Abstract
Aeromonas hydrophila is a gram-negative opportunistic pathogen in fish and humans. Many bacterial pathogens of animals and plants have been shown to inject anti-host virulence determinants into the hosts via a type III secretion system (TTSS). Degenerate primers based on lcrD family genes that are present in every known TTSS allowed us to locate the TTSS gene cluster in A. hydrophila AH-1. A series of genome walking steps helped in the identification of 25 open reading frames that encode proteins homologous to those in TTSSs in other bacteria. PCR-based analysis showed the presence of lcrD homologs (ascV) in all of the 33 strains of A. hydrophila isolated from various sources. Insertional inactivation of two of the TTSS genes (aopB and aopD) led to decreased cytotoxicity in carp epithelial cells, increased phagocytosis, and reduced virulence in blue gourami. These results show that a TTSS is required for A. hydrophila pathogenesis. This is the first report of sequencing and characterization of TTSS gene clusters from A. hydrophila. The TTSS identified here may help in developing suitable vaccines as well as in further understanding of the pathogenesis of A. hydrophila.
Aeromonas hydrophila, a normal inhabitant of the aquatic environment, is an opportunistic pathogen of a variety of aquatic and terrestrial animals, including humans (2, 41). A. hydrophila causes motile aeromonad septicemia, which is a major freshwater disease affecting aquaculture worldwide. In humans, the clinical symptoms include septicemia, wound infections, and gastroenteritis (21). A. hydrophila has been identified as an emerging cause of diarrhea, mostly in young children (1, 8). Janda and coworkers (22) also reported that A. hydrophila was responsible for 47% of septicemia cases in different regions in the United States. Therefore, the levels of A. hydrophila in drinking water and in food have been highlighted as a potential food safety issue (15).
The pathogenesis of A. hydrophila is multifactorial. These factors include O antigens, capsules (25, 47), the S layer (10), exotoxins such as hemolysins and enterotoxin (6, 19), and a repertoire of exoenzymes which digests cellular components such as proteases, amylases, and lipases (24, 27). These virulence determinants are involved sequentially in enabling the bacteria to colonize, gain entry, establish, replicate, and cause damage in host tissues and to evade the host defense system and spread, eventually killing the host. The mechanisms of action of most of these virulence factors remain unknown.
Recent studies have shown that the virulence mechanisms of various pathogens are highly similar (12). One of these mechanisms is the type III secretion system (TTSS), which plays crucial roles in host-pathogen interactions (7). The TTSS is found in many gram-negative animal and plant pathogens. This system can efficiently deliver anti-host virulence determinants into the host cells, directly interfering with and altering host processes. Recently, a fish pathogen, Aeromonas salmonicida subsp. salmonicida (referred to here as A. salmonicida), has been reported to have a functional TTSS located on a large thermolabile virulence plasmid (5, 39). Since motile aeromonads and A. salmonicida are in the same genus, it is possible that TTSSs are also present in other Aeromonas species, including A. hydrophila.
A. hydrophila AH-1 is pathogenic to several fish, such as the rainbow trout (50% lethal dose of 104.5 [J. M. Tomas, personal communication]) and blue gourami (LD50 of 105.6 [this study]). It belongs to the O:11 serotype, which is one of the four dominant serogroups (O:11, O:16, O:18, and O:34) that are associated with gastroenteritis and septicemia in clinical studies (23). In the present study, we successfully located the TTSS gene cluster in A. hydrophila AH-1 by using homology-based analysis. This is the first report of the cloning and characterization of a partial TTSS gene cluster in A. hydrophila AH-1. Further inactivation of two of the TTSS genes (aopB and aopD) resulted in a delayed cytotoxic effect on carp epithelial cells, increased phagocytosis, and reduced virulence in gourami fish, showing that a TTSS is required for A. hydrophila pathogenesis.
MATERIALS AND METHODS
Plasmids, bacterial strains, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. A. hydrophila strains were maintained on tryptic soy agar or in tryptic soy broth (Difco) at 25°C. Escherichia coli strains were maintained on Luria agar or in Luria broth (Difco) at 37°C. When required, media were supplemented with ampicillin (100 μg/ml), chloramphenicol (5 or 25 μg/ml), gentamicin (100 μg/ml), and colistin (6.25 μg/ml). The conjugal transfer of plasmids between A. hydrophila and E. coli strains was carried out by plate mating at 30°C for 36 h.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype and/or relevant propertya | Sourceb or reference |
|---|---|---|
| A. hydrophila strains | ||
| Aer-19 | O:11, virulent | Human, CDHS |
| Aer-27 | Virulent | Human, CDHS |
| Aer-184 | O:34, virulent | Human, CDHS |
| Aer-186 | O:16, virulent | Human, CDHS |
| Aer-205 | O:11, virulent | Human, CDHS |
| Aer-211 | O:18, virulent | Human, CDHS |
| ATCC 7966 | O:1, type strain | Milk, ATCC |
| AH-1 | O:11, virulent, Colr Cms | Fish, UM |
| aopB mutant | aopB insertion mutant from AH-1 | This study |
| aopD mutant | aopD insertion mutant from AH-1 | This study |
| AH-3 | O:34, virulent | Fish, UB |
| Ba5 | O:34, virulent | Fish, UM |
| JCM3968 | O:6 | Snake, JCM |
| JCM3973 | O:11 | Unknown, JCM |
| JCM3976 | O:14 | Frog, JCM |
| JCM3978 | O:16 | Unknown, JCM |
| JCM3980 | O:18 | Unknown, JCM |
| JCM3981 | O:19 | Unknown, JCM |
| JCM3983 | O:21 | Unknown, JCM |
| JCM3984 | O:22 | Unknown, JCM |
| JCM3985 | O:23 | Unknown, JCM |
| JCM3996 | O:34 | Unknown, JCM |
| L15 | O:51, avirulent | Fish, BAU |
| L31 | O:91, virulent | Fish, BAU |
| L36 | O:36, avirulent | Fish, BAU |
| LL1 | O:11, virulent | Fish, UM |
| PPD35/85 | O:7, avirulent | Fish, AVA |
| PPD11/90 | O:21, virulent | Fish, AVA |
| PPD64/90 | O:34, avirulent | Fish, AVA |
| PPD88/90 | O:16, avirulent | Fish, AVA |
| PPD45/91 | Avirulent | Fish, AVA |
| PPD70/91 | O:5, virulent | Fish, AVA |
| PPD122/91 | O:11, virulent | Fish, AVA |
| PPD134/91 | O:18, virulent | Fish, AVA |
| Xs91/4/1 | Virulent | Fish, China |
| E. coli strains | ||
| JM109 | Cols Amps | Promega |
| MC1061(λpir) | (λpir) thi thr-1 leu6 proA2 his-4 argE2 lacY1 galK2 ara14 xyl5 supE44, λpir | 34 |
| S17-1(λpir) | thi pro hsdR hsdM+recA[RP42-Tc::Mu-Km::Tn7 (Tpr Smr)Tra+] | 37 |
| Plasmids | ||
| pGEM-T Easy vector | Cloning vector; Apr | Promega |
| pFS100 | pGP704 suicide plasmid, λpir dependent; Kmr | 34 |
| pCM100 | pGP704 suicide plasmid, λpir dependent; Cmr | This study |
| pCM-AOPB | pCM with an internal fragment of aopB (605 bp) | This study |
| pCM-AOPD | pCM with an internal fragment of aopD (565 bp) | This study |
Virulent strains were defined as having a lower LD50 in blue gourami or rainbow trout (<106.5) than the avirulent strains (>107.5).
