Abstract
T-cell-mediated immunity plays a central role in the host response to Cryptosporidium parvum. Human T-cell clones (TCC) were isolated from peripheral blood mononuclear cells of five healthy donors with prior cryptosporidiosis by use of a C. parvum crude extract, two antigen fractions obtained by ion-exchange chromatography (IEC1 and IEC2), and two recombinant peptides (SA35 and SA40) from C. parvum sporozoites. The T-cell lines derived from the one recently infected donor had a higher proportion (26 to 38%) of T cells exhibiting the γ/δ T-cell receptor (γ/δ-TCR) than those from donors who had recovered from cryptosporidiosis several years earlier, suggesting that the γ/δ T-cell population is involved in the early stage of the infection. The specific TCC had the α/β-TCR, had the phenotype CD45RO+ CD4+ CD8−, and were characterized by either hyperproduction of gamma interferon (IFN-γ) alone, with a Th1 profile, or IFN-γ hyperproduction together with interleukin-4 (IL-4) or IL-5 production, with a Th0 profile. SA35, SA40, IEC1, and IEC2 may be considered good targets of the cellular response against C. parvum and may play a role in maintaining the T-cell-mediated memory response to this parasite. Furthermore, the SA35 and SA40 peptides may be regarded as immunodominant antigens involved in the maintenance of the T-cell response in healthy C. parvum-sensitized persons.
Cryptosporidium parvum is a protozoan parasite which mainly infects the epithelial intestinal cells of humans and animals and is transmitted by the ingestion of oocysts excreted in feces. In immunocompetent hosts, C. parvum infection induces self-limited diarrhea, yet in immunocompromised hosts, the infection can be persistent and severe and life-threatening diarrhea can ensue (10).
To date, no specific or effective therapy for cryptosporidiosis has been developed, although some anticryptosporidial effects have been observed for the aminoglycoside paromomycin (6, 11, 19) as well as for azithromycin and nitazoxamide (2, 13, 37). Indinavir, a protease inhibitor that is included as part of highly active antiretroviral therapy for persons infected with human immunodeficiency virus, has also shown a direct anticryptosporidial effect (29). Antibody therapy for C. parvum infection has been mainly proposed for persons with AIDS or with other immunodeficiencies. However, studies that have treated a significant number of persons with bovine colostrum from cows that were hyperimmunized with C. parvum oocysts have shown conflicting results (18, 22, 33, 42, 43). In light of these findings, the host cell immune response seems to be the only effective form of defense.
The immune response to C. parvum involves a complex interplay of both natural and acquired responses (14). Clinical observations have suggested that CD4+ T cells play a major role in the control of cryptosporidiosis (4, 12, 35). Studies of cytokine production in persons with cryptosporidiosis have demonstrated that gamma interferon (IFN-γ) is a key factor in the immune response and that infection is generally more severe in persons who are unable to produce IFN-γ (15, 16). Furthermore, IFN-γ seems to be necessary for controlling the infection rapidly and efficiently (25). With murine models, it was shown that the control of C. parvum infection requires not only CD4+ T cells (28), but also the major histocompatibility complex (MHC) class II molecules (1) and the intact CD40-CD154 signaling pathway (7, 20, 21). With SCID mice, non-antigen-specific CD4+-T-cell effector mechanisms, in combination with the innate arm of the immune system, have been shown to eradicate C. parvum infection (27).
Most of the studies that have attempted to identify C. parvum antigens involved in the immune response have analyzed antigens that are recognized by antibodies in the serum and feces of experimentally or naturally infected animals and persons (31, 34). Many glycoproteins of oocysts and surface proteins expressed at the sporozoite and merozoite stages are immunogenic in humans and are specifically recognized by convalescent-phase serum antibodies; thus, the most important antigens are considered to be those in the range of 14 to 200 kDa (8). However, little information is available on the C. parvum antigens that are capable of eliciting a T-cell response (5, 15, 38).
For the study of immune responses to microbial antigens, T-cell lines (TCL) and T-cell clones (TCC) can be useful, in that they provide direct evidence of the immunodominant epitopes of the parasite (32). In humans, since the host cell immune response is the only effective form of defense against C. parvum, the cell-mediated immunity needs to be characterized so we can identify the functional T cells associated with the control of the infection. The antigens capable of inducing a T-cell response and thus potentially related to protection should also be identified.
To this end, we studied the ability of five antigen fractions from C. parvum crude extract (CCE), obtained by ion-exchange chromatography (IEC), to induce proliferation in peripheral blood mononuclear cells (PBMC) from C. parvum-sensitized donors. We also tested the ability of four C. parvum peptides (A10, SA20, SA35, and SA40), representing antigenic proteins expressed at the sporozoite stage (39), to induce PBMC proliferation.
MATERIALS AND METHODS
Antigens and stimulants.
