Abstract
We recorded electroencephalogram (EEG; 6–9 Hz) and heart rate (HR) from infants at 5 and 10 months of age during baseline and performance on the looking A-not-B task of infant working memory (WM). Longitudinal baseline-to-task comparisons revealed WM-related increases in EEG power (all electrodes) and EEG coherence (medial frontal-occipital electrode pairs) at both ages. WM-related decreases in HR were only present at 5 months, and WM-related increases in EEG coherence became more localized by 10 months. Regression analyses revealed that baseline-to-task changes in psychophysiology accounted for variability in WM performance at 10, but not 5, months. HR and EEG power (medial frontal and lateral frontal electrodes) were unique predictors of variability in 10-month WM performance. These findings are discussed in relation to frontal lobe development, and represent the first comprehensive longitudinal analysis of age-related changes in the behavioral and psychophysiological correlates of WM.
Keywords: working memory, EEG power, EEG coherence, heart rate, infants
The frontal cortex is intricately linked to higher order cognitive processes, such as executive functions (i.e., working memory (WM), inhibitory control, cognitive flexibility; Kane & Engle, 2002; Osaka et al., 2003). During the first year, there are drastic changes in frontal lobe maturation: synaptogenesis, dendritic and axonal growth, myelination, increases in glucose metabolism (Chugani, Phelps, & Mazziotta, 1987; Deoni et al., 2011; Huttenlocher & Dabholkar, 1997; Tsekhmistrenko, Vasil’eva, Shumeiko, & Vologirov, 2004). Infant researchers have proposed that the maturation of the frontal cortex and associated neural circuitry late in the first year underlies the emergence of higher order cognitive processes (Colombo & Cheatham, 2006; Diamond, 1990). We are particularly interested in how the emergence of WM during the first year is related to frontal functioning.
WM research has emphasized the role of the prefrontal cortex within a distributed fronto-parietal neural network (e.g., Linden et al., 2003; Thomas et al., 1999). In the present study, we examined frontal functioning via measures of brain electrical activity (electroencephalogram (EEG) power), functional connectivity (EEG coherence), and cardiac activity (heart rate; HR) that were recorded during baseline and WM processing (i.e., looking A-not-B task). Infants were tested at 5 and 10 months because these ages represent distinct periods in WM development (Cuevas & Bell, 2010; Pelphrey et al., 2004) and in frontal lobe maturation. Thus, we could directly investigate whether measures of frontal functioning (a) were differentially activated during WM processing early and late in the first year, and (b) were associated with individual differences in WM early and late in the first year. In the following paragraphs, we first discuss infant WM tasks (i.e., A-not-B, delayed response (DR)), highlighting age-related changes in WM between 5 and 10 months. Next, we review the literature on the psychophysiological correlates of the A-not-B and DR tasks, focusing on infant EEG and HR research. Finally, we note gaps in the infant WM literature that the present study addressed.
Infant WM: Behavior
During the A-not-B task, infants watch as a desirable object is hidden in one of two possible locations, a brief delay follows, and then infants search for the object. Typically, the object is hidden in one location (A) until the infant searches correctly, and then the hiding location is switched (B). The A-not-B error occurs when infants reach to the incorrect location (A) on reversal trials (B). The A-not-B task is a variant of the DR task that has been used with human and nonhuman animals to examine WM (see Pelphrey & Reznick, 2003, for review). The tasks are methodologically identical (except that the reward hiding location is predetermined— independent of participant responding—for the DR task), with very similar age-related changes in performance (humans: Diamond & Doar, 1989; nonhuman primates: Diamond, 1990).
There are individual differences in WM performance throughout the first year (Diamond, 1985). Five months is the youngest age that DR and A-not-B data have been collected, and provides a relative “baseline” for WM performance—the majority of 5-month-olds perform poorly (e.g., Baird et al., 2002; Cuevas & Bell, 2010; Reznick, Morrow, Goldman, & Snyder, 2004). Performance on reversal trials (with a minimal delay between hiding and searching) is normally distributed at 8 months of age (Bell & Fox, 1992; Diamond, 1985). By 10 months, infants exhibit a “basic level” of WM performance—the majority of infants are successful on reversal trials with a minimal delay and some infants succeed with longer delays (Cuevas & Bell, 2010; Diamond, 1985; Matthews, Ellis, & Nelson, 1996).
Infant WM: Psychophysiology
Early research on the neural correlates of A-not-B and DR performance—lesion studies with adult and infant nonhuman primates—indicated that performance was mediated by the dorsolateral prefrontal cortex (Diamond, 1990; Diamond & Goldman-Rakic, 1989). Subsequent near-infrared spectroscopy and EEG research with human infants confirmed the contribution of the frontal areas to A-not-B performance (Baird et al., 2002; Bell, 2001, 2002, in press; Bell & Fox, 1992, 1997).
EEG
The 6–9 Hz frequency band has been used by infant EEG researchers to investigate both cognitive and emotional processing (Bell, 2001, in press; Fox, Henderson, Rubin, Calkins, & Schmidt, 2001; Orekhova, Stroganova, & Posikera, 2001). EEG power is a measure of the excitability of neuronal groups. For both infants and adults, task-related changes in cortical activation are considered indicative of cognitive processing (e.g., Bell, 2001; Klimesch, Doppelmayr, Schimke, & Ripper, 1997; Orekhova et al., 2001). Infants tested with the looking A-not-B task exhibit task-related increases in EEG power for all electrodes (Bell, 2001, 2002; Cuevas & Bell, 2011). By 4.5 years, children exhibit more localized changes in EEG power during WM processing (Bell & Wolfe, 2007).
Colombo and Cheatham (2006) have proposed that integrative frontal functioning (i.e., frontal coordination of specialized brain regions, presumably via neural circuitry) provides the foundation for higher order cognitive processes. EEG power, however, does not provide information about functional cortico-cortical connections during WM processing. EEG coherence is the frequency-dependent squared cross-correlation between two scalp electrode sites (range: 0–1) and is a measure of functional connectivity between two neuronal groups (Thatcher, Walker, & Giudice, 1987; see Pizzagalli, 2007, for review). If the activity at two electrode sites is synchronized then coherence values approach 1, and if there is no synchronization then coherence values approach 0.
Previous infant research has measured frontal EEG coherence to examine potential alterations in frontal functional connectivity during WM processing. These findings, however, were not as straightforward as the EEG power findings. Infants exhibited either minimal evidence of WM-related changes in frontal coherence (Bell, 2001; Cuevas & Bell, 2011) or different patterns of WM-related changes in frontal coherence as a function of performance and electrode pairing (Bell, in press). Additional research is necessary to determine whether infants exhibit task-related changes in frontal EEG coherence during WM processing, and whether these findings are consistent throughout the first year.
HR
Peripheral measures, such as HR, have also been used to examine infant cognition (see Reynolds & Richards, 2008, for review). The brain modulates HR via activation of the parasympathetic and sympathetic nervous systems. According to the neurovisceral integration model, the prefrontal cortex interacts with other cortical and subcortical areas via direct and indirect pathways to modulate autonomic activity (see Thayer & Lane, 2009, for review). This model is supported by pharmacological and neuroimaging evidence of prefrontal-autonomic associations (e.g., Ahern et al., 2001; Gianaros, Van Der Veen, & Jennings, 2004)
In general, HR increases during tasks requiring cognitive processing (i.e., information processing and manipulation) and HR decreases during attention to environmental stimuli (Lacey & Lacey, 1974). Accordingly, research with children and adults has revealed task-related increases in HR during cognitively challenging WM tasks (e.g., Gianaros et al., 2004; Wolfe & Bell, 2004). Two infant studies have examined HR changes during WM processing (Bell, in press; Cuevas & Bell, 2011), but only one revealed WM-related changes in HR. The direction of the WM-related change at 8 months of age varied as a function of WM performance; high performers exhibited task-related increases in HR and low performers exhibited task-related decreases in HR (Bell, in press). Thus, although HR is potentially related to WM processing, additional research is necessary to further understand how these measures are related throughout the first year.