ATCC, American Type Culture Collection; AVA, Agri-Food and Veterinary Authority, Singapore; BAU, Bogor Agricultural University of Indonesia; CDHS, California Department of Health Services; JCM, Japan Collection of Microorganisms; UB, University of Barcelona; UM, University of Montreal.
DNA manipulations and Southern hybridization.
Bacterial genomic DNA was extracted as described in the manuals for the genomic DNA isolation and purification kits from Promega. Plasmid DNA was extracted by using the QIAprep spin miniprep or Qiagen plasmid midi kit. Restriction endonuclease digestion was accomplished by standard methods (35). Southern blotting was performed with the digoxigenin DNA labeling kit (Roche Diagnostics GmbH). Transfers of DNA to nylon membranes (GeneScreen; NEN Research Products), hybridization conditions, and visualization were as recommended by the manufacturer's protocol.
PFGE.
A. hydrophila AH-1 cells were embedded in low-melting-point agarose (Bio-Rad) to prepare agar plugs by using the contour-clamped homogeneous electric field genomic DNA plug kit (Bio-Rad) according to the manufacturer's instructions. For PacI restriction digestion, agarose plugs were equilibrated with 500 μl of suitable restriction enzyme buffer for 30 min, exchanged with 400 μl of fresh buffer containing 10 U of PacI restriction enzyme, and incubated overnight at 37°C. DNA fragments were separated by using a Bio-Rad CHEF-DRII apparatus. Electrophoresis was carried out at 200 V and 14°C for 22 h, with pulse times ranging from 5 to 60 s. For detecting and determining the sizes of large plasmids in A. hydrophila AH-1, plugs were incubated with S1 nuclease and subjected to pulsed-field gel electrophoresis (PFGE) as described previously (3).
DNA sequencing and sequence analysis.
DNA sequencing was carried out on an Applied Biosystems PRISM 3100 genetic analyzer with an ABIPRISM BigDye terminator cycle sequencing kit (Applied Biosystems). The sequences were edited by using the manufacturer's software. Sequence assembly and further editing were carried out with Vector NTI DNA analysis software (InforMax). BLASTN, BLASTP, and BLASTX sequence homology analyses and a protein conserved-domain database analysis were performed by using the BLAST network server of the National Center for Biotechnology Information. Terminator programs from the Genetics Computer Group package on a VAX4300 were used to predict terminator sequences in the TTSS gene cluster.
Genome walking and general PCR analysis.
Advantage polymerase 2 (Clontech) was used for genome walking and general PCR. PCRs were carried out under the following conditions: one hold at 94°C for 2 min, followed by 32 cycles of 94°C for 20 s, 60°C for 30 s, and 72°C for 1 min. Genome walker libraries were constructed by using five restriction enzymes (DraI, EcoRV, PvuII, ScaI, and StuI). The cycling parameters for genome walking were as follows: 7 cycles of 15 s at 94°C and 4 min at 72°C, followed by 32 cycles of 15 s at 94°C and 3 min at 67°C. The amplified fragments were cloned into the pGEM-T Easy vector (Promega). The recombinant DNA molecules were transformed into competent E. coli JM109 cells and sequenced.
Construction of defined insertion mutants.
Mutants with defined insertions in aopB and aopD were constructed by using the protocol described earlier (34) with slight modifications. For better selection of insertion mutants of A. hydrophila AH-1, we removed the kanamycin resistance cassette in pFS100 and replaced it with the chloramphenicol resistance (Cmr) cassette from pACYC184 and generated the plasmid pCM100. Then, oligonucleotides aopB-F (5′-GTGGATATCTTGATCAATTGAGGAAGACGG-3′) and aopB-R (5′-GGAGATATCGGTACCAATATCAACTACCAG-3′) and oligonucleotides aopD-F (5′-GAAGATATCGATTCGAGCCTGCTGAGCAA-3′) and aopD-R (5′-GGAGATATCGCACTTCATCTTCCTTGGCATT-3′) were used to amplify internal fragments from the aopB and aopD genes, respectively. Amplified fragment was ligated to pGEM-T Easy vector (Promega) and transformed into E. coli JM109. The internal fragment was recovered by EcoRV restriction digestion (EcoRV sites in the primers are underlined) and finally ligated to EcoRV-digested and dephosphorylated pCM100 and transformed into E. coli MC1061(λpir), selecting Cmr to generate plasmid pCM-AOPB and pCM-AOPD (Table 1). The recombinant plasmid was isolated and transformed into E. coli S17-1(λpir). Plasmids pCM-AOPB and pCM-AOPD were transferred by conjugation to A. hydrophila AH-1 (colistin resistant [Colr]) to obtain defined mutants by selecting Cmr and Colr, and aopB and aopD insertion mutants, respectively, were generated. The insertion of plasmids on the chromosomes of these mutants was confirmed by both PCR with appropriate primers and Southern blot analysis with pCM100 suicide vectors, aopB, and aopD as probes.
