Abstract
Psychostimulants induce neuroadaptations in excitatory and fast inhibitory transmission in the ventral tegmental area (VTA). Mechanisms underlying drug-evoked synaptic plasticity of slow inhibitory transmission mediated by GABAB receptors and G protein-gated inwardly rectifying potassium (GIRK/Kir3) channels, however, are poorly understood. Here, we show that one day after methamphetamine (METH) or cocaine exposure, both synaptically-evoked and baclofen-activated GABABR-GIRK currents were significantly depressed in VTA GABA neurons, and remained depressed for 7 days. Presynaptic inhibition mediated by GABABRs on GABA terminals was also weakened. Quantitative immunoelectron microscopy revealed internalization of GABAB1R and GIRK2, which occurred coincident with dephosphorylation of Ser783 in GABAB2R, a site implicated in regulating GABABR surface expression. Inhibition of protein phosphatases recovered GABABR-GIRK currents in VTA GABA neurons of METH-injected mice. This psychostimulant-evoked impairment in GABABR signaling removes an intrinsic brake on GABA neuron spiking, which may augment GABA transmission in the mesocorticolimbic system.
INTRODUCTION
Changes in the motivation for drugs and natural rewards are central to the development of addiction (Koob and Volkow, 2010). The mesocorticolimbic dopamine (DA) system is the major brain reward circuit involved in translating motivations into goal-directed behaviors. Within this system, natural rewards increase activity of the ventral tegmental area (VTA) DA neurons, which primarily project to the nucleus accumbens (NAc), amygdala and medial prefrontal cortex (mPFC). Addictive drugs converge on the mesocorticolimbic DA system, however, producing long-lasting changes in DA levels and excitability of DA neurons (Koob and Volkow, 2010; Luscher and Malenka, 2011). One of the key pathways for controlling DA neuron excitability is through activation of a slow GABA-dependent inhibitory current, mediated by GABAB receptors (GABABRs) and G protein-gated inwardly rectifying potassium (GIRK/Kir3) channels (Johnson and North, 1992; Cruz et al., 2004; Labouèbe et al., 2007), and through an autoinhibitory pathway mediated by D2 dopamine receptors (D2Rs) and GIRK channels (Johnson and North, 1992; Beckstead et al., 2004). In vivo exposure to psychostimulants leads to reduced sensitivity of D2 autoreceptors, and increased DA neuron excitability (White and Wang, 1984; Henry et al., 1989; White, 1996), implicating GIRK channels in the response to addictive drugs (Lüscher and Slesinger, 2010). Consistent with this, mice lacking GIRK channels self-administer less cocaine (Morgan et al., 2003) and show reduced withdrawal after chronic exposure to morphine (Cruz et al., 2008). Furthermore, Girk2 transcripts in the mesocorticolimbic dopamine pathway are increased in some human cocaine addicts (Lehrmann et al., 2003). Although GIRK channels are implicated in the response to addictive drugs, the cellular mechanisms underlying drug-evoked changes in GIRK signaling are not well understood.
Accumulating evidence suggests that acquisition of addictive behaviors is learned and, similar to other learning and memory models, involves persistent changes in synaptic strength within the reward circuit and changes in DA neuron signaling (Koob and Volkow, 2010; Luscher and Malenka, 2011). Early drug-evoked neuroadaptations are thought to occur within the VTA and are critical for remodeling the reward circuit and facilitating the development of addiction. Lesion of VTA DA neurons blocks drug-dependent addictive behaviors (Roberts and Koob, 1982). Neuroadaptations have been described that occur 24h following exposure to addictive drugs in vivo. Systemic injection of a psychostimulant strengthens excitatory synapses in the VTA (White et al., 1995; Zhang et al., 1997; Ungless et al., 2001; Borgland et al., 2004; Argilli et al., 2008) through recruitment of GluA2-lacking AMPA receptors to the synapses (Bellone and Luscher, 2006; Argilli et al., 2008). Neuroadaptations in fast GABA transmission have also been reported; fast inhibitory currents mediated by GABAA receptors are impaired 24h after a single injection of morphine (Nugent et al., 2007) and the amplitudes of GABA-mediated synaptic currents are reduced in mice receiving several injections of cocaine (Liu et al., 2005). Chronic amphetamine enhances GABAB receptor transmission in the VTA during early withdrawal, but the cellular mechanism underlying this change is unknown (Giorgetti et al., 2002). Following chronic cocaine or morphine treatment, D1R stimulation decreases GABAB-GIRK currents in DA neurons but this occurs from a change in presynaptic GABA release (Bonci and Williams, 1996). In this study, we sought to characterize the early modulation of GABAB signaling by a single exposure to psychostimulants. We discovered that ~24h following intraperitoneal injection of methamphetamine (METH) or cocaine, GABAB receptor signaling in VTA GABA neurons is strongly and persistently impaired. This drug-evoked depression of GABABR-GIRK signaling involves de-phosphorylation of the GABAB receptor and changes in GABABR and GIRK channel trafficking. As a consequence, VTA GABA neuron firing is not affected by the GABABR agonist baclofen, suggesting GABAergic function may be augmented in the VTA with psychostimulants.
RESULTS
Psychostimulant-evoked plasticity in GABABR signaling in VTA
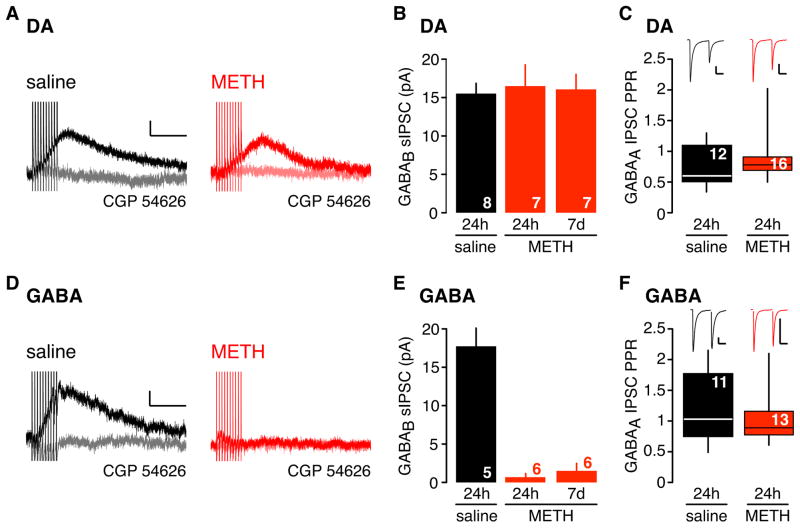
A single injection of psychostimulants enhances glutamatergic synaptic efficacy in the VTA 24h later (Ungless et al., 2001; Borgland et al., 2004; Argilli et al., 2008). We examined whether a single injection of psychostimulant also alters GABABR-GIRK signaling in the VTA. To test this, we injected C57BL/6 mice with methamphetamine (METH) at 2 mg/kg, a dose that elicits locomotor sensitization when administered repeatedly (Shimosato et al., 2001; Fukushima et al., 2007; Scibelli et al., 2010), and examined GABABR-GIRK signaling in the VTA 24h later. We first investigated the synaptically activated GABABR-GIRKs, commonly referred to as the slow inhibitory postsynaptic current (sIPSC), in acutely prepared VTA slices. High frequency (66 Hz) stimulation of GABA afferents induces a spillover of GABA that diffuses to perisynaptic GABAB receptors and elicits a slow outward K+ current (sIPSC) (Figure 1). The GABAB receptor antagonist, CGP 54626 (2 μM), inhibited the evoked current, confirming the identity of the GABAB sIPSC (Figure 1), similar to previous studies (Johnson and North, 1992; Bonci and Williams, 1996). In DA neurons, the GABAB sIPSC did not significantly change 24h following METH, compared to saline injection (Figure 1A,B). By contrast, the sIPSC was significantly smaller in GABA neurons (Figure 1D,E). Moreover, the sIPSC in GABA neurons remained depressed for at least 7 days (Figure 1E). Examination of the paired-pulse ratio for the fast GABAA-mediated IPSC revealed no difference in either DA or GABA neurons (Figure 1C,F), suggesting that the depression of the sIPSC in GABA neurons was not due to the inability of GABA terminals to release GABA.
