Abstract
Protective immunity in mice infected with Toxoplasma gondii is mainly mediated by NK cells, CD4 and CD8 T cells, and type 1 cytokines, such as gamma interferon (IFN-γ). To clarify the roles of NK cells and IFN-γ in protection against primary congenital toxoplasmosis, we used recombination activating gene 2 knockout (RAG-2−/−) mice, which lack T and B lymphocytes, in comparison with the wild-type BALB/c model. RAG-2−/− mice had a significantly lower risk of fetal toxoplasmosis than BALB/c mice (25 versus 63.9%; P = 0.003). This protection was associated with an increased number of maternal NK cells, IFN-γ secretion by spleen cells, and decreased parasitemia. In the RAG-2−/− mice, NK cell depletion increased both the rate of fetal infection, to 56.5% (P = 0.02), and the blood parasite burden. Conversely, in the BALB/c mice, this treatment did not modify maternofetal transmission or the blood parasite burden. Neutralization of IFN-γ in both infected RAG-2−/− and BALB/c mice decreased congenital Toxoplasma transmission, contrasting with an exacerbation of maternal infection. These data suggest that a partially protective immunity against congenital toxoplasmosis is achieved due to the increased number of NK cells in RAG-2−/− mice. However, it seems that IFN-γ enhances, directly or indirectly, the transplacental transmission.
Congenital toxoplasmosis poses a public health problem, being capable of causing fetal death and ocular and neurological sequelae in congenitally infected children. Congenital infection occurs only when mothers first encounter Toxoplasma gondii during pregnancy (19, 21). Resistance to T. gondii is mainly mediated by type 1 cytokines, such as gamma interferon (IFN-γ) and interleukin 2 (IL-2), whereas type 2 cytokines, such as IL-4 and IL-10, are associated with increased susceptibility to infection (10, 12). Susceptibility of the pregnant host to toxoplasmosis may be due to a type 2 cytokine bias that is maintained during gestation (26). A type 2 cytokine bias has been identified in the normal murine placenta and is associated with successful implantation, maintenance of early pregnancy, and suppression of local inflammatory responses (5, 13). Type 1 responses are downregulated during pregnancy in order to induce maternal tolerance of the semiallogeneic fetus (30, 32). This cytokine pattern of pregnancy enhances susceptibility to toxoplasmosis, together with the risk of placental infection and congenital transmission (26).
Cell-mediated immune responses involving CD4 and CD8 T cells and NK cells play a protective role in T. gondii primary infection (22, 23, 24, 28). However, the cellular and molecular mechanisms underlying protection against congenital toxoplasmosis are poorly understood. To elucidate this mechanism, it is of great importance to determine the cell immunities which are mainly responsible for protection against congenital Toxoplasma transmission. In the present study, we focused especially on the role of innate NK cells, and for this purpose, recombination activating gene knockout (RAG-2−/−) mice, which are B- and T-cell deficient, were used to evaluate the contribution of NK cells independently from B and T cells in protection against congenital Toxoplasma transmission. Congenital infection of the corresponding BALB/c wild-type strain shares some characteristics with the human infection (21).
Primary infection during the second third of gestation leads to a high percentage of infected fetuses in the BALB/c model. After infecting such mice with an avirulent cyst-forming strain of T. gondii, we determined the maternofetal transmission of parasites and the roles of NK cells and IFN-γ in congenital toxoplasmosis.
MATERIALS AND METHODS
Mice.
Mice were used at 6 to 8 weeks of age. RAG-2−/− mice (25) from the 10th backcross generation to BALB/c were kindly provided by Manfred Kopf (Former Institute of Immunology, Basel, Switzerland). BALB/c mice were purchased from Centre d’Elevage R. Janner (Le Genet-Saint-Isle, France). All mice were maintained under specific-pathogen-free conditions in barrier facilities at our laboratory. The mice were genotyped for RAG-2 alleles by PCR of tail biopsy specimens. The primers used to distinguish the mutant and RAG-2 loci were RAG-2 sense, 5′-TTG GGA GGA CAC TCA CTT AGT-3′, and neomycin sense, 5′-GGA GAA CCT GCG TGC AAT CC-3′, and antisense, 5′-GCA ACA TGT TAT CCA GTA GCC GGT-3′ (Life Technologies, Cergy Pontoise, France). Amplification of RAG-2 and neomycin gene fragments results in 605- and 700-bp products, respectively.
