Summary
The simian varicella virus (SVV) genome encodes ORF A, a truncated homolog of SVV ORF 4. The SVV ORF A was expressed as a 1.0 kb transcript in SVV-infected Vero cells. The ORF A promoter was active in infected Vero cells and was stimulated by the SVV immediate early gene ORF 62 product (IE62), a viral transactivator of SVV genes. The SVV ORF A did not transactivate SVV IE, early, or late gene promoters in transfected Vero cells and was unable to augment IE62-mediated transactivation of SVV promoters. A SVV mutant lacking the ORF A replicated as efficiently as wild-type SVV in infected Vero cells indicating that ORF A expression is not essential for in vitro replication.
Simian varicella virus (SVV) induces in non-human primates a natural disease which clinically resembles human chickenpox (varicella), caused by varicella-zoster virus (VZV) [2,4]. Epizootics of simian varicella, characterized by fever and vesicular skin rash, occur sporadically in facilities housing Old World monkeys [3]. Following resolution of the acute disease, SVV establishes latent infection of neural ganglia, and may subsequently reactivate to induce a secondary disease, just as VZV reactivates to cause herpes zoster [8, 9].
SVV and VZV are genetically related and the viral genomes are similar in size and structure and share extensive DNA homology [4]. The SVV genome encodes 69 distinct open reading frames (ORFs), each of which has homology to a corresponding VZV gene [6]. The only significant difference between the SVV and VZV genomes occurs at the genomic left end [10]. SVV DNA lacks a homolog of the VZV ORF 2, which encodes a membrane phosphoprotein that is dispensable for in vitro replication [17]. In addition, the SVV genome includes a 879 bp ORF A that is not present in VZV DNA.
The SVV ORF A encodes a putative 293 amino acid (aa) protein that is a truncated homolog of the 470 aa SVV ORF 4 [10]. The SVV ORF A does not include a region corresponding to the 177 aa at the amino end of SVV ORF 4, but shares 42% aa identity with the remaining 293 aa of ORF 4. In addition, the SVV ORF A has 49% aa identity to the homologous region of VZV ORF 4, an immediate early (IE) gene which encodes a transactivator of VZV IE, early, and late genes and which augments IE ORF 62 mediated transactivation of VZV genes [12, 13]. The function of the ORF A in viral replication and pathogenesis is unknown.
The SVV ORF A is reported to be expressed in SVV-infected BSC-1 cells and in tissues of acutely infected monkeys as detected by reverse-transcriptase PCR [10, 11]. This study confirms and further characterizes SVV ORF A gene expression in cell-culture and demonstrates that ORF A is not essential for in vitro viral replication.
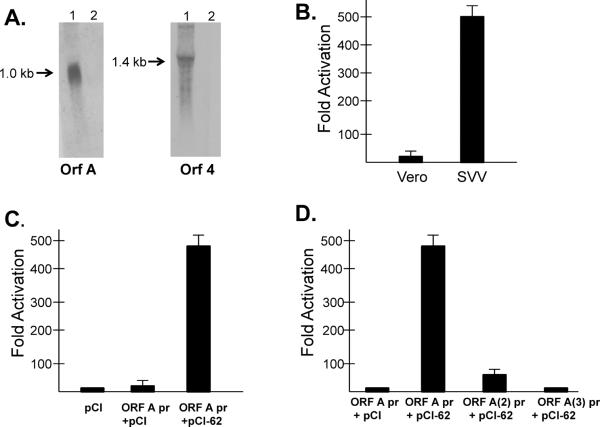
The expression of a SVV ORF A transcript in SVV-infected Vero cells was demonstrated by Northern blot hybridization using an ORF A DNA probe. SVV expresses a 1.0 kb ORF A RNA in SVV-infected Vero cells (Fig. 1A). The SVV ORF 4 is expressed as a 1.4 bp transcript as detected using a ORF 4 specific DNA probe. The ORF A probe did not detect the ORF 4 transcript in infected cells using stringent hybridization conditions.
Figure 1.