The Current Study
Integrative frontal functioning is posited to provide the foundation for higher order cognitive processes (Colombo & Cheatham, 2006; Luna & Sweeney, 2004). Although there is separate evidence of frontal maturation (e.g., Chugani et al., 1987; Deoni et al., 2011) and improvements in WM during the first year (e. g., Cuevas & Bell, 2010; Diamond, 1985; Matthews, et al., 1996), analysis of age-related changes in frontal functioning during WM processing is essential to our understanding of the emergence of integrated frontal functioning. Thus, in our first set of analyses, we asked whether measures of frontal functioning (EEG power, EEG coherence, HR) were differentially activated during WM processing early and late in the first year. Our sample size ensured sufficient statistical power to reveal task-related changes (and interactions with age) for all measures (see Cuevas & Bell, 2011). We hypothesized task-related increases in EEG power at multiple frontal and non-frontal electrode sites, with more localized activation at 10 months (see Bell & Wolfe, 2007). Although children exhibit WM-related increases in frontal EEG coherence and HR (Bell & Wolfe, 2007; Wolfe & Bell, 2004), inconsistent findings have emerged during infancy (Bell, 2001, in press; Cuevas & Bell, 2011).
Our second set of analyses focused on individual differences in task performance. During childhood, WM capacity is related to frontal myelination and frontal brain activity (Klingberg, Forssberg, & Westerberg, 2002; Nagy, Westerberg, & Klingberg, 2004; Wolfe & Bell, 2004, 2007). During infancy, Bell (in press) found that WM-related changes in EEG coherence (medial frontal-medial parietal) and HR are unique predictors of 8-month WM performance. However, EEG power was not measured, and only one EEG coherence pairing was included in the regression analysis because of the moderate sample size. It is unknown whether WM-related measures of EEG and HR are associated with individual differences in WM performance for infants younger or older than 8 months. In the present study, we asked if WM-related changes in EEG power, EEG coherence, and HR could predict concurrent WM performance at 5 and 10 months. The present study is the first study with a sufficient sample size to provide a comprehensive analysis of the contribution of multiple psychophysiological measures in predicting WM performance.
We hypothesized that EEG and HR measures will account for variability in 10-month WM. Five-month-olds, however, typically perform at near floor levels (Cuevas & Bell, 2010; Reznick et al., 2004), and there may not be sufficient variability in WM for psychophysiological variables to predict performance. If the 5-month model is significant, then we predict that psychophysiological measure will account for more variability in WM performance at 10 months than at 5 months. Finally, because of the well-established role of the frontal cortex in WM performance, we hypothesized that frontal EEG measures will account for a unique percentage of variance in 10-month WM performance.
Method
Participants
As part of a longitudinal study examining individual differences in the development of executive functions across early development, 410 healthy full-term infants (209 girls, 201 boys; 26 Hispanic, 383 Non-Hispanic, 1 Not reported; 315 Caucasian, 56 African American, 32 Multi Racial, 2 Asian, 1 Other, 4 Not Reported) were recruited by the two research locations (XXX, XX; YYY, YY), with each location recruiting half of the total sample. For parents who reported educational information (404 mothers, 392 fathers), 99% of mothers and 97% of fathers graduated from high school (6 % and 7% technical degree; 42% and 31% bachelor’s degree; 22% and 24% graduate degree; respectively). Mothers and fathers were approximately 29 and 32 years old (SD = 6 and 7), respectively, when the infants were born. Infants were recruited via commercial mailing lists, newspaper birth announcements, and word of mouth. All infants were born within 15 days of their calculated due dates and were healthy at the time of testing. Infants’ mean age (in days) was 162 (SD = 8) and 314 (SD = 11) at 5 and 10 months, respectively. Parents were paid for each laboratory visit.
Data were collected in both research locations using identical protocols. Research assistants from both locations were trained together by the second author on protocol administration, as well as on behavioral and psychophysiological coding. To ensure that identical protocol administration was maintained between the labs, the XX team periodically viewed DVD recordings and psychophysiology files collected by the YY lab. To ensure that identical coding criteria were maintained between labs, the XX lab provided reliability coding (percentage of trial-by-trial agreement for 20% of YY lab’s sample was 96.7% and 98.5% at 5 and 10 months, respectively) for behavioral data and verification of artifact screening for psychophysiology data collected and coded by the YY lab.
Procedure
EEG recording
EEG was recorded during baseline and during the looking A-not-B task. Recordings were made from 16 left and right scalp sites: frontal pole (Fp1, Fp2), medial frontal (F3, F4), lateral frontal (F7, F8), central (C3, C4), temporal (T7, T8), medial parietal (P3, P4), lateral parietal (P7, P8), and occipital (O1, O2). All electrode sites were referenced to Cz during recording. EEG was recorded using a stretch cap (Electro-Cap, Inc.) with electrodes in the 10/20 system pattern (Jasper, 1958; Pizzagalli, 2007). After the cap was placed on the infant’s head, recommended procedures regarding EEG data collection with infants were followed (Fox, Schmidt, Henderson, & Marshall, 2007; Pivik et al., 1993). Specifically, a small amount of abrasive was placed into each recording site and the scalp gently rubbed. Following this, conductive gel was placed in each site. Electrode impedances were measured and accepted if they were below 10K ohms.
The electrical activity from each lead was amplified using separate SA Instrumentation Bioamps (San Diego, CA) and bandpassed from .1 to 100 Hz. Activity for each lead was displayed on the monitor of an acquisition computer. The EEG signal was digitized on-line at 512 samples per second for each channel so that the data were not affected by aliasing. The acquisition software was Snapshot-Snapstream (HEM Data Corp.; Southfield, MI) and the raw data were stored for later analyses.
EEG analysis
EEG data were examined and analyzed using EEG Analysis System software developed by James Long Company (Caroga Lake, NY). First, the data were re-referenced via software to an average reference configuration (Lehmann, 1987). Average referencing, in effect, weighted all the electrode sites equally and eliminated the need for a noncephalic reference. Active (F3, F4, etc.) to reference (Cz) electrode distances vary across the scalp. Without the re-referencing, power values at each active site may reflect interelectrode distance as much as they reflect electrical potential. The average reference configuration requires that a sufficient number of electrodes be sampled and that these electrodes be evenly distributed across the scalp. Currently, there is no agreement concerning the appropriate number of electrodes (Davidson, Jackson, & Larson, 2000; Hagemann, Naumann, & Thayer, 2001; Luck, 2005), although the 10/20 configuration that we used does satisfy the requirement of even scalp distribution.
The re-referenced EEG data were artifact scored for eye blinks using Fp1 and Fp2 (Myslobodsky et al., 1989) and for gross motor movements and these artifact-scored epochs were eliminated from all subsequent analyses. The data then were analyzed with a discrete Fourier transform (DFT) using a Hanning window of 1-s width and 50% overlap. Power was computed for the 6–9 Hz frequency band. The power was expressed as mean square microvolts and the data were transformed using the natural log (ln) to normalize the distribution. Coherence between medial frontal and all other electrode sites within each hemisphere was computed for the 6–9 Hz band using an algorithm by Saltzberg, Burton, Burch, Fletcher, and Michaels (1986). We chose to only examine medial frontal pairings because of the well-established role of the frontal cortex during the A-not-B task and to limit the potential number of electrode pairings for statistical analysis.
HR recording
HR also was measured during baseline and the looking A-not-B task from two neonatal disposable electrodes using modified lead II (right collarbone and lower left rib; Stern, Ray, & Quigley, 2001), grounded at the scalp near electrode site Fz. The cardiac electrical activity was amplified using a SA Instrumentation Bioamp and the QRS complex was displayed on the acquisition computer monitor along with the EEG data. The cardiac signal was digitized at 512 samples per second. The acquisition software was Snapshot-Snapstream (HEM Data Corp.) and the raw data were stored for later analyses.
HR analysis
Heart data were examined and analyzed using IBI Analysis System software developed by James Long Company. First, R waves were detected and movement artifact, designated by the absence of at least three consecutive R waves, was scored. These artifact-scored epochs were eliminated from all cardiac calculations. HR was calculated as beats per minute (bpm).
