Abstract
In this report, we describe an investigation of the effects of Lutzomyia longipalpis sand fly salivary gland homogenates (SGH) on cytokine production and expression of costimulatory molecules on human monocytes, macrophages (Mφs), and dendritic cells (DCs). SGH of L. longipalpis induced an increase in interleukin-6 (IL-6), IL-8 and IL-12p40 production but a decrease in tumor necrosis factor alpha and IL-10 production by lipopolysaccharida (LPS)-stimulated monocytes. We also examined the expression of costimulatory molecules on the surface of monocytes, Mφs, and DCs. Whereas SGH affected the expression of these molecules on monocytes and Mφs, it had little effect on these molecules on DCs. However, when DCs were generated from human monocytes in the presence of SGH, SGH inhibited the expression of costimulatory molecules. In addition, a decrease in the maturation of DCs induced by CD40L was observed in the presence of SGH. Finally, preincubating SGH with human sera containing anti-SGH-specific antibodies abolished the effects of SGH on cytokine production by LPS-stimulated monocytes.
Members of the genus Leishmania are sand fly vector-transmitted protozoan parasites that cause leishmaniasis in the mammalian host. Within the mammalian host, Leishmania resides in phagocytic cells such as monocytes, Mφs, and DCs. Cell-mediated immunity is essential for resistance to the parasite. In the experimental-mouse model of infection, it has been found in general that type 1 T-cell responses (and the gamma interferon [IFN-γ], interleukin-12 [IL-12], tumor necrosis factor alpha [TNF-α], and inducible nitric oxide synthase [iNOS] or NO associated with them) are protective whereas type 2 T-cell responses (and the IL-4, IL-5, and IL-6 associated with them) exacerbate disease (reviewed in references 12, 21, and 35).
Sand flies inject the mammalian host with Leishmania in saliva. The saliva of blood-feeding arthropods contains a wide variety of molecules that modulate their hosts' hemostatic, inflammatory, and immune responses (39). When Lutzomyia longipalpis or Phlebotomus papatasi sand fly SGH were mixed with Leishmania major and injected into mice, the resulting cutaneous lesions were significantly larger than those seen on mice injected with L. major alone (5, 37, 38, 40, 44). To define the mechanism underlying this phenomenon, the effects of SGH on various immune functions were examined. SGH, whose components may have unique, synergistic, or antagonistic activities, have been reported to affect iNOS activity, the Th1-Th2 balance, and the persistence of neutrophils and eosinophils at sites of Leishmania infection (10, 20, 28, 29, 43). In addition, injecting sand fly saliva into normal but not scid mice induced IL-4 production at the dermis and in draining lymph nodes (5, 25). It has been suggested that SGH acts at the level of APCs rather than exerting a direct effect on T cells. Sand fly saliva affects APCs by decreasing antigen presentation, NO production, and Leishmania killing (15, 38). Maxadilan, the vasodilator of L. longipalpis salivary glands, also has potent immunosuppressive properties that lead to induction of IL-10 and IL-6 but inhibition of TNF-α production (7, 34). Taken together, these observations indicate that sand fly saliva modifies the early inflammatory response of the host to a phenotype permissive for infection with Leishmania.
In striking contrast to the results seen in sand fly saliva-naive mice, the disease-exacerbating effects of saliva were abolished in mice preexposed to saliva or the bites of noninfected sand flies. This was associated with a strong inflammatory response and with the production of IL-12 and IFN-γ at the site of inoculation (5, 18, 19). Furthermore, immunization of mice against a single component of saliva (a molecule of 15 kDa) also blunted the disease-enhancing effects of saliva. This was also true in B-cell-deficient mice, indicating that a cell-mediated inflammatory response to saliva provided most if not all the protective effects of this vaccine (42).
When sand flies feed on humans, they lacerate capillaries, forming a hemorrhagic pool of blood (29, 33). This creates a microenvironment where monocytes, Mφs, and DCs, among other cells, are exposed to salivary components injected by the sand flies. Little is known about the effects of sand fly saliva on human cells; however, evidence suggests that such interactions occur. Children living in an area where visceral leishmaniasis is endemic developed antibodies against components of L. longipalpis saliva (3), and this response was concurrent with the development of an anti-Leishmania response (14). Additionally, anti-Leishmania immune responses differed between groups of children who mount or do not mount an anti-sand fly saliva response (14), suggesting that sand fly saliva modifies the immune response to Leishmania in humans as it does in mice.
In order to explore the effects of SGH from L. longipalpis on the human immune response, we examined the effects of SGH on the expression of surface molecules (e.g., CD80 and CD86) important in the development of an immune response, as well as cytokine (e.g., interleukin) production by human monocytes, Mφs, or DCs treated with SGH and stimulated or not stimulated with LPS. Understanding how salivary products interfere with LPS-induced costimulatory molecule expression and cytokine production will help clarify the complex interactions that occur between sand fly saliva, Leishmania, and the human host when the host is infected with the parasite.
MATERIALS AND METHODS
Abbreviations used.
SGH, Lutzomyia longipalpis sand fly salivary gland homogenate; Mφs macrophages; DCs, dendritic cells; IL, interleukin; TNF, tumor necrosis factor; IFN, interferon; NOs, inducible nitric oxide synthase; APCs, antigen-presenting cells; ELISA, enzyme-linked immunosorbent assay; DTH, delayed type hypersensitivity; PBMC, peripheral blood mononuclear cells; PE, phycoerythrin; FITC, fluoroscein isothiocyanate; Ig, immunoglobulin; LPS, lipopoly saccharide.
