Abstract
Understanding the uptake and patterns of sexual partnerships of adolescent males reveals their risky behaviors that could persist into adulthood. Using venue-based sampling, we surveyed 671 male youth ages 15–19 from an urban Tanzanian neighborhood about their sexual partnerships during the past six months. The proportion of males who had ever had sex increased with age (21% at age 15; 70% at age 17; 94% at age 19), as did the proportion who engaged in concurrency (5% at age 15; 28% at age 17; 44% at age 19). Attendance at ≥2 social venues per day and meeting a sexual partner at a venue was associated with concurrency. Concurrency was associated with alcohol consumption before sex among 18–19 year olds and with not being in school among 15–17 year olds. We find that concurrency becomes normative over male adolescence. Venue-based sampling may reach youth vulnerable to developing risky sexual partnership patterns.
Keywords: youth, men, concurrency, HIV, venues
Introduction
Globally, one-third of all new HIV infections occur among youth 15–24 years of age. Of the estimated 5 million HIV-infected persons in 2008, 4 million lived in sub-Saharan Africa, where heterosexual intercourse is the primary mode of transmission.(1) Investigation of sexual partnership patterns among youth remains essential for developing youth-focused interventions that effectively reduce HIV risk behavior.
Understanding young men’s sexual partnership patterns is critical for reducing HIV and STI risk for young women in sub-Saharan Africa who have HIV/AIDS incidence three times that of men.(2,3) Men play a strategic role in HIV transmission to women because gender roles encourage men to control sexual interactions.(4) Adolescent males are a key population in which to assess sexual partnerships patterns because they are forming sexual partnership practices that will likely persist into adulthood.
One characteristic of sexual partnerships shown to contribute to the spread of sexually transmitted infections (STIs) is concurrent sexual partnerships (multiple sexual partnerships that overlap in time).(5–8) In contrast to serial monogamous partnerships, concurrent partnerships theoretically amplify STI/HIV transmission by decreasing the exposure time among multiple sexual contacts and increasing the likelihood of exposure to acute, highly transmissible infections.(9–11) The extent to which concurrency contributes to the generalized HIV epidemics in sub-Saharan Africa has been challenged, primarily due to inconclusive results of empirical studies that have employed diverse measures of concurrency and the differential effects of concurrency versus number of lifetime partners on HIV spread.(12–15) Nevertheless, concurrency involves a subgroup of multiple partnerships amenable to STI spread; assessing the prevalence of concurrency provides information to better understand sexual network formation and transmission potential within a population.(16) A recent study reported that concurrency reduction programs in sub-Saharan Africa that target the highest risk individuals, those with the greatest number of partners, would be the most effective and cost-effective at reducing HIV incidence.(17) Youth-focused concurrency reduction programs are currently being rolled out by USAID and others in sub-Saharan Africa.(18) These programs may benefit from identifying the factors associated with concurrency among high risk youth and targeting these factors to slow the HIV epidemic.
A number of studies have measured the prevalence of concurrency among adolescent males in sub-Saharan Africa. These studies have recruited male youth from a clinical trial (19) and household surveys (20–23) that may underestimate the concurrency prevalence by excluding groups that engage in high levels of risk behavior.(24) These prior studies have demonstrated that age at first sex is associated with concurrency, and the results for other factors such as respondent’s age were inconsistent.(20–23) Complicating comparison of these studies is the heterogeneity in their measurement of concurrency, including: 1) the method for measuring concurrency, involving either gathering dates of partnerships (19,23) or directly asking participants about overlapping partnerships (20–22) – referred to as the calendar and direct methods, respectively (25,26); 2) the recall period for partnerships, ranging from the most recent partnership (21) to 12 months (23) and lifetime sexual histories (19); and 3) the number of partners for which data are gathered, ranging from two (20) to twelve.(19) In 2010, the UNAIDS Reference Group on Estimates, Modelling, and Projections called for consistent measurement of concurrency, recommending that participants be asked how long ago they had sexual intercourse for the first and last time with up to three most recent partners from the past six months; overlapping dates indicate concurrent partnerships.(27) Only one of the previous studies among male adolescents younger than 18 years in sub-Saharan Africa used the UNAIDS method of assessing concurrency; however, this study collected information on overlapping dates for only three partners from the past 12, not 6, months.(23) In this paper we improve upon previous studies by using a method consistent with the UNAIDS recommendations for assessing concurrency, going beyond the recommendations by assessing an unlimited number of partners from the past six months, and surveying a sample of high risk young men in Tanzania.
Evidence from Tanzania suggests that men’s multiple sexual partnerships are associated with increased HIV risk. In a cohort of HIV testing clients age 18 years and older in Moshi, Tanzania, monogamous women who reported that their partners were unfaithful or who didn’t know if their partners were unfaithful were 36% more likely to be HIV-infected than women who reported that their partners were monogamous.(28) A report summarizing 10 years of population-based surveys in Tanzania suggests that the proportion of young men aged 15–19 who had casual sex in the past year increased over time (29), with the most recent survey indicating that 95.3% of sexually experienced men aged 15–19 reported casual sex in the past year.(30) Recent data also indicate that a greater proportion of young men who have no schooling are HIV positive, compared to those who have at least some schooling. Men without schooling are also more likely to have higher risk sex with multiple partners than men who had completed primary school or secondary school.(31)
We use the proximate-determinants framework as our conceptual model for capturing the multi-level determinants of concurrency among a sample of young Tanzanian men.(32) The framework orders the determinants of HIV infection from distal to proximal, with biological factors such as transmission efficiency being the most proximal. We define underlying determinants as demographic factors (e.g. age of participants) and the socioeconomic context (e.g. education level). Proximate determinants include sexual behaviors by individuals (e.g. age of sexual debut) or within partnerships (e.g. alcohol use before sex). The framework indicates that the proximate determinants are directly linked to biological determinants of HIV infection, including efficiency of transmission per contact, exposure of susceptible to infected persons, and duration of infectivity.(33)
We designed the Vijana Vijiweni Project to assess the multi-level determinants of sexual risk behavior and sexual partnership patterns of young men aged 15–19 who socialize in high risk venues within one ward (neighborhood) of Dar es Salaam, the city with the second highest HIV prevalence in Tanzania at 9%.(31) Our previous qualitative research suggested that most men who socialized in these venues were not in school and had informal jobs.(34) Our rationale for venue-based sampling originates from theories positing that the places where people congregate are regulated by social norms which influence people’s behavior in those places.(35–37) Social norms in some places may promote HIV risk behavior.(38) For example, research among South African adults indicates that attendance at certain places (e.g., bars) is associated with HIV risk behaviors such as frequently forming new sexual partnerships and drinking alcohol before sex. (39) Evidence supports the association between HIV and such venues in Tanzania. Research among women working in bars/hotels in 15 Tanzanian wards found that the women who had the highest HIV prevalence rates worked in wards where there were more guesthouses, or venues where patrons engage in commercial sex.(40) Qualitative research (41,42), including our own (34), demonstrates that Tanzanian young men engage in sexual risk behavior at particular venues, including local beer shops, guest houses, and “camps”, which we have previously described. Camps are urban gathering spaces for stable social networks of predominantly young men. Men who frequent the camps reported meeting new sexual partners at the camps, including sex workers from the nearby brothel and guest houses.(34)
Understanding the determinants of HIV risk behavior and sexual partnership patterns of young men who socialize in high risk venues may inform the design of interventions for high risk populations, such as youth who are not attending school.(43) We present findings from our cross-sectional survey of men ages 15–19 who socialized in venues known for meeting new sexual partners in a high risk ward of Dar es Salaam. We report the prevalence of concurrency among the male youth and associated underlying and proximate determinants.
