Abstract
This study characterized developmental emergence of individual alcohol use disorder (AUD) symptoms, and evaluated their ability as early indicators of progression into alcohol dependence (AD), conditional upon gender, parental alcohol dependence, early onset of drinking, and level of delinquent behavior at onset. The two parameters of interest were (a) likelihood of specific AUD symptom appearance once drinking has begun, and (b) primacy of symptom appearance as an indicator of likelihood for eventual move into diagnosis. We analyzed prospective data from a community sample of high risk youth from childhood to early adulthood. Symptoms that were at higher probability of being experienced at drinking onset and that could serve as good indicators for the early stage of disease progression were: persistent desire or unsuccessful efforts to control alcohol use (AD4), and continued use despite having persistent or recurrent interpersonal problems (AA4). Tolerance (AD1) may serve as an indicator for the intermediate stage of progression. Young people tended to be at an elevated risk for developing AD6 (activities given up), AD7 (physical/psychological problems), and AA3 (legal problems) in later years so these symptoms may be good indicators for later stages of progression. In addition to being male, an early onset drinker, or high in delinquent behavior, drinkers who experienced AA4 or AD1 as first symptoms were at higher risk for progression to AD. We also identified two high risk clusters: late onset drinkers with AA4 as first symptom, and children of alcoholics with AD1 as first symptom.
Keywords: alcohol use disorder, progression, prospective, longitudinal
Introduction
Temporal progression of alcohol use disorder (AUD) symptoms has been studied among members of Alcoholics Anonymous and treatment samples since the 1940’s (see Nelson, Heath, & Kessler, 1998 for a comprehensive review of the history). In recent decades, this line of research has been extended to the general population (Nelson et al., 1998; Nelson, Little, Heath, & Kessler, 1996), adolescent drinkers (Martin, Langenbucher, Kaczynski, & Chung, 1996), and community-residing older drinkers (Lemke, Schutte, Brennan, & Moos, 2005). One common goal of these studies is to characterize AUD in terms of a disease-progression model (Lemke et al., 2005), which specifies a temporal ordering of clusters of symptoms that may indicate the stages of disease progression. Such a model has the potential to improve screening of early cases, designs of preventive intervention, allocation of treatment resources, and timing of treatment. However, different patterns of progression have been identified by existing studies depending on the statistical methods, characteristics of the samples and the different ways in which AUD symptoms were asked. In addition, because of the cross-sectional design of existing studies, the accuracy of retrospective data at the symptom level remains a major concern, especially for individuals who had been drinking for some years at the time the data were collected. Thus, a prospective longitudinal design that follows youth from drinking initiation to the onset of AUD symptoms is needed for a better understanding of the developmental emergence of AUD symptoms.
Symptom-specific progression patterns to date have only been examined by one prospective study, the Early Developmental Stages of Psychopathology (EDSP) study, which followed 3021 community participants aged 14–24 (at baseline) in Germany across 4 waves over 10 years (Behrendt, Wittchen, Hofler, Lieb, Low, Rehm, et al., 2008). The EDSP study showed that the distributions of the duration of progression from first alcohol use to first alcohol dependence symptom were different for the seven DSM-IV alcohol dependence symptoms (see Table 2 for a comprehensive list of the AUD symptoms and acronyms). However, these curves may not be totally comparable because they were estimated with different levels of accuracy. In particular, the curves corresponding to the symptoms rarely endorsed as a first alcohol dependence symptom (e.g. AD2-withdrawal, AD6-activities given up) were based on only a handful of cases (range of 12 to 24). Moreover, the sample characteristics, involving a relatively high socioeconomic status region in Germany, may render the findings of only limited generalizability to the high risk youth population in the United States.
Table 2.
First symptom prevalence rates and lifetime prevalence rates of DSM-IV alcohol abuse (AA) and alcohol dependence (AD) symptoms.
| First symptom prevalence rates * |
Lifetime prevalence rates |
|
|---|---|---|
| AA1 Neglect roles | 7.73% ( 9) | 24.94% ( 8) |
| AA2 Hazardous use | 10.97% ( 6) | 34.66% ( 5) |
| AA3 Legal problems | 3.24% (11) | 12.72% (11) |
| AA4 Social/interpersonal problems | 19.70% ( 3) | 38.15% ( 3) |
| AD1 Tolerance | 25.94% ( 2) | 52.62% ( 2) |
| AD2 Withdrawal | 8.23% ( 8) | 18.45% (10) |
| AD3 Larger/longer | 32.92% ( 1) | 61.10% ( 1) |
| AD4 Quit/control | 19.20% ( 4) | 34.91% ( 4) |
| AD5 Time spent | 8.98% ( 7) | 30.67% ( 6) |
| AD6 Activities given up | 6.98% (10) | 18.70% ( 9) |
| AD7 Physical/psychological problems | 14.46%( 5) | 29.43% ( 7) |
| Any symptom | 75.81% | 75.81% |
Note: The number in the parenthesis is the rank order of the prevalence rate among the eleven symptoms (from the highest to the lowest).
Multiple symptoms could be identified as the first symptom if they were first reported in the same year. About 37% of the participants only had a single first symptom; 39% had 2–9 first symptoms. The most commonly observed combinations of multiple first symptoms were: AD1 & AD3 (2%); AA4 & AD3 (2%).
In addition to the issue of temporal progression of appearance, individual AUD symptoms may also have potential as early harbingers to predict the likelihood of progression to alcohol dependence. Such information can improve screening of high risk cases at an early stage.Nelson et al. (1996) found that people who reported AD2 (withdrawal) or AD6 (activity given up) as their first symptom were most likely to progress subsequently to alcohol dependence than those who reported other symptoms. This finding was based on retrospective data from a representative sample of the adult population in the U.S. However, prospective data from the EDSP study (Behrendt et al., 2008) showed that the risk for alcohol dependence was elevated for those with a completely different set of first symptoms: AD1 (tolerance), AD4 (quit/control), AD5 (time spent), or AD7 (physical/psychological problems). These inconsistent findings could be the result of the differences between the two studies in terms of sample characteristics, study designs, or analytic methods. One shared limitation of these two studies is that they did not control for some well-known risk factors such as early onset of drinking as they evaluated the association between first AUD symptoms and the risk for progression to alcohol dependence. Without taking into account important preexisting risk profiles, it is impossible to know if these first symptoms add any new information about risk.
Extant literature has identified risk factors for progression from alcohol use to alcohol dependence (or AUD). National epidemiologic surveys consistently found that men were at higher risk for progression to an alcohol dependence diagnosis than women (Keyes, Martins, Blanco, & Hasin, 2010; Wagner & Anthony, 2007). Such gender differences were also supported by prospective longitudinal data from a community sample (Hussong, Bauer, & Chassin, 2008). In addition to gender, national survey data showed that a higher density of family history of alcoholism was associated with higher risk of progression to alcohol dependence diagnosis (Dawson, 2000). Specifically, parental history of alcohol problems or AUD diagnosis was linked to a higher risk of progression to alcohol dependence symptoms or AUD diagnosis in adolescents (Bucholz, Heath, & Madden, 2000; Hussong et al., 2008). Another risk factor shown to be associated with progression to an alcohol dependence diagnosis in cross-sectional health surveys was early onset of drinking (Dawson, 2000; DeWit, Adlaf, Offord, & Ogborne, 2000). Prospective longitudinal data also supported this association (Behrendt, Wittchen, Hofler, Lieb, & Beesdo, 2009). Moreover, twin studies consistently found a common genetic factor, labeled as externalizing, underlying conduct disorder, antisocial personality disorder, and alcohol dependence (Kendler, Prescott, Myers, & Neale, 2003; Krueger, Hicks, Patrick, Carlson, Iacono, & McGue, 2002). In a prospective longitudinal study, adolescent externalizing behavior reported at the age of drinking onset was shown to be a significant risk factor for progression to AUD diagnosis (Hussong et al., 2008). The externalizing measure used in that study was a composite of delinquency and aggression subscales of the Child Behavior Checklist (Achenbach, 1991a). Recent work by our group (Mayzer, Fitzgerald, & Zucker, 2009) using the same instrument found that delinquent behavior predicted problem drinking better than aggression in a longitudinal design. Thus, delinquency may be a more fine grained risk factor than the broad band externalizing factor.