Cell culture and morphological changes induced by A. hydrophila.
All tissue culture reagents were obtained from Invitrogen. Epithelioma papillosum of carp (Cyprinus carpio) (EPC cells) (44) was grown in minimal essential medium with Hanks salts, 10 mM HEPES (pH 7.3), 2 mM glutamine, 0.23% NaHCO3, and 10% heat-inactivated fetal bovine serum (FBS) at 25°C in a 5% (vol/vol) CO2 atmosphere. Cells were grown in 75-cm2 flasks and split at least once a week by trypsin-EDTA treatment and dilution at 1:10 in fresh medium. Studies on morphological changes were conducted by seeding 5 × 105 fish cells into each well of a 24-well tissue culture plate (Falcon) and then proceeding as described previously (40). EPC monolayers were infected with A. hydrophila cells suspended in phosphate-buffered saline (PBS) (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.4 mM KH2PO4 [pH 7.2]) at a multiplicity of infection (MOI) (number of bacteria per cultured cell) of 1. Changes in the cell morphology were observed over a period of 6 h. The fish cells were photographed under an Axiovert 25CFL phase-contrast inverted microscope (Carl Zeiss) at a magnification of ×40 after 2.5 and 5.5 h of infection at 25°C.
Phagocyte isolation.
Healthy blue gourami (Trichogaster trichopterus Pallas) were obtained from a commercial fish farm and maintained in well-aerated, dechlorinated water at 25 ± 2°C. Phagocytes were isolated from the head kidney of naive gourami and purified by the procedure of Secombs (36). Purified phagocytic cells (4 × 106 to 5 × 106 cells/well) were allowed to adhere to 24- or 48-well tissue culture plates (Falcon) in L-15 medium (Sigma) supplemented with 5% FBS. After 3 h of incubation at 25°C in a 5% CO2 atmosphere, the cells were washed twice with Hanks balanced salt solution (HBSS) (Sigma) to remove unattached cells. The remaining monolayer of phagocytes was infected with the wild-type and mutant strains of A. hydrophila at an MOI of 10 in all of the experiments.
Microscopic examination and phagocytosis assay.
Glass coverslips were placed into each well of a 24-well tissue culture plate, and the wells were seeded with blue gourami phagocytes and incubated for 3 h at 25°C in a 5% CO2 atmosphere as described above. Three hours after infection, the phagocytes were washed three times with HBSS and later stained with Giemsa stain (Merck) for 30 min. After they were washed three times with PBS, the stained samples were examined under an Axiovert 25CFL phase-contrast inverted microscope (Carl Zeiss) at a magnification of ×100. For the phagocytosis assay, the phagocytes were infected for 3 h, washed twice with HBSS, and then incubated for 1.5 h in fresh L-15 medium supplemented with 5% FBS and 100 μg of gentamicin per ml. The gentamicin treatment killed extracellular bacteria but did not affect the viability of intracellular organisms. The phagocytes were then washed three times with HBSS to remove gentamicin and lysed with 1% (vol/vol) Triton X-100. This was followed by a 1-min incubation, which released intracellular bacteria. Intracellular bacteria were quantified on triplicate tryptic soy agar plates. The percentage of phagocytosis was calculated by dividing the viable bacterial count after gentamicin treatment by the initial bacterial count. Three independent experiments with duplicate wells were performed.
Studies of LD50 in fish.
Healthy blue gourami were obtained from a commercial fish farm, maintained in well-aerated dechlorinated water at 25 ± 2°C, and acclimatized to the laboratory conditions for at least 15 days. The fish were approximately 13 g each and were about 3 months old. Three groups of ten fish each were injected intramuscularly with 0.1 ml of PBS-washed bacterial cells adjusted to the required concentrations. The fish were monitored for mortality for 7 days, and LD50s were calculated by the method of Reed and Muench (31).
Statistical analysis.
All data were expressed as means ± standard errors of the means. Data were analyzed by a one-way analysis of variance and a Duncan multiple-range test (SAS software; SAS Institute). P values of <0.05 were considered significant.
Nucleotide sequence accession number.
The nucleotide sequence data for the A. hydrophila AH-1 TTSS gene cluster have been deposited in GenBank under accession no. AY394563.
RESULTS AND DISCUSSION
Sequencing and genetic organization of a TTSS gene cluster in AH-1.
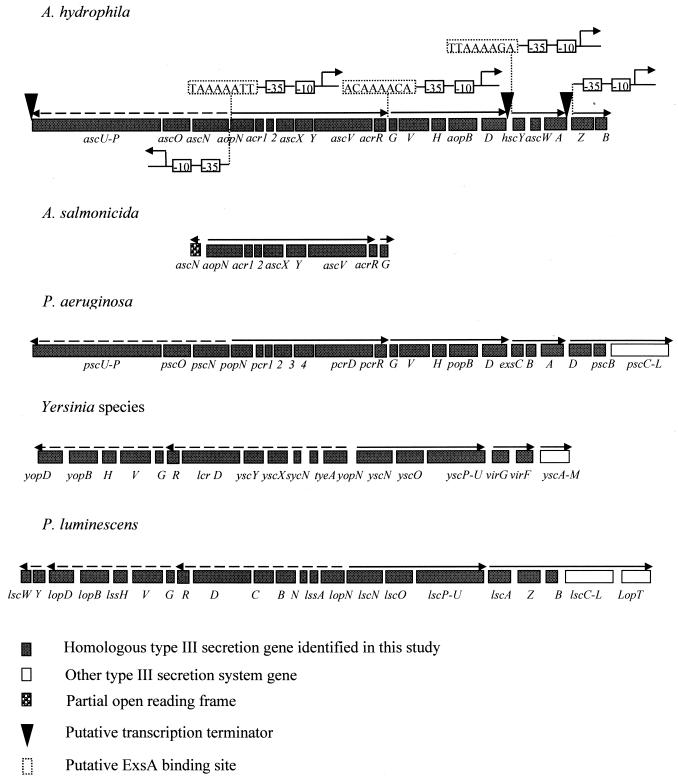
TTSSs are present in many gram-negative bacteria (13, 20). Various components of the type III secretion genes are highly conserved. lcrD homologs are one of those components that are present in all known TTSSs, and sequence similarities between individual members of the LcrD family vary between 36 and 66% (20). Multiple-sequence alignment of proteins of the LcrD family from A. salmonicida (5), Pseudomonas aeruginosa (46), and Yersinia enterocolitica (accession no. AF102990) helped in locating two regions which are highly conserved at both the amino acid and nucleotide levels. A pair of degenerate primers (ascV-F [5′-GTAARCAGATGAGTATCGATGG-3′] and ascV-R [5′-GAGACSCGGGTGACGATAAT-3′]) were designed, and the truncated lcrD-homologous gene (renamed ascV in this study; 331 bp in size) was successfully amplified in A. hydrophila AH-1. The 331-bp fragment showed 92 and 99% identity to the ascV gene of A. salmonicida at the nucleotide and amino acid levels, respectively. Subsequently, the TTSS gene cluster was partially sequenced by a series of genome walking steps. This led to the identification of 25 open reading frames (ORFs) whose products showed high homology with the TTSS proteins in A. salmonicida (5), Photorhabdus luminescens (42), P. aeruginosa (46), and Yersinia species (4, 26) (Table 2). Most of the putative TTSS proteins in A. hydrophila were designated according to the Yersinia nomenclature, which was also used to designate the ORFs of the A. salmonicida TTSS (Table 2). The genetic organization of this cluster is highly similar to that of P. aeruginosa (Fig. 1). It is also similar to those of A. salmonicida, P. luminescens, and Yersinia species but differs from the gene order in P. luminescens and Yersinia species, where the homologous ascU-aopD regions are inverted.
