Abstract
ATP-binding cassette (ABC) transporters are membrane proteins that efflux various compounds from cells, including chemotherapeutic agents, and are known to affect multidrug resistance. Recent reports disagree on whether ABCC11 is a risk factor for breast tumorigenesis, but its expression in breast cancer is poorly investigated. We hypothesized that both frequency and expression levels of ABC transporters in breast tumors would vary by cancer subtype, and be associated with prognosis. Here, we constructed a tissue microarray breast tumor samples from 281 patients, and analyzed expressions of ABCB1, ABCC1, ABCC11, and ABCG2 immunohistochemically. Breast cancer subtypes were determined by immunohistochemistry of estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 (HER2). Protein expression was correlated to clinicopathological characteristics, clinical follow-up, and pathological complete response to neoadjuvant chemotherapy. The tissue microarray comprised 191 luminal A (68.0%), 17 luminal B (6.0%), 27 HER2 (9.0%), and 46 triple-negative (16.4%) samples. ABCC1 and ABCC11 expressions were associated with significantly shorter disease-free survival (P = 0.027 and P = 0.003, respectively). ABCC1, ABCC11, and ABCG2, but not ABCB1, were expressed significantly more, and more frequently, in aggressive subtypes. Patients with HER2+ and triple-negative tumor subtypes that expressed high levels of ABCC11 had significantly worse disease-free survival (P = 0.017 and P < 0.001, respectively). We have shown, for the first time, that ABCC1, ABCC11 and ABCG2 are highly expressed in aggressive breast cancer subtypes, and that tumor ABCC11 expression is associated with poor prognosis.
Keywords: Breast cancer, ATP binding cassette transporters, ABCC11, tissue microarray, subtype
Introduction
Breast cancer is a heterogeneous disease [1]. DNA microarray profiling studies on breast cancer have identified distinct subtypes: luminal A, luminal B, human epidermal growth factor receptor 2 (HER2)-enriched, and triple-negative (which is sometimes further subdivided into the core-basal and five-negative subtypes) [2]. These subtypes are reportedly associated with differences in resistance to chemotherapy [3–5] and subsequent outcomes [6, 7]. Several mechanisms affect how cancer cells become resistant to cytotoxic drugs, which include efflux of the drug compound from cancer cells, and others such as mutation, overexpression of the drug’s targets, and drug inactivation [8].
The ATP-binding cassette (ABC) transporters are transmembrane proteins that use ATP to transport various molecules across extra- and intra-cellular membranes. This function is thought to have evolved as a xenobiotic protective mechanism [9]. Of the 49 human ABC transporters so far identified (which have been classified into seven subfamilies), ABCA2, ABCB1, ABCC1–6, ABCC11, and ABCG2 have been associated with chemoresistance in breast cancer [8]. Unfortunately, all clinical trials that have targeted ABC transporters failed to improve outcomes [10]. One explanation for this is that they all targeted ABCB1 (also known as MDR1, permeability glycoprotein 1 [P-glycoprotein or Pgp], and cluster of differentiation 243 [CD243]). This led us to hypothesize that other ABC transporters may be more important for drug resistance.
ABCC11 is a member of the ABCC1 (also known as MDR-associated protein) sub-family. A single nucleotide polymorphism (SNP) in the ABCC11 gene was shown to be responsible for “wet earwax” in humans [11]. Reports as to whether ABCC11 is a risk factor for breast tumorigenesis conflict; although this gene was originally shown to be a risk factor for development of breast cancer among Japanese women [12], it is reportedly not the case in Caucasian women [13, 14]. There has been no investigation of ABCC11 protein expression levels in breast tumors or their association with cancer subtype and prognosis. We hypothesized that both frequency and expression levels of ABC transporters (ABCB1, ABCC1, ABCC11, and ABCG2) in breast tumors would differ by cancer subtype and be associated with prognosis. Here, utilizing a tissue microarray newly constructed from 281 breast cancer samples, we analyzed the expression of these transporters in light of breast cancer subtype and prognosis, as well as investigating the effects of neoadjuvant chemotherapy.
Methods
Tissue sources and clinical characteristics
Tissues for this study were obtained from 281 patients treated in Yokohama City Medical Center, Japan, between 2006 and 2008, involving all stages of breast cancer. This study was approved by the Institutional Review Board of Yokohama City University, Kanagawa, Japan, and the patients gave their informed consent before their inclusion in the study. Core biopsy samples taken prior to treatment were obtained from 50 patients who received neoadjuvant chemotherapy (35 patients received anthracycline followed by taxane; 14 received anthracycline alone; and one received taxane alone). One hundred and eight patients received adjuvant chemotherapy after surgery (45 received anthracycline followed by taxane; 38 received anthracycline alone; 15 received taxane alone; and 10 received other regimens) and 208 patients received adjuvant hormonal therapy (tamoxifen and luteinizing hormone-releasing hormone-agonist for 61 premenopausal patients; tamoxifen or aromatase inhibitor for 147 postmenopausal patients). None of the tissues described here was obtained after any treatment. All the patients were followed up at least every 3 months after surgery. The mean observation period was 49 months (range: 28–60 months). The clinical characteristics are presented in Table 1.
