Abstract
Background
The association between daily protein intake and osteoporosis is still controversial and only a few studies have explored the issue in Korea. This study investigated the relationship between daily protein intake and the prevalence of osteoporosis in Korean adults.
Methods
This study analyzed data extracted from the Korean National Health and Nutrition Examination Survey 4. Participants were aged 19 years or older and had never been treated for osteoporosis. The percentage of calories coming from protein intake was assessed by 24-hour recall method, and participants were divided into three groups according to recommended daily dietary protein intake as a proportion of total daily calories (i.e., <10%, 10%-20%, and >20%). A lumbar or femur neck bone mineral density T-score less than -2.5 was indicative of the presence osteoporosis. The influence of daily protein intake on the prevalence of osteoporosis was analyzed.
Results
In both sexes, the group with the highest protein intake had significantly lower odds of developing lumber osteoporosis when compared to the group with the lowest protein intake, after adjusting for associated factors (females: odds ratio [OR], 0.618; 95% confidence interval [CI], 0.610 to 0.626; P for trend <0.001; males: OR, 0.695; 95% CI, 0.685 to 0.705; P for trend <0.001).
Conclusion
Sufficient daily protein intake lowered the prevalence of osteoporosis in Korean adults. Further prospective studies are necessary to verify the preventive effect of adequate protein intake on osteoporosis.
Keywords: Osteoporosis, Bone Density, Proteins
INTRODUCTION
Osteoporosis is a systemic skeletal disorder characterized by decreased bone mass with abnormal bone microstructure leading to an increased risk of fracture.1) As the older adult population increases, the incidence of osteoporosis and osteoporotic fractures have continued to increase.2,3) According to a recent study on osteoporosis in the Korean population, osteoporotic femoral neck fractures and accompanying mortality have increased.4)
Many reports have described the strong association between development of osteoporosis and smoking, excessive alcohol consumption, lack of physical activity, and low body weight. However, the association between dietary protein intake and bone metabolism is still controversial. Bones are composed of 30% protein, and because the amino acids in bone collagen that are necessary for bone metabolism cannot be reused, dietary protein intake is essential for bone metabolism.5,6) A recent prospective study of the association between protein intake and bone mineral density (BMD) reported that intake of animal protein is positively correlated with BMD in females.7) In contrast, other studies report that a high protein diet could have a harmful effect on bone health due to excessive acid from protein catabolism possibly forcing bones to act as a buffer, resulting in calcium leaching from bones.8-10) This is supported by the observation that the calcium content of urine increases after a high protein diet.11) However, other studies propose that high levels of calcium could overcome the detrimental effects of a high protein diet.6)
It is well-known that appropriate calcium and vitamin D intake is required to prevent and treat osteoporosis,12) but the relationship between dietary protein intake and osteoporosis remains unclear. This study investigated the association between osteoporosis and protein intake in a sample Korean population using data from a national nutrition survey.
METHODS
1. Participants
The data analyzed in this study were extracted from the Korean National Health and Nutrition Examination Survey IV (KNHANES IV, 2008-2009). The analysis included 6,952 subjects (3,100 male and 3,852 female), all of whom had available dual energy X-ray absorptiometry (DXA) results, were older than 19 years of age, and had not been previously treated for osteoporosis.