Figure 1. Absence of slow inhibitory postsynaptic currents in VTA GABA neurons 24h and 7d following in vivo METH exposure.
The slow inhibitory postsynaptic current (sIPSC) recorded from DA (A) and GABA (D) neurons in the VTA 24h following a saline (0.9%) or METH (2mg/kg) injection. The GABAB receptor antagonist CGP 54626 (2 μM) inhibited the sIPSC (light grey or light red trace). The GABABR-sIPSC is reduced in the VTA GABA neuron 24h following METH injection. Scale bars: 5pA, 200ms. B,E Bar graphs show mean amplitudes for sIPSC following saline or METH (24h and 7d later) in DA (B) (DA saline: 15.8 ± 1.5 pA, DA METH: 16.5 ± 2.8 pA, DA 7d METH: 16.1 ± 2.0 pA) and GABA (E) neurons (GABA saline: 17.8 ± 2.6 pA, GABA METH: 0.7 ± 0.5 pA, GABA 7d METH: 1.5 ± 1.0 pA). The sIPSC is significantly depressed 24h and 7d following a single injection of METH in GABA neurons (** P < 0.05 One-way ANOVA). C,F Box plots show GABAA receptor-mediated IPSC paired-pulse ratio (PPR) plotted for DA (C) and GABA (F) neurons in saline and METH injected mice (DA saline: 0.73 ± 0.10 pA, DA METH: 0.89 ± 0.09 pA, GABA saline: 1.20 ± 0.17 pA, GABA METH: 1.02 ± 0.11 pA, ns p>0.05, Mann-Whitney test). Line shows mean. Insets show representative traces for each condition. Scale bars: 100pA, 20ms. N (number of recordings) indicated on all graphs.
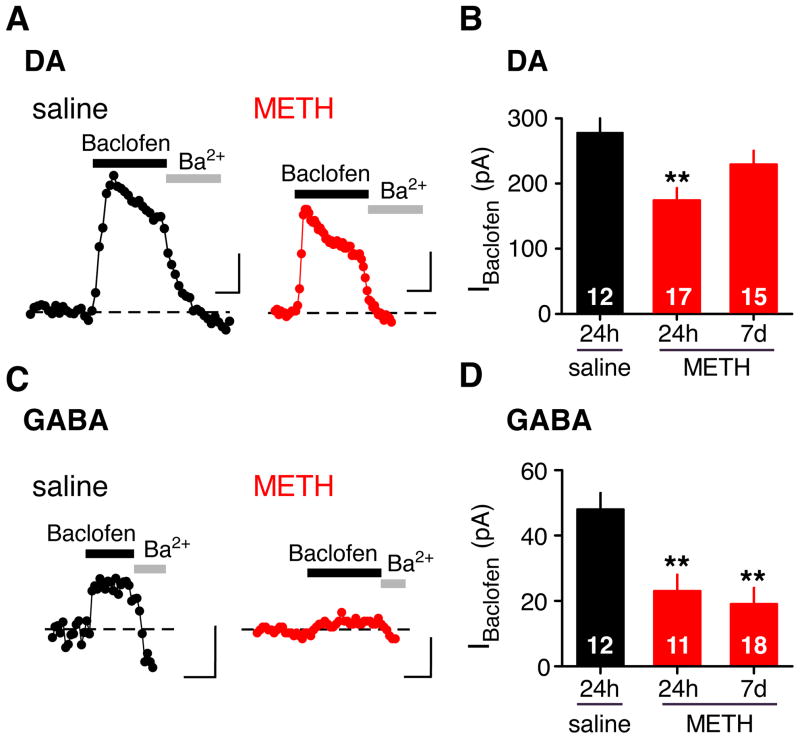
To investigate the effects of METH on synaptic and extra-synaptic GABAB receptors, the GABAB receptor agonist baclofen was applied to the bath. As described previously (Labouèbe et al., 2007), saturating doses of baclofen (300 μM for DA and 100 μM for GABA) elicited large and desensitizing GABABR-activated GIRK currents in DA neurons and small non-desensitizing currents in GABA neurons (Figure 2). All baclofen-activated currents were inhibited with the inwardly rectifying K channel inhibitor Ba2+ or the GABAB receptor antagonist (CGP 54626 – not shown). In contrast to the sIPSC recordings, there was a ~40% decrease in the GABABR-GIRK currents of DA neurons 24h following a METH injection (Figure 2A,B. However, this decrease in current was not apparent at 7d following METH injection (Figure 2B). By contrast, the baclofen-activated GIRK (IBaclofen) currents in GABA neurons were significantly depressed by ~55% 24h following a single METH injection and the reduced IBaclofen persisted for 7 days (Figure 2C,D). We next examined whether METH altered GABABR-GIRK signaling in other brain regions. There was no significant change in the sIPSC or IBaclofen in CA1 hippocampal pyramidal or GABAergic neurons 24h following METH (Supplemental Figure S1). We also measured the sIPSC and IBaclofen in pyramidal and GABAergic neurons of the prelimbic cortex, a target region of VTA DA cells, and observed no significant changes in GABAB-GIRK currents in METH injected mice (Supplemental Figure S1). Thus, a single exposure to METH triggered a profound and long-lasting depression in both the sIPSC and IBaclofen in GABA neurons of the VTA.
Figure 2. Reduced GABABR-GIRK currents in VTA GABA neurons 24h and 7d following in vivo METH exposure.
The baclofen-activated GIRK currents (IBaclofen) recorded from VTA DA (A) and GABA (C) neurons 24h following a saline (0.9%) or METH (2mg/kg) injection. Outward currents recorded at −50 mV are plotted as a function of time. IBaclofen is blocked by the inward rectifier inhibitor Ba2+ (1mM) or by the GABABR antagonist CGP 54626 (data not shown). Scale bars: 100pA (A) 50 pA (C), 100s. B, Bar graph shows average IBaclofen in DA neurons 24h following saline (DA saline: 278 ± 37 pA) or 24h and 7d following METH injection (DA METH: 174 ± 19, DA 7d METH: 229 ± 21 pA). D, Bar graph shows average IBaclofen in GABA neurons 24h following saline injection (GABA saline: 48.4 ± 5.3 pA) or 24h and 7d following METH injection (GABA METH: 22.9 ± 4.7, GABA 7d METH: 19.4 ± 4.5 pA,). Note significant decrease in IBaclofen in GABA neurons of METH-injected mice that persists for 7d. **P < 0.05 One-way ANOVA One-way ANOVA
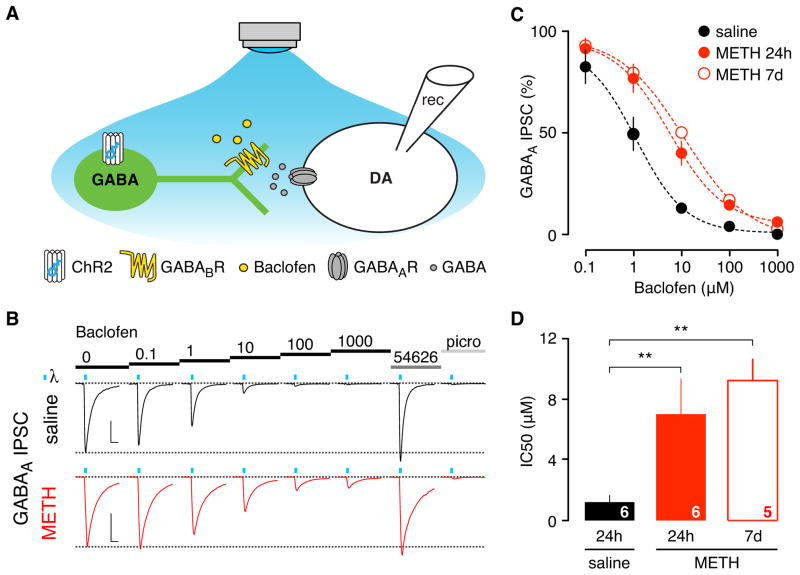
In addition to postsynaptic GABAB receptors, pre-synaptic GABAB receptors are also involved in reducing GABA release, typically through inhibition of voltage-gated Ca2+ channels (Padgett and Slesinger, 2010). To investigate whether in vivo exposure to METH altered GABAB receptor-dependent presynaptic inhibition, we used an optogenetic strategy to selectively stimulate GABA neurons in the VTA and measure the effect of baclofen on a light-evoked fast inhibitory post-synaptic current (IPSC) recorded in DA neurons (Figure 3). AAV virus expressing a double floxed-stopped ChR2-EYFP was stereotaxically injected into the VTA of mice expressing Cre recombinase in GABA neurons (GAD65-Cre) (Figure 3A, Supplemental Figure S2). After 21 days, neurons expressing ChR2-YFP were evident in horizontal slices of the VTA (Figure S2A). Prolonged blue light stimulation (400ms) elicited tetrodotoxin (TTX)-insensitive photocurrents in GABA neurons, whereas short light pulses (4ms) evoked picrotoxin- and TTX-sensitive fast IPSCs in DA neurons (Supplemental Figure S2B,C; Figure 3B). Bath application of baclofen (1 μM) depressed the light-evoked IPSC by ~50% in saline injected mice. By contrast, baclofen (1 μM) decreased the light-evoked IPSC by only ~20% in METH-injected mice (Figure 3B,C). Construction of dose-response curves revealed that GABAB receptor-dependent inhibition of presynaptic release was shifted significantly to higher agonist concentrations (Figure 3C), reflected by an increase in the IC50, which is the concentration of Baclofen needed to inhibit 50% of the light-induced current (Figure 3D). Similar to the change in postsynaptic GABABR-GIRK signaling, the reduced sensitivity of presynaptic GABABRs persisted for 7 days (Figure 3C,D). As a control, we examined GABABR-dependent presynaptic inhibition of glutamate release onto DA neurons, by measuring the amplitude of electrically evoked AMPA EPSC while applying increasing concentrations of Baclofen (Supplemental Figure S3). We found no significant change in the IC50 in METH injected mice, compared to saline controls. Taken together, these results demonstrate that a single in vivo injection of METH triggers a depression in GABAB receptor signaling in VTA GABA neurons, both presynaptically (inhibition of GABA release) and postsynaptically (activation of GIRK channels).