The current study examines the patterning of emergence of AUD symptoms, and evaluates their potential as early indicators for the progression to alcohol dependence. We analyze prospective data on alcohol use, symptoms, and diagnoses collected from a community sample of high risk youth followed from early childhood to early adulthood. The longitudinal study design covers the age range of highest probability for AUD symptom onset, 10–24 (Nelson et al., 1998). The following are the objectives of this study. First, we aim to characterize the longitudinal patterns of risk for developing individual AUD symptoms during the ten years after onset of regular drinking. Based on existing retrospective data on symptom onset from adolescent drinkers (Martin et al., 1996), we expect that young people will be at the highest risk for developing AD3 (larger/longer) and AA4 (social/interpersonal problems) at the onset of regular drinking; the risk for developing AD1 (tolerance) will not start high at the onset of regular drinking but will drastically increase for some years and then decrease. We also expect that young people will be at high risk for developing AD6 (activities given up) and AD7 (physical/psychological problems) in later years. The second objective is to investigate if there is a synergy (i.e. statistical interactions) between any pairs of precursive risk factors including being male, COA, an early onset drinker, and higher in delinquent behavior at drinking onset. We expect that the people who carry both risk factors in a pair will be more likely to develop into alcohol dependence than those who only qualify for one of the risk factors. The third objective is to evaluate the potential of individual AUD symptoms as first symptoms to predict future progression to alcohol dependence diagnosis, incrementally beyond the precursive risk structure already in place. Based on existing prospective data from young people (Behrendt et al., 2008), we expect AD1 (tolerance), AD4 (quit/control), AD5 (time spent), and AD7 (physical/psychological problems) to be important first symptom predictors. The fourth objective is to examine if the first symptoms identified as significant predictors for progression to alcohol dependence are particularly important warning signs for drinkers with certain precursive risk profiles (i.e. there are statistical interactions between precursive risk factors and first symptom predictors). Due to the exploratory nature of this set of analysis, we do not have any particular expectation for this objective.
Method
Design and Sample
The present study is part of the Michigan Longitudinal Study (MLS), an ongoing multi-wave prospective study of families at high risk for substance use disorder currently spanning more than twenty years (Zucker, Ellis, Bingham, Fitzgerald, & Sanford, 1996; Zucker, Fitzgerald, Refior, Puttler, Pallas, & Ellis, 2000). Participants were families ascertained through two interconnected population-based methods carried out in a 4-county-wide area in the Midwest. Ascertainment of the highest risk portion of the sample was by way of the father’s drunk driving conviction with a sufficiently high blood alcohol concentration (0.15% if a first conviction, 0.12% if multiple convictions) to virtually assure a later AUD diagnosis would be obtained. The remaining families were systematically recruited door-to-door in the same neighborhoods as the drunkdriver families, thus controlling for neighborhood ecological influences. Recent analyses from the MLS indicating significant neighborhood influences on long-term health outcomes in both parent and offspring suggest the importance of such a geographical control (Buu, DiPiazza, Wang, Puttler, Fitzgerald, & Zucker, 2009; Buu, Mansour, Wang, Refior, Fitzgerald, & Zucker, 2007). The recruitment protocol also required the father to be living with a 3–5 year old son (the male target child) and the boy’s biological mother. The study families were originally recruited as triads, but thereafter siblings within +/− eight years of the initial male target child were also recruited.
Participant families received extensive in-home assessments at baseline, and assessment waves thereafter took place at three-year intervals (denoted as T). During the critical period of alcohol use development in much of the U.S. population (ages 11–17; Wagner & Anthony, 2002), annual assessments (denoted as A) were also conducted. The study assessment schedule is demonstrated as follows:
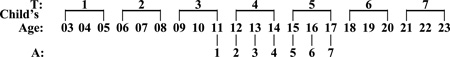
The present study includes those 401 children (all Caucasian, from 248 families who participated in the MLS) who had initiated regular drinking, defined by the use of alcohol at least once a month for 6 months, by T7 (ages 21–23). Forty-six percent of the families were recruited through drunk driving records and had at least one parent who met probable or definite Feighner diagnosis criteria of alcoholism (Feighner, Robins, Guze, Woodruff, Winokur, & Munoz, 1972). Twenty percent of the families were recruited through neighborhood canvassing and had a father (or both father and mother) who also met these diagnostic criteria. The remainder of the community ascertained group (34%) consisted of families in which neither the mother nor father made a lifetime substance use disorder diagnosis. The three family types represent a gradient of alcohol abuse/dependence and other substance use disorder vulnerability. Because this study is about progression from regular drinking to alcohol dependence, we have not included those 21 MLS offspring who had not started regular drinking by the T7 assessment wave (i.e. the latest wave that our analysis can reach), and thus did not have valid time-to-event data within the timeframe of our survival analysis. We have also excluded 139 participants without complete symptomatology data. The majority of them (86%) were older or younger siblings of the male target children who missed assessment at earlier waves or had not completed assessment at later waves. Some attrition analyses were conducted to compare these excluded participants with the participants who were included in the study and the results show that the two groups had about equal proportions of males (χ2=0.21, df=1, p>0.05) and of early onset drinkers (χ2=1.22, df=1, p>0.05), but the group that was excluded tended to have a higher proportion of COAs (χ2=5.88, df=1, p<0.05).
Measures
Onset ages of drinking and regular drinking
The Drinking and Drug History Questionnaire (DDHQ; Zucker, Fitzgerald, & Noll, 1990) and the Health and Daily Living Questionnaire (HDLQ) were used to determine the onset ages of drinking and regular drinking. The DDHQ incorporates items from national epidemiologic studies of drugs and alcohol (Johnston, Bachman, & O’Malley, 1979; Cahalan, Cisin, & Crossley, 1969), as well as from a structured clinical symptom questionnaire (Schuckit, 1978). All of the items have been extensively used in a variety of survey and clinical settings. They provide data on quantity, frequency, and consequences of substance use. The instrument was administered at T4-T7 (ages 12–23) and A1-A7 (ages 11–17). The HDLQ is a younger children’s version of the DDHQ with items modified to be relevant for children under the age of 12 but covering the same substantive areas. The HDLQ was administered at T2-T3 (ages 6–11). At each wave, the following two questions were consistently asked: Q1– “How old were you the first time you ever took a drink? Do not count the times when you were given a ’sip’ by an adult”; Q2– “Over the last 6 months,0 on the average, how many days a month have you had a drink?” We used the response to Q1 as the onset age of drinking with the test-retest reproducibility being 80% over a one year interval. When there was discrepancy across waves, we took the age reported at the earliest wave as the best estimate since it was the least retrospective account (Parra, O’Neill, & Sher, 2003). Adopting the commonly used cutoff in the literature (Hingson, Heeren, & Winter, 2006), we defined the early onset age for drinking as fourteen or younger. In order to make our survival analysis results comparable to other studies (e.g. Martin et al., 1996), we defined the onset age of regular drinking as the age at the earliest wave when the response to Q2 was larger than or equal to 1.
Onset ages of DSM-IV AUD symptoms
The onset age of each DSM-IV AUD symptom was derived from the age when the symptom was first reported among the following instruments: the DDHQ, HDLQ, Diagnostic Interview Schedule (DIS; Robins, Helzer, Croughan, & Ratcliff, 1980; Robins, Marcus, Reich, Cunningham, & Gallagher, 1996), and Diagnostic Interview Schedule for Children (DISC; Shaffer, Fisher, Lucas, Dulcan, & Schwab-Stone, 2000). The DDHQ and HDLQ were described above. The DIS and DISC are well validated and widely used diagnostic instruments that allow trained interviewers to gather extensive information about psychiatric, physical, alcohol-related, and drug-related symptoms. For substance use disorders, the test-retest reliability ranged from 0.53 to 0.86; and the validity related to the criterion, the WHO Schedules for Clinical Assessment in Neuropsychiatry (SCAN; Wing et al., 1990), ranged from 0.45 to 0.71. The DISC was administered at waves T3 through T5 (ages 9–17) and the DIS was administered at T6 and T7 (ages 18–23). The symptom that had the youngest onset age was designated as the first symptom. Multiple symptoms could be identified as the first symptom if they were first reported in the same year.