Pregnancy studies and Toxoplasma infection.
Female mice were mated and observed daily for vaginal-plug formation. The day the plug first appeared was designated day zero. An experimental group consisted of five to seven pregnant mice. A cyst suspension of the avirulent PRU strain of T. gondii was used to infect the pregnant females (31). The cysts were prepared from the brains of perorally inoculated chronically infected Swiss Webster (Centre d’Elevage R. Janner) female mice and prepared as previously described (2). For experimental infection, the mice received, by gavage, 20 PRU cysts (type II strain) in 100 μl of phosphate-buffered saline (PBS) on day 11 of gestation.
Detection of congenital infection.
In order to avoid possible T. gondii contamination of the pups through lactation, the pregnant females were sacrificed on the last day of gestation, day 18 (day 7 postinfection). The placentas and fetuses were removed under sterile conditions; homogenized separately in 1 ml of PBS, pH 7.2; and inoculated intraperitoneally into Swiss mice. Five weeks later, the Swiss mice were bled and tested for specific T. gondii antibodies (Abs) using an immunofluorescent assay. Briefly, sera diluted 1/25 in PBS were applied to slides containing formalin-fixed T. gondii tachyzoites (BioMérieux, Marcy l'Etoile, France) for 25 min at 37°C, and fluorescein isothiocyanate-labeled anti-mouse polyvalent immunoglobulin (immunoglobulin G [IgG], IgA, and IgM) conjugate (Sigma, St. Louis, Mo.) diluted 1/125 in PBS was then added for 25 min at 37°C.
Spleen cell culture.
Spleen cells were homogenized, and erythrocytes were lysed with Tris-NH4Cl (Sigma-Aldrich, Saint Quentin Fallavier, France). The recovered cells were washed with complete RPMI 1640 medium-HEPES spiked with 10% fetal calf serum, 1 mM sodium pyruvate, 2 mM l-glutamine, 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 0.25 μg of amphotericin B (Fungizone)/ml (Life Technology Ltd.). Splenocytes were cultured at a density of 4 × 106 per ml with medium alone in 96-well flat-bottom plates (Nunc, Roskilde, Denmark) or directly stained for flow cytometry (see below).
IFN-γ quantitation.
After 48 h of incubation, the spleen cell culture supernatants were harvested, pooled, and stored at −20°C until they were used. IFN-γ levels in the supernatants were determined by enzyme-linked immunosorbent assay with paired monoclonal Abs (MAbs) for IFN-γ (all from Pharmingen, San Diego, Calif.).
Fluorescence-activated cell sorter (FACS) analysis of leukocyte cell populations.
Splenocytes obtained from infected animals (106 cells) were incubated with 10 μl of CD32/CD16 Ab (Beckman Coulter, Villepinte, France) to block nonspecific binding sites. After 20 min of incubation at 4°C, the cells were incubated with 1 μg of phycoerythrin (PE)-labeled anti-CD4, anti-CD8, an isotype-PE control (Beckman Coulter), or PE-labeled anti-pan-NK cell DX5+ Ab (Clinisciences, Montrouge, France)/ml for 30 min at 4°C. The cells were washed twice with 0.1% fetal calf serum-PBS, fixed with 1% paraformaldehyde, and then analyzed in a FACSCalibur flow cytometer. Cell Quest software (Becton-Dickinson Immunocytometry Systems, Mountain View, Calif.) was used to analyze the data; 10,000 events were counted for each sample.
In vivo depletion of NK cells and IFN-γ.
A group of infected mice was depleted of NK cells by intraperitoneal injections of rabbit polyclonal anti-asialo GM1 Ab (Tebu, Le Perray en Yvelines, France). The dose recommended by the manufacturer is a minimum of 20 μl/mouse every 5 days. The Ab was administered on days −1 and +3 of infection at 30 μl per mouse diluted in 300 μl of PBS. Control infected mice were injected with control rabbit IgG (Euromedex, Souffelweyersheim, France) in the same manner.
