Abstract
Background
Limited comparative data are available on the outcomes between extended-release and standard-release tacrolimus when used de novo in kidney transplant recipients (KTRs).
Methods
We identified KTRs transplanted at our institution during 2009–10 routinely prescribed extended-release tacrolimus and compared them with those transplanted during 2008–09 prescribed standard-release tacrolimus. Graft function (eGFR by MDRD-7 equation) at 12 months post-transplant (primary outcome); new-onset diabetes and other cardiovascular risk factors, BK viremia incidence, acute rejection, and graft survival to 12 months (secondary outcomes) were compared by intent-to-treat analysis. Time-to-steady-state concentration and number of dose adjustments required to attain steady state were recorded.
Results
There were no important demographic differences between the extended-release (N = 106) and standard-release (N = 95) cohorts. The estimated glomerular filtration rate (eGFR) at 12 months was similar (58.8 ± 17 versus 59.2 ± 18 mL/min/1.73 m2, P = 0.307). There was no difference in new-onset diabetes (17 versus 20%, P = 0.581), BK viremia (10 versus 7%, P = 0.450), acute rejection (7 versus 16%, P = 0.067) or graft survival (97 versus 95%, P = 0.301). Time-to-steady state was similar (9.2 ± 1.1 versus 8.1 ± 4.7 days, P = 0.490) although extended-release patients required fewer adjustments to attain steady state (1.2 ± 1.7 [0–8] versus 1.7 ± 1.5 [0–7], P = 0.030) but a similar dose (7.2 ± 2.4 [2–17] versus 7 ± 2.7 [2–16] mg/day, P = 0.697).
Conclusion
De novo KTRs prescribed extended-release or standard-release tacrolimus demonstrate similar 12-month outcomes.
Keywords: complications, immunosuppression, outcome
Introduction
Tacrolimus is a widely used calcineurin inhibitor (CNI) immunosuppressant in kidney transplantation which is available in both standard-release (Prograf®, twice-daily tacrolimus, Astellas Pharma Inc, Tokyo, Japan) and extended-release (Advagraf®, once-daily tacrolimus, Astellas Pharma Inc., Tokyo, Japan) formulations. These two tacrolimus formulations are considered to be therapeutically equivalent [1]. Tacrolimus remains an immunosuppressant preferred by transplant recipients [2]. Compared with the standard-release formulation, the extended-release tacrolimus has been shown to provide bioequivalent drug exposure [3], efficacy and safety [4, 5] and so conversion of patients from twice-daily to once-daily CNI regimes is now plausible [6–8]. In contrast to conversion studies, comparative studies between the two formulations when used de novo in kidney transplant recipients (KTRs) are limited. The purpose of the present analysis, therefore, was to compare short-term de novo kidney transplant outcomes between these two tacrolimus formulations.
Materials and methods
St Michael's Hospital is a tertiary care medical-surgical center that provides post-transplant care to ∼1300 KTRs and performs ∼120 adult single-organ kidney transplants annually. During the period leading up to July 2009, standard immunosuppressive therapy in de novo transplant recipients included basiliximab (Simulect®), standard-release tacrolimus, mycophenolate mofetil (MMF, Cellcept®) and prednisone, with anti-thymocyte globulin (Thymoglobulin®) substituted for basiliximab in patients perceived to be at a higher immunological risk e.g. the peak panel-reactive antibody (PRA) titer>50% or in whom the donor-specific antibody was present. Starting from July 2009, the extended-release tacrolimus has been used instead of the standard-release formulation in all de novo patients, except for those participating in clinical trials or for whom cyclosporine was preferred a priori, e.g. those perceived to be at higher risk for new-onset diabetes after transplantation (NODAT). The initially prescribed total daily dose for both tacrolimus formulations is 0.10 mg/kg/day as per standard hospital protocol with adjustments based on trough levels first obtained on postoperative Day 2 or 3. In this investigator-initiated clinical Phase IV study, we identified all de novo recipients transplanted at our institution in the first year after change in the protocol from standard-release to extended-release tacrolimus, i.e. between July 2009 and July 2010 who were prescribed extended-release tacrolimus, and compared them with de novo recipients transplanted in the year immediately preceding the protocol change, i.e. between July 2008 and July 2009, who had been prescribed standard-release tacrolimus.