Data available for analysis
To be included in our final sample, it was necessary for infants to provide behavioral and psychophysiological data at either 5 or 10 months. At 5 months, 403 infants visited the laboratory: Behavioral data were available for 400 infants (i.e., losses due to video recording error and fussiness), EEG and HR electrodes were accepted by 397 infants, and psychophysiological data were available for 379 infants (i.e., 12 low-quality physiological recordings; 6 equipment failure). The psychophysiological data were screened for motor artifacts, and 313 five-month-olds had sufficient artifact-free EEG (≥ 10 dfts) and HR (≥ 10 s) data (baseline and task) for subsequent analyses.
At 10 months, 365 infants returned to the lab (i.e., 22 parents were busy/declined participation; 12 families could not be located; 9 families moved out of the local area; 2 infants were 12 months at visit). Behavioral data were available for 364 infants (i.e., loss due to fussiness), EEG and HR electrodes were accepted by 363 infants, and psychophysiological data were available for 353 infants (i.e., 7 low-quality physiological recordings; 2 equipment failure; 1 experimenter error). Thus, at 10 months, 306 infants had sufficient artifact-free EEG and HR data (baseline and task) for subsequent analyses.
Baseline
Baseline EEG and HR were recorded for 1 min while the infant sat on the mother’s lap. During the baseline recording, a research assistant manipulated a toy containing brightly colored balls on top of the testing table, 1.1 m in front of the infant. This procedure quieted the infant and yielded minimal eye movements and gross motor movements. Mothers were instructed not to talk to infants during the EEG/HR recording. Immediately after baseline, the recording of EEG and HR continued as the A-not-B task was administered.
Looking A-not-B task
Much like the reaching version of the infant A-not-B task (Diamond 1985; Diamond, Prevor, Callender, & Druin, 1997), the looking version requires the infant to constantly update memory of where the toy was hidden through a series of displacements and to inhibit looking back toward a previously rewarded hiding place (Bell & Adams, 1999). Thus, the infant "searched" for a hidden toy by making an eye movement to one of two possible hiding locations (e.g., Bell, 2002; Bell & Adams, 1999; Matthews et al., 1996; Pelphrey et al., 2004).
The testing apparatus for the looking task was a table measuring 90 cm (L)×60 cm (W)×75 cm (H) and the hiding sites were brightly colored plastic tubs measuring approximately 15 cm in diameter and 12 cm deep. The infant sat on the parent's lap 1.1 m from the edge of the testing table as the experimenter manipulated a noise-making toy, placed it on the table, and then simultaneously placed two (17.5 cm on either side of midline) bright orange or blue plastic tubs on the table (Diamond, Cruttenden, & Neiderman, 1994). One tub covered the toy, and the other covered the alternative hiding site.
The looking procedure is detailed in Bell and Adams (1999). After the toy was hidden, the infant's gaze to the hiding site was broken and brought to the experimenter’s face at midline by the experimenter calling the infant's name and asking, "Where's the toy?". The direction of the infant's first eye movement after being brought to midline was scored as either correct or incorrect. A video camera was placed behind and above the experimenter's head and focused so as to maintain a close-up view of the infant's face.
To begin, each infant was required to successfully make an eye movement toward a single tub completely covering the hidden toy to be declared competent at participating in the task. This criterion was met by all 10-month-olds and 139 five-month-olds. Noise-making toys were used to maintain the infant’s attention to the task during the hidings. Because infants were not allowed to manipulate the toys themselves, the experience they received from viewing the toy had to provide the impetus to continue to search for the toy. (Noise-making toys were silent when they were hidden.) Infants were rewarded for a correct eye movement with praise and clapping from the experimenter. After an incorrect eye movement, the experimenter sighed, blandly told the infant, “It’s over here.”, and showed the toy to the infant.
After the infant successfully looked for the hidden toy with one tub, the standard A-not-B procedure began. Our procedure was modeled after the standard two-location task commonly used in the developmental psychology literature; i.e., identical covers and backgrounds, two hiding locations horizontally oriented, and object hidden in the same location on all A trials and then hidden at the other location for the B trial (Marcovitch & Zelazo, 1999; Wellman, Cross, & Bartsch, 1986).
Initial side of hiding was counterbalanced among infants and the pattern of toy placement was determined by the infant’s performance. Two consecutive successful eye movements toward the same side (for example, toward the infant’s right) resulted in the toy then being hidden under the tub on the opposite side (toward the infant’s left; i.e., Right-Right-Left). Regardless of whether or not the infant was successful on the “reversal” trial, new “same-side” trials commenced at the reversal site and continued until two consecutive successful eye movements were executed, initiating another reversal (i.e., L-L-R). Thus, flawless performance by an infant would result in this pattern of trials: R-R-L-L-L-R-etc. In reality, most infants were not flawless in performance and needed multiple same-side trials in order to achieve two consecutive successful eye movements prior to reversal trials.
Infants who made an eye movement toward the correct tub on reversal trials in two out of three attempts were then tested with a delay. The delay was initiated during the time the infant's gaze to the hiding site was broken and brought to the experimenter’s face at midline. In the delay condition, the experimenter called the infant's name, counted out the delay period, and then asked, "Where's the toy?". Subsequent delays were initiated until the infant looked at the incorrect tub in two out of three reversal trials at any given delay. Delay was incremented in 2- second intervals. In reality, even during the “no delay” trials at the beginning of the assessment the infants experienced a brief delay as their gaze to the tub was broken and brought to midline. This brief delay was unavoidable because of the necessity of breaking the infant’s gaze so that the direction of their first eye movement could be determined. Assessment terminated when the infant made an eye movement toward the incorrect tub in two out of three reversal trials.
A research assistant coded each infant’s performance from a videotape/DVD of the laboratory session, and a second independent coder provided reliability coding for 19% and 24% of infants included in the final sample at 5 and 10 months, respectively. The percentage of trials in which the two coders agreed was 92.9% (5 months) and 98.3% (10 months). An event marker was used during electrophysiological recordings to indicate which portions of the electrophysiological record were associated with the most cognitively demanding sections of the looking task. The task-related EEG and HR used in data analyses was that associated with the use of WM (Bell, 2001, 2002). The task-related EEG and HR started with the covering of the hiding site(s) with the tubs, continued through the distraction and delay periods, and stopped when the experimenter lifted a tub prior to giving the infant appropriate verbal feedback. Thus, electrophysiological data not included in the analyses were those recorded when the experimenter was (a) manipulating the toy prior to hiding it and (b) giving appropriate feedback to the infant after the infant’s eye movement to one of the hiding sites. The artifact-free EEG and HR data from all trials, correct and incorrect, were used in the analyses. The average number of trials (combining nonreversal and reversal trials) from which electrophysiological data were collected was 5.18 (SD = 2.87) and 12.60 (SD = 3.92) per infant at 5 and 10 months, respectively.
Results
Longitudinal WM Processing Analyses: Behavior
We analyzed infants’ performance on nonreversal and reversal trials by calculating the proportion of correct responses for each trial type (e.g., Bell & Adams, 1999; Cuevas & Bell, 2010; Hofstadter & Reznick, 1996). For nonreversal trials, a repeated-measures analysis of variance (ANOVA)1 revealed a significant main effect for age, F(1, 110) = 14.51, p < .001, ηp2= .12, such that performance on nonreversal trials was higher at 10 months (M = .69, SD = .19) than at 5 months (M = .58, SD = .22). Infants were presented with reversal trials only after correctly searching on two consecutive nonreversal trials. This criterion was met by 52 (37%) and 292 (95%) infants at 5 and 10 months, respectively; 42 infants had reversal data at both ages. For reversal trials (5 months: M = .27, SD = .39; 10 months: M = .33, SD = .36), a repeated-measures ANOVA failed to reveal a significant main effect for age, F(1, 41) = .75, p = .392. Separate repeated-measures ANOVAs compared performance on reversal and nonreversal trials at 5 and 10 months, F(1, 51) = 50.65, p < .001, ηp2= .50 and F(1, 291) = 173.74, p < .001, ηp2= .37, respectively. At both ages, performance was higher on nonreversal trials (5 months: M = .75, SD = .18; 10 months: M = .68, SD = .19) as compared to reversal trials (5 months: M = .27, SD = .40; 10 months: M = .41, SD = .36).
