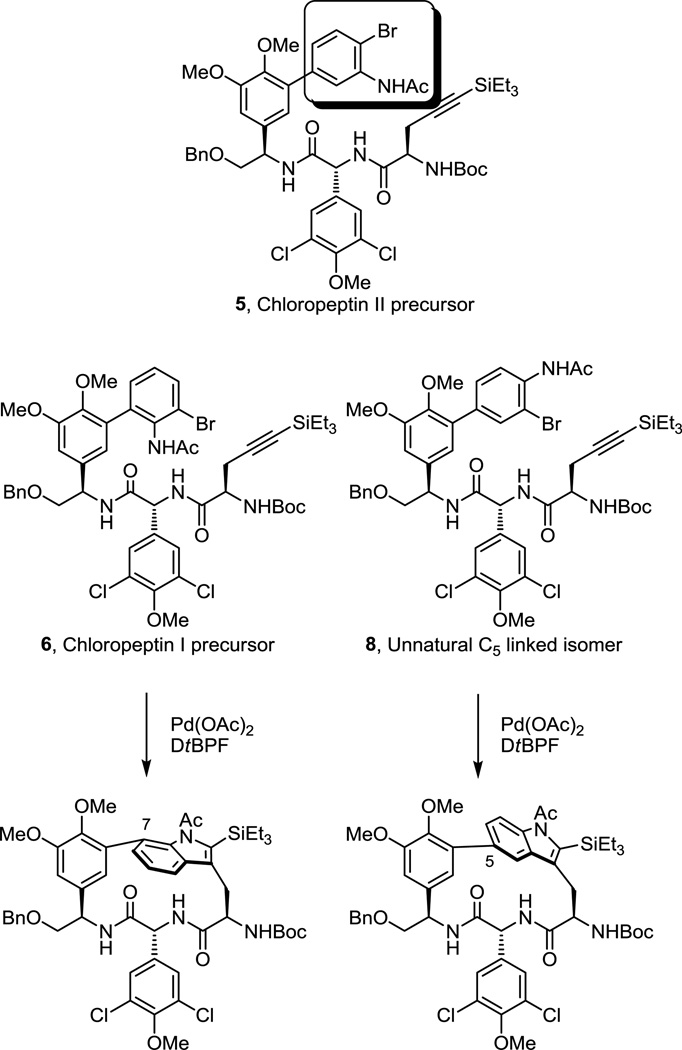
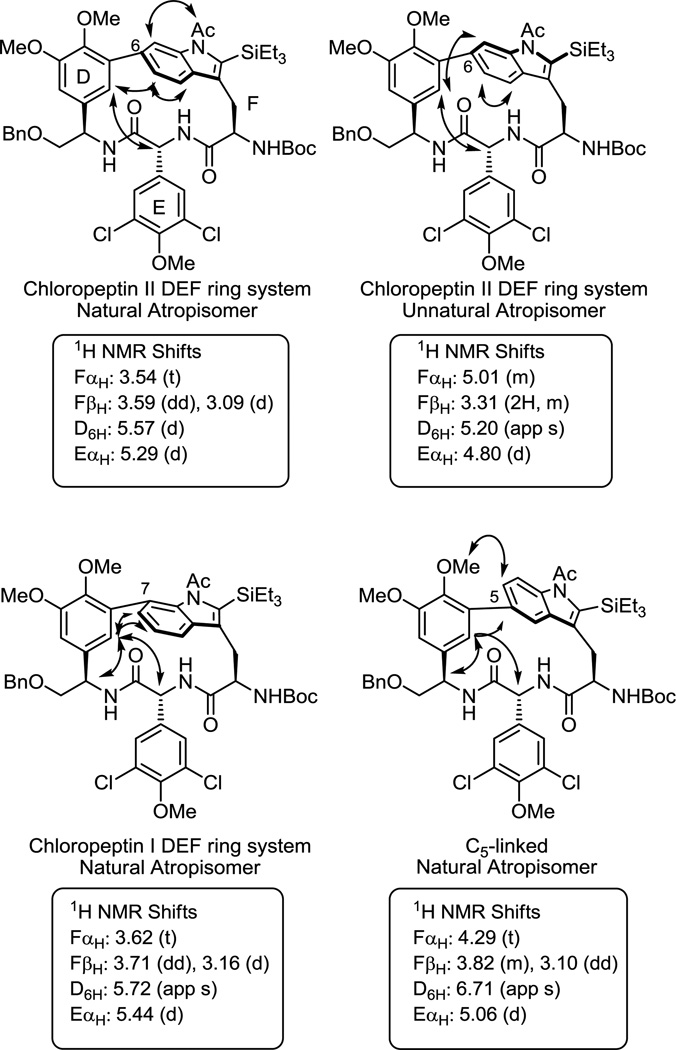
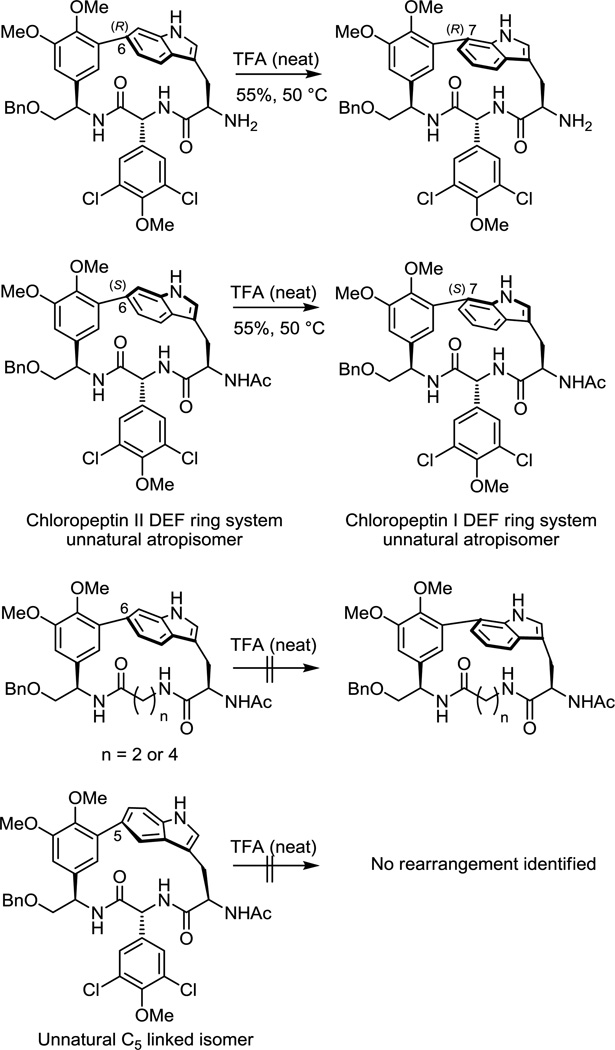
Chief among our initial studies was the extension of the cyclization reaction to the direct preparation of the indole C7-linked DEF ring system of chloropeptin I and its possible use in accessing the unnatural C5-linked isomer. Although previous work demonstrated that the 16–17-membered macrocycle present in chloropeptin II (complestatin) rearranges to the less strained DEF ring system of chloropeptin I upon exposure to acid providing access to both natural products by a single route,5 we were especially interested in establishing whether the macrocyclization method could be extended to the direct construction of the chloropeptin I DEF ring system. Modification of the aniline component of the substrate introduced by way of a Suzuki-Miyaura11 coupling with use of 2-bromo-6-iodoaniline would be expected to furnish the chloropeptin I ring system directly following macrocyclization. Thus, the requisite precursor 6 was prepared following the approach developed for the synthesis of 1 with the exception that 2-bromo-6-iodoaniline versus 2-bromo-5-iodoaniline was used in the early stage Suzuki-Miyaura coupling (Supporting Information). Exposure of the precursor 6 to the reaction conditions used in the first generation synthesis of 2 (Pd(OAc)2, DtBPF, Et3N in toluene:acetonitrile, 0.002 M, 110 °C, 1 h) provided the desired macrocycle 7 in good yield (66%). Much to our satisfaction, only a single atropisomer corresponding to the natural atropisomer configuration was observed (>20:1, detection limits) (Figure 3). Inspection of the chemical shifts of key 1H NMR signals12,13 of 7 indicate that the indole is in the same spatial orientation as that found in the natural product. The deshielded chemical shifts of D6H and EαH and especially the pronounced shielded chemical shift FαH are diagnostic of the natural (R)-atropisomer stereochemistry (Figure 4). Similarly, the distinct chemical shifts and diagnostic coupling of the two hydrogens of FβH of 7 are characteristic of the natural (R)-atropisomer. This stereochemistry was further established by observation of diagnostic NOEs between D6H/F5H, D6H/F6H, D6H/EαH, and D6H/DαH. Significantly, the Pd(0)-mediated macrocyclization reaction was extended to directly provide the chloropeptin I DEF ring system in good yield (66%) and with complete atropdiastereoselectivity (>20:1).
Figure 3.
Additional isomeric macrocyclization substrates.
Figure 4.
Diagnostic 1H NMR chemical shifts and NOE's for indole macrocycles (CDCl3).
The provocative suggestion from modeling studies conducted by others14 had suggested that a C5-linked indole might well account for the spectroscopic properties attributed to the natural chloropeptin II DEF ring system bearing a C6-linked indole. Although synthetic studies have confirmed the structure (C6-linked indole) and stereochemistry (R-atropisomer) of the chloropeptin II DEF ring system, the behavior and properties of this isomeric C5-linked indole macrocyclic ring system remained of interest to us. The requisite precursor 8 for macrocyclization was prepared following the approach developed for 1 enlisting 2-bromo-4-iodoaniline in the early stage Suzuki-Miyaura coupling reaction (Supporting Information). Exposure of this substrate to the macrocyclization conditions furnished the new C5-linked macrocycle 9 as a single atropisomer in excellent yield (71%) without reoptimization of the reaction parameters (Figure 3). Importantly, the chemical shifts of the key diagnostic hydrogens of 9 are significantly different and easily distinguishable from those of either atropisomer of those 2 and 6. Comprehensive NMR studies, including COSY and NOE, confirmed the structural and atropisomer stereochemical assignment of 9, adopting a “natural” (R)-configuration for this presently unnatural C5-linkage (Figure 4).