The primary outcome was graft function as assessed by the eGFR (mL/min/1.73 m2) determined by the Modification of Diet in Renal Diseases-7 (MDRD-7) equation at 12 months post-transplant. The secondary outcomes included graft function at Days 7 and 14, Months 1, 2, 3 and 6 post-transplant; and the incidence of acute rejection (AR), BK viremia, NODAT, and graft survival to 12 months post-transplant and cardiovascular risk factors at Month 12 including blood pressure (BP), fasting lipids, C-reactive protein (CRP), uric acid and urine albumin-to-creatinine ratio (ACR). Patients receiving dialysis were assigned an eGFR of 0 at that point of time, and patients experiencing graft loss were censored from future eGFR calculations. AR was defined by indication-based renal biopsy specimen determination according to Banff 1997 criteria, while NODAT was defined based on the Canadian Diabetes Association 2008 guidelines. Delayed graft function (DGF) was defined as the requirement for dialysis therapy within the first post-transplant week. All patients were routinely screened for BK viremia using a qualitative polymerase chain reaction assay once every 3 months, with additional tests ordered as needed based on clinical suspicion. A positive assay was reported as >1 × 103 copies/mL. Routine laboratory testing including renal function (serum creatinine), CRP, urine ACR and random blood glucose was assessed twice weekly to Month 3, weekly to Month 6 and once every 2 weeks to Month 12. Fasting blood glucose was measured monthly, fasting lipid profile once every 6 months and resting sitting BP using the BPTru® device at each clinic visit. Since data collection points included 1, 3, 6 and 12 months post-transplant, laboratory and clinical assessments were assigned to these times based on the closest measurement to the exact date ±2 weeks. All data are prospectively entered into a secure on-site electronic clinical database to readily facilitate access and retrieval.
In addition, we computed time-to-steady-state tacrolimus concentration, defined as the first attainment of consecutive trough levels of 5–10 ng/mL in keeping with our clinical practice, but also as 3–10 ng/mL in order to provide a comparison to possible variations in international practice. Steady state was defined as the tacrolimus level within the target range without any dose adjustment in the preceding 3 days, to correspond to at least five half-lives. Tacrolimus was administered orally once- or twice-daily depending on the formulation usually starting within 6 h of engraftment. Tacrolimus blood levels were measured using the liquid chromatography-mass spectrometry assay in the morning on a daily basis prior to initial hospital discharge (typically 6 to 7 days post-transplant), and along with other laboratory tests post-discharge (see above). The number of dosage adjustments prior to steady-state attainment was calculated from the date of first tacrolimus dose to the date at steady state and the corresponding dose (normalized to body weight) was recorded. We also performed a post-hoc comparison of tacrolimus dosing, concentrations and eGFR by ethnicity, comparing Caucasians with non-Caucasians. Non-adherence to tacrolimus was assessed by determining whether a patient had more than once failed to provide scheduled blood testing for tacrolimus concentrations, failed to appear for clinic visits without explanation or had two or more undetectable tacrolimus concentrations between the attainment of steady state and 12 months post-transplant.
The extended-release and standard-release tacrolimus cohorts were compared by independent Student t-test, chi-square test or Fisher's exact test as appropriate using an intent-to-treat analysis approach. The results are reported as mean ± standard deviation (SD) unless stated otherwise. For all tests, a P value of <0.05 was considered to be statistically significant. SAS version 9.2 (Cary, NC) was the statistical software used. Approval to conduct the study was obtained from the Research Ethics Board at St Michael's Hospital (Research Ethics Board, REB 11–111, May 26 2011).