Table 1.
Patients’ characteristics
| N | % | |
|---|---|---|
| Age | ||
| <65 | 197 | 70.1 |
| 65≦ | 80 | 28.5 |
| 4 | 1.4 | |
| Menstruation states | ||
| pre menopause | 87 | 31.0 |
| post menopause | 154 | 54.8 |
| NA | 40 | 14.2 |
| Estrogen Receptor | ||
| positive | 210 | 74.8 |
| negative | 71 | 25.2 |
| NA | 0 | 0.0 |
| Progesterone Receptor | ||
| positive | 162 | 42.7 |
| negative | 119 | 57.3 |
| NA | 0 | 0.0 |
| HER2 overexpression | ||
| present | 44 | 15.7 |
| absent | 237 | 84.3 |
| NA | 0 | 0.0 |
| Basal markers | ||
| basal | 34 | 12.1 |
| non basal | 235 | 83.4 |
| NA | 12 | 4.5 |
| Subtype | ||
| Luminal A | 191 | 68.0 |
| Luminal B | 17 | 6.0 |
| HER2 | 27 | 9.6 |
| Triple negative | 46 | 16.4 |
| (Core basal | 26 | 9.3) |
| (Five negative | 20 | 7.1) |
| Tumor stage | ||
| T1 | 123 | 43.8 |
| T2 | 122 | 43.4 |
| T3 | 11 | 3.9 |
| T4 | 19 | 6.8 |
| NA | 6 | 2.1 |
| Nodes | ||
| N0 | 150 | 53.4 |
| N1 | 83 | 29.5 |
| N2 | 23 | 8.2 |
| N3 | 11 | 3.9 |
| NA | 14 | 5.0 |
| Metastases | ||
| M0 | 259 | 92.2 |
| M1 | 6 | 2.1 |
| NA | 16 | 5.7 |
| TNM stage | ||
| 1 | 106 | 37.8 |
| 2 | 122 | 43.4 |
| 3 | 31 | 11.0 |
| 4 | 6 | 2.1 |
| NA | 16 | 5.7 |
| Observation time (days ) | 1458 ± 509* | |
Expressed as mean ± standard deviation
Tissue microarray
The tissue microarray was constructed by taking 3.0-mm cores from representative areas of surgical specimens from patients using a KIN-2 tissue arrayer (Azumaya, Tokyo, Japan), and re-embedding these cores into a gridded paraffin block. Tissue cores were excluded from the tissue microarray if they fail to adhere to the glass slide, did not include invasive carcinoma, or were a non-interpretable specimen.
Immunohistochemistry
Formalin-fixed paraffin-embedded tissue blocks were sliced into 5-μm sections. The sections were baked at 60°C, deparaffinized in xylene, and gradually rehydrated in ethanol. Sections were boiled in antigen retrieval solution (Funakoshi, Japan) for 30 minutes. Activity of endogenous peroxidase was blocked by 20 minutes of quenching in 0.3% H2O2 and methanol; the sections were then incubated in 5% rabbit serum for ABCB1 and ABCC1, or goat serum for ABCC11 and ABCG2. Immunohistochemical reactions were performed overnight at 4°C using monoclonal mouse antibodies against ABCB1 (C219; 1:100; Abcam, UK), monoclonal rat antibodies against ABCC1 (MRPr1; 1:40; Monosan, The Netherlands), polyclonal rabbit antibodies against ABCC11 (1:500) [15], or monoclonal mouse antibodies against ABCG2 (BXP-21; 1:100; Abcam). For the triple-negative subtype, cytokeratin 5/6 (D5/16 B4; Dako, Denmark) and epidermal growth factor receptor (EGFR; Roche Diagnostics K.K, Japan,) were used for subdivision into the core-basal or non-basal (five negative) subtypes. After washing, the slides were incubated with biotinylated antibodies (15 minutes, room temperature) and streptavidin-biotinylated peroxidase complex (15 minutes, room temperature). 3,3′-diaminobenzidine (Dako Japan, Tokyo, Japan) was used as the chromogen. All sections were counterstained with Meyer’s hematoxylin.
