Abstract
After transmission through the bite of female sand flies, Leishmania spp. can cause a broad spectrum of disease manifestations collectively known as leishmaniases. L. amazonensis is endemic in South America, where it causes cutaneous, diffuse cutaneous, and visceral leishmaniasis. In this study, we have provided evidence that salivary gland extracts (SGE) of Lutzomyia longipalpis enhances L. amazonensis infection. BALB/c mice infected intradermally in the ear with 105 metacyclic promastigotes of L. amazonensis together with SGE (equivalent to 0.5 gland) showed an early onset of disease and larger lesions that contained ∼3-log-units more parasites than did controls. To determine the potential mechanism underlying this enhancement, we assessed cytokine production via reverse transcriptase PCR and enzyme-linked immunosorbent assay. Mice coinjected with parasites and SGE displayed higher levels of interleukin-10 (IL-10) mRNA in the ear tissues, as well as higher levels of IL-10 in supernatants of restimulated draining lymph node (LN) cells, than did controls. Flow cytometric analysis revealed high frequencies of IL-10-producing CD4+ and CD8+ T cells in the draining LN of mice coinjected with the parasite and SGE. In addition, we examined bone marrow derived-macrophage cultures and detected increased IL-10 but decreased nitric oxide (NO) production in cells exposed to SGE prior to infection with L. amazonensis. Together, these results imply that the sand fly saliva facilitates Leishmania evasion of the host immune system by modulating IL-10 production.
Many of the most devastating human diseases are transmitted by insect vectors. There is a unique pairing in nature between pathogens and the vector arthropods that deliver them to a suitable host. Not only do the piercing mouthparts of the insects bypass the epithelial barrier, but also the coinjected salivary gland components include potent vasodilators, blood-clotting inhibitors, and immunomodulating factors that assist their pathogens in establishing successful infections (44). The first experimental evidence of transmission of leishmaniasis by the bite of sand flies was recorded by Shortt et al. in 1931 (50), who achieved the transmission of Leishmania donovani to hamsters by the bite of Phlebotomus argentipes. It is now known that all forms of leishmaniasis are transmitted by phlebotomine sand flies (46). After the transmission of Leishmania through the bite of female sand flies, metacyclic promastigotes can establish infection in cells of the mononuclear phagocyte lineage, within which the parasite exists as a nonmotile, aflagellated, obligatory intracellular form called the amastigote. The vector-host cycle continues once the sand fly feeds on the blood that contains the amastigotes, which transform to promastigotes and replicate extracellularly in the gut of the sand fly.
Leishmania infections can cause a broad spectrum of clinical manifestations, depending on the involved parasite species and the host immune status. The disease is endemic in 88 countries in the tropical and subtropical regions of the world, with at least 12 million people infected and 350 million people at risk of infection (http://www.who.int/emc/diseases/leish/omdex/html). Among the 30 Leishmania species that are known to cause human diseases, L. amazonensis causes primarily cutaneous leishmaniasis, with occasional reports of diffuse cutaneous and visceral leishmaniasis in South America (2, 3). The phlebotomine vectors of New World leishmaniasis in Central and South America belong to the genus Lutzomyia (46).
Murine models of cutaneous leishmaniasis are valuable for the study of disease pathogenesis (7) and vaccine development (29, 58). It is well documented that Laishmania major infection in susceptible BALB/c mice is linked to a strong Th2 response (high interleukin-4 [IL-4] but low gamma interferon [IFN-γ] production) while Th1-type cytokines are prominent in the self-healing mouse strains, such as C57BL/6 and C3H/HeJ mice (42, 47). It should be pointed out that the nature of the host immune response to L. amazonensis and pathogenic mechanisms of its associated diseases remain poorly understood. Studies from other laboratories, as well as ours, indicate that in contrast to L. major infection, there is no evident polarization in Th-cell differentiation in L. amazonensis-infected mice (12, 24, 40, 52). Although nitric oxide (NO) production is critical in the control of L. amazonensis infection (53), as it is also with L. major (19), a robust IL-4 production is not observed, even in highly susceptible BALB/c mice (24, 52). In addition, studies with specific gene-deficient mice have indicated that impaired IL-12 responsiveness during L. amazonensis infection is mediated by an IL-4-independent mechanism (25, 27). IL-10 has been previously implicated in disease progression and long-term persistence of Leishmania in both human and experimental animal infections (5, 10, 30, 37). IL-10 was initially identified as a product of Th2 cells, inhibiting Th1-cell proliferation, development, and function (15). It is also known to be synthesized by a variety of other cells, including macrophages (MΦ), monocytes, keratinocytes, dendritic cells, and mast cells (35). IL-10 can inhibit the production of several proinflammatory cytokines, including tumor necrosis factor alpha (TNF-α) and IL-1β, as well as NO, in monocytes and MΦs (9, 17, 41). At present, there is limited information about the role of IL-10 in L. amazonensis infection (25, 26).
It is generally agreed that injecting parasites by syringe does not entirely mimic the natural transmission of and host responses to the parasite because in nature parasites are delivered to the host via the saliva of the sand fly (44, 46). Recent experimental evidence indicates that the saliva of the vector strongly influences the evolution and outcome of leishmanial infection (6, 49, 55, 57). In the present study, we examined the role of Lutzomyia longipalpis saliva in L. amazonensis infection, using an ear infection system initially reported by Belkaid et al. (6). Using a low dose of metacyclic parasites inoculated with salivary gland extracts (SGE) into the ear dermis, we demonstrated that L. longipalpis saliva enhanced the infectivity of L. amazonensis parasites and that vector saliva assisted L. amazonensis infection by stimulating IL-10 production in macrophages and T cells.
MATERIALS AND METHODS
Parasite culture and antigen preparation.
Infectivity of L. amazonensis (MHOM/BR/77/LTB0016) was maintained by regular passage through BALB/c mice. Promastigotes were cultured at 23°C in 20% fetal bovine serum-supplemented Schneider's Drosophila medium (Life Technologies, Rockville, Md.). To prepare parasite antigen, promastigotes obtained from stationary-phase culture (108/ml) were subjected to three cycles of freezing-thawing in phosphate-buffered saline followed by a 45-min sonication in an ice bath and then were stored in aliquots at −70°C.
SGE.
L. longipalpis sand flies, which originated in the Lapinhã cave in Minas Gerais, Brazil, were reared in the laboratory as described by Modi and Tesh (34). Salivary glands were dissected from 4- to 6-day-old, non-blood-fed female flies and stored in HEPES buffer at −70°C. Immediately before in vivo and in vitro experiments, SGE were prepared by three cycles of freezing-thawing followed by a 45-min sonication. For in vitro experiments, SGE were sterilized using syringe filters (pore size, 0.2 μm).
Mice and infection.
Female BALB/c mice were purchased from Harlan Sprague-Dawley (Indianapolis, Ind.). The mice were maintained under specific-pathogen-free conditions and used for experiments at 7 to 8 weeks of age by methods approved by the Animal Care and Use Committee of the University of Texas Medical Branch (Galveston, Tex). Age-matched mice were infected intradermally at the dorsal sites of both ears with 105 L. amazonensis metacyclic promastigotes in 10 μl of phosphate buffered saline with or without SGE (equivalent to 0.5 gland) by using a 27.5-gauge needle. L. amazonensis metacyclic promastigotes were purified through negative selection with 3A1 monoclonal antibody (a gift from D. Sacks, National Institute of Allergy and Infectious Diseases) as previously described (13). Lesion diameter in the ear was monitored using a digital caliper (Control Co., Friendswood, Tex.). The Ear parasite load was determined using a limiting-dilution assay (7).