Finally, we combined performance across trial type to provide a comprehensive measure of WM performance that maximizes the amount of available data. (For this reason, total performance was also the measure of interest in subsequent regression analyses.) The total proportion of correct responses was determined by summing the number of correct responses on nonreversal and reversal trials and then dividing by the total number of trials. A repeated-measures ANOVA of total proportion of correct responses revealed a significant main effect for age, F(1, 110) = 14.72, p < .001, ηp2= .12. Total performance was higher at 10 months (M = .64, SD = .17) than at 5 months (M = .54, SD = .18).
Longitudinal WM Processing Analyses: Psychophysiology
Statistical analysis
Our first set of psychophysiological analyses consisted of repeated-measures multivariate analysis of variance (MANOVAs) with region, hemisphere, age, and condition as within-subjects factors. Of major interest were main effects and interactions involving age (development) and condition (baseline vs. WM processing) factors. Region was of interest for all power and coherence analyses as our EEG hypotheses were focused on specific scalp locations.
EEG power
A repeated-measures MANOVA was completed on the ln 6–9 Hz EEG power values. The within-subjects factors were age (i.e., 5 or 10 months), condition (i.e., baseline or task), region (i.e., frontal pole, medial frontal, lateral frontal, temporal, lateral parietal, medial parietal, or occipital), and hemisphere (i.e., left or right). For this overall MANOVA, there were main effects for age, F(1, 99) = 165.98, p < .001, ηp2= .63, condition, F(1, 99) = 73.11, p < .001, ηp2= .43, region, F(6, 94) = 247.13, p < .001, ηp2= .94, and hemisphere, F(1, 99) = 6.06, p = .016, ηp2= .06. There were also Age × Region, F(6, 94) = 16.72, p < .001, ηp2= .52, Condition × Region, F(6, 94) = 7.17, p < .001, ηp2= .31, Region × Hemisphere, F(6, 94) = 5.13, p < .001, ηp2= .25, and Age × Region × Hemisphere, F(6, 94) = 3.13, p = .008, ηp2= .17, interactions.
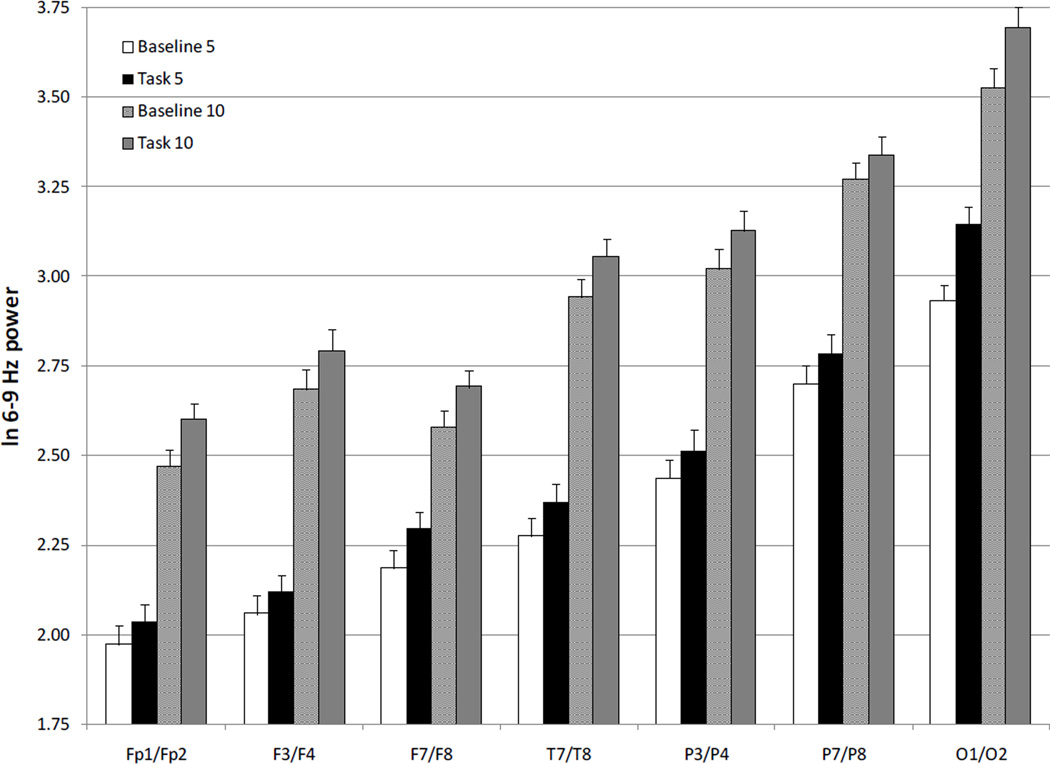
Post-hoc analyses, paired t tests (p < .05), were completed to examine the Condition × Region interaction, and thus determine whether task-related increases in EEG power would occur at numerous anterior and posterior scalp locations. As can be seen in Figure 1, there were task-related increases in power at all electrode sites. Furthermore, post-hoc analyses (p < .05) confirmed that there were age-related increases in power at all electrode sites (Figure 1).
Figure 1.
EEG ln power values (S.E.) at 6–9 Hz for baseline and working memory task at 5 and 10 months.
EEG coherence
A repeated-measures MANOVA was completed on the 6–9 Hz EEG coherence values. The within-subjects factors were age, condition, regional electrode pair (i.e., frontal pole-medial frontal, medial frontal-lateral frontal, medial frontal-temporal, medial frontal-medial parietal, medial frontal-lateral parietal, or medial frontal-occipital), and hemisphere. For this overall MANOVA, there were main effects for age, F(1, 99) = 20.03, p < .001, ηp2= .17, condition, F(1, 99) = 13.22, p < .001, ηp2= .12, and regional pair, F(5, 95) = 409.73, p < .001, ηp2= .96. There were also Age × Condition, F(1, 99) = 5.26, p = .024, ηp2= .05, Age × Regional Pair, F(5, 95) = 7.82, p < .001, ηp2= .29, Regional Pair × Hemisphere, F(5, 95) = 6.13, p < .001, ηp2= .24, and Age × Regional Pair × Hemisphere, F(5, 95) = 3.44, p = .007, ηp2= .15, interactions. The main effects and interactions involving age and condition were superseded by an Age × Condition × Regional Pair interaction, F(5, 95) = 4.03, p = .002, ηp2= .18.