Evaluation of staining
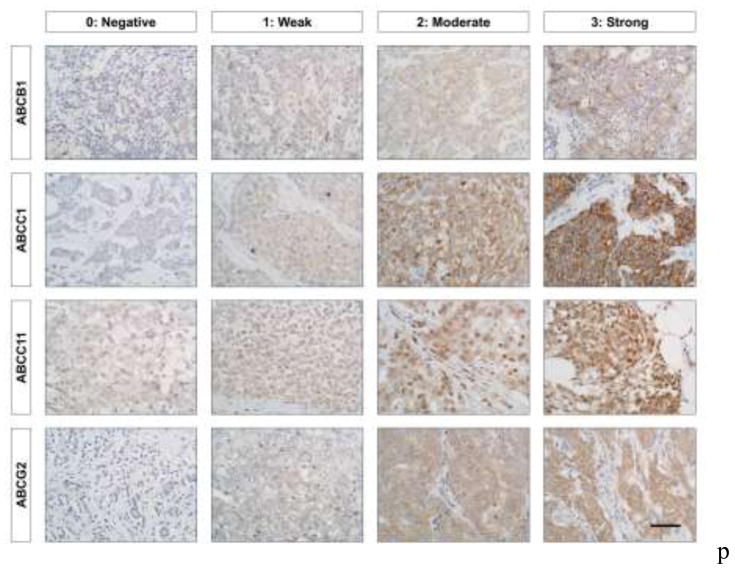
Staining results were assessed by two pathologists independently, using a 4-point scoring system as shown in Fig.1: 0 = invasive tumor cells present in the tissue core with no staining; 1 = invasive tumor cells present with weak staining intensity; 2 = invasive tumor cells present with strong staining intensity and < 30% of tumor cells stained or intermediate staining intensity in ≥ 30% of tumor cells; and 3 = invasive tumor cells present with strong staining in ≥30% of tumor cells. To evaluate positivity, both membranous and/or cytoplasmic staining scoring 2 or above was considered positive (high expression). CK5/6 and EGFR were considered positive when cytoplasmic and/or membranous staining of invasive carcinoma cells was observed, regardless of intensity.
Fig. 1.
4-point scoring system for ABCB1, ABCC1, ABCC11, and ABCG2 protein expression. Our tissue microarray contained 281 breast tumor tissues, and was stained with antibodies against ABCB1 (1:100), ABCC1 (1:40), ABCC11 (1:500), and ABCG2 (1:100). Stain intensity was graded as negative (0), weak (1), moderate (2), or strong (3). Representative images are shown under high magnification. Scale bar: 50 μm.
Genotyping
Genotyping of ABCC11 by the SmartAmp method was performed as previously reported [12].
Statistical analysis
Statistical analysis used SPSS 19.0 for Windows software (SPSS Inc., Chicago, IL). Correlations among the clinicopathologic parameters and each transporter were evaluated by the Pearson χ2 test, the Fisher exact test, and the Mann–Whitney test. Tukey-type multiple comparison analyses with the χ2 test and Mantel test were carried out to compare expression of each transporter among the subtypes. Patient outcomes were assessed by disease-free survival. Survival distributions were estimated by the Kaplan–Meier method; differences were compared using the log-rank test. The multivariate Cox proportional hazard regression method was used to determine the independent prognostic value. P < 0.05 was considered statistically significant.
Results
Characteristics of samples used for the tissue microarray
Subtypes of the 281 samples on the tissue microarray were determined using immunohistochemistry for the estrogen receptor (ER), progesterone receptor (PgR), and HER2, as previously reported [5, 16]. Patients’ and tumor characteristics used for the tissue microarray are summarized in Table 1. The numbers of cases of the respective subtypes were: luminal A (ER+ and HER2−): 191 (68.0%); luminal B (ER+ and HER2+): 17 (6.0%); HER2 (ER− and HER2+): 27 (9.6%); and triple-negative (ER− and HER2−): 46 (16.4%). Triple-negative tumors were further sub-divided into two groups, core-basal (CK5/6+ and/or EGFR+) and five-negative (CK5/6− and EGFR−). The core-basal subtype constituted 56.5% (26/46) of triple-negative tumors.
Associations between ABC transporter expression and clinical features of the tumors are shown in Table 2. ABCB1 was detected in 32.4% (91/277) of the tumors, ABCC1 in 39.1% (110/279), ABCC11 in 40.2% (113/259), and ABCG2 in 24.2% (68/278). There was no association between ABCB1 expression and any clinical features. ABCG2 was more frequently highly expressed in young premenopausal patients. High expressions of ABCC1 and ABCG2 were significantly more frequent in ER tumors than in ER+ ones (P = 0.001 and P = 0.006, respectively). There was no association between HER2 expression and ABC transporter expression.
Table 2.
