Abstract
Interstrand crosslinks (ICLs) are highly toxic DNA lesions that prevent transcription and replication by inhibiting DNA strand separation. Agents that induce ICLs were one of the earliest, and are still the most widely used, forms of chemotherapeutic drug. Only recently, however, have we begun to understand how cells repair these lesions. Important insights have come from studies of individuals with Fanconi anaemia (FA), a rare genetic disorder that leads to ICL sensitivity. Understanding how the FA pathway links nucleases, helicases and other DNA-processing enzymes should lead to more targeted uses of ICL-inducing agents in cancer treatment and could provide novel insights into drug resistance.
The use of DNA interstrand crosslinking agents as a tool for chemotherapy was born out of the horror of the use of chemical weapons in the Second World War. In December 1943, the US Merchant Ship S.S. John Harvey, which was anchored in the Italian harbour of Bari, was bombed in a German air raid. On board was a secret and deadly cargo of around 60 tonnes of sulphur mustard bombs, which detonated and contaminated the nearby city and surrounding area. After the ensuing chaos had passed, physicians carrying out autopsies noticed that the chemical had specifically attacked the victims’ white blood cells. With great insight, they hypothesized that these chemicals could have a more righteous action: for the treatment of leukaemia. In 1946, after the barbarity of the war had past, the first publication of ‘Nitrogen Mustard Therapy’ appeared1.
Sixty-eight years and a few chemical modifications later nitrogen mustards such as cyclophosphamide and melphalan are still front-line chemotherapeutic agents in the treatment of leukaemia, as well as solid tumours. Nitrogen mustards were joined in the clinical treatment of cancer by mitomycin C (MMC) in 1956, platinum compounds such as cisplatin in 1971 and psoralens in 1989. Although these drugs all showed remarkable anti-cancer properties, it was not known that they function by inducing interstrand crosslinks (ICLs) until well after their introduction as chemotherapy. Only within the past few years has it become clear how cells can recognize, repair and ultimately develop resistance to ICL-inducing agents, and how this allows tumour regrowth.
The separation of the two strands of a DNA double helix is essential for cellular processes such as replication and transcription. ICLs are extremely toxic to cells because they prevent this separation, by coordinated chemical reactions with bases on opposing strands. The resulting covalent linkage is usually irreversible. Covalent crosslinks between bases in the same DNA strand, which are known as intrastrand crosslinks, can also arise. Such damage can be bypassed by some DNA polymerases, making this type of lesion less toxic during replication than crosslinks between the two DNA strands.
The mechanism of action of all interstrand crosslinking drugs is broadly similar (BOX 1). Each ICL-inducing agent exhibits different base specificity and, as discussed below, the resulting structural changes in the DNA can result in rather different cellular responses. The structure of some of these drugs crosslinked to DNA bases, and their clinical usage, is highlighted in TABLE 1. In this Review we detail how complex networks of DNA repair processes are coordinated to repair ICLs. We also describe how defects in these repair processes can cause cancer but can also sensitize established cancers to ICL-based chemotherapy.
At a glance.
DNA interstrand crosslinks (ICLs) may arise following exposure to environmental mutagens, and are potently toxic when induced in large numbers by chemotherapeutic drugs. ICL-based chemotherapy is one of the most widely used forms of cancer treatment, particularly in the treatment of leukaemias.
Fanconi anaemia (FA) is a disorder that results in sensitivity to ICLs, which is caused by the mutation of one of at least 14 different genes. Although their functions have not been completely elucidated, there is substantial evidence to suggest that these genes, including the BRCA2 (also known as FANCD1) breast and ovarian cancer tumour suppressor, participate in a common pathway of ICL repair.
Homologous recombination (HR)-mediated repair of ICLs is promoted by the FA pathway. FA cells that are exposed to ICL-inducing agents, or chronically treated wild-type cells, may use alternative pathways of repair that lead to deleterious genetic aberrations such as radial chromosomes.
Structure-specific nucleases and translesion polymerases participate in the coordinated removal of the ICL and the resumption or completion of DNA replication. There is increasing evidence that non-replication-associated repair of ICLs also takes place — however, this is insufficient to remove all ICL damage.
The modulation of ICL repair could improve chemotherapy outcomes. For example, the dose-limiting toxicities of ICLs mostly affect the blood system, so increasing the ability to repair ICLs in blood cells could prevent anaemia phenotypes. Alternatively, targeted downregulation of ICL repair in tumours could improve ICL-mediated tumour killing.
Table 1. Crosslinking agents used in the clinic.
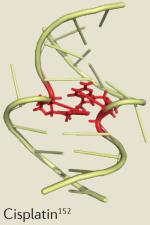
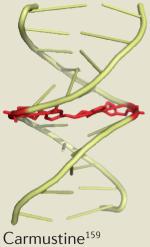
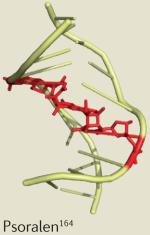
| Drug | Clinical application | Dose-limiting toxicity | Example solution structure |
|---|---|---|---|
| Platinums | |||
| Cisplatin | Testicular, ovarian and non-small-cell lung cancer |
Central nervous system, renal and gastrointestinal toxicity153 |
|
| Carboplatin | Ovarian cancer | Neutropenia154 | |
| Oxaliplatin | Colorectal cancer | Neuropathy135,155 | |
| Satraplatin | Prostate and breast cancer | Thrombocytopenia and neutropenia156,157 |
|
| Picoplatin | Phase II trials for relapsed lung cancer and Phase I and II trials for the treatment of solid tumours, and prostate, colorectal and small-cell lung cancer |
Thrombocytopenia and neutropenia158 |
|
| Nitrogen mustards | |||
| Cyclophosphamide | Lymphoma | Neutropenia160 |
|
| Melphalan | Multiple myeloma, melanoma and ovarian cancer |
Leukopenia and thrombocytopenia161 |
|
| Chlorambucil | Chronic lymphocytic leukaemia | Pancytopenia and neurotoxicity162 |
|
| Ifosfamide | Non-small-cell lung cancer | Leukopenia, thrombocytopenia and renal toxicity163 |
|
| Others | |||
| Mitomycin C | Oesophageal and bladder cancer | Leukopenia and thrombocytopenia165,166 |
|
| Psoralen plus ultraviolet A radiation |
Cutaneous T cell lymphoma | Dermatitis167,168 | |
| Pyrrolobenzodiazepines | Phase II trial for solid tumours | Fatigue and thrombocytopenia169 |
|
ICLs and cancer initiation
Although the clinical use of DNA crosslinking drugs in killing cancer cells is well established (FIG. 1), it is less clear whether DNA crosslinking presents a cause of cancer. One reason for this is that ICL-inducing drugs generally cause a wide variety of DNA lesions in addition to crosslinks. For example, cisplatin, the most widely used crosslinking drug, causes damage that is estimated to comprise of 90% intrastrand crosslinks (predominantly crosslinks between adjacent purine residues on the same strand of the DNA double helix) and less than 5% ICLs2. MMC and alkylating agents, such as the nitrogen mustards, also typically only cause 5–10% ICLs3. Furthermore, exposure to such drugs is generally in a clinical setting — there is very little evidence to suggest exposure to high levels of natural sources of ICL-inducing agents.
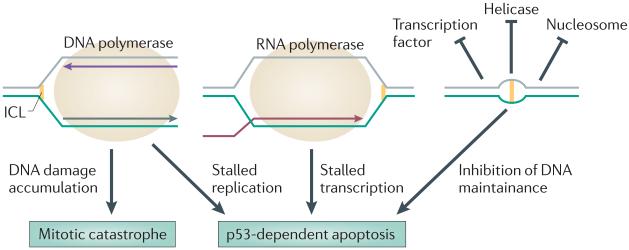
Figure 1. How ICLs kill tumour cells.
Interstrand crosslinks (ICLs) can block the progression of the replication fork by inhibiting the progression of the replisome. ICLs can stall transcription. ICLs may distort the structure of chromatin and prevent the access of DNA-interacting proteins. Tumour cell death can be induced by p53- and FAS ligand-dependent apoptosis (programmed cell death) or p53-independent mitotic catastrophe (death when apoptosis is absent but vital DNA integrity is lost).
The most well-characterized potential sources of ICLs in nature are the by-products of lipid peroxidation, including acrolein and crotonaldehyde R, β-unsaturated aldehydes4,5, the concentrations of which could increase with a high-fat diet or with alcoholism6,7. Other sources could include abasic sites, natural psoralens (such as those found in bergamot, celery and parsley) and even oestrogens8-12. Pre-cancer phenotypes such as hyperplasia and cell atypia are found to be more severe in mice that are treated with photoactivatable bifunctional psoralens (which are able to form crosslinks) than when closely related, but monofunctional, agents are used13; this is despite the fact that bifunctional agents cause a similar number of point mutations to monofunctional agents when assayed in vitro14. One interpretation is that, although ICLs inhibit strand separation, their repair proceeds via a pathway that causes point mutations but also other tumour-promoting lesions, such as deletions and translocations.
Sadly, the Italian inhabitants of Bari harbour went on to have a much higher incidence of cancers, specifically leukaemias, in the aftermath of the S.S. John Harvey bombing15. An increased incidence of acute myeloid leukaemia (AML) is also seen in patients who undergo therapeutic treatment with ICL-inducing drugs16,17. The blood seems to be specifically sensitive to both the carcinogenic and the chemotherapeutic effects of ICLs, and we have come some way in understanding the molecular details of these events by analysis of the genetic disorder known as Fanconi anaemia (FA).
Cancer that is associated with FA
FA is a rare inherited syndrome that is characterized by sub-fertility, congenital abnormalities, progressive bone marrow failure and a highly elevated risk of haematological and squamous cell cancers18. The disease is genetically heterogeneous, and 14 complementation groups have so far been identified after exhaustive genetic and functional complementation studies19,20. All of the products of the FA genes are thought to act in a common cellular pathway, and at least seven (FANCA, FANCB, FANCC, FANCE, FANCF, FANCG and FANCL) form a complex that is known as the FA core complex. Diagnosis of FA involves the cytogenetic analysis of lymphocytes that have been treated with ICL-inducing agents — FA-positive cells show highly increased levels of chromosome aberrations, including chromosome breakage and radial chromosome formation21. FA cells are also hypersensitive to ICL-inducing agents, and the management of cancer in patients with FA must strictly exclude treatment with these drugs.
Although the majority of reported cases of FA are due to mutations in FANCA (65%), FANCC (15%) or FANCG (10%) (see the Fanconi Anaemia Mutation Database; see Further information), the range of both clinical outcome and cancer susceptibility in each of the groups is broadly similar (except for the BRCA2 (also known as FANCD1) and partner and localizer of BRCA2 (PALB2; also known as FANCN) subgroups, as detailed below). Bone marrow failure is the most common symptom, and this can be readily treated with human leukocyte antigen (HLA)-matched transplant. In this regard, the autologous transplant of gene-corrected stem cells may be possible in the near future22. Unfortunately, many patients with FA develop AML before a stem cell transplant can take place, with a >700-fold risk of developing AML compared with the general population23. In one study, the actuarial risk of developing haematopoietic abnormalities was 98%, and the risk specifically for AML was 52% by the age of 40 years in patients with FA24. Solid tumours are also present at early onset and represent a greater risk in patients with FA, with the most common being head and neck squamous cell carcinoma (HNSCC; 700-fold elevated risk), as well as oesophageal (2,300-fold increased risk) and gynaecological (>180-fold increased risk) cancers23.
There are, however, some differences in the cancer susceptibilities that are associated with the various complementation groups. For example, patients with PALB2 or BRCA2 mutations seem to be prone to cancer earlier in life, and there is a high proportion of cases of medullo-blastoma in patients with FANCD1 (REFS 25,26). Cancer mortality was close to 100% by 5 years of age for children with biallelic BRCA2 mutations and in seven families with PALB2 mutations27,28. Other groups, such as those with mutations in FANCM, FANCL and RAD51C (also known as FANCO) have no cancer probands but this is probably due to the very low incidence of individuals with a mutation in these genes29-31. Within the most prevalent complementation group there have been descriptions of highly cancer-prone families who have been associated with particular FANCA mutations, but a larger population-based study has shown that the most prevalent ‘hot spots’ within FANCA are actually founder mutations25,32. Unfortunately, although the majority of the leukaemia predisposition is removed when patients receive donor transplants, patients with FA often go on to develop extremely aggressive HNSCCs with 40% cumulative incidence by the age of 40 years. Two-year survival rates in such individuals are less than 50%33.
