Abstract
Rationale
Activation of the mitochondrial ATP-sensitive potassium channel (mitoKATP) has been implicated in the mechanism of cardiac ischemic preconditioning, yet its molecular composition is unknown.
Objective
To use an unbiased proteomic analysis of the mitochondrial inner membrane to identify the mitochondrial K+ channel underlying mitoKATP.
Methods and Results
Mass spectrometric analysis was used to identify KCNJ1(ROMK) in purified bovine heart mitochondrial inner membrane and confirmed that ROMK mRNA is present in neonatal rat ventricular myocytes and adult hearts. ROMK2, a short form of the channel, is shown to contain an N-terminal mitochondrial targeting signal and a full length epitope-tagged ROMK2 colocalizes with mitochondrial ATP synthase β. The high-affinity ROMK toxin, tertiapin Q, inhibits mitoKATP activity in isolated mitochondria and in digitonin-permeabilized cells. Moreover, shRNA-mediated knockdown of ROMK inhibits the ATP-sensitive, diazoxide activated, component of mitochondrial thallium uptake. Finally, the heart-derived cell line, H9C2, is protected from cell death stimuli by stable ROMK2 overexpression, while knockdown of the native ROMK exacerbates cell death.
Conclusions
The findings support ROMK as the pore-forming subunit of the cytoprotective mitoKATP channel.
Keywords: ATP-sensitive potassium channel, mitochondria, apoptosis, preconditioning, ischemia, cytoprotection, renal outer medullary potassium channel
INTRODUCTION
The heart possesses an innate ability to protect itself against ischemic injury through a mechanism known as preconditioning1, whereby one or more cycles of brief ischemia and reperfusion trigger resistance to a subsequent prolonged ischemia. This infarct-sparing effect lasts for several hours (early preconditioning) and also re-emerges as a “second window” of protection that is present 24–72 hours after the initial preconditioning event2. Potassium channels were implicated as critical mediators of endogenous cardioprotection when it was observed that the effects of ischemic preconditioning were inhibited by K+ channel blockers3 and could be mimicked by openers of ATP-sensitive potassium (KATP) channels. The latter effect was independent of changes in the cardiac action potential4, 5. Moreover, the K+ channel opener diazoxide, an effective cardioprotective agent, is much more potent at enhancing K+ flux at the mitochondrial, rather than the sarcolemmal, membrane6 and it protects cells against injury while having little impact on surface membrane KATP current7, 8. Thus, mitoKATP, originally described through single-channel recordings of ATP-sensitive K+ currents in giant liver mitoplasts9, was linked to ischemic preconditioning10, ischemic postconditioning11, 12, and cytoprotection in general. Since then, numerous methods have been used to study mitoKATP in cells, mitochondria and tissues7, 13–15, but the low copy number of the channels in the mitochondrial membrane16, its fleeting activity in vitro17, and the confounding nonspecificity of available pharmacological agents10 and antibodies18, has hampered efforts to identify the channel at a molecular level, fostering persistent skepticism among some investigators as to the nature, and even existence, of mitoKATP19, 20.
Here, we employ an unbiased proteomic approach to identify KCNJ1(ROMK) in the mitochondrial inner membrane and demonstrate that ROMK channels localize to mitochondria, mediating ATP-sensitive K+ flux and conferring protection against cell death stimuli, consistent with mitoROMK being the pore-forming subunit of mitoKATP.
RESULTS
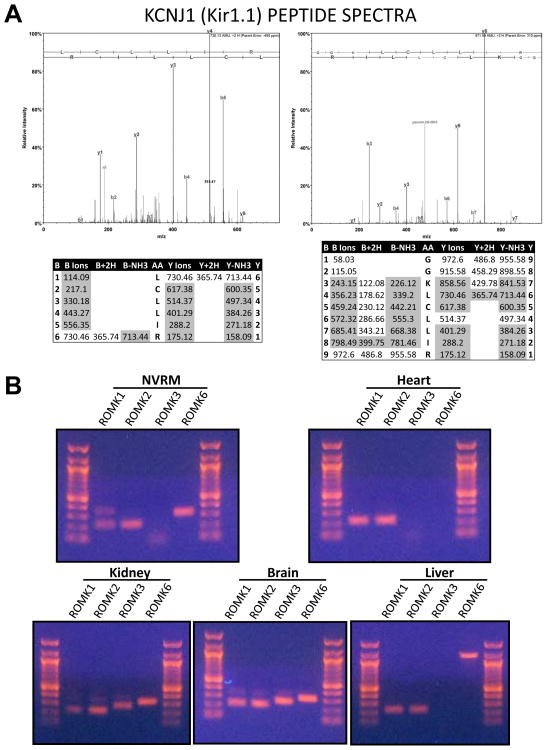
A large scale proteomic analysis of enriched mitochondrial inner membrane fractions from highly purified bovine mitochondria was undertaken to identify low abundance proteins that were underrepresented in previous mitochondrial studies. More than 20 million spectra were collected by two-dimensional liquid chromatography mass spectrometry (2DLC-MS/MS) and 964 proteins were identified with high confidence. The proteomic data were compared with previously published compendia of mitochondrial proteins21, 22. A total of 687 proteins matched either the Mitocarta database21 or the mitochondrial annotation of the Uniprot KnowledgeBase. However, we identified 186 additional proteins that were likely to be mitochondrial (high Maestro scores22), for which there was no previous mass spectral evidence in the heart. Two overlapping peptides, LCLLIR and GGKLCLLIR (Figure 1A), uniquely matched the predicted protein sequence of the bovine KCNJ1 gene product, Kir1.1 (the Renal Outer Medullary Kidney channel, ROMK). The identification was validated statistically (P>95%; Peptide Prophet23) and matching spectra had overlapping contiguous b- and y-ion series (Figure 1A). The ROMK channel is highly expressed in the kidney24, where the channel mediates K+ recycling in the thick ascending limb and K+ secretion in the cortical collecting duct of the nephron. Although expression levels were low in non-renal tissues, we confirmed by reverse transcriptase PCR (RT-PCR) that ROMK isoforms are present (Figure 1B) in neonatal rat ventricular myocytes (NRVM; ROMK1, 2, 6) and adult rat hearts (ROMK1, 2), as well as in brain (ROMK1, 2, 3, 6) and liver (ROMK1, 2) - all of which have been reported to have mitoKATP activity24. Of the isoforms found in the heart, the only difference at the protein level is that ROMK1 has an extra 19 amino acids at the N-terminus as compared to ROMK2 or ROMK625.
