Abstract
The S-layer glycoprotein is the sole component of the protein shell surrounding Haloferax volcanii cells. The deduced amino acid sequence of the S-layer glycoprotein predicts the presence of a C-terminal membrane-spanning domain. However, several earlier observations, including the ability of EDTA to selectively solubilize the protein, are inconsistent with the presence of a trans-membrane sequence. In the present report, sequential solubilization of the S-layer glycoprotein by EDTA and then with detergent revealed the existence of two distinct populations of the S-layer glycoprotein. Whereas both S-layer glycoprotein populations underwent signal peptide cleavage and N-glycosylation, base hydrolysis followed by mass spectrometry revealed that a lipid, likely archaetidic acid, modified only the EDTA-solubilized version of the protein. These observations are consistent with the S-layer glycoprotein being initially synthesized as an integral membrane protein and subsequently undergoing a processing event in which the extracellular portion of the protein is separated from the membrane-spanning domain and transferred to a waiting lipid moiety.
Keywords: Archaea, Haloferax volcanii, lipid modification, membrane protein, S-layer glycoprotein
1. INTRODUCTION
In the years when 16S rRNA sequence analysis was first employed to distinguish Archaea from Bacteria [1,2], other characteristic traits of Archaea were identified, including a unique cell wall composition (for reviews, see [3,4]). Lacking peptidoglycan, a major component of the bacterial cell wall [4,5], many Archaea are instead surrounded by a cell envelope composed solely of a protein-based surface (S)-layer1, a self-assembling two-dimensional crystalline array found in intimate association with the plasma membrane [6–9]. In the case of the haloarchaea Haloferax volcanii, the S-layer glycoprotein is the sole component of this structure [10]. Synthesized as an 827 amino acid precursor that includes a 34 amino acid residue N-terminal signal peptide, the Hfx. volcanii S-layer glycoprotein also includes a stretch of 20 hydrophobic residues near the C-terminus (amino acid residues 804–823) that is thought to serve as a trans-membrane domain, anchoring the protein to the membrane [11].
With a broad range of genetic tools available for working with Hfx. volcanii, the species has become an important model for addressing molecular questions in Archaea. In a widely employed protocol for the transformation of Hfx. volcanii cells, spheroplasts are generated upon incubation with 0.5 M EDTA-containing solution [12]. Such EDTA treatment releases the S-layer glycoprotein into the surrounding growth medium [11]. A similar effect is observed in cells grown in medium lacking magnesium [13]. EDTA-generated spheroplasts, however, regain the cup-shaped morphology of the native cells when magnesium is once again provided, presumably due to a restoration of the S-layer [14]. Although the precise requirement for magnesium in S-layer biogenesis is unclear, it has been shown that in the absence of magnesium, the S-layer glycoprotein fails to experience a maturation event that transpires on the external cell surface, possibly the addition of a lipid moiety attached to the mature protein [13,15].
Based on current understanding, it is difficult to envisage how the S-layer glycoprotein can be associated with the plasma membrane simultaneously in an EDTA-sensitive, magnesium-dependent manner and via a trans-membrane domain. With the aim of clarifying this seeming paradox, the present study more closely examined the Hfx. volcanii S-layer glycoprotein and its mode of membrane attachment. Such efforts reveal that two S-layer glycoprotein populations co-exist, with one requiring detergent for solubilization, presumably corresponding to S-layer glycoprotein anchored to the membrane via the C-terminal trans-membrane domain, and the other being lipid-modified and associated with the membrane in an EDTA-sensitive manner.
2. MATERIALS AND METHODS
2.1 Growth conditions
Haloferax volcanii strain H53 was grown in complete medium containing 3.4 M NaCl, 0.15 M MgSO4*7H20, 1 mM MnCl2, 4 mM KCl, 3 mM CaCl2, 0.3% (w/v) yeast extract, 0.5% (w/v) tryptone, 50 mM Tris-HCl, pH 7.2, at 37°C [16].
2.2 S-layer glycoprotein solubilization
Hfx. volcanii cells were grown to stationary phase (OD600 = 3.0) and harvested by centrifugation (10,900 xg, 10 min). The cell pellet was washed with 2 M NaCl, 50 mM Tris-HCl, pH 7.2 and resuspended in minimal medium to which EDTA was added at a final concentration of 50, 100, 150, 200, 300 or 500 mM, or not at all. The cells were incubated for 3–4 h at 37°C and harvested by centrifugation. The supernatant was dialyzed against 50 mM Tris-HCl, pH 7.2, and precipitated with 15% trichloroacetic acid (TCA). The pellet was washed with 2 M NaCl, 50 mM Tris-HCl, pH 7.2, and resuspended in the same buffer to which 1% Triton X-100 was added to a final concentration. After a 20 min incubation at room temperature, the samples were subjected to centrifugation, the supernatant was precipitated with 15% TCA. The TCA-precipitated samples were acetone-washed, incubated with sample buffer, separated on 7.5% SDS-PAGE and Coomassie-stained.