The origin of cancers in patients with FA has been a topic of debate, and given that some form of bone marrow failure precedes many sporadic leukaemias34, it is one that may apply to some sporadic cancers. Although Fanca−/−, Fancc−/−, Fancg−/−, Fancm−/− and Fancd2−/− mice all show mildly elevated tumour incidence35, no research undertaking has determined whether this incidence increases with low-dose exposure to agents such as MMC. Furthermore, laboratory animals that are raised in a highly controlled setting may not be exposed to environmental sources of ICLs. The severe genetic instability of FA cells (both mouse and human) exposed to interstrand crosslinking agents nonetheless suggests several potential routes to tumour development.
First, the genetic instability of FA cells would make them more prone to the translocation of oncogenes or the deletion of tumour suppressor genes. Interestingly, the incidence of point mutations seems to be somewhat decreased in FA cells, but genomic rearrangements, including a selection of specific and oncogenic translocations such as loss of 1q and 3q and monosomy on chromosome 7, are favoured36-38. It has been suggested that a single unrepaired ICL that arises spontaneously in DNA could be enough to induce an oncogenic translocation in an FA cell39,40.
Second, the induction of apoptosis by unrepaired DNA damage leads to increased cell death in the haemato poietic system of patients with FA and in knockout mouse models41,42. This leads to the depletion of the haematopoietic stem cell pool, which is analogous to the depletion that is seen in other DNA repair defective disorders43. The repeated proliferation of the remaining haematopoietic stem cells in order to maintain tissue homeostasis leads to the rapid selection of tumorigenic clones. At least in mice, such clones have increased resistance to apoptosis-inducing cytokines, and these tumorigenic clones can accumulate excessive translocations44. In this manner, the increased genomic instability and selective pressure for resistance to apoptosis may combine to drive tumorigenesis in patients with FA.
A third potential mechanism of tumour susceptibility in patients with FA has recently been suggested that may account for the unusually high incidence of HNSCCs45. The accumulation of unrepaired ICLs induces a transient arrest at the G2 phase of the cell cycle, presumably until the ICLs have been removed46. In FA cells, this G2 arrest is considerably extended and may make them more susceptible to Epstein–Barr virus (EBV) and human papilloma virus (HPV) infection — a potent source of oncogenic activation. These viruses preferentially integrate into regions of the genome that have stalled replication47. In a sample of 25 American patients with FA-associated head and neck cancers, 85% of tumours were found to be positive for HPV DNA48 (although a similar European follow-up study found only 10%49). If other studies provide support for the link between the failure to repair ICLs and HPV-associated HNSCC, it may also explain why young cancer patients who receive ICL-inducing chemotherapy have an increased incidence of head and neck cancers in later life42.
The failure to repair ICLs and the consequent tumorigenesis in individuals with FA indicate that the FANC genes are essential for an ICL repair pathway, especially as inherited or targeted defects in many DNA repair genes can lead to ICL sensitivity and, in many cases, cancer-prone disorders that are similar to FA (TABLE 2). FA has thus become a model disease for the understanding of ICL repair, and the FA pathway is now thought to involve the coordination of several repair systems, including homologous recombination (HR), nucleotide excision repair (NER) and translesion synthesis (TLS). The essence of the ICL repair pathway is highlighted in FIG. 2, and the various elements are discussed below.
Table 2. Genes associated with ICL sensitivity.
| Gene | Disease | Cancer types | Functions of gene product |
|---|---|---|---|
|
FANCA, FANCG, FANCF, FANCC, FANCE and FANCB |
FA | AML and HNSCC | Forms the FA core complex that is required to activate FANCL |
| FANCL | FA | None reported (FANCL mutations account for only two cases of FA) |
Ubiquitin ligase that monoubiquitylates FANCD2–FANCI |
| FANCD2 and FANCI | FA | AML and HNSCC | Binds DNA, promotes DNA damage signalling and the recruitment of repair enzymes |
|
USP1 and WDR48 (which encodes UAF1) |
Heterodimer required for the deubiquitylation of FANCD2 and the completion of ICL repair |
||
| BRIP1 (which encodes FANCJ) | FA | AML and breast* cancer | 3′-5′ DNA helicase with preference for branched DNA substrates |
| FAN1 | DNA 5′-3′ exonuclease, and 5′-flap endonuclease. Specifically binds the monoubiquitylated form of FANCD2 |
||
|
REV1, DNA polymerase ν and DNA polymerase ζ |
Translesion synthesis polymerases that are required for DNA synthesis at the site of ICLs |
||
| HELQ | Helicase function required for the completion of ICL repair | ||
| FANCM | FA | None reported (only two cases of FA with FANCM mutations) |
5′-3′ translocase and branch migration activity. Structure-specific DNA junction binding and recruitment of FA core complex and BLM. Checkpoint activation |
|
APITD1 (which encodes MHF1), STRA13 (which encodes MHF2) and C19orf40 (which encodes FAAP24) |
FANCM accessory factors | ||
| PALB2 (which encodes FANCN) | FA and BOC |
AML, medulloblastoma, neuroblastoma, breast and ovarian* cancer and pancreatic* cancer |
Stabilizes and promotes localization of BRCA2 to DNA damage sites |
| BRCA2 (which encodes FANCD1) | FA and BOC |
AML, ALL, medulloblastoma, breast and ovarian* cancer and prostate* cancer |
Required for loading of RAD51 recombinase onto DNA |
| BRCA1 | BOC | Breast and ovarian* cancer | Ubiquitin ligase activity towards histone H2A and CTIP |
| SLX4 (which encodes FANCP)–SLX1 | FA | HNSCC (one case reported) | Structure-specific endonuclease |
| ERCC4 (which encodes XPF)–ERCC1 | XP and XFE |
Skin cancer, HNSCC* and lung cancer* |
DNA 5′-flap endonuclease |
| MUS81-EME1 | DNA 3′-flap endonuclease | ||
| RAD51 | Recombinase that searches for homology in DNA templates and promotes strand exchange |
||
| RAD51C (which encodes FANCO) | FA and BOC |
None reported (four cases of FA with RAD51C mutations), breast and ovarian cancer* |
Required for HR |
| NBS1 | NBS | B cell lymphoma | Required for HR. Component of the MRE11-RAD50-NBS1 (MRN) complex |
| BLM | BS | A broad range of cancers are increased |
5′-3′ DNA helicase, inhibits RAD51 strand invasion and promotes dissolution of Holliday junction intermediates |
|
TOP3A, RMI1 and C16orf75 (which encodes RMI2) |
Required for Holliday junction resolution by BLM | ||
| ATR | SS | None reported | Senses accumulation of RPA-coated ssDNA. It is a kinase and phosphorylates many targets that are required to activate cell cycle checkpoints and promote repair |
ALL, acute lymphocytic leukaemia; AML, acute myeloid leukaemia; APITD1, apoptosis-inducing, TAF9-like domain 1; ATR, ataxia-telangiectasia and Rad3-related; BLM, Bloom’s syndrome RecQ-helicase like; BOC, breast and ovarian cancer susceptibility; BRIP1, BRCA1-interacting protein 1; BS, Bloom’s syndrome; FA, Fanconi anaemia; FAN1, Fanconi-associated nuclease 1; HELQ, helicase, POLQ-like; HNSCC, head and neck squamous cell carcinoma; HR, homologous recombination; ICL, interstrand crosslink; NBS, Nijmegen breakage syndrome; NBS1, nibrin; PALB2, partner and localizer of BRCA2; RMI, RecQ-mediated genome instability; SS, Seckel syndrome; ssDNA, single-stranded DNA; STRA13, stimulated by retinoic acid 13; TOP3A, topoisomerase IIIα; XFE, XPF/ERCC1 progeroid syndrome; UAF1, USP1-associated factor 1; USP1, ubiquitin specific peptidase 1; WDR48, WD repeat domain 48; XP, xeroderma pigmentosum.
Documented in heterozygotes only.
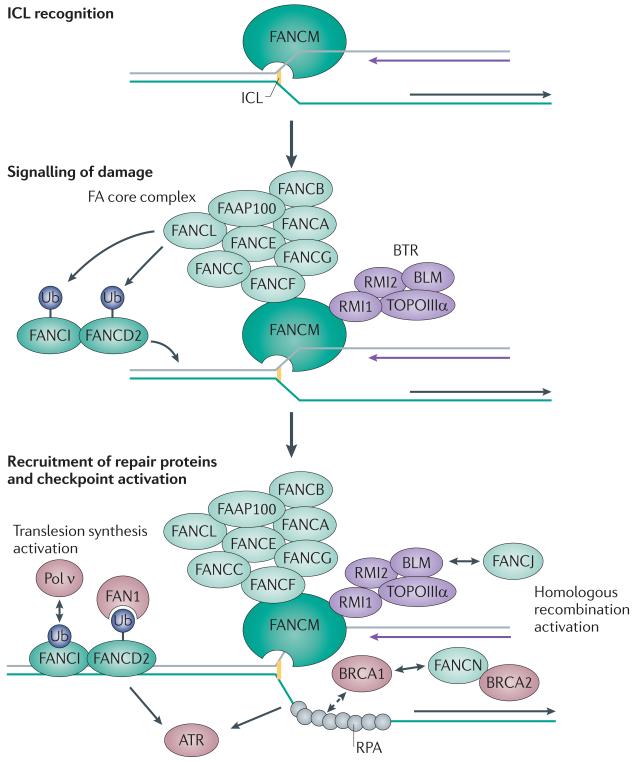
Figure 2. Activation of the Fanconi anaemia pathway coordinates DNA repair at an ICL.
Interstrand crosslink (ICL) recognition by FANCM and associated proteins leads to the recruitment of the Fanconi anaemia (FA) core complex and monoubiquitylation (Ub) of FANCD2–FANCI. Retention of FANCD2–FANCI in the chromatin is followed by the recruitment of nucleases and polymerases that are required for the repair process. FANCM also recruits the Bloom’s syndrome complex (BTR), and is involved in activating cell cycle checkpoints through the replication protein A (RPA)–ataxia telangiectasia and Rad3-related (ATR)–CHK1 signalling cascade. The BRCA1 and BRCA2 complexes initiate and regulate homologous recombination leading to the stabilization of the stalled replication fork. FAN1, Fanconi-associated nuclease 1; Pol ν, DNA polymerase ν; RMI, RecQ-mediated genome instability; TOPOIIIα, topoisomerase IIIα.
Activation of coordinated ICL repair
Seven of the FA gene products are known to form the FA core complex, which contains ubiquitin ligase activity owing to the FANCL component. Other proteins in the complex are orphans with no known functional domains but with many coiled-coil regions (reviewed in REF. 20). In some respects, the FA core complex is similar to the SKP1–CUL1–F-box (SCF) ubiquitin ligase complex, which is involved in cell cycle regulation. The FA core complex interacts with FANCM, which is a protein that belongs to the ERCC1/XPF family of structure-specific DNA-binding proteins50. ICLs that are induced during S phase activate the FA core complex to monoubiquitylate two further DNA-associated factors, FANCD2 and FANCI51,52, leading to their retention on chromatin53. In the absence of any one component of the FA core complex, ubiquitylation fails to take place — highlighting its importance as a central element of FA pathway activation.
ICL damage recognition requires FANCM when the damage is encountered by a replication fork54 (FIG. 2). As such, this mechanism of ICL repair is inefficient or non-existent in cells in G1 and in non-cycling cells55. The replication of DNA requires DNA unwinding ahead of the DNA polymerase, which is achieved by the helicase functions of the minichromosome maintenance complex (MCM2–7)56. However, replication stalls when the replisome encounters an ICL because the DNA strands cannot be separated owing to the covalent linkage between the two DNA strands57. The resulting structure is then recognized by FANCM, which contains both a degenerate inactive carboxy-terminal ERCC4 nuclease domain that binds to branched DNA structures in vitro58 and an internal domain that is required for interaction with the FA core complex59. FANCM obtains stability and specificity by interactions with another ERCC4 domain-containing protein, FAAP24, to form a FANCM–FAAP24 heterodimer58. Recruitment of the FA core complex to chromatin by FANCM–FAAP24 is thus limited to phases of the cell cycle in which replication is active, and recruitment is also limited by the regulated degradation of FANCM during mitosis55,59. Possibly because of the interaction of FANCM with the histone-like proteins MHF1 (also known as CENPS) and MHF2 (also known as CENPX), FANCM itself is constitutively associated with chromatin60,61. As the other FA proteins only enter the chromatin on replication stalling, it is likely that the interaction of FANCM and the FA core complex is further regulated. In support of this, FANCM or FAAP24 depletion leads to the loss of FANCD2-FANCI monoubiquitylation, despite normal FA core complex assembly58,59.