Figure 3. Reduced sensitivity of pre-synaptic GABAB receptor-mediated inhibition 24h and 7d following in vivo METH exposure.
A, Schematic shows channel rhodopsin 2 (ChR2) protein expressed selectively in VTA GABA neurons of GAD65-Cre mice. GABA neuron activity is induced by blue light, resulting in a fast GABAA receptor-mediated IPSCs recorded from VTA DA neuron. Baclofen impairs GABA release by acting on presynaptic GABAB receptors. B, Example traces of blue light-evoked IPSCs recorded 24h following saline or METH injection in presence of increasing concentrations of baclofen. Blue ticks indicate light stimulation (2× 4 ms). Basal IPSC amplitude recovers after application of CGP 54626 (2μM) and is subsequently blocked by picrotoxin (100μM). Scale bars: 200pA, 10ms. C, Dose response curves show reduced sensitivity for baclofen-dependent inhibition of fast IPSCs in METH injected mice after 24h and 7 days. D, Bar graph plots IC50 for indicated conditions (saline: 1.2 ± 0.4 μM, 24h METH: 7.1 ± 2.4 μM, 7d METH: 9.4 ± 1.4 μM, ** P < 0.05 One-way ANOVA).
Psychostimulant-evoked plasticity in GABABR involves dopamine
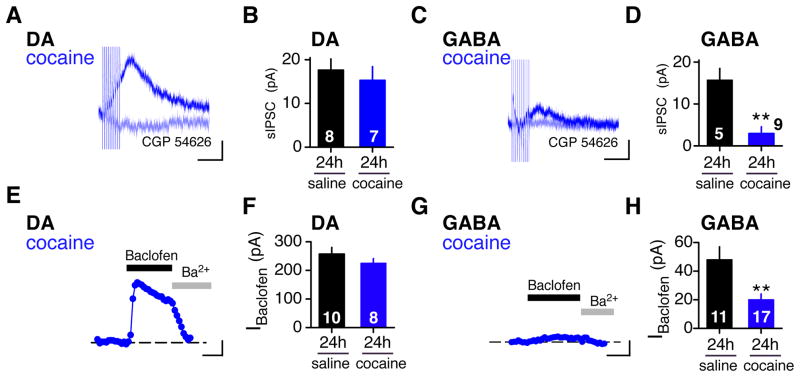
Cocaine is another psychostimulant that rapidly elevates DA levels within minutes after the injection. In contrast to METH, which is taken up by DA neurons and stimulates reverse transport of DA through the dopamine transporter (DAT), cocaine inhibits DAT from the extracellular side (Sulzer, 2011). We examined whether a single injection of cocaine would evoke a change in GABABR-GIRK signaling. Like METH, cocaine (15mg/kg) produced a significant decrease in the sIPSC in GABA neurons but not in DA neurons 24h later (Figure 4A–D). Similarly, IBaclofen was depressed in GABA neurons but not in DA neurons (Figure 4E–H). Thus, both cocaine and METH trigger a similar neuroadaptation in GABABR-GIRK signaling in GABA neurons of the VTA, suggesting that elevated DA may be an important step in inducing the GABABR-GIRK plasticity.
Figure 4. Depression of GABABR-GIRK signaling in VTA GABA neurons 24h following a single cocaine injection.
A–D, The sIPSC is reduced in VTA GABA (C) but not in DA (A) neurons 24h following a single cocaine (15mg/kg) injection. Scale bars: 5pA, 200ms. Only recording from cocaine-injected mice are shown. Light blue trace shows sIPSC recorded with CGP 54626 (2 μM). B,D Bar graphs show mean amplitudes for sIPSC 24h following saline or cocaine (DA saline: 17.6 ± 2.6 pA; DA cocaine: 15.3 ± 3.1 pA; GABA saline: 15.7 ± 2.8 pA, GABA METH: 2.9 ± 1.6 pA). E–H, The baclofen-induced GIRK current (IBaclofen) is reduced in VTA GABA (G) but not DA (E) neurons 24h following cocaine (15mg/kg) injection. Traces show current recorded at −50 mV with Baclofen (100 μM) or Ba2+ (1 mM). Scale bars: 50pA, 100s. F, Bar graph shows average IBaclofen in DA neurons 24h following saline or cocaine injection (F) (DA saline: 269 ± 27, DA cocaine: 225 ±16 pA). H, Bar graph shows average IBaclofen in GABA neurons 24h following saline or cocaine injection (GABA saline: 47.7 ± 8.9, GABA cocaine: 20.4 ± 3.8 pA). **P < 0.05 Student’s t-test.