2.3 Native gel electrophoresis
S-layer glycoprotein solubilized by either Triton X-100 or EDTA treatment was examined by native gel electrophoresis performed using 7.5 % Tris-glycine gels (pH 8.9) containing 0.5% Triton X-100 [17]. The gels were run at 70 V overnight at 4°C and the S-layer glycoprotein was visualized by Coommasie staining.
2.4 Liquid chromatography-electrospray ionization mass spectrometry (LCESI/MS)
LC-ESI/MS analysis of tryptic fragments of the S-layer glycoprotein was performed as described previously [18]. Triton X-100- and EDTA-solubilized Hfx. volcanii proteins were separated on 7.5% polyacrylamide gels and stained with Coomassie R-250 (Fluka, St. Louis MO). For in-gel digestion of each version of the protein, the relevant bands were excised, destained in 400 µl of 50% (vol/vol) acetonitrile (Sigma, St Louis, MO) in 40 mM NH4HCO3, pH 8.4, dehydrated with 100% acetonitrile, and dried using a SpeedVac drying apparatus. The S-layer glycoprotein was reduced with 10 mM dithiothreitol (Sigma) in 40 mM NH4HCO3 at 56°C for 60 min and then alkylated for 45 min at room temperature with 55 mM iodoacetamide in 40 mM NH4HCO3. The gel pieces were washed with 40 mM NH4HCO3 for 15 min, dehydrated with 100% acetonitrile, and SpeedVac-dried. The gel slices were rehydrated with 12.5 ng/µl of mass spectrometry (MS)-grade Trypsin Gold (Promega, Madison, WI) in 40 mM NH4HCO3. The protease-generated peptides were extracted with 0.1% (v/v) formic acid in 20 mM NH4HCO3, followed by sonication for 20 min at room temperature, dehydration with 50% (v/v) acetonitrile, and additional sonication. After three rounds of extraction, the gel pieces were dehydrated with 100% acetonitrile, dried completely with a SpeedVac, resuspended in 5% (v/v) acetonitrile containing 1% formic acid (v/v). The protein digests, infused using static nanospray Econotips (New Objective, Woburn, MA), were separated on-line by nano-flow reverse-phase liquid chromatography (LC) by loading onto a 150-mm by 75-µm (internal diameter) by 365-µm (external diameter) Jupifer pre-packed fused silica 5-µm C18 300Å reverse-phase column (Thermo Fisher Scientific, Bremen, Germany). The sample was eluted into the LTQ Orbitrap XL mass spectrometer (Thermo Fisher Scientific) using a 60-min linear gradient of 0.1% formic acid (v/v) in acetonitrile/0.1% formic acid (1:19, by volume) to 0.1% formic acid in acetonitrile/0.1% formic acid (4:1, by volume) at a flow rate of 300 nl/min.
2.5 Base hydrolysis of the S-layer glycoprotein
For base hydrolysis of the S-layer glycoprotein, comparable amounts of the protein, as determined by SDS-PAGE and Coomassie staining, were combined with 0.6 ml PBS, 1 ml CHCl3 and 2 ml methanol (MeOH) in a 15 ml glass tube with a teflon-lined cap to yield a single phase (CHCl3:MeOH:PBS = 1:2:0.8, v/v) solution. Following addition of 200 µl (15N) KOH and intermittent mixing by vortex for 5 min, the mixture was sonicated in a water bath for 15 min at room temperature. The sample was centrifuged (3,000 rpm) for 5 min at room temperature and the supernatant was transferred to a fresh 15 ml glass tube with a Teflon-lined cap. One ml CHCl3 and 1 ml PBS were added to yield a two-phase Bligh-Dyer mixture (CHCl3:MeOH:PBS = 2:2:1.8, v/v). After mixing, the sample was centrifuged (3,000 rpm) for 5 min at room temperature to separate the phases. The upper phase was removed and the lower CHCl3 phase was dried under a stream of nitrogen. The dried lipid samples were then dissolved in 120 µL CHCl3:MeOH (1:1; v/v) and analyzed by LC-ESI/MS.
For LC separation, an Ascentis Si HPLC column (5 µm, 25 cm × 2.1 mm) was used. Mobile phase A consisted of chloroform/methanol/aqueous ammonium hydroxide (800:195:5, v/v/v). Mobile phase B consisted of chloroform/methanol/water/ aqueous ammonium hydroxide (600:340:50:5, v/v/v/v). Mobile phase C consisted of chloroform/methanol/water/aqueous ammonium hydroxide (450:450:95:5, v/v/v/v). The elution program consisted of the following: 100% mobile phase A was held isocratically for 2 min and then linearly increased to 100% mobile phase B over 14 min and held at 100% B for 11 min. The LC gradient was then changed to 100% mobile phase C over 3 min and held at 100% C for 3 min, and finally returned to 100% A over 0.5 min and held at 100% A for 5 min. The total LC flow rate was 300 µl/min.