Human cells.
Human blood was obtained from healthy volunteers by Hemocentro do Estado da Bahia, Salvador, Bahia, Brazil. All individuals participated in this study after informed consent was obtained, and the Committee of Ethics of Centro de Pesquisa Gonçalo Moniz approved this study. PBMC were obtained by passage over Ficoll-Hypaque gradients (Sigma-Aldrich, St. Louis, Mo.). The cells were then washed and separated on multistep Percoll gradients (Amersham Pharmacia, São Paulo, Brazil). The light density fraction at the 40 to 50% interface was recovered and depleted of CD19+ and CD2+ cells by using magnetic beads coated with specific antibodies (Microbeads; Miltenyi Biotec, Inc., Sunnyvale, Calif.). Monocytes were characterized by flow cytometry (FACSort; Becton Dickinson, San Diego, Calif.), using anti-CD14 and HLA-DR antibodies conjugated to PE or FITC as well as isotype controls. The remaining cells (106) were cultured in RPMI 1640 medium supplemented with 2mM l-glutamine, penicillin (100 U/ml), gentamicin (100 μg/ml), and 10% heat-inactivated fetal bovine serum (HyClone, Ogden, Utah), termed complete medium. These cells were pretreated with SGH (0.5 pair of glands/ml) overnight and then incubated with LPS of E. coli serotype O11:B4 (Sigma-Aldrich) (30 pg/ml) for 24 or 48 h. The cultured cells and/or culture supernatants were harvested 24 or 48 h later. The cells were analyzed by flow cytometry to characterize cell surface molecule expression (CD80, CD86, HLA-DR, and CD14). The supernatants were kept at −20°C until tested for cytokine content by ELISA.
Human Mφs were obtained from human PBMC. PBMC were placed in 24-well plates (Costar, Corning, Corning, N.Y.) at a concentration of 5 × 106 cells/ml and incubated for 30 min at 37°C at 5% CO2. Nonadherent cells were removed from the plates, and adherent cells were cultured in complete medium for 7 days. The cells were pretreated with SGH (0.5 pair of glands/ml) overnight and stimulated with LPS for 24 or 48 h. Cells or culture supernatants were then harvested and analyzed by flow cytometry or by ELISA as described above.
Human DCs were generated from PBMC as previously described (32). Briefly, monocytes were obtained as detailed above and 3 × 105/ml cells were cultured at 37°C, under 5% CO2 in air, in RPMI 1640 medium plus 10% fetal bovine serum supplemented with granulocyte-Mφ colony-stimulating factor (50ng/ml) and IL-4 (1,000 U/ml). Portions (300 ml) medium and cytokines were replaced every 3 days. DCs were used after 8 days of culture. The cells were characterized by flow cytometry using antibodies against CD1a, CD14, CD80, CD86, HLA-DR, and CD83 conjugated to PE or FITC, as well as isotype controls. These cells were pretreated with SGH (1.0 pair of glands/ml) and stimulated with LPS (200 pg/ml) or CD40L (Ancell, Bayport, Minn.) at 2 μg/ml for 24 or 48 h. In some experiments, DCs were generated in the presence of SGH (1.0 pair of glands/ml). Cells were harvested to be analyzed by flow cytometry to determine their surface molecule expression, and supernatants were tested for cytokine content by ELISA.
Sand flies and preparation of SGH:
L. longipalpis sand flies were reared at Centro de Pesquisa Gonçalo Moniz, FIOCRUZ, as described elsewhere (26). Adult sand flies had free access to a 10% solution of sucrose. Salivary glands from 5-to 7-day old adult female flies were dissected and transferred to polypropylene vials, usually in groups of 20 pairs of glands in 20 μl of HEPES-saline. Salivary glands were kept at −75°C until needed, when they were disrupted by sonication using a Sonifier 450 homogenizer (Branson, Danbury, Conn.) (30). The SGH were centrifuged at 10,000 × g for 2 min, and the supernatants were then used for experiments.
Flow cytometry.
Cells (5 × 105) were resuspended in PAB (phosphate-buffered saline, 1% bovine serum albumin, 0.05% sodium azide) and blocked with 20% fetal bovine serum and Fc block (20 μg/ml of mouse Ig) for 30 min on ice. They cells were then labeled with monoclonal antibodies or respective controls for an additional 30 min. They were fixed with 1% paraformaldehyde in phosphate-buffered saline and analyzed by flow cytometry. We used the following antibodies (from Becton Dickinson) in these experiments PE- or FITC-labeled anti-human CD80 (clone L307.4, mouse IgG1); PE- or FITC-labeled anti-human CD86 (clone 233, FUN-1 mouse IgG1); cyanine-labeled anti-human CD40 (clone 5C-3, mouse IgG1); PE-labeled anti-human CD83 (clones HB15e); FITC-labeled anti-human HLA DR, DP, and DQ (clone TU 39, mouse IgG2a); PE-, FITC- or Cy-labeled mouse IgG1, κ chain (clone MOPC-21, isotype control); and FITC-labeled mouse IgG2a, κ chain (clone MOPC-173, isotype control).
Cytokine assays.
Levels of TNF-α, IL-6, IL-8, IL-10, and IL-12p40 in supernatants were determined by ELISA using commercial anti-cytokine antibody pairs (Becton Dickinson) as specified by the manufacturer. Human recombinant TNF-α, IL-6, IL-8, IL-10, and IL-12p40 (Pharmingen) were used to generate standard curves.
Human sera.