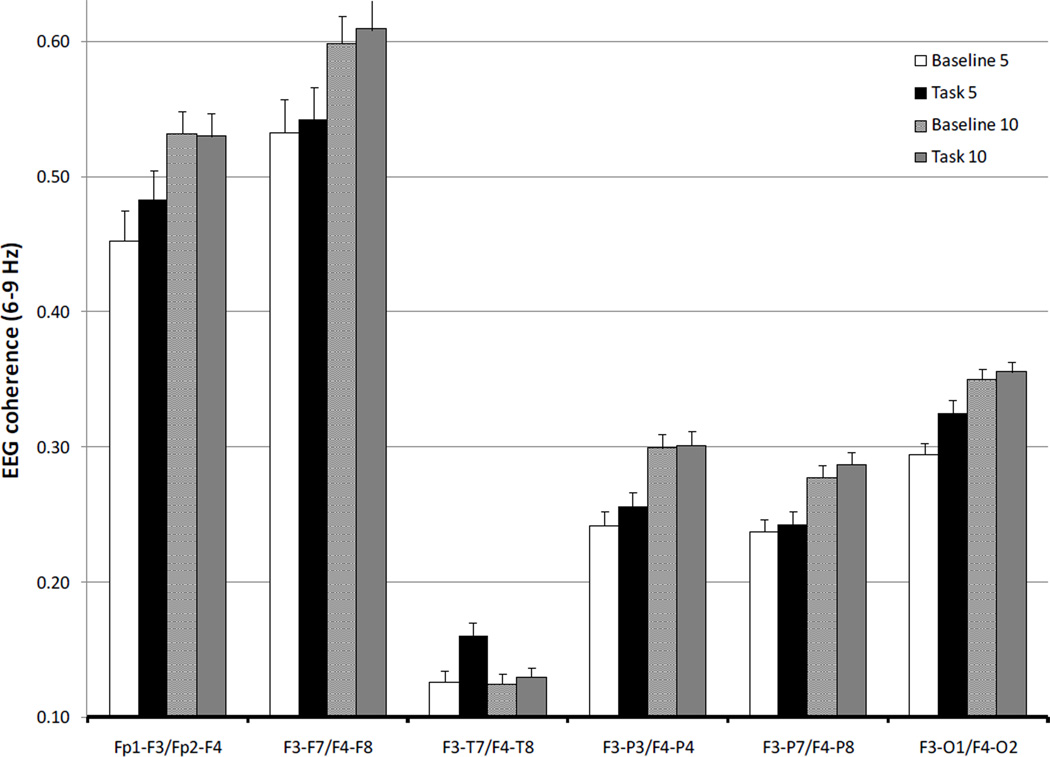
To examine the Age × Condition × Regional Pair interaction, separate MANOVAs (within-subjects factors: age, condition) were performed on the coherence values for each regional pair. Because there were no significant interactions involving both condition (our primary variable of interest) and hemisphere, we averaged regional pair coherence data across hemispheres to limit the number of statistical comparisons. The results of the regional pair MANOVAs are displayed in Table 1. Only main effects and interactions involving age and condition are highlighted. As can be seen in Figure 2, there were age-related increases in coherence values for all electrode pairs (ηp2= .06 – .18). In addition, there was a main effect for condition (ηp2= .06 – .15) for frontal pole-medial frontal, medial frontal-temporal, and medial frontal-occipital electrode pairs; coherence values were higher during task than during baseline (Figure 2).
Table 1.
Cognitive Processing Analysis of EEG Coherence: Summary of Multivariate Analyses F Values for Baseline and Task Activation Comparisons.
| df | Age | Condition | Age×Condition | |
|---|---|---|---|---|
| Fp1-F3/Fp2-F4 | 1, 110 | 6.93** | 6.49* | 6.14* |
| F3-F7/F4-F8 | 1, 109 | 6.36* | ||
| F3-T7/F4-T8 | 1, 109 | 6.36* | 19.59*** | 10.22** |
| F3-P3/F4-P4 | 1, 107 | 21.06*** | ||
| F3-P7/F4-P8 | 1, 106 | 23.23*** | ||
| F3-O1/F4-O2 | 1, 106 | 22.29*** | 14.21*** |
Note.
p ≤ .001;
p ≤ .01;
p ≤ .05.
Fp1/Fp2 = frontal pole; F3/F4 = medial frontal; F7/F8 = lateral frontal; T7/T8 = temporal; P3/P4 = medial parietal; P7/P8 = lateral parietal; O1/O2 = occipital. Only significant F-values are reported in the table.
Figure 2.
EEG coherence values (S.E.) at 6–9 Hz for baseline and working memory task at 5 and 10 months.
There was an Age × Condition interaction (ηp2= .05 – .09) for the frontal pole-medial frontal and medial frontal-temporal electrode pairs. Post-hoc analyses (p < .05) revealed that infants exhibited task-related increases in coherence for both electrode pairs at 5, but not 10, months of age (Figure 2).
HR
A repeated-measures MANOVA (within-subjects factors: age, condition) for HR revealed a main effect for age, F(1, 110) = 134.86, p < .001, ηp2= .55, and an Age × Condition interaction, F(1, 110) = 7.46, p = .007, ηp2= .06. HR was higher at 5 months (baseline: M = 135.94, SE = .94; task: M = 134.38, SE = .93) than at 10 months (baseline: M = 124.60, SE = .89; task: M = 125.05, SE = .85). Post-hoc analyses (p < .05) revealed that at 5 months, infants exhibit WM-related decreases in HR, but at 10 months, there were no WM-related changes in HR.
Regression Analyses
Our psychophysiological MANOVAs revealed a main effect of condition for the majority of psychophysiological measures. Accordingly, baseline-to-task change scores were calculated for each psychophysiological measure by subtracting the baseline value from the task value (i.e., task minus baseline). EEG power and coherence change scores were then averaged across hemispheres because there were no significant Condition × Hemisphere interactions. Thus, our final set of analyses involved the entry of EEG and HR baseline-to-task change scores as independent variables in multiple regression analyses predicting A-not-B performance (total proportion of correct responses2) at 5 and 10 months3.
5 months: psychophysiology predicting WM performance
A multiple regression analysis revealed that EEG power, EEG coherence, and HR baseline-to-task change scores did not account for significant variance in concurrent WM performance, F(14, 131) = 1.19, p = .293.
10 months: psychophysiology predicting WM performance
The results from a multiple regression analysis are displayed in Table 2. EEG power, EEG coherence, and HR baseline-to-task change scores accounted for 11% of variance in 10-month WM performance. An examination of the regression weights revealed that task-related changes in medial frontal and lateral frontal EEG power and HR accounted for unique variance in 10-month WM (Table 2).
Table 2.
Results of Regression Analysis Predicting 10-Month Working Memory Performance from 10-Month Psychophysiology.
| R | R2 | F | β | t | sr | sr 2 | |
|---|---|---|---|---|---|---|---|
| Dependent variable: Total proportion correct at 10 months | |||||||
| EEG Power (Baseline-to-Task Change) | .33 | .11 | 2.47** | ||||
| Fp1/Fp2 | .14 | 1.85 | .10 | ||||
| F3/F4 | .25 | 2.33* | .13 | .02 | |||
| F7/F8 | −.28 | −3.28*** | −.19 | .03 | |||
| T7/T8 | .02 | .25 | .01 | ||||
| P3/P4 | .00 | −.04 | .00 | ||||
| P7/P8 | .01 | .06 | .00 | ||||
| O1/O2 | −.11 | −1.46 | −.08 | ||||
| EEG Coherence (Baseline-to-Task Change) | |||||||
| Fp1-F3/Fp2-F4 | .03 | .41 | .02 | ||||
| F3-F7/F4-F8 | −.02 | −.27 | −.02 | ||||
| F3-T7/F4-T8 | .02 | .41 | .02 | ||||
| F3-P3/F4-P4 | −.09 | −1.22 | −.07 | ||||
| F3-P7/F4-P8 | .01 | .17 | .01 | ||||
| F3-O1/F4-O2 | −.01 | −.10 | −.01 | ||||
| ECG (Baseline-to-Task Change) | |||||||
| HR | .27 | 4.29*** | .24 | .06 |
Note:
p ≤ .001,
p ≤ .01,
p < .05.
Only sr2 values ≥ .01 are reported. n = 290.
Discussion
We used a longitudinal design to investigate the psychophysiological correlates (i.e., EEG power, EEG coherence, HR) of infant WM processing at 5 and 10 months of age. Analyses yielded valuable information regarding possible age-related changes in the psychophysiological correlates of WM. Regression analyses revealed that WM-related changes in psychophysiology accounted for variability in 10-month, but not 5-month, WM performance. These data provide a unique contribution to the infant WM literature; this is the first analysis of age-related changes in the psychophysiological measures that account for variability in concurrent WM performance.
WM Processing
The A-not-B task requires infants to constantly update their memory of where a desirable object was hidden through a series of displacements and to inhibit looking back toward a previously rewarded hiding place (Bell & Adams, 1999). We found that infants exhibited substantial improvements in WM between 5 and 10 months of age. These findings are consistent with previous longitudinal research (Cuevas & Bell, 2010; Diamond, 1985; Matthews et al., 1996) and confirm that the two ages selected for the present study represent considerably different levels of WM. Although the same guidelines were used for task administration at both ages, the extremely low levels of 5-month performance resulted in two related, but different, types of WM data. Overall, 5-month data represent WM during nonreversal trials, and 10-month data represent WM (and inhibitory processes) during reversal and nonreversal trials—most similar to the traditional A-not-B task. Thus, although WM processes are recruited during both reversal and nonreversal trials of the incremental-delay A-not-B task, additional inhibitory processes are likely recruited during reversal trials.