Results
There were no important differences in recipient or donor demographic characteristics, or relevant peri-transplant variables between the extended-release (N = 106) and standard-release tacrolimus (N = 95) cohorts (Table 1). The patients excluded from this study included those receiving an allograft from their identical twin who, therefore, did not require any immunosuppression (N = 2), those receiving cyclosporine (Neoral®) (N = 14), participants in clinical trials (N = 21), those with a stated intention to transfer to another center prior to 12 months (N = 5) and technical failures (N = 3). There were four temporary crossovers from the extended-release to standard-release tacrolimus formulations due to intensive care unit admission and two permanent conversions for insurance coverage reasons. All patients received induction therapy (anti-thymocyte globulin [ATG] or basiliximab) at the time of transplantation and were receiving tacrolimus, MMF and prednisone at initial hospital discharge. In the extended-release cohort, 25% received ATG, while 30% in the standard-release cohort received ATG (P = 0.450 for difference). Seven percent in the extended-release cohort received additional intravenous immunoglobulin (IVIG), while 16% in the standard-release cohort received IVIG (P = 0.229 for difference).
Table 1.
Baseline comparison of 201 KTRs prescribed de novo extended-release (N = 106) or standard-release (N = 95) tacrolimus
| Parameter | Extended-release tacrolimus (N = 106) | Standard-release tacrolimus (N = 95) | P value |
|---|---|---|---|
| Recipient age (years) | 53.4 ± 14 (22–76) | 52.6 ± 13.2 (21–77) | 0.660 |
| Donor age (years) | 45 ± 15.1 (4–81) | 44.9 ± 15.4 (8–75) | 0.940 |
| Gender M/F N (%) | 64(60)/42(40) | 52 (55)/43(45) | 0.419 |
| Dialysis duration (Years) | 4.3 ± 2.8 (0–11.0) | 5.3 ± 6.1 (0–15.0) | 0.139 |
| No. of transplants N (%) | |||
| 1 | 98(92) | 88(93) | 0.938 |
| 2 | 6(6) | 7(7) | 0.938 |
| >2 | 2(2) | 0(0) | 0.938 |
| Donor source | |||
| Live | 35(33) | 35(37) | 0.570 |
| Deceased | 71(67) | 60(63) | 0.570 |
| Body mass index (kg/m2) | 26.2 ± 5.2 (15.9–41.9) | 25.2 ± 4.6 (16–36.6) | 0.175 |
| Smoking N (%) | |||
| Previous | 31(29) | 32(34) | 0.498 |
| Current | 5(5) | 8(8) | 0.268 |
| Ethnicity N(%) | |||
| Caucasian | 59(55) | 51(54) | 0.778 |
| Black | 12(11) | 5(5) | 0.123 |
| East Asian | 16(15) | 20(21) | 0.271 |
| South Asian | 14(13) | 14(15) | 0.754 |
| Hispanic | 2(2) | 1(1) | 0.541 |
| Other | 3(3) | 4(4) | 0.439 |
| Cause of end-stage renal disease N (%) | |||
| Diabetes | 17(16) | 11(12) | 0.362 |
| Hypertension | 13(12) | 12(13) | 0.937 |
| Glomerulonephritis | 37(35) | 45(47) | 0.727 |