Young healthy adults from areas where leishmaniasis was not endemic, with negative serology to sand fly saliva and normal IgE levels, were accepted in our study so that we could obtain human sera containing high levels of antibodies to L. longipalpis saliva. These individuals underwent repeated experimental exposures to L. longipalpis bites, with sequential evaluation of their humoral immunity against the vector saliva. Individuals were exposed to 20 phlebotomines four times at 15-day intervals. All individuals participated in this study after informed consent was obtained, and the Committee of Ethics of Centro de Pesquisa Gonçalo Moniz approved this study. These sera showed high levels of IgG anti-L. longipalpis saliva antibodies. We also used human sera from normal volunteers who were not exposed to L. longipalpis as controls of the experiments.
Statistical analysis.
Comparison of cytokine levels in the presence or absence of SGH was done by a nonparametric Wilcoxon test. All tests were performed using Prism software (GraphPad Software, Inc., San Diego, Calif.). Differences were considered significant if P < 0.05.
RESULTS
SGH affects cytokine production by LPS-stimulated human monocytes.
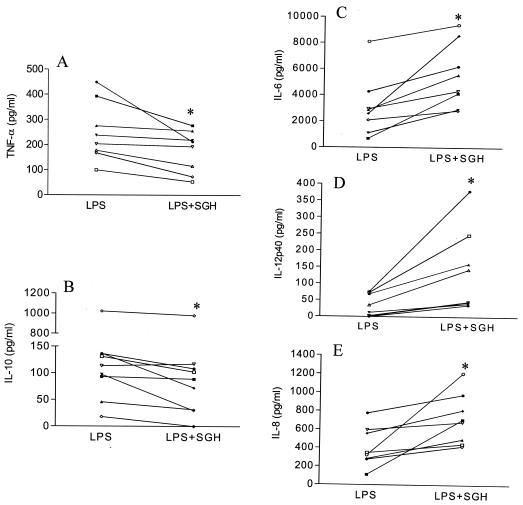
We first investigated whether L. longipalpis sand fly SGH would affect cytokine production by LPS-treated human monocytes. Monocytes were pretreated with SGH (0.5 pair of glands/ml) overnight and then treated with LPS. SGH significantly decreased LPS-induced TNF-α and IL-10 secretion by monocytes (Fig. 1A and B). Conversely, we observed a significant increase in the levels of IL-6, IL-8, and IL-12p40 production by LPS-stimulated monocytes (Fig. 1C to E).
FIG. 1.
Effect of SGH on LPS-stimulated monocytes Monocytes obtained from PBMC were incubated with SGH (0.5 pair of glands/ml) overnight and treated with LPS (30 pg/ml) for 24 h (TNF-α, IL-6, IL-8, and IL-12p40 measurements) or 48 h (IL-10 measurement). The supernatants were then analyzed for their content of TNF-α (A), IL-10 (B), IL-6 (C), IL-12p40 (D), or IL-8 (E) by ELISA. *, the results are statistically significant (P < 0.05). The data for individual donors are presented with background cytokine levels subtracted.
We also tested the effects of SGH on human Mφs and DCs. SGH had no significant effect on the LPS-induced secretion of TNF-α, IL-10, or IL-12 p40 by either cell type (data not shown).
Effects of SGH on the expression of costimulatory molecules on human monocytes and Mφs.
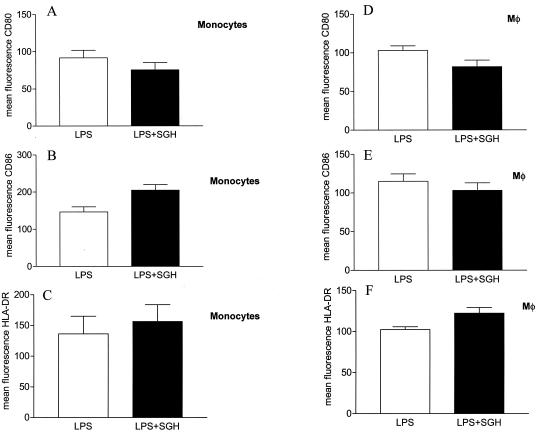
Since costimulatory molecules play an essential role in the activation and maintenance of T-cell responses, we analyzed the expression of CD80, CD86, CD40, and HLA-DR on SGH pretreated monocytes and Mφs. SGH decreased the expression of CD80 on the surface of LPS-stimulated monocytes and Mφs (Fig. 2A and D). On the other hand, SGH enhanced the expression of CD86 and HLA-DR but not of CD40 (data not shown) on the surface of LPS-stimulated monocytes (Fig. 2B and C). On Mφs, there was an increase in HLA-DR expression (Fig. 2F) but no alteration in CD86 expression (Fig. 2E). This effect was due to increased mean fluorescence intensity, not an increase in the percentage of positive cells.
FIG. 2.
Effect of SGH on costimulatory molecule expression on LPS-stimulated monocytes and Mφs. Monocytes and Mφs obtained from PBMC were incubated in the presence or absence of SGH (0.5 pair of glands/ml) plus LPS (see Materials and Methods) for 48 h. After this period, the cells were harvested, labeled with antibodies, and analyzed by flow cytometry. The mean fluorescence of costimulatory molecules is shown. (A to C) CD80, CD86, and HLA-DR on monocytes; (D to F) CD80, CD86, and HLA-DR on Mφs. The data shown are from four independent experiments, and standard errors are indicated.
SGH inhibits the expression of costimulatory molecules on human DCs if SGH is included in cultures in which the cells are derived.