Figure 1. Identification of KCNJ1/Kir1.1/ROMK in heart mitochondria and its expression in non-renal tissues.
A) Acquired MS/MS spectra were searched against a custom database of bovine protein reference sequences (NCBI), using the Mascot search algorithm (Matrix Sciences). Data were uploaded into Scaffold (Proteome Software) for statistical validation. 6 spectra matched to 2 overlapping peptides with Peptide ID probabilities of 95%. The cumulative Protein ID probability was >99%. Manual inspection of the spectral matches confirmed that all major peaks were assigned and that b- and y-ion matches formed contiguous and overlapping series. B) Expression of ROMK isoforms in NRVM and adult rat heart by RT-PCR. ROMK1, ROMK2 and ROMK6 were detected in NRVM. In adult rat heart, ROMK1 and ROMK2 were detected. ROMK3 and ROMK6 were undetectable in the heart. ROMK isoforms 1, 2, 3, and 6 were detected in kidney and similar isoforms were found in brain. ROMK expression in liver resembled that of heart, as only ROMK1 and ROMK2 were detected. The high molecular weight ROMK6 PCR product in the liver sample likely reflects residual genomic DNA contamination.
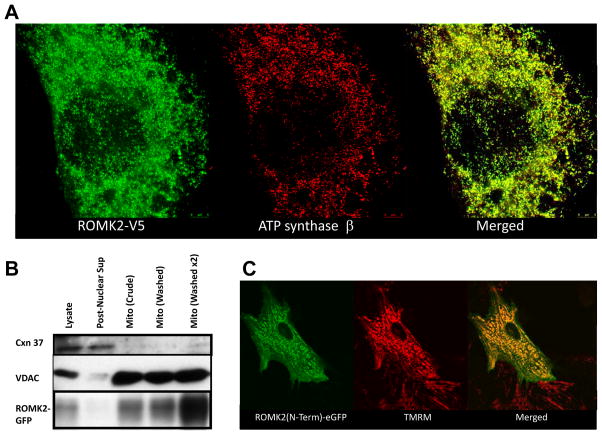
Intriguingly, bioinformatic analysis of the bovine ROMK sequence with mitochondrial localization algorithms indicated that trafficking to the mitochondrion was highly likely, yielding probabilities of 99.5%, 89.9%, and 99% with the Mitoprot II, Target P, and Mitopred algorithms, respectively. In a recent genome-wide ranking of mitochondrial localization likelihood in mouse and humans21, ROMK/KCNJ1 had the highest ranking of all the inward rectifier K+ channel (Kir) genes. Likewise, among Kir protein sequences in the UniprotKB rat database, ROMK2 (accession no. P35560-2) had the highest mitochondrial localization probability (99%) according to Mitopred; consequently, we focused on ROMK2 as the prime candidate. These predictions were confirmed experimentally. A ROMK2 construct containing a c-terminal tag (V5 epitope) was heterologously expressed in H9C2 cells, a rat embryonic heart-derived cell line. Cells transfected with ROMK2-V5 were fixed and subjected to immunofluorescence labeling with a V5-specific antibody and imaged using a dual color super-resolution stimulated-emission depletion fluorescence microscope (Leica TCS STED). ROMK2-V5 fluorescence (Alexa 488 secondary Ab) was highly correlated with the mitochondrial marker, ATP synthase β (Pacific Orange 568 secondary Ab), indicating subcellular localization of the channel in mitochondria. Similarly, specific mitochondrial enrichment of ROMK was demonstrated in Chinese Hamster Ovary (CHO) cells transiently transfected with a ROMK2-eGFP fusion protein; GFP signal increased in intensity with stepwise purification of mitochondrial membranes by differential centrifugation in concert with a mitochondrial marker (VDAC), varying inversely with a plasma membrane marker (connexin 37; Figure 2B). We also tested the prediction (from MitoProt II) that the first 24 amino acids of ROMK2 constituted a mitochondrial targeting sequence sufficient to impart mitochondrial targeting. The sequence MFKHLRKWVVTRFFGHSRQRARL was fused to the N-terminus of eGFP, transiently transfected into neonatal rat ventricular myocytes, and imaged in living cells (Figure 2C; two-photon laser scanning fluorescence microscopy). The eGFP signal predominantly co-localized with the mitochondrial membrane potential probe tetramethylrhodamine methyl ester (TMRM), confirming that the ROMK2 N-terminal signal confers mitochondrial protein targeting.
Figure 2. Targeting of ROMK2 to mitochondria.
A) The ROMK2 isoform, fused with a V5 tag on its C-terminus, was transiently expressed in H9C2 cells. Cells were fixed, permeabilized and incubated with antibodies against V5 (rabbit) and ATP synthase β, a mitochondrial marker. ROMK-V5 was subsequently stained with anti-rabbit secondary antibody conjugated to Alexa 488 (left panel), and anti-mouse secondary conjugated Pacific Orange 458 (middle panel). The right hand panel depicts the merged images. B) Subcellular fractions of CHO cells transiently expressing ROMK2-eGFP were labelled with connexin 37, VDAC, and eGFP antibodies as markers of plasma membrane, mitochondrial membrane, and ROMK2, respectively. Western blot shows co-enrichment of ROMK-eGFP and the mitochondria marker. All lanes were loaded with equal total protein amounts.
C) The predicted N-terminal mitochondrial targeting sequence of ROMK2 was fused to eGFP and imaged in living NRVM (left panel). It colocalized with tetramethylrhodamine methyl ester (TMRM), a mitochondrial membrane potential probe. The right hand panel depicts the merged images.