CCE and the purified recombinant antigens were obtained as previously described (15, 39). The A10 peptide (10 kDa) consists of 75 amino acids corresponding to positions 20 to 94 of the protein CpA10 (GenBank accession number AJ574898), and it is fused with a six-histidine tag. The peptides SA35 (35 kDa) and SA40 (40 kDa) represent antigenic portions of two different microneme proteins, namely Cpa135 and Gp900, respectively (3, 40). The SA20 peptide (20 kDa) (39), which is recognized by human immunoglobulin G (IgG), represents a portion of a nuclear C. parvum protein which has never been documented (F. Tosini, unpublished data).
The five antigen fractions (IEC1 to IEC5) were obtained from CCE by IEC. In brief, CCE samples (2.5 mg of total proteins/ml) were concentrated in PD10 columns (Amersham International, Buckinghamshire, United Kingdom) and loaded onto a 1-ml High Q column (Bio-Rad, Hercules, Calif.) which was equilibrated with 10 mM Tris-HCl, pH 8.0. Unretained proteins were eluted with the column buffer, and a linear gradient of NaCl was established (from 0 to 1 M in 30 ml of column buffer). The column was then eluted with 1 M NaCl in the same buffer. Proteins in the eluate were monitored at 280 nm. Five fractions were obtained.
For determination of the molecular mass of the antigen fractions, sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis was performed (23), and the fractions were transferred onto nitrocellulose (41). The reactive bands were identified after incubation with a rabbit anti-C. parvum IgG polyclonal antibody which recognizes all stages of C. parvum (36). However, only the proteins from IEC1, with sizes of 25, 30, 40 to 42, and 68 kDa, and from IEC2, with sizes of 35, 102, and 130 kDa, were selected for further analyses, since these were the two fractions that induced the strongest specific PBMC proliferation (Table 1). It cannot be ruled out that the SA40 and SA35 peptides are included, respectively, in IEC1 and IEC2, given that both of these fractions contain reactive bands with molecular masses compatible with these peptides. Recombinant interleukin-2 (rIL-2; Amersham International) and purified phytohemagglutinin (PHA; Murex Diagnostics S.A., Dartford, United Kingdom) were used as mitogens.
TABLE 1.
Proliferative response of PBMC from C. parvum-sensitized persons to CCE, five fractions isolated from CCE by IEC (IEC1, -2, -3, -4, and -5), and four recombinant peptides from C. parvum sporozoites (A10, SA20, SA35, and SA40)
| Stimulant | Concn (μg/ml) | PBMC proliferation (SI) for indicated donora
|
||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5b | ||
| None | 1 | 1 | 1 | 1 | 1 | |
| IL-2 | 100c | 160 | 216 | 254 | 194 | 105 |
| CCE | 5 | 76 | 96 | 80 | 40 | 22 |
| 50 | 123 | 176 | 235 | 188 | 105 | |
| IEC1 | 5 | 25 | 13 | ND | 30 | ND |
| 50 | 97 | 127 | 99 | 106 | 110 | |
| IEC2 | 5 | 19 | 5 | ND | 25 | ND |
| 50 | 89 | 115 | 92 | 87 | 78 | |
| IEC3 | 5 | 10 | 12 | ND | 11 | ND |
| 50 | 30 | 43 | 31 | 32 | 34 | |
| IEC4 | 5 | 1 | 2 | ND | ND | ND |
| 50 | 2 | 2 | 3 | 1 | 1 | |
| IEC5 | 5 | 2 | 2 | ND | ND | ND |
| 50 | 9 | 11 | 10 | 8 | 6 | |
| A10 | 1 | ND | 8 | ND | ND | ND |
| 10 | ND | 6 | 7 | 8 | 8 | |
| SA20 | 1 | ND | 1 | ND | ND | ND |
| 10 | ND | 1 | 1 | 1 | 1 | |
| SA35 | 1 | 19 | 28 | ND | 23 | ND |
| 5 | 24 | ND | ND | ND | ND | |
| 10 | 27 | 29 | ND | 28 | 30 | |
| SA40 | 1 | 14 | 22 | ND | 18 | ND |
| 5 | 19 | ND | ND | ND | ND | |
| 10 | 20 | 23 | ND | 25 | 27 | |
PBMC were harvested on day 7 of culture, and 0.5 μCi of [3H]thymidine was added during the last 18 h. The SI was calculated as the counts per minute in the presence of antigen divided by the counts per minute in the absence of antigen. ND, not done.
Donor with recent cryptosporidiosis.
International units per milliliter.
Donors.
The five C. parvum-sensitized immunocompetent donors were selected based on the reactivity of their PBMC to CCE (the donors are designated with the numbers 1 to 5). Four donors (donors 1 to 4) had acquired cryptosporidiosis >3 years earlier, whereas donor 5, who was healthy on the day that blood samples were taken, had acquired cryptosporidiosis 15 days earlier.
Generation of C. parvum-specific TCL and TCC.