Unlike the polyubiquitylation signals that are important for protein degradation, the monoubiquitylation of proteins alters their activity, localization or ability to form protein–protein interactions62,63. It is unknown whether monoubiquitylation of FANCD2–FANCI precedes chromatin binding, or merely stabilizes their interaction with damaged sites, but the vast majority of chromatin-associated FANCD2 is in the monoubiquity-lated form64. Recombinant FANCD2 or FANCI have both been shown to bind independently to branched DNA structures65,66, suggesting that they might be capable of associating with sites of damage, such as those recognized by FANCM65,66. One possibility is that FANCD2 and FANCI can transiently interact with damaged DNA, but that monoubiquitylation by the proximal FA core complex stabilizes their localization. Once the damage is repaired, the subsequent dissociation of the FA core complex would allow access to the deubiquitylating enzyme ubiquitin-specific peptidase 1 (USP1). Deactivation of the pathway following DNA damage removal seems to be equally important because the deletion of USP1 also causes an FA-like phenotype in mice67 (although USP1 does not seem to be associated with human disease).
Recently, two proteins were shown to specifically interact with the modified form of FANCD2: Fanconi-associated nuclease 1 (FAN1) and DNA polymerase ν (Pol ν; also known as POLN)68-71. Both proteins colocalize with FANCD2 in nuclear foci when cells are treated with agents such as MMC, but neither is required for monoubiquitylation. FAN1 has a UBZ4-type ubiquitin-binding zinc finger domain, which is required for interaction with modified FANCD2, as well as a VRR-nuclease domain. FAN1 degrades single-stranded DNA (ssDNA) or double-stranded DNA (dsDNA) exposed ends (5′-3′ exonuclease activity) and can cut DNA on the 5′ side of a ssDNA–dsDNA junction (known as 5′-flap endonuclease activity) and would therefore have an activity that is suited to cutting DNA that is adjacent to a stalled replication fork. Such cleavage is termed ‘unhooking’, and may convert the stalled fork into a double-strand break (DSB). This has been the most widely supported model for the initial step in ICL repair, and it can be adapted to include two replication forks encountering the ICL57. The stalling of a replication fork by MMC can trigger the initiation of a new replication fork from a nearby origin72, which would approach the ICL from the other side. The frequency of double replication fork encounters with ICLs has never been experimentally determined, but could be presumed to increase as cells complete replication in late S phase. In the comprehensive model for ICL repair depicted in FIG. 3, we present mechanisms for ICL repair at each stage of the cell cycle. Given that nuclease-mediated ICL unhooking is necessary in some form at all stages of the cell cycle, followed by bypass of the crosslinked base pair by TLS DNA polymerases, it is likely that repair will require the actions of various nucleases and DNA polymerases.
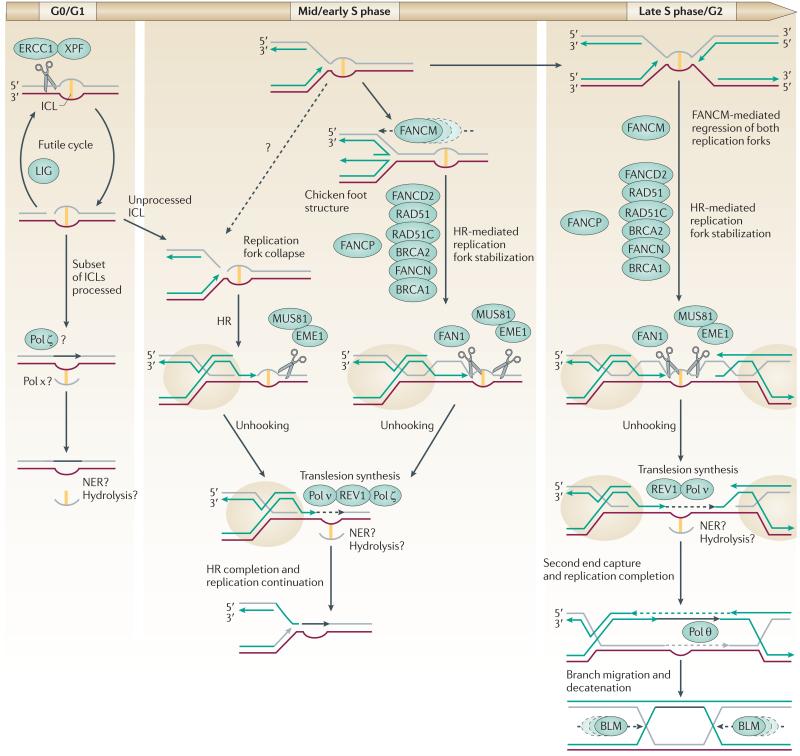
Figure 3. ICL repair at different stages of the cell cycle.
In cells in G1 phase of the cell cycle, nucleotide excision repair (NER) can remove a subset of interstrand crosslinks (ICLs). Excision by XPF–ERCC1 is followed by translesion synthesis, and excision of the ‘flipped out’ ICL. However, some lesions cannot be bypassed in this manner and a futile cycle of repair involving excision and ligation occurs. During S phase, a partially processed intermediate may be encountered by a replication fork, which leads to the collapse of the replication fork and the formation of a one-ended double-strand break (DSB). These collapses are probably avoided by FANCM-mediated fork regression and stabilization of the fork by the Fanconi anaemia (FA) pathway. This FA-stabilized homologous recombination (HR) intermediate (highlighted by beige circles) allows the nascent leading strand of DNA synthesis (indicated by a green arrow) to be extended by translesion synthesis polymerases (Pol; black dashed arrow). Unhooking of the ICL by coordinated incision by the 5′- and the 3′-flap endonucleases allows extension past the lesion. HR is then completed and replication can be re-established. If a second replication fork has encountered the ICL from the opposite direction, unhooking on the 3′ side cuts the leading strand template. The extension of the ‘left-hand’ leading strand would lead to its joining with the lagging strand of the right-hand fork, followed by second end capture and extension of the right-hand leading strand and the left-hand template strand. This process would complete replication without the re-initiation of lagging strand synthesis but would generate a double Holliday junction. This structure is resolved by the BLM-containing complex or may in some instances be cut by structure-specific nucleases (not shown). FAN1, Fanconi-associated nuclease 1.
Nucleases and polymerases in ICL repair
Hints regarding the importance of specific nucleases in ICL repair have come from individuals with biallelic mutations in ERCC4 (which encodes XPF), ERCC1 and SLX4. Hypomorphic mutations in ERCC4 cause the ultraviolet (UV) radiation-sensitive disorder xeroderma pigmentosum (XP), whereas biallelic ERCC4 or ERCC1 mutations lead to a premature ageing syndrome known as XPF/ERCC1 progeroid syndrome that causes sensitivity to ICLs73,74. SLX4 mutations cause a more typical FA phenotype, and the gene has recently been designated FANCP on the basis of the identification of FA families with biallelic mutations in this gene75,76. FAN1 and the heterodimeric nucleases MUS81–EME1, XPF–ERCC1 and SLX1–SLX4 are all required to prevent sensitivity to nitrogen mustards, MMC or platinum drugs50,77,78. These three endonucleases are specific for 3′-flap structures, which, combined with the 5′-flap activity of FAN1, could result in the cleavage of the DNA backbone on both sides of an ICL. Interestingly, SLX1–SLX4, MUS81–EME1 and XPF–ERCC1 have been shown to form a ‘supercomplex’ that can be co-immunoprecipitated from cells78; this makes it difficult to determine which particular nuclease promotes cleavage at an ICL. The possible involvement of these nucleases in downstream events, such as in the resolution of recombination intermediates, provides a further complication79-81.
One possibility is that these different nucleases exist to deal with diverse forms of DNA crosslinks — such as crosslinks that distort the DNA double helix, as well as those that do not distort the DNA double helix — and there may be particular requirements for different nucleases according to the stage in the cell cycle at which an ICL is encountered. For example, it has been shown that the amount of unhooking present in G1 phase is directly influenced by the degree of ICL-induced helical distortion82. XPF-ERCC1 could cleave DNA adjacent to ICLs that substantially distort the DNA double helix in G1, in much the same way as they cut adjacent to damaged nucleotides that distort DNA during NER50. SLX4 could also be recruited to distorting ICLs in G1 by its direct interaction with the mismatch repair (MMR) complex MUTSβ78,83,84.
The importance of helix distortion in ICL repair has been addressed using cell-free systems and plasmids containing different types of ICLs. Lesions that result in little helix distortion are only repaired in replication-competent extracts, whereas the repair of helix-distorting ICLs can also be detected in non-replicating plasmid DNA57,85. Genetic and toxicity studies suggest that even for highly distorting lesions, such as those that are induced by platinums, not all ICLs are repaired in G1 (REFS 86,87). One possibility is that these distorting lesions are not detected in the context of highly condensed heterochromatic DNA or in regions of the genome that are non-B-form DNA, such as upstream promoter elements88. Some of this damage could also undergo a futile cycle of repair, involving NER and TLS enzymes that mediate the nucleolytic cleavage of ICLs during G1 but are unable to complete the removal of the covalently linked bases87 (FIG. 3). These cleavage events would persist into S phase, leading to the direct collapse of the replication fork when it encounters the nicked DNA. HR would then repair this collapsed fork in a scenario that is similar to that in which canonical S phase ICL repair is initiated.
The encounter of an ICL by the replication fork leads to nuclease recruitment by mechanisms that are different from those that occur in G1. FAN1 is recruited to stalled replication forks by monoubiquitylated FANCD2 (REF. 70), and SLX4 could be recruited directly to the vicinity of DNA damage by its tandem UBZ domains76 that interact directly with K63-linked ubiquitin chains, which themselves represent markers of stalled replication76,89. Finally, MUS81 and XPF are relocalized as part of the SLX4 complex90. This regulated recruitment of nucleases by ubiquitylated proteins might suggest a mechanism that orients cleavage on the basis of the localization of the ubiquitylated protein in relation to the DNA substrate. In an environment such as that found at an ICL between two converging replication forks (FIG. 3), there are many potential nucleolytic substrates, so the careful regulation of DNA cleavage will be essential.
After unhooking, the nascent DNA strand must be extended past the lesion but normal replicative DNA polymerases are incapable of extension when the replisome is blocked by a crosslink91 (as opposed to the situation with base damage in which the MCM helicase and polymerase can be uncoupled92). The replication fork may be stabilized by HR (see below) and lesion bypass will require the TLS polymerases (reviewed in REF. 93).
Xenopus laevis extracts that are capable of replication have provided a useful model system for studying the repair of a single platinum crosslink, and these studies indicate that the replicative polymerase stalls about 24 nucleotides before the crosslink. At this site, a switch to TLS is presumed to take place, and this may involve Pol ν, which extends the nascent strand to within 1 base pair of the ICL before unhooking takes place57. Such a switch would be more difficult if the replication fork had collapsed much earlier owing to unhooking before replication. TLS requires the monoubiquitylation of the replication-associated factor proliferating cell nuclear antigen (PCNA) on K164, a step that also stimulates the activation of the FA pathway through FANCD2 recruitment94,95.
After unhooking, the deoxycytosine monophosphate (dCMP) transferase REV1 can insert a cytosine into the nascent strand opposite the unhooked ICL96,97. One of several other TLS polymerases may then be responsible for the extension of the nascent DNA beyond the crosslinked base pair still present in the DNA double helix. Although many of these polymerases can extend the nascent strand beyond the crosslink (reviewed in REF. 98), only depletion of Pol ζ seems to abrogate TLS extension in X. laevis extracts57. Consistent with this summary, only Pol ν, REV1 and Pol ζ are essential for ICL repair, and, owing to the error-prone nature of the reactions they promote, their involvement is potentially mutagenic71,99.
The role of HR in ICL repair
Extremely positive results have been obtained with the ICL-inducing agent carboplatin in early phase clinical trials for the treatment of triple-negative breast cancers — those that do not express HER2 (also known as ERBB2) or oestrogen and progesterone receptors and that are most likely to be associated with alterations in BRCA1 or BRCA2 (REF. 100). Platinum drugs are also beneficial when used as single agents in the treatment of BRCA1- and BRCA2-associated ovarian cancers101. The protein products of these two genes are important for HR-mediated repair of DNA damage. BRCA1, in combination with BRCA1-associated RING domain protein 1 (BARD1), exhibits ubiquitin ligase activity102 that is necessary for the proper localization of RAD51, a central player in HR; BRCA2 interacts directly with RAD51 and promotes its specific targeting to sites where recombination is initiated103,104. RAD51-deficient cells, like the tumour cell lines derived from patients with BRCA1 and BRCA2 alterations, are hypersensitive to ICL-inducing agents105,106. This observation further supports a role for HR in ICL repair.