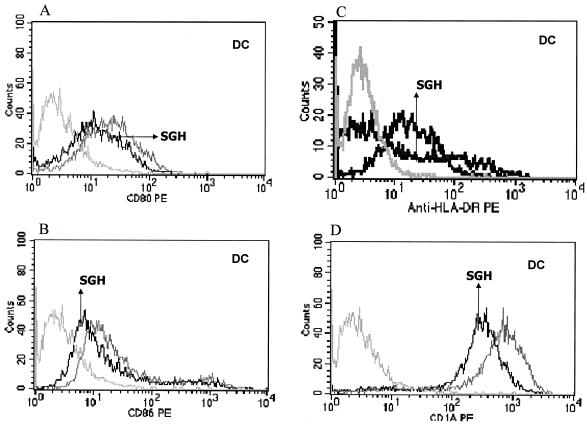
To confirm and extend our analysis of the influence of SGH on human APCs, we included SGH in cultures in which we derived human DCs (methods for derivation of DCs are described in Materials and Methods). Figure 3 shows that addition of SGH during the generation of DCs led to a decrease in CD80, CD86, HLA-DR, and CD1a expression in the cells as well as in the mean fluorescence (inhibition of 20.2, 16.6, 30.2, and 20.2% respectively), suggesting once again that L. longipalpis saliva can inhibit the expression of costimulatory molecules that are important for T-cell activation.
FIG. 3.
Effect of SGH (1 pair of glands/ml) on differentiation of human DCs from monocytes. During the generation of DCs from monocytes (see Materials and Methods), SGH was added to the cultures to determine the effect of saliva on the generation of these cells. After 7 days in culture, the cells were harvested and labeled with antibodies. (A) CD80; (B) CD86; (C) HLA-DR; (D) CD1a. The medium-gray line represents the expression of different molecules on the DC surfaces generated in the absence of SGH. The solid line represents the expression of different molecules on DC surfaces generated in the presence of SGH. The light-gray line represents cells stained with isotype antibody controls. The data shown are representative of five separate experiments.
Effect of SGH on DC maturation induced by CD40L stimulation.
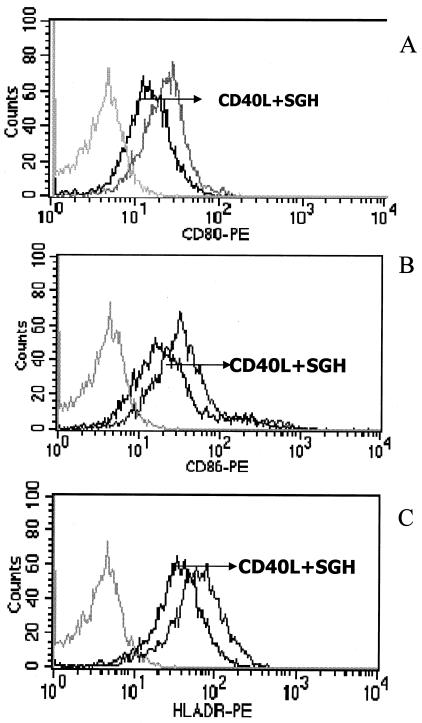
Next we analyzed the effect of SGH on DC maturation. No effect was seen when we stimulated DCs with LPS (200 pg/ml) in the presence or absence of SGH (data not shown). However, when we stimulated DCs with CD40L in the presence of SGH, we observed a decrease in the expression of CD80, CD86, and HLA-DR (Fig. 4).
FIG. 4.
Effect of SGH (1 pair of glands/ml) on the expression of costimulatory molecules on human DCs stimulated with CD40L. DCs obtained from PBMC (see Materials and Methods) were stimulated with CD40L (2 μg/ml) for 24 h. They were then harvested, labeled with antibodies, and analyzed by flow cytometry. (A) CD80; (B) CD86; (C) HLA-DR. The black line represents costimulatory molecule expression on cells pretreated with SGH and stimulated with CD40L; the gray line represents cells treated with CD40L; and the light grayline represents cells stained with isotype antibody controls. The data shown are representative of five separate experiments.
Anti-SGH antibodies neutralize the effects of SGH on LPS-stimulated monocytes.
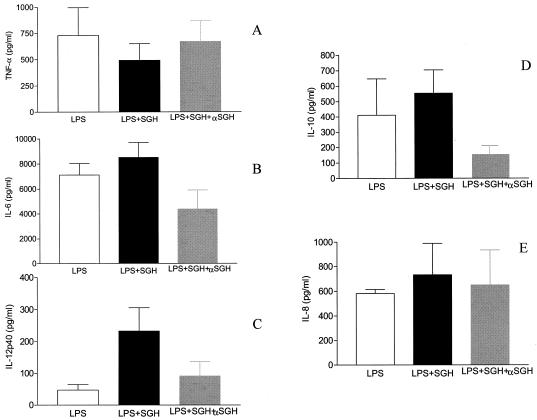
Pretreating SGH with immune serum abrogated both the inhibitory and stimulatory effects of SGH on LPS-stimulated human monocytes. Whereas SGH alone led to decreased production of TNF-α and IL-10, immune serum-pretreated samples exhibited levels similar to those in cultures without saliva (Fig. 5A and D). On the other hand, while SGH led to increased production of IL-6 and IL-12 p40, these effects disappeared when SGH was pretreated with immune serum (Fig. 5B and C). As controls, we pretreated SGH with normal human sera without anti-saliva antibodies. We did not observe any significant differences between this group and that treated with SGH plus LPS (data no shown).