Preparation of MΦ cultures.
Bone marrow-derived macrophages (BM-MΦ) were prepared as described by Stewart (54), and were cultured in Iscove's modified Dulbeco's medium supplemented with 2 mM l-glutamine, 10% fetal bovine serum, 1 mM sodium pyruvate, 5 × 10−5 M 2-mercaptoethanol (Sigma Chemical Co., St. Louis, Mo.), 100 U of penicillin per ml, and 100 μg of streptomycin sulfate per ml. Briefly, bone marrow was flushed with culture medium by using a 23-gauge needle and cells were dissociated by repeated flushing. The cells were seeded in either 24-well plates or 6-well plates (2 × 105 cell/ml) in a volume of 1 and 2 ml, respectively, in the presence of 30% (vol/vol) L-cells conditioned medium as a source of MΦ colony-stimulating factor. Nonadherent cells were discarded on day 3. Adherent cells composing a monolayer were maintained by changing the culture medium every other day for an additional 4 days.
To examine the effect of SGE in infection, cells were treated with medium containing a 0.5-gland equivalent of SGE/ml for 4 h prior to the addition of stationary-phase promastigotes at a parasite-to-MΦ ratio of 10:1. Infected MΦ cultures were kept at 33°C in the presence of 5% CO2 for up to 72 h. The optimal parasite dose, incubation temperature, and SGE concentration were determined in preliminary analyses. For experiments aimed at determining NO levels, control cells were treated with lipopolysaccharides (LPS; 100 ng/ml) at the infection step and used as positive controls. The number of intracellular parasites per 100 cells and the percentage of infected MΦ were evaluated at 18 to 72 h postinfection. For these experiments, cells were seeded in four-well chamber slides (Nalge Nunc International, Naperville, Ill.) and treated as described above. At the indicated time points, slides were stained with Diff-Quik (Dade Behring AG) and examined under a microscope (magnification, ×100).
RT-PCR.
Total RNA was extracted from infected mouse ears or MΦs by using Tri Reagent (Sigma) as specified by the manufacturer. Synthesis of the first-strand cDNA from 3 μg of total RNA was achieved using Moloney murine leukemia virus reverse transcriptase. (RT) (Epicentre). PCR was then performed using primers specific for IL-10, IL-4, and hypoxanthine-guanine phosphoribosyltransferase (HPRT), as described previously (43). Autoradiographs of PCR products were analyzed using a model GS-700 imaging densitometer and Molecular Analyst version 1.5 software (Bio-Rad Laboratories, Hercules, Calif.), and the results were normalized to the density of HPRT for each sample.
Measurement of cytokines by ELISA.
Draining lymph node (LN) cells were prepared from infected mice at 1, 2, and 4 weeks of infection and cultured in 24-well plates (2 × 106/ml). The cells were restimulated with L. amazonensis promastigote lysates at a concentration equivalent to 8 × 106/ml. Culture supernatants were harvested after 72 h of incubation and stored at −20°C until measurement. For in vitro BM-MΦ cultures, supernatants were collected at 48 h of infection. The sensitivity of the IL-10 enzyme-linked immunosorbent assay (ELISA) was 20 pg/ml. Background IL-10 levels were determined using the supernatants from unstimulated MΦs and were consistently between 50 and 300 pg/ml in all experiments.
Flow cytometric analysis for intracellular cytokines.
Reagents for staining cell surface markers and intracellular cytokines were purchased from BD Biosciences unless specified otherwise. Draining LN cells were collected from individual mice to prepare single-cell suspensions. Cells (2 × 106) were stimulated with whole parasite lysates (equivalent to 8 × 106 parasites) in 24-well plates for 72 h and then briefly stimulated with phorbol myristate acetate (50 ng/ml) and ionomycin (500 ng/ml) for 5 h in complete medium containing Golgi-Stop. The cells were incubated with anti-CD16/32 (clone 2.4G2) for 15 min to block nonspecific binding via the Fc receptors. They were stained with TriColor-labeled anti-CD4 (TC-CD4; Caltag Laboratories, San Jose, Calif.) and fluorescein isothiocyanate-labeled anti-CD8 (53.6.7), then fixed/permeabilized, and incubated with phycoerythrin-labeled anti-IL-10 (JES5-16E3), anti-IL-4 (BVD4-1D11), or anti-IFN-γ (XMG 1.2). Isotype controls were included in all the assays, as needed. Data were acquired using a FACScan instrument (BD Biosciences) and analyzed using CellQuest software (Becton Dickinson, San Jose, Calif.).
Nitrite assay.
The level of nitrite in the MΦ culture medium was determined by the Griess reaction using a nitrate/nitrite colorimetric assay kit (Cayman Chemical, Ann Arbor, Mich.). In brief, nitrate present in the sample was reduced to nitrite by the addition of NADPH in the presence of the enzyme nitrate reductase. End product NO2− was assayed by the Griess reaction. The absorbance was measured at 540 nm, and the NO2− concentration was determined by comparison with a standard curve of NaNO2 and expressed as micromoles per milliliter.
Statistical analysis.
Whenever variances were equal, the t test for paired data was used to assess differences between groups. If the assumptions for the t tests were violated, the Wilcoxon rank sum test was used instead, as in Table 1. All data from parasite numbers were log transformed before statistical analysis was conducted. Differences were considered significant at P < 0.05.
TABLE 1.
IL-10 mRNA levels relative to untreated MΦ culturesa
| Expt | Time (h) post-infection | Relative IL-10 mRNA level in BM-MΦ with:
|
|
|---|---|---|---|
| Parasite alone | Parasite + SGEb | ||
| 1 | 18 | 1.39 | 1.98 |
| 2 | 36 | 2.99 | 4.47 |
| 3 | 36 | 1.3 | 1.8 |
| 4 | 48 | 5.59 | 6.23 |
| 5 | 48 | 1.61 | 2.41 |
BM-MΦs were treated and infected as described in the legend to Fig. 5A. The optical intensity values of IL-10 transcripts were normalized with those of HPRT. Shown are values relative to untreated MΦ cultures from five independent experiments.
Significant differences (P < 0.05) between two experimental groups were calculated by the Wilcoxon rank sum test.
RESULTS
SGE of L. longipalpis enhances the infectivity of L. amazonensis parasites.