Between 5 and 10 months, infants exhibited age-related increases in EEG power and coherence as well as age-related decreases in HR. Similar developmental trends have been noted in other samples (e.g., Bar-Haim, Marshall, & Fox, 2000; Bell, 1998; Cuevas & Bell, 2011). In fact, age-related changes in baseline EEG are considered reflective of brain maturation (i.e., scalp electrical activity related to cortical activity; see Bell, 1998; Bell & Fox, 1994, for reviews).
Our EEG power findings replicated previous research (Cuevas & Bell, 2011); infants exhibited WM-related increases in EEG power (all electrodes) at both 5 and 10 months. At first glance, evidence of similar EEG power patterns at both 5 and 10 months might be considered counterintuitive: How can infants exhibit substantial improvements in WM performance but exhibit the same patterns of task-related change in EEG power? The WM task used in the present study, however, was a dynamic task (e.g., increasing delays between hiding and responding) that was designed to challenge infants’ WM skills regardless of individual differences in WM. In absolute terms, the WM task was more difficult (i.e., more reversal trials and longer delays) at 10 months than at 5 months. However, in relative terms, the task may have been equally taxing of infants’ WM processing at each age because the task was designed to capture infants’ optimal performance at each age. Thus, upon further consideration, evidence that infants between 5 and 10 months exhibit what appear to be the same patterns of change in EEG power when WM demands were “equally” taxed is not counterintuitive.
For EEG coherence, infants exhibited task-related increases for frontal pole-medial frontal, medial frontal-temporal, and medial frontal-occipital electrode pairs at 5 months, and by 10 months, task-related increases were only present for medial frontal-occipital electrode pairs. We interpret these findings to potentially represent (a) evidence of fronto-posterior functional connectivity which is essential to the development of executive abilities (Luna & Sweeney, 2004); and (b) increasing specificity in functional connectivity during WM processing at 10 months. During the first year, rapid changes in axonal myelination contribute to more efficient neural communication (see Richards, 2010, for review). Initial evidence of localized task-related changes in scalp-recorded EEG coherence at 10 months may reflect the cortex becoming more organized and more efficient (Thatcher, 1994). Likewise, visual attention research using cortical source localization has noted that infants exhibit more localized frontal sources between 4.5 and 7 months of age (Reynolds, Courage, & Richards, 2010).
In the present study, infants exhibited WM-related decreases in HR at 5 months—the same pattern noted for attention research with young infants (see Reynolds & Richards, 2008, for review), and no WM-related changes in HR at 10 months. WM-related increases in HR have been found for 8-month-olds with high WM performance, children, and adults (Bell, in press; Gianaros et al., 2004; Wolfe & Bell, 2004). The pattern of HR change at 5 months is consistent with 8-month-olds with low WM performance (Bell, in press). Together, these findings suggest that the direction of HR change during WM processing may be indicative of frontal functional development and thus WM processing. Perhaps our MANOVA analysis failed to reveal task-related changes in 10-month HR because these findings were not analyzed in relation to individual differences in WM performance. Accordingly, our regression analysis indicated differential changes in HR as a function of 10-month performance.
In sum, we found task-related increases in EEG power and coherence at 5 and 10 months as well as task-related decreases in HR at 5 months. These findings confirm that by 5 months, infants exhibit changes in psychophysiology during WM processing. WM research has found that by 4.5 years, children exhibit localized, as compared to widespread, task-related changes in EEG power (medial frontal) and coherence (medial frontal-lateral parietal and medial frontal-occipital pairs) (Bell & Wolfe, 2007). In the present study, WM-related changes in EEG power remained global (i.e., all electrodes) and WM-related changed in EEG coherence became more localized by 10 months.
Predicting Variability in WM
Previous WM research has found that infants exhibit different patterns of baseline and task psychophysiology when categorized as high and low performers (Bell, 2001, in press; Bell & Fox, 1992). Our study is the first examination of whether task-related changes in multiple psychophysiological measures predict concurrent WM performance at 5 and 10 months of age. Although WM processing analyses indicated that infants exhibited similar patterns of task-related changes in EEG power at 5 and 10 months, regression analyses revealed that psychophysiological measures accounted for significant variability in WM performance only at 10 months. We attribute the failure to predict 5-month WM performance from concurrent psychophysiological measures to two intricately linked factors: the maturation of the frontal lobe and low levels of WM performance. There is greater variability in WM performance and substantial maturation of the frontal lobe at 8 (Bell, in press) and 10 months (current study), when psychophysiological measures account for variability in WM performance.
Task-related changes in psychophysiology accounted for 11% of the variance in 10- month WM performance. Higher WM performance was associated with greater task-related increases in medial frontal power and HR as well as smaller task-related increases in lateral frontal power. Our medial frontal EEG findings are consistent with functional magnetic imaging evidence that children’s WM capacity is related to frontal brain activity (Klingberg et al., 2002). Furthermore, our EEG findings might be related to future localization of cortical activity during WM processing (see Bell & Wolfe, 2007); as the frontal cortex develops, “high WM” infants may exhibit more activation at medial frontal electrodes and less activation at lateral frontal electrodes. Accordingly, these findings provide initial evidence that individual differences in localization of task-related changes in EEG power may be associated with individual differences in infant WM. Our findings support our frontal EEG hypothesis and reveal that EEG and HR measures provide unique information about variability in 10-month WM performance.
Previous research has indicated that HR is a unique predictor of 8-month WM performance, such that high performers exhibited task-related increases in HR and low performers exhibited task-related decreases in HR (Bell, in press). Our HR data verify that this association is also present at 10 months. Thus, regression analyses revealed that at 10 months, task-related changes in HR were associated with WM performance although multivariate analyses failed to reveal task-related changes in HR when performance is not considered.
Although WM-related changes in psychophysiology accounted for variance in 10-month WM performance, this percentage was relatively low. Other factors such as temperament and attention, which rely on similar neural circuitry, may contribute to individual differences in WM performance. For instance, Wolfe and Bell (2007) found that temperament was related to 8- month WM performance, and that temperament and language were related to 4.5-year WM performance. Thus, future research should examine the contribution of additional constructs to WM performance.
In the present study, multivariate analyses provided evidence of localized task-related changes in EEG coherence by 10 months and regression analyses provided evidence that increased localization of baseline-to-task changes in EEG power is related to individual differences in 10-month WM performance. WM-related changes in EEG coherence, however, did not account for unique variability in 10-month WM performance. During childhood, development of WM capacity is related to frontal myelination (Nagy et al., 2004). Future research is necessary to determine whether different patterns of brain-behavior relations emerge after extensive myelination of frontal axons.
Conclusion
At 5 and 10 months, infants exhibited task-related changes in EEG during WM processing. These data provide initial evidence of increasing localization of WM processing during the first year. However, task-related changes in EEG power and HR accounted for variability WM performance at 10, but not 5, months. We interpret these findings to represent the increased role of the frontal lobe in WM processing during the first year.
We acknowledge, however, that although tasks are typically associated with specific cognitive processes, tasks are rarely “process pure.” Inevitably other processes (i.e., attention and inhibition for A-not-B task; Bell & Adams, 1999) are also recruited during task performance. The administration of a variety of WM tasks would permit the extraction of a latent variable that represents WM processes from other processes. Currently, infant WM is primarily measured via variants of the A-not-B/DR task, and additional measures of WM are essential to a multi-task approach. The findings of the present study can be used to guide the particular psychophysiological measures, infant population, and task parameters used in future single- and multi-task analyses of WM.
Acknowledgments
This research was supported by Grants HD049878 and HD043057 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) awarded to Martha Ann Bell. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or the National Institutes of Health. We are grateful to the families for their participation in our research and to our research teams at Virginia Polytechnic Institute and State University and the University of North Carolina at Greensboro for their assistance with data collection and coding.