Next, we determined whether ROMK plays a role in modulating mitochondrial K+ fluxes in isolated mitochondria, a cardiac-derived cell line (H9C2), and primary cultures of neonatal rat ventricular myocytes (NRVMs), employing a high affinity K+ channel toxin, pharmacological tools, and molecular methods.
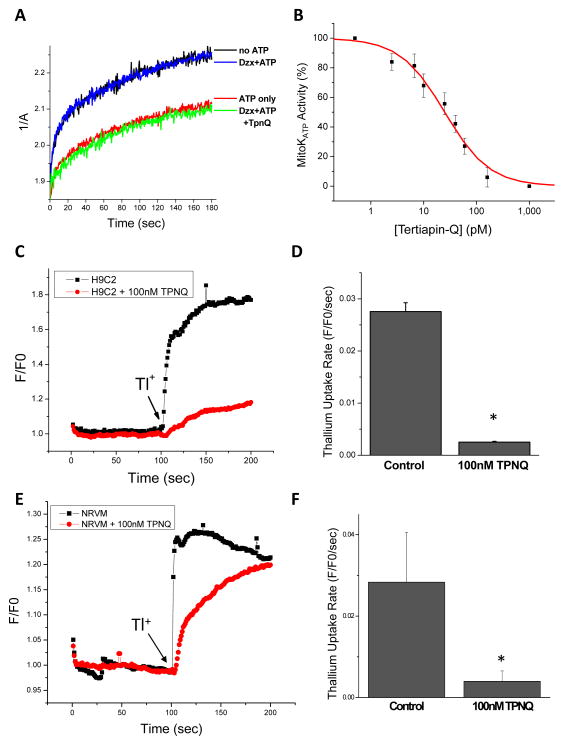
The classical mitoKATP assay, developed by Garlid and coworkers, employs the 90° light scattering property of isolated mitochondria as a readout of mitochondrial matrix volume26. It is based on the principle that the activation of K+ uptake into mitochondria is accompanied by the movement of osmotically-obligated water, counterbalanced by a K+/H+ exchanger27. The initial rate of mitochondrial swelling is inhibited by ATP, and this inhibition is reversed by the action of K+ channel opener compounds (e.g., cromakalim, diazoxide, etc.)13. Conversely, mitoKATP is inhibited by sulfonylureas or by 5-hydroxydecanoate28. The expected effects of ATP and diazoxide (30μmol/L; in the presence of ATP) on mitochondrial swelling in isolated rat heart mitochondria were observed (Figure 3A). Notably, Tertiapin Q, a stable variant of a peptide bee venom toxin that is a high affinity pore-binding blocker of surface membrane ROMK channels29, 30, abrogates the effect of diazoxide (Figure 3A) with subnanomolar potency (IC50= 25 pmol/L; Figure 3B). The native KATP sensitivity to Tertiapin Q was also investigated in H9C2 cells and in NRVMs by measuring the initial rates of thallium (Tl+) uptake (a surrogate for K+) into mitochondria in partially-permeabilized (digitonin-treated) cells using a fluorescent reporter assay (see Online Supplement: Methods). Tertiapin Q (100 nmol/L) reduced the rate of Tl+ uptake in both permeabilized H9C2 cells (Figure 3C and 3D) and NRVMs (Figure 3E and 3F). Tertiapin Q is highly selective for ROMK over Kir2.1 type inward rectifier K+ channels and is partially selective for ROMK over GIRK130 and KCa31 channels. GIRK channels have not been reported in mitochondria (and we found no mass spectrometric evidence for them) and although KCa channels are thought to be present in the mitochondrial inner membrane32, our experiments were performed in the absence of Ca2+.
Figure 3. Inhibition of mitoKATP activity with a ROMK toxin.
A) Swelling of isolated rat heart mitochondria in the presence of K+ and Pi measured spectrophotometrically as a change in 90° light scattering at 520nm excitation. The matrix volume increase is inhibited by ATP while the K+ channel opener diazoxide reverses the inhibition. Tertiapin Q (TPNQ), a stable variant of a bee venom toxin that inhibits of ROMK channels, potently suppressed mitoKATP. B) Concentration-response curve for TPNQ inhibition of mitoKATP in isolated rat heart mitochondria. C) Inhibition of thallium uptake by 100 nmol/L TPNQ in permeabilized H9C2 cells (Fluozin-2 indicator used in this experiment). D) Summary of TPNQ’s effect on initial thallium uptake rate in H9C2 cells (n=3 experiments) E) Inhibition of thallium uptake by 100 nmol/L TPNQ in permeabilized NRVM monolayers (Fluozin-2 indicator). F) Summary of TPNQ’s effect on initial thallium uptake rate in NRVMs (n=3). * denotes statistically significant differences between control and TPNQ treated samples (p<0.05).