PBMC were isolated from heparinized blood by centrifugation in a density gradient (Lymphoprep; Nyegaard, Oslo, Norway). TCL were generated by distributing PBMC in 24-well tissue culture plates (Costar Corporation, Cambridge, Mass.) at a concentration of 106 cells/ml in complete RPMI 1640 medium (GIBCO, Grand Island, N.Y.) supplemented with 1.6% l-glutamine (Sigma, Saint Louis, Mo.), 1% sodium pyruvate (Sigma), 1% antibiotic-antimycotic solution with 5% human serum (pooled AB sera), and 50 μg of soluble CCE. The cells were maintained at 37°C in a humidified atmosphere containing 5% CO2. Once a week, fresh medium with human serum and rIL-2 was added to the cell cultures. After 14 days of culturing, growing lines were tested for antigen specificity by use of a proliferation assay, and positive cells were pulsed with CCE, IEC1, IEC2, and selected recombinant peptides (i.e., SA35 and SA40) in the presence of 0.5 × 106 autologous irradiated PBMC/ml (6,000 rad) as antigen-presenting cells (APCs) and 20 IU of rIL-2/ml. The cultures were expanded, tested for antigen specificity, immunophenotyped, and then cloned by limiting dilution, as follows. The cells were individually seeded in 96-well U-bottom tissue culture plates (Costar) in the presence of 2 × 105 feeder cells (6,000-rad-irradiated PBMC from unrelated donors) in complete medium supplemented with 5% human serum, 10% fetal calf serum (FCS), 40 IU of rIL-2/ml, and 2 μg of PHA/ml. After 2 weeks, the cultures were tested for antigen specificity in a proliferation assay; reactive TCC were immunophenotyped and then expanded and maintained in complete medium containing rIL-2. Feeder cells with 1 μg of PHA/ml were added at 15- to 20-day intervals. TCC were always used at least 10 days after restimulation.
Epstein-Barr virus (EBV) transformation of B cells.
Autologous EBV-B-cell lines were prepared from the PBMC of the TCC donors by in vitro infection with EBV obtained from the supernatant of the EBV-producing marmoset line B95/8 (provided by Elena Giacomini, Istituto Superiore di Sanità). The transformed EBV cells were maintained in complete medium, with human serum replaced with 10% FCS.
Proliferation assays.
For PBMC proliferation, the cells were diluted in complete medium supplemented with a 1% antibiotic-antimycotic solution with 5% human serum. One hundred international units of rIL-2 per milliliter or 50 μg of CCE/ml was then added. PBMC proliferation was measured in 96-well flat-bottom microwell tissue culture plates in triplicate. The plates were incubated in 5% CO2 at 37°C, and PBMC were harvested on the 7th day; 0.5 μCi of [3H]thymidine (Amersham Life Science) was added to the culture for the last 18 h. The data were expressed as stimulation indexes (SI) (i.e., counts per minute in the presence of antigen divided by counts per minute in the absence of antigen). The antigens were used at a dilution of 1:10 in a dose-response test. In inhibition experiments, the proliferation specific for IEC1, IEC2, SA35, and SA40 was blocked by treatment of the cultures with decreasing concentrations of an anti-HLA-DR monoclonal antibody (MAb) (twofold dilutions from 1:16 to 1:64) (Pharmingen, San Diego, Calif.).
TCL and TCC (2 × 105/ml) and irradiated autologous PBMC (2 × 105 cells/ml) were incubated in complete medium with 5% human serum, in the presence of antigens, in 96-well flat-bottom tissue culture plates (Costar) with 2 × 105 autologous irradiated (9,000 rad) EBV-B cells/ml. Cultures without antigen were used as controls. Cultures were maintained for 3 days and treated with 0.5 μCi of [3H]thymidine/well during the last 18 h. TCC specificity was determined by proliferation with each antigen, as measured by [3H]thymidine incorporation. TCC reactivity was scored as follows: low, SI between 2 and 10; intermediate, SI between 11 and 20; high, SI of ≥21 (32).
Phenotypic analysis.
TCL and TCC were analyzed for the expression of surface markers by flow cytometry with a FACSCalibur flow cytometer (Becton Dickinson, San Jose, Calif.). Data were acquired by using CELL-Quest software (Becton Dickinson). The following MAbs labeled with fluorescein isothiocyanate (FITC) or phycoerythrin (PE) were used according to the manufacturer's instructions: CD3(FITC)/CD4(PE), CD3(FITC)/CD8(PE), TCR-αβ(FITC)/TCR-γδ(PE), and CD45RA(FITC)/CD45RO(PE) (all from Becton Dickinson). Cells incubated with FITC-conjugated and PE-conjugated mouse IgG1 or IgG2a served as isotype controls.
Stimulation of TCC for cytokine production and cytokine detection assays.