Materials and Methods
We conducted our study in Tandale, an impoverished ward of about 44,000 people in Dar es Salaam.(44,45) Elevated reports of illicit drug use and commercial sex in Tandale suggested an increased risk for transmitting and acquiring HIV infections.(46,47) We applied the PLACE (Priorities for Local AIDS Control Efforts) method to identify the venues where male youth meet new sexual partners.(48,49)
Eligibility
Because we wanted to describe the diversity of behaviors over the course of adolescence, the eligibility criteria for men included age 15–19 and able to provide informed consent. Sexual experience was not one of the eligibility criteria because we were also interested in what proportion of men in this age group lacked sexual experience.
Participant recruitment
The PLACE method involves a systematic sequence of procedures to identify venues where people at high risk for HIV infection meet new sexual partners. We conducted semi-structured interviews with 232 community informants and identified 83 venues where young men reportedly met new sexual partners, drank alcohol, and socialized.(34) At each venue, a venue representative was interviewed regarding the number of men who socialized at the venue and the busiest times for the venue, which is standard procedure for the PLACE method.(49)
Using the equiprobability sampling technique recommended by the PLACE method in which the probability of a unit being selected is equal to the probability of any other unit being selected, we selected 66 out of the 83 venues to reach our targeted sample size, a minimum of 600 men to detect significant differences in risk factors for concurrency.(49) The sampling technique involved: a) sorting the list of venues first by geographic area, and then by the number of men reported by the venue representative to be socializing at the site; b) generating a random number using statistical software and selecting the venue closest to the random number as the first for inclusion; and c) selecting every other venue in ascending order on the venue list. This process was repeated twice with the remaining venues to reach the targeted number of interviews.
Interviewers went to each selected venue during one of the busiest times to recruit men ages 15 to 19. Each interviewer documented the number of men socializing at the venue at the time of their arrival and departure. The interviewers approached all men socializing at the venue at the time they arrived. To each man they explained the purpose of the study, verified his age eligibility, and asked if he was willing to participate. Each interviewer documented the number of men who completed interviews, were ineligible, or declined. The age range of men who were approached for an interview was predominantly 15–24. Of these, 222 men were age ineligible and 66 men refused. Structured face-to-face surveys were administered to all men who were willing to participate and socializing at the selected venues at the time the interviewers arrived. Interviewers obtained oral consent and conducted the interview in a pre-identified private space near the venue. Respondents received a snack and drink.
Measures
Prior to implementation, we pre-tested and piloted the study questionnaires. All interviews were administered in Kiswahili by research interviewers who were Tanzanian college graduates and received training on the research protocol and ethics.
The survey assessed respondents’ demographic characteristics, including their employment status, where they slept the previous night (parent’s home or elsewhere), and the number of other venues they did or planned to attend on the interview day. Assessments of respondents’ sexual behavior included the number of partners in the past 12 months, past 6 months, and past four weeks and the age of first sex. Early age of sexual debut was defined as younger than age 15, the youngest age in our sample. Self-reported STI symptoms in the past four weeks included painful urination, unusual discharge, and sores.
Concurrency definition
We used the calendar method to assess concurrency, but unlike the UNAIDS recommendation, we gathered dates for an unlimited number of sexual partners (instead of three) within the past six months; the maximum number of partners reported by participants was six. For each partner, respondents provided the partner’s nickname or initials and dates of most recent and first sexual intercourse that were marked on a calendar by the interviewer. To minimize recall bias the UNAIDS Reference Group recommended prompting respondents with key event dates.(27) We marked 17 key event dates, including national and religious holidays, on the calendars and trained the interviewers to use them as prompts. Similar to other studies, if the participant could not remember an exact date, the first day of the month was used. If all dates fell within the same month they were not classified as concurrent.(50,51) Partnerships in which the date of first sex with one partner preceded the date of last sex with another partner were classified as concurrent.
Sexual partner grid
From the dates of last sex provided by the respondent, the interviewers identified the respondent’s three most recent partners from the past six months. Interviewers administered a series of 39 questions for each of the respondent’s three most recent partners. We did not ask detailed questions for partners beyond the most recent three to minimize respondent fatigue.
Most respondents reported having vaginal sex or vaginal and anal sex with their three most recent partners (these questions were not asked for additional partners). Two respondents reported that they had only anal sex (no vaginal sex) with their partners; these two respondents were dropped from analyses because they may have been men who had sex with men and we could not adequately describe concurrency among this population. The questions for each of the three most recent partners included the place where they first met the partner (venue, school, beach, etc.), the partner’s age, relationship type (main, casual), and whether they or their partner used alcohol before or during intercourse. We also measured condom use during the most recent vaginal sex with each of the three most recent partners. A review of data from Eastern and Southern Africa recommends measuring condom use at last sex for high risk and unmarried populations.(52) For analysis, partnership-level risk factors were constructed from three variables: drinking alcohol before sex; meeting a sex partner at a venue; and using a condom during most recent vaginal sex. To accommodate the information obtained from the three most recent sexual partners, responses for these three variables were categorized according to whether the respondent engaged in the risk behavior with at least one partner or none of the partners.