The process of HR during ICL repair requires the generation of a DSB, because RAD51 nucleoprotein filament formation requires a free DNA end and single-stranded regions in order to stimulate the search for homology on the adjacent DNA strand107. γH2AX is a biomarker of nuclear DSBs in patients receiving chemotherapy, and can be measured in hair follicles that are extracted at various times after treatment108. Several potential sources of DSBs exist during the processing of an ICL. As discussed above, the nuclease-mediated unhooking of an ICL can occur either before encountering the replication fork — leading to the collapse of the replication fork into a one-ended DSB — or after the switch to TLS and the progression of the replication fork to within 1 base pair of the ICL57. Alternatively, the stalled replication fork may regress to form a ‘chicken foot’ structure at the site of the ICL to generate a free dsDNA end (FIG. 3). FANCM has been shown to promote fork regression in vitro109, and chicken foot structures have been shown to exist in vivo and in vitro110,111. Replication fork stabilization after formation of a chicken foot structure occurs through the recombination of the nascent regressed arm into homologous sequences ahead of the fork (FIG. 3).
Although the process of HR at a two-ended DSB (such as that caused by ionizing radiation) is well characterized (reviewed in REF. 107), there are important differences when HR takes place at a stalled replication fork. First, the association of the replication complex with the nascent DNA molecules that are adjacent to the break may facilitate recombination with the correct template sequences rather than with related, but incorrect, target sequences. Second, the MRE11–RAD50–nibrin (NBS1; known as the MRN complex) nuclease, which is normally required for end resection, is inhibited by RAD51, probably because extensive resection at a replication fork is unnecessary or potentially deleterious112. The involvement of HR factors that function specifically in ICL repair indicate that there may also be important structural differences in the types of DNA intermediates that form during the repair process. For example, cells that are deficient in the RAD51-paralogue RAD51C (also known as FANCO) are sensitive to ICL-inducing agents, and this protein may have a more important role in the repair of a one-ended, rather than a two-ended, DSB113,114. RAD51C, like BRCA1, BRCA2 and BRCA1-interacting protein 1 (BRIP1; which encodes FANCJ), was recently shown to be a breast and ovarian cancer predisposition gene in heterozygous carriers, and it causes an FA-like syndrome when homozygously mutated31,115. The HR step of ICL repair (and probably other replication-fork stalling lesions) may thus be one of the most important tumour suppressive roles of BRCA1 and BRCA2.
The completion of HR requires ‘second end capture’ and extension of the template strand by a DNA polymerase. The identity of the polymerase that carries out this DNA synthesis step is currently unknown, but after encountering a single replication fork this polymerase will promote replication restart. In the ‘late-S phase model’, when two forks converge on an ICL (FIG. 3), second end capture and extension will lead to the completion of replication and the formation of a double Holliday junction (DHJ) that can be dissolved by the BTR complex (comprised of the BLM RecQ helicase, topoisomerase IIIα (TOPOIIIα), RecQ-mediated genome instability 1 (RMI1) and RMI2) in a reaction that avoids sister chromatid exchanges (SCEs)116,117. BLM is mutated in Bloom’s syndrome, a disorder that is similar to FA in many respects. Consistent with this, the BTR complex associates with the FA core complex, providing a direct link between the upstream signalling of ICL damage recognition and the downstream resolution of the HR products59,118. A characteristic feature of Bloom’s syndrome cells is an elevated frequency of SCEs, and these levels are further increased by treatment with MMC119. It was recently shown that, in the absence of BLM, DHJs are resolved by the MUS81–EME1, SLX1–SLX4 and GEN1 endonucleases that promote SCE formation81. Strikingly, genetic exchanges between homologous chromosomes are also highly elevated in BLM-deficient cells, resulting in loss of heterozygosity120, a hallmark feature of tumorigenesis. High doses of ICL-inducing agents in wild-type cells also lead to increased SCE levels121, suggesting that the nucleolytic cleavage pathway of Holliday junction resolution occurs when BLM function is normal but when the levels of DNA damage are saturating. By promoting the BLM-mediated pathway during ICL repair, there is less chance of both SCE formation and crossing over between homologous chromosomes. The high tumour incidence in patients with Bloom’s syndrome (which includes a broader range of cancer types than those associated with FA) reflects the importance of this component of the repair process.
Suppression of NHEJ reduces ICL sensitivity
Although HR promotes error-free DSB repair in S phase, an alternative mechanism that is known as non-homologous end joining (NHEJ) exists to repair broken DNA in all phases of the cell cycle. NHEJ uses a simple splice mechanism to rejoin the two free ends of DNA. The process is initiated by binding of the KU70–KU80 heterodimer to the free dsDNA ends and involves the activation of downstream steps by the binding of DNA-dependent protein kinase catalytic subunit (DNA-PKcs)122. DNA is processed to remove any 5′- or 3′-ssDNA tails, and the resulting end is directly rejoined with a similarly processed sequence by DNA ligase IV-XRCC4 (reviewed in REF. 123). Unlike HR, in which homologous sequences are used to proofread the repair process, NHEJ is capable of generating deletions, insertions and translocations when incorrect ends are rejoined.
Human cells that are defective for KU70, KU80, DNA-PKcs, DNA ligase IV or XRCC4, are insensitive to MMC, platinums or other ICL-inducing drugs, confirming studies in yeast and mice showing that the NHEJ pathway is not required for ICL repair124,125. However, recent reports have shown that the inhibition of the NHEJ pathway in cell lines derived from patients with FA can reduce the toxicity of ICL-inducing drugs. For example, in chicken or nematode knockout models, specific FA-like defects can be rescued by the co-deletion of KU70 or ligase IV126,127. Furthermore, in FANCA- and FANCD2-deficient human cells, the high sensitivity to MMC can be rescued through the simultaneous inhibition of NHEJ by the DNA-PKcs inhibitor NU7026 (REF. 127). Analysis of mitotic spreads from these cells shows that the atypical radial chromosomes that are seen in FA cells are only rarely found when NHEJ is inactivated. These observations indicate that a major function of the FA pathway in ICL repair is to suppress spurious ligation of ICL-induced DSBs between non-homologous chromosomes (FIG. 3).
For the repair of de novo DSBs (such as those caused by ionizing radiation) HR and NHEJ provide complementary functions, and co-inhibition of HR and NHEJ leads to increased cell death128-130. However, FA cells are not defective in HR per se, so the inhibition of NHEJ in FA cells still allows them to proliferate and repair DSBs (FIG. 4). This is because the FA pathway only promotes HR at stalled replication forks, by stabilizing the intermediate that is required for unhooking and TLS. HR can still occur if the replication fork is not stabilized, but the free DNA end that is generated is more likely to be bound by KU70–KU80, which has a very high affinity for such structures131,132. KU70–KU80 promotes NHEJ with the correct adjacent sequence only some of the time, but causes the formation of radial structures if it promotes ligation with sequences on another broken chromosome133. By inhibiting the NHEJ pathway, the less active, but also less toxic, FA-independent HR pathway can re-establish the replication fork (FIG. 4). Ongoing work aims to test the efficacy of NHEJ inhibitors in diluting the severity of FA-like phenotypes in FA mouse models, but these drugs could have a wider use in the protection from ICL toxicity in the blood of patients treated with chemotherapies.
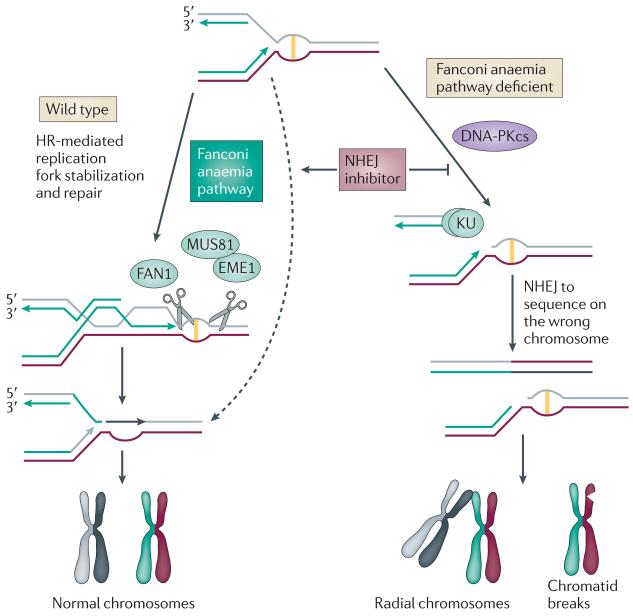
Figure 4. Suppression of ICL sensitivity by inhibition of non-homologous end Nature Reviews Cancer joining.
In wild-type cells, interstrand crosslinks (ICLs) can be repaired by homologous recombination (HR) or non-homologous end joining (NHEJ). The Fanconi anaemia (FA) pathway, however, promotes HR-dependent stabilization of the replication fork and DNA repair. In FA cells, double-strand breaks (DSBs) are frequently created that can be repaired by either HR or NHEJ. Because the DSB is one ended, NHEJ does not have a natural substrate to rejoin so these breaks can remain unrepaired (generating chromatid breaks) or may ligate with a DSB in a different chromosome (generating radial chromosomes). Suppression of NHEJ by deleting a component of this pathway or by using DNA-dependent protein kinase catalytic subunit (DNA-PKcs) inhibitors promotes the repair of the DSBs by FA-independent pathways of HR (dashed arrow).
Modulating ICL sensitivity: protect and destroy
As platinums and alkylating agents currently represent some of our most successful chemotherapeutic agents, the ability to change cell sensitivity to these drugs could have enormous therapeutic implications. As outlined in TABLE 1, the therapeutic window for ICL-based chemotherapy is restricted by dose-limiting toxicities in the blood: therefore, being able to specifically activate haematopoietic ICL resistance could theoretically raise the maximum tolerated dose. In patients with FA, a potential strategy to prevent developmental cytopenias may be the use of inhibitors of NHEJ (such as the DNA-PKcs inhibitor NU7026), but it remains to be seen whether such an approach would also protect the blood of wild-type patients undergoing chemotherapy. Current clinical practice involves the use of recombinant cytokines granulocyte colony-stimulating factor (G-CSF; known as filgrastim) and granulocyte-macrophage CSF (GM-CSF; known as sargramostim) to promote the mobilization of haematopoietic stem cells to try and recover the cells lost by ICL-induced apoptosis134. These agents are normally given in the recovery phase, and most ICL-induced cytopenias can be rescued in this manner. However, when given concurrently with crosslinking drugs, these cytokines may actually potentiate cytopenia135, as stem cells that are induced to proliferate may be more sensitive to DNA damage than quiescent cells136. It is therefore reasonable to suggest that the prevention of cytopenias may benefit from an understanding of how tumour cells become resistant to ICLs, and in applying this knowledge to promote resistance in the non-diseased cells of the blood.
One mechanism of resistance to ICL-inducing drugs is the accelerated removal of DNA adducts. Such activities have been detected in ovarian and testicular tumours that become resistant after chronic cisplatin exposure, and this is often associated with elevated levels of XPF–ERCC1, MUS81–EME1 and FA proteins137-141. FANCF-dependent resistance to cisplatin leads to increased cross-resistance to MMC139, and larger studies have identified a general pattern of cross-resistance after exposure to just one type of ICL-inducing drug142. The development of methods that increase ICL repair in normal tissue might lead to effective chemoprotection.
Conversely, as resistance to ICL-inducing drugs develops, targeted inactivation of ICL repair represents a tumour control strategy. An indirect mechanism of downregulating elevated ICL repair in tumour cells is mediated by the inhibition of extracellular signalling pathways, although little is understood about this mechanism. For example, the HER2 antibody trastuzumab can suppress the repair of cisplatin adducts in cell cultures143 and can potentiate cisplatin and melphalan treatments in breast cancer144-146. Trastuzumab may function indirectly to affect cell cycle checkpoints, and the direct targeting of checkpoint kinases has also shown some clinical promise.
The checkpoint kinase CHK1 suppresses exit from G2 phase into mitosis by phosphorylating several key cell cycle proteins147. This G2 cell cycle checkpoint is necessary to allow time to repair DNA damage, particularly when the G1 phase, p53-dependent cell cycle checkpoint is absent, as it is in most tumour cells. The absence of both cell cycle checkpoints allows p53-mutant tumour cells to enter mitosis even though they have extensive DNA damage. These cells die by a process known as mitotic catastrophe — death occurs not by the defined pathway of apoptosis, but by the loss of genetic material that is essential for survival148. This strategy may be particularly potent in re-establishing sensitivity to ICL-inducing drugs in tumours because the mechanism of death is apoptosis-independent and the catastrophe can be accentuated by larger amounts of unrepaired DNA damage. In this manner, strategies that include CHK1 inhibition, or MAPK inhibition (MAPK participates in a parallel pathway to CHK1 in G2) have shown promise in re-establishing cisplatin sensitivity in in vitro models149,150.