TCC (5 × 105) were cultured in 12- by 75-mm round-bottomed tubes (Costar) with the respective antigens (50 μg of CCE, IEC1, and IEC2 and 10 μg of SA35 and SA40) in the presence of 105 autologous 6,000-rad-irradiated EBV-B cells as APCs for 72 h. Cultures were maintained in 0.5 ml of complete medium with 10% FCS. Supernatants were harvested by centrifugation, divided into aliquots, and stored at −80°C before testing. Concentrations of IL-2, IL-4, IL-5, IL-10, and IFN-γ were measured by use of a commercial multiplex assay (Cytometric bead array; Becton Dickinson) according to the manufacturer's instructions. The detection limit of the assay was 20 pg/ml for each cytokine.
RESULTS
Proliferative response of C. parvum-sensitized immunocompetent donors.
Although all antigen preparations induced PBMC proliferation (Table 1), for each type of antigen, only those preparations inducing the highest levels of proliferation (i.e., CCE, IEC1, IEC2, SA35, and SA40) were selected for the remaining analyses.
The observed high level of proliferation in these donors suggests that there was an increase in the proportion of C. parvum-specific memory T cells and/or in the production of cytokines that induce T-cell growth.
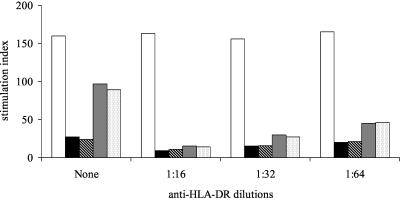
The PBMC proliferation in response to IEC1, IEC2, SA35, and SA40 was blocked by an anti-HLA-DR MAb in the tested donor (donor 1), and the extent of blocking was larger for lower MAb dilutions (Fig. 1). The proliferation was not influenced by protein heat inactivation at 100°C for 1 h (data not shown).
FIG. 1.
Inhibition of the proliferation of C. parvum-sensitized PBMC induced by two IEC fractions (IEC1 and IEC2) and the recombinant peptide SA35 with a MAb directed against MHC class II-specific HLA-DR. PBMC were harvested on day 7 of culture; 0.5 μCi of [3H]thymidine were added during the last 18 h of culture. The SI was calculated as counts per minute in the presence of antigen divided by counts per minute in the absence of antigen. White bars, IL-2; black bars, SA35 peptide; striped bars, SA40 peptide; dark gray bars, IEC1; light gray bars, IEC2.
Generation and isolation of antigen-specific TCL and TCC.
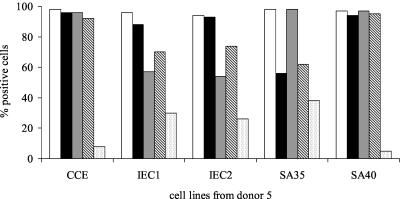
TCL constituted from 30 to 49% of the growing cells. When the cultures were expanded and immunophenotyped, most of the cells were found to have the α/β T-cell receptor (α/β-TCR) and the phenotype CD45RO+ CD4+ CD8−. Four of the 10 TCL from donor 5 constituted a higher percentage (26 to 38%) of cells exhibiting γ/δ-TCR than the TCL from the other donors (Fig. 2).
FIG. 2.
Phenotypic analysis of five representative TCL from donor 5: CCE; IEC1 and IEC2, fractions isolated from CCE by IEC; and SA35 and SA40, two recombinant peptides from C. parvum sporozoites. White bars, CD3+; black bars, CD4+; dark gray bars, CD45RO+; striped bars, α/β-TCR; light gray bars, γ/δ-TCR.
Only TCL from donors 1, 4, and 5 were cloned, and 50 TCC were selected from each donor. Most of the TCC showed high or medium reactivity to the respective antigen (Table 2). No proliferation of TCC was observed when APCs that were not primed with an antigen or antigens not related to C. parvum (e.g., Trichinella crude antigen extract) were used (data not shown). Ten CCE-specific TCC from one of the donors (donor 1) were also subjected to proliferation assays with the SA35 and SA40 antigens in the presence of autologous irradiated APCs. The SI values were ≥21 for five SA35-specific TCC and three SA40-specific TCC, 11 to 20 for three SA35-specific TCC and three SA40-specific TCC, and 2 to 10 for two SA35-specific TCC and four SA40-specific TCC. All TCC were identified as having the α/β-TCR and the phenotype CD45RO+ CD4+ CD8−.
TABLE 2.