Analysis
To eliminate bias due to non-response at the venues, each respondent’s data was weighted by the inverse of the response rate of the venue from which he was enrolled. Rates were calculated using AAPOR Response Rate 4.(53) Weights were normalized by multiplying the respondent-specific weights times a correction factor, the original sample divided by the total weighted sample.(54) We report weighted proportions for overall prevalence estimates.
We examined correlates of concurrency among sexually active respondents using multivariable logistic regression, stratified by two age categories, ages 15–17 and 18–19. Although we performed stratified analyses in these two age groups, we adjusted for age. Variables that were significant at α=.10 in unadjusted models were included in the multivariable models. Analyses were weighted to reduce potential bias in standard errors due to clustering on the dependent variable by venue. Because we sampled a large proportion of a finite number of venues without replacement, we applied the finite population correction. All data were analyzed using Stata 10.0 (Stata Corp, College Station, TX).
The study occurred between January and July 2008 and was approved by the Tanzanian National Institute of Medical Research, the local government, and the University of North Carolina at Chapel Hill Institutional Review Board.
Results
Venues
Participants were recruited from 66 venues where young men were known to meet new sexual partners. Alcohol was sold at 21% of the venues and at an additional 33% of the venues, patrons reportedly brought alcohol. The majority of the venues were called “camps” (n = 59); the remaining venues included bus stops (n=3), bars and “guest houses” where people can rent rooms for sex (n=3), and a store. 85% of the venues had been in operation for more than 2 years and the remaining 15% were camps that had primarily been in operation from 1 – 2 years. We extensively described the camps, permanent social venues for networks of mostly young men, in a previously published paper.(34)
Participant demographics and risk behaviors
Among the men approached who were eligible, 84% enrolled and the most common reason for refusal was “no time” (53%). The median response rate by venue was 100% of eligible men (range 44–100%). The average number of respondents at each venue who were age eligible was 11.
Overall, 64% of the sample reported always living in Tandale and 94% reported attending the venue where they were recruited at least twice per week. Half (55%) did not attend school at the time of the survey, and school completion rates were: 79% completed primary school, 5% completed secondary school, and 16% had not completed any level of schooling. Most men were unemployed (45%) or occasionally employed (19%), while 36% reported working full-time. Only 7 men in the sample had ever been married and all of these men were ages 18 or 19.
In Table 1, we report the frequencies, stratified by age, for young men’s demographic characteristics and risk behaviors. The proportions of men who were in school decreased with age, as did those who slept at their parents’ home the previous evening. With increasing age, the proportions of men who reported the following characteristics also increased: being employed and not a student (23% at age 15 and 76% at age 19); attending two or more other venues on the day of the interview (16% at age 15 and 39% at age 19); drinking alcohol with at least one sexual partner in the past six months (among sexually experienced men, 14% at age 15 and 52% at age 19); and meeting a sexual partner at a venue (among sexually experienced men, 14% at age 15 and 41% at age 19).
Table 1.
Demographic characteristics and risk behaviors of young men ages 15–19, Vijana Vijiweni Project, Dar es Salaam, 2008
| Characteristic | Age (n) | ||||
|---|---|---|---|---|---|
| 15 (108) | 16 (112) | 17 (120) | 18 (146) | 19 (177) | |
| Currently in school | 79% | 70% | 54% | 30% | 19% |
| Slept at parents’ home last night | 93% | 86% | 81% | 60% | 40% |
| Employed, not student | 23% | 33% | 31% | 53% | 76% |
| Total other venues attended | |||||
| No venues | 55% | 44% | 35% | 35% | 31% |
| 1 other venue | 29% | 27% | 24% | 26% | 30% |
| 2 or more other venues | 16% | 28% | 42% | 40% | 39% |
| Mean number of sexual partners in pasta | |||||
| Twelve months | 1.59 | 1.71 | 2.33 | 2.97 | 3.15 |
| Six months | 1.18 | 1.44 | 1.60 | 1.88 | 2.08 |
| Four weeks | 0.73 | 0.76 | 0.82 | 1.05 | 1.27 |
| Have at least one STI symptom in the past four weeks | 32% | 19% | 23% | 24% | 30% |
| Drank alcohol with ≥ 1 sexual partner of past 6 monthsa | 14% | 22% | 26% | 49% | 52% |
| Met a sexual partner at a venuea | 14% | 27% | 23% | 29% | 41% |
| No condom use at last sex with ≥ 1 sexual partner of past 6 monthsa | 92% | 84% | 72% | 71% | 70% |
Among those who had ever had sex
Sexual partnership patterns
Sexually experienced youth (including all ages) reported an average of 1.25 partners in the past six months (s.d. = 1.33; range =1–6) and 1.95 partners in the past 12 months (s.d. = 2.21; range = 1–24). The number of partners reported by sexually experienced respondents from the past 12 months and past 6 months was highly consistent (ϱ =.75). Among those men who had sex in the past six months, 88% reported having only casual sexual partners. Respondents’ last sex with their 3 most recent partners occurred during the 3 months prior to the interview 83% of the time. Six respondents reported that they had sex only once or twice with every partner; these partnerships were not considered concurrent.
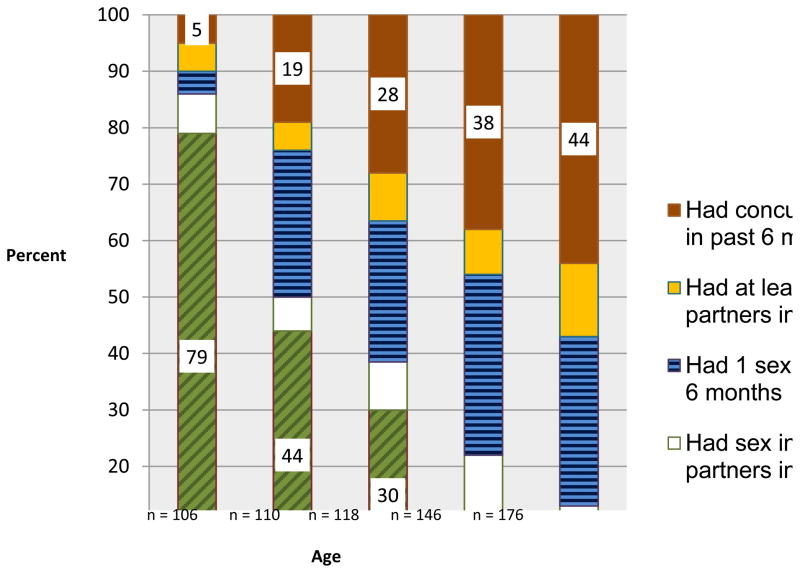
As illustrated in Figure 1, increasing levels of sexual risk behaviors corresponds to increasing age. Among 15 year old men, 79% had never had sex, compared to 6% of 19 year old men. Although the prevalence of concurrency among the overall sample within the past six months was 29% and among sexually experienced youth was 42%, it sharply increased by age. Furthermore, for each age group 16–19, approximately equal proportions were monogamous with one partner during the preceding six months. For youth with at least two partners, however, most had concurrent partnerships instead of consecutive partnerships. The prevalence of concurrency in the past six months increased remarkably with age from 5% of 15 year old men to 44% of 19 year old men.