ORF A gene expression. (A) Detection of ORF A and ORF 4 transcripts in SVV-infected Vero cells by Northern blot hybridization using DIG-labeled ORF A and ORF 4 DNA probes, respectively. (B) Activation of the ORF A promoter in SVV-infected Vero cells as indicated by transfection of a ORF A promoter-pGL3 construct into SVV-infected or uninfected Vero cells. (C) SVV IE62-mediated transactivation of the ORF A promoter. Vero cells were transfected with the pCI vector, the ORF A pr-GL3 plus the pCI vector, or with ORF A pr-pGL3 plus pCI-IE62. (D) Deletion analysis of the ORF A promoter. Vero cells were transfected with the ORF A pr-pGL3 or ORF A deletion mutants plus pCI-IE62. In all cases luciferase activity was detected in cell lysates at 48 hrs post-transfection. Experiments were conducted in triplicate. Fold activation is relative to the Vero (B) or pCI (C and D) transfected controls. Statistical P values are indicated. NS- statistically not significant.
SVV ORF A gene expression was further characterized by analysis of ORF A promoter activity. Initially, 326 bp upstream of the ORF A ATG start codon was cloned into the pGL3 vector (Promega Corp.) at a site upstream of the luciferase reporter gene. This SVVORFApr-pGL3 DNA construct was transfected into Vero cell monolayers and luciferase activity was determined in cell lysates at 48 hrs post-transfection. The results determined that the SVV ORF A promoter is active in SVV-infected Vero cells, but not in uninfected Vero cells (Fig. 1B).
The ORF A promoter is stimulated by the SVV ORF 62 IE gene product (IE62), a major viral transactivator of SVV IE, early, and late genes, as indicated by co-transfection of Vero cells with SVVORFApr-pGL3 and pCI-IE62, which expresses SVV IE62 from a human cytomegalovirus promoter/enhancer (Fig. 1C) [7, 14]. The essential sequences of the ORF A promoter required for IE62 stimulation were identified by promoter deletion analysis. Deletion of 127 bp [bp 199–326 upstream of the ATG start site, SVVORFA(2)pr-pGL3] resulted in a five-fold reduction in ORF A promoter activity (Fig. 1D). Analysis of this sequence identified putative binding sites for transcription factors known to stimulate SVV and VZV genes, including USF (located beginning at bp −314 relative to the ATG start site) and SP1 (bp −202) [14–16]. Deletion of 252 bp [bp 74– 326 bp upstream of the ATG start site, SVVORFA(3)pr-pGL3] resulted in additional reduction of ORF A promoter activity potentially due to loss of a putative TATA binding site at −183 bp upstream of the ATG start site. The results indicate that sequences between 74 and 326 bp upstream of the ATG start site are critical for IE62-mediated transactivation of the ORF A promoter.
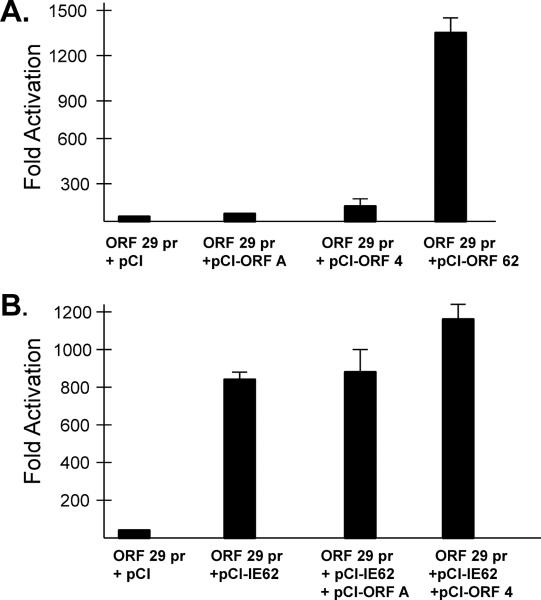
The ability of the ORF A protein expressed from a HCMV promoter to transactivate other SVV promoters in transfected Vero cells was evaluated using a luciferase reporter assay. As a positive control, the SVV IE62 strongly transactivated the SVV ORF 29 early gene promoter (Fig 2A). In addition, the SVV IE62 transactivated its own ORF 62 IE promoter and the glycoprotein E (gE, ORF 68) late gene promoter (data not shown). In contrast the, SVV ORF A was not able to transactivate the SVV ORF 29 promoter (Fig. 2A), nor the ORF 62 or gE promoters (data not shown), under the same conditions. In addition, the ORF A product was not able augment SVV IE62 mediated transactivation of the SVV ORF 29 promoter in Vero cells co-transfected with pCI-ORF A and pCI-IE62 (Fig 2B). The SVV ORF 4 was a weak transactivator of the SVV ORF 29 promoter (two fold, but not statistically significant, Fig. 2A), but was able to significantly stimulate IE62 mediated transactivation of the ORF 29 promoter (Fig. 2B).