Antigen recognition of C. parvum-specific TCC (50 clones from each donor) in the presence of autologous irradiated PBMC as APCs
| Donor | TCC specificity | TCC reactivity (no. of TCC)a
|
||
|---|---|---|---|---|
| Low | Medium | High | ||
| 1 | CCE | 1 | 2 | 7 |
| IEC1 | 2 | 1 | 7 | |
| IEC2 | 1 | 2 | 7 | |
| SA35 | 3 | 5 | 2 | |
| SA40 | 2 | 5 | 3 | |
| 4 | CCE | 0 | 4 | 6 |
| IEC1 | 0 | 4 | 6 | |
| IEC2 | 0 | 4 | 6 | |
| SA35 | 1 | 6 | 3 | |
| SA40 | 0 | 5 | 5 | |
| 5 | CCE | 1 | 0 | 9 |
| IEC1 | 1 | 4 | 5 | |
| IEC2 | 1 | 4 | 5 | |
| SA35 | 2 | 4 | 4 | |
| SA40 | 1 | 4 | 5 | |
Reactivity was evaluated as follows: low, SI of 2 to 10; intermediate, SI of 11 to 20; high, SI of ≥21.
Cytokine production by TCC.
All TCC tested were characterized by the hyperproduction of IFN-γ (Table 3). Different patterns of cytokine production were observed as follows: there was a Th1 pattern with IFN-γ production and a Th0 pattern with production of IFN-γ, IL-4 (or IL-5), and a small amount of IL-10. The amount of IL-2 produced was very small (data not shown), suggesting that this cytokine was consumed by TCRs.
TABLE 3.
Production of IL-4, IL-5, IL-10, and IFN-γ in supernatants of representative C. parvum-specific TCC from three C. parvum-sensitized persons
| Donor | TCC specificity | Concentration of cytokine (pg/ml)
|
Phenotype | |||
|---|---|---|---|---|---|---|
| IL-4 | IL-5 | IL-10 | IFN-γ | |||
| 1 | CCE | 134 | ≤20 | 58 | 1,224 | Th0 |
| SA35 | ≤20 | ≤20 | ≤20 | 952 | Th1 | |
| SA35 | ≤20 | ≤20 | ≤20 | 2,229 | Th1 | |
| SA35 | ≤20 | 500 | 53 | ≥3,000 | Th0 | |
| SA35 | ≤20 | 270 | ≤20 | ≥3,000 | Th0 | |
| SA40 | 42 | ≤20 | 122 | ≥3,000 | Th0 | |
| SA40 | ≤20 | 300 | ≤20 | 2,500 | Th0 | |
| SA40 | ≤20 | ≤20 | ≤20 | ≥3,000 | Th1 | |
| SA40 | ≤20 | 168 | 80 | 407 | Th0 | |
| SA40 | ≤20 | ≤20 | ≤20 | ≥3,000 | Th1 | |
| 4 | CCE | ≤20 | ≤20 | ≤20 | 415 | Th1 |
| IEC 1 | ≤20 | ≤20 | ≤20 | 550 | Th1 | |
| IEC 1 | ≤20 | 438 | ≤20 | ≥3,000 | Th0 | |
| 5 | CCE | ≤20 | ≤20 | ≤20 | 2,175 | Th1 |
| SA35 | ≤20 | 174 | ≤20 | 1,000 | Th0 | |
| SA35 | ≤20 | ≤20 | ≤20 | ≥3,000 | Th1 | |
| SA35 | ≤20 | ≤20 | ≤20 | ≥3,000 | Th1 | |
| SA35 | ≤20 | ≤20 | ≤20 | 580 | Th1 | |
DISCUSSION
Cell-mediated immunity has been shown to play the most important role in the host's defense against the acute and chronic phases of C. parvum infection (10). The results of the present study show that C. parvum-specific CD4+ T cells taken from donors who had recovered from cryptosporidiosis and generated in vitro by stimulation with SA35, SA40, IEC1 and IEC2 are characterized either by hyperproduction of IFN-γ alone, with a predominant Th1 profile of cytokine production, or by IFN-γ hyperproduction together with IL-4 or IL-5 production, with a Th0 profile. Thus, these peptides and antigen fractions can be considered good targets of the cell-mediated immune response against C. parvum, and they contribute to maintaining the T-cell-mediated memory response. Moreover, the finding that CCE-specific TCC were highly reactive to SA35 and SA40 peptides indicates that these two recombinant antigens can be regarded as immunodominant in the T-cell response in healthy C. parvum-sensitized persons. This seems to have been confirmed by the finding that a high proportion of CCE-generated TCC were reactive to SA35 and, although to a lesser degree, to SA40. The SA35 and SA40 peptides have also been shown to elicit a humoral immune response (39), which together with the cell-mediated immune response, could prevent C. parvum infection.