In other experiments, infected mice were depleted of IFN-γ by intraperitoneal injection of 1 mg of rat anti-mouse IFN-γ MAb (XMG1.2; Pharmingen) on days −1 and +3 of infection (6, 7, 15). Control infected RAG-2−/− mice were treated with a control rat IgG MAb (Pharmingen) under the same conditions. In preliminary experiments, the number of pups and fetal outcome were not altered by Ab treatment compared to the control group.
Detection of T. gondii DNA in blood.
Primers and a SYBR Green PCR reagent kit were purchased from Eurogentec (Angers, France).
Preparation of DNA templates for PCR.
Blood samples from control infected mice and infected mice treated with anti-asialo GM1 or anti-IFN-γ were collected on day 7 postinfection. For positive control, T. gondii tachyzoites (RH strain) were used and were obtained as follows. After peritoneal lavage of mice inoculated with the RH strain, parasites collected from the mouse ascitic fluid were washed and resuspended in PBS. The concentration of tachyzoites was determined with a phase-contrast microscope using a Neubauer counting chamber. DNA was extracted using the QIAmp DNA Blood Mini Kit isolation kit (Qiagen, Courtaboeuf, France), eluted with 200 μl of Qiagen elution buffer, and stored at −20°C.
PCR primer design.
The pair of primers was based on the nucleotide sequence of the 18S rRNA gene (GenBank no. L24381). The primers were 5′-GGC ATT CCT CGT TGA TT-3′ (sense) and 5′-CCT TGG CCG ATA GGT CTA GG-3′ (antisense).
Quantitative real-time PCR.
SYBR Green PCR was set up in a 25-μl final volume (96-sample trays) containing 1× PCR buffer, 3.5 mM MgCl2, 200 μM (each) deoxynucleoside triphosphate, each primer at a concentration of 1 μM, 0.625 U of Taq polymerase, a 1:66,000 dilution of SYBR Green 1, and 5 μl of extracted DNA. One 10-fold serial dilution of T. gondii DNA was prepared as a positive control, with parasite concentrations ranging from 5,000 to 0.05 per 5 μl. The series of 10-fold dilutions was included in each amplification run. The parasite count for a given mouse blood sample was calculated by interpolation from the standard curve. After initial activation of Hot Gold Star DNA polymerase at 95°C for 10 min, 40 PCR cycles of 15 s at 95°C followed by 1 min at 55°C were performed on an ICycler (Bio-Rad, Nanterre, France) in accordance with the Eurogentec recommendation. Fluorescence was detected in the last step of each cycle. Specificity of the products was assured by melting curve analysis (20). The melting temperature for all mouse blood samples was 80.5°C, which was consistent with that produced by the positive control. The quantitative results were expressed by determination of the threshold cycle of detection, which marked the cycle when the fluorescence of a given sample significantly exceeded the baseline signal. Real-time PCR was repeated three times under identical conditions. Each experiment was performed in duplicate.
Statistical analysis.
Differences in the percentages of infected fetuses between the groups were analyzed using Fisher's exact test. Cell numbers, IFN-γ levels, and blood parasite burdens were compared by Student's t test. Differences were deemed significant when P was <0.05.
RESULTS
The congenital Toxoplasma transmission rate is lower in RAG-2−/− mice than in BALB/c mice.
First, we compared the congenital Toxoplasma transmission rates in RAG-2−/− and BALB/c control mice. The mice were infected perorally with 20 cysts of the PRU strain of T. gondii, and the infected placentas and fetuses were detected by mouse subinoculation on day 18 of gestation (day 7 postinfection). The rate of fetal infection was lower in RAG-2−/− mice than in BALB/c mice (7 of 28 fetuses positive [25%] versus 23 of 36 fetuses positive [63.9%]; P = 0.003). In both the RAG-2−/− mice and BALB/c mice, all placentas were infected in all of the tests. However, this cannot be interpreted, since it may simply reflect the presence of blood parasites found in all of the dams on day 7 postinfection (data not shown). Thus, despite the absence of B and T cells in RAG-2−/− mice, the fetuses are better protected than their BALB/c counterparts against Toxoplasma transmission.
NK cell numbers are higher in infected RAG-2−/− mice than in BALB/c mice.