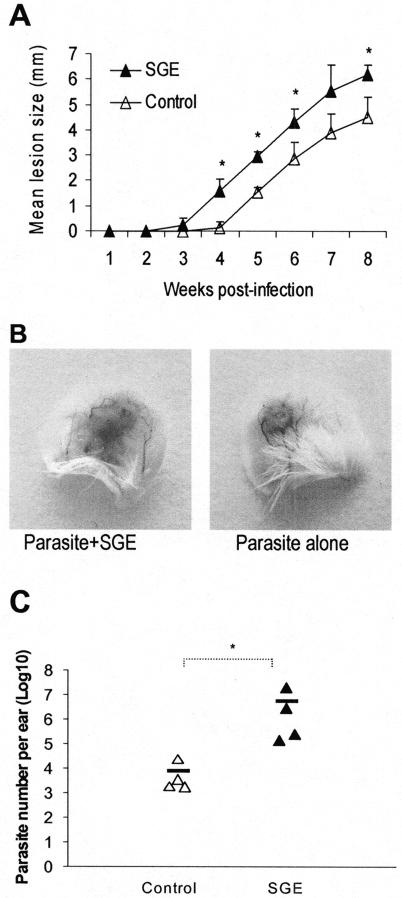
To evaluate the effect of sand fly saliva in L. amazonensis infection, we injected 105 metacyclic promastigotes into the ear dermis of BALB/c mice with and without L. longipalpis SGE (equivalent to 0.5 gland per injection). Measurable lesions were seen at 3 weeks in the SGE group and at 5 to 6 weeks in the control group (Fig. 1A). During the 8-week observation period, the lesions in the SGE group were significantly larger than those in the control group (Fig. 1A and B, P < 0.05). To exclude the possibility that SGE directly elicits additional inflammatory responses without affecting the parasite, we determined the parasite load in infected ears by using a limiting-dilution assay. At 8 weeks of infection, the average parasite number in the SGE group was 3 log units higher than that in the controls (Fig. 1C, P < 0.05). These data indicated that L. longipalpis saliva significantly enhanced the infectivity of L. amazonensis parasites.
FIG. 1.
Sand fly saliva enhances the infectivity of L. amazonensis promastigotes. (A) BALB/c mice (six to eight per group) were infected in both ears via intradermal inoculation of 105 metacyclic promastigotes alone (▵) or with a 0.5-gland equivalent of SGE (▴). The course of lesion development was monitored weekly. Lesion sizes (in millimeters) are expressed as means and standard deviations for each group. Shown is one representative of two independent experiments with similar results. (B) Photomicrographs of mouse ears at 8 weeks postinfection demonstrate enhancement of infection via sand fly saliva. (C) Ear parasite burdens at 8 weeks were determined via a limiting-dilution assay. Each triangle represents one individual mouse, and the dash represents the mean for each group. Note that there was an approximately 3-log-unit increase in parasite number in the SGE group. *, P < 0.05.
SGE promotes IL-10 and IL-4 production in draining LN cells.
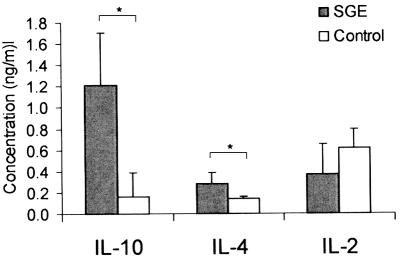
A number of scenarios can occur as a result of the SGE coinjection, contributing to enhanced infectivity of the parasite. To understand whether an altered T-cell response is one of them, we examined the production of several cytokines (IL-2, IL-4, IL-6, IL-10, TNF-α, and IFN-γ) following restimulation of T cells with parasite antigens. At 1, 2, and 4 weeks of infection, draining LN cells were collected and stimulated in vitro with parasite lysates. Culture supernatants were harvested at 24 h (for IL-2) or 72 h (for other cytokines) and measured by ELISA. Consistent with previous studies of coinjecting sand fly saliva with L. major and L. braziliensis (6, 32, 33), we detected increased production of IL-4 in the SGE group compared to the infection controls at 1 week postinfection (Fig. 2, P < 0.05). Of note, we also found a significant increase in IL-10 production (Fig. 2, P < 0.05). Surprisingly, the increase in IL-4 and IL-10 production in the SGE group was very brief, being seen at 1 week postinfection but not at other time points examined. This disparity in cytokine production did not extend to IL-2 (Fig. 2) or other cytokines such as IL-6, TNF-α, and IFN-γ (data not shown).
FIG. 2.
Sand fly saliva enhances L. amazonensis infection by promotion of a Th2-type response. BALB/c mice (seven or eight per group) were infected in both ears as described in the Legend to Fig. 1. At 1 week postinfection, draining LN cells (2 × 106/ml) were harvested and stimulated with L. amazonensis antigen (equivalent to 8 × 106 parasites/ml) for 24 or 72 h. Cytokine levels in the supernatants were determined by ELISA. Shown are the mean and standard deviation for each group. Data for IL-10 are representative of three independent experiments, while data for IL-4 and IL-2 are representative of two independent experiments. *, P < 0.05.
To validate the cytokine profiles and to identify the source of the cytokines, we collected draining LN cells from individual mice at 1 week of infection and stimulated them with parasite lysates for 3 days and then briefly with phorbol myristate acetate-ionomycin. The cells were stained for intracellular IL-4, IL-10, and IFN-γ, as well as surface expression of CD4 and CD8, and then analyzed by flow cytometry. As shown in Fig. 3, higher percentages of IL-10-producing cells were detected in CD4+ and CD8+ T-cell subsets in mice coinjected with parasite and SGE than in the infection controls (P < 0.01). The frequencies of IL-4- and IFN-γ-expressing cells were very low in both groups of mice (data not shown). Together, these results suggest that an early and brief induction of IL-10 and, to a lesser extent, of IL-4 resulting from SGE coinjection is sufficient to alter T-cell-mediated immune responses and exacerbate disease progression.
FIG. 3.
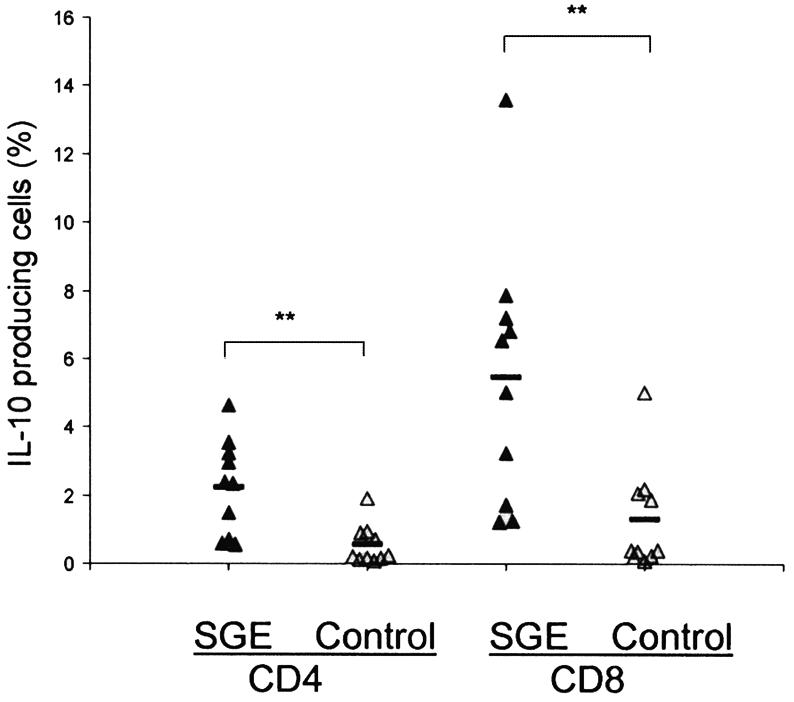
Coinjection of SGE increases the frequency of IL-10-producing T cells in draining LN. At 1 week of infection, draining LN cells were collected, stimulated with L. amazonensis antigen for 72 h, and stained for intracellular IL-10, IL-4, and IFN-γ (not included in the figure), along with CD4 and CD8 markers. Each triangle symbol represents one individual mouse, and the dash represents the mean for each group. **, P < 0.001.