Footnotes
To account for the possible influence of skew, all behavioral analyses were confirmed with nonparameteric analyses (i.e., Friedman’s two-way ANOVA for related samples).
Regression analyses reveal the same pattern of findings if ordinal scale scores (e.g., Bell & Fox, 1992; Cuevas & Bell, 2010), which vary as a function of delay, are used as the dependent measure of performance at 5 months, F(14, 131) = 1.28, p = .251, and 10 months, F(14, 289) = 3.13, p < .001. At 10 months, baseline-to-task changes in frontal pole, medial frontal, and lateral frontal EEG power and HR accounted for 14% of the variance in ordinal scale scores.
A longitudinal regression analysis of 5-month psychophysiology predicting 10-month WM performance was not significant, F(14, 108) = 1.09, p = .373. However, interpretation of this is analysis is difficult because (a) 5- and 10-month WM performance are not correlated and (b) 5-month psychophysiology does not predict 5-month WM performance.
Contributor Information
Kimberly Cuevas, Department of Psychology, Virginia Polytechnic Institute and State University.
Martha Ann Bell, Department of Psychology, Virginia Polytechnic Institute and State University.
Stuart Marcovitch, Department of Psychology, University of North Carolina at Greensboro.
Susan D. Calkins, Department of Human Development and Human Studies and Department of Psychology, University of North Carolina at Greensboro
References
- Ahern GL, Sollers JJ, Lane RD, Labiner DM, Herring AM, Weinand ME, Hutzler R, Thayer JF. Heart rate and heart rate variability changes in the intracarotid sodium amobarbital (ISA) test. Epilepsia. 2001;42:912–921. doi: 10.1046/j.1528-1157.2001.042007912.x. [DOI] [PubMed] [Google Scholar]
- Baird AA, Kagan J, Gaudette T, Walz KA, Hershlag N, Boas DA. Frontal lobe activation during object permanence: Data from near-infrared spectroscopy. NeuroImage. 2002;16:1120–1126. doi: 10.1006/nimg.2002.1170. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Marshall PJ, Fox NA. Developmental changes in heart period and high-frequency heart period. Developmental Psychobiology. 2000;37:44–56. doi: 10.1002/1098-2302(200007)37:1<44::aid-dev6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Bell MA. The ontogeny of the EEG during infancy and childhood: Implications for cognitive development. In: Garreau B, editor. Neuroimaging in child neuropsychiatric disorders. Berlin: Springer-Verlag; 1998. pp. 97–111. [Google Scholar]
- Bell MA. Brain electrical activity associated with cognitive processing during a looking version of the A-not-B task. Infancy. 2001;2:311–330. doi: 10.1207/S15327078IN0203_2. [DOI] [PubMed] [Google Scholar]
- Bell MA. Power changes in infant EEG frequency bands during a spatial working memory task. Psychophysiology. 2002;39:450–458. doi: 10.1017.S0048577201393174. [DOI] [PubMed] [Google Scholar]
- Bell MA. A psychobiological perspective of working memory performance at 8 months of age. Child Development. doi: 10.1111/j.1467-8624.2011.01684.x. in press. [DOI] [PubMed] [Google Scholar]
- Bell MA, Adams SE. Comparable performance on looking and reaching version of the A-not-B task at 8 months of age. Infant Behavior and Development. 1999;22:221–235. [Google Scholar]
- Bell MA, Fox NA. The relations between frontal brain electrical activity and cognitive development during infancy. Child Development. 1992;63:1142–1163. [PubMed] [Google Scholar]
- Bell MA, Fox NA. Brain development over the first year of life: Relations between electroencephalographic frequency and coherence and cognitive and affective behaviors. In: Dawson G, Fischer KW, editors. Human behavior and the developing brain. New York: Guilford; 1994. pp. 314–345. [Google Scholar]
- Bell MA, Fox NA. Individual differences in object permanence at 8 months: Locomotor experience and brain electrical activity. Developmental Psychobiology. 1997;31:287–297. [PubMed] [Google Scholar]
- Bell MA, Wolfe CD. Brain reorganization from infancy to early childhood: Evidence from EEG power and coherence during working memory tasks. Developmental Neuropsychology. 2007;31:21–38. doi: 10.1207/s15326942dn3101_2. [DOI] [PubMed] [Google Scholar]
- Chugani HT, Phelps ME, Mazziotta JC. Positron emission tomography study of human brain functional development. Annals of Neurology. 1987;22:487–497. doi: 10.1002/ana.410220408. [DOI] [PubMed] [Google Scholar]
- Colombo J, Cheatham CL. The emergence and basis of endogenous attention in infancy and early childhood. In: Kail RV, editor. Advances in child development and behavior. vol 34. Amsterdam: Elsevier; 2006. pp. 283–322. [DOI] [PubMed] [Google Scholar]
- Cuevas K, Bell MA. Developmental progression of looking and reaching performance on the A-not-B task. Developmental Psychology. 2010;46:1363–1371. doi: 10.1037/a0020185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas K, Bell MA. EEG and ECG from 5 to 10 months of age: Developmental changes in baseline activation and cognitive processing during a working memory task. International Journal of Psychophysiology. 2011;80:119–128. doi: 10.1016/j.ijpsycho.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, Jackson DC, Larson CL. Human electroencephalography. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. 2nd ed. Cambridge, UK: Cambridge University Press; 2000. pp. 27–52. [Google Scholar]
- Deoni SCL, Mercure E, Blasi A, Gasston D, Thomson A, Johnson M, Murphy DGM. Mapping infant brain myelination with magnetic resonance imaging. The Journal of Neuroscience. 2011;31:784–791. doi: 10.1523/JNEUROSCI.2106-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. Development of the ability to use recall to guide action, as indicated by infants’ performance on AB. Child Development. 1985;56:868–883. [PubMed] [Google Scholar]
- Diamond A. The development and neural bases of memory function as indexed by the AB and delayed response tasks in human infants and infant monkeys. In: Diamond A, editor. The development and neural bases of higher cognitive functions. New York: New York Academy of Sciences Press; 1990. pp. 267–317. [DOI] [PubMed] [Google Scholar]
- Diamond A, Cruttenden L, Neiderman D. AB with multiple wells: 1. Why are multiple wells sometimes easier than two wells? 2. Memory or memory + inhibition? Developmental Psychology. 1994;30:192–205. [Google Scholar]
- Diamond A, Doar B. The performance of human infants on a measure of frontal cortex function, the delayed response task. Developmental Psychobiology. 1989;22:271–294. doi: 10.1002/dev.420220307. [DOI] [PubMed] [Google Scholar]
- Diamond A, Goldman-Rakic PS. Comparison of human infants and rhesus monkeys on Piaget’s A-not-B task: Evidence for dependence on dorsolateral prefrontal cortex. Experimental Brain Research. 1989;74:24–40. doi: 10.1007/BF00248277. [DOI] [PubMed] [Google Scholar]
- Diamond A, Prevor M, Callender G, Druin DP. Prefrontal cortex cognitive deficits in children treated early and continuously for PKU. Monographs of the Society for Research in Child Development. 1997;62 (4, Serial No 252). [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: Psychophysiological and behavioral influences across the first four years of life. Child Development. 2001;72:1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Fox NA, Schmidt LA, Henderson HA, Marshall P. Cacioppo JT, Tassinary LG, Berntson GG. Handbook of psychophysiology. (3rd ed.) Cambridge, UK: Cambridge University Press; 2007. Developmental psychophysiology: Conceptual and methodological issues; pp. 453–481. [Google Scholar]
- Gianaros PJ, Van Der Veen FM, Jennings JR. Regional cerebral blood flow correlates with heart period and high-frequency heart period variability during working-memory tasks: Implications for the cortical and subcortical regulation of cardiac autonomic activity. Psychophysiology. 2004;41:521–530. doi: 10.1111/1469-8986.2004.00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemann D, Naumann E, Thayer JF. The quest for the EEG reference revisited: A glance from brain asymmetry research. Psychophysiology. 2001;38:847–857. [PubMed] [Google Scholar]