Concluding remarks
Over the past decade, rapid advances have been made in understanding how ICL damage is repaired following chemotherapy. We now know that ICL repair involves a complex coordination of NER, HR and TLS, as well as the suppression of NHEJ. But information is still missing, and this has meant that the translation of knowledge into the improved clinical use of ICL-damaging agents remains in its infancy. Some investment has been made in improving cell killing by these drugs, such as for use in BRCA1-deficient breast cancers101, but the core dose-limiting toxicity of ICL-inducing agents — the damage to the haematopoietic system that was ironically first seen in the casualties of the Bari disaster nearly 70 years ago — has generally been ignored. Overcoming this toxicity will allow greater therapeutic windows, and reduced side effects in patients. Increased tumour cell killing by using higher doses of ICL-inducing drugs may also limit the development of tumour resistance. Further investigation into FA, and other model diseases, may uncover the last remaining players in ICL repair, but a concerted effort to understand the precise mechanistic details of events that occur when the FA pathway is activated will undoubtedly provide key insights regarding the use of DNA crosslinking agents in chemotherapy.
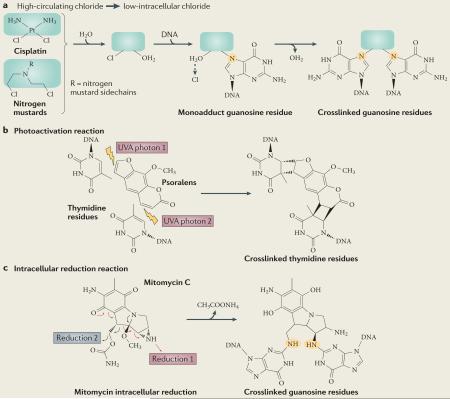
Box 1. Mechanism of interstrand crosslink formation by commonly used chemotherapeutic agents.
The mechanisms of action of the four main classes of crosslinking drugs (alkylating agents, platinums, mitomycin C and furocoumarins, and psoralens) are broadly similar in that two chemically active leaving groups are required. In bifunctional nitrogen mustards and platinum compounds these leaving groups are acquired inside the cell by the sequential displacement of two chloride ions by water molecules (see part a of the figure). This activated form of the drug can react with bases on each DNA strand, most commonly the N7-position of guanosine or adenosine (highlighted in orange in the figure)151. Mitomycin C and psoralens are natural products originally derived from fungal sources that form DNA crosslinks more directly. Mitomycin C and psoralens both contain planar rings that are activated by photon-mediated cycloaddition or cycloreduction, respectively, to attack DNA bases on opposing strands (see parts b and c of the figure). Monoadducts, in which the agent remains linked to one rather than two DNA strands, can also form if a water molecule accepts the second free leaving group. Psoralen is particularly useful for targeted tumour therapy, as its crosslinking ability is only activated in response to treatment with ultraviolet A (UVA) radiation.
Acknowledgements
This research was supported by a grant to A.J.D. and S.C.W. from the Fanconi Anaemia Research Fund. Work in S.C.W.’s laboratory is supported by the European Research Council, the Louis-Jeantet foundation, the Breast Cancer Campaign, the Swiss Bridge Foundation and Cancer Research UK.
Glossary
- Lipid peroxidation
The oxidation of fats and oils to form radicals capable of crosslinking DNA. It can occur in foods before they are eaten or can take place in the body.
- Radial chromosome
A structure thought to result from the fusion of the broken arms of non-homologous chromosomes. Such chromosomes cannot be properly segregated in most cells, resulting in either chromosome breakage or a failure in cell division.
- Homologous recombination
(HR). RAD51-mediated pairing and exchange of genetic information between homologous DNA sequences.
- Nucleotide excision repair
(NER). Nucleolytic removal of a damaged nucleotide by sequential action of 5′- and 3′-endonucleases followed by polymerase-mediated filling of the resulting gap.
- Translesion synthesis
(TLS). A DNA damage tolerance process that allows replication past DNA lesions. If the normal replicative polymerase cannot insert a base owing to damage in the template strand, it is often replaced by a lower fidelity translesion polymerase.
- Replisome
The active and assembled structure that contains the enzymes required for DNA replication.
- Mismatch repair
(MMR). A strand-specific mechanism for editing mismatched bases inserted in the daughter strand during replication. This damage is repaired by recognition of the deformity caused by the mismatch.
- Futile cycle
A DNA repair process that is repeatedly initiated, but a subsequent step re-establishes the damaged state.
- Sister chromatid exchanges
(SCEs). An exchange of genetic information between the two daughter strands of a replicated chromosome. This in itself does not result in loss of genetic information, but high SCE levels indicate that other recombination events such as the exchange of genetic material between homologous chromosomes could also occur and so lead to a loss of heterozygosity.
- Cytopenias
Loss of one or multiple types of white or red blood cells, which is often caused by the depletion of stem cell pools.
- Cell cycle checkpoints
A control mechanism that prevents cell cycle continuation in the presence of damaged, unreplicated or incompletely segregated DNA.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
DATABASES
National Cancer Institute Drug Dictionary: http://www.cancer.gov/drugdictionary cisplatin | cyclophosphamide | filgrastim | melphalan | MMC |sargramostim | trastuzumab
FURTHER INFORMATION
Stephen C. West’s homepage: http://science.cancerresearchuk.org/sci/genrecombi/
Fanconi Anaemia Mutation Database: http://www.rockefeller.edu/fanconi/
References
- 1.Goodman LS, et al. Nitrogen mustard therapy. Use of methyl-bis(β-chloroethyl)amine hydrochloride and tris(β-chloroethyl)amine hydrochloride for Hodgkin’s disease, lymphosarcoma, leukemia and certain allied and miscellaneous disorders. J. Am. Med. Assoc. 1946;132:126–132. doi: 10.1001/jama.1946.02870380008004. [DOI] [PubMed] [Google Scholar]
- 2.Eastman A. Reevaluation of interaction of cis-dichloro(ethylenediamine)platinum(II) with DNA. Biochemistry. 1986;25:3912–3915. doi: 10.1021/bi00361a026. [DOI] [PubMed] [Google Scholar]
- 3.Gargiulo D, Kumar GS, Musser SS, Tomasz M. Structural and function modification of DNA by mitomycin C. Mechanism of the DNA sequence specificity of mitomycins. Nucleic Acids Symp. Ser. 1995:169–170. [PubMed] [Google Scholar]
- 4.Kozekov ID, et al. DNA interchain cross-links formed by acrolein and crotonaldehyde. J. Am. Chem. Soc. 2003;125:50–61. doi: 10.1021/ja020778f. [DOI] [PubMed] [Google Scholar]
- 5.Stone MP, et al. Interstrand DNA cross-links induced by α, β-unsaturated aldehydes derived from lipid peroxidation and environmental sources. Acc. Chem.Res. 2008;41:793–804. doi: 10.1021/ar700246x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brooks PJ, Theruvathu JA. DNA adducts from acetaldehyde: implications for alcohol-related carcinogenesis. Alcohol. 2005;35:187–193. doi: 10.1016/j.alcohol.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Folmer V, Soares JC, Gabriel D, Rocha JB. A high fat diet inhibits δ-aminolevulinate dehydratase and increases lipid peroxidation in mice (Mus musculus) J. Nutr. 2003;133:2165–2170. doi: 10.1093/jn/133.7.2165. [DOI] [PubMed] [Google Scholar]
- 8.Ljunggren B. Severe phototoxic burn following celery ingestion. Arch. Dermatol. 1990;126:1334–1336. [PubMed] [Google Scholar]
- 9.Manderfeld MM, Schafer HW, Davidson PM, Zottola EA. Isolation and identification of antimicrobial furocoumarins from parsley. J. Food Prot. 1997;60:72–77. doi: 10.4315/0362-028x-60.1.72. [DOI] [PubMed] [Google Scholar]
- 10.Ashwood-Smith MJ, Poulton GA, Barker M, Mildenberger M. 5-Methoxypsoralen, an ingredient in several suntan preparations, has lethal, mutagenic and clastogenic properties. Nature. 1980;285:407–409. doi: 10.1038/285407a0. [DOI] [PubMed] [Google Scholar]
- 11.Dutta S, Chowdhury G, Gates KS. Interstrand cross-links generated by abasic sites in duplex DNA. J. Am. Chem. Soc. 2007;129:1852–1853. doi: 10.1021/ja067294u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bennetts LE, et al. Impact of estrogenic compounds on DNA integrity in human spermatozoa: evidence for cross-linking and redox cycling activities. Mutat. Res. 2008;641:1–11. doi: 10.1016/j.mrfmmm.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Dunnick JK, Forbes PD, Davies RE, Iverson WO. Toxicity of 8-methoxypsoralen, 5-methoxypsoralen, 3-carbethoxypsoralen, or 5-methylisopsoralen with ultraviolet radiation in the hairless (HRA/Skh) mouse. Toxicol. Appl. Pharmacol. 1987;89:73–80. doi: 10.1016/0041-008x(87)90177-3. [DOI] [PubMed] [Google Scholar]
- 14.Sanderson BJ, Shield AJ. Mutagenic damage to mammalian cells by therapeutic alkylating agents. Mutat. Res. 1996;355:41–57. doi: 10.1016/0027-5107(96)00021-8. [DOI] [PubMed] [Google Scholar]
- 15.Pechura CM, Rall DP. Veterans at Risk: The Health Effects of Mustard Gas and Lewisite. National Academy Press; Washington D. C.: 1993. [PubMed] [Google Scholar]
- 16.Tucker MA, Coleman CN, Cox RS, Varghese A, Rosenberg SA. Risk of second cancers after treatment for Hodgkin’s disease. N. Engl. J. Med. 1988;318:76–81. doi: 10.1056/NEJM198801143180203. [DOI] [PubMed] [Google Scholar]
- 17.Travis LB, et al. Risk of leukemia after platinum-based chemotherapy for ovarian cancer. N. Engl. J. Med. 1999;340:351–357. doi: 10.1056/NEJM199902043400504. [DOI] [PubMed] [Google Scholar]; This study examined the incidence of leukaemia in more than 28,000 patients with ovarian cancer who were treated with cisplatin and found a fourfold higher incidence compared with those who received radiotherapy.
- 18.Fanconi G. Familial constitutional panmyelocytopathy, Fanconi’s anemia (F. A.). I. Clinical aspects. Semin. Hematol. 1967;4:233–240. [PubMed] [Google Scholar]
- 19.Niedernhofer LJ, Lalai AS, Hoeijmakers JH. Fanconi anemia (cross)linked to DNA repair. Cell. 2005;123:1191–1198. doi: 10.1016/j.cell.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Alpi AF, Patel KJ. Monoubiquitylation in the Fanconi anemia DNA damage response pathway. DNA Repair. 2009;8:430–435. doi: 10.1016/j.dnarep.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 21.Auerbach AD. A test for Fanconi’s anemia. Blood. 1988;72:366–367. [PubMed] [Google Scholar]; This paper describes the chromosome breakage test for FA that is still widely used.
- 22.Raya A, et al. Disease-corrected haematopoietic progenitors from Fanconi anaemia induced pluripotent stem cells. Nature. 2009;460:53–59. doi: 10.1038/nature08129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenberg PS, Greene MH, Alter BP. Cancer incidence in persons with Fanconi anemia. Blood. 2003;101:822–826. doi: 10.1182/blood-2002-05-1498. [DOI] [PubMed] [Google Scholar]
- 24.Butturini A, et al. Hematologic abnormalities in Fanconi anemia: an International Fanconi Anemia Registry study. Blood. 1994;84:1650–1655. [PubMed] [Google Scholar]; A large cohort study that determined that the risk of developing haematological malignancies in patients with FA is greater than 50%.