| Polycystic kidney disease | 12(11) | 9(9) | 0.669 |
| Interstitial nephritis | 6(5) | 2(2) | 0.178 |
| Obstructive uropathy | 5(5) | 6(6) | 0.618 |
| Unknown | 16(15) | 10(10) | 0.335 |
| Peak panel reactive antibody (%) | 21.7 ± 32.2 (0–100) | 27.9 ± 35.1 (0–100) | 0.189 |
| Cold ischemia time (h) | 10.8 ± 5.5 (2.8–30.1) | 10.4 ± 3.5 (1.5–14.7) | 0.771 |
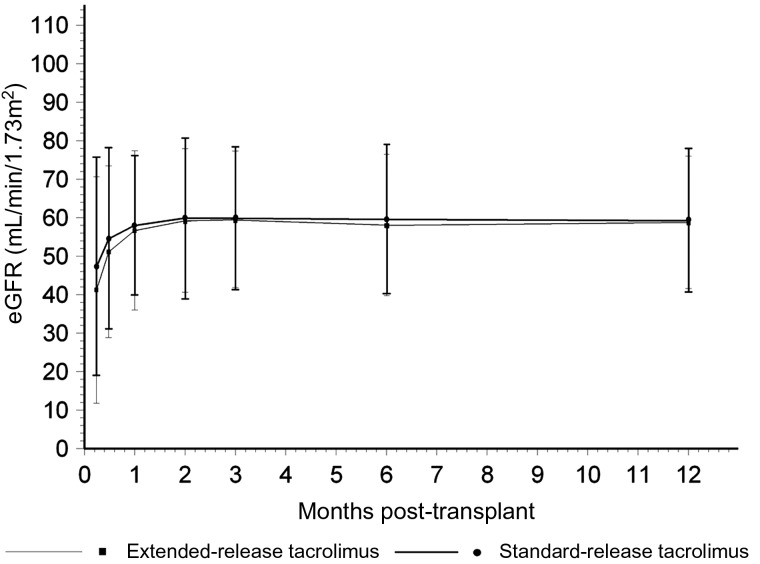
The eGFR at 12 months was similar in the extended-release and standard-release cohorts (58.8 ± 17 versus 59.2 ± 18 mL/min/1.73 m2 respectively, P = 0.307). There were also no significant differences in eGFR at earlier post-transplant times (Figure 1). DGF rates were similar (19 versus 13%, P = 0.227) and graft survival rates were similar (97 versus 95%, P = 0.301) at 12 months. Patient survival was also similar in both the groups (97%). Biopsy-proven acute rejection (AR) rates were similar: 7% in the extended-release and 16% in the standard-release tacrolimus cohorts (P = 0.067). Time-to-AR was similar (132 ± 95 [11–252] versus 127 ± 103 [6–338] days, P = 0.911). There was one steroid-resistant AR in the extended-release and two in the standard-release cohort. Most AR episodes in both cohorts were Banff Grade IA; there were one grade II three grade IIA AR in the standard-release cohort. One AR in the standard-release cohort was positive for C4d staining on biopsy. The BK viremia rate (10 versus 7%, P = 0.450) and time to initial BK viremia were similar (112 ± 36 [83–213] versus 159 ± 71 [77–293] days, P = 0.081). There was no demonstration of BK virus infection by kidney biopsy, and there were no graft losses due to BK virus infection over 12 months of follow-up in either cohort. NODAT rates were similar in the two cohorts (17 versus 20%, P = 0.581). Time-to-NODAT was also similar (123 ± 148 [3–365] versus 106 ± 179 [3–365] days, P = 0.747). There was also no significant difference between the two cohorts in other cardiovascular risk factors. At 12 months in the extended-release and standard-release cohorts, BP was 129 ± 16/76 ± 10 versus 133 ± 16/78 ± 9 mmHg (P = 0.257), total cholesterol 4.3 ± 0.6 versus 4.6 ± 1.4 mmol/L (P = 0.361), low density lipoprotein cholesterol 2.3 ± 0.6 versus 2.6 ± 1.2 mmol/L (P = 0.436), triglycerides 1.4 ± 0.4 versus 1.8 ± 0.8 mmol/L (P = 0.104), CRP 4.4 ± 7.2 versus 6.3 ± 13.7 mg/L (P = 0.329), uric acid 393 ± 100 versus 376 ± 80 µmol/L (P = 0.216) and urine ACR 10 ± 21 versus 8 ± 12 mg/mmol (P = 0.573). There was also no difference in the number of antihypertensive medications (1 versus 0.9, P = 0.557) or patients receiving statins (53 versus 56%, P = 0.645) at 12 months.
Fig. 1.