TABLE 2.
Putative A. hydrophila AH-1 proteins and their homologs in other bacteria
| A. hydrophila protein | Homolog (I/S)a in:
|
Putative function | |||
|---|---|---|---|---|---|
| A. salmonicida | P. luminescens | Yersinia spp. | P. aeruginosa | ||
| AscU | LscU (75/84) | YacU (69/81) | PscU (68/81) | Regulation of secretion | |
| AscT | LscT (54/63) | YscT (51/62) | Unknown | ||
| AscS | LscS (82/90) | YscS (77/89) | PscS (78/90) | Unknown | |
| AscR | LscR (78/87) | YscR (77/84) | PscR (79/87) | Unknown | |
| AscQ | LscQ (47/58) | YscQ (44/60) | PscQ (47/59) | Unknown | |
| AscP | LscP (36/53) | YscP (39/57) | PscP (34/51) | Regulation of secretion | |
| AscO | LscO (40/47) | YscO (37/53) | PscO (42/53) | Regulation of secretion | |
| AscN | AscN (NDb) | LscN (90/90) | YscN (87/90) | PscN (83/90) | ATP synthase |
| AopN | AopN (86/91) | LopN (66/78) | YopN (51/66) | PopN (59/74) | Regulation of translocation |
| Acr1 | Acr1 (90/94) | LssA (71/86) | TyeA (54/74) | Pcr1 (57/75) | Translocation apparatus |
| Acr2 | Acr2 (81/89) | LssN (63/78) | SycN (60/75) | Pcr2 (46/62) | Chaperone |
| AscX | AscX (80/89) | LssB (59/73) | YscX (50/70) | Pcr3 (46/61) | Type III secretion apparatus |
| AscY | AscY (58/62) | LssC (46/52) | YscY (37/43) | Pcr4 (46/54) | Type III secretion apparatus |
| AscV | AscV (86/88) | LssD (78/84) | LcrD (76/82) | PcrD (74/82) | Type III secretion apparatus |
| AcrR | AcrR (60/70) | LssR (51/64) | LcrR (58/74) | PcrR (47/61) | Unknown |
| AcrG | AcrG (43/56) | LssG (43/56) | LcrG (47/63) | PcrG (46/55) | Regulation of low-calcium response |
| AcrV | LssV (42/59) | LcrV (41/63) | PcrV (32/48) | Protective antigen, anti-host factor | |
| AcrH | LssH (58/69) | LcrH (61/75) | PcrH (61/73) | Chaperone | |
| AopB | LopB (36/50) | YopB (32/48) | PopB (36/51) | Translocation apparatus | |
| AopD | LopD (49/66) | YopD (45/61) | PopD (47/64) | Translocation apparatus | |
| HscY | LscY (67/80) | ExsC (56/80) | Unknown | ||
| AscW | LscW (38/58) | YscW (35/55) | ExsB (33/52) | Regulation of secretion | |
| AscA | LscA (75/82) | VirF (62/76) | ExsA (64/77) | Transcriptional activator | |
| AscZ | LscZ (41/54) | ExsD (33/51) | Unknown | ||
| AscB | LscB (54/67) | YscB (45/64) | PscB (44/60) | Unknown | |
I, percent identity; S, percent similarity.
ND, not determined due to incomplete sequence.
FIG. 1.
Genetic organizations of TTSSs in A. hydrophila and other bacteria. Arrows indicate the proposed directions of transcription.
Further sequence analysis of this TTSS gene cluster identified five putative promoter regions (Fig. 1), three of which possess ExsA binding sites (TxAAAAxA) that are similar to those in A. salmonicida (5) and P. aeruginosa (18). In P. aeruginosa, these consensus sequences are bound by ExsA, a transcriptional activator of exoenzyme S regulon (18). These three promoters are located upstream of aopN, acrG, and hscY. We also have identified AscA, which shows 77% similarity to ExsA (Table 2) and which may regulate the transcription of these operons in the AH-1 TTSS cluster. Further work is required to confirm this hypothesis. The other two putative promoters are upstream of ascN and ascZ, without the characteristic ExsA consensus sequences being identified. Terminator analysis showed the presence of three possible transcription terminators. They are downstream of ascU (ΔG, −3.30 kcal/mol), aopD (ΔG, −18.60 kcal/mol), and ascA (ΔG, −6.40 kcal/mol) (Fig. 1). Based on the analysis described above, we speculate that there may be five operons in the AH-1 TTSS gene cluster. They are ascN to ascU, aopN to aopD, acrG to aopD, hscY to ascA, and ascZ and beyond.
The TTSS is located on the AH-1 chromosome.