SGE enhances IL-10 gene expression in the ear tissues of L. amazonensis-infected mice.
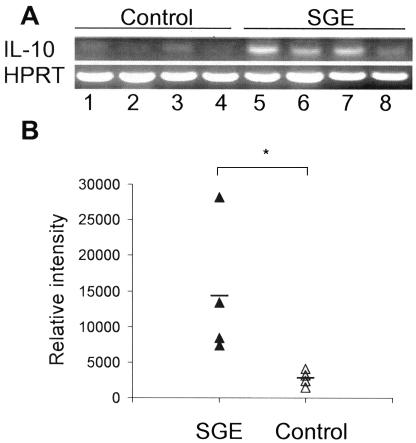
In many infection systems, local as well as systemic responses can skew cytokine profiles and hence the outcome of the infection (48). To examine whether the observed events in draining LNs are associated with local events, we assessed the immune response in ear tissues. Total RNA was extracted from ear tissues of individual mice at 1, 2, and 4 weeks of infection, and RT-PCR was performed to evaluate the expression of IL-10, IL-4, and HPRT. As with IL-10 production in draining LNs, the increase in IL-10 gene expression in the ear tissues was transient. The intensity of normalized IL-10 levels in the SGE group was about two- to threefold higher than in the infection controls at 1 week postinfection (Fig. 4, P < 0.05). No significant differences were detected at later time points (data not shown). IL-4 gene expression was not detected in two experimental groups at the tested time points (data not shown). These results are consistent with our previous studies with IL-10- and IL-4-deficient mice (25), suggesting a role for IL-10, but not IL-4, in the susceptibility of mice to L. amazonensis infection.
FIG. 4.
Coinjection of sand fly SGE significantly increases IL-10 expression in infected ear tissues. BALB/c mice (four per group) were injected in both ears with metacyclic promastigotes of L. amazonensis in the presence or absence of SGE. (A) Total RNA was isolated from ear tissues at 1 week postinfection for subsequent RT-PCR analysis. (B) Levels of IL-10 were normalized to those of HPRT and are expressed as relative intensity. Each triangle symbol represents an individual mouse, and the dash represents the mean for each group. Shown is one representative of two independent experiments. *, P < 0.05.
SGE alters IL-10 and NO production, but not parasite infection, in cultured MΦs.
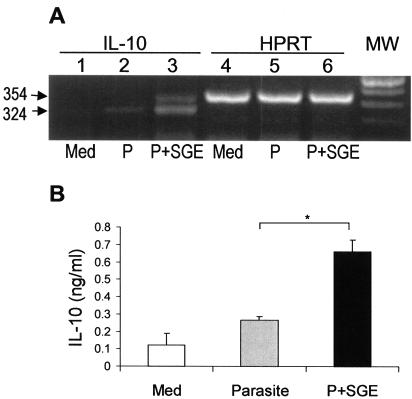
MΦs play a central role in the immune response to Leishmania infection, serving as the main targets for parasite replication as well as the effector cells for intracellular killing. Given the dramatic increase in tissue parasite load in mice coinjected with SGE (Fig. 1), we asked whether saliva has a direct effect on MΦ functions. To examine whether SGE alters IL-10 production by MΦ, we infected BM-MΦs in the presence and absence of SGE (equivalent to 0.5 gland/ml). Total RNA was harvested at 18, 36, and 48 h postinfection, and RT-PCR was carried out to assess mRNA abundance for IL-10 and HPRT. A representative PCR result at 36 h postinfection is shown in Fig. 5A. Overall, the levels of IL-10 mRNA were significantly higher in cells pretreated with SGE than in the infection controls (Table 1, P < 0.05). Likewise, the levels of IL-10 detected by ELISA were significantly higher in the supernatants from SGE-treated, L. amazonensis-infected cells (Fig. 5B, P < 0.05). The direct effect of SGE on IL-10 production in the absence of parasites was evaluated in several in vitro experiments. However, the levels of IL-10 in MΦ treated with saliva alone (0.19 ± 0.15 ng/ml) did not reach statistical significance in comparison to the medium controls. These results suggest additional use of the salivary components by the parasite in altering the host immune system.
FIG. 5.
Sand fly SGE enhances IL-10 production in L. amazonensis-infected MΦ cultures. (A) BM-MΦs (5 × 105/well) were left untreated (Med), infected with promastigotes at a parasite-to-cell ratio of 10:1 (P), or treated with SGE (equivalent to 0.5 gland/ml) for 4 h prior to infection (P+SGE). Total RNA was extracted from cells at 36 h postinfection for detection of IL-10 and HPRT mRNA by RT-PCR analysis. Shown is one representative of five independent experiments with similar results, which were summarized in Table 1. (B) Culture supernatants were harvested at 48 h postinfection for detection of IL-10 by ELISA. Data are expressed as mean and standard deviation of four replicate determinations for each group. Shown is one representative of five independent experiments. *, P < 0.05. MW, molecular weight markers.
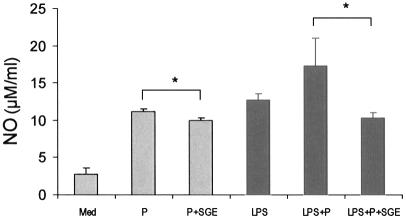
Intracellular killing of Leishmania parasites by murine MΦs depends mainly on the production of reactive oxygen intermediates and reactive nitrogen intermediates, in particular NO (8, 19, 60). NO production in MΦs is catalyzed by an inducible nitric oxide synthase, which is activated by a range of immunological stimuli or microbial products, such as TNF-α and LPS (8). To examine the effect of SGE on NO induction, we determined NO levels in BM-MΦ cultures. Following treatment with SGE (0.5 gland/ml), MΦs were infected with stationary-phase promastigotes (10 parasites per cell) in the presence or absence of LPS (100 ng/ml). Consistent with previous reports (33, 61), the addition of SGE significantly suppressed parasite-induced NO production (Fig. 6, P < 0.05). While cells infected with parasites and activated with LPS produced the highest levels of NO (17.3 ± 3.7 μM/ml), this production was also significantly suppressed by SGE (10.3 ± 0.6 μM/ml) (P < 0.05).
FIG. 6.
Pretreatment with sand fly SGE reduces NO production by L. amazonensis-infected MΦs. BM-MΦ were cultured in 24-well plates and treated with SGE (0.5 gland/ml) for 4 h prior to infection with stationary-phase promastigotes. Infection was performed at a 10:1 parasite-to-cell ratio in the presence or absence of LPS (100 ng/ml). Supernatants were collected at 48 h postinfection to evaluate the level of nitrite. Results are presented as mean and standard deviation of four replicates for each group. Shown is a representative of three independent experiments. *, P < 0.05.