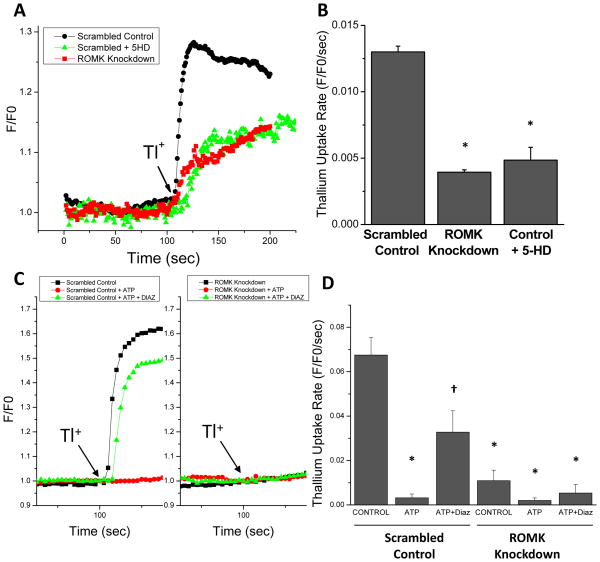
The Tertiapin Q experiments provided some indication that ROMK may be a component of the native mitoKATP; nevertheless, we sought molecular evidence that ROMK channels participate in mitochondrial K+ uptake directly. Therefore, we established a stable H9C2 cell line expressing two short hairpin RNA (shRNA) constructs targeted to the core exon of the ROMK channel and determined the effect of ROMK knockdown on the initial rates of Tl+ uptake into mitochondria in partially-permeabilized (digitonin-treated) cells. The amount of ROMK expression was reduced by 72% on the mRNA level, as confirmed by quantitative real-time PCR, compared to a negative control scrambled shRNA (0.021ng ROMK/ng 18s in control vs. 0.005ng ROMK/ng 18s in knockdown cells). Knockdown of the native ROMK protein was also confirmed by western blot (see Online Supplement Figure III). Knocking down endogenous ROMK expression resulted in a 70% decrease in the Tl+ uptake rate in H9C2 cells, measured in the absence of ATP, compared to the scrambled shRNA negative controls (Figure 4A and 4B). The degree of suppression of Tl+ uptake was similar to that observed for control cells treated with the mitoKATP inhibitor 5-hydroxydecanoate (5-HD; Figure 4A and 4B). In addition, Tl+ uptake was inhibited by 1 mmol/L ATP and this inhibition was reversed by 10 μM diazoxide in control cells (Figure 4C and 4D) or by the K+ ionophore valinomycin (see Online Supplement Figures I and II). However, 10 μM diazoxide did not reverse the ATP inhibition of Tl+ flux when the native ROMK was knocked down by stable shRNA expression, indicating that ROMK is a necessary component of the ATP-sensitive, diazoxide-activated, mitochondrial K+ channel.
Figure 4. Suppression of mitochondrial thallium uptake by ROMK knockdown.
A) H9C2 cells were permeabilized with digitonin and mitochondrial thallium uptake (2 mmol/L; ATP-free), was determined as the initial rate of change in fluorescence intensity, normalized to the baseline K+- and Tl+- free fluorescence, of a mitochondrial matrix-localized thallium-binding reporter, BTC-AM (F/F0). Three cell populations were examined: i) a stable cell line expressing scrambled control shRNA, ii) the same control cells treated with 500 μM 5-HD, and iii) a stable cell line expressing ROMK shRNA. B) Summary of initial thallium uptake rates for each group (n=3 experiments). * denotes statistically significant differences between control and TPNQ treated samples (p<0.05). C) Thallium uptake in the presence of (i) 1 mmol/L ATP and (ii) 1 mmol/L ATP with 10μM diazoxide in permeabilized H9C2 cells pre-loaded with Fluozin-2 indicator (Left panel: control cells; Right panel: ROMK knockdown cells). D) Summary of initial thallium uptake rates for each group (n=3 for each group other than control cells with ATP [n=4] and knockdown cells with ATP [n=5]). Statistically significant differences were determined by 2-way ANOVA followed by Tukey’s test (p<0.05): * denotes a significant difference compared to scrambled control cells alone; † difference compared to scrambled control cells with ATP.
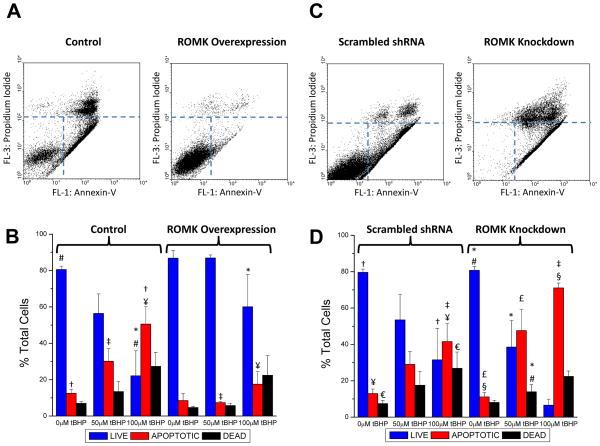
The potential cytoprotective role of ROMK against oxidative stress-induced cell injury was examined by increasing or decreasing the level of ROMK expression in H9C2 cell lines and treating them with tert-butyl hydroperoxide (tBHP). Flow cytometry was used to count the number of apoptotic (Annexin-V positive) and necrotic (propidium iodide (PI) positive: indicates loss of surface membrane integrity) cells after incubation with tBHP. After 22 hours of tBHP (100μmol/L) treatment, survival was markedly enhanced in the stable ROMK2-V5-overexpressing cell line compared to control cells, clearly evident as an increased population of living cells in the lower left quadrant of the fluorescence dot plot (Figure 5A; right panel). Summary data from five paired experiments in which cells were treated with either 50 or 100 μmol/L tBHP shows the protective effect of ROMK overexpression; the dose dependent decrease in live cells and increase in necrotic and apoptotic cells with tBHP treatment was blunted by ROMK (Figure 5B). Conversely, H9C2 cells in which the native ROMK was knocked down by stable expression of shRNA displayed increased sensitivity to tBHP-induced necrosis and apoptosis compared to a cell line expressing a negative control scrambled shRNA (Figures 5C and 5D).
Figure 5. ROMK2 overexpression protects against necrotic/apoptotic cell death induced by oxidative stress - ROMK knockdown exacerbates cell death.