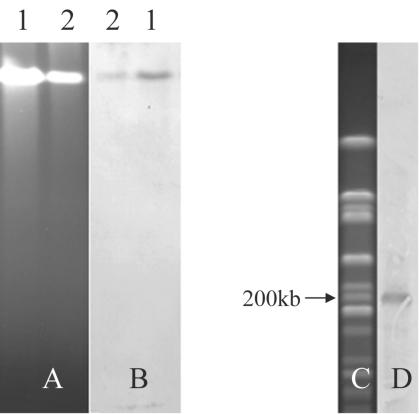
TTSS gene clusters can be either on a plasmid or on the bacterial chromosome (30). PFGE of bacterial DNA of A. hydrophila AH-1 was carried out to investigate whether it contained any plasmids. The PFGE results showed that the DNA cut with S1 nuclease was brighter than the uncut DNA (Fig. 2A). Dodd and Pemberton (9) also reported that the treatment of A. hydrophila JMP636 genomic DNA with S1 nuclease produced a highly intense band compared to untreated DNA, indicating the digestion of circular DNA by S1 nuclease. PFGE of an S1 nuclease-treated A. hydrophila AH-1 genomic DNA plug showed only one band as an uncut genomic DNA, indicating that there may not be a large plasmid in AH-1. We further prepared a genomic DNA plug digested with PacI and carried out Southern blot analysis with the ascV probe. The results revealed that the ascV probe hybridized with S1 nuclease-cut genomic DNA, uncut genomic DNA, and a 200-kb PacI-digested fragment (Figs. 2B and D). A 20-kb plasmid was detected by using a plasmid purification kit which was capable of purifying plasmids of up to 150 kb. This small plasmid was not detected in PFGE, which could be due to the low copy number of this plasmid. ascV probe did not hybridize with this 20-kb plasmid (data not shown). These results indicate that the TTSS gene cluster is located on the chromosome of A. hydrophila AH-1, which is similar to the case for other pathogens such as P. aeruginosa (46) and P. luminescens (42). A. hydrophila AH-1 is unlike A. salmonicida, in which the TTSS is located on a large thermolabile plasmid of approximately 140 kb (39).
FIG. 2.
Location of the ascV gene as determined by PFGE and Southern blot analysis. (A) PFGE of S1 nuclease-treated (lane 1) and intact (lane 2) genomic plugs. (B) Southern blot analysis with ascV as a probe against the gel in panel A. (C and D) Gel showing a PacI-digested genomic DNA plug from AH-1 (C) and Southern blot with ascV as a probe against the gel in panel C.
Distribution of TTSSs in A. hydrophila.
In several gram-negative pathogens, TTSSs are encoded by pathogenicity islands, which are present in virulent strains but absent from avirulent strains (7, 43). Hence, the distribution of TTSS gene clusters among 33 A. hydrophila strains isolated from different sources, such as fish and humans, from various geographical locations was surveyed (Table 1). These isolates represented diverse serotypes with various levels of virulence. The same pair of degenerate primers (ascV-F and ascV-R) was used to detect the ascV gene in all 33 strains. PCR results showed that a 331-bp DNA fragment was present in all of the A. hydrophila strains. FlhA, an essential component of the flagellar export apparatus, has also shown some similarity to the LcrD family protein in the TTSS (20). Hence, DNA sequencing was performed to confirm that these 33 PCR products were highly similar to ascV but not to flhA. All of the sequences showed high identity to ascV of A. hydrophila AH-1, with amino acid and nucleotide identities ranging from 83 to 100% and from 73 to 99%, respectively. Our results indicate, therefore, that a partial or an entire TTSS gene cluster may be present in all 33 A. hydrophila strains that we examined.
Similarly, the TTSS has also been shown to be ubiquitously present in both clinical and environmental isolates of P. aeruginosa (11). Hence, the presence of TTSS genes may not correlate with virulence for some of the bacteria. On the other hand, the TTSSs in nonpathogenic strains of A. hydrophila may not be functional. Pierson and Falkow (29) have reported the presence of nonfunctional invA-homologous genes in nonpathogenic Y. enterocolitica.
Construction of mutants and LD50 studies.
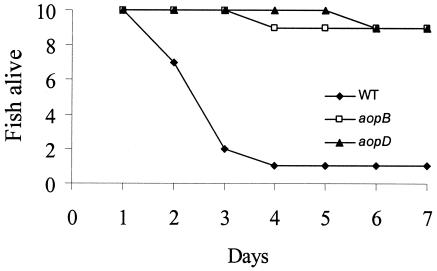
To ascertain that the TTSS in A. hydrophila AH-1 was functional, we carried out insertional mutagenesis of aopB and aopD. They are the homologs of yopB and yopD, respectively, in Y. enterocolitica, which may form the translocation apparatus for functional type III secretion (20). Disruptions in yopB and/or yopD reduced virulence in mice (16, 17). In A. hydrophila AH-1, both aopB and aopD mutants showed growth rates in tryptic soy broth that were similar to that of the wild type (data not shown). The LD50 of AH-1 by intramuscular injection into blue gourami was estimated to be 105.6, while those of the aopB and aopD mutants were 106.6 and 106.4, respectively, which was about 1 log unit higher than that of the wild type. When blue gourami were inoculated with the same dosage (106), most of the fish injected with aopB and aopD mutants recovered within 4 days and displayed no marked skin ulceration at the injection site. However, 8 out of 10 fish injected with the wild type died within 3 days (Fig. 3). To confirm the stability of the insertional aopB and aopD mutant genes, bacteria were isolated from live or dead fish inoculated with these mutants, all of which showed resistance to chloramphenicol. PCR with appropriate primers for these mutants also confirmed the stability of these mutants. These results strongly indicate that the TTSS plays an important role in the pathogenesis of A. hydrophila.
FIG. 3.
aopB and aopD mutants show a decrease in the pathogenesis of blue gourami infections. The number of fish alive after being injected with 106 CFU of A. hydrophila is plotted against the number of days that it took for the fish to die. The fish were monitored for 7 days. WT, wild-type AH-1.
Delayed cytotoxic effect of aopB and aopD mutants on EPC cells.