The expression of ABC transporters and clinical features
| ABCB1
|
p Value | ABCC1
|
p Value | ABCC11
|
p Value | ABCG2 | p Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| negative | positive | NA | negative | positive | NA | negative | positive | NA | negative | positive | NA | |||||
| N (%) | 186 (66.2%) | 91 (32.4%) | 4 (1.4%) | 169 (60.2%) | 110 (39.1%) | 2 (0.7%) | 146 (52.0%) | 113 (40.2%) | 22 (7.8%) | 210 (74.7%) | 68 (24.2%) | 3 (1.1%) | ||||
| Age | ||||||||||||||||
| <65 | 125 (63.5%) | 68 (34.5%) | 4 (2%) | 0.14 | 119 (60.4%) | 76 (38.6%) | 2 (1%) | 0.54 | 97 (49.2%) | 83 (42.1%) | 17 (8.7%) | 0.13 | 138 (70.0%) | 56 (28.4%) | 3 (1.6%) | <0.01 |
| 65≦ | 58 (72.5%) | 22 (27.5%) | 0 | 49 (61.2%) | 31 (38.8%) | 0 | 47 (58.8%) | 28 (35.0%) | 5 (6.2%) | 69 (86.3%) | 11 (13.7%) | 0 | ||||
| Menstruation status | ||||||||||||||||
| pre menopause | 55 (63.2%) | 29 (33.3%) | 3 (3.5%) | 0.40 | 51 (58.6%) | 36 (41.4%) | 0 | 0.32 | 41 (47.2%) | 37 (42.5%) | 9 (10.3%) | 0.35 | 54 (62.1%) | 31 (35.6%) | 2 (2.3%) | <0.01 |
| post menopause | 104 (67.5%) | 49 (31.8%) | 1 (0.7%) | 95 (61.7%) | 57 (37.0%) | 2 (1.3%) | 81 (52.6%) | 63 (40.9%) | 10 (6.5%) | 132 (85.7%) | 22 (14.3%) | 0 | ||||
| Estrogen receptor | ||||||||||||||||
| negative | 50 (70.4%) | 21 (29.6%) | 0 | 0.61 | 30 (42.3%) | 39 (54.9%) | 2 (2.8%) | <0.01 | 37 (52.1%) | 27 (38.0%) | 7 (9.9%) | 0.65 | 43 (60.6%) | 27 (38.0%) | 1 (1.4%) | <0.01 |
| positive | 135 (64.5%) | 70 (33.5%) | 4 (2.0%) | 139 (66.5%) | 70 (33.5%) | 0 | 108 (51.7%) | 86 (41.1%) | 15 (7.2%) | 166 (79.4%) | 41 (19.6%) | 2 (1.0%) | ||||
| Progesterone receptor | ||||||||||||||||
| negative | 78 (65.5%) | 38 (31.8%) | 3 (2.7%) | 0.78 | 63 (52.9%) | 54 (45.4%) | 2 (1.7%) | 0.06 | 57 (47.9%) | 49 (41.2%) | 13 (10.9%) | 0.55 | 81 (68.0%) | 35 (29.4%) | 3 (2.6%) | 0.15 |
| positive | 107 (66.5%) | 53 (32.9%) | 1 (0.6%) | 106 (65.8%) | 55 (34.2%) | 0 | 88 (54.7%) | 64 (40.0%) | 9 (5.3%) | 128 (79.5%) | 33 (20.5%) | 0 | ||||
| HER2 expression | ||||||||||||||||
| absent | 155 (66.0%) | 77 (32.8%) | 3 (1.2%) | 1.00 | 146 (62.1%) | 88 (37.4%) | 1 (0.5%) | 0.18 | 123 (52.4%) | 97 (41.2%) | 15 (6.4%) | 1.00 | 179 (76.1%) | 55 (23.4%) | 1 (0.5%) | 0.33 |
| present | 29 (65.9%) | 14 (31.8%) | 1 (2.3%) | 22 (50.0%) | 21 (47.7%) | 1 (2.3%) | 21 (47.7%) | 16 (36.4%) | 7 (15.9%) | 29 (65.9%) | 13 (29.5%) | 2 (4.6%) | ||||
Expression of ABCC1 and ABCC11 is associated with poor patient survival
We compared expression of each transporter and patient disease-free survival (Figure 2). In the entire study group, patients with ABCC1+ or ABCC11+ tumors had significantly shorter disease-free survival compared to patients with corresponding ABCC1− or ABCC11− tumors (P = 0.027 or P = 0.003, respectively).
Fig. 2.

Kaplan–Meier disease-free survival curves according to expression of ABCB1 (A), ABCC1 (B), ABCC11 (C), and ABCG2 (D). The thick bold line indicates positivity; and the light gray line indicates negativity, for the respective transporters. Only the ABCC1+ and ABCC11+ groups showed significantly improved survival (P = 0.025 and P = 0.005, respectively).
ABC transporters are more frequently highly expressed in aggressive subtypes of breast cancer
Because breast cancer subtypes are associated with different clinical behaviors [2], we further analyzed clinical outcomes according to cancer subtype and ABC transporter expression. Expression of each transporter according to breast cancer subtype is shown in Figure 3. The percentage of patients whose tumors expressed ABCB1 did not differ among the subtypes. ABCC1 and ABCG2 were more frequently highly expressed in triple-negative subtype, especially in the core-basal subtype, compared with the luminal A subtype, whereas highly expressed ABCC11 was more common in HER2-enriched, core-basal, and luminal A subtypes. Although core-basal tumors tended to express ABC transporters more often than five-negative tumors did, only ABCC11 showed significantly more frequent high expression in the core-basal subtype. Semi-quantification of ABC transporters expression is shown in Figure 3B. ABCC1, ABCC11, and ABCG2 were more highly expressed in HER2-enriched and/or the core-basal subtypes, which is consistent with frequency data shown in Figure 3A.