2. Data Collection
BMD was measured by DXA (DISCOVERY-W fan-beam densitometer, Hologic Inc., Bedford, MA, USA). Average BMD of the lumbar spine (L1-L4), and BMD of the femoral neck were measured. Subjects with a BMD less than -2.5 standard deviations of the normal adult value (T-score ≤ -2.5) were diagnosed with osteoporosis. The percentage of calories coming from protein was assessed by 24-hour recall method. The optimal protein intake for Korean adults is 7% to 20% of total calories. Participants were divided into three groups based on their daily protein intake (<10%, 10%-20%, and >20%).13)
Variables used to analyze socioeconomic status and lifestyle included age, education, household income, alcohol intake, smoking status, and physical activity level. Education level was divided into three groups (<6, 7-12, and >12 years) and household income by quartiles. An individual that drank more than one cup of alcohol per month was considered to consume alcohol. Only current smokers were included in the smoker group. Based on the International Physical Activity Questionnaire, walking, moderate intensity, and vigorous physical activity were calculated in minutes per week as follows: time spent sitting multiplied by average metabolic equivalent (MET) cost (walking, 3.3 MET; moderate intensity, 4.0; and vigorous, 8.0; MET-min per week). Individuals with more than 600 MET-min per week were considered minimally active. Individuals who participated in vigorous physical activity more than three days per week and had more than 1,500 MET-min per week, or individuals with any combination of physical activity and more than 6,000 MET-min per week were categorized as the health enhancing physical activity group. Individuals who did not belong to either of these two groups were classified as inactive.14)
Obesity was defined as a body mass index (BMI) ≥25 kg/m2, per the Asian Pacific criterion of obesity.15) Thyroid disease, menopause and hormone therapy were included in the analysis as concomitant medical conditions.
3. Statistical Analysis
According to the sample design of KNHANES IV, statistical analysis was performed with consideration given to sampling units, stratified variables, and weighted values. Categorical variables and continuous variables were analyzed by chi-square test and Student t-test. Binary logistic regression analysis was performed to determine the association between protein intake and prevalence of osteoporosis. Other variables associated with prevalence of osteoporosis were adjusted via three