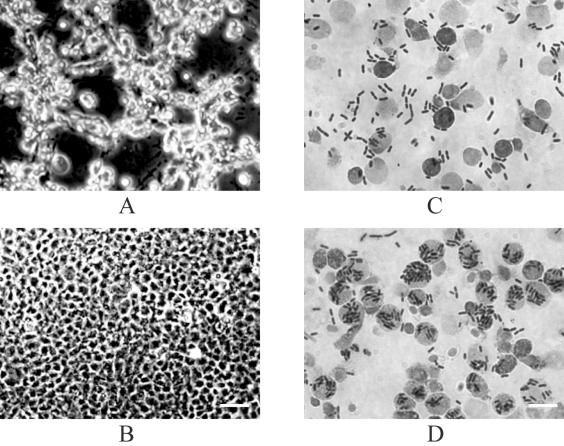
EPC cells were infected with AH-1 and the aopB and aopD mutants. Upon infection with A. hydrophila AH-1, the EPC cells underwent a series of cytopathic changes similar to those of another strain, PPD134/91, as described in an earlier study (40). The cells infected with AH-1 progressively detached from one another, elongated to form long spindles, became rounded, and eventually detached from one another and also from the well. At 2.5 h postinfection, approximately 50% of the rounded EPC cells remained attached to the tissue culture plate (Fig. 4A), while EPC cells infected with either the aopB or aopD mutant showed no significant morphological changes (Fig. 4B) compared to the uninfected control (data not shown). An uninfected monolayer of EPC cells appeared as a smooth sheet with the cells adhering tightly to their neighbors. EPC cells infected with the aopB or aopD mutant started to show morphological changes at 3 h postinfection. By 5.5 h postinfection, only 50% of the rounded EPC cells remained. Mutation of aopB or aopD therefore delayed cytotoxic changes to the EPC cells. There may be other factors mediating cytotoxicity in A. hydrophila. Virulence factors such as aerolysin and hemolysin (45) and serine protease (32) have been reported to be involved in the cytotoxicity for different cultured cells. This may explain the delayed onset of the cytotoxic effect of aopB and aopD mutants of AH-1.
FIG. 4.
Micrographs of EPC cells (A and B) and blue gourami phagocytes (C D) infected with A. hydrophila AH-1 (A and C) and the aopB mutant (B and D). Phase-contrast micrographs of EPC monolayers were infected with A. hydrophila strains at 2.5 h postinfection (MOI of 1). Giemsa-stained bright-field micrographs of blue gourami phagocytes were infected with A. hydrophila strains at 3 h postinfection (MOI of 10). The aopB and aopD mutants had similar results in both EPC cell and phagocyte experiments. Bars, 50 μm (B) and 10 μm (D); panels A and C are at the same magnifications as panels B and D, respectively.
Phagocytosis assay.
Phagocytes are the primary defense barriers in any host, and bacteria have to either avoid or overcome the phagocyte-mediated killing to establish themselves in the host. We carried out a microscopic examination of phagocytes infected with AH-1 and the mutants. After 3 h of infection with AH-1, most of the bacteria were outside the phagocytes, with a few of them inside (Fig. 4C). On the other hand, most of the aopB and aopD mutants were inside the phagocytes (Fig. 4D). These results may indicate that the wild-type (AH-1) bacteria may avoid or inhibit phagocytosis but that the mutants fail to avoid phagocytosis, thereby becoming ingested.
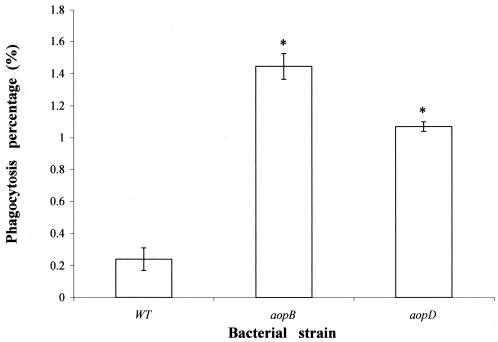
To analyze further whether the wild-type bacteria resist phagocytosis, an internalization assay was carried out. After infection of the phagocytes with AH-1 and the aopB and aopD mutants for 3 h, the monolayer was treated with gentamicin for 1.5 h to kill all of the extracellular bacteria. The results clearly showed that the mutants had a 4- to 6-times-higher ingestion rate than the wild type (Fig. 5). Wild-type AH-1 was resistant to phagocytosis, with only 0.24% of bacteria within the phagocytes. However, the aopD and aopB mutants showed increased phagocytosis, with 1.1 and 1.5% of bacteria within the phagocytes, respectively. These results indicate that mutations in aopB and aopD affect the phagocytosis and may play an important role in antiphagocytosis.
FIG. 5.
Phagocytosis assay. The percent phagocytosis of gourami phagocytes was calculated after infection with wild-type A. hydrophila (AH-1) (WT) or mutants (aopB and aopD). Results are expressed as the representative mean ± standard error of the mean from duplicate wells in triplicate experiments. Asterisks indicate a significant difference from results obtained with A. hydrophila AH-1 (P < 0.05).
In Y. enterocolitica, YopB and YopD, the homologs of AopB and AopD, have also been shown to be essential for cytotoxicity and antiphagocytosis (16, 17). They may form the pore on the host cell membrane to deliver effectors such as YopE and YopH, which cause cytotoxicity and antiphagocytosis, to the respective target host cells (14, 28, 33, 38). From the results of cell culture assays, we speculate that AopB and AopD of AH-1 may acting in a manner similar to those of Y. enterocolitica. The TTSS of AH-1 may also use AopB and AopD as translocon components to deliver similar effectors into host cells, mediating antiphagocytosis and cytotoxicity. The natures of these effectors produced by AH-1 are being investigated. These results will help in a better understanding of the roles played by the TTSS in A. hydrophila pathogenesis.
Conclusions.