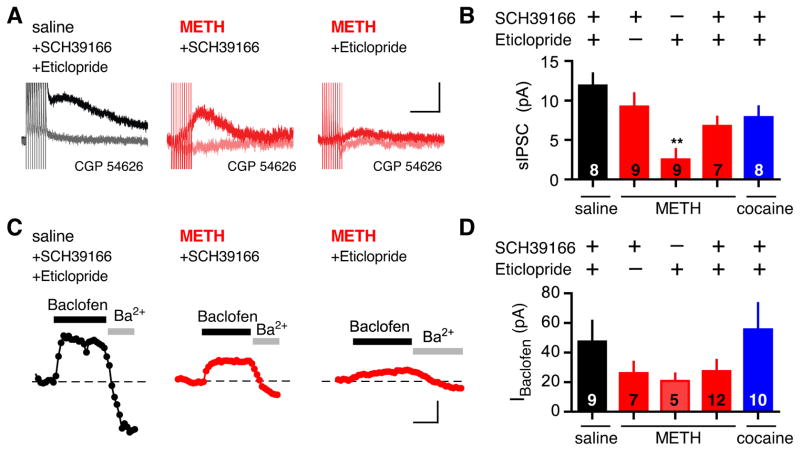
Dopamine stimulates two classes of DA receptors, D1-like and D2-like receptors, in the brain (White, 1996). D1-like receptor antagonists block sensitization to psychostimulants (Kalivas and Stewart, 1991), reduce self-administration of cocaine (Caine et al., 2007) and prevent psychostimulant-induced changes in glutamatergic signaling in the VTA (Argilli et al., 2008; Brown et al., 2010). To test the requirement for DA receptors, we injected an antagonist for D1-like (SCH39166) or D2-like (Eticlopride) receptors with METH (Figure 5). The sIPSC recorded from mice injected with METH and SCH39166 (0.3 mg/kg) was not significantly different from saline (Figure 5A,B). By contrast, coinjection of Eticlopride (0.1 mg/kg) with METH did not attenuate the METH-dependent decrease in sIPSC. For macroscopic GABABR-GIRK currents, coinjection of METH and SCH39166 also partially blocked the METH-dependent decrease in IBaclofen (Figure 5C,D). Interestingly, coinjection of Eticlopride with METH attenuated the METH-dependent decrease in IBaclofen (Figure 5C,D), in contrast to the effect of Eticlopride on the sIPSC. This could reflect a difference in synaptically and extra-synaptically activated GABAB receptors. For cocaine, coinjection of METH or cocaine with both SCH39166 and Eticlopride partially recovered the sIPSC, compared to saline-injected controls (Figure 5B,D). Together, these pharmacological experiments clearly implicate DA and the D1-like receptor in mediating the psychostimulant-dependent depression in GABABR-GIRK signaling in VTA GABA neurons, similar to the plasticity changes in excitatory synapses in VTA DA neurons following cocaine (Argilli et al., 2008).
Figure 5. D1-like receptor antagonist blocks METH-induced depression in GABABR-GIRK signaling.
A, The sIPSC recorded from VTA GABA neurons in a VTA slice from mice coinjected with saline and D1-like receptor antagonist (SCH39166; 0.3 mg/kg)/D2-like receptor antagonist (Eticlopride; 0.1 mg/kg), METH and SCH39166, or METH and Eticlopride. The GABAB receptor antagonist CGP 54626 (2 μM) inhibited the sIPSC. Scale bar: 5pA, 200ms. B, Bar graph shows the average sIPSC in VTA GABA neurons (saline + SCH39166/Eticlopride: 11.9 ± 1.6pA; METH + SCH39166: 9.2 ± 1.7pA; METH + Eticlopride: 2.5 ± 1.4pA; cocaine + SCH39166/Eticlopride 6.8 ± 1.2pA; ** P < 0.05 vs saline One-way ANOVA). C, IBaclofen recorded from mice coinjected with saline and SCH39166/Eticlopride, METH and SCH39166, or METH and Eticlopride. Scale bar: 50pA, 100s. H, Bar graph shows the average IBaclofen (saline + SCH39166/Eticlopride: 44.4 ± 12.5pA; METH + SCH39166: 27.3 ± 4.1pA; METH + Eticlopride: 23.0 ± 9.7pA; METH + SCH39166/Eticlopride: 27.3 ± 7.8pA; cocaine + SCH39166/Eticlopride 55.7 ± 17.8pA; P > 0.05 not significant from saline).
Cellular mechanism underlying depression of GABABR-GIRK signaling
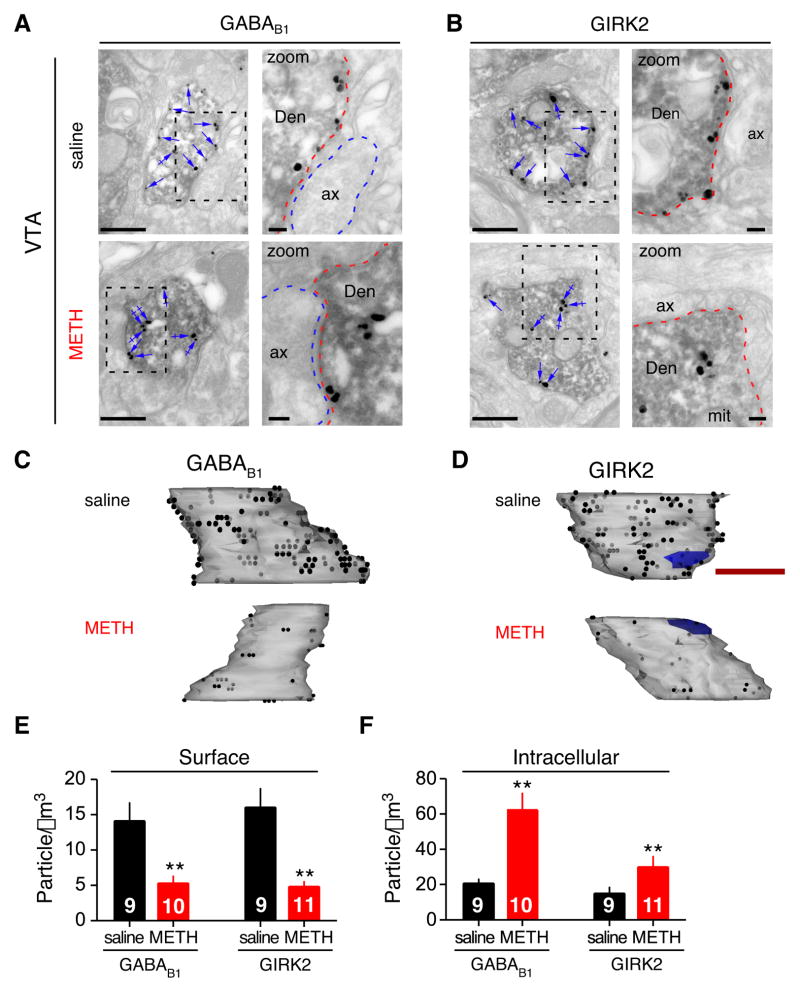
A reduction in the amplitude of GABAB-GIRK currents could involve a change in G protein coupling (Nestler et al., 1990; Labouèbe et al., 2007), desensitization of GABAB receptors (Taniyama et al., 1991; Gonzalez-Maeso et al., 2003), and/or internalization of the receptor-channel (Fairfax et al., 2004; Guetg et al., 2010; Maier et al., 2010; Terunuma et al., 2010). To investigate the latter possibility, we used quantitative immuno-electron microscopy to study the sub-cellular distribution of GABAB receptors and GIRK channels in saline and METH injected mice. In serial ultrathin sections through the VTA, GABA neurons were identified using antibodies against GAD65/67 and secondary antibodies coupled to horseradish peroxidase (HRP), generating a dark reaction product in GABA neurons (Figure 6A,B). VTA sections were also labeled with immunogold particles using specific antibodies for GABAB1 or GIRK2 (Kulik et al., 2003; Koyrakh et al., 2005). In single ultrathin sections (Figure 6A,B), both GABAB1 and GIRK2 were expressed predominantly at the plasma membrane of GABA neuron dendrites (Den; arrows) following saline injection. By contrast, 24h following a METH injection, there was a reduction in plasma membrane associated GABAB1 and an increase at intracellular sites (Figure 6A, crossed arrows). Similarly, there was a reduction in GIRK2 protein on the plasma membrane and an increase in intracellular compartments in GABA neurons following METH treatment (Figure 6B). To quantify these changes, we generated a three dimensional reconstruction of the dendrite using the serial ultrathin sections (Figure 6C,D) and then counted gold particles on the plasma membrane and in the cytoplasm. Calculating the surface and intracellular densities (Figure 6E,F) revealed that 24h following METH injection there was a significant reduction (~60–70%) in plasma membrane-associated GABAB1 and GIRK2, with a concomitant increase in the intracellular-associated GABAB1 and GIRK2 (~50–65%). By contrast, we did not observe a significant change in immunogold particle labeling of plasma membrane staining for GIRK2 and GABAB1 in GAD65/67-negative neurons (GIRK2: 0.924 ± 0.032 particles/μm2 saline vs 0.843 ± 0.054 METH, n=21 and GABAB1: 1.042 ± 0.043 saline vs. 0.922 ± 0.050 GABAB1; P > 0.05). Interestingly, the reduction in plasma membrane-associated GIRK2 and GABAB1 parallels the ~50% depression in baclofen-induced GABABR-GIRK currents (Figure 2F). Moreover, the relative decreases in GABAB1 and GIRK2 protein on the plasma membrane are very similar, suggesting the GABAB receptor and GIRK channel may internalize as a signaling complex from the plasma membrane (Boyer et al., 2009). Taken together, these data demonstrate that 24h after a single injection of METH both GABAB receptor and GIRK channel protein levels are reduced on the plasma membrane of GABA neurons, providing a reasonable explanation for depressed GABABR-GIRK currents in those neurons.