Given the evident effect of SGE on IL-10 and NO production, we then examined the possibility that SGE could directly enhance parasite uptake or intracellular growth. After treatment with SGE (0.5 gland/ml) for 4 h, BM-MΦs were infected with promastigotes at different parasite-to-cell ratios (2:1 to 10:1) in triplicate. At different time points, cells were stained with Dif-Quik and counted microscopically (at least 500 cells per well). At an infection dose of 4:1, the average infection rates were 23.9 and 24.4% whereas the average parasite numbers per 100 infected MΦ were 365 and 367 at 48 h postinfection in the presence and absence of SGE, respectively. Overall, we found that SGE did not significantly change the infection rate or intracellular growth of the parasite (data not shown).
DISCUSSION
This study has indicated that L. longipalpis SGE can significantly enhance the infectivity of L. amazonensis metacyclic promastigotes in BALB/c mice. Coinjection of parasites with SGE results in an earlier onset of symptoms and more extensive lesion development with higher parasite burden than that of the infection controls (Fig. 1). The exacerbated course of infection is accompanied by an early but brief increase in IL-10 production in both the draining LN cells and ear tissues (Fig. 2 to 4). When parasite infection was initiated in the presence of SGE, BM-MΦs became compromised in their levels of NO production and produced higher levels of IL-10 (Fig. 5 and 6). Furthermore, this early and transient increase in IL-10 production may significantly skew the type of immune responses and promote disease progression in L. amazonensis-infected mice. These observations are important in our understanding of the pathogenic mechanisms of cutaneous leishmaniasis caused by this New World parasite species.
Although promastigotes of many Leishmania species can readily grow in various cell-free culture media, the virulence of these promastigotes and the host immune response to them vary significantly. Titus and Ribeiro have demonstrated that sand fly saliva dramatically enhances the infectivity of L. major in mice and that this enhancement is specific to the saliva of phlebotomine but of other blood-feeding arthropods (57). The effect of sand fly saliva in murine models of cutaneous leishmaniasis has been subsequently documented at different settings for infection with L. major and L. braziliensis (6, 18, 28, 33, 49, 55); however, the precise mechanism(s) underlying this enhancement has not been fully elucidated yet.
Sand fly saliva contains various substances that can alter the local hemostasis, enabling the fly to take the blood meal without clotting from its mammalian host (44, 46). The natural vector for L. amazonensis is Lutzomyia flaviscutellata; however, rearing this species in laboratories is very difficult. L. longipalpis is capable of transmitting L. amazonensis under experimental conditions (31, 62), and its saliva components have been extensively investigated at the level of molecular genetics and protein chemistry, as has the host immune response to the saliva (62). One of the defined proteins of L. longipalpis salivary contents, maxadilan, has been shown to exacerbate L. major infection to a degree comparable to that for the whole saliva, while vaccination against maxadilan can protect mice against infection with L. major (36). In addition to maxadilan, other sand fly salivary components can alter host immune responses (28, 45, 46, 58). It has been suggested that sand fly saliva can modulate host immune responses in a nonspecific manner, suppressing immune responses to sheep red blood cells in vivo and T-cell responsiveness to concanavalin A stimulation in vitro (56). The immunosuppressive effect of saliva for L. longipalpis and P. papatasi (an Old World species) in L. major infection is associated with enhanced IL-4 production, since this effect can be abrogated in IL-4-deficient mice or in wild-type mice treated with anti-IL-4 monoclonal antibody (6, 32, 33). The importance of IL-4 in L. major infection is further supported by the observations that the frequency of epidermal cells producing Th2 cytokines, mainly IL-4 and IL-5, is significantly increased in the presence of the salivary gland sonicates (6). In contrast to these reports, however, Kamhawi et al. have recently demonstrated that the severity of disease in IL-4-deficient mice infected by bites of L. major-infected P. papatasi sand flies is not reduced in comparison with wild-type controls (29), suggesting that IL-4 may not play a major role in modifying the outcome of L. major infection. Along these lines, it has been reported that following the bites of infected sand flies, epidermal cells produce low levels of IL-4 and no detectable levels of IL-5 (18). It is known that the contribution of IL-4 in L. major infection varies, depending on the infection systems (39). Other immunomodulatory mechanisms of sand fly saliva are associated with the inhibition of MΦ functions, including antigen presentation and IFN-γ-induced nitric oxide synthase gene expression and NO production (22, 61). In light of our data that enhanced production of IL-4 was only transiently observed in draining LN cells of mice coinoculated with parasites and SGE (Fig. 2) but not in the lesions, we speculate that IL-4 is not the main modulator in saliva-assisted disease enhancement in this study. Our previous studies with IL-4-deficient mice also support this conclusion, since we found that IL-4 does not contribute to the infection with L. amazonensis because phenotypes of infected IL-4-deficient mice were identical to those of the wild-type controls, as judged by lesion sizes, tissue parasite burdens, and levels of cytokine/chemokine gene expression (25).
IL-10 is a cytokine produced by a number of cell types and has diverse immunomodulatory properties in mice and humans (9, 35). It can suppress many effector functions of monocytes and MΦ, including the release of proinflammatory monokines and chemokines, as well as the production of NO and H2O2 (35). The role of IL-10 in promoting disease progression following L. major infection is supported by a significant reduction in lesion development in IL-10-deficient BALB/c mice (30) and enhanced susceptibility of transgenic mice with overexpression of IL-10 in antigen-presenting cells (21). At concentrations above 10 ng/ml, IL-10 can almost completely inhibit the killing of intracellular Leishmania organisms, while the addition of anti-IL-10 neutralizing antibody or IL-10-specific antisense oligonucleotides leads to an enhanced killing of parasites after stimulation with either IFN-γ or IL-7 (59). It has been suggested that IL-10-blocking agents could be administrated as a possible approach to compensate for conventional therapy to achieve an efficient, sterile cure for human visceral or cutaneous leishmaniasis (5, 30, 38).
At present, there is limited information on the role of IL-10 in sand fly-mediated enhancement of cutaneous leishmaniasis in animals. It has been reported that enhanced disease in mice coinfected with L. braziliensis and L. longipalpis saliva or with L. major and P. papatasi saliva is not correlated with increased IL-10 production (32, 33). However, in other studies it was observed that L. longipalpis maxadilan significantly induced IL-10 production in vitro and in vivo (11, 51). In the present study, we also found a significant increase in the expression of IL-10 in ear tissues and in draining LN cells of mice coinfected with SGE; however, these changes were observed only at 1 week postinfection. It appears that both CD4+ and CD8+ T cells contribute to the enhanced production of IL-10 in the SGE group by the first week of infection (Fig. 3). Furthermore, MΦs pretreated with SGE and then infected with L. amazonensis promastigotes produced high levels of IL-10 (Fig. 5, Table 1). These results provide strong evidence that exposure to sand fly saliva can skew the initial cellular responses to the parasites and the course of leishmaniasis, leading to enhanced disease progression.