Estimated GFR by the Modification of Diet in Renal Disease Equation-7 over 12 months in the extended-release (N = 106) and standard-release (N = 95) tacrolimus cohorts (P = NS for difference).
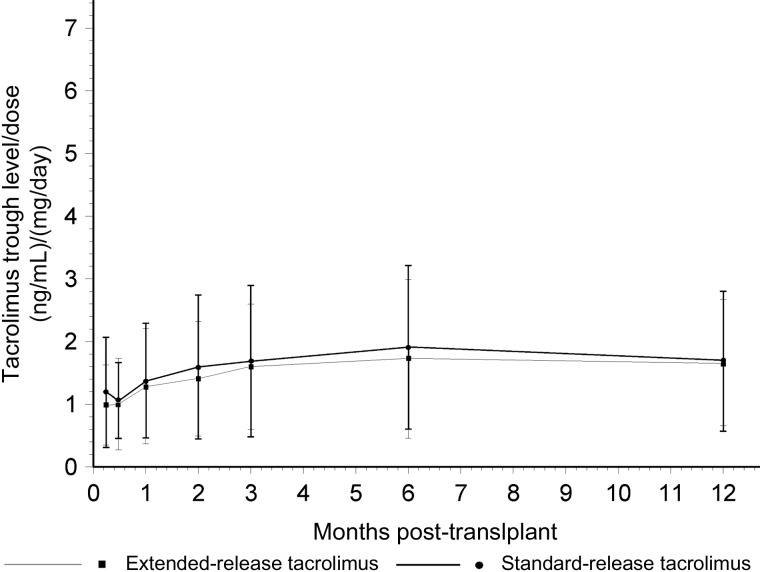
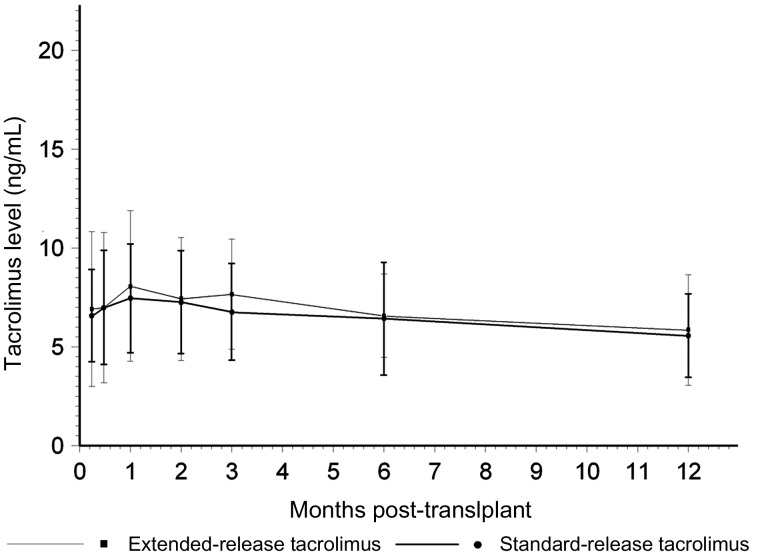
Time to steady state was similar between the extended-release and standard-release cohorts (9.2 ± 1.1 versus 8.1 ± 4.7 days, P = 0.490), but the extended-release cohort required fewer dosage adjustments to attain this steady state (1.2 ± 1.7 [0–8] versus 1.7 ± 1.5 [0–7], P = 0.030) when defined as a range of 5–10 ng/mL. Dose requirement to attain this steady state was similar (7.2 ± 2.4 [2–17] versus 7 ± 2.7 [2–16] mg/day, P = 0.697). Likewise, when the steady state was defined as 3–10 ng/mL, the time-to-steady state was similar (6.7 ± 6.1 versus 8.1 ± 4.7 days, P = 0.201), fewer dose adjustments were needed (0.9 ± 1.3 [0–7] versus 1.7 ± 1.5 [0–7], P < 0.0001) and dose requirement was similar (6.8 ± 2.2 [1–15] versus 7 ± 2.7 [2–16] mg/day, P = 0.514). The median time to steady state was 5 days in the extended-release and 8 days in the standard-release cohort. Figure 2 provides a comparison between the two cohorts expressed as trough concentration divided by dose over 12 months. No significant difference was noted. Figure 3 provides the average tacrolimus concentration maintained over 12 months in each group. No significant differences were seen at any point in time. The total number of tacrolimus dose adjustments to 12 months was 5.5 (range 0–12) in the extended-release and 5.1 (range 1–11) in the standard-release cohorts. Other immunosuppressive drug exposure was similar in the two cohorts. The median dose of mycophenolate mofetil was 1500 mg/day and the mean dose of prednisone 6 mg/day in both the groups at 12 months.