A) Representative flow cytometry fluorescence dot plots for Annexin-V and Propidium Iodide (PI) labelling in control and ROMK2 overexpressing H9C2 cells subjected to 100μM tertbutyl hydroperoxide (tBHP) treatment for 22 hours. The three populations selected to quantify live, apoptotic (Annexin positive), and dead (PI positive) cells are illustrated by the dotted lines. B) Summary of the percentages of live, apoptotic, and dead cells from paired experiments of control and ROMK2-overexpressing cells (ROMK2-OE) subjected to no tBHP, 50μM and 100μM tBHP. Statistically significant differences between groups were determined by 2-way ANOVA followed by Tukey’s test (p<0.05): # live control cells vs. tBHP treatment; † apoptotic control cells vs. tBHP treatment; * live control cells vs. live ROMK2-OE cells at 100μM tBHP; ‡ apoptotic control cells vs. apoptotic ROMK2-OE at 50μM tBHP; ¥ - apoptotic control cells vs. apoptotic ROMK2-OE at 100μM tBHP. C) Representative flow cytometry fluorescence dot plots for Annexin-V and PI labeling in scrambled shRNA control and ROMK knockdown cell lines subjected to 50μM tBHP incubation for 22 hours. D) Summary of the percentages of live, apoptotic, and dead cells from paired experiments of scrambled control and ROMK knockdown cells (ROMK-KD) subjected to no tBHP, 50μM and 100μM tBHP. Statistical significance (p<0.05): # live control cells vs. tBHP treatment; † apoptotic control cells vs. tBHP treatment; € dead control cells vs. tBHP treatment; * live ROMK-KD vs. tBHP treatment; § apoptotic ROMK-KD cells vs. tBHP treatment; ‡ apoptotic control cells vs. apoptotic ROMK-KD at 100μM tBHP.
DISCUSSION
Canonical KATP channels serve to couple membrane K+ conductance to the metabolic state of the cell. They typically consist of four K+-selective pore-forming subunits from the Kir6.x family of inward rectifiers and four auxiliary regulatory subunits from the sulfonylurea receptor family (SUR1/2). The precise Kir and SUR isoform composition of KATP channels varies by tissue, consistent with unique roles in the regulation of processes ranging from insulin secretion, to action potential modulation, to vascular tone. The composition of the mitochondrial KATP channel, however, has remained elusive, and dominant negative suppression33 or genetic knockout of Kir6.x channels has been ineffective in suppressing mitoKATP responses34, 35. Moreover, antibody-based identification of mitochondrial channel has been confounded by non-specific binding of the available reagents18.
Apart from the abundant voltage-dependent anion channel (VDAC) of the mitochondrial outer membrane, proteomic efforts, thus far, have failed to unequivocally identify any ion channels in the inner membrane. The present approach differed from previous studies primarily by increasing the amount of mitochondrial starting material and increasing the degree of fractionation at the level of the membrane, in order to decrease the complexity of the peptide mixture analyzed by 2DLC-MS/MS. This allowed us to find more low abundance proteins, including ROMK, which was the only K+ channel to be identified with high confidence. From the peptide evidence in hand, it is not currently possible to determine which of the ROMK protein isoforms is present, since the peptides were from the common exon expressed in all ROMK channels25. The data do not preclude the possibility that other K+ channels may be present, however, since a negative result by mass spectrometry cannot be taken as evidence of absence.
Confirmation of the ability of the ROMK2 leader sequence to target eGFP to the mitochondria, and localization of the full length ROMK2 to mitochondria were enough to motivate further investigation of mitoROMK properties; however, there were also several known characteristics of the ROMK channel that heightened our interest in ROMK as a candidate for mitoKATP. In addition to its having the highest probably of mitochondrial targeting (>95%), as determined by multiple intracellular targeting algorithms, ROMK contains a Walker A type ATP binding motif on its C-terminus and, similar to KATP channels, it is both activated and inhibited by ATP24, 36. Channel activity of excised membrane patches containing ROMK channels rapidly runs down when Mg2+ is present. This effect can be reversed by applying MgATP or the catalytic subunit of protein kinase A, suggesting phosphorylation-dependent activation36. The ROMK channel, and particularly ROMK2, is inhibited by millimolar concentrations of MgATP (K1/2=2.3 mmol/L) 36. This inhibition is independent of ATP hydrolysis - moreover, mutations in the Walker site of ROMK2 shift the MgATP inhibition curve36. Like other KATP channels, ROMK can be activated by PIP237, a feature that has also been documented for mitoKATP38, and it is regulated by pH39 and protein kinase C40. In addition, it has been reported that ROMK2 may be intrinsically sensitive to the KATP inhibitor glibenclamide (which also inhibits mitoKATP 28) even in the absence of SUR coexpression41. Finally, ROMK can interact with members of the ATP binding cassette family of proteins, including CFTR42 and SUR43. For example, when coexpressed in Xenopus oocytes, ROMK2, but not ROMK1 or ROMK3, was shown to physically associate with SUR2B to form glibenclamide-sensitive channels44.
Circumstantial evidence aside, the fact that Tertiapin Q, a high affinity ROMK toxin30, could inhibit diazoxide-induced K+-specific swelling in isolated mitochondria, as well as ATP-sensitive mitochondrial Tl+ uptake in partially-permeabilized cells, provides the first evidence that ROMK is a component of native mitoKATP. Moreover, 5-HD inhibits, and diazoxide activates, a similar component of the Tl+ influx pathway that is suppressed by ROMK knockdown, providing additional support for the conclusion that ROMK is a component of mitoKATP.
Future studies will be required to determine potential binding partners for native or heterologously expressed ROMK channels in the heart; however, a recent study found a short (55kDa) splice variant of SUR that displays mitochondrial localization45. We did not identify any SUR peptides in our proteomic dataset, but SUR coexpression or concomitant knockdown strategies are possible. Similarly, recent studies have implicated connexin 43 in the modulation of mitoKATP channels46, 47: the molecular identification of mitoROMK and the assay techniques developed herein enable a detailed investigation of the proteins and regulatory interactions required for cell protection. Because it is well accepted that ROMK channels can be readily targeted to the surface membrane to fulfill their primary functional role in the kidney, additional work will also be necessary to define how ROMK channels are targeted to different sites in different tissues including the heart, brain, liver or skeletal muscle - tissues where mitoKATP has been documented.
A limitation of the present study is that the necessary tools required for exploring the role of mitoROMK in the intact animal, a prerequisite for assessing its impact on ischemic pre- or postconditioning, are not yet at hand. Also, while the K+ selectivity and conductance of ROMK channels localized to the surface membrane are well characterized, more work will be required to determine if the channel expressed in mitochondria possesses similar biophysical properties.