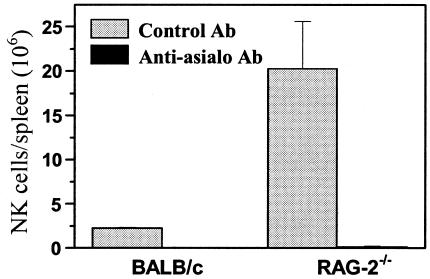
We then examined spleen cells on day 7 postinfection to assess the cell numbers in RAG-2−/− mice. To obtain absolute numbers of each subset (CD4+, CD8+, and DX5+), percentages determined by FACS analysis were multiplied by the mean total number of spleen cells in each group. As expected, no CD4+ or CD8+ T cells were detected in either uninfected or infected RAG-2−/− mice (data not shown). As shown in Fig. 1, the spleens of infected RAG-2−/− mice contained nine times as many NK cells as those of infected BALB/c mice (P = 0.04).
FIG. 1.
Numbers of NK cells in spleens of T. gondii-infected RAG-2−/− and BALB/c mice on day 18 of gestation (day 7 postinfection). Spleen cell suspensions were harvested, stained for pan-NK cell DX5+ expression, and analyzed by FACScan. The values represent the means plus standard deviations of three mice in each group. Similar results were obtained in two independent experiments.
Depletion of NK cells partially abrogates protection against fetal Toxoplasma infection in RAG-2−/− mice but not in BALB/c mice.
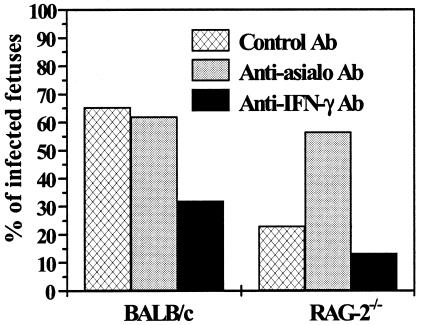
To evaluate the contribution of NK cells in the absence of B and T cells in congenital Toxoplasma transmission, we depleted NK cells in infected animals. FACS analysis demonstrated at least 98% depletion of pan-NK cells in the spleens of the mice (Fig. 1). The results for maternofetal transmission of T. gondii are shown in Fig. 2. In the RAG-2−/− mice, NK cell depletion significantly increased the proportion of parasite transmission to the fetuses (13 of 23 fetuses positive [56.5%]; P = 0.02) in comparison with RAG-2−/− mice treated with control Ab. In contrast, in BALB/c mice, NK cell depletion did not modify parasite transmission in comparison with BALB/c mice treated with control Ab (18 of 29 fetuses positive [62%] versus 17 of 26 fetuses positive [65.3%]; P = 1). This result indicates that NK cells play a pivotal role in protection against congenital toxoplasmosis in RAG-2−/− mice but not in BALB/c mice.
FIG. 2.
Effect of depletion of NK cells (anti-asialo GM1 Ab) or IFN-γ (XGM1.2 Ab) on maternofetal transmission of T. gondii in RAG-2−/− and BALB/c mice. The presence or absence of fetal infection was assessed on day 18 of gestation (day 7 postinfection). The values represent the mean percentages of infected fetuses and are representative of three independent experiments.
T. gondii infection increases IFN-γ production by splenocytes from infected RAG-2−/− mice.
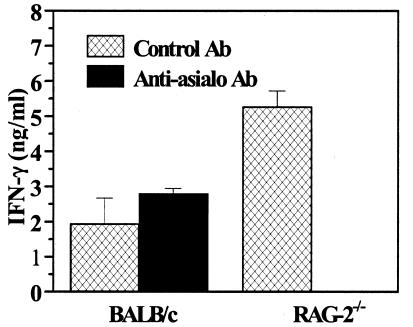
We investigated whether the protection against congenital Toxoplasma transmission in the RAG-2−/− mice correlated with IFN-γ production by spleen cells. As shown in Fig. 3, spleen cells from infected RAG-2−/− mice, cultured for 48 h, released significantly more IFN-γ than those from infected BALB/c mice (P = 0.002). The anti-asialo-GM1 Ab treatment abolished IFN-γ production by spleen cells from infected RAG-2−/− mice. In contrast, NK cell depletion did not significantly modify IFN-γ production by spleen cells from BALB/c mice (P = 0.09).
FIG. 3.