steps. Step 1 included age, height, and obesity. Age was dichotomized by 50. Step 2 included the same variables as step 1, plus thyroid disease in males and menopause and hormone therapy in females. Step 3 included the variables of step 2, plus household income, education level, physical activity, alcohol intake, and smoking status. Stratified groups according to dietary protein intake as a proportion of total calories consumed were selected as dummy variables, and a trend analysis of osteoporosis prevalence in the stratified groups was performed using multiple linear regression at each step. In all tests, a P-value < 0.05 was considered significant. All statistical analyses were conducted using the Stata/SE ver. 10.0 (Stata Co., College Station, TX, USA) package.
RESULTS
1. Subject Characteristics at Baseline
With the weighed value, a total of 35,256,072 subjects were included. Of the 17,964,433 males, 523,817 subjects (2.9%) had lumbar osteoporosis. Of the 17,291,639 females, 1,622,181 (9.3%) had osteoporosis. Both males and females in the osteoporosis group were older and had a lower household incomes and education levels than the normal BMD group. Moderate intensity physical activity was more common in those with osteoporosis, but vigorous activity was less common compared to the normal BMD group. Weight, height, BMI, total calories consumed, protein intake, and calcium intake were also lower in the osteoporosis group compared to the normal BMD group. Additionally, there were significant differences in concomitant medical conditions between groups (Table 1).
Table 1.
Subject characteristics at baseline
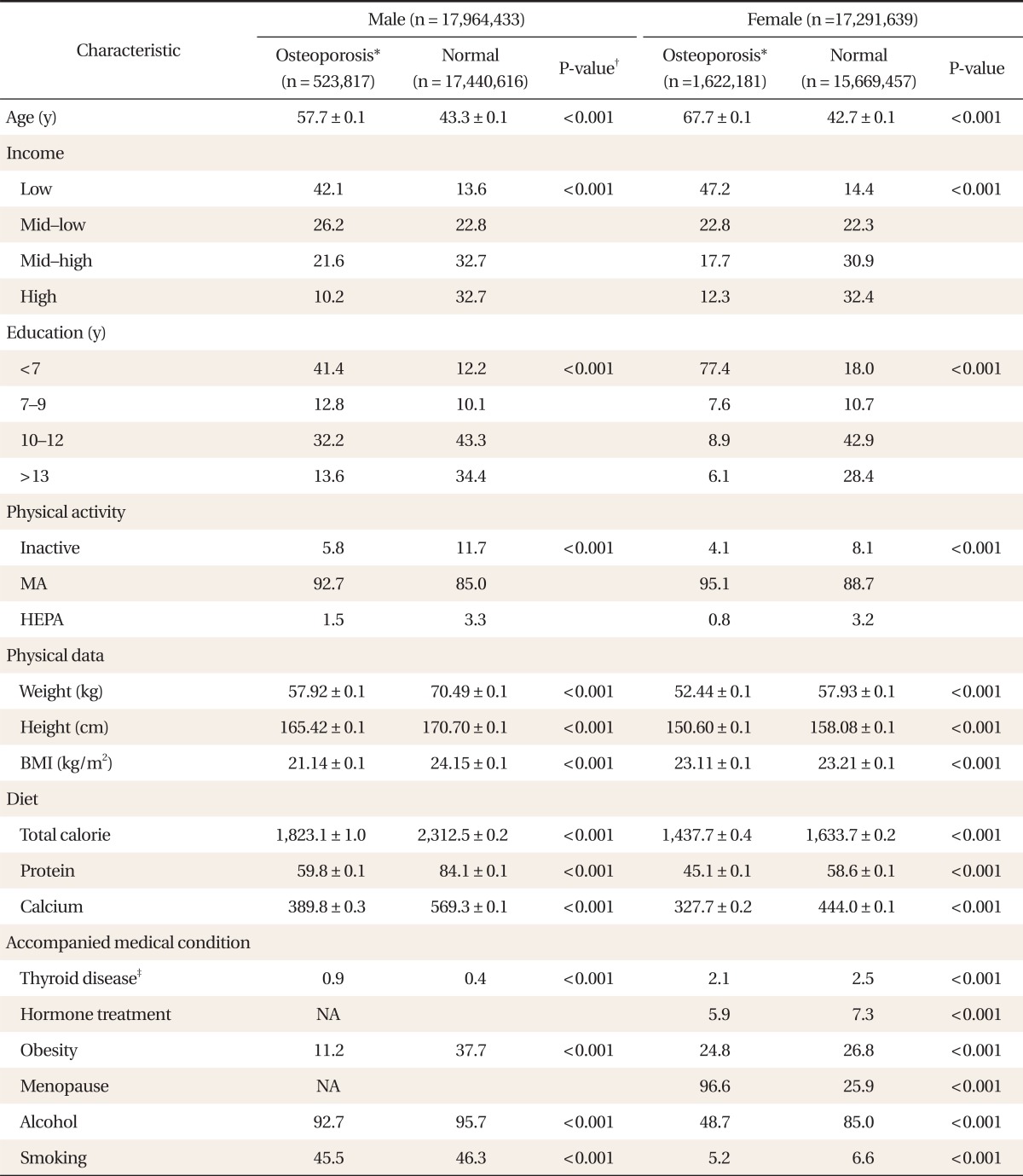
Values are presented as mean ± SD or estimated proportion (%). BMI was calculated as weight in kilograms divided by height in meters squared.
MA: minimally active, HEPA: health enhancing physical activity, BMI: body mass index, NA: not available.
*Lumbar T score ≤ -2.5 was used to classify the normal and osteoporosis groups. †Categorical data were analyzed by Pearson chi-squared test and numerical data were analyzed by two independent sample t-test. ‡Current prevalence of any thyroid disorders.
2. Prevalence of Osteoporosis and Dietary Protein in Females
Osteoporosis prevalence determined by lumbar and femoral neck T-scores showed the high protein proportion group to be statistically less likely to have osteoporosis in all statistical adjustment steps and an increase in osteoporosis prevalence as dietary protein proportion increased (Table 2).
Table 2.