DSM-IV alcohol abuse or dependence diagnoses and onset ages
A doctoral level clinical psychologist conducted year-by-year DSM-IV alcohol abuse and alcohol dependence diagnoses utilizing information from the DIS (DISC for younger ages), and the DDHQ (HDLQ for younger ages). When discrepancies were observed between the two instruments, the more severe pattern was taken as the best estimate of actual functioning. At baseline, lifetime DSM-IV alcohol abuse or dependence diagnoses for the parents were also obtained by combining information from the DIS, DDHQ, and the Short Michigan Alcohol Screening Test (SMAST; Selzer, Vinokur, & van Rooijen, 1975). Inter-rater reliability was established by having another clinical psychologist blindly diagnose a subset of the protocols; Kappa was 0.81. Participants were categorized as children of alcoholic (COA), if at least one of the biological parents met a lifetime DSM-IV alcohol dependence diagnosis.
Delinquent behavior at drinking onset
The participants rated their own behavior on the Youth Self-Report (YSR; Achenbach, 1991b), which is a widely used and recognized empirical measure of child behavior problems with excellent reliability and validity. One week test-retest reliability coefficients for the various scales on the measure ranged from 0.50 to 0.80, with 0.72 being reported for the delinquent behavior subscale. The validity of the measure was established by its ability to discriminate between clinical and nonclinical samples. The total score of the items on the delinquent behavior subscale was standardized according to the national norm (with a mean 50 and a standard deviation 10). The YSR was administered at T4-T5 and A1-A7. The subscale score at the wave closest to the onset age of drinking was used as a risk factor for progression to alcohol dependence in the survival analysis.
Statistical Analysis
In order to achieve the first objective of this study, the hazard function, which assesses the chance (risk) that an individual who has not yet developed the symptom will experience the symptom at the next instant, was used to characterize developmental emergence of individual AUD symptoms. Graphing the hazard function of each symptom along the number of years since the onset of regular drinking, we can identify developmental variations in pattern of emergence for a particular symptom, as well as the highest probability periods for its emergence. We estimated the hazard function using a kernel smoothing method implemented in the R package muhaz. Technical details such as the global and local bandwidth selection algorithms and the boundary kernel formulations are described in Mueller and Wang (1994).
Cox regression (Cox, 1972) was employed to examine individual AUD symptoms’ predictive ability for progression to alcohol dependence because this methodology is designed to handle censored data which in our study involved the subset of participants who had not made an alcohol dependence diagnosis by early adulthood. It is a semiparametric method that does not impose distributional assumptions about the event time and therefore is quite robust. Due to parental or sibling influences on alcohol use, the time-to-event data collected from siblings within the same family tended to be correlated and thus violated the independence assumption of an ordinary Cox regression model. A well-known consequence of such violation is that the standard errors of the regression coefficients tend to be underestimated and therefore the corresponding hypothesis tests tend to be liberal. Thus, we adopted the robust sandwich estimator (Lee, Wei, & Amato, 1992) for the standard errors, which is implemented in the SAS PROC PHREG (SAS Institute Inc., 2008), to adjust for the undesirable effect of correlated data. We first fitted our baseline model with well-known precursive risk factors for progression to AUD including being male, COA, an early onset drinker, and higher in delinquent behavior at drinking onset. In order to achieve the second objective of this study, to investigate if there is a synergy between these precursive risk factors, we tested for all possible interaction terms (conditional on the baseline model) and only retained the significant ones (p<0.05).
The third objective of the study is to evaluate the potential of individual AUD symptoms as first symptoms to predict future progression to alcohol dependence diagnosis, incrementally beyond the precursive risk structure already in place. To achieve this objective, a binary variable was created for each AUD symptom to indicate if it was first appearing. For those who reported multiple first symptoms in the same year, their values on the corresponding variables were all coded as 1. Conditional on the precursive risk factors and their significant interaction terms, we entered these binary symptom appearance variables into the model one by one and only retained the ones that were significant predictors (p<0.05).
The fourth objective is to examine if the first symptoms identified as significant predictors for progression to alcohol dependence are particularly important warning signs for drinkers with certain precursive risk profiles. Given the precursive risk factors, their interaction terms retained, and the significant first symptom indicators identified above, we entered the interaction terms between these two sets of predictors one by one and only retained significant interaction terms (p<0.05). We transformed the Cox regression coefficients to hazard ratios for the predictors in the final model. For binary predictors such as COA status, the hazard ratio can be straightforwardly interpreted as the relative risk of the high risk group in comparison to the control group. For continuous predictors such as delinquent behavior, we standardized the original variable with the sample mean and standard deviation so that the corresponding hazard ratio can be interpreted as the change in relative risk with a standard deviation increase. In order to facilitate the interpretation of the interaction terms in the final model, we calculated the survival function along the number of years from onset of regular drinking to alcohol dependence using the fitted model. The survival functions for people with different profiles of risk were graphed to show that they had different levels of risk for progression to alcohol dependence.
Results
Descriptive Statistics of Participants’ Background
Table 1 lists the means and standard deviations of study participants’ demographic and psychopathology background variables. Their mean ages at the first and last symptomatology assessments were 10 and 22, respectively. The majority of the participants were male (73%) and children of alcoholics (64%; defined as children with either parent meeting lifetime DSM-IV alcohol dependence diagnosis). On average, the mean level of education across both parents was 14 years. The average annual family income was $35K, with a large variation among study families (SD=$16K). Their average delinquency score at drinking onset was 4 points higher than the population mean (i.e. 50). On average, the youth started regular drinking at the age of 16. Fifty-three percent of them had their first drink at the age of 14 or younger. By T7 (ages 21–23), 54% of the participants had met DSM-IV alcohol abuse diagnosis with the mean onset age at 17, while 23% had met DSM-IV alcohol dependence diagnosis with the mean onset age at 18.
Table 1.
Descriptive statistics of demographic and psychopathology background (N=401).
| Variable | Mean | Std. Dev. |
|---|---|---|
| Age at first symptomatology assessment | 10.40 | 2.09 |
| Age at last symptomatology assessment | 21.72 | 1.59 |
| Gender (male) | 72.82% | 2.22% |
| Children of Alcoholics (COA) | 64.34% | 2.39% |
| Average parental education (years) | 13.59 | 1.80 |
| Family income at baseline (dollars in the 1980’s) | 34,950 | 16,423 |
| Delinquency T-score at drinking onset | 53.73 | 5.44 |
| Age at onset of regular drinking | 16.21 | 2.08 |
| Early onset of drinking (age of first drink <=14) | 52.87% | 2.49% |
| Lifetime DSM-IV alcohol abuse diagnosis* | 53.87% | 2.49% |
| Lifetime DSM-IV alcohol dependence diagnosis* | 23.19% | 2.11% |
| Lifetime DSM-IV AUD diagnosis | 61.10% | 2.43% |
| Age at onset of DSM-IV alcohol abuse diagnosis | 17.06 | 2.08 |
| Age at onset of DSM-IV alcohol dependence diagnosis | 17.98 | 1.96 |
| Age at onset of DSM-IV AUD diagnosis | 17.02 | 2.05 |
The alcohol abuse and alcohol dependence diagnoses were coded independently.