3. RESULTS
3.1 Hfx. volcanii contains two distinct versions of the S-layer glycoprotein
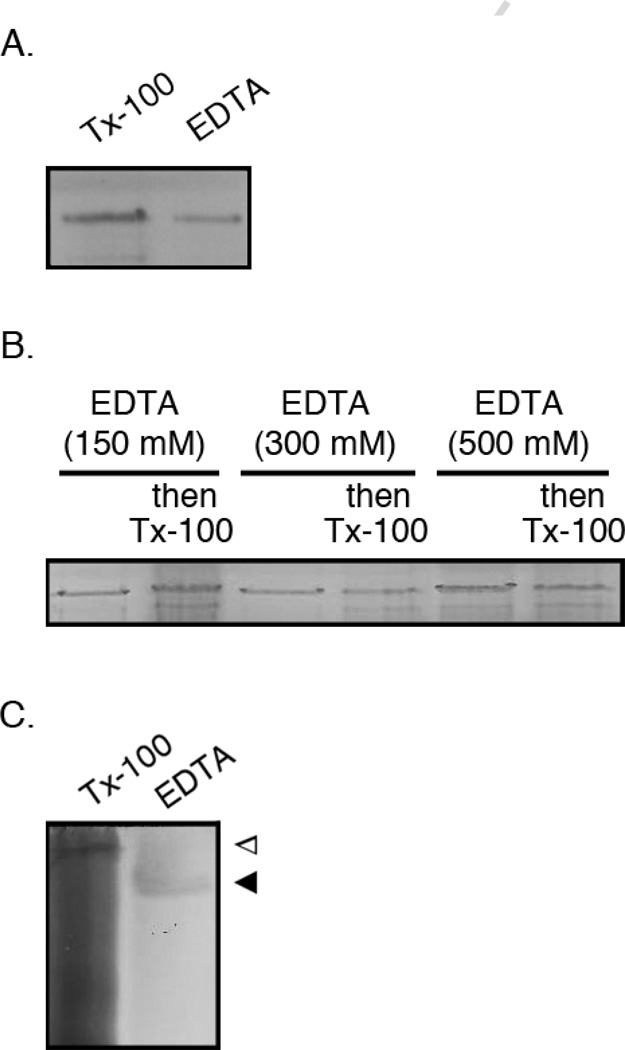
The Hfx. volcanii S-layer glycoprotein can be released from the cell surface by either treatment with Triton X-100 or following chelation with EDTA (Fig 1A). Towards determining whether both treatments solubilized the same S-layer glycoprotein population, Hfx. volcanii cells were grown in minimal medium lacking tryptone or yeast extract [11], treated with increasing amounts of EDTA (0–500 mM) for 3–4 h, and collected by centrifugation. Whereas the resulting supernatant was subjected to dialysis prior to TCA-precipitation and SDS-PAGE, the cell pellet was washed to remove the chelator and challenged with 1% Triton X-100. After centrifugation and TCA-precipitation, any detergent-solubilized proteins were also separated by SDS-PAGE. The minimal medium in which the Hfx. volcanii cells were grown contains 150 mM Mg2+ (as well as 3 mM Ca2+ and 1 mM Mn2+) [16]. Yet, even following a challenge with 500 mM EDTA (i.e. a 3.3-fold excess of chelator over the concentration of divalent ions), the subsequent detergent challenge still led to the release of S-layer glycoprotein (Fig 1B). These results thus point to the existence of distinct EDTA- and detergent-sensitive forms of the Hfx. volcanii S-layer glycoprotein.
Fig 1.
Distinct versions of the Hfx. volcanii S-layer glycoprotein are solubilized by EDTA or Triton X-100. A. Hfx. volcanii cells were challenged with 1% Triton X-100 (Tx-100) or incubated with 500 mM EDTA and the supernatant was collected. Both samples were precipitated with 15% TCA, separated by 7.5% SDS-PAGE and subjected to Coomassie staining. B. Hfx. volcanii cells were incubated with increasing concentrations of EDTA (as indicated) and collected by centrifugation. The supernatant was removed and the pellet was washed and incubated with 1% Triton X-100. The supernatants and solubilized pellets samples were precipitated with 15% TCA, separated by 7.5% SDS-PAGE and subjected to Coomassie staining. C. Hfx. volcanii cells (10 ml) were challenged with EDTA for 3 h and collected by centrifugation. After dialysis to clear the EDTA, a 1 ml aliquot of the growth medium was TCA-precipitated. The pelleted cells were washed and resuspended in 1 ml lysis buffer containing 1% Triton X-100. A 100 µl aliquot of the lysate was TCAprecipitated. After acetone washes, both TCA-precipitated samples were dissolved in non-reducing native gel electrophoresis sample buffer (17) and loaded onto a 7.5% native gel containing 0.5% Triton X-100 and Coomassie-stained. The open arrowhead depicts the position of Triton X-100-solubilized S-layer glycoprotein, while the full arrowhead depicts the position of EDTA-solubilized S-layer glycoprotein.