Figure 1.
Raw proportions indicating types of sexual experience stratified by age of young men, Vijana Vijiweni Project, Dar es Salaam, Tanzania, 2008
Correlates of concurrency
We stratified the logistic regressions by age (15–17 and 18–19; Table 2) to identify factors associated with concurrency among sexually experienced men. Because the effect of employment was not significantly different from age at first sex in chi-square analysis, it was excluded from subsequent models.
Table 2.
Factors associated with engagement in concurrent partnerships among men aged 15–17 and 18–19 who had at least one sexual partner in past 6 months, Vijana Vijiweni Project, Dar es Salaam, Tanzania, 2008
| Characteristic |
15–17 year olds (N=142)b |
18–19 year olds (N=268)c |
||||||
|---|---|---|---|---|---|---|---|---|
| n | % Reported Concurrencya | Adjusted Model | n | % Reported Concurrencya | Adjusted Model | |||
| OR (95% CI) | p | OR (95% CI) | p | |||||
| Demographics | ||||||||
| Age | ||||||||
| 15 | 14 | 42 | n.s. | 114 | 50 | .74 (.54–1.02) | .07 | |
| 16/18 | 55 | 39 | n.s. | 153 | 50 | |||
| 17/19 | 73 | 45 | ||||||
| Education | ||||||||
| In school | 81 | 33 | 1.85 (1.13–3.02) | .02* | 57 | 45 | . | n.s. |
| Not in school | 61 | 54 | 208 | 51 | ||||
| Where slept last night | ||||||||
| At parents’ home | 121 | 42 | 117 | 38 | 2.05 (1.50–2.80) | <.001* | ||
| Not at parents’ home | 21 | 47 | n.s | 148 | 58 | |||
| Individual risk factors | ||||||||
| Age at first sex | ||||||||
| ≥15 | 79 | 31 | 1.91 (1.24–2.94) | <.001* | 195 | 43 | 2.38 (1.62–3.50) | <.001* |
| <15 | 63 | 57 | 69 | 68 | ||||
| Other venues attended | ||||||||
| None | 41 | 28 | 77 | 40 | n.s. | |||
| One | 37 | 46 | 2.24 (1.19–4.21) | .01 | 73 | 49 | 1.58 (.99–2.53) | |
| Two or more | 55 | 51 | 1.68 (0.91–3.09) | .10* | 101 | 57 | .06* | |
| Recent STI symptoms | ||||||||
| None | 109 | 39 | 1.70 (.92–3.12) | .09* | 187 | 41 | ||
| At least one | 33 | 53 | 77 | 70 | n.s.* | |||
| Total sexual partners past six months | ||||||||
| 1 or 2 sex partners | 125 | 35 | 17.90 (4.31–74.33) | <.001* | 171 | 29 | 9.58 (6.31–14.54) | <.001* |
| >2 sex partners | 17 | 94 | 96 | 86 | ||||
| Partnership-level risk factors | ||||||||
| Drank alcohol at least a few times before sex | ||||||||
| With no partners | 109 | 40 | 132 | 31 | ||||
| With 1 or more partners | 33 | 51 | n.s. | 135 | 67 | 2.07 (1.45–2.94) | <.001* | |
| Met sexual partners at venue | ||||||||
| None | 103 | 37 | 1.70 (.90– 3.22) | 161 | 37 | 1.45 (1.05– 2.00) | ||
| One or more partners | 39 | 56 | .10* | 106 | 69 | .03* | ||
| Condom use at last sex | ||||||||
| With all partners | 30 | 34 | 77 | 45 | ||||
| Didn’t use with at least one partner | 109 | 46 | n.s.* | 185 | 52 | n.s.* | ||
p≤.10 in unadjusted model
Weighted percentages.
Adjusted model included 141 men with data on all variables.
Adjusted model included 263 men with data on all variables.
Ages 15–17
For men aged 15–17, having had concurrent sexual partnerships in the past six months was significantly associated with the following factors in unadjusted logistic regression models (Table 2): not being school; early age of sexual debut; attending at least two more venues on the day of the interview; having had at least one STI symptom in the past four weeks; having more than two sexual partners in the past six months; meeting one or more sexual partners at a venue; and not using condoms at last sex with at least one partner from the past six months (versus using condoms at last sex with all partners from the past six months).
In the multivariable adjusted model for men aged 15–17, not being in school, experiencing sexual debut at age 15 or younger, attending two or more venues on the day of the interview, having two or more sex partners in the past six months, having at least one STI symptom, and meeting a sexual partner at a venue persisted to be associated with concurrency. Men in this age group who did not attend school were 1.85 times (95% CI: 1.13–3.02) as likely as men who did attend school to report concurrency in the past six months. Compared to men who attended no other venues on the day of the interview, those who attended one other venue on the day of the interview were 2.24 times (95% CI: 1.19–4.21) more likely to have reported concurrency.
Ages 18–19
Among men aged 18–19, the following factors were significantly associated with concurrency in the unadjusted logistic regression models (Table 2): not having slept at parents’ home the previous night; experiencing sexual debut at age 15 or younger; attending two more venues on the day of the interview; reporting at least one STI symptom in the past four weeks; having more than two sexual partners within the past six months; drinking alcohol at least a few times before sex with one or more partners (compared to none of the past three partners); meeting at least one sexual partner at a venue; and not using a condom at last sex with at least one partner.