FIG. 5.
Neutralization of the effects by of SGH by human sera that contain anti-salivary antibodies. Monocytes were obtained from PBMC and were stimulated with LPS (30 pg/ml) or pretreated with SGH overnight and stimulated with LPS (30 pg/ml). In addition, in some cultures, SGH was first incubated with human sera for 40 min at 37°C. This treated SGH was then incubated with monocytes overnight, and the cells were stimulated with LPS. At 24 or 48 h later, the supernatants of the cultures were harvested and tested for their content of the indicated cytokines by ELISA. (A) TNF-α; (B) IL-6; (C) IL-12p40; (D) IL-10. The white bars represent cytokine production by cells stimulated with LPS alone; the black bars represent cytokine production in the presence of SGH, and the gray bars represent cytokine production when the SGH was preincubated with the antiserum and this mixture was used to pretreat the cells followed by stimulation with LPS. The data shown are from four independent experiments, and standard errors are indicated. As controls, SGH were pretreated with normal human sera (without anti-saliva antibodies), and no significant differences were observed between this group and that treated with SGH plus LPS (data not shown).
DISCUSSION
It is now clear that saliva of many different arthropod vectors of disease, including sand flies (18), contains immunomodulatory molecules that can affect the immune response at several levels (13). Moreover, it has been shown with both Old World (P. papatasi) and New World (L. longipalpis) sand flies that saliva can dramatically enhance infection by several species of Leishmania (13, 18). It has been suggested that the molecule in L. longipalpis saliva that enhances infection is the vasodilator/immunomodulator maxadilan, since maxadilan alone can enhance infection with L. major to the same degree as whole saliva can (27).
Most of the work with sand fly saliva has utilized experimental animals (principally mice). A common finding in this experimental-mouse work is that sand fly saliva inhibits TNF-α production while it augments the secretion of IL-6 (34). Since comparatively little work has been done examining the effects of sand fly saliva on human cells, we examined the effects of SGH on human monocytes, Mφs, and DCs with respect to cytokine secretion and expression of surface molecules important in the development of an immune response. We show that L. longipalpis SGH augments IL-6, IL-8, and IL-12p40 production while it inhibits TNF-α and IL-10 production by human monocytes (Fig. 1). The intensity of the stimulatory or inhibitory effects was distinct among different donors and was negligible in some of them. Considering the variation of the human population, the dispersion observed in our results is not unexpected and in fact has been observed and reported in many publications dealing with studies of humans.
In the mouse model of leishmaniasis, the proinflammatory cytokine TNF-α has been examined extensively because of its potential effector function. Treatment with TNF-α results in a reduction of the lesion size and parasitic burden (22, 23, 41). In addition, treatment with a neutralizing anti-TNF-α antibody leads to a transient aggravation of the disease (9, 37, 41). Recently, it has been shown that resistant C57BL/6 mice that lack the TNF-α gene developed visceral leishmaniasis when infected with L. major, which led to rapid death of the animals (45).
IL-6 is a cytokine synthesized by T cells and Mφs. It has pleiotropic effects on diverse cell types and is recognized for its proinflammatory properties (17). Pretreatment of human Mφs with IL-6 induced a dose- and time-dependent suppression of IFN-γ and TNF-α activation of Mφs and thus induced inhibition of killing of Leishmania amazonensis (16). IL-6 alone had no effect on Mφ viability or intracellular L. amazonensis growth, but it down modulated the ability of TNF-α to enhance the oxidative capacity of treated Mφs and thus the ability of the Mφs to kill the parasite. The data presented here show that SGH down regulates TNF-α but enhances IL-6 production by human monocytes (Fig. 1). Therefore, through its ability to modify components of the innate immune response (TNF-α and IL-6), sand fly saliva may enhance the survival of Leishmania in humans infected with the parasite.
L. longipalpis SGH also augmented IL-8 secretion by human monocytes (Fig. 1). Interestingly, Leishmania parasites also the augment production of IL-8 by human monocytes (2). IL-8 is strongly chemotactic for neutrophils, and neutrophils are plentiful in early cutaneous lesions of Leishmania. It is unknown how an increase in the influx of neutrophils into leishmanial lesions might affect the evolution of those lesions. Neutrophils can engulf Leishmania, but they are very short-lived cells. However, it has recently been reported (1) that infecting neutrophils with L. major prevented the cells from apoptosis, substantially extending the lifetime of the cells and hence survival of the parasite within the cells.
Finally, we found that L. longipalpis SGH augmented IL-12p40 synthesis while inhibiting IL-10 secretion by human monocytes (Fig. 1). IL-12 is known to be critical for the development of cell-mediated/Th1 T-cell responses. Indeed, IL-12 positively regulates the production of IFN-γ while IL-10 negatively regulates it; thus, the three cytokines closely regulate each other's production (4, 27, 34). Since SGH augments IL-12 but inhibits IL-10, this suggests that components of SGH may promote the development of a cell-mediated response. In fact, it has been known for decades that the saliva of Old World P. papatasi sand flies can induce DTH responses in humans (a condition known as harara [36]). It has been proposed that a DTH response to saliva might be beneficial to the sand fly since blood flow at DTH sites is enhanced and thus the sand fly can complete its blood meal more rapidly (6). Although a New World counterpart of harara has not been reported, it is possible that such a clinical condition exists. More work is required to determine whether New World sand fly saliva can induce DTH responses to itself.