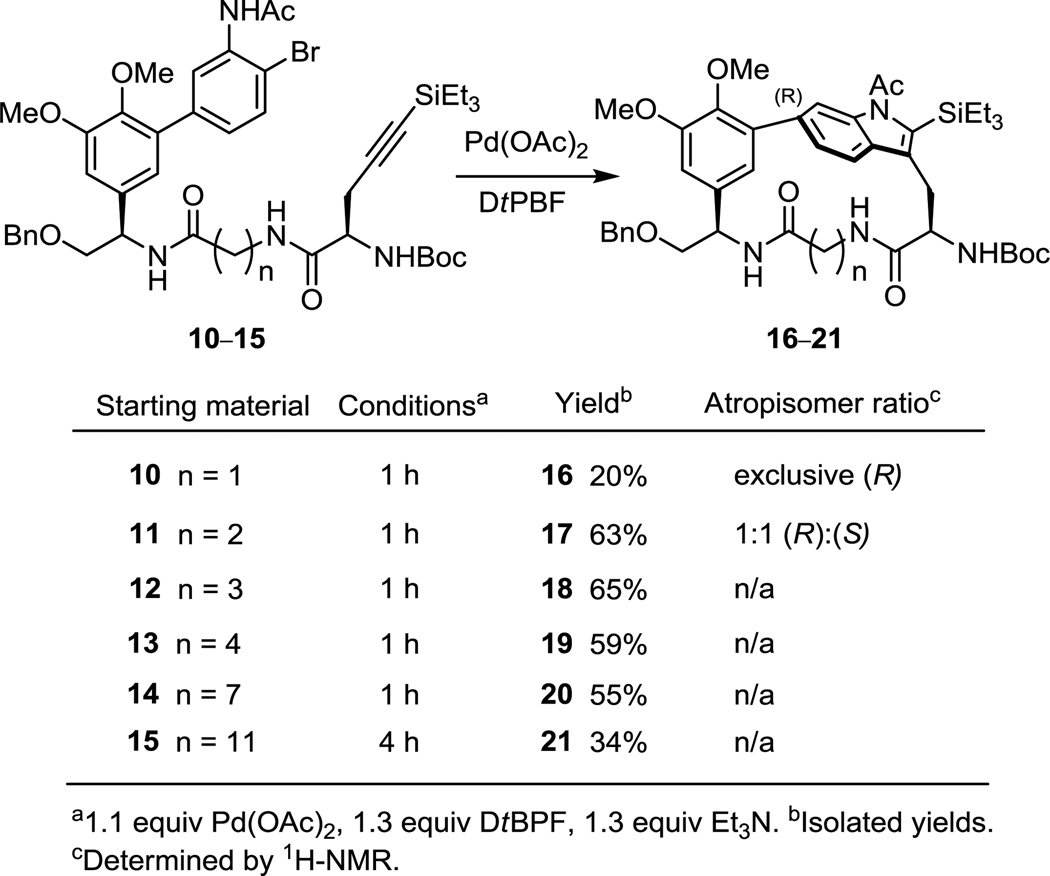
In addition to probing the site of biaryl linkage to the indole, the examination of a series of systematically altered peptide-derived substrates further revealed the generality of the observations, established the scope of ring sizes accessible on complex substrates, shed further light on the structural features contributing to the success of the macrocyclization of 2, and allowed us to probe the impact of ring size on the unique chemical and conformational properties of the chloropeptin DEF ring systems. Simplification of the peptide backbone with incorporation of commercially available, unsubstituted amino acids for the chlorinated arylglycine E-subunit provided ample opportunity for this exploration. In particular, and by increasing the backbone by a single methylene unit at a time, the resulting modified skeletons allowed us to probe the presence and interconversion of atropisomers as well as their propensity for participation in the unusual acid-catalyzed ring expansion rearrangement responsible for the direct conversion of chloropeptin II to chloropeptin I. The synthesis of the cyclization substrates 10–15 are provided in the Supporting Information and the results of their examination are summarized in Figure 5. Most notably, when glycine was incorporated in place of the chlorinated arylglycine E subunit, the macrocyclization yield providing 16 (n = 1) was substantially reduced, and the macrocyclic product bears only the natural (R)-configuration. Since 16 (n = 1) and 2 are macrocycles of the same ring size and indole linkage site, the reduced yield for the closure of 10 versus 1 suggests the 3,5-dichloro-4-hydroxyphenylglycine side chain of 1 may help preorganize the substrate in a compact conformation favorable to cyclization to provide 2. When the glycine unit was extended by one carbon (β-alanine), the macrocyclization yield substantially improved without additional optimization and interestingly, a 1:1 atropisomer ratio was observed. While the individual atropisomers of 17 (n = 2) were easily separated by silica gel chromatography, they were found to slowly interconvert at room temperature, and after 8 h at 50 °C, a 1:1 thermodynamic mixture was reestablished. Given that the atropisomers of the natural products chloropeptin I and II or their individual DEF ring systems do not interconvert even at temperatures as high as 180 °C,5a,12a it is remarkable that this insertion of one additional methylene unit (now a 17–18-membered vs 16–17-membered macrocycle) allows slow room temperature atropisomer interconversion. Performing the annulation on substrates with increasingly longer linker lengths of n = 3, 4 and 7 provided the macrocyclic indoles in good yields without further optimization and with no observance of atropisomers. Increasing the linker length by an additional four methylene units (n = 11) to form a 28-membered macrocycle resulted in slightly lower yield; however, the reaction only needed to be warmed for an additional 3 h, and no additional dilution was required. Notably, no intermolecular reaction was detected under any reaction conditions.