The factors that remained associated with concurrency for men aged 18–19 in the adjusted model included not having slept at parents’ home the previous night, sexual debut before age 15, attending two or more venues on the day of the interview, having more than two sexual partners in the past six months, drinking alcohol before sex, and meeting at least one sexual partner at a venue. Those who did not sleep at their parents’ home were 2.05 times (95% CI: 1.50–2.80) more likely to have had a concurrent partnership than young men who slept at their parents’ home the previous evening. Those who attended two or more other venues on the day of the interview were 1.58 times (95% CI: .99–2.53) more likely to report concurrency than those who attended no other venues. Those 18 and 19 year old men who had at least one partnership in which alcohol was consumed at least a few times before or during sex were 2.07 times (95% CI: 1.45–2.94) more likely to report concurrency than those for whom none of their three most recent partnerships involved alcohol consumption. For men who had more than two sexual partners in the past six months, the likelihood of concurrency increased nearly ten-fold.
Discussion
We documented a shift over the course of adolescence in the sexual partnership patterns of nearly 700 male youths ages 15–19 who socialized in venues in an impoverished urban, high risk neighborhood in Tanzania. The six month cumulative prevalence of concurrency was 29% overall and 42% among sexually experienced youths. With increasing age, youth gained sexual experience, and the prevalence of concurrency increased steadily from 19%, 28%, 38%, and 44% for 16, 17, 18, and 19 year-olds respectively (Figure 1). Moreover, for the youth who had at least two partners in the preceding six months, few had sequential monogamous partnerships (22%); more than three-quarters had concurrent partnerships. These findings suggest that as the male youth transition from early to late adolescence/early adulthood, having concurrent sexual partners becomes normative.
The young men’s median age of first sex, 16.3 years, was lower than the national estimate of 17.7 years, and the proportion who had multiple partners in the past year (47%) was markedly higher than the national proportion for their age (26%).(55) Furthermore, the six month cumulative prevalence of concurrency among sexually experienced youths was substantially higher than other studies of male youth in sub-Saharan Africa that ranged between 20–38% over time periods of past 12 months and past three years.(20–23) Data from a case-control study of male circumcision of Kenyan men ages 18–24 using the calendar method to assess concurrency revealed that 63% had a concurrent partnership in their lifetime.
The variability of concurrency estimates for young men in sub-Saharan Africa highlights the importance of using a consistent measurement of concurrency. Our method conforms with the UNAIDS guidelines.(27) We used the calendar method for the past six months, and did not restrict the number of partners for dates of sex, whereas the other studies of male youth in Africa assessed concurrency with only three partners from the past twelve months (23) or with the direct method or lifetime history. Despite the measurement issues, results from these studies underscore our finding that as young men transition into adulthood, concurrency becomes normative.(19)
We surveyed men at a key period of time in their life when they are developing their sexual partnership patterns. The median age of first marriage for men in Tanzania is 24.3.(30) Of the men we surveyed, nearly half the 19 year olds were engaging in concurrent sexual partnerships, and this proportion gradually increased over ages 15 to 19. Because these men all socialized in the same venues, it is possible that the younger men were socially influenced by the behaviors of their older peers. Peer pressure to engage in risky sexual activities may be more pronounced for young men than young women (56,57) and men’s interactions with peers likely shape their sexual attitudes and behaviors.(58) Future studies of young men who socialize in high risk venues may investigate this hypothesis. Young men who engage in high risk sexual behaviors are often elusive to prevention programs because they may not be in school or accessible through health centers. Our study suggests that it may be strategic to target male social networks that congregate in high risk venues and change their social norms to promote less risky sexual behaviors.
Previous studies of concurrency among young men in sub-Saharan Africa have been predominantly drawn from household surveys in South Africa, including a national household survey of South African youth aged 15–24 (23) and a rural household survey of adults aged 15–49 from KwaZulu-Natal.(20) Other similar studies include men aged 18–24 recruited for a clinical trial in Kisumu, Kenya (19) and a household survey among men aged 15–49 in 11 populous districts in Botswana.(22) Household surveys may underestimate the concurrency prevalence by excluding groups that engage in high levels of risk behavior.(24) We specifically recruited a population of young men aged 15–19 that engaged in HIV risk behavior by identifying social venues where this age group met new sexual partners and interviewing the venue patrons.(48,49)
Prior research on young men’s concurrency from sub-Saharan Africa demonstrated inconclusive results regarding the association between respondent’s age and likelihood of concurrency.(20–22) We present stratified models by age to account for the developmental differences among youth transitioning through adolescence, a rapid time of growth into early adulthood. In both age groups, concurrency was associated with early sexual debut, consistent with other studies of young men in Africa.(19–22) In the younger age group, not being in school was associated with concurrency. Interventions targeting out-of-school youth are needed in sub-Saharan Africa, home to the largest proportion of out-of-school youth in the world.(43,59) Out-of-school youth may be more mobile than in-school youth and, as such, it may be more difficult to keep them engaged in community-based interventions.(60) Identifying venues like camps where out-of-school youth socialize regularly may be important for sustaining the involvement of this population in HIV prevention interventions.(34)
In terms of individual risk factors, we found that among both age groups having more than two sexual partners in the past six months and attending multiple social venues on the day of the interview was associated with concurrency. Possible explanations for the latter result include the presence of venue-based social norms that promoted concurrency or men’s increased exposure to available sexual partners when they socialize at multiple venues. Most young men in our study were not in school, worked informally, and regularly attended the venues. A substantial proportion of the men reported a recent STI symptom. Therefore, venue-based sampling approaches may be a strategic tool for reaching such groups of men for STI prevention and treatment interventions.
Regarding sexual partnership-level risk factors, for both age groups, having met at least one sexual partner at a venue was associated with concurrency. This finding confirms that venues are sources of access to sexual partners, as presupposed by the PLACE method, and suggests that this access may contribute to HIV risk behavior.(48) Among men aged 18–19, reports of alcohol consumption at least a few times with one or more partners more than doubled their odds of concurrency, while this factor was insignificant for the younger men. It may be that 18–19 year old men have greater access to resources for purchasing alcohol. Other studies from sub-Saharan Africa, including from Tanzania (61), document that alcohol use is associated with higher sexual risk behaviors.(62) Interventions to reduce concurrency among men aged 18–19 should consider the role that alcohol plays in promoting concurrent sexual partnerships.