It should be mentioned that maxadilan, the immunomodulator from L. longipalpis salivary glands, has been reported to have some of the same effects (e.g., the ability to inhibit TNF-α but enhance IL-6 secretion) on both mouse (34) and human (31) cells. However, maxadilan does not enhance IL-12p40 secretion; rather, it inhibits the production of both IL-12p40 and IFN-γ by human PBMC treated with maxadilan and infected with L. major (31). Although the two systems are not entirely comparable, this suggests either that maxadilan is capable under certain circumstances (the experimental systems employed here) of enhancing IL-12p40 production or that there is an immunomodulator(s) other than maxadilan present in the saliva of L. longipalpis sand flies that is capable of doing so.
We also examined the effects of SGH on the expression of cell surface molecules on APCs that are important to the development of an immune response. In leishmaniasis these molecules are critical for the development of a protective T-cell response. For instance, class II MHC molecules are required for the activation of CD4 T cells and protection against infection with L. major (24). Moreover, costimulatory molecules (e.g., CD80 and CD86) also influence the immune response to L. major (8, 11). Thus, changes in the expression of MHC class II, CD80 or CD86 can alter the T-cell response. SGH influenced the expression of all of these molecules on the three APC types examined; monocytes, Mφs, and DCs. Perhaps the most striking effect was the ability of SGH to enhance HLA-DR expression (Fig. 2C and F).
Finally, we have recently reported that anti-sand fly salivary antibodies develop in the serum of Brazilian children living in areas where leishmaniasis is endemic. The appearance of these antibodies was correlated with the development of anti-leishmanial DTH. That is, it appeared that anti-sand fly salivary gland immunity (which presumably would neutralize the immunomodulatory effects of sand fly saliva) allows the host to develop protective immunity against Leishmania (14). To directly test this hypothesis that the anti-saliva antibodies could neutralize the immunomodulatory properties of saliva, we tested whether the antisera would block the ability of SGH to modulate cytokine responses. In all cases tested (Fig. 5), the effects of SGH were blocked.
The data presented here represent the first report that SGH from L. longipalpis sand flies affects the phenotype and functions of cells of the human immune system. This is important information since although it is clear from work with experimental animals that sand fly saliva can affect the immune response and that targeting components of the salivary glands can protect against leishmaniasis (13, 18, 27, 42), little is known about the effect of sand fly saliva on human cells and thus whether a sand fly-based vaccine would protect humans. The data showing that sera from humans who live in areas where leishmaniasis is endemic can neutralize the immunomodulatory effects of saliva are particularly encouraging. These data suggest that humans can develop antibodies that block the effects of saliva and that an appropriate vaccine should accelerate the development of these antibodies in the vaccinated host and thus protect against leishmaniasis. As to what target should be used for this vaccine, it has been shown that maxadilan in L. longipalpis saliva can protect mice from infection with Leishmania (27). However, the data presented here suggest that there may be another immunomodulator in L. longipalpis saliva that promotes the secretion of IL-12p40 and inhibits IL-10 synthesis (Fig. 1). Since humans can generate antibodies that can block the IL-12-enhancing effect of saliva (Fig. 5), it would be interesting to find which molecule(s) is responsible for IL-12 enhancement. Such a molecule(s) might be another vaccine target since it would be predicted to inherently stimulate the development of a Th1 response, which is protective in leishmanial infections. Moreover, since humans naturally produce antibodies to the IL-12 enhancer, individuals who receive this vaccine would be naturally boosted when they are bitten by sand flies.
Acknowledgments
We thank Ana Cristina Bahia and Deboraci Prates for technical assistance with the insect colony.
This work is supported by grant AI30639 from the National Institutes of Health. A.B. M.B. and C.B. are investigators from the Brazilian Research National Council (CNPq).
Editor: B. B. Finlay
REFERENCES
- 1.Aga, E., D. M. Katschinski, G. van Zandbergen, H. Laufs, B. Hansen, K. Muller, W. Solbach, and T. Laskay. 2002. Inhibition of the spontaneous apoptosis of neutrophil granulocytes by the intracellular parasite Leishmania major. J. Immunol. 169:898-905. [DOI] [PubMed] [Google Scholar]
- 2.Badolato, R., D. L. Sacks, D. Savoia, and T. Musso. 1996. Leishmania major: infection of human monocytes induces expression of IL-8 and MCAF. Exp. Parasitol. 82:21-26. [DOI] [PubMed] [Google Scholar]