Prevalence Rates of DSM-IV Alcohol Use Disorder Symptoms
Table 2 shows first symptom prevalence rates and lifetime prevalence rates of individual AUD symptoms. The former was the percentage of participants whose first symptom was the particular symptom. The latter was defined as the percentage of participants who had reported the symptom during this study. As expected, the lifetime prevalence rates were much higher than the first symptom prevalence rates but the differences in rank order of the 11 prevalence rates were within +/−1 except for two symptoms: AD2 (withdrawal) and AD7 (physical/psychological problems). These two symptoms tended to have higher prevalence rates as first symptom. Over 50% of the participants had experienced AD3 (larger/longer) and AD1 (tolerance) by the age of 23, whereas fewer than 20% had reported AA3 (legal problems), AD2 (withdrawal), and AD6 (activities given up). Overall, 76% of the participants had developed at least one AUD symptom during the study period. This indicates that less than a quarter of the atrisk youth who had started regular drinking were free from any symptomatology.
Hazard Functions of DSM-IV Alcohol Use Disorder Symptoms
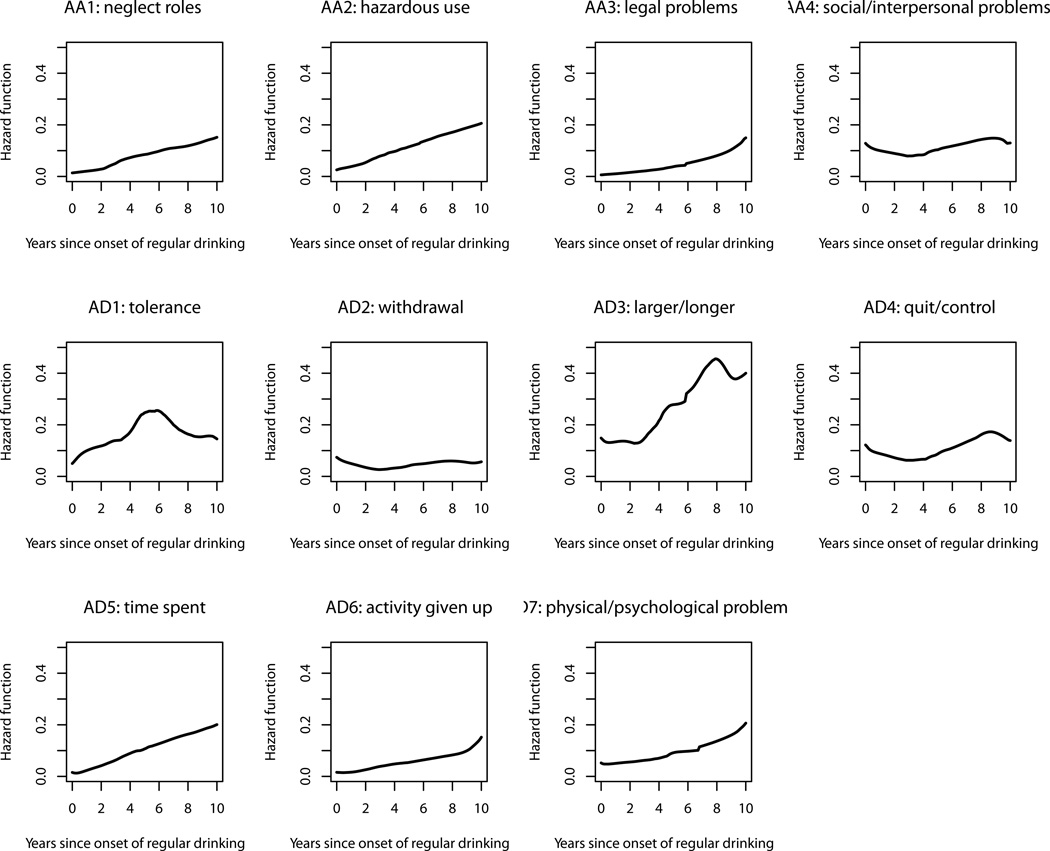
Figure 1 depicts the longitudinal change in risk for each of the DSM-IV AUD symptoms during the 10 years following onset of regular drinking (we did not graph the hazard functions beyond 10 years because the sparse time-to-event data would result in poor accuracy after that point). The risk for AD3 (larger/longer) was the highest among all the 11 symptoms throughout the entire period and was particularly accelerated from the 3rd to the 8th year. The symptom AD1 (tolerance), carrying the second highest risk, was like a heavy-tailed bell shape with elevated risk around the 5th–6th years. Although AA4 (social/interpersonal problems) and AD4 (quit/control) belong to different sets of diagnostic criteria under the DSM-IV taxonomy, their risk followed similar patterns – starting with elevated risk, decreasing slowly and gradually rising again after the 4th year. The risk for a group of symptoms, including AA1 (neglect roles), AA2 (hazardous use) and AD5 (time spent), tended to have a low initial value and increased linearly year by year. The symptoms AA3 (legal problems), AD6 (activity given up) and AD7 (physical/psychological problem) formed another group that had low risk in earlier years but heightened risk in later years. Moreover, the risk for AD2 (withdrawal) tended to stay low and flat throughout the entire period.
Figure 1.
Hazard functions for progression from onset of regular drinking to onset of individual alcohol use disorder symptoms.
Cox Regression Model of Progression to Alcohol Dependence Diagnosis
Table 3 shows Cox regression models of the effects of risk factors on progression from onset of regular drinking to onset of DSM-IV alcohol dependence diagnosis. As described in the Method section, we first fitted our baseline model (i.e. Model A) using the 4 risk factors that have been found to be associated with progression to AUD diagnosis including being male, a COA, an early onset drinker, and having a higher level of delinquent behavior around drinking onset. All of them, except COA status, significantly predicted later progression to alcohol dependence. To investigate if there is a synergy between these precursive risk factors, we tested for all possible interaction terms one by one (conditional on Model A) and found none of them significant. Conditional on the precursive risk factors in Model A, we added a binary variable for each of the 11 AUD symptoms that indicated if the particular symptom was experienced as the first symptom one by one. Model B was the resultant model with the two significant binary variables corresponding to AA4 and AD1 retained. The Wald χ2 test result shows that Model B fitted the data better than Model A (χ2=18.96, df=2, p<0.05). Conditional on the risk factors in Model B, we tested the interactions between the set of precursive risk factors and the two retained first symptom predictors one by one, resulting in Model C with the two significant interaction terms (early onset of drinking × AA4 as first symptom; COA status × AD1 as first symptom). Based on the Wald χ2 test result (χ2=8.39, df=2, p<0.05), Model C had a better fit than Model B and thus became our final model.
Table 3.
Cox regression models of the effects of risk factors on progression from onset of regular drinking to onset of DSM-IV alcohol dependence diagnosis.
| Predictor | Model A | Model B | Model C | |
|---|---|---|---|---|
| Coefficient (std error) |
Coefficient (std error) |
Coefficient (std error) |
Hazard ratio | |
| Male | 0.64* | 0.64* | 0.66* | 1.94 |
| (0.25) | (0.26) | (0.26) | ||
| Children of alcoholic | 0.31 | 0.42 | 0.04 | 1.04 |
| (0.27) | (0.29) | (0.30) | ||
| Early onset of drinking | 0.60* | 0.51* | 0.82* | 2.26 |
| (0.28) | (0.30) | (0.35) | ||
| Delinquent behavior at drinking onset |
0.22* | 0.20* | 0.22* | 1.24 |
| (0.07) | (0.08) | (0.07) | ||
| AA4 as first symptom | 0.89* | 1.88* | 6.54 | |
| (0.23) | (0.45) | |||
| AD1 as first symptom | 0.68* | −0.09 | 0.92 | |
| (0.24) | (0.47) | |||
| Early onset of drinking | −1.16* | 0.31 | ||
| ×AA4 as first symptom | (0.50) | |||
| COA status | 1.01* | 2.75 | ||
| ×AD1 as first symptom | (0.52) | |||
| Model comparison | A vs. B | B vs. C | ||
| Wald χ2 test | χ2(2)=18.96* | χ2(2)=8.39* | ||
p<0.05
In Table 3, we also transformed the Cox regression coefficients under Model C to hazard ratios in order to facilitate interpretations of the results. The risk of progression to alcohol dependence among male youth was 1.94 times the risk of female youth. Among the people who did not experience AA4 (social/interpersonal problems) as the first symptom, early onset drinkers tended to carry higher risk (2.26 times) than late onset drinkers. For a one standard deviation increase in delinquency score measured around drinking onset, the risk for later progression into alcohol dependence was heightened about 1.24 times. Among late onset drinkers, the risk for those who had AA4 as the first symptom was 6.54 times the risk for those who did not experience AA4 as first symptom.