A limitation of this study is that it is cross-sectional. A longitudinal study could clarify whether the pattern we observed of increasing risk behavior with increasing age is a progression of risk behavior by individuals over time and could test the direction of the observed associations. Another limitation is that we could not evaluate recall bias. However, in our sample, respondents’ last sex with their three most recent partners occurred predominantly during the three months prior to the interview; this time period has been recommended as an appropriate recall period for sexual behavior.(63) Our data may be limited in that we surveyed men about their risk behaviors with only their three most recent partners and they may have engaged in these risk behaviors with additional partners from the past six months. However, the majority of men who reported more than three partners in the past six months engaged in the partnership-level risk factors we examined. Men may also have exaggerated their number of sexual partners.(64) We included 39 detailed questions about each of the three most recent sexual partners to minimize this bias. Finally, although our findings may be generalizable to men who socialize at venues in this neighborhood and others like it, they are not generalizable to all youth in Tanzania. We estimate that we surveyed almost 25% of men aged 15–19 who resided in the Tandale neighborhood (population = 2,452).(45)
Conclusions
Identifying and targeting young men who engage in high risk sexual behaviors remains a challenge for HIV prevention programs. Concurrency is an important characteristic of sexual network formation in a population and is one indicator of sexual risk behavior. Research is needed to understand the mechanisms through which venue-based social networks promote young men’s risky sexual behavior. Investigation of such patterns might lead to the development of HIV prevention interventions for high risk, hard-to-reach populations of young men at a point in their transition to adulthood that may slow the spread of HIV to future generations.
Acknowledgments
The Vijana Vijiweni Project was funded through grants from the National Institute of Mental Health (R21MH 080577) and the University of North Carolina at Chapel Hill’s Center for AIDS Research and Injury Prevention Research Center. The first author’s participation in the project was funded by the University of North Carolina at Chapel Hill’s C.V. Starr International Award and Dissertation Travel Award from the Center for Global Initiatives, as well as a National Research Service Award for pre-doctoral training from the University of North Carolina at Chapel Hill’s Center for Infectious Diseases (NRSA-AI 7001-32).
References
- 1.UNAIDS. Outlook Breaking News: Young people are leading the HIV prevention revolution. 2010. [Google Scholar]
- 2.Gouws E, Stanecki KA, Lyerla R, Ghys PD. The epidemiology of HIV infection among young people aged 15–24 years in southern Africa. AIDS. 2008 Dec;22(Suppl 4):S5–16. doi: 10.1097/01.aids.0000341773.86500.9d. [DOI] [PubMed] [Google Scholar]
- 3.Pettifor AE, Rees HV, Kleinschmidt I, Steffenson AE, MacPhail C, Hlongwa-Madikizela L, et al. Young people’s sexual health in South Africa: HIV prevalence and sexual behaviors from a nationally representative household survey. AIDS. 2005 Sep 23;19(14):1525–1534. doi: 10.1097/01.aids.0000183129.16830.06. [DOI] [PubMed] [Google Scholar]
- 4.Barker G, Ricardo C. The World Bank Social Development Papers: Conflict Prevention & Reconstruction June, 2005;Paper No. 26. Oct 25, 2010. Young Men and the Construction of Masculinity in sub-Saharan Africa: Implications for HIV/AIDS, Conflict, and Violence. [Google Scholar]
- 5.Koumans EH, Farley TA, Gibson JJ, Langley C, Ross MW, McFarlane M, et al. Characteristics of persons with syphilis in areas of persisting syphilis in the United States: sustained transmission associated with concurrent partnerships. Sex Transm Dis. 2001;28(9):497. doi: 10.1097/00007435-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Potterat JJ, Zimmerman-Rogers H, Muth SQ, Rothenberg RB, Green DL, Taylor JE, et al. Chlamydia Transmission: Concurrency, Reproduction Number, and the Epidemic Trajectory. Am J Epidemiol. 1999;150(12):1331. doi: 10.1093/oxfordjournals.aje.a009965. [DOI] [PubMed] [Google Scholar]
- 7.Guwatudde D, Wabwire-Mangen F, Eller LA, Eller M, McCutchan F, Kibuuka H, et al. Relatively Low HIV Infection Rates in Rural Uganda, but with High Potential for a Rise: A Cohort Study in Kayunga District, Uganda. PLoS ONE. 2009;4(1) doi: 10.1371/journal.pone.0004145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helleringer S, Kohler HP, Kalilani-Phiri L. The association of HIV serodiscordance and partnership concurrency in Likoma Island (Malawi) AIDS. 2009 Jun 19;23(10):1285–1287. doi: 10.1097/QAD.0b013e32832aa85c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morris M, Kretzschmar M. Concurrent partnerships and the spread of HIV. AIDS. 1997 Apr;11(5):641–648. doi: 10.1097/00002030-199705000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Pilcher CD, Tien HC, Eron JJ, Jr, Vernazza PL, Leu SY, Stewart PW, et al. Brief but efficient: acute HIV infection and the sexual transmission of HIV. J Infect Dis. 2004 May 15;189(10):1785–1792. doi: 10.1086/386333. [DOI] [PubMed] [Google Scholar]