- Hofstadter M, Reznick JS. Response modality affects human infant delayed-response performance. Child Development. 1996;67:646–658. [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. The Journal of Comparative Neurology. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Jasper HH. The 10/20 international electrode system. EEG and Clinical Neurophysiology. 1958;10:371–375. [Google Scholar]
- Kane MJ, Engle RW. The role of the prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: An individual-differences perspective. Psychonomic Bulletin & Review. 2002;9:637–671. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Schimke H, Ripper B. Theta synchronization and alpha desynchronization in a memory task. Psychophysiology. 1997;34:169–176. doi: 10.1111/j.1469-8986.1997.tb02128.x. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Forssberg H, Westerberg H. Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. Journal of Cognitive Neuroscience. 2002;14:1–10. doi: 10.1162/089892902317205276. [DOI] [PubMed] [Google Scholar]
- Lacey BC, Lacey JI. Studies on heart rate and other bodily processes in sensorimotor behavior. In: Obrist PA, Black AH, Brener J, DiCara LV, editors. Cardiovascular psychophysiology: Current issues in response, mechanisms, biofeedback, and methodology. Chicago: Aldine; 1974. pp. 538–564. [Google Scholar]
- Lehmann D. Principles of spatial analysis. In: Gevins AS, Remond A, editors. Handbook of electroencephalography and clinical neuropsychology, rev. ser. Vol 1: Methods of analysis of brain electrical and magnetic signals. Amsterdam: Elsevier; 1987. pp. 309–354. [Google Scholar]
- Linden DEJ, Bittner RA, Muckli L, Waltz JA, Kriegeskorte N, Goebel R, Munk MHJ. Cortical capacity constraints for visual working memory: dissociation of fMRI load effects in a fronto-parietal network. NeuroImage. 2003;20:1518–1530. doi: 10.1016/j.neuroimage.2003.07.021. [DOI] [PubMed] [Google Scholar]
- Luck SJ. An Introduction to the Event-Related Potential Technique. Cambridge, MA: MIT Press; 2005. [Google Scholar]
- Luna B, Sweeney JA. The emergence of collaborative brain function: FMRI studies of the development of response inhibition. Annals of the New York Academy of Sciences. 2004;1021:296–309. doi: 10.1196/annals.1308.035. [DOI] [PubMed] [Google Scholar]
- Marcovitch S, Zelazo PD. The A-not-B error: Results from a logistic meta-analysis. Child Development. 1999;70:1297–1313. [Google Scholar]
- Matthews A, Ellis AE, Nelson CA. Development of preterm and full-term infant ability on AB, recall memory, transparent barrier detour, and means-end tasks. Child Development. 1996;67:2658–2676. [PubMed] [Google Scholar]
- Myslobodsky MS, Coppola R, Bar-Ziv J, Karson C, Daniel D, van Praag H, Weinberger DR. EEG asymmetries may be affected by cranial and brain parenchymal asymmetries. Brain Topography. 1989;1:221–228. doi: 10.1007/BF01129599. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. Journal of Cognitive Neuroscience. 2004;16:1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- Orekhova EV, Stroganova TA, Posikera IN. Alpha activity as an index of cortical inhibition during sustained internally controlled attention in infants. Clinical Neurophysiology. 2001;112:740–749. doi: 10.1016/s1388-2457(01)00502-8. [DOI] [PubMed] [Google Scholar]
- Osaka M, Osaka N, Kondo H, Morishita M, Fukuyama H, Aso T, Shibasaki H. The neural basis of individual differences in working memory capacity: An fMRI study. NeuroImage. 2003;18:789–797. doi: 10.1016/s1053-8119(02)00032-0. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Reznick JS. Working memory in infancy. Advances in Child Development. 2003;31:173–227. doi: 10.1016/s0065-2407(03)31005-5. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Reznick JS, Goldman BD, Sasson N, Morrow J, Donahoe A, Hodgson K. Development of visuospatial short-term memory in the second half of the 1st year. Developmental Psychology. 2004;40:836–851. doi: 10.1037/0012-1649.40.5.836. [DOI] [PubMed] [Google Scholar]
- Pivik RT, Broughton RJ, Coppola R, Davidson RJ, Fox NA, Nuwer MR. Guidelines for the recording and quantitative analysis of electroencephalographic activity in research contexts. Psychophysiology. 1993;30:547–558. doi: 10.1111/j.1469-8986.1993.tb02081.x. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA. Electroencephalography and high-density electrophysiological source localization. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. 3rd ed. Cambridge, UK: Cambridge University Press; 2007. pp. 56–84. [Google Scholar]
- Reynolds GD, Courage ML, Richards JE. Infant attention and visual preference: Converging evidence from behavior, event-related potentials, and cortical source localization. Developmental Psychology. 2010;46:886–904. doi: 10.1037/a0019670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds GD, Richards JE. Infants heart rate: A developmental psychophysiological perspective. In: Schmidt LA, Segalowitz SJ, editors. Developmental psychophysiology: Theory, systems, and methods. Cambridge, MA: Cambridge University Press; 2008. pp. 173–212. [Google Scholar]
- Reznick JS, Morrow JD, Goldman BD, Snyder J. The onset of working memory in infants. Infancy. 2004;6:145–154. [Google Scholar]
- Richards JE. Attention in the brain and early infancy. In: Johnson S, editor. Neoconstructivism: The new science of cognitive development. Oxford: Oxford University Publishing; 2010. pp. 3–31. [Google Scholar]
- Saltzberg B, Burton WD, Jr, Burch NR, Fletcher J, Michaels R. Electrophysiological measures of regional neural interactive coupling. Linear and nonlinear dependence relationships among multiple channel electroencephalographic recordings. International Journal of Bio-medical Computing. 1986;18:77–87. doi: 10.1016/0020-7101(86)90050-4. [DOI] [PubMed] [Google Scholar]
- Stern RM, Ray WJ, Quigley KS. Psychophysiological Recording. 2nd ed. Oxford: Oxford University Press; 2001. [Google Scholar]
- Thatcher RW. Cyclic cortical reorganization: Origins of human cognitive development. In: Dawson G, Fischer KW, editors. Human behavior and the developing brain. New York: Guilford; 1994. pp. 232–266. [Google Scholar]
- Thatcher RW, Walker RA, Giudice S. Human cerebral hemispheres develop at different rates and ages. Science. 1987;236:1110–1113. doi: 10.1126/science.3576224. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. Claude Bernard and the heart-brain connection: Further elaboration of a model of neurovisceral integration. Neuroscience and Biobehavioral Reviews. 2009;33:81–88. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Thomas KM, King SW, Franzen PL, Welsh TF, Berkowitz AL, Noll DC, Casey BJ. A developmental functional MRI study of spatial working memory. NeuroImage. 1999;10:327–338. doi: 10.1006/nimg.1999.0466. [DOI] [PubMed] [Google Scholar]
- Tsekhmistrenko TA, Vasil’eva VA, Shumeiko NS, Vologirov AS. Quantitative changes in the fibroarchitectonics of the human cortex from birth to the age of 12 years. Neuroscience and Behavioral Physiology. 2004;34:983–988. doi: 10.1023/b:neab.0000042657.80112.7b. [DOI] [PubMed] [Google Scholar]
- Wellman HM, Cross D, Bartsch K. Infant search and object permanence: A meta-analysis of the A-not-B error. Monographs of the Society for Research in Child Development. 1986;51 (3, Serial No 214). [PubMed] [Google Scholar]
- Wolfe CD, Bell MA. Working memory and inhibitory control in early childhood: Contributions from physiology, temperament, and language. Developmental Psychobiology. 2004;44:68–83. doi: 10.1002/dev.10152. [DOI] [PubMed] [Google Scholar]
- Wolfe CD, Bell MA. The integration of cognition and emotion during infancy and early childhood: Regulatory processes associated with the development of working memory. Brain and Cognition. 2007;65:3–13. doi: 10.1016/j.bandc.2006.01.009. [DOI] [PubMed] [Google Scholar]