Perhaps most importantly, the results demonstrate that mitoROMK confers protection against cell death, and even provides a basal level of protection against oxidative stress in the absence of preconditioning stimuli. In this respect, the data confirm the general idea that mitochondrial K+ channels, such as mitoKATP or mitoKCa, are cytoprotective. Identification of mitoROMK provides, for the first time, a molecular target for mechanistic and therapeutic investigation of this important cell survival pathway.
Supplementary Material
Novelty and Significance.
What is Known?
Mitochondrial ATP-sensitive potassium channels (mitoKATP) have been implicated in the mechanism of cardiac preconditioning.
The molecular composition of mitoKATP has not been determined, limiting advancement in understanding its role in cytoprotection.
What New Information Does This Article Contribute?
ROMK/KCNJ1, an ATP-sensitive potassium channel, was identified in the inner mitochondrial membrane using a proteomic approach.
An isoform of ROMK contains a mitochondrial targeting motif at its N-terminus.
Inhibition of ROMK with the honey bee venom toxin, tertiapin-Q, inhibits the classical mitochondrial swelling response attributed to mitoKATP opening and also inhibits mitochondrial ATP-sensitive thallium (Tl+; a surrogate for K+) uptake.
Genetic knockdown of ROMK also suppresses mitochondrial ATP-sensitive Tl+ uptake.
Overexpression of ROMK protects H9C2 cells from oxidant-induced cell death, while knockdown of ROMK renders them susceptible to cell death.
A resident mitochondrial isoform of ROMK is the likely pore forming subunit of the long-sought cardioprotective mitoKATP channel.
Several lines of evidence support the existence of a mitochondrial ATP-sensitive K+ channel (mitoKATP), which has been linked to the mechanism of cardiac preconditioning through the actions of pharmacological agents that preferentially interact with the mitochondrial, as opposed to the sarcolemmal, channel. The lack of a molecular identification of mitoKATP, and nonspecific actions of the compounds employed, has restricted progress in understanding mitoKATP-mediated cytoprotection. Here, we report that an isoform of a renal outer medullary K+ channel, ROMK, is present in the mitochondrial inner membrane and mediates mitochondrial ATP-sensitive K+ flux. MitoKATP activity in isolated mitochondria or permeabilized cells was potently inhibited by the ROMK toxin, tertiapin-Q, and genetic knockdown of ROMK also suppressed mitochondrial Tl+ uptake. Finally, ROMK overexpression protected cells from oxidant-induced cell death and knockdown of the native channel exacerbated death. Establishing ROMK as a pore-forming subunit of mitoKATP overcomes a significant barrier in the field of cardioprotection, and opens the door for future studies to examine the mechanism of mitoKATP action, find binding partners of the channel, and develop more potent and specific modulators of cardioprotection.
Acknowledgments
SOURCES OF FUNDING
This work was supported by P01HL081427 and R01HL108917 (B.O’R.) and P01HL36573 (K.D.G.).
We would like to thank Marjan Gucek and Robert N. Cole of the Johns Hopkins Mass Spectrometry Core Facility for their important contributions to this work. We would also like to thank Dr Eduardo Marbán for providing the inspiration and early support for this project.
Non-standard Abbreviations
- MitoKATP
Mitochondrial ATP-sensitive potassium channel
- MitoROMK
Mitochondrial renal outer medullary potassium channel
- SUR
Sulfonylurea receptor
- tBHP
tert-butyl hydroperoxide
- TPNQ
Tertiapin-Q
Footnotes
DISCLOSURES
None
References
- 1.Murry C, Jennings R, Reimer K. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 2.Kuzuya T, Hoshida S, Yamashita N, Fuji H, Oe H, Hori M, Kamada T, Tada M. Delayed effects of sublethal ischemia on the acquisition of tolerance to ischemia. Circ Res. 1993;72:1293–1299. doi: 10.1161/01.res.72.6.1293. [DOI] [PubMed] [Google Scholar]
- 3.Gross GJ, Auchampach JA. Blockade of ATP-sensitive potassium channels prevents myocardial preconditioning in dogs. Circ Res. 1992;70:223–233. doi: 10.1161/01.res.70.2.223. [DOI] [PubMed] [Google Scholar]
- 4.Yao Z, Gross GJ. Effects of the KATP channel opener bimakalim on coronary blood flow, monophasic action potential duration, and infarct size in dogs. Circulation. 1994;89:1769–1775. doi: 10.1161/01.cir.89.4.1769. [DOI] [PubMed] [Google Scholar]
- 5.Grover GJ, D’Alonzo AJ, Parham CS, Darbenzio RB. Cardioprotection with the KATP opener cromakalim is not correlated with ischemic myocardial action potential duration. J Cardiovasc Pharmacol. 1995;26:145–152. doi: 10.1097/00005344-199507000-00023. [DOI] [PubMed] [Google Scholar]
- 6.Garlid KD, Paucek P, Yarov-Yarovoy V, Murray HN, Darbenzio RB, D’Alonzo AJ, Lodge NJ, Smith MA, Grover GJ. Cardioprotective effect of diazoxide and its interaction with mitochondrial ATP-sensitive K+ channels. Possible mechanism of cardioprotection. Circ Res. 1997;81:1072–1082. doi: 10.1161/01.res.81.6.1072. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Sato T, O’Rourke B, Marbán E. Mitochondrial ATP-dependent potassium channels: novel effectors of cardioprotection? Circulation. 1998;97:2463–2469. doi: 10.1161/01.cir.97.24.2463. [DOI] [PubMed] [Google Scholar]