Although it is known that CCE acts as a T-cell recall antigen (16), it has been difficult to determine which components are involved in the T-cell response, given the complexity of CCE, which contains a variety of proteins and nonprotein components, both with and without antigenic properties. It also remained to be determined which of the various C. parvum antigens are involved in the stimulation of the T-cell response. In a murine model of C. parvum infection, an 11-kDa antigen was reported to be immunodominant in the humoral response (45). A 190-kDa recombinant antigen from the C. parvum oocyst wall was shown to induce proliferation in human PBMC (16). The Cp23 antigen, present in the sporozoite and merozoite stages of C. parvum, was shown to be an important target of the cell-mediated immune response in a murine model of infection (5). C. parvum-specific proliferation was obtained in stimulated splenic T cells from mice immunized with a 15-kDa recombinant C. parvum antigen (38). The finding that the main profile of cytokine production in CD4 T cells was Th1, as was found in another study (17), may indicate that this population plays an important role in inducing defense mechanisms against C. parvum infection through the activation of a set of anticryptosporidial effectors, as observed for other infections (i.e., toxoplasmosis and schistosomiasis) (26). We also found a Th0 profile which showed the production of both IFN-γ and IL-4, IL-5, and IL-10. However, we hypothesized that the Th0 profile would eventually switch to a Th1 profile, given that a high level of IFN-γ production was found for both profiles and that the production of IL-4, IL-5, and IL-10 was low.
That a higher proportion of γ/δ T cells was found for donor 5, who had recovered from cryptosporidiosis only recently, than for the other donors, who had recovered years earlier (Fig. 2), is consistent with the hypothesis that γ/δ T cells are involved in the early stage of C. parvum infection; in fact, the role of γ/δ T cells as effector cells early in the immune response has been shown for a wide variety of infectious agents (24, 30). Moreover, in a murine model of C. parvum infection, γ/δ T cells have been shown to contribute to controlling the infection in the early stages (9, 44).
To the best of our knowledge, this is the first study in which human C. parvum-specific TCC have been isolated and characterized. The TCC were found to be constituted by populations of α/β-TCR CD45RO+ CD4+ CD8− T cells; the Th1 profile probably represents the predominant cytokine production profile; and SA35, SA40, IEC1, and IEC2 were found to be T-cell-stimulating C. parvum antigens.
Acknowledgments
We are grateful to R. Riganò for her support in the generation of T-cell clones. We are also grateful to D. Tonanzi for his invaluable technical assistance.
This work was supported by a grant from Istituto Superiore di Sanità, Ministero della Salute (National AIDS Project contract 50E).
Written informed consent was obtained from all blood donors.
Editor: B. B. Finlay
REFERENCES
- 1.Aguirre, S. A., P. H. Mason, and L. E. Perryman. 1994. Susceptibility of major histocompatibility complex (MHC) class I- and MHC class II-deficient mice to Cryptosporidium parvum infection. Infect. Immun. 62:697-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amadi, B., M. Mwiya, J. Musuku, A. Watuka, S. Sianongo, A. Ayoub, and P. Kelly. 2002. Effect of nitazoxanide on morbidity and mortality in Zambian children with cryptosporidiosis: a randomised controlled trial. Lancet 360:1375-1380. [DOI] [PubMed] [Google Scholar]
- 3.Barnes, D. A., A. Bonnin, J. X. Huang, L. Gousset, J. Wu, J. Gut, P. Doyle, J. F. Dubremetz, H. Ward, and C. Petersen. 1998. A novel multi-domain mucin-like glycoprotein of Cryptosporidium parvum mediates invasion. Mol. Biochem. Parasitol. 96:93-110. [DOI] [PubMed] [Google Scholar]
- 4.Blanshard, C., A. M. Jackson, D. C. Shanson, N. Francis, and B. G. Gazzard. 1992. Cryptosporidiosis in HIV-seropositive patients. Q. J. Med. 85:813-823. [PubMed] [Google Scholar]
- 5.Bonafonte, M. T., M. L. Smith, and J. R. Mead. 2000. A 23-kDa recombinant antigen of Cryptosporidium parvum induces a cellular immune response on in vitro stimulated spleen and mesenteric lymph node cells from infected mice. Exp. Parasitol. 96:32-41. [DOI] [PubMed] [Google Scholar]