Fig. 3.
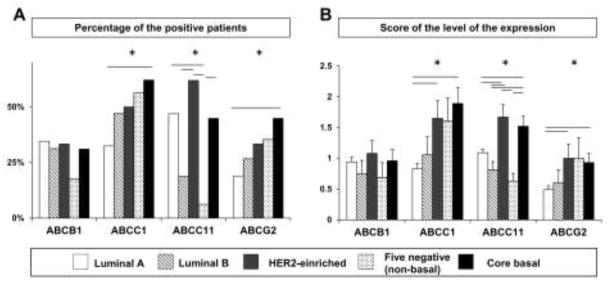
Frequency (A) and intensity (B) of high ABC transporter expression classified by subtype, including luminal A (open columns), luminal B (hatched columns), HER2-enriched (gray columns), five-negative (dotted columns), and core-basal (filled columns). (A) Percentage of patients who showed high expression of each transporter. (B) Semi-quantification of expression level of each transporter, using a 4-point scoring system.
Patients whose tumors expressed high levels of ABCC11 tended towards decreased pathological complete responses to neoadjuvant chemotherapy
We next investigated whether there was any association between the “wet earwax” genotypes and ABCC11 expression. Figure 4A and B show the relationship between ABCC11 genotypes and ABCC11 expression in breast cancer tissues. ABCC11 expression did not differ among the wet earwax genotype (538G/G+538G/A) and the dry earwax genotypes (538A/A), in either normal breast tissues or breast cancer tissues.
Fig. 4.
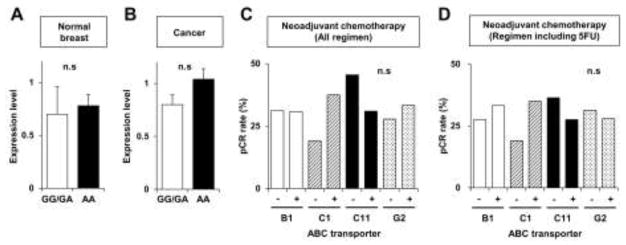
Semi-quantification of ABCC11 expression levels in normal breast tissue (A) and cancer tissue (B) in patients carrying 538G/G, 538G/A (white open column, GG/GA, wet earwax phenotype), and 538A/A alleles (black filled column, AA, dry earwax phenotype). (C, D) Pathological complete response ratios to neoadjuvant chemotherapy of all regimens (C) and regimens including 5-FU (D). Bars indicate ABCB1 (white columns), ABCC1 (hatched columns), ABCC11 (black columns), and ABCG2 (dotted columns).
As ABCC11 is known to efflux fluoropyrimidines (5-FU) in vitro [17], assessment of responses of ABCC11+ tumors to 5-FU-based regimens could be particularly valuable. Analysis of the association between ABC transporter expression and pathological complete response to neoadjuvant chemotherapy showed no statistically significant differences, regardless of regimen, but patients whose cancers expressed high levels of ABCC11 tended to have decreased pathological complete responses to neoadjuvant chemotherapy (Figure 4C and D).
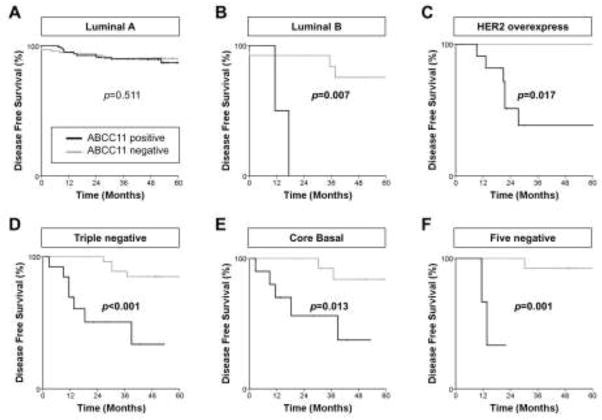
ABCC11+ tumors show worse prognoses among aggressive breast cancer subtypes
Because patients with ABCC1+ or ABCC11+ tumors tend to have poor prognoses, we investigated prognosis according to subtype. Patients with ABCC1+ tumors ended to have worse prognoses for luminal A tumors, but not significantly so (P = 0.096). Interestingly, patients with ABCC11+ tumors had significantly worse prognoses than did patients with ABCC11− tumors, except for the luminal A subtype, which is known to have a better prognosis than the other subtypes (Figure 5A–F).
Fig. 5.
Kaplan–Meier disease-free survival curve according to the subtype of breast cancer: luminal A (A), luminal B (B), HER2-enriched (C), triple-negative (D), core-basal (E), and five-negative (F). The thick bold line indicates ABCC11+, and the light gray line indicates ABCC11−.