This study indicates that an early increase in IL-10 production makes mice more susceptible to L. amazonensis infection. Several mechanisms are likely to be responsible for this enhancement. First, IL-10, can act to prevent antigen-specific T-cell proliferation and cytokine production (9, 41) by down-regulating the surface expression of class II major histocompatibility complex MHC and costimulatory molecules, such as CD80/B7.1, CD86/B7.2, and intercellular cell adhesion molecule 1 (ICAM-1), on antigen-presenting cells, mostly dendritic cells (9, 22). Second, sand fly saliva may modulate host immune responses to the parasite at the level of dendritic cell maturation and T-cell responsiveness by virtue of IL-10 production. At present, there are no reports of whether sand fly saliva has any direct effect on dendritic cells. Since the natural transmission requires deposition of the parasites into the dermal layer, the responsiveness of Langerhans' cells and dendritic cells to parasite infection in the presence of sand fly saliva warrants active investigation. Although the molecular basis of how sand fly saliva modulates IL-10 production remains unclear, available evidence suggests that increased levels of cyclic AMP (in the case of L. Longipalpis) (20) and 5′-AMP and adenosine (in the case of Phlebotomus) (45) are responsible for many activities of sand fly saliva, including vasodilatory activity and suppression of cytokine signaling pathways and cytokine expression in human leukocytes (20, 45). While these components promote IL-10 promotion, they suppress TNF-α, NO, and IL-12 production in murine MΦs (14, 16, 23, 45). Therefore, it is not surprising to find a direct correlation between high IL-10 production and L. amazonensis infection in the presence of L. longipalpis saliva.
Saliva-mediated uptake of promastigotes and intracellular growth of the parasites are documented in MΦ infection of L. infantum with P. papatasi saliva (64) but not in coinfection of L. major with P. papatasi saliva (22). We did not detect increased infection rates or parasite numbers per infected MΦ following in vitro treatment with SGE (data not shown). Possible explanations for this discrepancy may involve the differences in parasite species and dose or culture conditions used in these studies. It is also highly possible that saliva-mediated disease development can be achieved by more than one mechanism, including enhanced recruitment of immature target cells (MΦ and dendritic cells) for infection and suppression of their killing mechanisms. Sand fly saliva has chemotactic effect on murine MΦ (1, 64). Availability of MΦ at the site of inoculation is a crucial factor for the preliminary establishment of Leishmania infections in the skin, because parasites that fail to invade MΦ are quickly eliminated by the cytotoxic activity of NK cells, neutrophils, and eosinophils in the vertebrate host.
In summary, this study demonstrates that L. longipalpis saliva significantly enhances L. amazonensis infection and provides evidence for the first time that this enhancement correlates with an increased production of IL-10 by T cells and MΦs. It remains to be investigated whether IL-10 is the sole mediator by which sand fly saliva assists Leishmania infection or whether IL-10 acts with other mediators such as transforming growth factor β (4, 63). At this stage, it remains unclear whether there are differences in the nature or kinetics of cellular recruitment at the site of parasite injection in the presence or absence of sand fly saliva. Further studies will be directed at verifying the molecular basis of this enhanced infection and the mechanisms by which sand fly saliva modulates host immune responses to the parasite.
Acknowledgments
We thank Jiaxiang Ji, Hai Qi, and Joseph Masterson for helpful discussions and Mardelle Susman for assisting with the manuscript preparation.
This work was supported in part by NIH grants AI43003 to L. Soong and AI39540 to G. Lanzaro.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Anjili, C. O., P. A. Mbati, R. W. Mwangi, J. I. Githure, J. O. Olobo, L. L. Robert, and D. K. Koech. 1995. The chemotactic effect of Phlebotomus duboscqi (Diptera: Psychodidae) salivary gland lysates to murine monocytes. Acta Trop. 60:97-100. [DOI] [PubMed] [Google Scholar]
- 2.Barral, A., R. Badaro, M. Barral-Netto, G. Grimaldi, Jr., H. Momem, and E. M. Carvalho. 1986. Isolation of Leishmania mexicana amazonensis from the bone marrow in a case of American visceral leishmaniasis. Am. J. Trop. Med. Hyg. 35:732-734. [DOI] [PubMed] [Google Scholar]
- 3.Barral, A., D. Pedral-Sampaio, G. Grimaldi Junior, H. Momen, D. McMahon-Pratt, A. Ribeiro de Jesus, R. Almeida, R. Badaro, M. Barral-Netto, E. M. Carvalho, et al. 1991. Leishmaniasis in Bahia, Brazil: evidence that Leishmania amazonensis produces a wide spectrum of clinical disease. Am. J. Trop. Med. Hyg. 44:536-546. [DOI] [PubMed] [Google Scholar]
- 4.Barral-Netto, M., A. Barral, C. E. Brownell, Y. A. Skeiky, L. R. Ellingsworth, D. R. Twardzik, and S. G. Reed. 1992. Transforming growth factor-beta in leishmanial infection: a parasite escape mechanism. Science 257:545-548. [DOI] [PubMed] [Google Scholar]
- 5.Belkaid, Y., K. F. Hoffmann, S. Mendez, S. Kamhawi, M. C. Udey, T. A. Wynn, and D. L. Sacks. 2001. The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti-IL-10 receptor antibody for sterile cure. J. Exp. Med. 194:1497-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belkaid, Y., S. Kamhawi, G. Modi, J. Valenzuela, N. Noben-Trauth, E. Rowton, J. Ribeiro, and D. L. Sacks. 1998. Development of a natural model of cutaneous leishmaniasis: powerful effects of vector saliva and saliva preexposure on the long-term outcome of Leishmania major infection in the mouse ear dermis. J. Exp. Med. 188:1941-1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belkaid, Y., S. Mendez, R. Lira, N. Kadambi, G. Milon, and D. Sacks. 2000. A natural model of Leishmania major infection reveals a prolonged “silent” phase of parasite amplification in the skin before the onset of lesion formation and immunity. J. Immunol. 165:969-977. [DOI] [PubMed] [Google Scholar]
- 8.Bogdan, C., M. Rollinghoff, and A. Diefenbach. 2000. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr. Opin. Immunol. 12:64-76. [DOI] [PubMed] [Google Scholar]
- 9.Bogdan, C., Y. Vodovotz, and C. Nathan. 1991. Macrophage deactivation by interleukin 10. J. Exp. Med. 174:1549-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bourreau, E., G. Prevot, J. Gardon, R. Pradinaud, and P. Launois. 2001. High intralesional interleukin-10 messenger RNA expression in localized cutaneous leishmaniasis is associated with unresponsiveness to treatment. J. Infect. Dis. 184:1628-1630. [DOI] [PubMed] [Google Scholar]