- 25.Faivre L, et al. Association of complementation group and mutation type with clinical outcome in Fanconi anemia. Blood. 2000;96:4064–4070. [PubMed] [Google Scholar]
- 26.Hirsch B, et al. Association of biallelic BRCA2/FANCD1 mutations with spontaneous chromosomal instability and solid tumors of childhood. Blood. 2004;103:2554–2559. doi: 10.1182/blood-2003-06-1970. [DOI] [PubMed] [Google Scholar]
- 27.Reid S, et al. Biallelic mutations in PALB2 cause Fanconi anemia subtype FA-N. and predispose to childhood cancer. Nature Genet. 2007;39:162–164. doi: 10.1038/ng1947. [DOI] [PubMed] [Google Scholar]
- 28.Alter BP, Rosenberg PS, Brody LC. Clinical and molecular features associated with biallelic mutations in FANCD1/BRCA2. J. Med. Genet. 2007;44:1–9. doi: 10.1136/jmg.2006.043257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ali AM, et al. Identification and characterization of mutations in FANCL gene: a second case of Fanconi anemia belonging to FA-L complementation group. Hum. Mutat. 2009;30:E761–E770. doi: 10.1002/humu.21032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meetei AR, et al. A human ortholog of archaeal DNA repair protein Hef is defective in Fanconi anemia complementation group M. Nature Genet. 2005;37:958–963. doi: 10.1038/ng1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaz F, et al. Mutation of the RAD51C gene in a Fanconi anemia-like disorder. Nature Genet. 2010;42:406–409. doi: 10.1038/ng.570. [DOI] [PubMed] [Google Scholar]
- 32.Castella M, et al. Origin, functional role, and clinical impact of Fanconi anemia FANCA mutations. Blood. 2011;117:3759–3769. doi: 10.1182/blood-2010-08-299917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kutler DI, et al. High incidence of head and neck squamous cell carcinoma in patients with Fanconi anemia. Arch. Otolaryngol. Head Neck Surg. 2003;129:106–112. doi: 10.1001/archotol.129.1.106. [DOI] [PubMed] [Google Scholar]
- 34.Hofmann WK, Koeffler HP. Myelodysplastic syndrome. Annu. Rev. Med. 2005;56:1–16. doi: 10.1146/annurev.med.56.082103.104704. [DOI] [PubMed] [Google Scholar]
- 35.Tischkowitz M, Winqvist R. Using mouse models to investigate the biological and physiological consequences of defects in the Fanconi anaemia/breast cancer DNA repair signalling pathway. J. Pathol. 2011 Mar 9; doi: 10.1002/path.2903. doi:10.1002/path.2903. [DOI] [PubMed] [Google Scholar]
- 36.Tonnies H, et al. Clonal chromosomal aberrations in bone marrow cells of Fanconi anemia patients: gains of the chromosomal segment 3q26q29 as an adverse risk factor. Blood. 2003;101:3872–3874. doi: 10.1182/blood-2002-10-3243. [DOI] [PubMed] [Google Scholar]
- 37.Quentin S, et al. Myelodysplasia and leukemia of Fanconi anemia are associated with a specific pattern of genomic abnormalities that includes cryptic RUNX1/AML1 lesions. Blood. 2011;117:e161–e170. doi: 10.1182/blood-2010-09-308726. [DOI] [PubMed] [Google Scholar]
- 38.Sala-Trepat M, Boyse J, Richard P, Papadopoulo D, Moustacchi E. Frequencies of HPRT- lymphocytes and glycophorin A variants erythrocytes in Fanconi anemia patients, their parents and control donors. Mutat. Res. 1993;289:115–126. doi: 10.1016/0027-5107(93)90137-5. [DOI] [PubMed] [Google Scholar]
- 39.Jonnalagadda VS, Matsuguchi T, Engelward BP. Interstrand crosslink-induced homologous recombination carries an increased risk of deletions and insertions. DNA Repair. 2005;4:594–605. doi: 10.1016/j.dnarep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 40.Hinz JM, Nham PB, Salazar EP, Thompson LH. The Fanconi anemia pathway limits the severity of mutagenesis. DNA Repair. 2006;5:875–884. doi: 10.1016/j.dnarep.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 41.Ridet A, et al. Deregulated apoptosis is a hallmark of the Fanconi anemia syndrome. Cancer Res. 1997;57:1722–1730. [PubMed] [Google Scholar]
- 42.Bassal M, et al. Risk of selected subsequent carcinomas in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J. Clin. Oncol. 2006;24:476–483. doi: 10.1200/JCO.2005.02.7235. [DOI] [PubMed] [Google Scholar]
- 43.Nijnik A, et al. DNA repair is limiting for haematopoietic stem cells during ageing. Nature. 2007;447:686–690. doi: 10.1038/nature05875. [DOI] [PubMed] [Google Scholar]; This paper describes a direct link between the failure of DNA repair and the depletion of haematopoietic stem cell pools.
- 44.Haneline LS, et al. Retroviral-mediated expression of recombinant Fancc enhances the repopulating ability of fancc−/− hematopoietic stem cells and decreases the risk of clonal evolution. Blood. 2003;101:1299–1307. doi: 10.1182/blood-2002-08-2404. [DOI] [PubMed] [Google Scholar]
- 45.Park JW, et al. Deficiencies in the Fanconi anemia DNA damage response pathway increase sensitivity to HPV-associated head and neck cancer. Cancer Res. 2010;70:9959–9968. doi: 10.1158/0008-5472.CAN-10-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ceccaldi R, et al. Spontaneous abrogation of the G2 DNA damage checkpoint has clinical benefits but promotes leukemogenesis in Fanconi anemia patients. J. Clin. Invest. 2011;121:184–194. doi: 10.1172/JCI43836. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper describes a molecularly targeted therapy for FA that unfortunately promotes leukaemogenesis, supporting the hypothesis that intrinsic cancer-promoting mutations occur prior to cytopenia phenotypes.
- 47.Wilke CM, et al. FRA3B extends over a broad region and contains a spontaneous HPV16 integration site: direct evidence for the coincidence of viral integration sites and fragile sites. Hum. Mol. Genet. 1996;5:187–195. doi: 10.1093/hmg/5.2.187. [DOI] [PubMed] [Google Scholar]
- 48.Kutler DI, et al. Human papillomavirus DNA and p53 polymorphisms in squamous cell carcinomas from Fanconi anemia Patients. J. Natl Cancer Inst. 2003;95:1718–1721. doi: 10.1093/jnci/djg091. [DOI] [PubMed] [Google Scholar]
- 49.van Zeeburg HJ, et al. Clinical and molecular characteristics of squamous cell carcinomas from Fanconi anemia patients. J. Natl Cancer Inst. 2008;100:1649–1653. doi: 10.1093/jnci/djn366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ciccia A, McDonald N, West SC. Structural and functional relationships of the XPF/MUS81 family of proteins. Annu. Rev. Biochem. 2008;77:259–287. doi: 10.1146/annurev.biochem.77.070306.102408. [DOI] [PubMed] [Google Scholar]
- 51.Garcia-Higuera I, et al. Interaction of the Fanconi anemia proteins and BRCA1 in a common pathway. Mol. Cell. 2001;7:249–262. doi: 10.1016/s1097-2765(01)00173-3. [DOI] [PubMed] [Google Scholar]; This paper demonstrates a direct link between the FA pathway and the BRCA1 and BRCA2 pathway.
- 52.Smogorzewska A, et al. Identification of the FANCI protein, a monoubiquitinated FANCD2 paralog required for DNA repair. Cell. 2007;129:289–301. doi: 10.1016/j.cell.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matsushita N, et al. A FancD2-monoubiquitin fusion reveals hidden functions of Fanconi anemia core complex in DNA repair. Mol. Cell. 2005;19:841–847. doi: 10.1016/j.molcel.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 54.Niedernhofer LJ. The Fanconi anemia signalosome anchor. Mol. Cell. 2007;25:487–490. doi: 10.1016/j.molcel.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 55.Kim JM, Kee Y, Gurtan A, D’Andrea AD. Cell cycle-dependent chromatin loading of the Fanconi anemia core complex by FANCM/FAAP24. Blood. 2008;111:5215–5222. doi: 10.1182/blood-2007-09-113092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Remus D, et al. Concerted loading of Mcm2-7 double hexamers around DNA during DNA replication origin licensing. Cell. 2009;139:719–730. doi: 10.1016/j.cell.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raschle M, et al. Mechanism of replication-coupled DNA interstrand crosslink repair. Cell. 2008;134:969–980. doi: 10.1016/j.cell.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]; Complete reconstitution of ICL repair in vitro using X leavis egg extracts.
- 58.Ciccia A, et al. Identification of FAAP24, a Fanconi anemia core complex protein that interacts with FANCM. Mol. Cell. 2007;25:331–343. doi: 10.1016/j.molcel.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 59.Deans AJ, West SC. FANCM connects the genome instability disorders Bloom’s Syndrome and Fanconi Anemia. Mol. Cell. 2009;36:943–953. doi: 10.1016/j.molcel.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 60.Singh TR, et al. MHF1-MHF2, a histone-fold-containing protein complex, participates in the Fanconi anemia pathway via FANCM. Mol. Cell. 2010;37:879–886. doi: 10.1016/j.molcel.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yan Z, et al. A histone-fold complex and FANCM form a conserved DNA-remodeling complex to maintain genome stability. Mol. Cell. 2010;37:865–878. doi: 10.1016/j.molcel.2010.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pavri R, et al. Histone H2B monoubiquitination functions cooperatively with FACT to regulate elongation by RNA polymerase II. Cell. 2006;125:703–717. doi: 10.1016/j.cell.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 63.van der Horst A, et al. FOXO4 transcriptional activity is regulated by monoubiquitination and USP7/HAUSP. Nature Cell Biol. 2006;8:1064–1073. doi: 10.1038/ncb1469. [DOI] [PubMed] [Google Scholar]
- 64.Wang X, Andreassen PR, D’Andrea AD. Functional interaction of monoubiquitinated FANCD2 and BRCA2/FANCD1 in chromatin. Mol. Cell. Biol. 2004;24:5850–5862. doi: 10.1128/MCB.24.13.5850-5862.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Longerich S, San Filippo J, Liu D, Sung P. FANCI binds branched DNA and is monoubiquitinated by UBE2T-FANCL. J. Biol. Chem. 2009;284:23182–23186. doi: 10.1074/jbc.C109.038075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roques C, et al. MRE11-RAD50-NBS1 is a critical regulator of FANCD2 stability and function during DNA double-strand break repair. EMBO J. 2009;28:2400–2413. doi: 10.1038/emboj.2009.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim JM, et al. Inactivation of murine Usp1 results in genomic instability and a Fanconi anemia phenotype. Dev. Cell. 2009;16:314–320. doi: 10.1016/j.devcel.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates the importance of ubiquitylation and deubiquitylation in ICL repair.
- 68.Smogorzewska A, et al. A genetic screen identifies FAN1, a Fanconi anemia-associated nuclease necessary for DNA interstrand crosslink repair. Mol. Cell. 2010;39:36–47. doi: 10.1016/j.molcel.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kratz K, et al. Deficiency of FANCD2-associated nuclease KIAA1018/FAN1 sensitizes cells to interstrand crosslinking agents. Cell. 2010;142:77–88. doi: 10.1016/j.cell.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 70.MacKay C, et al. Identification of KIAA1018/FAN1, a DNA repair nuclease recruited to DNA damage by monoubiquitinated FANCD2. Cell. 2010;142:65–76. doi: 10.1016/j.cell.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moldovan G-L, et al. DNA polymerase POLN participates in cross-link repair and homologous recombination. Mol. Cell. Biol. 2010;30:1088–1096. doi: 10.1128/MCB.01124-09. [DOI] [PMC free article] [PubMed] [Google Scholar]; References 68, 69, 70 and 71 describe the importance of monoubiquitylated FANCD2 and FANCI in the recruitment of the essential DNA repair enzymes FAN1 and POLN.