Further support for the existence of sub-populations of the S-layer glycoprotein was obtained when the EDTA- and Triton X-100-solubilized versions of the protein were examined by native gel electrophoresis, using gels containing 0.5% Triton X-100. In such gels, the embedded detergent molecules bind to exposed hydrophobic domains of the protein, resulting in retarded migration on the gel, relative to the same protein either lacking that hydrophobic domain or else presenting a different hydrophobic region [17]. Indeed, the same gel system has been previously employed in the study of Hfx. volcanii S-layer glycoprotein processing [13]. Now, when the EDTA- and Triton X-100-solubilized versions of the S-layer glycoprotein were subjected to such analysis, that version of the protein solubilized by Triton X-100 migrated slower in the detergent-containing native gel than did the same protein released from the cell by EDTA treatment (Fig 1C). As such, the two versions of the S-layer glycoprotein differ in terms of their hydophobicity.
3.2 EDTA-solubilized S-layer glycoprotein is lipid-modified
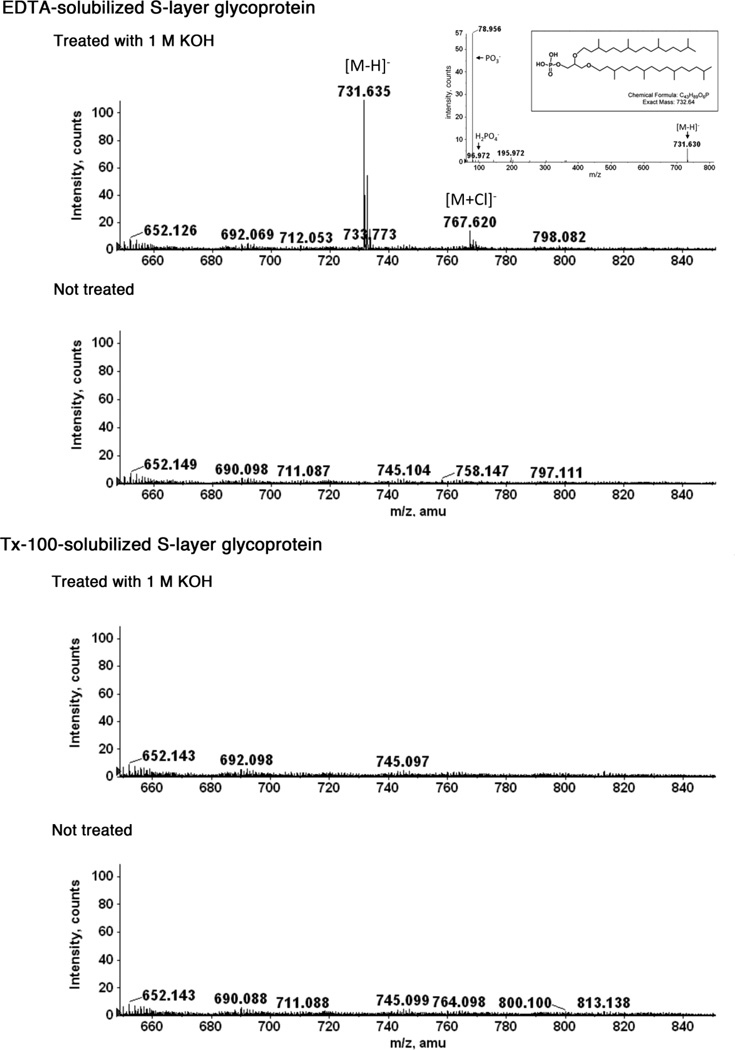
Earlier efforts determined that the Hfx. volcanii S-layer glycoprotein is subject to lipid modification in a magnesium-dependent manner on the external cell surface, as reflected by the incorporation of radiolabeled isoprene precursors into the Hfx. volcanii S-layer glycoprotein [13,15]. To assess whether either or both the EDTA- and Triton X-100-solubilized versions of the S-layer glycoprotein are lipid-modified, comparable amounts of S-layer glycoprotein released from Hfx. volcanii cells by each protocol were subjected to base hydrolysis with 1 M KOH. The presence of any released lipid was then determined by LC-ESI MS. Base hydrolysis of the EDTA-solubilized S-layer glycoprotein (Fig 2, top pair of panels, upper panel) led to the release of material detected as a monoisotopic [M-H]− peak at m/z 731.635. This released lipid (732.635 Da) likely corresponds to archaetidic acid (calculated mass 732.64 Da), as supported by MS/MS analysis (Fig 2, uppermost panel, inset). The chloride adduct ion is observed at m/z 767.620. No such peaks appeared when a mock hydrolysis was performed in the absence of 1 M KOH (Fig 2, top pair of panels, lower panel). Likewise, no released lipid was detected following similar base treatment of Triton X-100-solubilized S-layer glycoprotein (Fig 2, bottom pair of panels, upper panel). These results thus reveal that whereas a lipid anchor modifies the EDTA-solubilized form of the S-layer glycoprotein, no such moiety is attached to the Triton X-100-solubilized version of the same protein.