- 11.Morris M. Concurrent partnerships and syphilis persistence: new thoughts on an old puzzle. Sex Transm Dis. 2001 Sep;28(9):504–507. doi: 10.1097/00007435-200109000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Lurie MN, Rosenthal S. The concurrency hypothesis in sub-Saharan Africa: convincing empirical evidence is still lacking. Response to Mah and Halperin, Epstein, and Morris. AIDS Behav. 2010 Feb;14(1):34. doi: 10.1007/s10461-009-9640-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lurie MN, Rosenthal S. Concurrent partnerships as a driver of the HIV epidemic in sub-Saharan Africa? The evidence is limited. AIDS and Behavior. 2010;14(1):17–24. doi: 10.1007/s10461-009-9583-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sawers L, Stillwaggon E. Concurrent sexual partnerships do not explain the HIV epidemics in Africa: a systematic review of the evidence. Journal of the International AIDS Society. 2010;13(34) doi: 10.1186/1758-2652-13-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanser F, Bärnighausen T, Hund L, Garnett GP, McGrath N, Newell M. Effect of concurrent sexual partnerships on rate of new HIV infections in a high-prevalence, rural South African population: a cohort study. The Lancet. 2011 Jul 16–22;378(9787):247–255. doi: 10.1016/S0140-6736(11)60779-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Padian NS, Manian S. The concurrency debate: time to put it to rest. Lancet. 2011 Jul 16;378(9787):203–204. doi: 10.1016/S0140-6736(11)60974-4. [DOI] [PubMed] [Google Scholar]
- 17.Enns EA, Brandeau ML, Igeme TK, Bendavid E. Assessing effectiveness and cost-effectiveness of concurrency reduction for HIV prevention. Int J STD AIDS. 2011 Oct;22(10):558–567. doi: 10.1258/ijsa.2011.010322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eaton JW, Case KK. Secretariat of the UNAIDS Reference Group on Estimates, Modelling, and Projections. Consultation on concurrent sexual partnerships: recommendations from a meeting of the UNAIDS Reference Group on Estimates, Modelling, and Projections; Nairobi, Kenya. April 20–21st 2009; 2009. [Google Scholar]
- 19.Mattson CL, Bailey RC, Agot K, Ndinya-Achola JO, Moses S. A nested case-control study of sexual practices and risk factors for prevalent HIV-1 infection among young men in Kisumu, Kenya. Sex Transm Dis. 2007 Oct;34(10):731–736. doi: 10.1097/01.olq.0000261335.42480.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrison A, Cleland J, Frohlich J. Young people’s sexual partnerships in KwaZulu-Natal, South Africa: patterns, contextual influences, and HIV risk. Stud Fam Plann. 2008 Dec;39(4):295–308. doi: 10.1111/j.1728-4465.2008.00176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mah TL. Prevalence and Correlates of Concurrent Sexual Partnerships Among Young People in South Africa. Sex Transm Dis. 2010 Feb;37(2):105–108. doi: 10.1097/OLQ.0b013e3181bcdf75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carter MW, Kraft JM, Koppenhaver T, Galavotti C, Roels TH, Kilmarx PH, et al. “A bull cannot be contained in a single kraal”: concurrent sexual partnerships in Botswana. AIDS Behav. 2007 Nov;11(6):822–830. doi: 10.1007/s10461-006-9203-6. [DOI] [PubMed] [Google Scholar]
- 23.Steffenson AE, Pettifor AE, Seage GR, 3rd, Rees HV, Cleary PD. Concurrent sexual partnerships and human immunodeficiency virus risk among South African youth. Sex Transm Dis. 2011 Jun;38(6):459–466. doi: 10.1097/OLQ.0b013e3182080860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rothenberg R, Potterat J, Gisselquist D. Concurrency and sexual transmission. AIDS. 2002 Mar 8;16(4):678–9. doi: 10.1097/00002030-200203080-00026. author reply, 679–80. [DOI] [PubMed] [Google Scholar]
- 25.Nelson SJ, Manhart LE, Gorbach PM, Martin DH, Stoner BP, Aral SO, et al. Measuring sex partner concurrency: it’s what’s missing that counts. Sex Transm Dis. 2007 Oct;34(10):801–807. doi: 10.1097/OLQ.0b013e318063c734. [DOI] [PubMed] [Google Scholar]
- 26.Mah TL, Halperin DT. Concurrent Sexual Partnerships and the HIV Epidemics in Africa: Evidence to Move Forward. AIDS Behav. 2010;14(1):11–16. doi: 10.1007/s10461-008-9433-x. [DOI] [PubMed] [Google Scholar]
- 27.Modelling, and Projections: Working Group on Measuring Concurrent Sexual Partnerships, UNAIDS Reference Group on Estimates. HIV: consensus indicators are needed for concurrency. The Lancet. 2010 Feb 20;375(9715):621–622. doi: 10.1016/S0140-6736(09)62040-7. [DOI] [PubMed] [Google Scholar]
- 28.Landman KZ, Ostermann J, Crump JA, Mgonja A, Mayhood MK, Itemba DK, et al. Gender differences in the risk of HIV infection among persons reporting abstinence, monogamy, and multiple sexual partners in northern Tanzania. PloS one. 2008;3(8):e3075. doi: 10.1371/journal.pone.0003075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorgens M, Kalinga R. The HIV epidemic in Tanzania Mainland: Where have we come from, where is it going, and how are we responding? 2008 Nov 6;2008 [Google Scholar]
- 30.National Bureau of Statistics (NBS) [Tanzania], ORC Macro. Tanzania Demographic and Health Survey 2010. 2010. [Google Scholar]
- 31.Tanzania Commission for AIDS (TACAIDS), Zanzibar AIDS Commission (ZAC), National Bureau of Statistics (NBS), Office of the Chief Government Statistician (OCGS), Macro International Inc. Tanzania HIV/AIDS and Malaria Indicator Survey 2007–08. 2008. [Google Scholar]
- 32.Boerma JT, Weir SS. Integrating demographic and epidemiological approaches to research on HIV/AIDS: the proximate-determinants framework. J Infect Dis. 2005 Feb 1;191(Suppl 1):S61–7. doi: 10.1086/425282. [DOI] [PubMed] [Google Scholar]
- 33.Royce RA, Sena A, Cates W, Jr, Cohen MS. Sexual transmission of HIV. N Engl J Med. 1997 Apr 10;336(15):1072–1078. doi: 10.1056/NEJM199704103361507. [DOI] [PubMed] [Google Scholar]
- 34.Yamanis T, Maman S, Mbwambo J, Earp JA, Kajula L. Social venues that protect against and promote HIV risk for young men in Dar es Salaam, Tanzania. Social Science & Medicine. 2010;71(9):1601–1609. doi: 10.1016/j.socscimed.2010.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bourdieu P, Nice R. Distinction: A social critique of the judgement of taste. Cambridge, MA: Harvard University Press; 1987. [Google Scholar]
- 36.Poland B, Greene L, Rootman I. Settings for Health Promotion: Linking Theory and Practice. Newbury Park, CA: Sage Publications; 2001. [Google Scholar]
- 37.Latkin C, Knowlton A. Micro-social approaches to HIV prevention: a social ecological perspective. AIDS Care. 2005;17(Suppl 1):S102–S113. doi: 10.1080/09540120500121185. [DOI] [PubMed] [Google Scholar]