- 8.Sato T, Sasaki N, Seharaseyon J, O’Rourke B, Marbán E. Selective pharmacological agents implicate mitochondrial but not sarcolemmal K(ATP) channels in ischemic cardioprotection. Circulation. 2000;101:2418–2423. doi: 10.1161/01.cir.101.20.2418. [DOI] [PubMed] [Google Scholar]
- 9.Inoue I, Nagase H, Kishi K, Higuti T. ATP-sensitive K+ channel in the mitochondrial inner membrane. Nature. 1991;352:244–247. doi: 10.1038/352244a0. [DOI] [PubMed] [Google Scholar]
- 10.O’Rourke B. Evidence for mitochondrial K+ channels and their role in cardioprotection. Circ Res. 2004;94:420–432. doi: 10.1161/01.RES.0000117583.66950.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang X-M, Proctor JB, Cui L, Krieg T, Downey JM, Cohen MV. Multiple, brief coronary occlusions during early reperfusion protect rabbit hearts by targeting cell signaling pathways. J Am Coll Cardiol. 2004;44:1103–1110. doi: 10.1016/j.jacc.2004.05.060. [DOI] [PubMed] [Google Scholar]
- 12.Mykytenko J, Reeves J, Kin H, Wang N-P, Zatta A, Jiang R, Guyton R, Vinten-Johansen J, Zhao Z-Q. Persistent beneficial effect of postconditioning against infarct size: role of mitochondrial KATP; channels during reperfusion. Basic Res Cardiol. 2008;103:472–484. doi: 10.1007/s00395-008-0731-2. [DOI] [PubMed] [Google Scholar]
- 13.Garlid KD, Paucek P, Yarov-Yarovoy V, Sun X, Schindler PA. The mitochondrial KATP channel as a receptor for potassium channel openers. J Biol Chem. 1996;271:8796–8799. doi: 10.1074/jbc.271.15.8796. [DOI] [PubMed] [Google Scholar]
- 14.Debska G, Kicinska A, Skalska J, Szewczyk A, May R, Elger CE, Kunz WS. Opening of potassium channels modulates mitochondrial function in rat skeletal muscle. Biochim Biophys Acta. 2002;1556:97–105. doi: 10.1016/s0005-2728(02)00340-7. [DOI] [PubMed] [Google Scholar]
- 15.Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, Ziman BD, Wang S, Ytrehus K, Antos CL, Olson EN, Sollott SJ. Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest. 2004;113:1535–1549. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bajgar R, Seetharaman S, Kowaltowski AJ, Garlid KD, Paucek P. Identification and properties of a novel intracellular (mitochondrial) ATP-sensitive potassium channel in brain. J Biol Chem. 2001;276:33369–33374. doi: 10.1074/jbc.M103320200. [DOI] [PubMed] [Google Scholar]
- 17.Wojtovich AP, Brookes PS. The endogenous mitochondrial complex II inhibitor malonate regulates mitochondrial ATP-sensitive potassium channels: Implications for ischemic preconditioning. Biochim et Biophys Acta. 2008;1777:882–889. doi: 10.1016/j.bbabio.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foster DB, Rucker JJ, Marbán E. Is Kir6. 1 a subunit of mitoKATP? Biochem Biophys Res Comm. 2008;366:649–656. doi: 10.1016/j.bbrc.2007.11.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Das M, Parker JE, Halestrap AP. Matrix volume measurements challenge the existence of diazoxide/glibencamide-sensitive KATP channels in rat mitochondria. J Physiol. 2003;547:893–902. doi: 10.1113/jphysiol.2002.035006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanley PJ, Daut J. K(ATP) channels and preconditioning: a re-examination of the role of mitochondrial K(ATP) channels and an overview of alternative mechanisms. J Mol Cell Cardiol. 2005;39:17–50. doi: 10.1016/j.yjmcc.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Pagliarini DJ, Calvo SE, Chang B, Sheth SA, Vafai SB, Ong S-E, Walford GA, Sugiana C, Boneh A, Chen WK, Hill DE, Vidal M, Evans JG, Thorburn DR, Carr SA, Mootha VK. A Mitochondrial Protein Compendium Elucidates Complex I Disease Biology. Cell. 2008;134:112–123. doi: 10.1016/j.cell.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calvo S, Jain M, Xie X, Sheth SA, Chang B, Goldberger OA, Spinazzola A, Zeviani M, Carr SA, Mootha VK. Systematic identification of human mitochondrial disease genes through integrative genomics. Nat Genet. 2006;38:576–582. doi: 10.1038/ng1776. [DOI] [PubMed] [Google Scholar]
- 23.Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem. 2002;74:5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 24.Ho K, Nichols CG, Lederer WJ, Lytton J, Vassilev PM, Kanazirska MV, Hebert SC. Cloning and expression of an inwardly rectifying ATP-regulated potassium channel. Nature. 1993;362:31–38. doi: 10.1038/362031a0. [DOI] [PubMed] [Google Scholar]
- 25.Kondo C, Isomoto S, Matsumoto S, Yamada M, Horio Y, Yamashita S, Takemura-Kameda K, Matsuzawa Y, Kurachi Y. Cloning and functional expression of a novel isoform of ROMK inwardly rectifying ATP-dependent K+ channel, ROMK6 (Kir1.1f) FEBS letters. 1996;399:122–126. doi: 10.1016/s0014-5793(96)01302-6. [DOI] [PubMed] [Google Scholar]
- 26.Beavis AD, Brannan RD, Garlid KD. Swelling and contraction of the mitochondrial matrix. I. A structural interpretation of the relationship between light scattering and matrix volume. J Biol Chem. 1985;260:13424–13433. [PubMed] [Google Scholar]
- 27.Li XQ, Hegazy MG, Mahdi F, Jezek P, Lane RD, Garlid KD. Purification of a reconstitutively active K+/H+ antiporter from rat liver mitochondria. J Biol Chem. 1990;265:15316–15322. [PubMed] [Google Scholar]