Fig 2.
EDTA-solubilized Hfx. volcanii S-layer glycoprotein is lipid-modified. As described in the Materials and Methods, equivalent amounts of EDTA-solubilized (top pair of panels) and Triton X-100-solubilized (bottom pair of panels) S-layer glycoprotein were treated with 1 M KOH or not (upper and lower panels in each pair, respectively). Such analysis reveals that an entity detected as a monoisotopic [M-H]− peak at m/z 731.635, corresponding in mass to archaetidic acid, was only released from the EDTA-solubilized S-layer glycoprotein following base hydrolysis (uppermost panel; the inset shows MS/MS analysis of the m/z 731.635 peak, the chemical structure of archaetidic acid and its calculated mass).
3.3 Both Triton X-100- and EDTA-solubilized S-layer glycoproteins undergo signal peptide cleavage and N-glycosylation
In addition to the lipid modification reported here, the S-layer glycoprotein is known to experience a variety of processing events, including signal peptide cleavage and Nglycosylation [11]. To begin understanding the relationship between the various post-translational modifications to which the protein is subjected, both the EDTA- and Triton X-100-solubilized versions of the Hfx. volcanii S-layer glycoprotein were analyzed by LC-ESI MS.
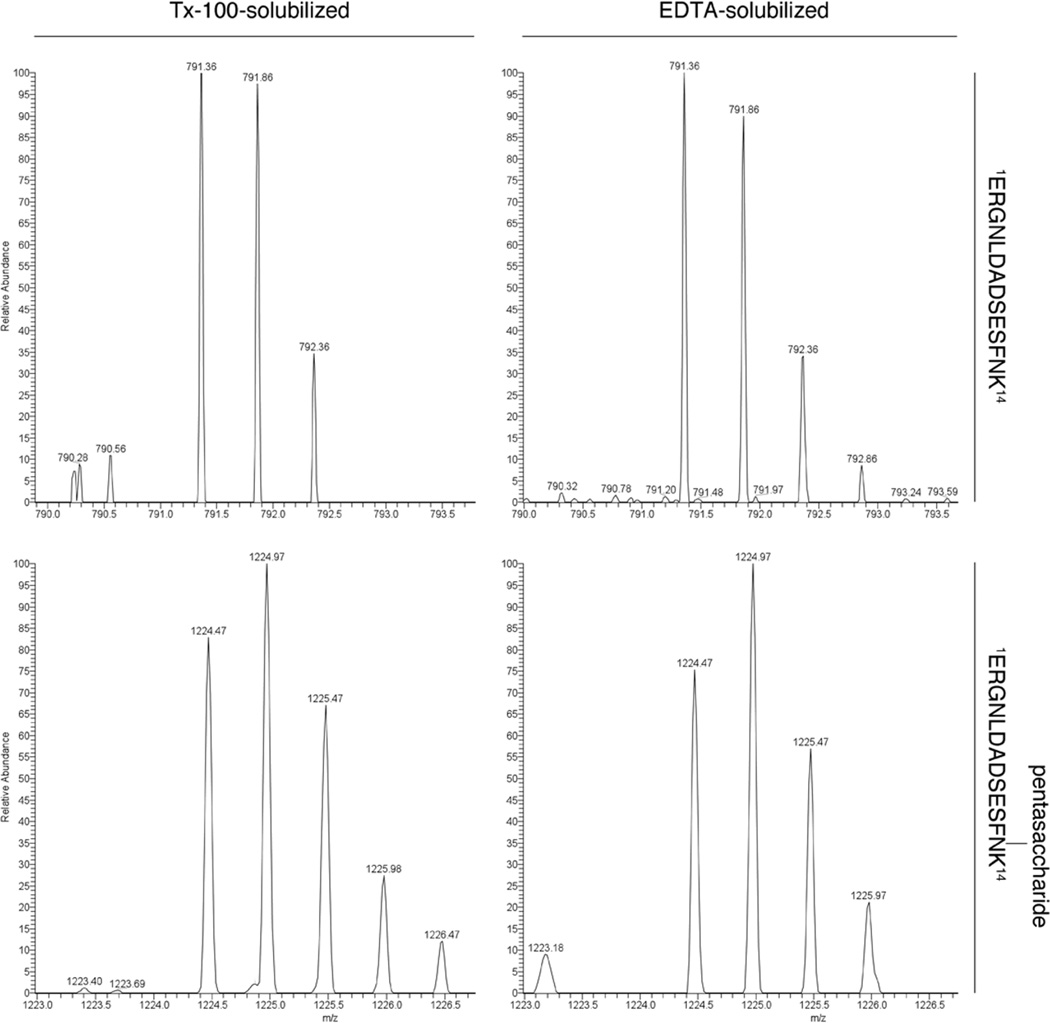
LC-ESI MS analysis of the S-layer glycoprotein solubilized by either Triton X-100 or EDTA revealed a doubly-charged [M+2H]2+ ion peak at m/z 791.36, corresponding to the N-terminal 1ERGNLDADSESFNK14 tryptic fragment (Fig 3, upper left and right panels, respectively). Thus, the signal peptide is no longer attached to either version of the S-layer glycoprotein. In addition, LC-ESI MS analysis of the S-layer glycoprotein solubilized by either treatment revealed a doubly-charged [M+2H]2+ ion peak at m/z 1224.97, corresponding to the same N-terminal 1ERGNLDADSESFNK14 tryptic fragment bearing a pentasaccharide at the Asn-13 position [19] (Fig 3, lower left and right panels, respectively). Accordingly, it can be concluded that both versions of the S-layer glycoprotein experience N-glycosylation.
Fig 3.
Both EDTA- and Triton X-100-solubilized Hfx. volcanii S-layer glycoprotein lack a signal peptide and are N-glycosylated. LC-ESI MS analysis of the S-layer glycoprotein solubilized by either Triton X-100 (Tx-100) or EDTA (left and right columns, respectively) reveal doubly-charged [M+2H]2+ ion peaks at m/z 791.36 (upper panels) and 1224.97 (lower panels), corresponding to the N-terminal 1ERGNLDADSESFNK14 tryptic fragment and the same peptide modified by a pentasaccharide at Asn-13, respectively.
4. DISCUSSION
In direct contact with outside world, the archaeal S-layer must not only provide structural rigidity to cell, it must also offer protection from the harsh environments that Archaea often encounter. To date, amongst the most detailed studies on archaeal S-layers and S-layer glycoproteins have focused on halophilic species, although a high-resolution structural analysis of these components from the methanogen, Methanosarcina acetivorans, appeared recently [20]. In Hfx. volcanii, it was proposed that the S-layer comprises regularly repeated units composed of six S-layer glycoproteins organized into a 4.5 nm-thick dome-shaped pore, with an open center that expands as one approaches the membrane [10]. In addition, the Hfx. volcanii S-layer glycoprotein is known to undergo various post-translational modifications, including signal peptide cleavage, N-glycosylation, O-glycosylation and lipid modification [8,21]. Presently, however, little is know of how these protein-processing events affect S-layer behavior or architecture.