- 3.Barral, A., E. Honda, A. Caldas, J. Costa, V. Vinhas, E. D. Rowton, J. G. Valenzuela, R. Charlab, M. Barral-Netto, and J. M. Ribeiro. 2000. Human immune response to sand fly salivary gland antigens: a useful epidemiological marker? Am. J. Trop. Med. Hyg. 62:740-745. [DOI] [PubMed] [Google Scholar]
- 4.Belkaid, Y., K. F. Hoffmann, S. Mendez, S. Kamhawi, M. C. Udey, T. A. Wynn, and D. L. Sacks. 2001. The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti-IL-10 receptor antibody for sterile cure. J. Exp. Med. 194:1497-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belkaid, Y., S. Kamhawi, G. Modi, J. Valenzuela, N. Noben-Trauth, E. Rowton, J. Ribeiro, and D. L. Sacks. 1998. Development of a natural model of cutaneous leishmaniasis: powerful effects of vector saliva and saliva preexposure on the long-term outcome of Leishmania major infection in the mouse ear dermis. J. Exp. Med. 188:1941-1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belkaid, Y., J. G. Valenzuela, S. Kamhawi, E. Rowton, D. L. Sacks, and J. M. Ribeiro. 2000. Delayed-type hypersensitivity to Phlebotomus papatasi sand fly bite: an adaptive response induced by the fly? Proc. Natl. Acad. Sci. USA 97:6704-6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bozza, M., M. B. Soares, P. T. Bozza, A. R. Satoskar, T. G. Diacovo, F. Brombacher, R. G. Titus, C. B. Shoemaker, and J. R. David. 1998. The PACAP-type I receptor agonist maxadilan from sand fly saliva protects mice against lethal endotoxemia by a mechanism partially dependent on IL-10. Eur. J. Immunol. 28:3120-3127. [DOI] [PubMed] [Google Scholar]
- 8.Brown, J. A., R. G. Titus, N. Nabavi, and L. H. Glimcher. 1996. Blockade of CD86 ameliorates Leishmania major infection by down-regulating the Th2 response. J. Infect. Dis. 174:1303-1308. [DOI] [PubMed] [Google Scholar]
- 9.de Kossodo, S., G. E. Grau, J. A. Louis, and I. Muller. 1994. Tumor necrosis factor alpha (TNF-alpha) and TNF-beta and their receptors in experimental cutaneous leishmaniasis. Infect. Immun. 62:1414-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donnelly, K. B., H. C. Lima, and R. G. Titus. 1998. Histologic characterization of experimental cutaneous leishmaniasis in mice infected with Leishmania braziliensis in the presence or absence of sand fly vector salivary gland lysate. J. Parasitol. 84:97-103. [PubMed] [Google Scholar]
- 11.Elloso, M. M., and P. Scott. 1999. Expression and contribution of B7-1 (CD80) and B7-2 (CD86) in the early immune response to Leishmania major infection. J. Immunol. 162:6708-6715. [PubMed] [Google Scholar]
- 12.Etges, R., and I. Muller. 1998. Progressive disease or protective immunity to Leishmania major infection: the result of a network of stimulatory and inhibitory interactions. J. Mol. Med. 76:372-390. [DOI] [PubMed] [Google Scholar]
- 13.Gillespie, R. D., M. L. Mbow, and R. G. Titus. 2000. The immunomodulatory factors of bloodfeeding arthropod saliva. Parasite Immunol. 22:319-331. [DOI] [PubMed] [Google Scholar]
- 14.Gomes, R. B., C. Brodskyn, C. I. de Oliveira, J. Costa, J. C. Miranda, A. Caldas, J. G. Valenzuela, M. Barral-Netto, and A. Barral. 2002. Seroconversion against Lutzomyia longipalpis saliva concurrent with the development of anti-Leishmania chagasi delayed-type hypersensitivity. J. Infect. Dis. 186:1530-1534. [DOI] [PubMed] [Google Scholar]
- 15.Hall, L. R., and R. G. Titus. 1995. Sand fly vector saliva selectively modulates macrophage functions that inhibit killing of Leishmania major and nitric oxide production. J. Immunol. 155:3501-3506. [PubMed] [Google Scholar]
- 16.Hatzigeorgiou, D. E., S. He, J. Sobel, K. H. Grabstein, A. Hafner, and J. L. Ho. 1993. IL-6 down-modulates the cytokine-enhanced antileishmanial activity in human macrophages. J. Immunol. 151:3682-3692. [PubMed] [Google Scholar]
- 17.Heinrich, P. C., I. Behrmann, S. Haan, H. M. Hermanns, G. Muller-Newen, and F. Schaper. 2003. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 374:1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamhawi, S. 2000. The biological and immunomodulatory properties of sand fly saliva and its role in the establishment of Leishmania infections. Microbes Infect. 2:1765-1773. [DOI] [PubMed] [Google Scholar]
- 19.Kamhawi, S., Y. Belkaid, G. Modi, E. Rowton, and D. Sacks. 2000. Protection against cutaneous leishmaniasis resulting from bites of uninfected sand flies. Science 290:1351-1354. [DOI] [PubMed] [Google Scholar]
- 20.Katz, O., J. N. Waitumbi, R. Zer, and A. Warburg. 2000. Adenosine, AMP, and protein phosphatase activity in sandfly saliva. Am. J. Trop. Med. Hyg. 62:145-150. [DOI] [PubMed] [Google Scholar]
- 21.Launois, P., F. Tacchini-Cottier, C. Parra-Lopez, and J. A. Louis. 1998. Cytokines in parasitic diseases: the example of cutaneous leishmaniasis. Int. Rev. Immunol. 17:157-180. [DOI] [PubMed] [Google Scholar]
- 22.Liew, F. Y., Y. Li, and S. Millott. 1990. Tumor necrosis factor-alpha synergizes with IFN-gamma in mediating killing of Leishmania major through the induction of nitric oxide. J. Immunol. 145:4306-4310. [PubMed] [Google Scholar]
- 23.Liew, F. Y., C. Parkinson, S. Millott, A. Severn, and M. Carrier. 1990. Tumour necrosis factor (TNF alpha) in leishmaniasis. I. TNF alpha mediates host protection against cutaneous leishmaniasis. Immunology 69:570-573. [PMC free article] [PubMed] [Google Scholar]