IFN-γ production by spleen cells on day 18 of gestation (day 7 postinfection). The supernatants of spleen cells cultured for 48 h in RPMI were harvested and analyzed for the presence of IFN-γ by enzyme-linked immunosorbent assay. Each bar represents the mean plus standard deviation of triplicate measures of supernatants from three mice and is representative of three independent experiments.
Neutralization of IFN-γ decreases the risk of fetal Toxoplasma infection.
We then depleted IFN-γ in both RAG-2−/− and BALB/c mice to analyze the role of this cytokine in congenital Toxoplasma transmission. Administration of anti-IFN-γ MAb decreased the risk of fetal Toxoplasma infection in RAG-2−/− mice relative to RAG-2−/− mice treated with a control Ab, albeit not significantly (4 of 30 fetuses positive [13.3%] versus 8 of 35 fetuses positive [22.9%]) (Fig. 2) and significantly decreased maternofetal parasite transmission in BALB/c mice to 32% (8 of 25 fetuses positive; P = 0.02) (Fig. 2) in comparison with BALB/c mice treated with control Ab.
Depletion of NK cells or IFN-γ in RAG-2−/− mice enhanced the maternal blood parasite burden.
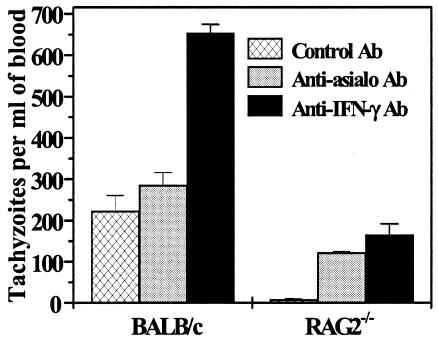
In order to evaluate the influence of the blood parasite load on fetal infection, we quantified parasitemia using quantitative real-time PCR. The relative abundance of the 18S rRNA gene, a genetic marker for T. gondii, was determined on day 7 postinfection in the blood. In RAG-2−/− mice, NK cell depletion significantly increased parasitemia compared to RAG2−/− mice treated with control Ab (P < 0.0001). Conversely, in BALB/c mice, depletion of NK cells did not modify parasitemia in comparison with BALB/c mice treated with control Ab. The blood parasite loads in both mouse groups (RAG2−/− and BALB/c) treated with anti-IFN-γ were significantly higher than those in their control groups (mice treated with control Ab) (P ≤ 0.0005) (Fig. 4). This result indicates that IFN-γ neutralization in both mouse groups and NK cell depletion in RAG-2−/− mice were associated with an increase in maternal blood infection. It is important to note that the parasitemia in RAG-2−/− mice was always lower than in their wild-type counterparts.
FIG. 4.
Maternal blood parasite burden measured by quantitative real-time PCR on day 18 of gestation (day 7 postinfection). The data represent the means plus standard deviations of three mice. Similar results were obtained in two independent experiments.
DISCUSSION
NK cells are involved in innate immunity against a broad range of intracellular pathogens, and especially T. gondii, in the earliest stage of infection (1, 9, 17). However, the role of NK cells in transplacental Toxoplasma transmission is unknown. The findings of the present study suggest that an increased NK cell number in infected RAG-2−/− mice, which may compensate for the lack of T and B cells, was associated with increased fetal protection. When we depleted NK cells in the mice, we observed an increased fetal Toxoplasma infection rate in RAG-2−/− mice, in contrast to BALB/c mice, in which this treatment did not statistically modify the percentage of T. gondii-infected fetuses. As previous studies showed no cross-reactivity of anti-asialo GM1 Abs to other placental cells (18), these data suggest that NK cells, the number of which is increased in RAG-2−/− mice, play a pivotal role in controlling transplacental Toxoplasma transmission. The decreased levels of NK cells in BALB/c mice may have had a favorable role in transplacental T. gondii transmission. A threshold NK cell level is required to enable partial protection against primary congenital toxoplasmosis. Given their capacity to respond to external stimuli without prior sensitization, NK cells are effectors of innate resistance, forming the first line of defense against infection (24). In human congenital toxoplasmosis, elevated circulating NK cell numbers have been linked to protection (16).