In the current model of the Hfx. volcanii S-layer, the predicted C-terminal trans-membrane domain of each S-layer glycoprotein is thought to anchor the repeating unit structure to the membrane. Yet, previous experiments demonstrating the incorporation of radiolabelled precursors of isoprene [15], the basic building block of archaeal lipids [22], and the release of the S-layer glycoprotein from the membrane upon incubation with EDTA [12] point to the existence of a distinct mode of S-layer glycoprotein attachment to the Hfx. volcanii membrane. Here, sequential extraction, differential electrophoretic migration and mass spectrometry showed that this is indeed the case. One sub-population of the S-layer glycoprotein, apparently corresponding to the trans-membrane domain-containing version of the protein, required detergent for its solubilization. A second sub-population, solubilized by a divalent ion chelator, was shown to contain an attached lipid, likely archaetidic acid, the archaeal version of phosphatidic acid, the core of eukaryal and bacterial membrane phosopholipids [23]. The existence of lipid-modified Hfx. volcanii S-layer glycoprotein thus explains the earlier observation of post-translational lipid-related maturation event that the protein undergoes following translocation across the plasma membrane [13,15].
Presently, the biosynthetic steps leading to S-layer glycoprotein lipid modification are not known. One can hypothesize that the S-layer glycoprotein is initially synthesized with a C-terminal trans-membrane domain and following proteolytic cleavage upstream of the trans-membrane domain, the truncated protein would be transferred to a waiting lipid anchor. Recently, bioinformatics support for the existence of such a pathway was provided with the proposed identification of both an archaeal enzyme suspected of catalyzing this cleavage/transfer reaction (archaeosortase A or ArtA) and the target protein motif (PGF-Cterminal) recognized by this enzyme [24]. It is thought that this system would parallel that protein processing system found in Gram-positive bacteria, where sortases recognize a carboxyl terminal sequence motif immediately upstream of a C-terminal membrane-spanning domain in the target protein, cleave the protein as this site and then covalently attach the released proteins to the cell wall upon their transfer to a waiting lipid moiety [25]. In the case of the Hfx. volcanii S-layer glycoprotein, the PGF motif thought to be recognized by ArtA is present just upstream of the predicted C-terminal trans-membrane domain. A similar motif is detected at the comparable position in the Hbt. salinarum S-layer glycoprotein, also shown to undergo lipid modification [15,24,26].