Figure 5.
Cyclic peptide macrocyclizations.
Thus, peptide macrocyclization using a Larock indole annulation proved general, rivaling the power of the more conventional Suzuki or Stille cross-coupling protocols. It is of special note that it surpassed the effectiveness of either the Suzuki or Stille coupling macrocyclizations enlisted in the synthesis of chloropeptin II or I DEF ring systems12,15 providing high yields and complete control of the atropisomer stereochemistry.5 However, and like the observations made also with the chloropeptin Stille or Suzuki macrocyclizations,12,15 effective ring closure was only observed with use of stoichiometric palladium reagent presumably due to macrocycle sequestration of the transition metal. As detailed in the next section, reactions requiring catalytic palladium reagent were found to be effective with more conventional macrocyclization substrates.
With a variety of new macrocyclic scaffolds assembled bearing the embedded macrocyclic indole, studies designed to probe the unusual acid-catalyzed ring rearrangement of chloropeptin II to chloropeptin I were conducted. Mindful that strain release in going from the 16–17-membered macrocycle in chloropeptin II to the macrocycle featured in chloropeptin I drives the reaction, systematic studies with the macrocycles bearing larger ring sizes as well as the unnatural C5-linked isomer of the chloropeptin DEF ring system were conducted. In previous work, we demonstrated that indole free NH (vs N-acetyl) is necessary for the reaction, and that the N-acetyl protecting group used during the course of the synthesis of chloropeptin II tamed the inherent reactivity (Figure 6).5 Further, the rearrangement proceeds smoothly on the isolated DEF ring system where the substrate contains the indole free NH and an unprotected D-ring C4 phenol.12a On the DEF chloropeptin II macrocyclic ring system bearing phenol methyl ethers, the requisite rearrangement precursors were prepared from the macrocyclization products by removing the indole N-acetyl and triethylsilyl groups, and protecting primary amine as its N-acetyl derivative. Both the natural and unnatural atropisomers rearrange to the corresponding ring expanded macrocycle with retention of stereochemistry further illustrating that the D-ring C4 free phenol is not required for observation of the rearrangement. With the ring expanded macrocycles derived from 17 and 19 (n = 2 and n = 4), no rearranged product was detected upon their exposure to neat TFA at 50 °C for 15 min or TFA at room temperature for 16 h, conditions known to promote the rearrangement (Figure 6). Rather, only recovered starting material was observed and eventually complex mixtures of unidentified compounds were observed upon extended exposure to the more forceful reaction conditions. These results suggest that these ring systems do not possess sufficient or the same inherent strain found in the chloropeptin II system to drive the rearrangement reaction. In the case of the unnatural C5-linked isomer, treatment with neat TFA did not affect a clean rearrangement. Only recovered starting material was observed at room temperature (16 h), and complex mixtures devoid of detectable rearranged product were observed at elevated temperatures (50 °C, 5–20 min).