Figure 6. Reduced surface expression of GABAB1 receptors and GIRK2 channels in GABA neurons of METH injected mice.
A,B, Pre-embedding double-labeling electron microscopy reveals GABAB1 and GIRK2 expression in GABAergic neurons of the VTA 24h following saline or METH injection. GABA neurons were identified by GAD65/67-HRP immunoreactivity (labeled Den). Immunogold particles identify GABAB1 or GIRK2. A reduction in immunogold particles against GIRK2 or GABAB1 along the plasma membrane (arrows) was clearly detected in GAD65/67 positive dendrites following METH injection; an increase in immunogold particles against GIRK2 and GABAB1 at intracellular sites can also be seen (crossed arrows). Right panels in A,B show zoom of boxed area. Den: dendrite; ax: axon; mit: mitochondria. Scale bars: 0.5 μm. C,D Three-dimensional reconstructions of dendrites from serial electron micrographs. Note decrease in surface expression of GABAB1 receptors (C) and GIRK2 (D) following METH treatment. Black dots represent immunogold particles on the front surface and grey dots show immunogold particles on the reverse side of the dendrite. Blue regions are excitatory synapses. Note immunogold particles are abundantly distributed over the dendritic plasma membrane in control mice. Scale bars: 0.5 μm. E,F Bar graphs show quantification of immunogold particles in reconstructed GAD65/67 positive dendrites for plasma membrane associated (GABAB1 saline: 16.0 ± 2.7, GABAB1 METH: 4.8 ± 0.8, GIRK2 saline: 14.1 ± 2.6, GIRK2 METH: 5.3 ± 1.0 particles/μm3) and intracellular particles (GABAB1 saline: 15 ± 3.6, GABAB1 METH: 29.9 ± 6.2, GIRK2 saline: 20.7 ± 2.6, GIRK2 METH: 62.3 ± 10.5 particles/μm3). Immunogold particles against both GABAB1 and GIRK2 are significantly reduced at the dendritic plasma membrane and increased at intracellular sites 24h after METH injection (**P<0.05 Student t-test).
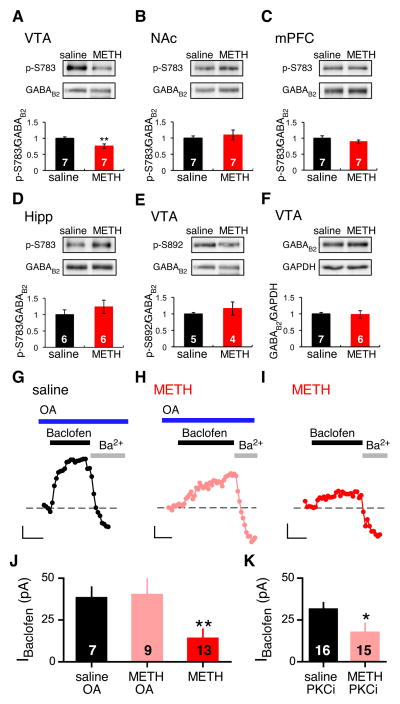
The quantitative immunogold electron microscopy data suggested that METH treatment induced internalization of the receptor and channel. The phosphorylation status of the GABAB receptor is important for regulating surface expression of the receptor (Fairfax et al., 2004; Koya et al., 2009; Guetg et al., 2010; Terunuma et al., 2010). We therefore examined whether phosphorylation of the GABAB receptor could play a role in mediating the METH-dependent depression. We examined the phosphorylation of S783 (p-S783) in GABAB2 because dephosphorylation is associated with reduced surface expression of GABAB receptors in neurons (Terunuma et al., 2010). Protein isolated from tissue punches of the VTA, NAc, hippocampus or mPFC from saline- and METH-injected mice (24h) were examined using a phospho-specific antibody for phosphorylated S783 in GABAB2 (Dobi et al., 2010). Remarkably, METH injection led to a ~25% reduction in phosphorylation of GABAB2-S783 in the VTA (Figure 7A). This change in p-S783 compares to a METH-induced ~50% reduction in IBaclofen in GABA neurons (Figure 2D). However, the VTA tissue punches contain a mixture of cell types that express GABAB receptors, which likely accounts for the smaller change in GABAB2-p-S783. By contrast, there was no change in GABAB2-p-S783 in the NAc, mPFC or hippocampus from METH injected mice (Figure 7B–D). Examination of p-S892, a different phosphorylation site on GABAB2 (Fairfax et al., 2004), revealed no change in phospho-specific labeling of GABAB2-p-S892 in METH-injected mice, indicating the effect of METH was unique to GABAB2-S783 (Figure 7E). Lastly, there was no apparent change in the levels GABAB2 receptor protein (Figure 7F), suggesting little METH-dependent degradation of receptor.
Figure 7. Role of de-phosphorylation of GABABRs in METH-dependent depression of GABABR-GIRK currents in GABA neurons.
A–D Western blots using phospho-specific antibody for p-S783 in GABAB2 and total GABAB2 in tissue punches of VTA, NAc, PFC and hippocampus from saline and METH injected mice (6–7 mice per group). Bar graphs show quantification of western blots normalized to GABAB2 levels. Note significant decrease in p-S783 in VTA (**P<0.05 Student’s t-test). E, Western blot and quantification for p-S892 in GABAB2 and total GABAB2 in VTA. F, Western blot and quantification for total GABAB2 and GAPDH in VTA. G–I, Intracellular application of OA but not PKC inhibitor recovered IBaclofen in METH-injected mice. Representative recordings of IBaclofen in GABA neurons from saline and METH injected mice are shown with 100nM okadaic acid (OA) included in patch electrode (OApipet) (Vm = −50 mV). Scale bars: 10pA, 100s. J, Bar graph shows average IBaclofen for saline-injected/OApipet (38.3 ± 6.3pA), METH-injected/OApipet (40.2 ± 9.4pA), and METH-injected (14.1 ± 5.6 pA). **P < 0.05 vs saline using one-way ANOVA. K, For control, a PKC inhibitor (PKC(19–36)), 1 μM) included in the pipet (PKCipipet) did produce significant recovery of IBaclofen (saline+ PKCipipet: 31.5 ± 3.9 pA; METH+ PKCipipet: 17.5 ± 5.7pA; * P < 0.05 Student’s t-test).
Dephosphorylation of GABAB2-p-S783 has previously been shown to be regulated by protein phosphatase 2A (PP2A) (Terunuma et al., 2010), raising the possibility that in vivo exposure to METH enhances the phosphatase activity in VTA GABA neurons. To address this, we examined the effect of acutely inhibiting PP1/PP2A phosphatases with okadaic acid (100nM, OA). In saline-injected mice, there was no significant difference in the amplitude of IBaclofen with OA in the pipet, suggesting basal activity of PP1/PP2A does not significantly regulate GABABR-GIRKs (Figure 7G–J). In METH-injected mice, however, intracellular application of OA promoted recovery of the IBaclofen (Figure 7H,J). Note the slow time course of activation for IBaclofen in the presence of OA in METH injected mice. This increase could reflect insertion of GABAB receptors and GIRK channels on the plasma membrane or restoration of functional G protein coupling. For control, we examined the effect of PKC(19–36), a peptide inhibitor of PKC (Figure 7K). Unlike OA, the presence of PKC inhibitor in the pipet did not restore IBaclofen, similar to the effect of METH alone. Taken together, these findings suggest that in vivo exposure to METH triggers a phosphatase-dependent down-regulation of GABABRs and GIRK channels from the plasma membrane of GABA neurons, which results in reduced GABABR-GIRK signaling and accumulation of GABAB receptor complexes in intracellular compartments.