- 72.Woodward AM, et al. Excess Mcm2–7 license dormant origins of replication that can be used under conditions of replicative stress. J. Cell Biol. 2006;173:673–683. doi: 10.1083/jcb.200602108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Niedernhofer LJ, et al. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature. 2006;444:1038–1043. doi: 10.1038/nature05456. [DOI] [PubMed] [Google Scholar]
- 74.Jaspers NG, et al. First reported patient with human ERCC1 deficiency has cerebro-oculo-facio-skeletal syndrome with a mild defect in nucleotide excision repair and severe developmental failure. Am. J. Hum. Genet. 2007;80:457–466. doi: 10.1086/512486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stoepker C, et al. SLX4, a coordinator of structure-specific endonucleases, is mutated in a new Fanconi anemia subtype. Nature Genet. 2011;43:138–141. doi: 10.1038/ng.751. [DOI] [PubMed] [Google Scholar]
- 76.Kim Y, et al. Mutations of the SLX4 gene in Fanconi anemia. Nature Genet. 2011;43:142–146. doi: 10.1038/ng.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hanada K, et al. The structure-specific endonuclease MUS81-EME1 promotes conversion of interstrand DNA crosslinks into double-strands breaks. EMBO J. 2006;25:4921–4932. doi: 10.1038/sj.emboj.7601344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Svendsen JM, et al. Mammalian BTBD12/SLX4 assembles a Holliday junction resolvase and is required for DNA repair. Cell. 2009;138:63–77. doi: 10.1016/j.cell.2009.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Al-Minawi AZ, et al. The ERCC1/XPF endonuclease is required for completion of homologous recombination at DNA replication forks stalled by inter-strand cross-links. Nucleic Acids Res. 2009;37:6400–6413. doi: 10.1093/nar/gkp705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Niedernhofer LJ, et al. The structure-specific endonuclease ERCC1-XPF is required for targeted gene replacement in embryonic stem cells. EMBO J. 2001;20:6540–6549. doi: 10.1093/emboj/20.22.6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wechsler T, Newman S, West SC. Aberrant chromosome morphology in human cells defective for Holliday junction resolution. Nature. 2011;471:642–646. doi: 10.1038/nature09790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Smeaton MB, et al. Distortion-dependent unhooking of interstrand cross-links in mammalian cell extracts. Biochemistry. 2008;47:9920–9930. doi: 10.1021/bi800925e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fink D, et al. The role of DNA mismatch repair in platinum drug resistance. Cancer Res. 1996;56:4881–4886. [PubMed] [Google Scholar]
- 84.Zhao J, Jain A, Iyer RR, Modrich PL, Vasquez KM. Mismatch repair and nucleotide excision repair proteins cooperate in the recognition of DNA interstrand crosslinks. Nucleic Acids Res. 2009;37:4420–4429. doi: 10.1093/nar/gkp399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ben-Yehoyada M, et al. Checkpoint signaling from a single DNA interstrand crosslink. Mol. Cell. 2009;35:704–715. doi: 10.1016/j.molcel.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sarkar S, Davies AA, Ulrich HD, McHugh PJ. DNA interstrand crosslink repair during G1 involves nucleotide excision repair and DNA polymerase zeta. EMBO J. 2006;25:1285–1294. doi: 10.1038/sj.emboj.7600993. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper describes an important ICL repair mechanism that is active in G1 phase of the cell cycle.
- 87.Mu D, et al. DNA interstrand cross-links induce futile repair synthesis in mammalian cell extracts. Mol. Cell. Biol. 2000;20:2446–2454. doi: 10.1128/mcb.20.7.2446-2454.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wong B, Chen S, Kwon JA, Rich A. Characterization of Z.-DNA as a nucleosome-boundary element in yeast Saccharomyces cerevisiae. Proc. Natl Acad. Sci. USA. 2007;104:2229–2234. doi: 10.1073/pnas.0611447104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ulrich HD, Walden H. Ubiquitin signalling in DNA replication and repair. Nature Rev. Mol. Cell Biol. 2010;11:479–489. doi: 10.1038/nrm2921. [DOI] [PubMed] [Google Scholar]
- 90.Munoz IM, et al. Coordination of structure-specific nucleases by human SLX4/BTBD12 is required for DNA repair. Mol. Cell. 2009;35:116–127. doi: 10.1016/j.molcel.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 91.Harder HC, Smith RG, Leroy AF. Template primer inactivation by cis- and trans-dichlorodiammine platinum for human DNA polymerase α, β, and Rauscher murine leukemia virus reverse transcriptase, as a mechanism of cytotoxicity. Cancer Res. 1976;36:3821–3829. [PubMed] [Google Scholar]
- 92.Byun TS, Pacek M, Yee MC, Walter JC, Cimprich KA. Functional uncoupling of MCM helicase and DNA polymerase activities activates the ATR-dependent checkpoint. Genes Dev. 2005;19:1040–1052. doi: 10.1101/gad.1301205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lange SS, Takata K, Wood RD. DNA polymerases and cancer. Nature Rev. Cancer. 2011;11:96–110. doi: 10.1038/nrc2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Howlett NG, Harney JA, Rego MA, Kolling FW, Glover TW. Functional interaction between the Fanconi Anemia D2 protein and proliferating cell nuclear antigen (PCNA) via a conserved putative PCNA interaction motif. J. Biol. Chem. 2009;284:28935–28942. doi: 10.1074/jbc.M109.016352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Geng L, Huntoon CJ, Karnitz LM. RAD18-mediated ubiquitination of PCNA activates the Fanconi anemia DNA repair network. J. Cell Biol. 2010;191:249–257. doi: 10.1083/jcb.201005101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Masuda Y, Ohmae M, Masuda K, Kamiya K. Structure and enzymatic properties of a stable complex of the human REV1 and REV7 proteins. J. Biol. Chem. 2003;278:12356–12360. doi: 10.1074/jbc.M211765200. [DOI] [PubMed] [Google Scholar]
- 97.Hlavin EM, Smeaton MB, Noronha AM, Wilds CJ, Miller PS. Cross-link structure affects replication-independent DNA interstrand cross-link repair in mammalian cells. Biochemistry. 2010;49:3977–3988. doi: 10.1021/bi902169q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ho TV, Scharer OD. Translesion DNA synthesis polymerases in DNA interstrand crosslink repair. Environ. Mol. Mutagen. 2010;51:552–566. doi: 10.1002/em.20573. [DOI] [PubMed] [Google Scholar]
- 99.Mirchandani KD, McCaffrey RM, D’Andrea AD. The Fanconi anemia core complex is required for efficient point mutagenesis and Rev1 foci assembly. DNA Repair. 2008;7:902–911. doi: 10.1016/j.dnarep.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Silver DP, et al. Efficacy of neoadjuvant nisplatin in triple-negative breast cancer. J. Clin. Oncol. 2010;28:1145–1153. doi: 10.1200/JCO.2009.22.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cass I, et al. Improved survival in women with BRCA-associated ovarian carcinoma. Cancer. 2003;97:2187–2195. doi: 10.1002/cncr.11310. [DOI] [PubMed] [Google Scholar]; References 100 and 101 highlight the improved efficacy of cisplatin in the treatment of BRCA1-deficient and triple-negative breast cancer.
- 102.Wu-Baer F, Lagrazon K, Yuan W, Baer R. The BRCA1/BARD1 heterodimer assembles polyubiquitin chains through an unconventional linkage involving lysine residue K6 of ubiquitin. J. Biol. Chem. 2003;278:34743–34746. doi: 10.1074/jbc.C300249200. [DOI] [PubMed] [Google Scholar]
- 103.Jensen RB, Carreira A, Kowalczykowski SC. Purified human BRCA2 stimulates RAD51-mediated recombination. Nature. 2010;467:678–683. doi: 10.1038/nature09399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thorslund T, et al. The breast cancer tumor suppressor BRCA2 promotes the specific targeting of RAD51 to single-stranded DNA. Nature Struct. Mol. Biol. 2010;17:1263–1265. doi: 10.1038/nsmb.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bhattacharyya A, Ear US, Koller BH, Weichselbaum RR, Bishop DK. The breast cancer susceptibility gene BRCA1 is required for subnuclear assembly of RAD51 and survival following treatment with the DNA cross-linking agent cisplatin. J. Biol. Chem. 2000;275:23899–23903. doi: 10.1074/jbc.C000276200. [DOI] [PubMed] [Google Scholar]
- 106.Ito M, et al. RAD51 siRNA delivered by HVJ envelope vector enhances the anti-cancer effect of cisplatin. J. Gene Med. 2005;7:1044–1052. doi: 10.1002/jgm.753. [DOI] [PubMed] [Google Scholar]
- 107.West SC. Molecular views of recombination proteins and their control. Nature Rev. Mol. Cell Biol. 2003;4:435–445. doi: 10.1038/nrm1127. [DOI] [PubMed] [Google Scholar]
- 108.Fong PC, et al. Inhibition of poly(ADP-Ribose) polymerase in tumors from BRCA mutation carriers. N. Engl. J. Med. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 109.Gari K, Décaillet C, Delannoy M, Wu L, Constantinou A. Remodeling of DNA replication structures by the branch point translocase FANCM. Proc. Natl Acad. Sci. USA. 2008;105:16107–16112. doi: 10.1073/pnas.0804777105. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work elegantly describes how FANCM may function to remodel a replication fork stalled at an ICL.
- 110.Koster DA, Palle K, Bot ES, Bjornsti MA, Dekker NH. Antitumour drugs impede DNA uncoiling by topoisomerase I. Nature. 2007;448:213–217. doi: 10.1038/nature05938. [DOI] [PubMed] [Google Scholar]
- 111.Sogo JM, Lopes M, Foiani M. Fork reversal and ssDNA accumulation at stalled replication forks owing to checkpoint defects. Science. 2002;297:599–602. doi: 10.1126/science.1074023. [DOI] [PubMed] [Google Scholar]
- 112.Hashimoto Y, Chaudhuri AR, Lopes M, Costanzo V. RAD51 protects nascent DNA from MRE11-dependent degradation and promotes continuous DNA synthesis. Nature Struct. Mol. Biol. 2010;17:1305–1311. doi: 10.1038/nsmb.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lio YC, Schild D, Brenneman MA, Redpath JL, Chen DJ. Human RAD51C deficiency destabilizes XRCC3, impairs recombination, and radiosensitizes S/G2-phase cells. J. Biol. Chem. 2004;279:42313–42320. doi: 10.1074/jbc.M405212200. [DOI] [PubMed] [Google Scholar]
- 114.Lio YC, Mazin AV, Kowalczykowski SC, Chen DJ. Complex formation by the human RAD51B and RAD51C DNA repair proteins and their activities in vitro. J. Biol. Chem. 2003;278:2469–2478. doi: 10.1074/jbc.M211038200. [DOI] [PubMed] [Google Scholar]
- 115.Meindl A, et al. Germline mutations in breast and ovarian cancer pedigrees establish RAD51C as a human cancer susceptibility gene. Nature Genet. 2010;42:410–414. doi: 10.1038/ng.569. [DOI] [PubMed] [Google Scholar]
- 116.Wu L, Hickson ID. The Bloom’s syndrome helicase suppresses crossing over during homologous recombination. Nature. 2003;426:870–874. doi: 10.1038/nature02253. [DOI] [PubMed] [Google Scholar]; This paper describes the mechanism by which BLM suppresses SCE during the repair of replication-associated DNA damage.
- 117.Wu L, Hickson ID. DNA helicases required for homologous recombination and repair of damaged replication forks. Annu. Rev. Genet. 2006;40:279–306. doi: 10.1146/annurev.genet.40.110405.090636. [DOI] [PubMed] [Google Scholar]
- 118.Meetei AR, et al. A multiprotein nuclear complex connects Fanconi anemia and Bloom syndrome. Mol. Cell. Biol. 2003;23:3417–3426. doi: 10.1128/MCB.23.10.3417-3426.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper demonstrates that the proteins associated with FA and Bloom’s syndrome participate in a common nuclear complex.
- 119.Chaganti RS, Schonberg S, German J. A manyfold increase in sister chromatid exchanges in Bloom’s syndrome lymphocytes. Proc. Natl Acad. Sci. USA. 1974;71:4508–4512. doi: 10.1073/pnas.71.11.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Luo G, et al. Cancer predisposition caused by elevated mitotic recombination in Bloom mice. Nature Genet. 2000;26:424–429. doi: 10.1038/82548. [DOI] [PubMed] [Google Scholar]
- 121.Latt SA. Sister chromatid exchange formation. Annu. Rev. Genet. 1981;15:11–55. doi: 10.1146/annurev.ge.15.120181.000303. [DOI] [PubMed] [Google Scholar]
- 122.Gottlieb TM, Jackson SP. The DNA-dependent protein kinase: requirement for DNA ends and association with Ku antigen. Cell. 1993;72:131–142. doi: 10.1016/0092-8674(93)90057-w. [DOI] [PubMed] [Google Scholar]
- 123.Lieber MR, Ma Y, Pannicke U, Schwarz K. Mechanism and regulation of human non-homologous DNA end-joining. Nature Rev. Mol. Cell Biol. 2003;4:712–720. doi: 10.1038/nrm1202. [DOI] [PubMed] [Google Scholar]
- 124.Frankenberg-Schwager M, et al. Cisplatin-mediated DNA double-strand breaks in replicating but not in quiescent cells of the yeast Saccharomyces cerevisiae. Toxicology. 2005;212:175–184. doi: 10.1016/j.tox.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 125.Collins AR. Mutant rodent cell lines sensitive to ultraviolet light, ionizing radiation and cross-linking agents: a comprehensive survey of genetic and biochemical characteristics. Mutat. Res. 1993;293:99–118. doi: 10.1016/0921-8777(93)90062-l. [DOI] [PubMed] [Google Scholar]
- 126.Pace P, et al. Ku70 corrupts DNA repair in the absence of the Fanconi anemia pathway. Science. 2010;329:219–223. doi: 10.1126/science.1192277. [DOI] [PubMed] [Google Scholar]
- 127.Adamo A, et al. Preventing nonhomologous end joining suppresses DNA repair defects of Fanconi anemia. Mol. Cell. 2010;39:25–35. doi: 10.1016/j.molcel.2010.06.026. [DOI] [PubMed] [Google Scholar]; References 126 and 127 show that an important function of the FA pathway is to suppress NHEJ at sites of ICLs.