This is the first report on sequencing and characterization of a TTSS gene cluster in A. hydrophila. The products of most ORFs of this gene cluster showed high homology to TTSS proteins of other pathogens, such as A. salmonicida, P. aeruginosa, and Yersinia species, indicating that they may have evolved from a common ancestor. The detection of ascV by PCR and sequencing analysis with 33 A. hydrophila strains revealed that the TTSS may be present in all of the strains we examined, irrespective of their pathogenic or nonpathogenic nature. Ongoing work is being conducted to see whether the functional TTSS is limited to disease but not environmental isolates of A. hydrophila. Insertional inactivation of aopB or aopD delayed the cytotoxic effect in EPC cells and increased uptake by phagocytes significantly. LD50 assays of mutants also showed about 1-log-unit increase in LD compared to the wild type. The above-mentioned biological activities clearly demonstrate that the TTSS is functional in A. hydrophila AH-1. Complementation of aopB and aopD mutants and creation of mutants with mutations in other TTSS genes will help in understanding their roles in cytotoxicity, antiphagocytosis, and virulence in fish. The identification of a TTSS in A. hydrophila is an important discovery for unlocking the pathogenesis of this bacterium. This will allow us to understand the intimate host-bacterium interactions in order to develop suitable strategies to overcome diseases caused by A. hydrophila.
Acknowledgments
This work was supported in part by the Biomedical Research Council of Singapore (BMRC), A*STAR, to K. Y. Leung and by the Plan Nacional de I + D grants (Ministerio de Ciencia y Tecnología, Spain) and Generalitat de Catalunya to J. M. Tomas.
We are grateful to Michael Janda, California Department of Health Services, for providing us some of the A. hydrophila isolates and to X. H. Wang and Y. L. Lau for their help with cell culture and PFGE.
Editor: B. B. Finlay
REFERENCES
- 1.Agger, W. A., J. D. McCormick, and M. J. Gurwith. 1985. Clinical and microbiological features of Aeromonas hydrophila-associated diarrhea. J. Clin. Microbiol. 21:909-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Austin, B., and C. Adams. 1996. Fish pathogens, p. 197-243. In B. Austin, M. Altwegg, P. J. Gosling, and S. W. Joseph (ed.), The genus Aeromonas. John Wiley and Sons, New York, N.Y.
- 3.Barton, B. M., G. P. Harding, and A. J. Zuccarelli. 1995. A general method for detecting and sizing large plasmids. Anal. Biochem. 226:235-240. [DOI] [PubMed] [Google Scholar]
- 4.Bergman, T., K. Erickson, E. Galyov, C. Persson, and H. Wolf-Watz. 1994. The lcrB (yscN/U) gene cluster of Yersinia pseudotuberculosis is involved in Yop secretion and shows high homology to the spa gene clusters of Shigella flexneri and Salmonella typhimurium. J. Bacteriol. 176:2619-2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burr, S. E., K. Stuber, T. Wahli, and J. Frey. 2002. Evidence for a type III secretion system in Aeromonas salmonicida subsp. salmonicida. J. Bacteriol. 184:5966-5970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakraborty, T., M. A. Montenegro, S. C. Sanyal, R. Helmuth, E. Bulling, and K. N. Timmis. 1984. Cloning of enterotoxin gene from Aeromonas hydrophila provides conclusive evidence of production of a cytotoxic enterotoxin. Infect. Immun. 46:435-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cornelis, G. R., and F. Van Gijsegem. 2000. Assembly and function of type III secretory systems. Annu. Rev. Microbiol. 54:735-774. [DOI] [PubMed] [Google Scholar]
- 8.Deodhar, L. P., K. Saraswathi, and A. Varudkar. 1991. Aeromonas species and their association with human diarrheal disease. J. Clin. Microbiol. 29:853-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dodd, H. N., and J. M. Pemberton. 1998. Construction of a physical and preliminary genetic map of Aeromonas hydrophila JMP636. Microbiology 144:3087-3096. [DOI] [PubMed] [Google Scholar]
- 10.Dooley, J. S. G., and T. J. Trust. 1988. Surface protein composition of Aeromonas hydrophila strains virulent for fish: identification of a surface array protein. J. Bacteriol. 170:499-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feltman, H., G. Schulert, S. Khan, M. Jain, L. Peterson, and A. R. Hauser. 2001. Prevalence of type III secretion genes in clinical and environmental isolates of Pseudomonas aeruginosa. Microbiology 147:2659-2669. [DOI] [PubMed] [Google Scholar]
- 12.Finlay, B. B., and S. Falkow. 1997. Common themes in microbial pathogenicity revisited. Microbiol. Mol. Biol. Rev. 61:136-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galan, J. E., and A. Collmer. 1999. Type III secretion machines: bacterial devices for protein delivery into host cells. Science 284:1322-1328. [DOI] [PubMed] [Google Scholar]
- 14.Håkansson, S., K. Schesser, C. Persson, E. E. Galyov, R. Rosqvist, F. Homble, and H. Wolf-Watz. 1996. The YopB protein of Yersinia pseudotuberculosis is essential for the translocation of Yop effector proteins across the target cell plasma membrane and displays a contact dependent membrane disrupting activity. EMBO J. 15:5812-5823. [PMC free article] [PubMed] [Google Scholar]
- 15.Handfield, M., P. Simard, M. Couillard, and R. Letarte. 1996. Aeromonas hydrophila isolated from food and drinking water: hemagglutination, hemolysis, and cytotoxicity for a human intestinal cell line (HT-29). Appl. Environ. Microbiol. 62:3459-3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartland, E. L., A. M. Bordun, and R. M. Robin-Browne. 1996. Contribution of YopB to virulence of Yersinia enterocolitica. Infect. Immun. 64:2308-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartland, E. L., S. P. Green, W. A. Phillips, and R. M. Robins-Browne. 1994. Essential role of YopD in inhibition of the respiratory burst of macrophages by Yersinia enterocolitica. Infect. Immun. 62:4445-4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hovey, A. K., and D. W. Frank. 1995. Analyses of the DNA-binding and transcriptional activation properties of ExsA, the transcriptional activator of the Pseudomonas aeruginosa exoenzyme S regulon. J. Bacteriol. 177:4427-4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howard, S. P., S. Macintyre, and J. T. Buckley. 1996. The genus Aeromonas, p. 267-286. In B. Austin, M. Altwegg, P. J. Gosling, and S. Joseph (ed.), Toxin. John Wiley and Sons, Singapore.
- 20.Hueck, C. J. 1998. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62:379-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janda, J. M. 2001. Aeromonas and Plesiomonas, p. 1237-1270. In M. Sussman (ed.), Molecular medical microbiology. Academic Press, New York, N.Y.