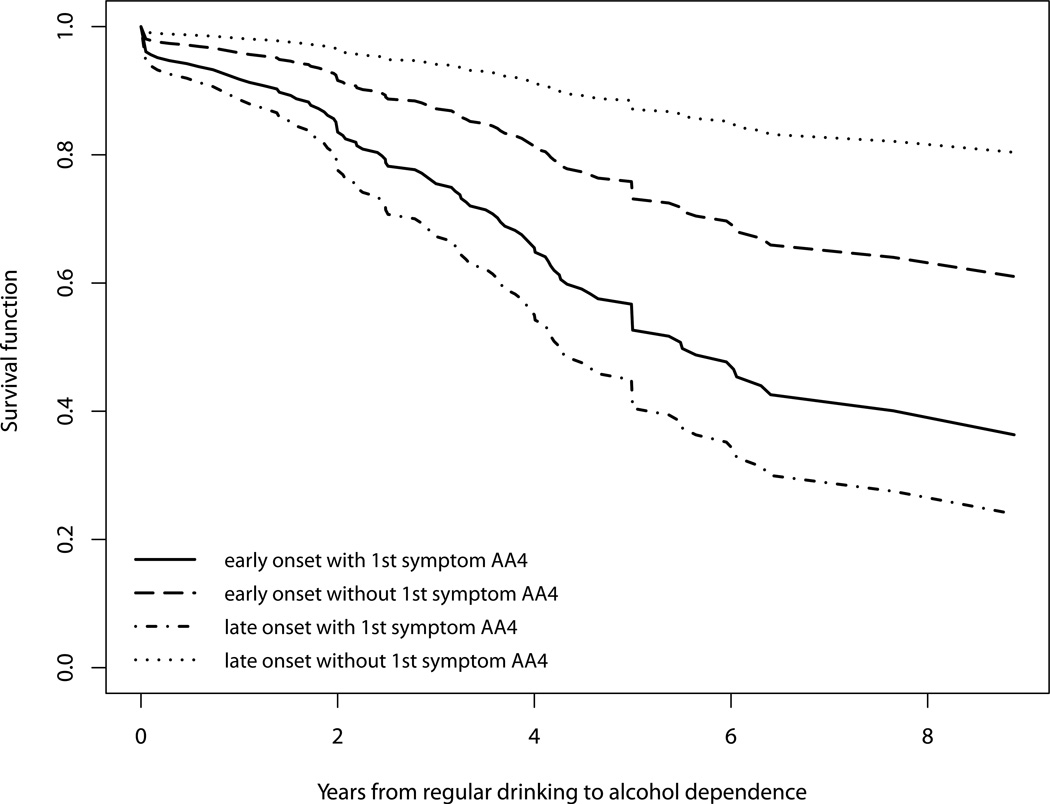
To facilitate understanding of the developmental patterning of the occurrence of alcohol dependence as a function of the interaction between early onset of drinking and the appearance of AA4 as the first symptom, four survival curves corresponding to all combinations of these two binary variables are shown in Figure 2. The late onset drinkers with AA4 as the first symptom showed the highest risk for developing alcohol dependence over time. Early onset drinkers who experienced AA4 as the first symptom carried the second highest risk for developing alcohol dependence thereafter. The remaining two groups, who did not experience AA4 as the first symptom, had lower risk with the late onset drinkers without AA4 as the first symptom having the lowest risk for later progression into alcohol dependence.
Figure 2.
Survival functions for progression from onset of regular drinking to onset of alcohol dependence based on early drinking onset status and experience of social/interpersonal problems (AA4) as first symptom.
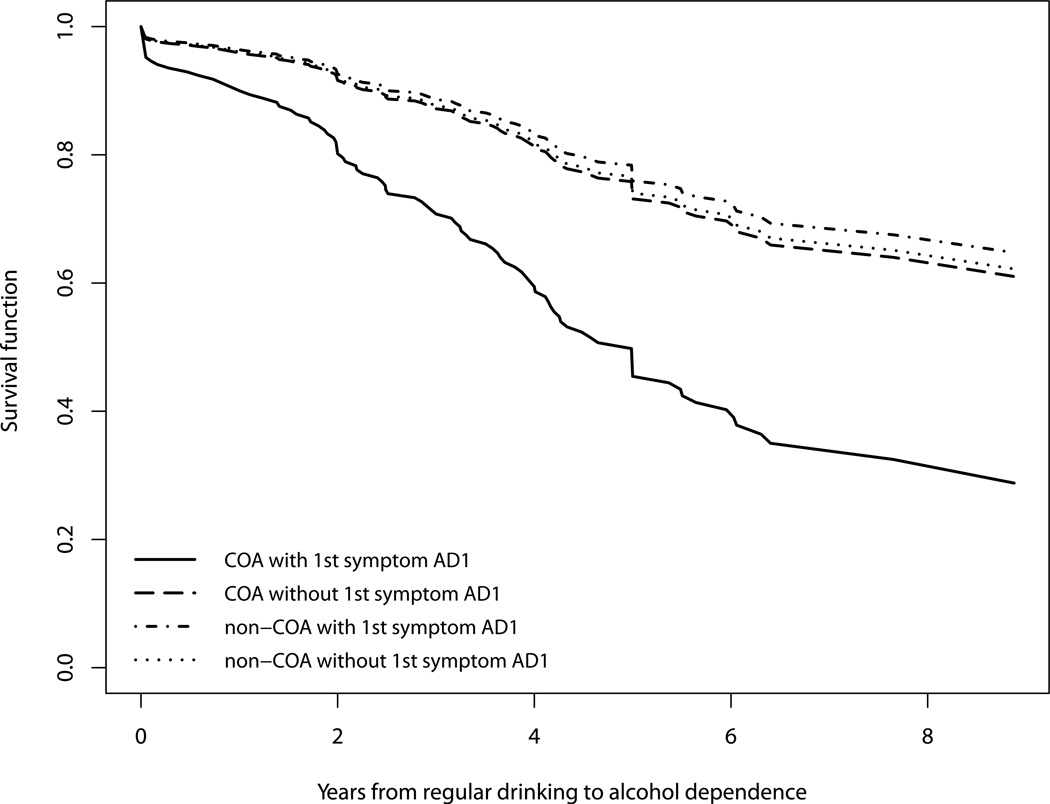
Figure 3 depicts the interaction between COA status and the appearance of AD1 as the first symptom with four survival curves corresponding to all combinations of these two binary variables. The group with both positive COA status and AD1 as the first symptom was at the highest risk for progression into alcohol dependence throughout the entire period of ten years after onset of regular drinking. The other groups were all at approximately equal and lower level risk, as demonstrated by the three flat and overlapping survival curves.
Figure 3.
Survival functions for progression from onset of regular drinking to onset of alcohol dependence based on COA status and experience of tolerance (AD1) as first symptom.
Discussion
Prevalence Rates of DSM-IV Alcohol Use Disorder Symptoms
As expected, the lifetime prevalence rate for every AUD symptom based on our high risk youth sample is much higher than the one reported in a national sample (Saha, Chou, & Grant, 2006). However, when we compare the rank order of the 11 prevalence rates in our sample with the national data, the majority of them are very close (within +/− 2). The only exceptions are that AA4 (social/interpersonal problems) is more common among this young adult sample (ranked the third) than in the adult population (ranked the eighth), whereas AD2 (withdrawal) is less common in our sample (ranked the tenth) than in the adult population (ranked the fifth). According to Wagner, Lloyd, and Gil (2002), adolescent drinkers tend to have heavy drinking episodes in social contexts so they are more likely to have social/interpersonal problems than adult drinkers. On the other hand, adolescent drinkers are less likely to demonstrate the withdrawal symptom which is usually associated with long-term alcohol use.