- 24.Locksley, R. M., S. L. Reiner, F. Hatam, D. R. Littman, and N. Killeen. 1993. Helper T cells without CD4: control of leishmaniasis in CD4-deficient mice. Science 261:1448-1451. [DOI] [PubMed] [Google Scholar]
- 25.Mbow, M. L., J. A. Bleyenberg, L. R. Hall, and R. G. Titus. 1998. Phlebotomus papatasi sand fly salivary gland lysate down-regulates a Th1, but up-regulates a Th2, response in mice infected with Leishmania major. J. Immunol. 161:5571-5577. [PubMed] [Google Scholar]
- 26.Modi, G. B., and R. B. Tesh. 1983. A simple technique for mass rearing Lutzomyia longipalpis and Phlebotomus papatasi (Diptera: Psychodidae) in the laboratory. J. Med. Entomol. 20: 568-569. [DOI] [PubMed] [Google Scholar]
- 27.Morris, R. V., C. B. Shoemaker, J. R. David, G. C. Lanzaro, and R. G. Titus. 2001. Sandfly maxadilan exacerbates infection with Leishmania major and vaccinating against it protects against L. major infection. J. Immunol. 167:5226-5230. [DOI] [PubMed] [Google Scholar]
- 28.Paranhos, M., W. C. dos Santos, I. Sherlock, G. G. Oliveira, and L. C. de Carvalho. 1993. Development of eosinophilia in dogs intradermically inoculated with sand fly saliva and Leishmania (Leishmania) chagasi stationary-phase promastigotes. Mem. Inst. Oswaldo Cruz 88:249-251. [DOI] [PubMed] [Google Scholar]
- 29.Ribeiro, J. M. 1995. Blood-feeding arthropods: live syringes or invertebrate pharmacologists? Infect. Agents Dis. 4:143-152. [PubMed] [Google Scholar]
- 30.Ribeiro, J. M., E. D. Rowton, and R. Charlab. 2000. Salivary amylase activity of the phlebotomine sand fly, Lutzomyia longipalpis. Insect Biochem. Mol. Biol. 30:271-277. [DOI] [PubMed] [Google Scholar]
- 31.Rogers, K. A., and R. G. Titus. 2003. Immunomodulatory effects of maxadilan and Phlebotomus papatasi sand fly salivary gland lysates on human primary in vitro immune responses. Parasite Immunol. 25:127-134. [DOI] [PubMed] [Google Scholar]
- 32.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179:1109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shortt, H., and C. Swaminath. 1928. The method of feeding of Phebotomus argentipes with relation to its bearing on the transmission of kala azar. Indian J. Med. Res. 15:827-836. [Google Scholar]
- 34.Soares, M. B., R. G. Titus, C. B. Shoemaker, J. R. David, and M. Bozza. 1998. The vasoactive peptide maxadilan from sand fly saliva inhibits TNF-alpha and induces IL-6 by mouse macrophages through interaction with the pituitary adenylate cyclase-activating polypeptide (PACAP) receptor. J. Immunol. 160:1811-1816. [PubMed] [Google Scholar]
- 35.Solbach, W., and T. Laskay. 2000. The host response to Leishmania infection. Adv. Immunol. 74:275-317. [DOI] [PubMed] [Google Scholar]
- 36.Theodor, O. 1935. A study of the reaction to phlebotomus bites with some remarks of “harara”. Trans. R. Soc. Trop. Med. Hyg. 29:273-284. [Google Scholar]
- 37.Theodos, C. M., L. Povinelli, R. Molina, B. Sherry, and R. G. Titus. 1991. Role of tumor necrosis factor in macrophage leishmanicidal activity in vitro and resistance to cutaneous leishmaniasis in vivo. Infect. Immun. 59:2839-2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Theodos, C. M., and R. G. Titus. 1993. Salivary gland material from the sand fly Lutzomyia longipalpis has an inhibitory effect on macrophage function in vitro. Parasite Immunol. 15:481-487. [DOI] [PubMed] [Google Scholar]
- 39.Titus, R., and J. Ribeiro. 1990. The role of vector saliva in transmission of arthropod-borne diseases. Parasitol. Today 6:157-159. [DOI] [PubMed] [Google Scholar]
- 40.Titus, R. G., and J. M. Ribeiro. 1988. Salivary gland lysates from the sand fly Lutzomyia longipalpis enhance Leishmania infectivity. Science 239:1306-1308. [DOI] [PubMed] [Google Scholar]
- 41.Titus, R. G., B. Sherry, and A. Cerami. 1989. Tumor necrosis factor plays a protective role in experimental murine cutaneous leishmaniasis. J. Exp. Med. 170:2097-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valenzuela, J. G., Y. Belkaid, M. K. Garfield, S. Mendez, S. Kamhawi, E. D. Rowton, D. L. Sacks, and J. M. Ribeiro. 2001. Toward a defined anti-Leishmania vaccine targeting vector antigens: characterization of a protective salivary protein. J. Exp. Med. 194:331-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waitumbi, J., and A. Warburg. 1998. Phlebotomus papatasi saliva inhibits protein phosphatase activity and nitric oxide production by murine macrophages. Infect. Immun. 66:1534-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Warburg, A., E. Saraiva, G. C. Lanzaro, R. G. Titus, and F. Neva. 1994. Saliva of Lutzomyia longipalpis sibling species differs in its composition and capacity to enhance leishmaniasis. Philos. Trans. R. Soc. London Ser. 345:223-230. [DOI] [PubMed] [Google Scholar]
- 45.Wilhelm, P., U. Ritter, S. Labbow, N. Donhauser, M. Rollinghoff, C. Bogdan, and H. Korner. 2001. Rapidly fatal leishmaniasis in resistant C57BL/6 mice lacking TNF. J. Immunol. 166:4012-4019. [DOI] [PubMed] [Google Scholar]