- 22.Janda, J. M., L. S. Guthertz, R. P. Kokka, and T. Shimada. 1994. Aeromonas species in septicemia: laboratory characteristics and clinical observations. Clin. Infect. Dis. 19:77-83. [DOI] [PubMed] [Google Scholar]
- 23.Kokka, R. P., J. M. Janda, L. S. Oshiro, M. Altwegg, T. Shimada, R. Sakazaki, and D. J. Brenner. 1991. Biochemical and genetic characterization of autoagglutinating phenotypes of Aeromonas species associated with invasive and noninvasive disease. J. Infect. Dis. 163:890-894. [DOI] [PubMed] [Google Scholar]
- 24.Leung, K. Y., and R. M. W. Stevenson. 1988. Tn5-induced protease-deficient strains of Aeromonas hydrophila with reduced virulence for fish. Infect. Immun. 56:2639-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merino, S., X. Rubires, A. Aguillar, J. F. Guillot, and J. M. Tomas. 1996. The role of the O-antigen lipopolysaccharide on the colonization in vivo of the germfree chicken gut by Aeromonas hydrophila serogroup O:34. Microb. Pathog. 20:325-333. [DOI] [PubMed] [Google Scholar]
- 26.Michiels, T., J. C. Vanooteghem, C. Lambert de Rouvroit, B. China, A. Gustin, P. Boudry, and G. R. Cornelis. 1991. Analysis of virC, an operon involved in the secretion of Yop proteins by Yersinia enterocolitica. J. Bacteriol. 173:4994-5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pemberton, J. M., S. P. Kidd, and R. Schmidt. 1997. Secreted enzymes of Aeromonas. FEMS Microbiol. Lett. 152:1-10. [DOI] [PubMed] [Google Scholar]
- 28.Persson, C., R. Nordfelth, A. Holmstrom, S. Håkansson, R. Rosqvist, and H. Wolf-Watz. 1995. Cell-surface-bound Yersinia translocate the protein tyrosine phosphatase YopH by a polarized mechanism into the target cell. Mol. Microbiol. 18:135-150. [DOI] [PubMed] [Google Scholar]
- 29.Pierson, D. E., and S. Falkow. 1990. Nonpathogenic isolates of Yersinia enterocolitica do not contain functional inv-homologous sequences. Infect. Immun. 58:1059-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plano, G. V., J. B. Day, and F. Ferracci. 2001. Type III export: new uses for an old pathway. Mol. Microbiol. 40:284-293. [DOI] [PubMed] [Google Scholar]
- 31.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty percent end points. Am. J. Hyg. 27:493-497. [Google Scholar]
- 32.Rodriguez, L. A., A. E. Ellis, and T. P. Nieto. 1992. Purification and characterization of an extracellular metalloprotease, serine protease and haemolysin of Aeromonas hydrophila strain B32: all are lethal for fish. Microb. Pathog. 13:17-24. [DOI] [PubMed] [Google Scholar]
- 33.Rosqvist, R., K. E. Magnusson, and H. Wolf-Watz. 1994. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 13:964-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubires, X., F. Saigi, N. Pique, N. Climent, S. Merino, S. Alberti, J. M. Tomas, and M. Regue. 1997. A gene (wbbL) from Serratia marcescens N28b(O4) complements the rfb-50 mutation of Escherichia coli K-12 derivatives. J. Bacteriol. 179:7581-7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 36.Secombs, C. J. 1990. Isolation of salmonid macrophages and analysis of their killing activity, p. 137-154. In J. S. Stolen, T. C. Fletcher, D. P. Anderson, B. S. Roberson, and W. B. Van Muiswinkel (ed.), Techniques in fish immunology. SOS Publications, Fair Haven, N.J.
- 37.Simon, R., U. Priefer, and A. Puhler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 38.Sory, M. P., A. Boland, I. Lambermont, and G. R. Cornelis. 1995. Identification of the YopE and YopH domains required for secretion and internalization into the cytosol of macrophages, using the cyaA gene fusionapproach. Proc. Natl. Acad. Sci. USA 92:11998-12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stuber, K., S. E. Burr, M. Braun, T. Wahli, and J. Frey. 2003. Type III secretion genes in Aeromonas salmonicida subsp. salmonicida are located on a large thermolabile virulence plasmid. J. Clin. Microbiol. 41:3854-3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan, E., K. W. Low, W. S. F. Wong, and K. Y. Leung. 1998. Internalization of Aeromonas hydrophila by fish epithelial cells can be inhibited with a tyrosine kinase inhibitor. Microbiology 144:299-307. [DOI] [PubMed] [Google Scholar]
- 41.Thune, R. L., L. A. Stanley, and R. K. Cooper. 1993. Pathogenesis of gram-negative bacterial infections in warm water fish. Annu. Rev. Fish Dis. 3:37-68. [Google Scholar]
- 42.Waterfield, N. R., P. J. Daborn, and R. H. ffrench-Constant. 2002. Genomic islands in Photorhabdus. Trends Microbiol. 10:541-545. [DOI] [PubMed] [Google Scholar]
- 43.Winstanley, C., and C. A. Hart. 2001. Type III secretion systems and pathogenicity islands. J. Med. Microbiol. 50:116-126. [DOI] [PubMed] [Google Scholar]
- 44.Wolf, K., and J. A. Mann. 1980. Poikilotherm vertebrate cell lines and viruses: a current listing for fishes. In Vitro 16:168-179. [DOI] [PubMed] [Google Scholar]
- 45.Wong, C. Y., M. W. Heuzenroeder, and R. L. Flower. 1998. Inactivation of two haemolytic toxin genes in Aeromonas hydrophila attenuates virulence in a suckling mouse model. Microbiology 144:291-298. [DOI] [PubMed] [Google Scholar]
- 46.Yahr, T. L., L. M. Mende-Mueller, M. B. Friese, and D. W. Frank. 1997. Identification of type III secreted products of the Pseudomonas aeruginosa exoenzyme S regulon. J. Bacteriol. 179:7165-7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang, Y. L., E. Arakawa, and K. Y. Leung. 2002. Novel Aeromonas hydrophila PPD134/91 genes involved in O-antigen and capsule biosynthesis. Infect. Immun. 70:2326-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]