- 11.Bozza, M., M. B. Soares, P. T. Bozza, A. R. Satoskar, T. G. Diacovo, F. Brombacher, R. G. Titus, C. B. Shoemaker, and J. R. David. 1998. The PACAP-type I receptor agonist maxadilan from sand fly saliva protects mice against lethal endotoxemia by a mechanism partially dependent on IL-10. Eur. J. Immunol. 28:3120-3127. [DOI] [PubMed] [Google Scholar]
- 12.Colmenares, M., S. Kar, K. Goldsmith-Pestana, and D. McMahon-Pratt. 2002. Mechanisms of pathogenesis: differences amongst Leishmania species. Trans. R. Soc. Trop. Med. Hyg. 96(Suppl. 1):S3-S7. [DOI] [PubMed] [Google Scholar]
- 13.Courret, N., E. Prina, E. Mougneau, E. M. Saraiva, D. L. Sacks, N. Glaichenhaus, and J. C. Antoine. 1999. Presentation of the Leishmania antigen LACK by infected macrophages is dependent upon the virulence of the phagocytosed parasites. Eur. J. Immunol. 29:762-773. [DOI] [PubMed] [Google Scholar]
- 14.Eigler, A., B. Siegmund, U. Emmerich, K. H. Baumann, G. Hartmann, and S. Endres. 1998. Anti-inflammatory activities of cAMP-elevating agents: enhancement of IL-10 synthesis and concurrent suppression of TNF production. J. Leukoc. Biol. 63:101-107. [DOI] [PubMed] [Google Scholar]
- 15.Fiorentino, D. F., M. W. Bond, and T. R. Mosmann. 1989. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J. Exp. Med. 170:2081-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gasperini, S., L. Crepaldi, F. Calzetti, L. Gatto, C. Berlato, F. Bazzoni, A. Yoshimura, and M. A. Cassatella. 2002. Interleukin-10 and cAMP-elevating agents cooperate to induce suppressor of cytokine signaling-3 via a protein kinase A-independent signal. Eur. Cytokine Netw. 13:47-53. [PubMed] [Google Scholar]
- 17.Gazzinelli, R. T., I. P. Oswald, S. L. James, and A. Sher. 1992. IL-10 inhibits parasite killing and nitrogen oxide production by IFN-gamma-activated macrophages. J. Immunol. 148:1792-1796. [PubMed] [Google Scholar]
- 18.Gillespie, R. D., M. L. Mbow, and R. G. Titus. 2000. The immunomodulatory factors of bloodfeeding arthropod saliva. Parasite Immunol. 22:319-331. [DOI] [PubMed] [Google Scholar]
- 19.Green, S. J., M. S. Meltzer, J. B. Hibbs, Jr., and C. A. Nacy. 1990. Activated macrophages destroy intracellular Leishmania major amastigotes by an l-arginine-dependent killing mechanism. J. Immunol. 144:278-283. [PubMed] [Google Scholar]
- 20.Grevelink, S. A., J. Osborne, J. Loscalzo, and E. A. Lerner. 1995. Vasorelaxant and second messenger effects of maxadilan. J. Pharmacol. Exp. Ther. 272:33-37. [PubMed] [Google Scholar]
- 21.Groux, H., F. Cottrez, M. Rouleau, S. Mauze, S. Antonenko, S. Hurst, T. McNeil, M. Bigler, M. G. Roncarolo, and R. L. Coffman. 1999. A transgenic model to analyze the immunoregulatory role of IL-10 secreted by antigen-presenting cells. J. Immunol. 162:1723-1729. [PubMed] [Google Scholar]
- 22.Hall, L. R., and R. G. Titus. 1995. Sand fly vector saliva selectively modulates macrophage functions that inhibit killing of Leishmania major and nitric oxide production. J. Immunol. 155:3501-3506. [PubMed] [Google Scholar]
- 23.Hasko, G., C. Szabo, Z. H. Nemeth, V. Kvetan, S. M. Pastores, and E. S. Vizi. 1996. Adenosine receptor agonists differentially regulate IL-10, TNF-alpha, and nitric oxide production in RAW 264.7 macrophages and in endotoxemic mice. J. Immunol. 157:4634-4640. [PubMed] [Google Scholar]
- 24.Ji, J., J. Sun, H. Qi, and L. Soong. 2002. Analysis of T helper cell responses during infection with Leishmania amazonensis. Am. J. Trop. Med. Hyg. 66:338-345. [DOI] [PubMed] [Google Scholar]
- 25.Ji, J., J. Sun, and L. Soong. 2003. Impaired expression of inflammatory cytokines and chemokines at early stages of infection with Leishmania amazonensis. Infect. Immun. 71:4278-4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones, D. E., M. R. Ackermann, U. Wille, C. A. Hunter, and P. Scott. 2002. Early enhanced Th1 response after Leishmania amazonensis infection of C57BL/6 interleukin-10-deficient mice does not lead to resolution of infection. Infect. Immun. 70:2151-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones, D. E., L. U. Buxbaum, and P. Scott. 2000. IL-4-independent inhibition of IL-12 responsiveness during Leishmania amazonensis infection. J. Immunol. 165:364-372. [DOI] [PubMed] [Google Scholar]
- 28.Kamhawi, S. 2000. The biological and immunomodulatory properties of sand fly saliva and its role in the establishment of Leishmania infections. Microbes Infect. 2:1765-1773. [DOI] [PubMed] [Google Scholar]
- 29.Kamhawi, S., Y. Belkaid, G. Modi, E. Rowton, and D. Sacks. 2000. Protection against cutaneous leishmaniasis resulting from bites of uninfected sand flies. Science 290:1351-1354. [DOI] [PubMed] [Google Scholar]
- 30.Kane, M. M., and D. M. Mosser. 2001. The role of IL-10 in promoting disease progression in leishmaniasis. J. Immunol. 166:1141-1147. [DOI] [PubMed] [Google Scholar]
- 31.Killick-Kendrick, R., A. J. Leaney, P. D. Ready, and D. H. Molyneux. 1977. Leishmania in phlebotomid sandflies. IV. The transmission of Leishmania mexicana amazonensis to hamsters by the bite of experimentally infected Lutzomyia longipalpis. Proc. R. Soc. London Ser. B 196:105-115. [DOI] [PubMed] [Google Scholar]
- 32.Lima, H. C., and R. G. Titus. 1996. Effects of sand fly vector saliva on development of cutaneous lesions and the immune response to Leishmania braziliensis in BALB/c mice. Infect. Immun. 64:5442-5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mbow, M. L., J. A. Bleyenberg, L. R. Hall, and R. G. Titus. 1998. Phlebotomus papatasi sand fly salivary gland lysate down-regulates a Th1, but up-regulates a Th2, response in mice infected with Leishmania major. J. Immunol. 161:5571-5577. [PubMed] [Google Scholar]
- 34.Modi, G. B., and R. B. Tesh. 1983. A simple technique for mass rearing Lutzomyia longipalpis and Phlebotomus papatasi (Diptera: Psychodidae) in the laboratory. J. Med. Entomol. 20:568-569. [DOI] [PubMed] [Google Scholar]
- 35.Moore, K. W., R. de Waal Malefyt, R. L. Coffman, and A. O'Garra. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19:683-765. [DOI] [PubMed] [Google Scholar]
- 36.Morris, R. V., C. B. Shoemaker, J. R. David, G. C. Lanzaro, and R. G. Titus. 2001. Sandfly maxadilan exacerbates infection with Leishmania major and vaccinating against it protects against L. major infection. J. Immunol. 167:5226-5230. [DOI] [PubMed] [Google Scholar]