Although withdrawal typically occurs after a fairly sustained period of heavy drinking, 8% of the sample reported experiencing it as the first symptom. We took a closer look at these records and found that only 3% of them experienced withdrawal as the single first symptom; the other 5% also experienced other symptom(s) such as tolerance in the same year and for such people, our study cannot differentiate if withdrawal actually happened before or after the other symptom(s). Moreover, young people are likely to confuse hangover with withdrawal due to the wording of structured interview schedules so the prevalence rate we observed could be inflated because of measurement error (Caetano & Babor, 2006).
Hazard Functions of DSM-IV Alcohol Use Disorder Symptoms
Studying the developmental emergence of AUD symptoms during the early years of drinking has the potential to improve screening of early cases as well as the design of preventive intervention. However, the data from adolescents or young adults at the symptom level are sparse in the literature.Martin et al. (1996) drew hazard functions of AUD symptoms in a 4-year window following the onset of regular drinking using retrospective data from adolescent drinkers. Our study has extended their work by collecting prospective data with a broader 10- year window that provides a clearer picture of the longitudinal changes in risk for individual symptoms. In general, we found similar trends to the ones in their study. Young people are at highest risk for experiencing AD3 (larger/longer), AA4 (social/interpersonal problems), and AD4 (quit/control) when they first start regular drinking. Because the risk for AD3 stays high in the entire 10 year period, it may not serve as a good indicator for the early stage of disease progression like the other two symptoms. In fact, another way of interpreting this is simply that it describes the normative increases in binge drinking known to occur during this developmental period. Young people do not tend to experience AD1 (tolerance) until a couple of years after onset; with an extended window, our study also specifies that tolerance is most likely to occur in the middle section of the 10 year window so it may serve as an indicator for the intermediate stage. Moreover, young people tend to be at elevated risk for developing AD6 (activities given up), AD7 (physical/psychological problems), and AA3 (legal problems) in later years so these symptoms may be good candidates to indicate later stages of progression.
Although there have been attempts to cluster AUD symptoms based on their temporal progression patterns, and to label each cluster as a stage of alcohol dependence (Chung, Langenbucher, McCrady, Epstein, & Cook, 2002; Langenbucher & Chung, 1995; Martin et al., 1996; Nelson et al., 1996, 1998), such efforts are unlikely to result in a commonly accepted theory. First, the resultant stages and labels vary across studies depending on the sample characteristics and statistical methodology. Second, according to a review of existing studies (Lemke et al., 2005), only a small portion of individual-level symptom sequences matched the expected sequence that was based on the group-level analysis. This implies that individual differences in temporal progression patterns were large. Preliminary analysis of our prospective data verified the same phenomenon. That is why we refrained from taking this approach. Instead, we examined the risk of developing each individual symptom as a function of drinking duration. As shown in Figure 1, although each symptom tends to have a higher chance of occurring in a certain period, it is still possible for it to be experienced by some people during other times. These individual differences could stem from the different personal, social and cultural contexts in which individual drinkers are situated. These contexts could change over time with moving, maturing, help seeking and other plausible explanations. They also could be a result of as yet not understood patterns of interaction among symptoms.
Martin, Chung, and Langenbucher (2008) conducted a comprehensive review of various findings that question the validity of the two separate DSM-IV diagnoses of alcohol abuse and alcohol dependence. They found that (1) the two criterion sets do not differ in prevalence, severity, or age of onset; (2) the 11 AUD criteria can be best modeled as a single dimension rather than two dimensions. However, these findings are all based on cross-sectional data or longitudinal data spanning only 1–2 years. The unique contribution of this study is that we provide the first set of prospective data to delineate longitudinal changes in symptom-specific risk during the ten years after onset of regular drinking. The symptom-level hazard rates in Figure 1 demonstrate that the 4 alcohol abuse symptoms do not have progression patterns that are distinct from the 7 alcohol dependence symptoms. In fact, some of them have similar change patterns to some of the dependence symptoms. For example, AA1 (neglect roles) and AA2 (hazardous use) follow a linearly increasing trend like AD5 (time spent). Moreover, unlike what the adult model based on retrospective data would suggest (Langenbucher & Chung, 1995; Nelson et al., 1996), the majority of the abuse symptoms do not have escalated risk in the beginning years of regular drinking. AA4 (social/interpersonal problems) is the only abuse symptom that carries a high risk around the onset of regular drinking.
The Potential of First AUD Symptoms as Early Indicators for Progression to Alcohol Dependence
Our study replicates the findings of previous studies (as reviewed in the Introduction) by showing that males, early onset drinkers, and youth with higher delinquent behavior at drinking initiation are at a higher risk for progression to diagnosis. Perhaps one reason why we did not find the COA status a significant predictor as previous studies did is that about 30% of our non- COA families actually had a family history of alcoholism in grandparents, according to the data from the biological parents of the participants. Children from such families may be at risk for developing alcohol dependence although their parents were not alcoholics. As a result, the difference between the COA group and the non-COA group might have been weakened. The other reason is that the COA status analysis involved controlling for three other variables (male, early drinking onset, and delinquency) which themselves are means by which COA risk is transmitted. Once this variance is removed, there is not enough residual variance that is significant.
Conditional on the precursive risk factors, we identified the first symptoms, AA4 (social/interpersonal problems) and AD1 (tolerance), as significant predictors for progression to alcohol dependence. In addition to AD1, the EDSP study identified three alcohol dependence symptoms (AD4, AD5, & AD7) as significant first symptom predictors for later progression to alcohol dependence diagnosis (Behrendt et al., 2008). Our preliminary analysis found all but one (AD4) of these first symptoms to be significant predictors, if those precursive risk factors were not included in the model. This analysis thus clarifies that after adjusting for the effects of gender, COA status, early onset status, and delinquent behavior, AD1 is the only significant first symptom predictor among all the dependence symptoms. Since the EDSP study did not examine the four abuse symptoms as first symptom predictors, these data are the first to indicate that one of the abuse symptoms (i.e. AA4) may also be a useful screening criterion for high risk cases. One possible confounding factor for AA4 is the amount/frequency of drinking because those experiencing social/interpersonal problems early may tend to drink the most and are also most likely to progress to AD. We compared the group of participants who had experienced social/interpersonal problems as the first symptom and the other group who had not and found that they did not differ on either the average number of drinking days per month (t=0.76, p>0.05) or the average number of drinks per day (t=1.59, p>0.05). Thus, we decided that it is not necessary to control for these variables in our models.
Contrary to the results based on retrospective data from a representative sample of the adult population (Nelson et al., 1996), our results – based on prospective data from a high risk community sample – do not indicate that AD2 (withdrawal) or AD6 (activities given up) are significant predictors for progression to dependence. In fact, both the EDSP study and ours have shown that very few young adults have either symptom as their initial one. Figure 1 also indicates that the risk for developing both of these symptoms is low within the first 10 years after onset of regular drinking. Thus, these symptoms may not be useful as screening criteria for high risk cases at an early stage, especially among adolescents or young adults. As the MLS continues to collect data in later adulthood, we may be able to test the potential of these two first symptoms as predictors for progression to alcohol dependence at an older age.