In addition to experimentally confirming the process described above, other aspects of lipid modification of the Hfx. volcanii S-layer glycoprotein require further study. For instance, the ratio of the two versions of the protein as a function of growth stage or environmental conditions is not known. Likewise, the importance of lipid modification for S-layer architecture and self-assembly should be considered. The ease with which Hfx. volcanii can be genetically manipulated will likely assist future studies addressing these and related questions.
5. CONCLUSIONS
In conclusion, the present study offers a solution to the apparent paradox whereby the Hfx. volcanii S-layer glycoprotein is simultaneously associated with the membrane in an EDTA-sensitive, magnesium-dependent manner and via a trans-membrane domain. It appears that two S-layer glycoprotein populations co-exist, one requiring detergent for its solubilization, presumably corresponding to S-layer glycoprotein anchored to the membrane via the C-terminal trans-membrane domain, and the other being lipid-modified and associated with the membrane in an EDTA-sensitive manner.
Highlights.
The S-layer glycoprotein solely comprises the Haloferax volcanii surface layer
Sequential EDTA and Triton X-100 treatment expose two distinct forms of the protein
Both S-layer glycoprotein forms undergo signal peptide cleavage and N-glycosylation
Only EDTA-solubilized S-layer glycoprotein is lipid-modified
ACKNOWLEDGEMENTS
J.E. is supported by grants from the Israel Science Foundation (8/11) and the US Army Research Office (W911NF-11-1-520). The mass spectrometry facility in the Department of Biochemistry of the Duke University Medical Center and Z.G. are supported by the LIPID MAPS Large Scale Collaborative Grant number GM-069338 from NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations used: liquid chromatography-electrospray ionization mass spectrometry, LC-ESI/MS; methanol, MeOH; surface layer, S-layer; trichloroacetic acid, TCA.
REFERENCES
- 1.Fox GE, Magrum LJ, Balch WE, Wolfe RS, Woese CR. Classification of methanogenic bacteria by 16S ribosomal RNA characterization. Proc. Natl. Acad. Sci. USA. 1977;74:4537–4541. doi: 10.1073/pnas.74.10.4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woese CR, Fox GE. Phylogenetic structure of the prokaryotic domain: the primary kingdoms. Proc. Natl. Acad. Sci. USA. 1977;74:5088–5090. doi: 10.1073/pnas.74.11.5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kandler O, König H H. Cell envelopes of archaea: Structure and chemistry. In: Kates M, Kusher DJ, Matheson AT, editors. The biochemistry of archaea (archaebacteria) Amsterdam: Elsevier; 1993. pp. 223–259. [Google Scholar]
- 4.Kandler O, König H H. Cell wall polymers in Archaea (Archaebacteria) Cell. Mol. Life Sci. 1998;54:305–308. doi: 10.1007/s000180050156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kandler O, König H H. Chemical composition of the peptidoglycan-free cell walls of methanogenic bacteria. Arch. Microbiol. 1978;118:141–152. doi: 10.1007/BF00415722. [DOI] [PubMed] [Google Scholar]
- 6.Engelhardt H, Peters J. Structural research on surface layers: a focus on stability, surface layer homology domains, and surface layer-cell wall interactions. J. Struct. Biol. 1998;124:276–302. doi: 10.1006/jsbi.1998.4070. [DOI] [PubMed] [Google Scholar]
- 7.Sára M M, Sleytr UB. S-Layer proteins. J. Bacteriol. 2000;182:859–868. doi: 10.1128/jb.182.4.859-868.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eichler J. Facing extremes: Archaeal surface-layer (glyco-) proteins. Microbiology. 2003;149:3347–3351. doi: 10.1099/mic.0.26591-0. [DOI] [PubMed] [Google Scholar]
- 9.Konig H, Claus H, Akca E E. In: Cell wall structures of mesophilic, thermophilic and hyperthermophilic archaea. Seckbach J, editor. Origins, Kluwer, Dordrecht: 2004. pp. 283–298. [Google Scholar]