Fig. 2.
Tacrolimus trough concentration (ng/mL) divided by dose (mg/day) over 12 months in the extended-release (N = 106) and standard release (N = 95) tacrolimus cohorts (P = NS for difference).
Fig. 3.
Tacrolimus trough concentration (ng/mL) over 12 months in the extended-release (N = 106) and standard-release (N = 95) tacrolimus cohorts (P = NS for difference).
In the post-hoc comparisons by ethnicity, there were 59 Caucasians and 47 non-Caucasians in the extended-release cohort. At 1 month post-transplant, the tacrolimus dose was 0.10 ± 0.05 mg/kg/day (range 0.02–0.28) in the Caucasians versus 0.12 ± 0.05 mg/kg/day (0.02–0.29) in non-Caucasians (P = 0.009 for difference). At 12 months, the doses were 0.05 ± 0.04 (0.01–0.17) mg/kg/day versus 0.09 ± 0.06 (0.02–0.36) mg/kg/day respectively (P = 0.006 for difference). Similar differences were noted at other times during the 12-month follow-up period. There were however no differences in tacrolimus concentrations or eGFR (data not shown). There were 51 Caucasians and 44 non-Caucasians in the standard-release cohort. At 1 month post-transplant the tacrolimus dose was 0.09 ± 0.04 mg/kg/day (range 0.03–0.20) in the Caucasians versus 0.11 ± 0.05 mg/kg/day (0.04–0.23) in non-Caucasians (P = 0.021 for difference). At 12 months the doses were 0.06 ± 0.03 (0.01–0.17) mg/kg/day versus 0.07 ± 0.04 (0.02–0.18) mg/kg/day respectively (P = 0.017 for difference). Similarly, the differences were noted at other times during the 12-month follow-up period but there were no differences in tacrolimus concentrations or eGFR (data not shown). There were 14/106 patients (13%) in the extended-release cohort and 19/95 (20%) in the standard-release cohort who met the definition for non-adherence to tacrolimus over the 12 month period.
Discussion
This single-center retrospective cohort analysis comparing important 12-month post-transplant outcomes in near-contemporaneous cohorts receiving the extended-release or standard-release tacrolimus formulations demonstrates that these are equally efficacious and safe, and allows for equivalent dosing requirements. The use of the extended-release formulation also allowed for fewer dosage adjustments prior to attaining steady-state concentrations. The extended-release formulation can be used in sensitized recipients, in the early post-transplant period when graft function has not yet been fully established and also during DGF. Adjunct immunosuppression can be maintained similarly with both preparations. Thus, it would seem reasonable to propose that the de novo prescription of the extended-release formulation to KTRs may be considered as an acceptable option for immunosuppressive therapy, in addition to a strategy of conversion from the standard-release to extended-release formulation at varying times post-transplant that has been shown in other studies [4–6].