In addition to the effects of individual precursive risk factors and first AUD symptoms, we found a significant interaction between early onset of drinking and AA4 as the first symptom (social/interpersonal problems) and another significant interaction between COA status and AD1 as the first symptom (tolerance). Figure 2 shows that within each drinking onset group, experiencing social/interpersonal problems as the first symptom increased the risk for progression into alcohol dependence. This increase in risk is substantially greater for the late onset drinkers than for the early onset drinkers. Early onset of drinking has commonly been associated with socially deviant and undercontrolled behavior (Mayzer et al., 2009; Zernicke, Cantrell, Finn, & Lucas, 2010). In our sample, the early onset drinkers were twice as likely to report social/interpersonal problems as the first symptom as the late onset drinkers (χ2=14.68, df=1, p<<.05). Thus, for early onset drinkers having social problems as the first symptom does not add as much information about risk as it does for late onset drinkers. On the other hand, for late onset drinkers, experiencing social problems early indicates vulnerability for alcoholspecific undercontrol, which appears to be an early harbinger that they are not able to restrain their drinking. Figure 3 illustrates the other interaction effect and shows that children of alcoholics with tolerance as the first symptom are at a particularly high risk for progression into alcohol dependence by comparison to all the other groups. There is some evidence from neuroimaging studies that self monitoring of internal states is deficient in those COAs at highest risk for the development of alcohol problems in late adolescence and early adulthood (Heitzeg, Nigg, Yau, Zubieta & Zucker, 2008). The presence of tolerance would reinforce this vulnerability because it would likewise provide less internal cueing that would ordinarily lead to the regulation of consumption. Moreover, experiencing tolerance as the first symptom may be a sign of a low level of response to alcohol which has been shown to be particularly prevalent among COAs and a strong predictor of future AUD by a decade long prospective data (Schuckit, 1994).
Strengths and Limitations
This study has some unique strengths that contribute to our understanding of the developmental emergence of AUD symptoms and their potential as early indicators for progression to alcohol dependence. First, alcohol use and alcohol symptoms were assessed prospectively throughout the critical developmental period for the emergence of AUD using combined information from self-report questionnaires and in-home diagnostic interview across 6 triennial waves (ages 6–23) and 7 annual assessments (ages 11–17). Thus, potential bias due to recall errors or motivational revision of past behavior was minimized. Such gains in accuracy are critical when characterizing symptomatology progression in people with an early onset of drinking (Parra et al., 2003). Second, the potential of AUD symptoms as first symptoms to predict future progression to alcohol dependence diagnosis was examined conditioning on well-known risk factors, so that we can evaluate if first symptoms can be used to improve the screening of high risk cases at an early stage given preexisting conditions. Testing potential interactions between first symptom predictors and precursive risk factors also enables us to understand better if these first symptoms are particularly important warning signs for drinkers with certain risk background.
The study also has several weaknesses. The major weakness involves generalizability of the findings to the larger population. The sample consisted of only Caucasian youth. In addition, because of the MLS design, men recruited into the study needed to reside with their son and his biological mother at the time of initial recruitment and at least one son needed to be between the ages of 3 and 5 at study entry. Also by design, the study was far more heavily seeded with alcoholic families than is true of the general population. These inclusionary criteria reduce external validity, since results may not be generalized to populations of lower risk, involving youth from racial minorities, or those raised in a less coupled relationship during their early development.
On the other hand, the recruitment protocol allows us to observe the longitudinal development of high risk children in the family context from early childhood to adulthood so that we can develop a complete picture of the progression from the onset of drinking to the onset of AUD symptoms to the onset of AUD diagnosis. Furthermore, the population based nature of the sample, and its recruitment focus on largely lower middle to lower class communities, makes results highly generalizable to the high risk community settings out of which so many alcoholics in treatment emerge. In the recent decade, our study has recruited an additional sample of both African American and Hispanic families using parallel recruitment criteria. Analyses of future data from this cohort of youth from racial minorities during the same critical developmental period will extend our understanding beyond the Caucasian youth population.
We should note that our original annual assessment protocol only covered ages 11 to 17, with the annual assessment extended to the mid-twenties only more recently. Therefore, the precision in establishing onset ages for events happening during ages 18–23 is not as good as those that occurred during earlier ages. As we collect more data from younger families, we will be able to develop higher precision in assessing the developmental period from late adolescence to young adulthood.
Conclusion
This study shows that the youth who experiences AA4 (social/interpersonal problems) or AD1 (tolerance) as the first symptom tends to be at high risk for progression into alcohol dependence, conditional on important precursive risk factors including being male, COA, an early onset drinker, and higher in delinquent behavior at drinking onset. Experiencing AA4 as the first symptom is a particularly important warning sign for late onset drinkers, whereas the first symptom AD1 reveals high risk for progression into alcohol dependence among children of alcoholics. Future intervention work may target these two high risk groups. Moreover, analyses of future data from our minority sample and extended annual assessment may enhance our knowledge about potential differences in symptomatology development across ethnic groups and the developmental emergence of AUD symptoms from late adolescence to young adulthood.
Acknowledgements
This work was supported by NIAAA Grants K01 AA-16591 to A. Buu and R37 AA-07065 to R. A. Zucker
References
- Achenbach TM. Manual for the child behavior checklist /4-18 and 1991 profile. Burlington, VT: Department of Psychiatry, University of Vermont; 1991a. [Google Scholar]
- Achenbach TM. Manual for the youth self-report and 1991 profile. Burlington, VT: Department of Psychiatry, University of Vermont; 1991b. [Google Scholar]
- Behrendt S, Wittchen H-U, Höfler M, Lieb R, Low NCP, Rehm J, Beesdo K. Risk and speed of transitions to first alcohol dependence symptoms in adolescents: A 10-year longitudinal community study in Germany. Addiction. 2008;103:1638–1647. doi: 10.1111/j.1360-0443.2008.02324.x. [DOI] [PubMed] [Google Scholar]
- Behrendt S, Wittchen HU, Höfler M, Lieb R, Beesdo K. Transitions from first substance use to substance use disorders in adolescence: Is early onset associated with a rapid escalation? Drug and Alcohol Dependence. 2009;99:68–78. doi: 10.1016/j.drugalcdep.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Heath AC, Madden PAF. Transitions in drinking in adolescent females: Evidence from the Missouri Adolescent Female Twin Study. Alcoholism: Clinical and Experimental Research. 2000;24:914–923. [PubMed] [Google Scholar]
- Buu A, DiPiazza C, Wang J, Puttler LI, Fitzgerald HE, Zucker RA. Parent, family, and neighborhood effects on the development of child substance use and other psychopathology from preschool to the start of adulthood. Journal of Studies on Alcohol and Drugs. 2009;70:489–498. doi: 10.15288/jsad.2009.70.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buu A, Mansour M, Wang J, Refior SK, Fitzgerald HE, Zucker RA. Alcoholism effects on social migration and neighborhood effects on alcoholism over the course of 12 years. Alcoholism: Clinical and Experimental Research. 2007;31:1545–1551. doi: 10.1111/j.1530-0277.2007.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caetano R, Babor TF. Diagnosis of alcohol dependence in epidemiological surveys: An epidemic of youthful alcohol dependence or a case of measurement error? Addiction. 2006;101:111–114. doi: 10.1111/j.1360-0443.2006.01599.x. [DOI] [PubMed] [Google Scholar]