Figure 6.
Acid-catalyzed rearrangement studies.
Carbon Chain-based Macrocycles
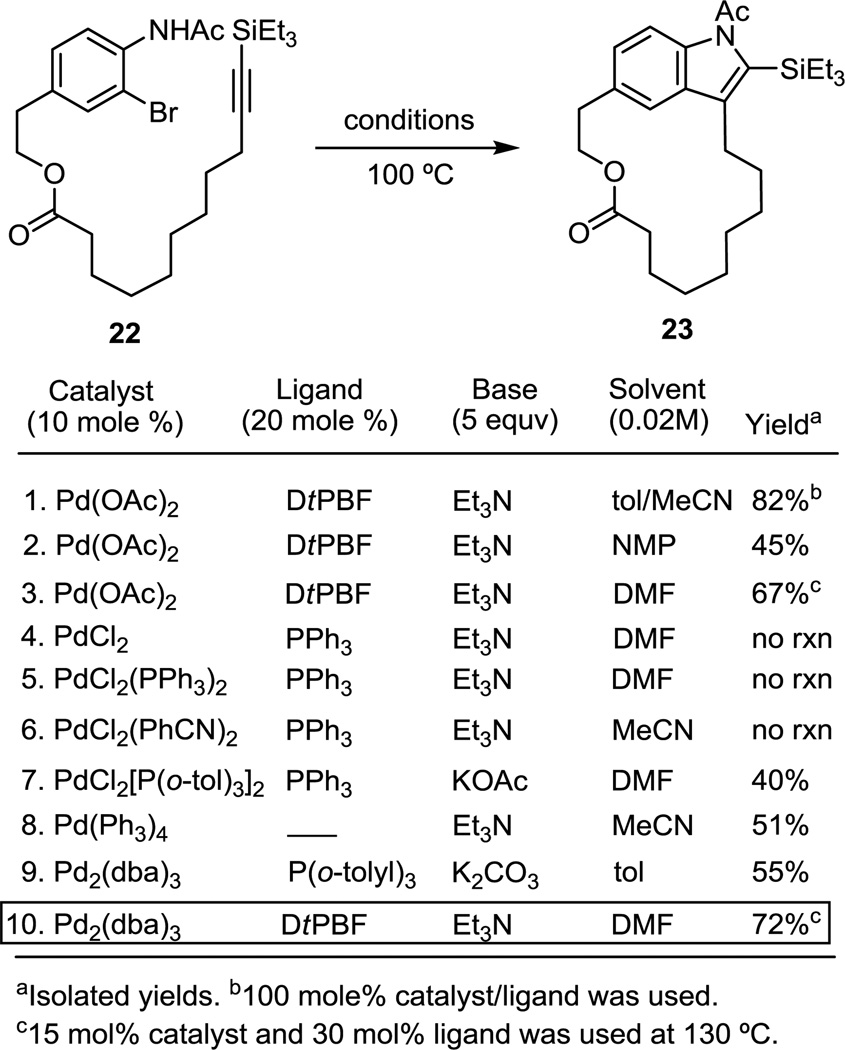
The extension of the reaction to the synthesis of macrocycles lacking the repetitive amide bonds of peptides and the development of a catalytic variant of the reaction were first explored with substrate 22. The investigations of the catalytic indole macrocyclization commenced with the catalyst system that was employed stoichiometrically in our total synthesis of the chloropeptins.5 The stoichiometric reaction provided an excellent yield (82%) of the desired macrocycle 23 under these conditions, performing even better than the peptide-derived substrates (Figure 7, entry 1). Moreover, the reaction provided good yields of the product using 10 mol % of Pd(OAc)2 (Figure 7, entry 2). Optimization efforts exploring various solvents [NMP (56%), DMSO (52%), MeCN (61%), toluene (53%), DMF (67%)], added base (vs Et3N), ligands or temperatures further improved the yield of the reaction, and indicated that macrocyclization of such substrates, even when conducted with catalytic palladium, is much less sensitive to the reaction conditions. However, recovery of the starting material without reduction of the aryl bromide prompted us to examine other catalyst systems known to facilitate Pd-catalyzed transformations.16 Additives, such as silver nitrate or silver carbonate, known to enhance the rate and yield of the cross coupling of organic halides with some organometallic reagents, simply led to the decomposition of the starting material.17 Although the substrate was inert towards many candidate palladium catalysts including PdCl2, PdCl2(PhCN)2 or PdCl2(PPh3)2, other readily accessible catalysts including PdCl2(PPh3)2, Pd(PPh3)4 and Pd2(dba)3 supported the macrocyclization reaction effectively (Figure 7). However, we were most pleased to find improved results using a Pd2(dba)3 catalyst system that was also remarkably successful in a challenging Suzuki coupling reaction in our vancomycin synthetic studies (Figure 7, entry 9), arising from its unusually rapid oxidative addition to a hindered and electron-rich aryl bromide.3c,d Further optimization of this catalyst system revealed that DtBPF was the best ligand of those examined and that DMF was the most effective solvent examined, although others were also effective. Use of K2CO3 often provided the products in unreliable yields, partially due to hydrolysis of the ester and indole acetamide. Organic bases such as Et3N that are soluble under these reaction conditions typically provided the desired product in improved yields. With the use of 15 mol % of the catalyst with the DtBPF ligand in DMF (0.02 M) at 130 °C for 3 h, the reaction provided the desired indole macrocycle in 72% yield (Figure 7, entry 10). Notably and as detailed later, the improved catalyst system worked effectively with hindered substrates for which the original Pd(OAc)2 catalyst was ineffective.
Figure 7.
Optimization of catalytic Pd(0)-mediated macrocyclization.