Figure 2.
Transactivation assays. (A) Transactivation of the SVV ORF 29 promoter by SVV IE-62, but not ORF A. Vero cells were co-transfected with the ORF29pr-pGL3 and also with pCI, pCI-ORFA, pCI-ORF4, or pCI-62. (B) Stimulation of SVV IE62 mediated transactivation of the ORF 29 promoter. Vero cells were co-transfected with the ORF29pr-pGL3 and pCI-IE62 and also with pCI-ORFA, or pCI-ORF4. In all cases luciferase activity was detected in cell lysates at 48 hrs post-transfection. Experiments were conducted in triplicate. Fold activation is relative to the pCI transfected controls. Statistical P values are indicated. NS- statistically not significant.
The inability of the SVV ORF A to transactivate SVV promoters may be related to the lack of an amino terminus corresponding to the VZV ORF 4, which has been shown to be critical for VZV ORF 4 transactivation of VZV promoters and for augmenting IE62 transactivation [13]. In addition, the SVV ORF A lacks a nuclear localization signal that is essential for VZV ORF 4 transactivation [10].
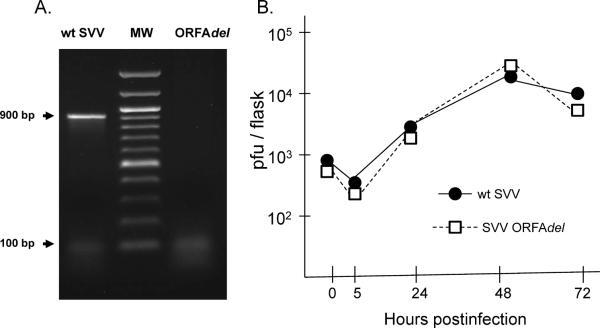
To determine the importance of the SVV ORF A for viral replication in cell culture, the ORF A was deleted from the SVV genome using the SVV cosmid system combined with the recA assisted restriction endonuclease cleavage method (RARE) [1, 5]. PCR analysis employing PCR primers flanking the ORF A confirmed deletion of 754 bp (SVV nt 1715– 2468) from within the 879 bp SVV ORF A (nt 1573– 2452) plus 16 upstream bp (Fig. 3A). Viral growth analysis demonstrated that the SVV ORFAdel replicated as efficiently as wild-type SVV in infected Vero cells (Fig. 3B). In addition, the median viral plaque size for SVVorfAdel (0.50 ± 0.06 mm) was similar to that of wild-type SVV (0.48 ± 0.08 mm) based upon plaque diameter in CV-1 cell monolayers at 72 hrs postinfection.
Figure 3.
Effect of ORF A deletion on viral replication in vitro. (A) Confirmation of the 754 bp ORF A deletion in SVVORFAdel by PCR using primers flanking ORF A (nt 1526–2651). MW- 1 kb molecular size marker. (B) Viral growth analysis of wt SVV and SVVORFAdel. Vero cell cultures (25 cm2 flasks) were infected with 800 pfu of cell-free virus and viral titer at various times postinfection was determined by plaque assay on CV-1 cell monolayers.
This study demonstrates SVV ORF A gene is expressed in acutely infected Vero cells as a 1.0 kb transcript and is actively stimulated by the SVV IE62 transactivator. However, ORF A expression is not essential for efficient viral replication in cell culture. The results do not support ORF A as a viral transactivator of other SVV genes and the function of ORF A in viral replication is unknown. While the ORF A may be a consequence of a truncated duplication of the ORF 4, the conservation of this gene within various SVV isolates derived from wide-spread epizootics suggests a role in viral pathogenesis [10]. The SVV ORF A is expressed in tissues of acutely infected monkeys [10, 11]. In addition, ORF A transcripts were detected in 3 of 16 neural ganglia derived from latently infected rhesus monkeys [11] . Further study of the SVVORFAdel mutant in the simian varicella model may be useful in determining the role of the SVV ORF A in viral pathogenesis, latency, and reactivation.
Acknowledgements
This work was supported by Public Health Service Grant AI052373 of the National Institutes of Health. I thank Lisa Mullis for technical assistance.