- Cahalan D, Cisin I, Crossley H. American Drinking Practice: A National Study of Drinking Behavior and Attitudes. New Brunswick, NJ: Publications Division, Rutgers Center for Alcohol Studies; 1969. [Google Scholar]
- Chung N, Langenbucher J, McCrady B, Epstein E, Cook S. Use of survival analyses to examine onset and staging of DSM-IV alcohol symptoms in women. Psychology of Addictive Behaviors. 2002;16:236–242. [PubMed] [Google Scholar]
- Cox DR. Regression models and life tables (with discussion) Journal of the Royal Statistical Society B. 1972;34:187–220. [Google Scholar]
- Dawson DA, et al. The link between family history and early onscoholism: Earlier initiation of drinking or more rapid development of dependence? Journal of Studies on Alcohol. 2000;61:637–646. doi: 10.15288/jsa.2000.61.637. [DOI] [PubMed] [Google Scholar]
- DeWit DJ, Adlaf EM, Offord DR, Ogborne AC. Age at first alcohol use: A risk factor for the development of alcohol disorders. The American Journal of Psychiatry. 2000;157:745–750. doi: 10.1176/appi.ajp.157.5.745. [DOI] [PubMed] [Google Scholar]
- Feighner JP, Robins E, Guze SB, Woodruff RA, Winokur G, Munoz R. Diagnostic criteria for use in psychiatric research. Archives of General Psychiatry. 1972;26:57–63. doi: 10.1001/archpsyc.1972.01750190059011. [DOI] [PubMed] [Google Scholar]
- Heitzeg MM, Nigg JT, Yau W-YW, Zubieta J-K, Zucker RA. Affective circuitry and risk for alcoholism in late adolescence: Differences in frontostriatal responses between vulnerable and resilient children of alcoholic parents. Alcoholism: Clinical and Experimental Research. 2008;32:414–426. doi: 10.1111/j.1530-0277.2007.00605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Winter MR. Age at drinking onset and alcohol dependence. Archives of Pediatrics and Adolescent Medicine. 2006;160:739–746. doi: 10.1001/archpedi.160.7.739. [DOI] [PubMed] [Google Scholar]
- Hussong A, Bauer D, Chassin L. Telescoped trajectories from alcohol initiation to disorder in children of alcoholic parents. Journal of Abnormal Psychology. 2008;117:63–78. doi: 10.1037/0021-843X.117.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, Bachman JG, O'Malley PM. Drugs and The class of '78: Behaviors, Attitudes, and Recent National Trends. Washington, DC: National Institute on Drug Abuse, Division of Research U.S. Department of Health, Education, and Welfare; 1979. [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of General Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Keyes KM, Martins SS, Blanco C, Hasin DS. Telescoping and gender differences in alcohol dependence: New evidence from two national surveys. The American Journal of Psychiatry. 2010;167:969–976. doi: 10.1176/appi.ajp.2009.09081161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior and personality: Modeling the externalizing spectrum. Journal of Abnormal Psychology. 2002;111:411–424. [PubMed] [Google Scholar]
- Langenbucher JW, Chung T. Onset and staging of DSM-IV alcohol dependence using mean age and survival-hazard methods. Journal of Abnormal Psychology. 1995;104:346–354. doi: 10.1037//0021-843x.104.2.346. [DOI] [PubMed] [Google Scholar]
- Lee EW, Wei LJ, Amato DA. Cox-type regression analysis for large numbers of small groups of correlated failure time observations. In: Klein JP, Goel P, editors. Survival Analysis: State of the Art. Boston, MA: Kluwer Academic Publishers; 1992. pp. 237–248. [Google Scholar]
- Lemke S, Schutte KK, Brennan PL, Moos RH. Sequencing the lifetime onset of alcohol-related symptoms in older adults: Is there evidence of disease progression? Journal of Studies on Alcohol. 2005;66:756–765. doi: 10.15288/jsa.2005.66.756. [DOI] [PubMed] [Google Scholar]
- Martin CS, Chung T, Langenbucher JW. How should we revise diagnostic criteria for substance use disorders in the DSM-V? Journal of Abnormal Psychology. 2008;117:561–575. doi: 10.1037/0021-843X.117.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CS, Langenbucher JW, Kaczynski NA, Chung T. Staging in the onset of DSM-IV alcohol symptoms in adolescents: Survival/hazard analyses. Journal of Studies on Alcohol. 1996;57:549–558. doi: 10.15288/jsa.1996.57.549. [DOI] [PubMed] [Google Scholar]
- Mayzer R, Fitzgerald HE, Zucker RA. Anticipating problem drinking risk from preschoolers’ antisocial behavior: Evidence for a common delinquency-related diathesis model. Journal of American Academy of Child and Adolescent Psychiatry. 2009;48:820–827. doi: 10.1097/CHI.0b013e3181aa0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller HG, Wang JL. Hazard rates estimation under random censoring with varying kernels and bandwidths. Biometrics. 1994;50:61–76. [PubMed] [Google Scholar]
- Nelson CB, Heath AC, Kessler RC. Temporal progression of alcohol dependence symptoms in the U.S. household population: Results from the National Comorbidity Survey. Journal of Consulting and Clinical Psychology. 1998;66:474–483. doi: 10.1037//0022-006x.66.3.474. [DOI] [PubMed] [Google Scholar]
- Nelson CB, Little RJA, Heath AC, Kessler RC. Patterns of DSM-III-R alcohol dependence symptom progression in a general population survey. Psychological Medicine: A Journal of Research in Psychiatry and the Allied Sciences. 1996;26:449–460. doi: 10.1017/s0033291700035534. [DOI] [PubMed] [Google Scholar]
- Parra GR, O'Neill SE, Sher KJ. Reliability of self-reported age of substance involvement onset. Psychology of Addictive Behaviors. 2003;17:211–218. doi: 10.1037/0893-164X.17.3.211. [DOI] [PubMed] [Google Scholar]
- Robins L, Helzer J, Croughan J, Ratcliff KS. The NIMH Diagnostic Interview Schedule: its history, characteristics and validity. St. Louis, MO: Washington University School of Medicine; 1980. [Google Scholar]
- Robins L, Marcus L, Reich W, Cunningham R, Gallagher T. Diagnostic Interview Schedule Version IV (DIS-IV) St. Louis, MO: Washington University School of Medicine; 1996. [Google Scholar]
- Saha TD, Chou SP, Grant BF. Toward an alcohol use disorder continuum using item response theory: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychological Medicine. 2006;36:931–941. doi: 10.1017/S003329170600746X. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. SAS/STAT® 9.2 user’s guide. Cary, NC: SAS Institute Inc; 2008. [Google Scholar]
- Schuckit MA. Research Questionnaire. San Diego, CA: Alcoholism Treatment Program, V.A. Medical Center, University of California; 1978. [Google Scholar]
- Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. The American Journal of Psychiatry. 1994;151:184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Selzer ML, Vinokur A, van Rooijen L. A self-administered Short Michigan Alcoholism Screening Test (SMAST) Journal of Studies on Alcohol. 1975;36:117–126. doi: 10.15288/jsa.1975.36.117. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Wagner EF, Lloyd DA, Gil AG. Racial/ethnic and gender differences in the incidence and onset age of DSM-IV alcohol use disorder symptoms among adolescents. Journal of Studies on Alcohol. 2002;63:609–619. doi: 10.15288/jsa.2002.63.609. [DOI] [PubMed] [Google Scholar]
- Wagner FA, Anthony JC. From first drug use to drug dependence: Developmental periods of risk for dependence upon marijuana, cocaine, and alcohol. Neuropsychopharmacology. 2002;26:479–488. doi: 10.1016/S0893-133X(01)00367-0. [DOI] [PubMed] [Google Scholar]
- Wagner FA, Anthony JC. Male-female differences in the risk of progression from first use to dependence upon cannabis, cocaine, and alcohol. Drug and Alcohol Dependence. 2007;86:191–198. doi: 10.1016/j.drugalcdep.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Wing JK, Babor T, Brugha T, Burke J, Cooper JE, Giel R, et al. SCAN-Schedules for Clinical Assessment in Neuropsychiatry. Archives of General Psychiatry. 1990;47:589–593. doi: 10.1001/archpsyc.1990.01810180089012. [DOI] [PubMed] [Google Scholar]
- Zernicke KA, Cantrell H, Finn PR, Lucas J. The association between earlier age of first drink, disinhibited personality, and externalizing psychopathology in young adults. Addictive Behaviors. 2010;35:414–418. doi: 10.1016/j.addbeh.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RA, Ellis DA, Fitzgerald HE, Bingham CR, Sanford K. Other evidence for at least two alcoholisms: II. Life course variation in antisociality and heterogeneity of alcoholic outcome. Development and Psychopathology. 1996;8:831–848. [Google Scholar]
- Zucker RA, Fitzgerald HE, Noll RB. Drinking and Drug History Version 4. East Lansing, MI: Michigan State University; 1990. [Google Scholar]
- Zucker RA, Fitzgerald HE, Refior SK, Puttler LI, Pallas DM, Ellis DA. The clinical and social ecology of childhood for children of alcoholics: Description of a study and implications for a differentiated social policy. In: Fitzgerald HE, Lester BM, Zuckerman BS, editors. Children of Addiction. New York, NY: Garland Press; 2000. pp. 109–142. [Google Scholar]