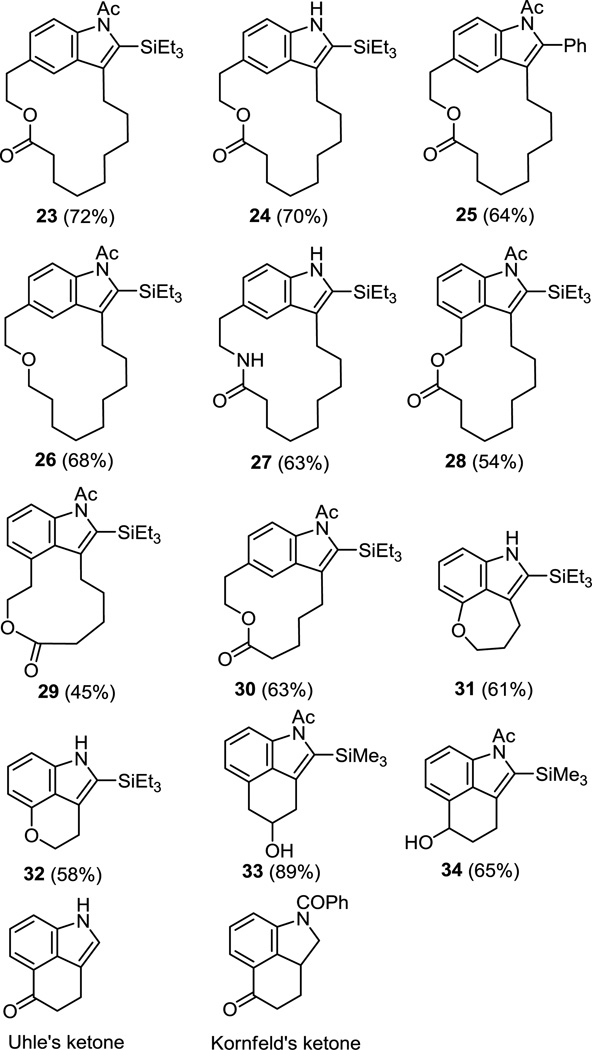
Without individual optimization, the scope of catalytic macrocyclization reaction was examined with the preparation of a variety of macrocyclic indoles from their corresponding 2-bromoanilines or acetamides (Figure 8). The reaction was found to be compatible with a variety of ring sizes and with a range of tethers linked by an ester, ether or amide. Exemplified are substrates in which the bromide and the tethering groups are in the meta positions to provide the 16- and 12-membered macrocycles in good yield (Figure 8, examples 23–27 and 30). Even substrates bearing hindered, electron-rich ortho disubstituted aryl bromides were effective participants in the macrocyclization reaction with the reactive Pd2(dba)3 derived catalyst, providing the products in good yields (Figure 8, examples 28–29). The use of a substrate bearing a free aniline or its acetamide derivative did not meaningfully impact or alter the success of the macrocyclization reaction (Figure 8, 23 vs 24).
Figure 8.
Scope of catalytic macrocyclization and cyclization reaction.
In addition to macrocyclization, the catalytic indole annulation was also extended to an intramolecular cyclization reaction. Despite the synthetic potential of such reactions, we were not able to locate an example and found only a single report of an intramolecular Larock-inspired indole annulation in which a mechanistically distinct alkyne amidopalladation was observed with alkynes tethered by N-acylation of a 2-chloroaniline.18 Thus, a variety of aryl bromide substrates leading to six- and seven-membered ring fused indole derivatives were also examined and provided the cyclized products in excellent yields (Figure 8, examples 31–34). The substrates chosen represent significant challenges for implementation because of the hindered, electron-rich character of the starting aryl bromide. Despite the potential of slow oxidative addition to the electron-rich ortho disubstituted aryl bromides and like the macrocyclization observations, no detrimental effect was observed during the cyclization of these compounds. Presumably, this may be attributed to a combination of the unique Pd2(dba)3 derived catalyst reactivity3d and its coordination to the proximal polar amine or amide of the substrates. In this respect, it is noteworthy that the use of even stoichiometric Pd(OAc)2 instead of the more reactive and catalytic Pd2(dba)3 for the preparation of the ether linked tricycles 31 and 32 did not provide the desired cyclization products.
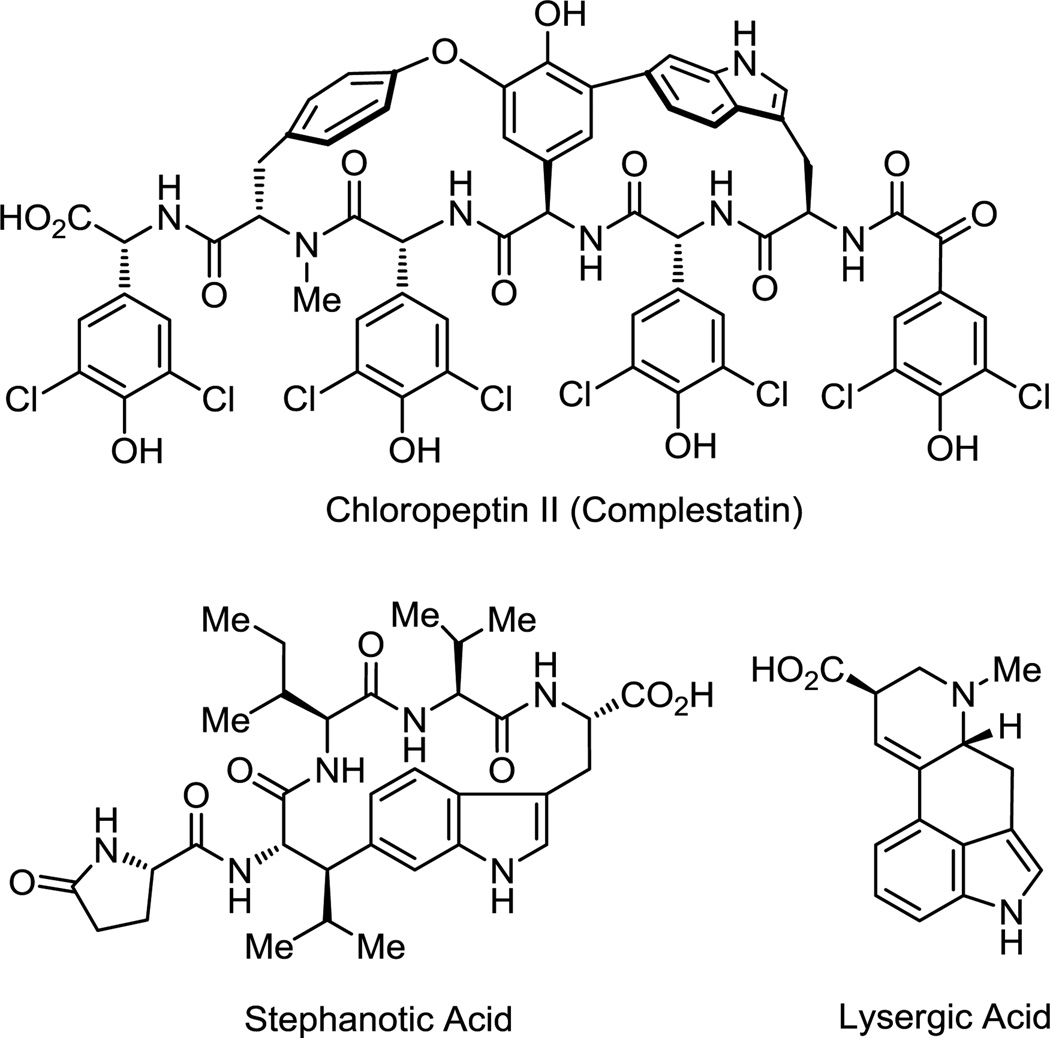
Fused indoles substituted at the 3 and 4 positions comprise a large family of biologically active natural and unnatural products and can potentially be accessed using the current strategy (Figure 9).19 For example, tricyclic indole 34 serves as a synthetic precursor to Uhle’s20 and Kornfeld’s21 ketones that have been extensively utilized in the synthesis of ergot alkaloids including lysergic acid and its derivatives, complementing its use in the syntheses of macrocyclic indoles such as chloropeptin II,5 kapakahines,22 and potentially stephanotic acid.1l–t
Figure 9.