- 10.Kessel M, Wildehaber I, Cohen S, Baumeiser W. Three-dimensional structure of the regular surface glycoprotein layer of Halobacterium volcanii from the Dead Sea. EMBO J. 1988;7:1549–1554. doi: 10.1002/j.1460-2075.1988.tb02974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sumper M, Berg E, Mengele R, Strobel I. Primary structure and glycosylation of the S-layer protein of Haloferax volcanii. J. Bacteriol. 1990;172:7111–7118. doi: 10.1128/jb.172.12.7111-7118.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cline SW, Lam WL, Charlebois RL, Schalkwyk LC, Doolittle WF. Transformation methods for halophilic archaebacteria. Can. J. Microbiol. 1989;35:148–152. doi: 10.1139/m89-022. [DOI] [PubMed] [Google Scholar]
- 13.Eichler J. Post-translational modification unrelated to protein glycosylation follows translocation of the S-layer glycoprotein across the plasma membrane of the haloarchaeon Haloferax volcanii. Eur. J. Biochem. 2001;268:4366–4373. doi: 10.1046/j.1432-1327.2001.02361.x. [DOI] [PubMed] [Google Scholar]
- 14.Charlebois RL, Lam WL, Cline SW, Doolittle WF. Characterization of pHV2 from Halobacterium volcanii and its use in demonstrating transformation of an archaebacterium. Proc. Natl. Acad. Sci. USA. 1987;84:8350–8354. doi: 10.1073/pnas.84.23.8530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Konrad Z, Eichler J. Lipid modification of proteins in Archaea: attachment of a mevalonic acid-based lipid moiety to the surface-layer glycoprotein of Haloferax volcanii follows protein translocation. Biochem. J. 2002;366:959–964. doi: 10.1042/BJ20020757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mevarech M, Werczberger R. Genetic transfer in Halobacterium volcanii. J. Bacteriol. 1985;162:461–462. doi: 10.1128/jb.162.1.461-462.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toutant JP, Roberts WL, Murray NR, Rosenberry TL. Conversion of human erythrocyte acetylcholinesterase from an amphiphilic to a hydrophilic form by phosphatidylinositol-specific phospholipase C and serum phospholipase D. Eur. J. Biochem. 1989;180:503–508. doi: 10.1111/j.1432-1033.1989.tb14674.x. [DOI] [PubMed] [Google Scholar]
- 18.Calo D, Guan Z, Eichler J. Glyco-engineering in Archaea: differential N-glycosylation of the S-layer glycoprotein in a transformed Haloferax volcanii strain. Microb. Biotechnol. 2011;4:461–470. doi: 10.1111/j.1751-7915.2011.00250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abu-Qarn M, Yurist-Doutsch S, Giordano A, Trauner A, Morris HR, Hitchen P, Dell A, Eichler J. Haloferax volcanii AglB and AglD are involved in N-glycosylation of the S-layer glycoprotein and proper assembly of the surface layer. J. Mol. Biol. 2007;14:1224–1236. doi: 10.1016/j.jmb.2007.10.042. [DOI] [PubMed] [Google Scholar]
- 20.Arbing MA, Chan S, Shin A, Phan T, Ahn CJ, Rohlin L, Gunsalus RP. Structure of the surface layer of the methanogenic archaean Methanosarcina acetivorans. Proc. Natl. Acad. Sci. USA. 2012;109:18112–18117. doi: 10.1073/pnas.1120595109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jarrell KF, Jones GM, Kandiba L, Nair DB, Eichler J. S-layer glycoproteins and flagellins: Reporters of archaeal post-translational modifications. Archaea. 2010;2010:612948. doi: 10.1155/2010/612948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kates M. Biology of halophilic bacteria, Part II. Membrane lipids of extreme halophiles: biosynthesis, function and evolutionary significance. Experientia. 1993;49:1027–1036. doi: 10.1007/BF01929909. [DOI] [PubMed] [Google Scholar]
- 23.Nishimura Y, Eguchi T. Biosynthesis of archaeal membrane lipids: digeranylgeranylglycerophospholipid reductase of the thermoacidophilic archaeon Thermoplasma acidophilum. J. Biochem. 2006;139:1073–1081. doi: 10.1093/jb/mvj118. [DOI] [PubMed] [Google Scholar]
- 24.Haft DH, Payne SH, Selengut JD. Archaeosortases and exosortases are widely distributed systems linking membrane transit with posttranslational modification. J. Bacteriol. 2012;194:36–48. doi: 10.1128/JB.06026-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ton-That H, Marraffini LA, Schneewind O. Protein sorting to the cell wall envelope of Gram-positive bacteria. Biochim. Biophys. Acta. 2004;1694:269–278. doi: 10.1016/j.bbamcr.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 26.Kikuchi A, Sagami H, Ogura K. Evidence for covalent attachment of diphytanylglyceryl phosphate to the cell-surface glycoprotein of Halobacterium halobium. J. Biol. Chem. 1999;274:18011–18016. doi: 10.1074/jbc.274.25.18011. [DOI] [PubMed] [Google Scholar]