Loss of GABABR-dependent inhibition of VTA GABA neuron firing
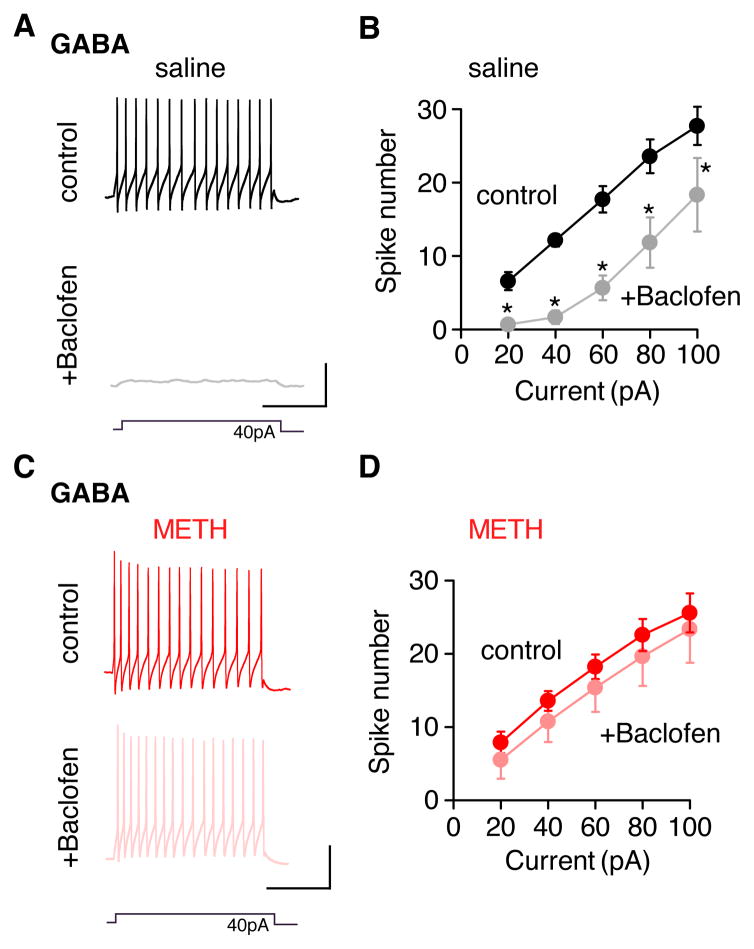
To investigate the functional consequence of reduced GABABR-GIRK currents in GABA neurons of METH-injected mice, we examined the effect of baclofen on the induced firing rate of GABA neurons (Figure 8). We predicted that a loss of GABABR-GIRK signaling would attenuate GABABR-mediated suppression of firing in GABA neurons. To test this, a series of current steps (20–100pA) were injected to elicit a train of action potentials in GABA neurons (Figure 8A,B). In saline-injected and METH-injected mice, the input-output (I-O) plot shows a linear increase in firing rate with larger current injections (Figure 8B,D). As expected, baclofen (100 μM) significantly suppressed firing in GABA neurons of saline-injected mice, decreasing the slope of the I-O curve (Figures 8A,B). By contrast, a saturating dose of baclofen (100 μM) did not significantly change the I-O curve in METH-injected mice (Figures 8B,C). These results demonstrate that a loss of GABABR-GIRK currents in GABA neurons removes an important ‘brake’ on GABA neuron firing in the VTA.
Figure 8. METH-injected mice lack GABABR-dependent inhibition of VTA GABA neuron firing.
A,C Current clamp recordings show spiking of VTA GABA neurons elicited by a current injection (40 pA) in saline (A) and METH (C) injected mice 24h later. Spiking recorded in the absence (top trace) and presence (bottom trace) of 100 μM baclofen. Scale bars: 250ms, 40mV. B,D Input-output graphs show spike number in GABA neurons plotted as a function of current injection for saline (B, N=7) and METH (D, N (Henry et al., 1989)=7) injected mice. Average spike number increases with current injections and is significantly suppressed by 100 μM baclofen in saline-injected mice (B). *P<0.05 Two Way Repeated Measure ANOVA. In METH-injected mice (D), Baclofen does not significantly suppress spiking (ns, P>0.05).
DISCUSSION
Drug-evoked synaptic plasticity can cause persistent modifications of neural circuits that eventually lead to addiction. We report here that a single dose of METH or cocaine is sufficient to significantly weaken the ability of GABAB receptors to control VTA GABA neuron firing when measured ex vivo 24h later. As such, this adaptive change is not likely sufficient to cause addiction, but rather represents a building block of the adaptations that underlie addictive behavior with repetitive exposure. Studying the effect of a single injection of drug enabled us to systematically probe the mechanism underlying the plasticity of the slow IPSC. We discovered the methamphetamine-induced loss of the slow IPSC arises from a reduction in the GABABR-GIRK currents, due to changes in protein trafficking, and is accompanied by a significant decrease in the sensitivity of presynaptic GABAB receptors in GABA neurons of the VTA. In contrast, GABA neurons of the hippocampus and prelimbic cortex did not show similar changes in GABAB-GIRK signaling, suggesting the GABABRs in the VTA are uniquely targeted by psychostimulants.
The psychostimulant-evoked reduction of GABAB-GIRK currents in VTA GABA neurons could arise from a change in G protein coupling (Nestler et al., 1990; Labouèbe et al., 2007) or internalization of the receptor-channel (Gonzalez-Maeso et al., 2003; Fairfax et al., 2004; Guetg et al., 2010; Maier et al., 2010; Terunuma et al., 2010). In support of the latter possibility, quantitative immunogold electron microscopy revealed a significant reduction in surface expression of GABAB receptors and GIRK channels in GABA neurons of METH-injected mice, coincident with a decrease in phosphorylation of GABABRs. In cortical and hippocampal neurons, a balance of AMP-activated protein kinase (AMPK)-dependent phosphorylation of GABAB2-S783 and PP2A-dependent dephosphorylation governs postendocytic sorting of GABAB receptors (Terunuma et al., 2010). The persistence of the GABAB-GIRK depression and the rapid recovery with phosphatase inhibitors suggest the balance of surface and internalized GABAB receptors in GABA neurons might be controlled by a molecular switch in a phosphatase, perhaps akin to the autophosphorylation switch in CaMKII (Lucchesi et al., 2011), or through an endogenous regulator of protein phosphatase activity (Guo et al., 1993). It remains possible that other kinases are also involved; both PKA- and CaMKII-dependent phosphorylation have been implicated in stabilization of GABAB1 on the plasma membrane (Couve et al., 2002; Guetg et al., 2010). Interestingly, total protein levels of GABAB2 receptors levels were not significantly changed in METH-injected mice, suggesting the internalized pool of receptors was not redirected to a degradation pathway, in contrast to activity-dependent degradation of GABAB receptors observed in cortex (Terunuma et al., 2010). If phosphorylation controls surface expression of GABAB receptors, then what controls the surface expression of GIRK channels? CaMKII-dependent phosphorylation of GIRK2 has been implicated in stabilizing GIRK2 channels on the plasma membrane of hippocampal neurons (Chung et al., 2009). In these neurons, protein phosphatase-1-mediated dephosphorylation promotes GIRK channel recycling and increases surface expression (Chung et al., 2009); therefore, a phosphatase inhibitor would be expected to reduce GIRK expression on the plasma membrane. An alternative explanation is that GIRK channels internalize via association with GABAB receptors in a macromolecular signaling complex. Previous studies have shown that both GPCRs and GIRK channels are physically close (Lavine et al., 2002; Nobles et al., 2005; Riven et al., 2006; Fowler et al., 2007) and can traffic together through intracellular compartments (Clancy et al., 2007).