Key natural products containing macrocyclic and cyclic indoles.
Conclusions
Recently, we reported the total synthesis of the chloropeptin family of natural products, including chloropeptin II (complestatin),5 chloropeptin I,5 complestatin A and complestatin B (neuroprotectin A and B),13b bicyclic peptides bearing a tryptophan-derived indole embedded in the macrocycle. Central to the design of the approach, a Pd(0)-mediated intramolecular Larock indole annulation reaction was developed for macrocyclization to furnish the eastern DEF ring system in both first and second generation total syntheses. The key macrocyclization reactions proceeded in superb yields with excellent atropselectivity, demonstrating exceptional functional group tolerance. Herein, we disclose a systematic study of this Pd(0)-mediated Larock indole annulation for macrocyclization that define the scope and generality of the reaction for peptide-derived substrates, its superb functional group tolerance, it use in directly accessing the chloropeptin I as well as chloropeptin II DEF ring system and key isomers, its utility for macrocyclization of more conventional carbon chain-based macrocycles, its extension to the intramolecular cyclization of more common ring sizes, and factors impacting reactivity and catalysis. Combined, the studies define a powerful method complementary to the Stille or Suzuki cross-coupling reaction for cyclization or macrocyclization reactions.23
Supplementary Material
Acknowledgments
We gratefully acknowledge the financial support of the National Institutes of Health (CA041101), the Skaggs Institute for Chemical Biology, and fellowship support from the National Science Foundation (S.P.B., 2008–2011).
Footnotes
Associated Content
Supporting Information. Full experimental details. This material is available free of charge via the internet at http://pubs.acs.org/
Notes
The authors declare no competing financial interest.
References
- 1.(a) Review: Marsault E, Peterson ML. J. Med. Chem. 2011;54:1961. doi: 10.1021/jm1012374. Kaneko I, Fearon DT, Austen KF. J. Immunol. 1980;124:1194. Tachikawa K, Hasumi K, Endo A. Thromb. Haemost. 1997;77:137. Tachikawa K, Hasumi K, Endo A. Thrombosis Res. 1997;87:571. doi: 10.1016/s0049-3848(97)00186-2. Kaneko I, Kamoshida K, Takahashi S. J. Antibiot. 1989;42:236. doi: 10.7164/antibiotics.42.236. Seto H, Fujioka T, Furihata K, Kaneko I, Takahashi S. Tetrahedron Lett. 1989;30:4987. Matsuzaki K, Ikeda H, Ogino T, Matsumoto A, Woodruff HB, Tanaka H, Omura S. J. Antibiot. 1994;47:1173. doi: 10.7164/antibiotics.47.1173. Tanaka H, Matsuzaki K, Nakashima H, Ogino T, Matsumoto A, Ikeda H, Woodruff HB, Omura S. J. Antibiot. 1997;50:58. doi: 10.7164/antibiotics.50.58. Matsuzaki K, Ogino T, Sunazuka T, Tanaka H, Omura S. J. Antibiot. 1997;50:66. doi: 10.7164/antibiotics.50.66. Naruse N, Tenmyo O, Kobaru S, Hatori M, Tomita K, Hamagishi Y, Oki T. J. Antibiot. 1993;46:1804. doi: 10.7164/antibiotics.46.1804. Naruse N, Oka M, Konishi M, Oki T. J. Antibiot. 1993;46:1811. doi: 10.7164/antibiotics.46.1812. Leung T-WC, Williams DH, Barna JCJ, Foti S, Oelrichs PB. Tetrahedron. 1986;42:3333. Kahn SD, Booth PM, Waltho JP, Williams DH. J. Org. Chem. 1989;54:1901. doi: 10.1021/jo004021n. Kahn SD, Booth PM, Waltho JP, Williams DH. J. Org. Chem. 2000;65:8406. doi: 10.1021/jo004021n. Morita H, Shimbo K, Shigemori H, Kobayashi J. Bioorg. Med. Chem. Lett. 2000;10:469. doi: 10.1016/s0960-894x(00)00029-9. Kobayashi J, Suzuki H, Shimbo K, Takeya K, Morita H. J. Org. Chem. 2001;66:6626. doi: 10.1021/jo0103423. Suzuki H, Morita H, Iwasaki S, Kobayashi J. Tetrahedron. 2003;59:5307. Suzuki H, Morita H, Shiro M, Kobayashi J. Tetrahedron. 2004;60:2489. Morita H, Suzuki H, Kobayashi J. J. Nat. Prod. 2004;67:1628. doi: 10.1021/np049858i. Yoshikawa K, Tao S, Arihara T. J. Nat. Prod. 2000;63:540. doi: 10.1021/np990532x. Singh SB, Jayasuriya H, Salituro GM, Zink DL, Shafiee A, Heimbuch B, Silverman KC, Lingham RB, Genilloud O, Teran A, Vilella D, Felock P, Hazuda D. J. Nat. Prod. 2001;64:874. doi: 10.1021/np000632z. Kobayashi H, Shin-Ya K, Furhata K, Nagai K, Suzuki K-I, Hayakawa Y, Seto H, Yun B-S, Ryoo I-J, Kim J-S, Kim C-J, Yoo I-D. J. Antibiot. 2001;54:1019. doi: 10.7164/antibiotics.54.1019. Kobayashi H, Shin-Ya K, Nagai K, Suzuki K-I, Hayakawa Y, Seto H, Yun B-S, Ryoo I-J, Kim J-S, Kim C-J, Yoo I-D. J. Antibiot. 2001;54:1013. doi: 10.7164/antibiotics.54.1013. Kohno J, Koguchi Y, Nishio M, Nakao K, Kuroda M, Shimizu R, Ohnuki T, Komatsubara S. J. Org. Chem. 2000;65:990. doi: 10.1021/jo991375+. Koguchi Y, Kohno J, Nishio M, Takahashi K, Okuda T, Ohnuki T, Komatsubara S. J. Antibiot. 2000;53:105. doi: 10.7164/antibiotics.53.105.