Renal function in the extended-release group as assessed by the eGFR at 12 months (58.8 ± 17 mL/min/1.73m2) was similar in this study to earlier Phase III trials (58.6 ± 17 and 55.1 ± 16 mL/min/1.73 m2) [1, 8]. Likewise, AR rates were similar in the two cohorts, but notably similar or lower than in the de novo Phase III clinical trial from which higher immunological risk patients were excluded and induction therapy avoided [1]. The AR rates were similar to an earlier Phase III trial in which basiliximab was used and patients with a high PRA titer included [8]. AR was mild and typically responsive to added corticosteroid therapy. BK viremia rates although similar in the present two cohorts were higher than in a previous study [1], possibly due to differences in adjunct immunosuppression and our implementation of a systematic surveillance policy. NODAT rates were similar between the two cohorts as well as similar to that previous trial [1] despite possible population differences. There were also no significant differences in various traditional and novel cardiovascular risk factors. These findings taken together may suggest that the extended-release tacrolimus formulation is likely to be safe for de novo use in a routine clinical practice setting.
Our reason for switching from standard-release to extended-release tacrolimus at our institution was the prospect of improved patient adherence, although a limitation of the present study is that we did not formally study patient adherence to tacrolimus through methods such as pill counts. Greater patient adherence has the potential to improve patient outcomes or at the very least results in less acute rejection from sub-therapeutic immunosuppressant exposure [9]. On the other hand, concern has been raised from conversion studies about early sub-therapeutic exposure associated with the extended-release preparation and the consequent need for increased dosing [10, 11]. It is judicious to monitor drug exposure closely when switching preparations since tacrolimus is a designated critical dose drug [12]. Concern has been raised about possible under-dosing when standard-release tacrolimus is switched to extended-release, and that it may not be possible to identify patients at risk for under-dosing preemptively [13]. Our data indicate that when used de novo in the context of our monitoring protocol, there are no safety signals related to under-dosing, and in fact, although there was a trend towards a higher dose requirement with extended-release tacrolimus early post-transplant, fewer dosage adjustments were needed. We speculate that a higher dose requirement with extended-release tacrolimus was not demonstrated in the current study because of either sample size or possible differences in population characteristics determined by factors such as ethnic heterogeneity. Less frequent early dose adjustment with extended-release tacrolimus may have been due to its longer release time and greater attentiveness to avoiding adjustments prior to steady-state attainment. Despite this, the total number of adjustments to maintain steady state was likely not different at 12 months due to the greater total number of outpatient measurements and adjustments involved. Nonetheless, it raises the interesting possibility of a lesser requirement for tacrolimus concentration measurements that could be explored in future studies. Likewise, the significant difference in dosing requirements noted between Caucasians and non-Caucasians with both tacrolimus preparations raises interesting mechanistic hypotheses worthy of further study such as ethnic differences in CYP3A5 genotypes [14].
Similar to previous studies, the current study is limited by its open-label design necessarily imposed by the retrospective analysis of prospectively collected data. The number of patients studied was relatively small, formal pharmacokinetic testing was not performed, and the cohorts were not truly contemporaneous although they were close enough to each other in the transplant date to avoid an era effect. These data by virtue of being from a de novo setting complement previous conversion studies in stable patients from routine clinical practice [4]. A revision of the need for frequent tacrolimus blood concentration measurements is a provocative proposal that can be evaluated, while longer-term follow-up of de novo KTRs prescribed extended-release tacrolimus will provide further reassurance of its efficacy and safety.
Acknowledgements
Funding. This study was funded through the competitive Investigator-Initiated Grant Request Program, Astellas Canada. Our funders had no role in the study design, analysis or decision to present the findings from this study.
Conflict of interest statement
J.S.Z. and G.V.R.P. have received consultant honoraria from Astellas Canada. The other authors do not have any conflicts of interest to declare.