We measured the tachyzoite load in maternal blood samples taken from the mice by quantitative real-time PCR. Our results show that blood from NK cell-depleted RAG-2−/− mice contains significantly more tachyzoites per milliliter than blood from RAG-2−/− mice treated with control Ab. These results indicate that NK cells protect the adult and are involved in protective immunity in congenital toxoplasmosis. RAG-2−/− mice do not have Abs; therefore, NK cells may act by means of their cytotoxic activity on infected T. gondii cells (4). Furthermore, Hauser and Tsai (8) have shown that acute Toxoplasma infection in mice induces spleen NK cells that are cytotoxic for T. gondii in vitro.
NK cells also play multiple roles in host-parasite interactions, through their production of cytokines. T. gondii infection triggers an increase in NK cells and is associated with IFN-γ production (11), which protects against acute toxoplasmosis in mice (29). In our model, IFN-γ secretion by spleen cells from infected RAG-2−/− mice was higher than in uninfected RAG-2−/− mice, suggesting that a systemic type 1 cytokine response is associated with protection against congenital Toxoplasma transmission. When we depleted NK cells in RAG-2−/− mice, IFN-γ production was abolished. These findings suggest that NK cells are the main producers of IFN-γ in RAG-2−/− mice and that NK cells could protect fetuses due to the anti-T. gondii activity of this cytokine in the maternal blood.
We therefore investigated the role of IFN-γ in our model. Surprisingly, IFN-γ neutralization in both RAG-2−/− and BALB/c mice decreased maternofetal transmission of Toxoplasma. When we measured parasitemia in mice, it was revealed that the blood parasite load in both infected RAG-2−/− and BALB/c mice treated with anti-IFN-γ was significantly higher than that in mice treated with control Ab. The decreased congenital Toxoplasma transmission in mice treated with anti-IFN-γ Ab despite the increase in maternal blood Toxoplasma infection is puzzling. One possible explanation for our observation is that IFN-γ facilitates the transplacental passage of Toxoplasma. Indeed, the presence of IFN-γ receptors in murine trophoblasts, at the maternofetal interface, has been demonstrated (3). It is possible that signaling through this receptor enhances the uptake or delivery of the parasite by these cells, for example, by stimulating phagocytic activity without parasite neutralization. Another possibility is an enhancement of adhesion receptor expression by IFN-γ on the trophoblast cell surface, which might enhance transplacental passage of Toxoplasma. Tumor necrosis factor alpha, IL-2β, and IFN-γ were found to be the most potent inducers of intercellular adhesion molecule 1 expression (27, 33). Moreover, Maubert et al. (14) showed, in human malaria-infected placentas, that expression of intercellular adhesion molecule 1 and chondroitin 4 sulfate on the trophoblast cell surface was upregulated by tumor necrosis factor alpha, a phenomenon possibly involved in placental sequestration of the parasite. Investigations of the roles of these processes for transmission of T. gondii are under way
Based on our observations, we propose that an increased number of NK cells in RAG-2−/− mice partially protects against congenital Toxoplasma transmission. NK cells and IFN-γ are important for reducing maternal infection by inhibition of tachyzoite replication. However, it seems that IFN-γ enhances, directly or indirectly, the transplacental transmission of T. gondii.
Acknowledgments
We gratefully acknowledge Dominique Wachsmann for critical reading of the manuscript.