- 2.Larock RC, Yum EK. J. Am. Chem. Soc. 1991;113:6689. [Google Scholar]
- 3. Kahne D, Leimkuhler C, Lu W, Walsh CT. Chem. Rev. 2005;105:425–448. doi: 10.1021/cr030103a. Boger DL. Med. Res. Rev. 2001;21:356. doi: 10.1002/med.1014. For related ongoing synthetic studies, see: Boger DL, Miyazaki S, Kim SH, Wu JH, Loiseleur O, Castle SL. J. Am. Chem. Soc. 1999;121:3226. Boger DL, Miyazaki S, Kim SH, Wu JH, Castle SL, Loiseleur O, Jin Q. J. Am. Chem. Soc. 1999;121:10004. Boger DL, Kim SH, Miyazaki S, Strittmatter H, Weng J-H, Mori Y, Rogel O, Castle SL, McAtee JJ. J. Am. Chem. Soc. 2000;122:7416. Boger DL, Kim SH, Mori Y, Weng J-H, Rogel O, Castle SL, McAtee JJ. J. Am. Chem. Soc. 2001;123:1862. doi: 10.1021/ja003835i. McComas CC, Crowley BM, Boger DL. J. Am. Chem. Soc. 2003;125:9314. doi: 10.1021/ja035901x. Crowley BM, Mori Y, McComas CC, Tang D, Boger DL. J. Am. Chem. Soc. 2004;126:4310. doi: 10.1021/ja039795a. Crowley BM, Boger DL. J. Am. Chem. Soc. 2006;128:2885. doi: 10.1021/ja0572912. Xie J, Pierce JG, James RC, Okano A, Boger DL. J. Am. Chem. Soc. 2011;133:13946. doi: 10.1021/ja207142h. Xie J, Okano A, Pierce JG, James RC, Stamm S, Crane CM, Boger DL. J. Am. Chem. Soc. 2012;134:1284. doi: 10.1021/ja209937s. James RC, Pierce JG, Okano A, Xie J, Boger DL. ACS Chem. Biol. 2012;7:797. doi: 10.1021/cb300007j.
- 4.(a) Boger DL, Mathvink RJ. J. Am. Chem. Soc. 1990;112:4008. [Google Scholar]; (b) Boger DL, Mathvink RJ. J. Org. Chem. 1992;57:1429. [Google Scholar]
- 5.(a) Shimamura H, Breazzano SP, Garfunkle J, Kimball FS, Trzupek JD, Boger DL. J. Am. Chem. Soc. 2010;132:7776. doi: 10.1021/ja102304p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Garfunkle J, Kimball FS, Trzupek JD, Takazawa S, Shimamura H, Tomishima M, Boger DL. J. Am. Chem. Soc. 2009;131:16036. doi: 10.1021/ja907193b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen M, Li G, Lu BZ, Hossain A, Roschangar F, Farina V, Senanayake CH. Org. Lett. 2004;6:4129. doi: 10.1021/ol048114t. [DOI] [PubMed] [Google Scholar]
- 7.(a) Larock RC, Yum EK, Refvik MD. J. Org. Chem. 1998;63:7652. [Google Scholar]; (b) Ma C, Liu X, Li X, Flippen-Anderson J, Yu S, Cook JM. J. Org. Chem. 2001;66:4525. doi: 10.1021/jo001679s. [DOI] [PubMed] [Google Scholar]; (c) Jeschke T, Wensbo D, Annby U, Gronowitz S, Cohen LA. Tetrahedron Lett. 1993;34:6471. [Google Scholar]
- 8. Stille JK, Tanaka M. J. Am. Chem. Soc. 1987;109:3785. (b) Review: Farina V, Krishnamurthy V, Scott WJ. Org. React. 1997;50:1–652. (c) Review: Duncton MA, Pattenden G. J. Chem. Soc. Perkin Trans. 1. 1999:1235. Smith AB, III, Ott GR. J. Am. Chem. Soc. 1996;118:13095. Smith AB, III, Condon SM, McCauley JA, Leazer JL, Jr, Leahy JW, Maleczka RE., Jr J. Am. Chem. Soc. 1995;117:5407. (f) Review: Nicolaou KC, Bulger PG, Sarlah D. Angew. Chem. Int. Ed. 2005;44:4442. doi: 10.1002/anie.200500368. Shair MD, Yoon T-Y, Mosny KK, Chou TC, Danishefsky SJ. J. Am. Chem. Soc. 1996;118:9509.