Association between proportion of daily protein intake and prevalence of osteoporosis in females
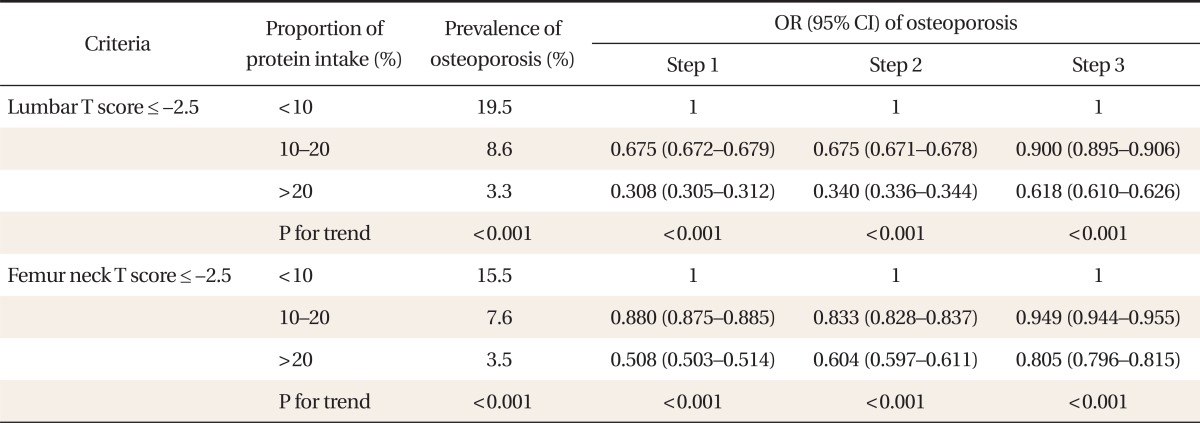
Step 1: adjusted for age, height, and obesity, Step 2: adjusted for thyroid disease, menopausal status and hormone therapy, plus all the variables in step 1, Step 3: adjusted for income of family, education, physical activity, alcohol, daily calcium intake (mg/d), and smoking plus all the variables in step 2.
OR: odds ratio, CI: confidence interval.
3. Prevalence of Osteoporosis and Dietary Protein Proportion in Males
Prevalence of lumbar osteoporosis showed the high protein proportion group to be statistically less likely to have osteoporosis in all statistical adjustment steps and an increase in osteoporosis prevalence as dietary protein proportion increased. However, femur neck osteoporosis showed statistical significance only in step 1 and 2 (Table 3).
Table 3.
Association between proportion of daily protein intake and prevalence of osteoporosis in males
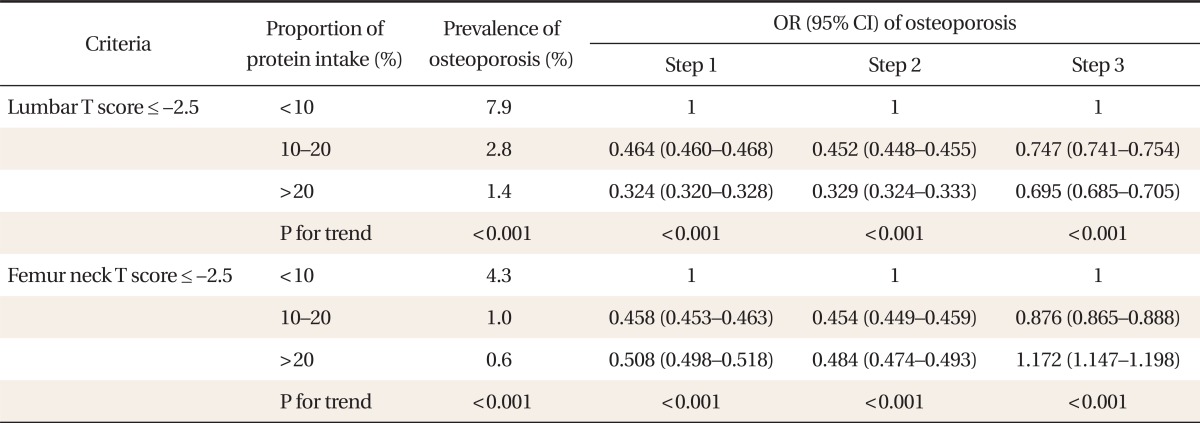
Step 1: adjusted for age, height, and obesity, Step 2: adjusted for age, obesity, height, and thyroid disease, Step 3: adjusted for income of family, education, physical activity, alcohol, daily calcium intake (mg/d), and smoking plus all the variables in step 2.