- 6.Cacciò, S., E. Pinter, R. Fantini, I. Mezzaroma, and E. Pozio. 2002. Human infection with Cryptosporidium felis: case report and literature review. Emerg. Infect. Dis. 8:85-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cosyns, M., S. Tsirkin, R. Jones, R. Flavell, H. Kikutani, and A. R. Hayward. 1998. Requirement of CD40-CD40 ligand interaction for elimination of Cryptosporidium parvum from mice. Infect. Immun. 66:603-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crabb, J. H. 1998. Antibody-based immunotherapy of cryptosporidiosis. Adv. Parasitol. 40:121-149. [DOI] [PubMed] [Google Scholar]
- 9.Eichelberger, M. C., P. Suresh, and J. E. Rehg. 2000. Protection from Cryptosporidium parvum infection by gamma delta T cells in mice that lack alpha beta T cells. Comp. Med. 50:270-276. [PubMed] [Google Scholar]
- 10.Farthing, M. J. G. 2000. Clinical aspects of human cryptosporidiosis, p. 50-74. In F. Petry (ed.), Cryptosporidiosis and microsporidiosis. Contribution to microbiology, vol. 6. Karger, Basel, Switzerland. [DOI] [PubMed] [Google Scholar]
- 11.Fichtenbaum, C. J., D. J. Ritchie, and W. G. Powderly. 1993. Use of paromycin for treatment of cryptosporidiosis in patients with AIDS. Clin. Infect. Dis. 16:298-300. [DOI] [PubMed] [Google Scholar]
- 12.Flanigan, T., C. Whalen, J. Turner, R. Soave, J. Toerner, D. Havlir, and D. Kotler. 1992. Cryptosporidium infection and CD4 counts. Ann. Intern. Med. 116:840-842. [DOI] [PubMed] [Google Scholar]
- 13.Giacometti, A., F. Burzacchini, O. Cirioni, F. Barchiesi, M. Dini, and G. Scalise. 1999. Efficacy of treatment with paromomycin, azithromycin and nitazoxanide in a patient with disseminated cryptosporidiosis. Eur. J. Clin. Microbiol. Infect. Dis. 18:885-889. [DOI] [PubMed] [Google Scholar]
- 14.Gomez Morales, M. A., and E. Pozio. 2002. Humoral and cellular immunity against Cryptosporidium infection. Curr. Drug Targets Immun. Endocr. Metabol. Disord. 2:291-301. [DOI] [PubMed] [Google Scholar]
- 15.Gomez Morales, M. A., C. M. Ausiello, A. Guarino, F. Urbani, M. I. Spagnuolo, C. Pignata, and E. Pozio. 1996. Severe, protracted intestinal cryptosporidiosis associated with interferon gamma deficiency: pediatric case report. Clin. Infect. Dis. 22:848-850. [DOI] [PubMed] [Google Scholar]
- 16.Gomez Morales, M. A., C. M. Ausiello, F. Urbani, and E. Pozio. 1995. Crude extract and recombinant protein of Cryptosporidium parvum oocysts induce proliferation of human peripheral blood mononuclear cells in vitro. J. Infect. Dis. 172:211-216. [DOI] [PubMed] [Google Scholar]
- 17.Gomez Morales, M. A., G. La Rosa, A. Ludovisi, A. M. Onori, and E. Pozio. 1999. Cytokine profile induced by Cryptosporidium antigen in peripheral blood mononuclear cells from immunocompetent and immunosuppressed persons with cryptosporidiosis. J. Infect. Dis. 179:967-973. [DOI] [PubMed] [Google Scholar]
- 18.Greenberg, P. C., and J. Cello. 1996. Treatment of severe diarrhea caused by Cryptosporidium parvum with oral bovine immunoglobulin concentrate in patients with AIDS. J. Acquir. Immune Defic. Syndr. Hum. Retrovir. 13:348-354. [DOI] [PubMed] [Google Scholar]
- 19.Hamour, A. A., A. Bonnington, B. Hawthorne, and E. G. Wilkins. 1993. Successful treatment of AIDS related cryptosporidial sclerosing cholangitis. AIDS 7:1449-1451. [DOI] [PubMed] [Google Scholar]
- 20.Hayward, A. R., L. Levy, F. Facchetti, L. Notarangelo, H. D. Ochs, A. Etzioni, J. Y. Bonnefoy, M. Cosyns, and A. Weinberg. 1997. Cholangiopathy and tumors of the pancreas, liver, and biliary tree in boys with X-linked immunodeficiency with hyper-IgM. J. Immunol. 158:977-983. [PubMed] [Google Scholar]
- 21.Hayward, A. R., M. Cosyns, M. Jones, and E. M. Ponnuraj. 2001. Marrow-derived CD40-positive cells are required for mice to clear Cryptosporidium parvum infection. Infect. Immun. 69:1630-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunt, E., Q. Fu, M. U. Armstrong, D. K. Rennix, D. W. Webster, J. A. Galanko, W. Chen, E. M. Weaver, R. A. Argenzio, and J. M. Rhoads. 2002. Oral bovine serum concentrate improves cryptosporidial enteritis in calves. Pediatr. Res. 51:370-376. [DOI] [PubMed] [Google Scholar]
- 23.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 24.Lagler, H., M. Willheim, F. Traunmuller, et al. 2003. Cellular profile of cytokine production in a patient with visceral leishmaniasis: gammadelta+ T cells express both type 1 cytokines and interleukin-10. Scand. J. Immunol. 57:291-295. [DOI] [PubMed] [Google Scholar]
- 25.Lean, I., V. McDonald, and R. C. G. Pollok. 2002. The role of cytokines in the pathogenesis of Cryptosporidium infection. Curr. Opin. Infect. Dis. 15:229-234. [DOI] [PubMed] [Google Scholar]