- 9.(a) White JD, Tiller T, Ohba Y, Porter WJ, Jackson RW, Wang S, Hanselmann R. Chem. Commun. 1998:79. doi: 10.1021/jo0104429. [DOI] [PubMed] [Google Scholar]; (b) Miyaura N, Ishiyama T, Sasaki H, Ishikara M, Satoh M, Suzuki A. J. Am. Chem. Soc. 1989;111:314. [Google Scholar]; (c) Ohe T, Miyaura N, Suzuki A. J. Org. Chem. 1993;58:2201. [Google Scholar]; (d) Mohr PJ, Halcomb RL. J. Am. Chem. Soc. 2003;125:1712. doi: 10.1021/ja0296531. [DOI] [PubMed] [Google Scholar]; (e) Kallan NC, Halcomb RL. Org. Lett. 2000;2:2687. doi: 10.1021/ol0062345. [DOI] [PubMed] [Google Scholar]
- 10.(a) Jayasuriya H, Salituro GM, Smith SK, Heck JV, Gould SJ, Singh SB, Homnick CF, Holloway MK, Pitzenberger SM, Patane MA. Tetrahedron Lett. 1998;39:2247. [Google Scholar]; (b) Hegde VR, Dai P, Patel M, Gullo VP. Tetrahedron Lett. 1998;39:5683. [Google Scholar]
- 11.Miyaura N, Suzuki A. Chem. Rev. 1995;95:2457. [Google Scholar]
- 12.(a) Shinohara T, Deng H, Snapper ML, Hoveyda AH. J. Am. Chem. Soc. 2005;127:7334. doi: 10.1021/ja051790l. [DOI] [PubMed] [Google Scholar]; (b) Deng H, Jung J-K, Liu T, Kuntz KW, Snapper ML, Hoveyda AH. J. Am. Chem. Soc. 2003;125:9032. doi: 10.1021/ja030249r. [DOI] [PubMed] [Google Scholar]
- 13.(a) Gouda H, Matsuzaki K, Tanaka H, Hirono S, Omura S, McCauley JA, Sprengeler PA, Furst GT, Smith AB., III J. Am. Chem. Soc. 1996;118:13087. [Google Scholar]; (b) Breazzano SP, Boger DL. J. Am. Chem. Soc. 2011;133:18495. doi: 10.1021/ja208570q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Private communication, Professor F. Pichierri, Department of Applied Chemistry, Tohoku University.
- 15.(a) Wang Z, Bois-Choussy M, Jia Y, Zhu J. Angew. Chem. Int. Ed. 2010;49:2018. doi: 10.1002/anie.200906797. [DOI] [PubMed] [Google Scholar]; (b) Jia Y, Bois-Choussy M, Zhu J. Angew. Chem. Int. Ed. 2008;47:4167. doi: 10.1002/anie.200800599. [DOI] [PubMed] [Google Scholar]; (c) Jia Y, Bois-Choussy M, Zhu J. Org. Lett. 2007;9:2401. doi: 10.1021/ol070889p. [DOI] [PubMed] [Google Scholar]; (d) Yamada Y, Akiba A, Arima S, Okada C, Yoshida K, Itou F, Kai T, Satou T, Takeda K, Harigaya Y. Chem. Pharm. Bull. Jpn. 2005;53:1277. doi: 10.1248/cpb.53.1277. [DOI] [PubMed] [Google Scholar]; (e) Smith AB, III, Chruma JJ, Han Q, Barbosa J. Bioorg. Med. Chem. Lett. 2004;14:1697. doi: 10.1016/j.bmcl.2004.01.056. [DOI] [PubMed] [Google Scholar]; (f) Elder AM, Rich DH. Org. Lett. 1999;1:1443. doi: 10.1021/ol990990x. [DOI] [PubMed] [Google Scholar]; (g) Roussi G, Zamora EG, Carbonnelle A-C, Beugelmans R. Heterocycles. 1999;51:2041. [Google Scholar]; (h) Kai T, Kajimoto N, Konda Y, Harigaya Y, Takayanagi H. Tetrahedron Lett. 1999;40:6289. [Google Scholar]; (i) Gurjar MK, Tripathy NK. Tetrahedron Lett. 1997;38:2163. [Google Scholar]
- 16. Beletskaya IP, Cheprakov AV. Chem. Rev. 2000;100:3009. doi: 10.1021/cr9903048. and the references cited therein.
- 17.Abelman MM, Oh T, Overman LE. J. Org. Chem. 1987;52:4130. [Google Scholar]
- 18.Liu J, Shen M, Zhang Y, Li G, Khodabocus A, Rodriguez S, Qu B, Farina V, Senanayake CH, Lu BZ. Org. Lett. 2006;8:3573. doi: 10.1021/ol061440j. [DOI] [PubMed] [Google Scholar]
- 19. Endo Y, Ohno M, Hirano M, Itai A, Shudo K. J. Am. Chem. Soc. 1996;118:1841. Panaccione DG, Tapper BA, Lane GA, Davies E, Fraser K. J. Agric. Food Chem. 2003;51:6429. doi: 10.1021/jf0346859. (c) Hapalindoles: Moore RE, Cheuk C, Patterson GML. J. Am. Chem. Soc. 1984;106:6456. Mohd Nor SM, Xu Z, Ye T. Bioactive Macrocyclic Natural Products. In: Majumdar KC, Chattopadhyay SK, editors. Heterocycles in Natural Product Synthesis. Weinheim, Germany: Wiley-VCH; 2011. pp. 569–611. (e) Review: Gulder T, Baran PS. Nat. Prod. Rep. 2012;29:899. doi: 10.1039/c2np20034a.
- 20.Uhle FC. J. Am. Chem. Soc. 1949;71:761. doi: 10.1021/ja01171a001. [DOI] [PubMed] [Google Scholar]
- 21.Kornfeld EC, Fornefeld EJ, Kline GB, Mann MJ, Morrison DE, Jones RG, Woodward RB. J. Am. Chem. Soc. 1956;78:3087. [Google Scholar]
- 22.Newhouse T, Lewis CA, Eastman KJ, Baran PS. J. Am. Chem. Soc. 2010;132:7119. doi: 10.1021/ja1009458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.(a) Boger DL, Panek JS. Tetrahedron Lett. 1984;25:3175. [Google Scholar]; (b) Boger DL, Duff SR, Panek JS, Yasuda M. J. Org. Chem. 1985;50:5782. [Google Scholar]; (c) Boger DL, Panek JS, Duff SR, Yasuda M. J. Org. Chem. 1985;50:5790. [Google Scholar]; (d) Boger DL, Patel M. Tetrahedron Lett. 1987;28:2499. [Google Scholar]; (e) Boger DL, Patel M. J. Org. Chem. 1988;53:1405. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.