Discussion
Different subtypes of breast cancer have different biological behaviors, including responses to systemic and local therapies [3–5] and subsequent clinical outcomes [6, 7]. The two hormone receptor-negative subtypes, triple-negative and HER2-enriched, have poor outcomes compared with the luminal subtypes. Among the triple-negative subtypes, the core-basal subtype, which responds poorly to cytotoxic chemotherapy, has the worst prognosis. Thus, there is a particular need to elucidate drug resistance mechanisms for this subtype. Expression of ABC transporters is reportedly related to chemoresistance [9]. Some ABC transporters, namely ABCB1, ABCC1, and ABCG2, have been identified as MDR proteins in breast cancer, which contribute to drug resistance via ATP-dependent drug efflux pumps [8]. Because ABCB1 effluxes drugs important for breast cancer—anthracyclines (doxorubicin, epirubicin, and daunomycin) and taxanes (paclitaxel, docetaxel)—ABC transporter inhibitors were the subjects of several widely anticipated clinical trials. Unfortunately, these agents proved disappointing [8, 18]. The vast majority of clinical trials targeting ABC transporters focused on ABCB1 (the most investigated ABC transporter) but data that associates patients’ clinicopathological factors with ABCB1 expression tends to conflict [10]. This led us to investigate expression of multiple ABC transporters that are associated with MDR, in the context of different breast cancer subtypes. We felt this information would be particularly relevant for the triple-negative subtype.
Patient characteristics and our tissue microarray staining data generally agree with previous reports [10, 19, 20]. The proportion of breast cancer subtypes may differ among different races or geographic populations; e.g., prevalence of the luminal A subtype may be higher, and the triple-negative subtype may be lower, in Asian women than in Western women [19]. The demographics of our tissue microarray are consistent with the prevalence among Japanese women. Leonessa et al. reported that the detection rate of ABCB1 and ABCC1 in untreated tumors by immunohistochemistry was 40% (range: 0–100%) and 49% (range: 20–100%), respectively, with no clear association between ABCB1 and hormone receptors [10]. In agreement, our results also showed no association between ABCB1 expression and clinical features.
Among ABC transporters, ABCC11 is at relatively early stages of investigation. ABCC11 is lipophilic anion pump that can confer resistance to chemotherapeutic agents such as methotrexate and 5-FU [17]. We previously reported that a SNP in ABCC11 is associated with the risk of developing breast cancer among Japanese women [12], although the association of ABCC11 with breast cancer risk is unclear in Caucasian and European women [13, 14]. These reports mentioned host factors that might differ among races and thus modify the impact of this gene on breast cancer risk. ABCC11 mRNA is reportedly over-expressed in breast tumors and breast cancer cell lines [9, 21, 22], but few studies discuss expression of the ABCC11 protein in human tumors [23]. Although the breast cancer risk conferred by the SNP in ABCC11 is not within the scope of this study, we did not see significant differences in breast cancer prognosis by SNP genotype in our samples.
Core-basal and HER2-enriched subtypes are associated with poor clinical outcome [5]. In our series, high expressions of ABCC1 and ABCG2 were more common in aggressive subtypes such as core-basal. Strikingly, high expression of ABCC11 was more frequent and intense in both the HER2-enriched and core-basal subtypes, which implies that ABCC11 may promote the aggressive behavior of these subtypes. Indeed, ABCC11 has been shown to export not only drugs but also other factors that affect cancer biology. In agreement, our results show that patients with high tumor expression of ABCC11 have worse outcomes, particularly among the HER2-enriched and core-basal subtypes. This is the first study to show such an association.
Reportedly, ABCC11 expression is related to sensitivity and resistance to chemotherapy [17, 24–26]. In our data, only ABCC11, but not other transporters, tended to correlate with neoadjuvant chemotherapy response. Interestingly, this was true of chemotherapy regimens that both did and did not include 5-FU, which suggests that ABCC11 possesses unidentified supportive functions for drug resistance other than simple drug efflux. For example, we reported that ABCC1 and ABCG2 in breast cancer cells export sphingosine-1-phosphate [27], a bioactive lipid mediator known to affect drug resistance; we cannot exclude the possibility that ABCC11 possesses such a function. In that case, ABCC11 could become a new target in suppressing drug resistance. Interestingly, it has been suggested that ABCB1 and ABCG2 may affect the role of cancer stem cells in drug resistance [8]. Although we do not currently have data on this relationship, it is intriguing to speculate that the worse prognosis of ABCC11-expressing tumors may be related to cancer stem cells.
Our study is limited in that it is a retrospective analysis of prospectively collected breast tumor samples, and that it shows only association of these transporters with breast cancer prognosis. To evaluate adequately the role of ABCC11 in breast cancer drug resistance, further studies of the mechanism of resistance are needed.
In conclusion, this is the first demonstration that ABCC11 expression in breast cancer is associated with aggressive subtypes and poor disease-free survival.
Acknowledgments
The authors thank Dr. Harry D. Bear (Virginia Commonwealth University) for his critical review and valuable input to improve this manuscript. Kazuaki Takabe is supported by the US National Institutes of Health (R01CA160688, K12HD055881) and a Susan G. Komen for the Cure Investigator Initiated Research Grant and Career Catalyst Research Grant (KG090510).