- 37.Murphy, M. L., U. Wille, E. N. Villegas, C. A. Hunter, and J. P. Farrell. 2001. IL-10 mediates susceptibility to Leishmania donovani infection. Eur. J. Immunol. 31:2848-2856. [DOI] [PubMed] [Google Scholar]
- 38.Murray, H. W., C. M. Lu, S. Mauze, S. Freeman, A. L. Moreira, G. Kaplan, and R. L. Coffman. 2002. Interleukin-10 (IL-10) in experimental visceral leishmaniasis and IL-10 receptor blockade as immunotherapy. Infect. Immun. 70:6284-6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noben-Trauth, N., W. E. Paul, and D. L. Sacks. 1999. IL-4- and IL-4 receptor-deficient BALB/c mice reveal differences in susceptibility to Leishmania major parasite substrains. J. Immunol. 162:6132-6140. [PubMed] [Google Scholar]
- 40.Qi, H., V. Popov, and L. Soong. 2001. Leishmania amazonensis-dendritic cell interactions in vitro and the priming of parasite-specific CD4+ T cells in vivo. J. Immunol. 167:4534-4542. [DOI] [PubMed] [Google Scholar]
- 41.Ralph, P., I. Nakoinz, A. Sampson-Johannes, S. Fong, D. Lowe, H. Y. Min, and L. Lin. 1992. IL-10, T lymphocyte inhibitor of human blood cell production of IL-1 and tumor necrosis factor. J. Immunol. 148:808-814. [PubMed] [Google Scholar]
- 42.Reiner, S. L., and R. M. Locksley. 1995. The regulation of immunity to Leishmania major. Annu. Rev. Immunol. 13:151-177. [DOI] [PubMed] [Google Scholar]
- 43.Reiner, S. L., S. Zheng, D. B. Corry, and R. M. Locksley. 1993. Constructing polycompetitor cDNAs for quantitative PCR. J. Immunol. Methods 165:37-46. [DOI] [PubMed] [Google Scholar]
- 44.Ribeiro, J. M. 1995. Blood-feeding arthropods: live syringes or invertebrate pharmacologists? Infect. Agents Dis. 4:143-152. [PubMed] [Google Scholar]
- 45.Ribeiro, J. M., O. Katz, L. K. Pannell, J. Waitumbi, and A. Warburg. 1999. Salivary glands of the sand fly Phlebotomus papatasi contain pharmacologically active amounts of adenosine and 5′-AMP. J. Exp. Biol. 202:1551-1559. [DOI] [PubMed] [Google Scholar]
- 46.Sacks, D., and S. Kamhawi. 2001. Molecular aspects of parasite-vector and vector-host interactions in leishmaniasis. Annu. Rev. Microbiol. 55:453-483. [DOI] [PubMed] [Google Scholar]
- 47.Sacks, D., and N. Noben-Trauth. 2002. The immunology of susceptibility and resistance to Leishmania major in mice. Nat. Rev. Immunol. 2:845-858. [DOI] [PubMed] [Google Scholar]
- 48.Salazar-Mather, T. P., T. A. Hamilton, and C. A. Biron. 2000. A chemokine-to-cytokine-to-chemokine cascade critical in antiviral defense. J. Clin. Investig. 105:985-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Samuelson, J., E. Lerner, R. Tesh, and R. Titus. 1991. A mouse model of Leishmania braziliensis braziliensis infection produced by coinjection with sand fly saliva. J. Exp. Med. 173:49-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shortt, H. E., R. Smith, C. Swaminath, and K. Krishnan. 1931. Transmission of Indian kala-azar by the bite of Phlebotomus argentipes. Indian J. Med. Res. 18:1373-1375. [PubMed] [Google Scholar]
- 51.Soares, M. B., R. G. Titus, C. B. Shoemaker, J. R. David, and M. Bozza. 1998. The vasoactive peptide maxadilan from sand fly saliva inhibits TNF-alpha and induces IL-6 by mouse macrophages through interaction with the pituitary adenylate cyclase-activating polypeptide (PACAP) receptor. J. Immunol. 160:1811-1816. [PubMed] [Google Scholar]
- 52.Soong, L., C. H. Chang, J. Sun, B. J. Longley, Jr., N. H. Ruddle, R. A. Flavell, and D. McMahon-Pratt. 1997. Role of CD4+ T cells in pathogenesis associated with Leishmania amazonensis infection. J. Immunol. 158:5374-5383. [PubMed] [Google Scholar]
- 53.Soong, L., J. C. Xu, I. S. Grewal, P. Kima, J. Sun, B. J. Longley, Jr., N.H. Ruddle, D. McMahon-Pratt, and R. A. Flavell. 1996. Disruption of CD40-CD40 ligand interactions results in an enhanced susceptibility to Leishmania amazonensis infection. Immunity 4:263-273. [DOI] [PubMed] [Google Scholar]
- 54.Stewart, C. C. 1981. Murine mononuclear phagocytes from bone marrow, p. 5. In D. O. Adams, P. J. Edelson, and H. Koren (ed.), Methods for studying mononuclear phagocytes. Academic Press, Inc., New York, N.Y.
- 55.Theodos, C. M., J. M. Ribeiro, and R. G. Titus. 1991. Analysis of enhancing effect of sand fly saliva on Leishmania infection in mice. Infect. Immun. 59:1592-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Titus, R. G. 1998. Salivary gland lysate from the sand fly Lutzomyia longipalpis suppresses the immune response of mice to sheep red blood cells in vivo and concanavalin A in vitro. Exp. Parasitol. 89:133-136. [DOI] [PubMed] [Google Scholar]
- 57.Titus, R. G., and J. M. Ribeiro. 1988. Salivary gland lysates from the sand fly Lutzomyia longipalpis enhance Leishmania infectivity. Science 239:1306-1308. [DOI] [PubMed] [Google Scholar]
- 58.Valenzuela, J. G., Y. Belkaid, M. K. Garfield, S. Mendez, S. Kamhawi, E. D. Rowton, D. L. Sacks, and J. M. Ribeiro. 2001. Toward a defined anti-Leishmania vaccine targeting vector antigens: characterization of a protective salivary protein. J. Exp. Med. 194:331-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vieth, M., A. Will, K. Schroppel, M. Rollinghoff, and A. Gessner. 1994. Interleukin-10 inhibits antimicrobial activity against Leishmania major in murine macrophages. Scand. J. Immunol. 40:403-409. [DOI] [PubMed] [Google Scholar]
- 60.von Stebut, E., Y. Belkaid, B. Nguyen, M. Wilson, D. L. Sacks, and M.C. Udey. 2002. Skin-derived macrophages from Leishmania major-susceptible mice exhibit interleukin-12- and interferon-gamma-independent nitric oxide production and parasite killing after treatment with immunostimulatory DNA. J. Investig. Dermatol. 119:621-628. [DOI] [PubMed] [Google Scholar]
- 61.Waitumbi, J., and A. Warburg. 1998. Phlebotomus papatasi saliva inhibits protein phosphatase activity and nitric oxide production by murine macrophages. Infect. Immun. 66:1534-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Warburg, A., E. Saraiva, G. C. Lanzaro, R. G. Titus, and F. Neva. 1994. Saliva of Lutzomyia longipalpis sibling species differs in its composition and capacity to enhance leishmaniasis. Philos. Trans. R. Soc. London Ser B. 345:223-230. [DOI] [PubMed] [Google Scholar]
- 63.Wilson, M. E., T. J. Recker, N. E. Rodriguez, B. M. Young, K. K. Burnell, J. A. Streit, and J. N. Kline. 2002. The TGF-beta response to Leishmania chagasi in the absence of IL-12. Eur. J. Immunol. 32:3556-3565. [DOI] [PubMed] [Google Scholar]
- 64.Zer, R., I. Yaroslavski, L. Rosen, and A. Warburg. 2001. Effect of sand fly saliva on Leishmania uptake by murine macrophages. Int. J. Parasitol. 31:810-814. [DOI] [PubMed] [Google Scholar]