- 38.Fichtenberg CM, Ellen JM. Moving from core groups to risk spaces. Sex Transm Dis. 2003;30(11):825. doi: 10.1097/01.OLQ.0000097141.29899.7F. [DOI] [PubMed] [Google Scholar]
- 39.Weir SS, Morroni C, Coetzee N, Spencer J, Boerma JT. A pilot study of a rapid assessment method to identify places for AIDS prevention in Cape Town, South Africa. Sex Transm Infect. 2002 Apr;78(Suppl 1):i106–13. doi: 10.1136/sti.78.suppl_1.i106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ao TT, Sam NE, Masenga EJ, Seage GR, 3rd, Kapiga SH. Human immunodeficiency virus type 1 among bar and hotel workers in northern Tanzania: the role of alcohol, sexual behavior, and herpes simplex virus type 2. Sex Transm Dis. 2006 Mar;33(3):163–169. doi: 10.1097/01.olq.0000187204.57006.b3. [DOI] [PubMed] [Google Scholar]
- 41.Mziray JC. Boys’ views on sexuality, girls, and pregnancies. In: Rwebangira MK, Liljestrom R, editors. Haraka, Haraka... Look before you leap. Stockholm, Sweden: Nordiska Afrikainstitutet; 1998. pp. 144–163. [Google Scholar]
- 42.Setel PW. A Plague of Paradoxes: AIDS, Culture and Demography in Northern Tanzania. Chicago: The University of Chicago Press; 1999. [Google Scholar]
- 43.Stroeken K, Remes P, De Koker P, Michielsen K, Van Vossole A, Temmerman M. HIV among out-of-school youth in Eastern and Southern Africa: a review. AIDS Care. 2011 Jul; doi: 10.1080/09540121.2011.596519. in press. [DOI] [PubMed] [Google Scholar]
- 44.Tanzania Research on Poverty Alleviation. Tanzania Poverty and Human Development Report. 2005. [Google Scholar]
- 45.National Bureau of Statistics, Tanzania. 2002 Population and Housing Census. 2002. [Google Scholar]
- 46.McCurdy SA, Ross MW, Kilonzo GP, Leshabari MT, Williams ML. HIV/AIDS and injection drug use in the neighborhoods of Dar es Salaam, Tanzania. Drug Alcohol Depend. 2006 Apr;82(Suppl 1):S23–7. doi: 10.1016/s0376-8716(06)80004-9. [DOI] [PubMed] [Google Scholar]
- 47.Dar es Salaam City Council. City Profile for Dar Es Salaam, United Republic of Tanzania. 2004 Nov;2004 [Google Scholar]
- 48.Weir SS, Pailman C, Mahlalela X, Coetzee N, Meidany F, Boerma JT. From people to places: focusing AIDS prevention efforts where it matters most. AIDS. 2003 Apr 11;17(6):895–903. doi: 10.1097/01.aids.0000050809.06065.e0. [DOI] [PubMed] [Google Scholar]
- 49.MEASURE Evaluation Project. PLACE: Priorities for Local AIDS Control Efforts. A Manual for Implementing the PLACE Method. 2005 Oct;2005:MS-05-13. [Google Scholar]
- 50.Doherty IA, Minnis A, Auerswald CL, Adimora AA, Padian NS. Concurrent partnerships among adolescents in a Latino community: The Mission District of San Francisco, California. Sex Transm Dis. 2006 Dec 21; doi: 10.1097/01.olq.0000251198.31056.7d. [DOI] [PubMed] [Google Scholar]
- 51.Kelley SS, Borawski EA, Flocke SA, Keen KJ. The role of sequential and concurrent sexual relationships in the risk of sexually transmitted diseases among adolescents. J Adolesc Health. 2003 Apr;32(4):296–305. doi: 10.1016/s1054-139x(02)00710-3. [DOI] [PubMed] [Google Scholar]
- 52.Reynolds HW, Luseno WK, Speizer IS. The measurement of condom use in four countries in East and Southern Africa. AIDS Behav. 2012 May;16(4):1044–1053. doi: 10.1007/s10461-012-0146-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.The American Association for Public Opinion Research. Standard Definitions: Final Dispositions of Case Codes and Outcome Rates for Surveys. 2008. [Google Scholar]
- 54.Kaplan D, Ferguson AJ. On the utilization of sample weights in latent variable models. Structural Equation Modeling. 1999;6(4):305. [Google Scholar]
- 55.National Bureau of Statistics (NBS) [Tanzania], ORC Macro. Tanzania Demographic and Health Survey 2004–05. 2005. [Google Scholar]
- 56.Gardner M, Steinberg L. Peer Influence on Risk Taking, Risk Preference, and Risky Decision Making in Adolescence and Adulthood: An Experimental Study. Dev Psychol. 2005 Jul;41(4):625–635. doi: 10.1037/0012-1649.41.4.625. [DOI] [PubMed] [Google Scholar]
- 57.MacPhail C, Campbell C. ‘I think condoms are good but, aai, I hate those things’: condom use among adolescents and young people in a Southern African township. Soc Sci Med. 2001 Jun;52(11):1613–1627. doi: 10.1016/s0277-9536(00)00272-0. [DOI] [PubMed] [Google Scholar]
- 58.Agadjanian V. Men’s Talk about “Women’s Matters”: gender, communication, and contraception in urban Mozambique. Gender Soc. 2002 Apr;16(2):194–215. [Google Scholar]
- 59.Harrison A, Newell ML, Imrie J, Hoddinott G. HIV prevention for South African youth: which interventions work? A systematic review of current evidence. BMC Public Health. 2010 Feb 26;10:102. doi: 10.1186/1471-2458-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cowan FM, Pascoe SJ, Langhaug LF, Mavhu W, Chidiya S, Jaffar S, et al. The Regai Dzive Shiri project: results of a randomized trial of an HIV prevention intervention for youth. AIDS. 2010 Oct 23;24(16):2541–2552. doi: 10.1097/QAD.0b013e32833e77c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fisher JC, Cook PA, Kapiga SH. Alcohol use before sex and HIV risk: situational characteristics of protected and unprotected encounters among high-risk African women. Sex Transm Dis. 2010 Sep;37(9):571–578. doi: 10.1097/OLQ.0b013e3181dbafad. [DOI] [PubMed] [Google Scholar]
- 62.Kalichman S, Simbayi L, Kaufman M, Cain D, Jooste S. Alcohol use and sexual risks for HIV/AIDS in Sub-Saharan Africa: systematic review of empirical findings. Prevention Science. 2007 Jun 01;8(2):141–151. doi: 10.1007/s11121-006-0061-2. [DOI] [PubMed] [Google Scholar]
- 63.Noar SM, Cole C, Carlyle K. Condom use measurement in 56 studies of sexual risk behavior: review and recommendations. Arch Sex Behav. 2006 Jun;35(3):327–345. doi: 10.1007/s10508-006-9028-4. [DOI] [PubMed] [Google Scholar]
- 64.Nnko S, Boerma JT, Urassa M, Mwaluko G, Zaba B. Secretive females or swaggering males? An assessment of the quality of sexual partnership reporting in rural Tanzania. Soc Sci Med. 2004 Jul;59(2):299–310. doi: 10.1016/j.socscimed.2003.10.031. [DOI] [PubMed] [Google Scholar]