Footnotes
Ethical standards
This study was approved by the Institutional Review Board of Yokohama City University, Kanagawa, Japan.
Conflicts of Interest
The authors declare that they have no conflict of interests.
References
- 1.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22(8):1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 3.Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, Ollila DW, Sartor CI, Graham ML, Perou CM. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13(8):2329–2334. doi: 10.1158/1078-0432.ccr-06-1109. [DOI] [PubMed] [Google Scholar]
- 4.Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K, Hess KR, Stec J, Ayers M, Wagner P, Morandi P, Fan C, Rabiul I, Ross JS, Hortobagyi GN, Pusztai L. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005;11(16):5678–5685. doi: 10.1158/1078-0432.ccr-04-2421. [DOI] [PubMed] [Google Scholar]
- 5.Blows FM, Driver KE, Schmidt MK, Broeks A, van Leeuwen FE, Wesseling J, Cheang MC, Gelmon K, Nielsen TO, Blomqvist C, Heikkila P, Heikkinen T, Nevanlinna H, Akslen LA, Begin LR, Foulkes WD, Couch FJ, Wang X, Cafourek V, Olson JE, Baglietto L, Giles GG, Severi G, McLean CA, Southey MC, Rakha E, Green AR, Ellis IO, Sherman ME, Lissowska J, Anderson WF, Cox A, Cross SS, Reed MW, Provenzano E, Dawson SJ, Dunning AM, Humphreys M, Easton DF, Garcia-Closas M, Caldas C, Pharoah PD, Huntsman D. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med. 2010;7(5):e1000279. doi: 10.1371/journal.pmed.1000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Eystein Lonning P, Borresen-Dale AL. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98(19):10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, Demeter J, Perou CM, Lonning PE, Brown PO, Borresen-Dale AL, Botstein D. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100(14):8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dean M. ABC transporters, drug resistance, and cancer stem cells. J Mammary Gland Biol Neoplasia. 2009;14(1):3–9. doi: 10.1007/s10911-009-9109-9. [DOI] [PubMed] [Google Scholar]
- 9.Szakacs G, Annereau JP, Lababidi S, Shankavaram U, Arciello A, Bussey KJ, Reinhold W, Guo Y, Kruh GD, Reimers M, Weinstein JN, Gottesman MM. Predicting drug sensitivity and resistance: profiling ABC transporter genes in cancer cells. Cancer cell. 2004;6(2):129–137. doi: 10.1016/j.ccr.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 10.Leonessa F, Clarke R. ATP binding cassette transporters and drug resistance in breast cancer. Endocr Relat Cancer. 2003;10 (1):43–73. doi: 10.1677/erc.0.0100043. [DOI] [PubMed] [Google Scholar]
- 11.Yoshiura K, Kinoshita A, Ishida T, Ninokata A, Ishikawa T, Kaname T, Bannai M, Tokunaga K, Sonoda S, Komaki R, Ihara M, Saenko VA, Alipov GK, Sekine I, Komatsu K, Takahashi H, Nakashima M, Sosonkina N, Mapendano CK, Ghadami M, Nomura M, Liang DS, Miwa N, Kim DK, Garidkhuu A, Natsume N, Ohta T, Tomita H, Kaneko A, Kikuchi M, Russomando G, Hirayama K, Ishibashi M, Takahashi A, Saitou N, Murray JC, Saito S, Nakamura Y, Niikawa N. A SNP in the ABCC11 gene is the determinant of human earwax type. Nat Genet. 2006;38(3):324–330. doi: 10.1038/ng1733. [DOI] [PubMed] [Google Scholar]
- 12.Ota I, Sakurai A, Toyoda Y, Morita S, Sasaki T, Chishima T, Yamakado M, Kawai Y, Ishidao T, Lezhava A, Yoshiura K, Togo S, Hayashizaki Y, Ishikawa T, Endo I, Shimada H. Association between breast cancer risk and the wild-type allele of human ABC transporter ABCC11. Anticancer Res. 2010;30 (12):5189–5194. [PubMed] [Google Scholar]
- 13.Beesley J, Johnatty SE, Chen X, Spurdle AB, Peterlongo P, Barile M, Pensotti V, Manoukian S, Radice P, Chenevix-Trench G. No evidence for an association between the earwax-associated polymorphism in ABCC11 and breast cancer risk in Caucasian women. Breast Cancer Res Treat. 2011;126(1):235–239. doi: 10.1007/s10549-010-1292-2. [DOI] [PubMed] [Google Scholar]
- 14.Lang T, Justenhoven C, Winter S, Baisch C, Hamann U, Harth V, Ko YD, Rabstein S, Spickenheuer A, Pesch B, Bruning T, Schwab M, Brauch H. The earwax-associated SNP c.538G>A (G180R) in ABCC11 is not associated with breast cancer risk in Europeans. Breast Cancer Res Treat. 2011;129(3):993–999. doi: 10.1007/s10549-011-1613-0. [DOI] [PubMed] [Google Scholar]