References
- 1.Kramer BK, Charpentier B, Backman L, et al. Tacrolimus once daily (ADVAGRAF) versus twice daily (PROGRAF) in de-novo renal transplantation: a randomized phase III study. Am J Transplant. 2010;10:2632–2643. doi: 10.1111/j.1600-6143.2010.03256.x. [DOI] [PubMed] [Google Scholar]
- 2.Prasad GV, Nash MM, McFarlane PA, et al. A numerical scale comparison of renal transplant recipient experience with and opinions about calcineurin inhibitors. Nephron Clin Pract. 2004;97:35–40. doi: 10.1159/000078398. [DOI] [PubMed] [Google Scholar]
- 3.Hardinger KL, Park JM, Schnitzler MA, et al. Pharmacokinetics of tacrolimus in kidney transplant recipients: twice daily versus once daily dosing. Am J Transplant. 2004;4:621–625. doi: 10.1111/j.1600-6143.2004.00383.x. [DOI] [PubMed] [Google Scholar]
- 4.Guirado L, Cantarell C, Franco A, et al. for the GREAT Study Group. Efficacy and safety of conversion from twice-daily to once-daily tacrolimus in a large cohort of stable kidney transplant recipients. Am J Transplant. 2011;11:1965–1971. doi: 10.1111/j.1600-6143.2011.03571.x. [DOI] [PubMed] [Google Scholar]
- 5.Lauzurica R, Morales JM, van Hooff J. Renal function and safety in stable kidney transplant recipients converted from immediate-release to extended-release tacrolimus. Transplant Int. 2012;25:48–55. doi: 10.1111/j.1432-2277.2011.01366.x. [DOI] [PubMed] [Google Scholar]
- 6.Kolonko A, Chudek J, Wiecek A. Improved kidney graft function after conversion from twice daily tacrolimus to a once daily extended-release formulation. Transplant Proc. 2011;43:2950–2953. doi: 10.1016/j.transproceed.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 7.Alloway R, Steinberg S, Khalil K, et al. Two years postconversion from a Prograf-based regimen to a once-daily tacrolimus extended-release formulation in stable kidney transplant recipients. Transplantation. 2007;83:1648–1651. doi: 10.1097/01.tp.0000264056.20105.b4. [DOI] [PubMed] [Google Scholar]
- 8.Silva HT, Yang HC, Abouljoud M, et al. One-year results with extended-release tacrolimus/MMF, tacrolimus/MMF and cyclosporine/MMF in de-novo kidney transplant recipients. Am J Transplant. 2007;7:595–608. doi: 10.1111/j.1600-6143.2007.01661.x. [DOI] [PubMed] [Google Scholar]
- 9.Undre NA, van Hooff J, Christiaans M, et al. Low systemic exposure to tacrolimus correlates with acute rejection. Transplant Proc. 1999;31:296–298. doi: 10.1016/s0041-1345(98)01633-9. [DOI] [PubMed] [Google Scholar]
- 10.de Jonge H, Kuypers DR, Verbeke K, et al. Reduced C0 concentrations and increased dose requirements in renal allograft recipients converted to the novel once-daily tacrolimus formulation. Transplantation. 2010;90:523–529. doi: 10.1097/TP.0b013e3181e9feda. [DOI] [PubMed] [Google Scholar]
- 11.Hougardy JM, Broeders N, Kianda M, et al. Conversion from Prograf to Advagraf among kidney transplant recipients results in sustained decrease in tacrolimus exposure. Transplantation. 2011;91:566–569. doi: 10.1097/TP.0b013e3182098ff0. [DOI] [PubMed] [Google Scholar]
- 12.Harrison JJ, Schiff JR, Coursol CJ, et al. Generic immunosuppression in solid organ transplantation : a Canadian perspective. Transplantation. 2012;93:657–665. doi: 10.1097/TP.0b013e3182445e9d. [DOI] [PubMed] [Google Scholar]
- 13.Hougardy J-M, de Jonge H, Kuypers D, et al. The once-daily formulation of tacrolimus: A step forward in kidney transplantation? Transplantation. 2012;93:241–243. doi: 10.1097/TP.0b013e31823aa56e. [DOI] [PubMed] [Google Scholar]
- 14.Wang P, Mao Y, Razo J, et al. Using genetic and clinical factors to predict tacrolimus dose in renal transplant recipients. Pharmacogenomics. 2010;11:1389–1402. doi: 10.2217/pgs.10.105. [DOI] [PubMed] [Google Scholar]