Psychostimulants, like METH and cocaine, generally lead to elevations in DA (Sulzer, 2011) that signals through two classes of GPCRs, D1-like and D2-like receptors. Activation of D1-like receptors is required for inducing locomotor sensitization (Kalivas and Stewart, 1991), for establishing self-administration of cocaine (Caine et al., 2007) and for potentiating excitatory synapses with psychostimulants (Argilli et al., 2008; Brown et al., 2010). Supporting a role for D1-like receptors, coinjection of a D1-like receptor antagonist significantly attenuated the psychostimulant-dependent depression of GABABR-GIRK currents in VTA GABA neurons. We also observed some effects of the D2-like antagonist and cannot completely rule out a component of D2-like receptor activation in the depression of GABAB-GIRK signaling. Recently, an acute cocaine-induced weakening of baclofen-induced GIRK currents in VTA DA neurons was found to be sensitive to D2-like but not D1-like receptor antagonists (Arora et al., 2011). In addition to DA, other neurotransmitters may be involved in the psychostimulant-dependent depression of GABABR-GIRK signaling. For example, acetylcholine levels in the VTA also increase following a single METH injection (Dobbs and Mark, 2008), and neuropeptides, such as hypocretin/orexin, BDNF, and CRF could be also involved in the response to addictive drugs (Wang et al., 2005; Borgland et al., 2006; Hyman et al., 2006; Pu et al., 2006). Conditional knockouts or selective pharmacological experiments will be needed to pinpoint the neurotransmitters involved in the psychostimulant-dependent depression of GABABR-GIRK responses in VTA GABA neurons.
How may the psychostimulant-evoked depression in GABAB-GIRK signaling in VTA GABA neurons alter the physiology of the VTA and contribute to addiction? DA neurons fire in two modes, tonic and phasic, with phasic firing leading to higher DA levels (Cooper, 2002). A balance of NMDAR activation and GABABR signaling controls tonic vs phasic firing, and activation of GABAB receptors plays an important role in reducing phasic firing in VTA DA neurons (Erhardt et al., 2002). The VTA GABA neurons provide a local source of GABA for controlling the firing of VTA DA neurons (Grace and Bunney, 1985; Johnson and North, 1992; Tan et al., 2010). Recent electron microscopy studies have confirmed synaptic contacts between local GABA and DA neurons within the VTA (Omelchenko and Sesack, 2009). In the present study, we demonstrate using optogenetic tools the functionality of these GABAergic synapses. The depression of GABABR-GIRK signaling in somatodendritic regions along with the reduced sensitivity of GABABRs in presynaptic GABA terminals of VTA GABA neurons would markedly impair an intrinsic ‘brake’ on GABA release several days after a single injection of METH. Together, these pre and postsynaptic neuroadaptations are predicted to increase GABA-mediated inhibition of VTA DA neurons. In line with this model, other groups have reported psychostimulant-evoked neuroadaptations in GABABR-signaling that lead to enhanced GABAergic transmission in the VTA (Giorgetti et al., 2002), the dorsolateral septal nucleus (Shoji et al., 1997) and the NAc (Xi et al., 2003). Similarly, chronic morphine increases the sensitivity of GABAB receptors on glutamatergic terminals in the VTA, which would further enhance the inhibition of DA neurons mediated by augmented GABA release (Manzoni and Williams, 1999).
The enhanced GABAergic inhibition of VTA DA neurons may represent an attempt to restore balance in activity of the VTA circuit and therefore GABABR-GIRK adaptation may be considered a form of synaptic scaling. Neuroadaptive changes in GABABR-GIRK signaling for reestablishing balance in neural circuits have been described in other model systems. In a mouse model of succinic semialdehyde dehydrogenease (SSADH) deficiency, an autosomal recessive disorder of GABA catabolism that leads to elevated synaptic GABA, GABABR-GIRK currents are significantly depressed in cortical neurons (Vardya et al., 2010). On the other hand, the GABABR-mediated IPSC in hippocampal pyramidal neurons is enhanced in response to potentiation of excitatory synaptic transmission (Huang et al., 2005). The level of inhibition mediated by GABABR-GIRK currents may be tightly tuned to changes in neuronal excitability.
The downregulation of GABAB receptor signaling in VTA GABA neurons occurs in parallel with other plastic changes in VTA DA neurons, such as the redistribution of AMPAR and NMDARs (White et al., 1995; Zhang et al., 1997; Ungless et al., 2001; Borgland et al., 2004; Argilli et al., 2008; Mameli et al., 2011) and alterations of fast GABAergic transmission (Nugent et al., 2007). As proposed above, the drug-evoked depression of GABABR signaling in GABA neurons removes a “brake” on GABA neuron firing that may enhance GABA-mediated inhibition of DA neurons and potentially reduce reward perception (Koob and Volkow, 2010; Luscher and Malenka, 2011). However, repeated psychostimulant administration leads to increases in the firing rates of VTA DA neurons (White and Wang, 1984; Henry et al., 1989; White, 1996), partly through reduced sensitivity of D2 autoreceptors (White, 1996). Thus, the increase in GABA-mediated inhibition of VTA DA neurons, while efficient at first, may eventually be inadequate to suppress the potentiating effects of psychostimulants on VTA DA neurons. Clearly, additional experiments will be needed to better understand how the adaptation of GABABR-GIRK signaling affects VTA GABA neuron function and, more generally, the role of the slow GABAB-mediated inhibition in drug-evoked remodeling of the mesocorticolimbic circuitry.
In conclusion, we have identified a novel, molecular switch in GABAB receptor signaling that occurs in response to a single in vivo exposure to psychostimulant – this depression of GABABR-GIRK signaling persists for days after the injection. This cellular memory trace of drug exposure is encoded in a phosphorylation-dependent depression of GABAB receptor signaling in VTA GABA neurons, which may augment GABA transmission in the mesocorticolimbic system.
MATERIALS AND METHODS
Animals
C57BL/6 mice were purchased from Harlan laboratories or bred in-house, and housed under constant temperature and humidity on a 12h light-dark cycle (light 6am-6pm) with free access to food and water. GAD67-GFP is a knock-in mouse that was kindly provided by Dr. Y. Yanagawa. Pitx3-GFP is a knock-in mouse that was kindly provided by Dr. M. Li. All procedures were performed in the light cycle using IACUC approved protocols for animal handling at the Salk Institute and the University of Geneva.
Drug treatment
Male and female mice (P15–35) were injected intraperitoneally with 0.9% saline (control), 2 mg/kg methamphetamine (METH) or 15 mg/kg cocaine using a 15 gauge insulin syringe and injection volume < 200ul to minimize stress. Experimental procedures were performed 24h-7d later. Methamphetamine and cocaine were purchased from Sigma.
Electrophysiology in acute slices
24h or 7 days following i.p. injections, mice were euthanized and horizontal slices from midbrain (250 μm) were prepared in ice cold artificial cerebral spinal fluid (ACSF) (see Supplemental Methods for details). Neurons were visualized with IR camera Gloor Instrument PCO or Dage-MTI IR-1000) on an Olympus scope (BX50 or BX51) and whole-cell patch-clamp recordings (Axopatch 200B or Multiclamp 700A amplifier) were made from neurons in the VTA, identified as the region medial to the medial terminal nucleus of the accessory optical tract. GABA neurons were identified by the absence of Ih current, a small capacitance (<20pF) and a fast spontaneous firing rate (5–10Hz). In contrast DA neurons have an Ih current, large capacitance (20–50 pF) and slow spontaneous firing (1–3Hz). Pitx3-GFP mice expressing GFP in DA neurons (Zhao et al., 2004) and GAD67-GFP mice expressing GFP in GABA neurons (Tamamaki et al., 2003) were used to confirm electrophysiological identification. The internal solution for measuring baclofen-activated GABAB currents contained (in mM) potassium gluconate (140), NaCl (4), MgCl2 (2), EGTA (1.1), HEPES (5), Na2ATP (2), sodium creatine phosphate (5) and Na3GTP (0.6), pH 7.3 with KOH. For GABAB sIPSCs, the internal solution contained (in mM) K-gluconate 140, KCl 5, MgCl2 2, EGTA 0.2, HEPES 10, Na2ATP 4, Creatine-phosphate 10 and Na3GTP 0.3. To measure GABAA currents, the internal solution contained (in mM) K-gluconate 30, KCl 100, MgCl2 4, creatine phosphate 10, Na2 ATP 3.4, Na3 GTP 0.1, EGTA 1.1 and HEPES 5.