This work was supported in part by Université Louis Pasteur de Strasbourg and the Centre Hospitalier Universitaire de Strasbourg.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Bancroft, G. J., R. D. Schreiber, G. C. Bosma, M. J. Bosma, and E. R. Unanue. 1987. A T cell-independent mechanism of macrophage activation by interferon-gamma. J. Immunol. 139:1104-1107. [PubMed] [Google Scholar]
- 2.Brinkmann, V., S. D. Sharma, and J. S. Remington. 1986. Different regulation of the L3T4-T cell subset by B cells in different mouse strains bearing the H-2K haplotype. J. Immunol. 137:2291-2297. [PubMed] [Google Scholar]
- 3.Chen, H. L., R. Kamath, J. L. Pace, S. W. Russel, and J. S. Hunt. 1994. Expression of the interferon-gamma receptor gene in mouse placentas is related to stage of gestation and is restricted to specific subpopulations of trophoblast cells. Placenta 15:109-121. [DOI] [PubMed] [Google Scholar]
- 4.Dannemann, B. R., V. A. Morris, F. G. Araujo, and J. S. Remington. 1989. Assessment of human natural killer and lymphokine-activated killer cell cytotoxicity against Toxoplasma gondii trophozoites and brain cysts. J. Immunol. 143:2684-2691. [PubMed] [Google Scholar]
- 5.Delassus, S., G. C. Coutinho, C. Saucier, S. Darche, and P. Kourilsky. 1994. Differential cytokine expression in maternal blood and placenta during murine gestation. J. Immunol. 152:2411-2420. [PubMed] [Google Scholar]
- 6.Gazzinelli, R. T., F. Hakim, S. Hieny, G. M. Shearer, and A. Sher. 1991. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-gamma production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J. Immunol. 146:286-292. [PubMed] [Google Scholar]
- 7.Haque, S., J. Franck, H. Dumon, L. H. Kasper, and A. Haque. 1999. Protection against lethal toxoplasmosis in mice by an avirulent strain of Toxoplasma gondii: stimulation of IFN-gamma and TNF-alpha response. Exp. Parasitol. 93:231-240. [DOI] [PubMed] [Google Scholar]
- 8.Hauser, W. E., Jr., and V. Tsai. 1986. Acute toxoplasma infection of mice induces spleen NK cells that are cytotoxic for T. gondii in vitro. J. Immunol. 136:313-319. [PubMed] [Google Scholar]
- 9.Hunter, C. A., C. S. Subauste, V. H. Van Cleave, and J. S. Remington. 1994. Production of gamma interferon by natural killer cells from Toxoplasma gondii-infected SCID mice: regulation by interleukin-10, interleukin-12, and tumor necrosis factor alpha. Infect. Immun. 62:2818-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunter, C. A., Y. Suzuki, C. S. Subauste, and J. S. Remington. 1996. Cells and cytokines in resistance to Toxoplasma gondii. Curr. Top. Microbiol. Immunol. 219:113-125. [DOI] [PubMed] [Google Scholar]
- 11.Kasper, L. H., T. Matsuura, and I. A. Khan. 1995. IL-7 stimulates protective immunity in mice against the intracellular pathogen, Toxoplasma gondii. J. Immunol. 155:4798-4804. [PubMed] [Google Scholar]
- 12.Khan, I. A., T. Matsuura, and L. H. Kasper. 1994. Interleukin-12 enhances murine survival against acute toxoplasmosis. Infect. Immun. 62:1639-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin, H., T. R. Mosmann, L. Guilbert, S. Tuntipopipat, and T. G. Wegmann. 1993. Synthesis of T helper 2-type cytokines at the maternal-fetal interface. J. Immunol. 151:4562-4573. [PubMed] [Google Scholar]
- 14.Maubert, B., L. J. Guilbert, and P. Deloron. 1997. Cytoadherence of Plasmodium falciparum to intercellular adhesion molecule 1 and chondroitin-4-sulfate expressed by the syncitiotrophoblast in the human placenta. Infect. Immun. 65:1251-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muller, I., P. Kropf, R. J. Etges, and J. A. Louis. 1993. Gamma interferon response in secondary Leishmania major infection: role of CD8+ T cells. Infect. Immun. 61:3730-3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nigro, G., J. Piazze, R. Paesano, T. Mango, S. Provvedi, O. Capuano, and L. Pollastrini. 1999. Low levels of natural killer cells in pregnant women transmitting Toxoplasma gondii. Prenat. Diagn. 19:401-404. [PubMed] [Google Scholar]
- 17.Orange, J. S., B. Wang, C. Terhorst, and C. A. Biron. 1995. Requirement for natural killer cell-produced interferon-gamma in defense against murine cytomegalovirus infection and enhancement of this defense pathway by interleukin 12 administration. J. Exp. Med. 182:1045-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Redline, R. W., and C. Y. Lu. 1989. Localization of fetal major histocompatibility complex antigens and maternal leukocytes in murine placenta. Implications for maternal-fetal immunological relationship. Lab. Investig. 61:27-36. [PubMed] [Google Scholar]
- 19.Remington, J. S., R. McLeod, and G. Desmonts. 1994. Toxoplasmosis, p. 141-267. In J. S. Remington and O. J. Klein (ed.), Infectious diseases of the fetus and new-born infant, 4th ed. W. B. Saunders Co., Philadelphia, Pa.