- 28.Jaburek M, Yarov-Yarovoy V, Paucek P, Garlid KD. State-dependent inhibition of the mitochondrial KATP channel by glyburide and 5-hydroxydecanoate. J Biol Chem. 1998;273:13578–13582. [PubMed] [Google Scholar]
- 29.Jin W, Lu Z. A Novel High-Affinity Inhibitor for Inward-Rectifier K+ Channels. Biochemistry. 1998;37:13291–13299. doi: 10.1021/bi981178p. [DOI] [PubMed] [Google Scholar]
- 30.Jin W, Lu Z. Synthesis of a Stable Form of Tertiapin: A High-Affinity Inhibitor for Inward-Rectifier K+ Channels. Biochemistry. 1999;38:14286–14293. doi: 10.1021/bi991205r. [DOI] [PubMed] [Google Scholar]
- 31.Kanjhan R, Coulson EJ, Adams DJ, Bellingham MC. Tertiapin-Q Blocks Recombinant and Native Large Conductance K+ Channels in a Use-Dependent Manner. J Pharmacol Exper Ther. 2005;314:1353–1361. doi: 10.1124/jpet.105.085928. [DOI] [PubMed] [Google Scholar]
- 32.Xu W, Liu Y, Wang S, McDonald T, Van Eyk JE, Sidor A, O’Rourke B. Cytoprotective role of Ca2+-activated K+ channels in the cardiac inner mitochondrial membrane. Science. 2002;298:1029–1033. doi: 10.1126/science.1074360. [DOI] [PubMed] [Google Scholar]
- 33.Seharaseyon J, Ohler A, Sasaki N, Fraser H, Sato T, Johns DC, O’Rourke B, Marbán E. Molecular Composition of Mitochondrial ATP-sensitive Potassium Channels Probed by Viral Kir Gene Transfer. J Molec Cell Cardiol. 2000;32:1923–1930. doi: 10.1006/jmcc.2000.1226. [DOI] [PubMed] [Google Scholar]
- 34.Miki T, Suzuki M, Shibasaki T, Uemura H, Sato T, Yamaguchi K, Koseki H, Iwanaga T, Nakaya H, Seino S. Mouse model of Prinzmetal angina by disruption of the inward rectifier Kir6.1. Nat Med. 2002;8:466–472. doi: 10.1038/nm0502-466. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki M, Sasaki N, Miki T, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, Seino S, Marbán E, Nakaya H. Role of sarcolemmal KATP channels in cardioprotection against ischemia/reperfusion injury in mice. J Clin Invest. 2002;109:509–516. doi: 10.1172/JCI14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McNicholas CM, Yang Y, Giebisch G, Hebert SC. Molecular site for nucleotide binding on an ATP-sensitive renal K+ channel (ROMK2) Am J Physiol. 1996;271:F275–285. doi: 10.1152/ajprenal.1996.271.2.F275. [DOI] [PubMed] [Google Scholar]
- 37.Wojtovich AP, Williams DM, Karcz MK, Lopes CMB, Gray DA, Nehrke KW, Brookes PS. A Novel Mitochondrial KATP Channel Assay. Circ Res. 2010;106:1190–1196. doi: 10.1161/CIRCRESAHA.109.215400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bednarczyk P, Dołowy K, Szewczyk A. New properties of mitochondrial ATP-regulated potassium channels. J Bioenerg Biomem. 2008;40:325–335. doi: 10.1007/s10863-008-9153-y. [DOI] [PubMed] [Google Scholar]
- 39.McNicholas CM, MacGregor GG, Islas LD, Yang Y, Hebert SC, Giebisch G. pH-dependent modulation of the cloned renal K+ channel, ROMK. Am J Physiol. 1998;275:F972–981. doi: 10.1152/ajprenal.1998.275.6.F972. [DOI] [PubMed] [Google Scholar]
- 40.Lin D, Sterling H, Lerea KM, Giebisch G, Wang WH. Protein kinase C (PKC)-induced phosphorylation of ROMK1 is essential for the surface expression of ROMK1 channels. J Biol Chem. 2002;277:44278–44284. doi: 10.1074/jbc.M203702200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Konstas AA, Dabrowski M, Korbmacher C, Tucker SJ. Intrinsic sensitivity of Kir1.1 (ROMK) to glibenclamide in the absence of sur2b. J Biol Chem. 2002;277:21346. doi: 10.1074/jbc.M202005200. [DOI] [PubMed] [Google Scholar]
- 42.Liu X, Singh BB, Ambudkar IS. ATP-dependent activation of K(Ca) and ROMK-type K(ATP) channels in human submandibular gland ductal cells. J Biol Chem. 1999;274:25121–25129. doi: 10.1074/jbc.274.35.25121. [DOI] [PubMed] [Google Scholar]
- 43.Welling PA, Ho K. A comprehensive guide to the ROMK potassium channel: form and function in health and disease. Am J Physiol. 2009;297:F849. doi: 10.1152/ajprenal.00181.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanemoto M, Vanoye CG, Dong K, Welch R, Abe T, Hebert SC, Xu JZ. Rat homolog of sulfonylurea receptor 2B determines glibenclamide sensitivity of ROMK2 in Xenopus laevisoocyte. Am J Physiol. 2000;278:F659. doi: 10.1152/ajprenal.2000.278.4.F659. [DOI] [PubMed] [Google Scholar]
- 45.Ye B, Kroboth SL, Pu J-L, Sims JJ, Aggarwal NT, McNally EM, Makielski JC, Shi N-Q. Molecular Identification and Functional Characterization of a Mitochondrial Sulfonylurea Receptor 2 Splice Variant Generated by Intraexonic Splicing. Circ Res. 2009;105:1083–1093. doi: 10.1161/CIRCRESAHA.109.195040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rottlaender D, Boengler K, Wolny M, Michels G, Endres-Becker J, Motloch LJ, Schwaiger A, Buechert A, Schulz R, Heusch G, Hoppe UC. Connexin 43 acts as a cytoprotective mediator of signal transduction by stimulating mitochondrial KATP channels in mouse cardiomyocytes. J Clin Invest. 2010;120:1441–1453. doi: 10.1172/JCI40927. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Heinzel FR, Luo Y, Li X, Boengler K, Buechert A, Garcia-Dorado D, Di Lisa F, Schulz R, Heusch G. Impairment of diazoxide-induced formation of reactive oxygen species and loss of cardioprotection in connexin 43 deficient mice. Circ Res. 2005;97:583–586. doi: 10.1161/01.RES.0000181171.65293.65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.