- 26.Levitz, S. M., H. L. Mathews, and J. W. Murphy. 1995. Direct antimicrobial activity of T cells. Immunol. Today 16:387-391. [DOI] [PubMed] [Google Scholar]
- 27.Lukin, K., M. Cosyns, T. Mitchell, M. Saffry, and A. R. Hayward. 2000. Eradication of Cryptosporidium parvum infection by mice with ovalbumin-specific T cells. Infect. Immun. 68:2663-2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDonald, V., and G. J. Bancroft. 1994. Mechanisms of innate and acquired resistance to Cryptosporidium parvum infection in SCID mice. Parasite Immunol. 16:315-320. [DOI] [PubMed] [Google Scholar]
- 29.Mele, R., M. A. Gomez Morales, F. Tosini, and E. Pozio. 2003. Indinavir reduces Cryptosporidium parvum infection in both in vitro and in vivo models. Int. J. Parasitol. 33:757-764. [DOI] [PubMed] [Google Scholar]
- 30.Moretto, M., B. Durell, J. D. Schwartzman, and I. A. Khan. 2001. Gamma delta T cell-deficient mice have a down-regulated CD8+ T cell immune response against Encephalitozoon cuniculi infection. J. Immunol. 166:7389-7397. [DOI] [PubMed] [Google Scholar]
- 31.Moss, D. M., C. L. Chappell, P. C. Okhuysen, et al. 1998. The antibody response to 27-, 17-, and 15-kDa Cryptosporidium antigens following experimental infection in humans. J. Infect. Dis. 178:827-833. [DOI] [PubMed] [Google Scholar]
- 32.Nisini, R., G. Romagnoli, M. J. Gomez, et al. 2001. Antigenic properties and processing requirements of 65-kilodalton mannoprotein, a major antigen target of anti-Candida human T-cell response, as disclosed by specific human T-cell clones. Infect. Immun. 69:3728-3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nord, J., P. Ma, D. DiJohn, S. Tzipori, and C. O. Tacket. 1990. Treatment with bovine hyperimmune colostrum of cryptosporidial diarrhea in AIDS patients. AIDS 4:581-584. [DOI] [PubMed] [Google Scholar]
- 34.Okhuysen, P. C., C. L. Chappell, C. R. Sterling, W. Jakubowski, and H. L. DuPont. 1998. Susceptibility and serologic response of healthy adults to reinfection with Cryptosporidium parvum. Infect. Immun. 66:441-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pozio, E., G. Rezza, A. Boschini, et al. 1997. Clinical cryptosporidiosis and human immunodeficiency virus (HIV)-induced immunosuppression: findings from a longitudinal study of HIV-positive and HIV-negative former injection drug users. J. Infect. Dis. 176:969-975. [DOI] [PubMed] [Google Scholar]
- 36.Ranucci, L., H. M. Muller, G. La Rosa, et al. 1993. Characterization and immunolocalization of a Cryptosporidium parvum protein containing repeated amino acids motifs. Infect. Immun. 61:2347-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rossignol, J. F. A., A. Ayoub, and M. S. Ayers. 2001. Treatment of diarrhea caused by Cryptosporidium parvum: a prospective randomized, double-blind, placebo-controlled study of nitazoxanide. J. Infect. Dis. 184:103-106. [DOI] [PubMed] [Google Scholar]
- 38.Sagodira, S., S. Iochmann, M. N. Mevelec, I. Dimier-Poisson, and D. Bout. 1999. Nasal immunization of mice with Cryptosporidium parvum DNA induces systemic and intestinal immune responses. Parasite Immunol. 21:507-516. [DOI] [PubMed] [Google Scholar]
- 39.Tosini, F., S. M. Cacciò, A. Tamburrini, G. La Rosa, and E. Pozio. 1999. Identification and characterisation of three antigenic proteins from Cryptosporidium parvum sporozoites using a DNA library expressing poly-histidine tagged peptides. Int. J. Parasitol. 29:925-933. [DOI] [PubMed] [Google Scholar]
- 40.Tosini, F., A. Agnoli, R. Mele, H. M. A. Gomez Morales, and E. Pozio. A new modular protein of Cryptosporidium parvum, with ricin B and LCCL domains, expressed in the sporozoite invasive stage. Mol. Biochem. Parasitol., in press. [DOI] [PubMed]
- 41.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tzipori, S., D. Roberton, and C. Chapman. 1986. Remission of diarrhoea due to cryptosporidiosis in an immunodeficient child treated with hyperimmune bovine colostrum. Br. Med. J. 293:1276-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tzipori, S., D. Roberton, D. A. Cooper, and L. White. 1987. Chronic cryptosporidial diarrhoea and hyperimmune cow colostrum. Lancet ii:344-345. [DOI] [PubMed] [Google Scholar]
- 44.Waters, W. R., and J. A. Harp.1996. Cryptosporidium parvum infection in T-cell receptor (TCR)-alpha- and TCR-delta-deficient mice. Infect. Immun. 64:1854-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whitmire, W. M., and J. A. Harp. 1990. In vitro murine lymphocyte blastogenic responses to Cryptosporidium parvum. J. Parasitol. 76:450-452. [PubMed] [Google Scholar]