- 15.Toyoda Y, Sakurai A, Mitani Y, Nakashima M, Yoshiura K, Nakagawa H, Sakai Y, Ota I, Lezhava A, Hayashizaki Y, Niikawa N, Ishikawa T. Earwax, osmidrosis, and breast cancer: why does one SNP (538G>A) in the human ABC transporter ABCC11 gene determine earwax type? FASEB J. 2009;23(6):2001–2013. doi: 10.1096/fj.09-129098. [DOI] [PubMed] [Google Scholar]
- 16.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S, Deming SL, Geradts J, Cheang MC, Nielsen TO, Moorman PG, Earp HS, Millikan RC. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295(21):2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 17.Guo Y, Kotova E, Chen ZS, Lee K, Hopper-Borge E, Belinsky MG, Kruh GD. MRP8, ATP-binding cassette C11 (ABCC11), is a cyclic nucleotide efflux pump and a resistance factor for fluoropyrimidines 2′,3′-dideoxycytidine and 9′-(2′-phosphonylmethoxyethyl)adenine. J Biol Chem. 2003;278(32):29509–29514. doi: 10.1074/jbc.M304059200. [DOI] [PubMed] [Google Scholar]
- 18.Leonard GD, Fojo T, Bates SE. The role of ABC transporters in clinical practice. Oncologist. 2003;8 (5):411–424. doi: 10.1634/theoncologist.8-5-411. [DOI] [PubMed] [Google Scholar]
- 19.Toi M, Ohashi Y, Seow A, Moriya T, Tse G, Sasano H, Park BW, Chow LW, Laudico AV, Yip CH, Ueno E, Ishiguro H, Bando H. The Breast Cancer Working Group presentation was divided into three sections: the epidemiology, pathology and treatment of breast cancer. Jpn J Clin Oncol. 2010;40(Suppl 1):i13–18. doi: 10.1093/jjco/hyq122. [DOI] [PubMed] [Google Scholar]
- 20.Xiang L, Su P, Xia S, Liu Z, Wang Y, Gao P, Zhou G. ABCG2 is associated with HER-2 expression, lymph node metastasis and clinical stage in breast invasive ductal carcinoma. Diagn Pathol. 2011;6:90. doi: 10.1186/1746-1596-6-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bera TK, Lee S, Salvatore G, Lee B, Pastan I. MRP8, a new member of ABC transporter superfamily, identified by EST database mining and gene prediction program, is highly expressed in breast cancer. Mol Med. 2001;7 (8):509–516. [PMC free article] [PubMed] [Google Scholar]
- 22.Honorat M, Mesnier A, Vendrell J, Guitton J, Bieche I, Lidereau R, Kruh GD, Dumontet C, Cohen P, Payen L. ABCC11 expression is regulated by estrogen in MCF7 cells, correlated with estrogen receptor alpha expression in postmenopausal breast tumors and overexpressed in tamoxifen-resistant breast cancer cells. Endocr Relat Cancer. 2008;15(1):125–138. doi: 10.1677/erc-07-0189. [DOI] [PubMed] [Google Scholar]
- 23.Sosonkina N, Nakashima M, Ohta T, Niikawa N, Starenki D. Down-regulation of ABCC11 protein (MRP8) in human breast cancer. Exp Oncol. 2011;33 (1):42–46. [PubMed] [Google Scholar]
- 24.Oguri T, Bessho Y, Achiwa H, Ozasa H, Maeno K, Maeda H, Sato S, Ueda R. MRP8/ABCC11 directly confers resistance to 5-fluorouracil. Mol Cancer Ther. 2007;6(1):122–127. doi: 10.1158/1535-7163.mct-06-0529. [DOI] [PubMed] [Google Scholar]
- 25.Toyoda Y, Ishikawa T. Pharmacogenomics of human ABC transporter ABCC11 (MRP8): potential risk of breast cancer and chemotherapy failure. Anticancer Agents Med Chem. 2010;10 (8):617–624. doi: 10.2174/187152010794473975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park S, Shimizu C, Shimoyama T, Takeda M, Ando M, Kohno T, Katsumata N, Kang YK, Nishio K, Fujiwara Y. Gene expression profiling of ATP-binding cassette (ABC) transporters as a predictor of the pathologic response to neoadjuvant chemotherapy in breast cancer patients. Breast Cancer Res Treat. 2006;99(1):9–17. doi: 10.1007/s10549-006-9175-2. [DOI] [PubMed] [Google Scholar]
- 27.Takabe K, Kim RH, Allegood JC, Mitra P, Ramachandran S, Nagahashi M, Harikumar KB, Hait NC, Milstien S, Spiegel S. Estradiol induces export of sphingosine 1-phosphate from breast cancer cells via ABCC1 and ABCG2. J Biol Chem. 2010;285(14):10477–10486. doi: 10.1074/jbc.M109.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]