References
- 1.Ferrin LJ, Camerini-Otero RD. Selective cleavage of human DNA: RecA-assisted restriction endonuclease [RARE] cleavage. Science. 1991;254:1494–1497. doi: 10.1126/science.1962209. [DOI] [PubMed] [Google Scholar]
- 2.Gray WL. Simian varicella: a model for human varicella-zoster virus infections. Rev Med Virol. 2004;14:363–381. doi: 10.1002/rmv.437. [DOI] [PubMed] [Google Scholar]
- 3.Gray WL. Simian varicella in Old World monkeys. Comp Med. 2008;58:22–30. [PMC free article] [PubMed] [Google Scholar]
- 4.Gray WL. Simian varicella: molecular virology. Curr Top Microbiol Immunol. 2010;342:291–308. doi: 10.1007/82_2010_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gray WL, Mahalingam R. A cosmid-based system for inserting mutations and foreign genes into the simian varicella virus genome. J Virol Meth. 2005;130:89–94. doi: 10.1016/j.jviromet.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Gray WL, Starnes B, White MW, Mahalingam R. The DNA sequence of the simian varicella virus genome. Virology. 2001;284:123–130. doi: 10.1006/viro.2001.0912. [DOI] [PubMed] [Google Scholar]
- 7.Mahalingam R, Gilden DH, Wellish M, Pugazhenthi S. Transactivation of the simian varicella virus (SVV) open reading frame (ORF) 21 promoter by SVV ORF 62 is upregulated in neuronal cells but downregulated in non-neuronal cells by SVV ORF 63 protein. Virology. 2006;345:244–250. doi: 10.1016/j.virol.2005.08.045. [DOI] [PubMed] [Google Scholar]
- 8.Mahalingam R, Smith D, Wellish M, Wolf W, Dueland AN, Cohrs R, Soike K, Gilden D. Simian varicella virus DNA in dorsal root ganglia. Proc. Natl Acad Sci USA. 1991;88:2750–2752. doi: 10.1073/pnas.88.7.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahalingam R, Traina-Dorge V, Wellish M, Lorino R, Sanford R, Ribka EP, Alleman SJ, Brazeau E, Gilden DH. Simian varicella reactivation in cynomolgus monkeys. Virology. 2007;368:50–59. doi: 10.1016/j.virol.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 10.Mahalingam R, White T, Wellish M, Gilden D, Gray WL. Sequence analysis of the leftward end of simian varicella virus (EcoRI- I fragment) reveals the presence of an 8-bp repeat flanking the unique long segment and an 881-bp open reading frame that is absent in the varicella-zoster virus genome. Virology. 2000;274:420–428. doi: 10.1006/viro.2000.0465. [DOI] [PubMed] [Google Scholar]
- 11.Meyer C, Kerns A, Barron A, Steblow DN, Messaoudi I. Simian varicella virus gene expression during acute and latent infection of rhesus monkeys. J Neurovirol. 2011;17:600–612. doi: 10.1007/s13365-011-0057-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moriuchi H, Moriuchi M, Smith HA, Cohen JI. Varicella-zoster virus open reading frame 4 protein is functionally distinct from and does not complement its herpes simplex virus type 1 homolog, ICP27. J Virol. 1994;68:1987–1992. doi: 10.1128/jvi.68.3.1987-1992.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moriuchi M, Moriuchi H, Debrus D, Piette J, Cohen JI. The acidic amino-terminal region of varicella-zoster virus open reading frame 4 protein is required for transactivation and can functionally replace the corresponding region of herpes simplex virus ICP27. Virology. 1995;208:376–382. doi: 10.1006/viro.1995.1164. [DOI] [PubMed] [Google Scholar]
- 14.Ou Y, Gray WL. The simian varicella virus gene 28 and 29 promoters share a common USF binding site and are induced by IE62 transactivation. J Gen Virol. 2006;87:1501–1508. doi: 10.1099/vir.0.81645-0. [DOI] [PubMed] [Google Scholar]
- 15.Ruyechan WT. Roles of cellular transcription factors in VZV replication. In: Abendroth A, Arvin AM, Moffat JF, editors. Varicella-zoster virus. Springer; Heidelberg: 2010. pp. 43–65. [DOI] [PubMed] [Google Scholar]
- 16.Ruyechan WT, Peng H, Yang M, Hay J. Cellular factors and IE62 activation of VZV promoters. J Med Virol. 2003;70:S90–S94. doi: 10.1002/jmv.10328. [DOI] [PubMed] [Google Scholar]
- 17.Sato H, Pesnicak L, Cohen JI. Varicella-zoster virus open reading frame 2 encodes a membrane phosphoprotein that is dispensible for viral replication and for establishment of latency. J Virol. 2002;76:3575–3578. doi: 10.1128/JVI.76.7.3575-3578.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]