OR: odds ratio, CI: confidence interval.
DISCUSSION
The aim of this study was to determine the association between dietary protein as a proportion of total daily calories consumed and the prevalence of osteoporosis using KNHANES IV. The group with the highest proportion of protein consumed had the lowest odds of having lumbar osteoporosis in both males and females. These results are consistent with previous studies of older adults. In these studies, the groups with the lowest protein intake also had the lowest BMD.7) A previous domestic study on post-menopausal women also found a positive correlation between protein intake and lumbar BMD.16) However, these studies were delimited to female and elderly study populations.17) The present study included young adults as well as the elderly and therefore the results can be applied to a wider range of the population.
Although it is well known that dietary protein has an important influence on bone health, the exact mechanism by which it impacts bone health is still controversial. In contrast to the present study, other studies have indicated that high protein intake results in bone loss given that bones are used as a buffer in protein catabolism, and the resulting excessive acid leaches calcium from bones.8-10) This opinion is supported by the observation that calcium content in urine increased after a high protein diet.11) It has also been proposed that production of insulin-like growth factor-1 (IGF-1) could decrease as protein intake increases, and decreased IGF-1 could have adverse effects on calcium and phosphate metabolism. High protein intake could load the kidneys which then results in higher urine calcium concentration. However, one report showed that higher urine calcium concentrations do not affect development of osteoporosis and instead cause an increase in calcium absorption through an increase in parathyroid hormone secretion with enhanced calcidiol 1 alpha hydroxylation as a compensatory mechanism. A study performed in the US focused on the association between protein intake and bone fracture in 85,900 females over the course of 12 years and reported that subjects who ingested more than 95 g of protein a day had a higher risk of forearm fracture than subjects who ingested less than 68 g a day.18) In another study of 40,000 Norwegian females, animal protein and femur neck fractures were not significantly associated, but females who had a lower calcium intake with high protein intake had a higher risk of fracture.19) However those studies were all performed in Caucasian female populations. Therefore, they may not be generalizable to Korean populations.
In this study, men that ingested the highest proportion of protein had a higher risk of femoral neck osteoporosis after adjusting for all confounding variables. This finding could be explained by the difference between cortical bones and spongy bones. Spongy bone occupies 70% to 100% of the lumbar spine, whereas cortical bone occupies 75% of the femoral neck.20)
In addition, a previous study on the association between animal protein consumption and the incidence of femoral neck fracture in 16 countries showed that populations with a higher animal protein consumption had a higher risk of femoral fracture, which seems consistent with the results of this study.3) Most nutrients have a detrimental effect when they are ingested excessively. In the same way, the excessive protein intake in this study could have a detrimental impact on BMD.6,21)
One limitation of this study is that it is cross sectional in design. However, this is the first report focused on an adult Korean population. In conclusion, this study suggests that sufficient dietary protein intake is associated with lower prevalence of osteoporosis. Further prospective studies on the protective effects of a protein diet on osteoporosis are needed.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1–129. [PubMed] [Google Scholar]