- 128.Gurley KE, Kemp CJ. Synthetic lethality between mutation in ATM and DNA-PKcs during murine embryogenesis. Curr. Biol. 2001;11:191–194. doi: 10.1016/s0960-9822(01)00048-3. [DOI] [PubMed] [Google Scholar]
- 129.Ferreira MG, Cooper JP. Two modes of DNA double-strand break repair are reciprocally regulated through the fission yeast cell cycle. Genes Dev. 2004;18:2249–2254. doi: 10.1101/gad.315804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang H, et al. Non-homologous end-joining of ionizing radiation-induced DNA double-stranded breaks in human tumor cells deficient in BRCA1 or BRCA2. Cancer Res. 2001;61:270–277. [PubMed] [Google Scholar]
- 131.Yun MH, Hiom K. CtIP-BRCA1 modulates the choice of DNA double-strand-break repair pathway throughout the cell cycle. Nature. 2009;459:460–463. doi: 10.1038/nature07955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Blier PR, Griffith AJ, Craft J, Hardin JA. Binding of Ku protein to DNA. Measurement of affinity for ends and demonstration of binding to nicks. J. Biol. Chem. 1993;268:7594–7601. [PubMed] [Google Scholar]
- 133.Bunting SF, et al. 53BP1 inhibits homologous recombination in Brca1-deficient cells by blocking resection of DNA breaks. Cell. 2010;141:243–254. doi: 10.1016/j.cell.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pierelli L, et al. Erythropoietin addition to granulocyte colony-stimulating factor abrogates life-threatening neutropenia and increases peripheral-blood progenitor-cell mobilization after epirubicin, paclitaxel, and cisplatin combination chemotherapy: results of a randomized comparison. J. Clin. Oncol. 1999;17:1288–1295. doi: 10.1200/JCO.1999.17.4.1288. [DOI] [PubMed] [Google Scholar]
- 135.Locke F, Clark JI, Gajewski TF. A phase II study of oxaliplatin, docetaxel, and GM-CSF in patients with previously treated advanced melanoma. Cancer Chemother. Pharmacol. 2010;65:509–514. doi: 10.1007/s00280-009-1057-y. [DOI] [PubMed] [Google Scholar]
- 136.Viale A, et al. Cell-cycle restriction limits DNA damage and maintains self-renewal of leukaemia stem cells. Nature. 2009;457:51–56. doi: 10.1038/nature07618. [DOI] [PubMed] [Google Scholar]
- 137.Selvakumaran M, Pisarcik DA, Bao R, Yeung AT, Hamilton TC. Enhanced cisplatin cytotoxicity by disturbing the nucleotide excision repair pathway in ovarian cancer cell lines. Cancer Res. 2003;63:1311–1316. [PubMed] [Google Scholar]
- 138.Ferry KV, Hamilton TC, Johnson SW. Increased nucleotide excision repair in cisplatin-resistant ovarian cancer cells: role of ERCC1-XPF. Biochem. Pharmacol. 2000;60:1305–1313. doi: 10.1016/s0006-2952(00)00441-x. [DOI] [PubMed] [Google Scholar]
- 139.Taniguchi T, et al. Disruption of the Fanconi anemia-BRCA pathway in cisplatin-sensitive ovarian tumors. Nature Med. 2003;9:568–574. doi: 10.1038/nm852. [DOI] [PubMed] [Google Scholar]; This paper shows that FANCF methylation can promote ovarian cancer tumorigenesis, but that demethylation and re-expression of the protein can suppress sensitivity to ICL-inducing agents.
- 140.Dabholkar M, et al. ERCC1 and ERCC2 expression in malignant tissues from ovarian cancer patients. J. Natl Cancer Inst. 1992;84:1512–1517. doi: 10.1093/jnci/84.19.1512. [DOI] [PubMed] [Google Scholar]
- 141.Tomoda Y, et al. Functional evidence for EME1 as a marker of cisplatin resistance. Int. J. Cancer. 2009;124:2997–3001. doi: 10.1002/ijc.24268. [DOI] [PubMed] [Google Scholar]
- 142.Torres-Garcia SJ, Cousieneau L, Caplan S, Panasci L. Correlation of resistance to nitrogen mustards in chronic lymphocytic leukemia with enhanced removal of melphalan-induced DNA cross-links. Biochem. Pharmacol. 1989;38:3122–3123. doi: 10.1016/0006-2952(89)90025-7. [DOI] [PubMed] [Google Scholar]
- 143.Pietras RJ, et al. Antibody to HER-2/neu receptor blocks DNA repair after cisplatin in human breast and ovarian cancer cells. Oncogene. 1994;9:1829–1838. [PubMed] [Google Scholar]
- 144.Goncalves A, et al. High-dose alkylating agents with autologous hematopoietic stem cell support and trastuzumab in ERBB2 overexpressing metastatic breast cancer: a feasibility study. Anticancer Res. 2005;25:663–667. [PubMed] [Google Scholar]
- 145.Hurley J, et al. Docetaxel, cisplatin, and trastuzumab as primary systemic therapy for human epidermal growth factor receptor 2-positive locally advanced breast cancer. J. Clin. Oncol. 2006;24:1831–1838. doi: 10.1200/JCO.2005.02.8886. [DOI] [PubMed] [Google Scholar]
- 146.Pegram MD, et al. Results of two open-label, multicenter phase II studies of docetaxel, platinum salts, and trastuzumab in HER2-positive advanced breast cancer. J. Natl Cancer Inst. 2004;96:759–769. doi: 10.1093/jnci/djh133. [DOI] [PubMed] [Google Scholar]
- 147.Sanchez Y, et al. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science. 1997;277:1497–1501. doi: 10.1126/science.277.5331.1497. [DOI] [PubMed] [Google Scholar]
- 148.Castedo M, et al. Cell death by mitotic catastrophe: a molecular definition. Oncogene. 2004;23:2825–2837. doi: 10.1038/sj.onc.1207528. [DOI] [PubMed] [Google Scholar]
- 149.Reinhardt HC, Aslanian AS, Lees JA, Yaffe MB. p53-deficient cells rely on ATM- and ATR-mediated checkpoint signaling through the p38MAPK/ MK2 pathway for survival after DNA damage. Cancer Cell. 2007;11:175–189. doi: 10.1016/j.ccr.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Koniaras K, Cuddihy AR, Christopoulos H, Hogg A, O’Connell MJ. Inhibition of Chk1-dependent G2 DNA damage checkpoint radiosensitizes p53 mutant human cells. Oncogene. 2001;20:7453–7463. doi: 10.1038/sj.onc.1204942. [DOI] [PubMed] [Google Scholar]
- 151.Eastman A. Characterization of the adducts produced in DNA by cis-diamminedichloroplatinum(II) and cis-dichloro(ethylenediamine)platinum(II) Biochemistry. 1983;22:3927–3933. doi: 10.1021/bi00285a031. [DOI] [PubMed] [Google Scholar]
- 152.Huang H, Zhu L, Reid BR, Drobny GP, Hopkins PB. Solution structure of a cisplatin-induced DNA interstrand cross-link. Science. 1995;270:1842–1845. doi: 10.1126/science.270.5243.1842. [DOI] [PubMed] [Google Scholar]; The structure of cisplatin-crosslinked DNA reveals marked differences to the models that were previously posed, eventually leading to the design of new platinum drugs.
- 153.Knox RJ, Friedlos F, Lydall DA, Roberts JJ. Mechanism of cytotoxicity of anticancer platinum drugs: evidence that cis-diamminedichloroplatinum(II) and cis-diammine-(1,1-cyclobutanedicarboxylato) platinum(II) differ only in the kinetics of their interaction with DNA. Cancer Res. 1986;46:1972–1979. [PubMed] [Google Scholar]
- 154.Vasey PA, et al. Phase III randomized trial of docetaxel-carboplatin versus paclitaxel-carboplatin as first-line chemotherapy for ovarian carcinoma. J. Natl Cancer Inst. 2004;96:1682–1691. doi: 10.1093/jnci/djh323. [DOI] [PubMed] [Google Scholar]
- 155.Pasetto LM, D’Andrea MR, Rossi E, Monfardini S. Oxaliplatin-related neurotoxicity: how and why? Crit. Rev. Oncol. Hematol. 2006;59:159–168. doi: 10.1016/j.critrevonc.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 156.Sternberg CN, et al. Phase III trial of satraplatin, an oral platinum plus prednisone vs. prednisone alone in patients with hormone-refractory prostate cancer. Oncology. 2005;68:2–9. doi: 10.1159/000084201. [DOI] [PubMed] [Google Scholar]
- 157.Smith JW, et al. Results of a phase II open-label, nonrandomized trial of oral satraplatin in patients with metastatic breast cancer. Breast Cancer Res. Treat. 2009;118:361–367. doi: 10.1007/s10549-009-0410-5. [DOI] [PubMed] [Google Scholar]
- 158.Eckardt JR, et al. Phase II study of picoplatin as second-line therapy for patients with small-cell lung cancer. J. Clin. Oncol. 2009;27:2046–2051. doi: 10.1200/JCO.2008.19.3235. [DOI] [PubMed] [Google Scholar]; References 156, 157 and 158 describe promising Phase II and Phase III trials of new cisplatin derivatives that seem to have a lower toxicity in non-target organs than the currently used platinum drugs.
- 159.Webba da Silva M, et al. Solution structure of a DNA duplex containing mispair-aligned N4C-ethyl-N4C interstrand cross-linked cytosines. Biochemistry. 2002;41:15181–15188. doi: 10.1021/bi026368l. [DOI] [PubMed] [Google Scholar]
- 160.Morley A, Stohlman F., Jr. Cyclophosphamide-induced cyclical neutropenia. An animal model of a human periodic disease. N. Engl. J. Med. 1970;282:643–646. doi: 10.1056/NEJM197003192821202. [DOI] [PubMed] [Google Scholar]
- 161.Facon T, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet. 2007;370:1209–1218. doi: 10.1016/S0140-6736(07)61537-2. [DOI] [PubMed] [Google Scholar]
- 162.Rai KR, et al. Fludarabine compared with chlorambucil as primary therapy for chronic lymphocytic leukemia. N. Engl. J. Med. 2000;343:1750–1757. doi: 10.1056/NEJM200012143432402. [DOI] [PubMed] [Google Scholar]
- 163.Ahn JB, et al. Effect of vinorelbine, ifosfamide, and cisplatin combination chemotherapy in advanced non-small-cell lung cancer. Am. J. Clin. Oncol. 2000;23:622–628. doi: 10.1097/00000421-200012000-00020. [DOI] [PubMed] [Google Scholar]
- 164.Spielmann HP, Dwyer TJ, Hearst JE, Wemmer DE. Solution structures of psoralen monoadducted and cross-linked DNA oligomers by NMR spectroscopy and restrained molecular dynamics. Biochemistry. 1995;34:12937–12953. [PubMed] [Google Scholar]
- 165.Stoll DB, Lavin PT, Engstrom PF. Hematologic toxicity of cisplatin and mitomycin in combination for squamous cell carcinoma of esophagus. Am. J. Clin. Oncol. 1985;8:231–234. doi: 10.1097/00000421-198506000-00007. [DOI] [PubMed] [Google Scholar]
- 166.Hussain SA, et al. Long-term results of a phase II study of synchronous chemoradiotherapy in advanced muscle invasive bladder cancer. Br. J. Cancer. 2004;90:2106–2111. doi: 10.1038/sj.bjc.6601852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wolff K. Side-effects of psoralen photochemotherapy (PUVA) Br. J. Dermatol. 1990;122:117–125. doi: 10.1111/j.1365-2133.1990.tb02889.x. [DOI] [PubMed] [Google Scholar]
- 168.Querfeld C, et al. Long-term follow-up of patients with early-stage cutaneous T-cell lymphoma who achieved complete remission with psoralen plus UV-A monotherapy. Arch. Dermatol. 2005;141:305–311. doi: 10.1001/archderm.141.3.305. [DOI] [PubMed] [Google Scholar]
- 169.Janjigian YY, et al. A phase I trial of SJG-136 (NSC#694501) in advanced solid tumors. Cancer Chemother. Pharmacol. 2010;65:833–838. doi: 10.1007/s00280-009-1088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]