- 20.Ririe, K. M., R. P. Rasmussen, and C. T. Wittwer. 1997. Product differentiation by analysis of DNA melting curves during the polymerase chain reaction. Anal. Biochem. 15:154-160. [DOI] [PubMed] [Google Scholar]
- 21.Roberts, C. W., and J. Alexander. 1992. Studies on a murine model of congenital toxoplasmosis: vertical disease transmission only occurs in BALB/c mice infected for the first time during pregnancy. Parasitology 104:19-23. [DOI] [PubMed] [Google Scholar]
- 22.Scharton-Kersten, T., H. Nakajima, G. Yap, A. Sher, and W. J. Leonard. 1998. Infection of mice lacking the common cytokine receptor gamma-chain (gamma(c)) reveals an unexpected role for CD4+ T lymphocytes in early IFN-gamma-dependent resistance to Toxoplasma gondii. J. Immunol. 160:2565-2569. [PubMed] [Google Scholar]
- 23.Scorza, T., S. D'Souza, M. Laloup, J. Dewit, J. De Braekeleer, H. Verschueren, M. Vercammen, K. Huygen, and E. Jongert. 2003. A GRA1 DNA vaccine primes cytolytic CD8+ T cells to control acute Toxoplasma gondii infection. Infect. Immun. 71:309-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scott, P., and G. Trinchieri. 1995. The role of natural killer cells in host-parasite interactions. Curr. Opin. Immunol. 7:34-40. [DOI] [PubMed] [Google Scholar]
- 25.Shinkai Y., G. Rathbun, K. P. Lam, E. M. Oltz, V. Stewart, M. Mendelsohn, J. Charron, M. Datta, F. Young, and A. M. Stall. 1992. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 68:855-867. [DOI] [PubMed] [Google Scholar]
- 26.Shirahata, T., N. Muroya, C. Ohta, H. Goto, and A. Nakane. 1992. Correlation between increased susceptibility to primary T. gondii infection and depressed production of gamma interferon in pregnant mice. Microbiol. Immunol. 36:81-91. [DOI] [PubMed] [Google Scholar]
- 27.Shrikant, P., I. Y. Chung, M. E. Ballestas, and E. N. Benveniste. 1994. Regulation of intercellular adhesion molecule-1 gene expression by tumor necrosis factor-alpha, interleukin-1 beta, and interferon-gamma in astrocytes. J. Neuroimmunol. 51:209-220. [DOI] [PubMed] [Google Scholar]
- 28.Subauste, C. S., L. Dawson, and J. S. Remington. 1992. Human lymphokine-activated killer cells are cytotoxic against cells infected with Toxoplasma gondii. J. Exp. Med. 176:1511-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki, Y., M. A. Orellana, R. D. Schreiber, and J. S. Remington. 1988. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science 240:516-518. [DOI] [PubMed] [Google Scholar]
- 30.Tangri, S., T. G. Wegmann, H. Lin, and R. Raghupathy. 1994. Maternal anti-placental reactivity in natural, immunologically-mediated fetal resorptions. J. Immunol. 152:4903-4911. [PubMed] [Google Scholar]
- 31.Thouvenin, M., E. Candolfi, O. Villard, J. P. Klein, and T. Kien. 1997. Immune response in a murine model of congenital toxoplasmosis: increased susceptibility of pregnant mice and transplacental passage of T. gondii are type-2 dependent. Parasitologia 39:279-283. [PubMed] [Google Scholar]
- 32.Wegmann, T. G., H. Lin, L. Guilbert, and T. R. Mosmann. 1993. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol. Today 14:353-356. [DOI] [PubMed] [Google Scholar]
- 33.Xiao, J., M. Garcia-Lloret, B. Winkler-Lowen, R. Miller, K. Simpson, and L. J. Guilbert. 1997. ICAM-1-mediated adhesion of peripheral blood monocytes to the maternal surface of placental syncytiotrophoblasts: implications for placental villitis. Am. J. Pathol. 150:1845-1860. [PMC free article] [PubMed] [Google Scholar]