- 2.Strassberger C, Unger L, Weber AT, Defer A, Bonnaire FA. Management of osteoporosis-related bone fractures: an integrated concept of care. Arch Orthop Trauma Surg. 2010;130:103–109. doi: 10.1007/s00402-009-0989-3. [DOI] [PubMed] [Google Scholar]
- 3.Abelow BJ, Holford TR, Insogna KL. Cross-cultural association between dietary animal protein and hip fracture: a hypothesis. Calcif Tissue Int. 1992;50:14–18. doi: 10.1007/BF00297291. [DOI] [PubMed] [Google Scholar]
- 4.Yoon HK, Park C, Jang S, Jang S, Lee YK, Ha YC. Incidence and mortality following hip fracture in Korea. J Korean Med Sci. 2011;26:1087–1092. doi: 10.3346/jkms.2011.26.8.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao JJ, Nielsen FH. Acid diet (high-meat protein) effects on calcium metabolism and bone health. Curr Opin Clin Nutr Metab Care. 2010;13:698–702. doi: 10.1097/MCO.0b013e32833df691. [DOI] [PubMed] [Google Scholar]
- 6.Heaney RP. Excess dietary protein may not adversely affect bone. J Nutr. 1998;128:1054–1057. doi: 10.1093/jn/128.6.1054. [DOI] [PubMed] [Google Scholar]
- 7.Promislow JH, Goodman-Gruen D, Slymen DJ, Barrett-Connor E. Protein consumption and bone mineral density in the elderly: the Rancho Bernardo Study. Am J Epidemiol. 2002;155:636–644. doi: 10.1093/aje/155.7.636. [DOI] [PubMed] [Google Scholar]
- 8.Barzel US. The skeleton as an ion exchange system: implications for the role of acid-base imbalance in the genesis of osteoporosis. J Bone Miner Res. 1995;10:1431–1436. doi: 10.1002/jbmr.5650101002. [DOI] [PubMed] [Google Scholar]
- 9.Kerstetter JE, Mitnick ME, Gundberg CM, Caseria DM, Ellison AF, Carpenter TO, et al. Changes in bone turnover in young women consuming different levels of dietary protein. J Clin Endocrinol Metab. 1999;84:1052–1055. doi: 10.1210/jcem.84.3.5552. [DOI] [PubMed] [Google Scholar]
- 10.Barzel US, Massey LK. Excess dietary protein can adversely affect bone. J Nutr. 1998;128:1051–1053. doi: 10.1093/jn/128.6.1051. [DOI] [PubMed] [Google Scholar]
- 11.Kerstetter JE, Allen LH. Dietary protein increases urinary calcium. J Nutr. 1990;120:134–136. doi: 10.1093/jn/120.1.134. [DOI] [PubMed] [Google Scholar]
- 12.Korean Society of Bone Metabolism. Physician's guide for diagnosis and treatment of osteoporosis. Seoul: Korean Society of Bone Metabolism; 2011. [Google Scholar]
- 13.Korean Nutrition Society. Dietary reference intakes for Koreans. Seoul: Korean Nutrition Society; 2010. [Google Scholar]
- 14.Oh JY, Yang YJ, Kim BS, Kang JH. Validity and reliability of Korean version of international physical activity questionnaire (IPAQ) short form. J Korean Acad Fam Med. 2007;28:532–541. [Google Scholar]
- 15.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 16.The Study Group of Menopause. The influence of dairy product and protein intake on bone mineral density in Korean postmenopausal women. J Korean Soc Menopause. 2007;13:45–59. [Google Scholar]
- 17.Kerstetter JE, Looker AC, Insogna KL. Low dietary protein and low bone density. Calcif Tissue Int. 2000;66:313. doi: 10.1007/s002230010062. [DOI] [PubMed] [Google Scholar]
- 18.Feskanich D, Willett WC, Stampfer MJ, Colditz GA. Protein consumption and bone fractures in women. Am J Epidemiol. 1996;143:472–479. doi: 10.1093/oxfordjournals.aje.a008767. [DOI] [PubMed] [Google Scholar]
- 19.Meyer HE, Pedersen JI, Loken EB, Tverdal A. Dietary factors and the incidence of hip fracture in middle-aged Norwegians. A prospective study. Am J Epidemiol. 1997;145:117–123. doi: 10.1093/oxfordjournals.aje.a009082. [DOI] [PubMed] [Google Scholar]
- 20.Yang S. Basic science of bone densitometry; Proceedings of the 2008 Conference of the Korean Society of Osteoporosis; 2008 Nov 16; Seoul, Korea. Guri: Korean Society of Osteoporosis; 2008. [Google Scholar]
- 21.Ginty F. Dietary protein and bone health. Proc Nutr Soc. 2003;62:867–876. doi: 10.1079/PNS2003307. [DOI] [PubMed] [Google Scholar]