For the sIPSC, the evoked synaptic recordings were isolated in presence of APV (100μM), NBQX (10μM) and sulpiride (200nM) for GABAAR IPSC, and PTX (100μM) for GABABR sIPSC. The stimulation electrode consisted of a saline-filled monopolar glass pipette, placed caudally to the cell being recorded. GABAAR paired-pulse ratio (PPR) was assessed by applying two pulses at 50 ms interval, every 10 seconds, whereas the GABABR sIPSCs were evoked by applying a train of 10 electrical pulses at 66Hz, once every 20–40 seconds. For IBaclofen, currents were recorded, filtered at 1 kHz and digitized at 5 kHz (Axon pClamp 8). Cells were clamped at −50 or −60 mV (membrane voltages were corrected for liquid junction potential; −15.7mV). For some recordings, a voltage ramp from +60mV to −100 mV was delivered at 1Hz. Cell membrane resistance and approximate access resistance were measured with a 200ms 10mV hyperpolarizing step. All electrophysiological chemicals for electrophysiology were purchased from Sigma; drugs purchased from Tocris. We did not observe any differences with wild-type mice and Pitx3-GFP or GAD67-GFP; therefore we have pooled the data. Data are expressed as mean ± s.e.m. and statistical significance (P<0.05) determined by one-way ANOVA with Holm-Sidak post hoc test, or Student’s t-test. All measurements made at ~33° C.
Optogenetic Experiment
AAVx-ChR2 flox virus (produced in the Vector Core Facility at the University of North Carolina) was injected into 3 week-old GAD65-Cre mice (kindly provided by Dr. Gero Miesenböck). Anesthesia was induced and maintained with isoflurane (Baxter AG, Vienna, Austria) at 5% and 1%, respectively. The animal was placed in a stereotaxic frame (Angle One; Leica, Germany) and craniotomies were performed bilaterally over the VTA using stereotaxic coordinates (AP −3.4, ML ±0.8, DV 4.4). Injections of AAV-ChR2 flox were carried out using graduated pipettes (Drummond Scientific Company, Broomall, PA), broken back to a tip diameter of 10–15 μm, at a rate of ~ 100nl min−1 for a total volume of 500nl. In all experiments the virus was allowed a 3 weeks to incubate before any other procedures were carried out. Fast GABAA IPSCs in DA cells were isolated in presence of kynurenic acid (2mM) and evoked by applying 2 consecutive 4ms blue-light (Thorlad – 472nm LED) flashes at 50ms interval to the slice, every 10 seconds. Recordings were as described above.
Antibodies
A rabbit polyclonal antibody anti-Glutamate Decarboxylase 65 & 67 (AB1511, Millipore, Billerica, MA, USA), anti-GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-phospho S783-GABAB2 (p-S783) (Terunuma et al., 2010), anti-phospho S892-GABAB2 (p-S892) (Couve et al., 2001) were used. A monoclonal antibody anti-GABAB1 (Clone N93A/49, NeuroMab, Davis, CA, USA) and anti-GABAB2 (Clone N81/37, NeuroMab, Davis, CA, USA) were used. A guinea-pig polyclonal antibody anti-GIRK2 (Aguado et al., 2008) was used.
Immunoelectron microscopy
A similar procedure to that described earlier (Luján et al., 1996; Koyrakh et al., 2005) was used. See on-line Supplemental Methods for details on procedure and quantitation.
Western blotting
Tissue punches from VTA, NAc, hippocampus and mPFC obtained from saline and METH injected mice were lysed in 20 mM Tris-HCl, pH 8.0, 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, 10 mM NaF, 2 mM Na3VO4, 10 mM Na4P2O7, 10 μg/mL leupeptin, 1 μg/mL aprotinin, 10 μg/mL antipain and 250 μg/mL 4-(2-Aminoethl) benzenesulfonyl fluoride hydrochloride. Soluble material was then subjected to immunoblotting with anti-GABAB2, anti-phospho S783-GABAB2 (p-S783), anti-phospho S892-GABAB2 (p-S892), anti-GAPDH, and detected by SuperSignal West Dura Chemiluminescent Substrate (Thermo Scientific, Rockford, IL, USA). The luminescence images were captured by Luminescent image analyzer (LAS3000, Fujifilm) and the intensity of bands were measured by Multi gauge (ver. 3, Fujifilm).
Supplementary Material
Acknowledgments
We thank all members of the Slesinger and Lüscher laboratories, as well as G.O. Hjelmstad for comments on the manuscript. This work was supported by grants from the Spanish Ministry of Education and Science (BFU-2009-08404/BFI; RL) and CONSOLIDER (CSD2008-00005; RL), NINDS (NS048045, NS051195, NS056359 and NS054900; SJM) and NIDA (DA019022; PAS & CL).
References
- Argilli E, Sibley DR, Malenka RC, England PM, Bonci A. Mechanism and time course of cocaine-induced long-term potentiation in the ventral tegmental area. J Neurosci. 2008;28:9092–9100. doi: 10.1523/JNEUROSCI.1001-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora D, Hearing M, Haluk DM, Mirkovic K, Fajardo-Serrano A, Wessendorf MW, Watanabe M, Lujan R, Wickman K. Acute cocaine exposure weakens GABA(B) receptor-dependent G-protein-gated inwardly rectifying K+ signaling in dopamine neurons of the ventral tegmental area. J Neurosci. 2011;31:12251–12257. doi: 10.1523/JNEUROSCI.0494-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead MJ, Grandy DK, Wickman K, Williams JT. Vesicular dopamine release elicits an inhibitory postsynaptic current in midbrain dopamine neurons. Neuron. 2004;42:939–946. doi: 10.1016/j.neuron.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Bellone C, Luscher C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat Neurosci. 2006;9:636–641. doi: 10.1038/nn1682. [DOI] [PubMed] [Google Scholar]
- Bonci A, Williams JT. A Common Mechanism Mediates Long-Term Changes in Synaptic Transmission after Chronic Cocaine and Morphine. Neuron. 1996;16:631–639. doi: 10.1016/s0896-6273(00)80082-3. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Malenka RC, Bonci A. Acute and Chronic Cocaine-Induced Potentiation of Synaptic Strength in the Ventral Tegmental Area: Electrophysiological and Behavioral Correlates in Individual Rats. J Neurosci. 2004;24:7482–7490. doi: 10.1523/JNEUROSCI.1312-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Boyer SB, Clancy SM, Terunuma M, Revilla-Sanchez R, Thomas SM, Moss SJ, Slesinger PA. Direct interaction of GABAB receptors with M2 muscarinic receptors enhances muscarinic signaling. J Neurosci. 2009;29:15796–15809. doi: 10.1523/JNEUROSCI.4103-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MT, Bellone C, Mameli M, Labouebe G, Bocklisch C, Balland B, Dahan L, Lujan R, Deisseroth K, Luscher C. Drug-driven AMPA receptor redistribution mimicked by selective dopamine neuron stimulation. PLoS One. 2010;5:e15870. doi: 10.1371/journal.pone.0015870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Thomsen M, Gabriel KI, Berkowitz JS, Gold LH, Koob GF, Tonegawa S, Zhang J, Xu M. Lack of self-administration of cocaine in dopamine D1 receptor knock-out mice. J Neurosci. 2007;27:13140–13150. doi: 10.1523/JNEUROSCI.2284-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HJ, Qian X, Ehlers M, Jan YN, Jan LY. Neuronal activity regulates phosphorylation-dependent surface delivery of G protein-activated inwardly rectifying potassium channels. Proc Natl Acad Sci USA. 2009;106:629–634. doi: 10.1073/pnas.0811615106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy SM, Boyer SB, Slesinger PA. Coregulation of Natively Expressed Pertussis Toxin-Sensitive Muscarinic Receptors with G-Protein-Activated Potassium Channels. J Neurosci. 2007;27:6388–6399. doi: 10.1523/JNEUROSCI.1190-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper DC. The significance of action potential bursting in the brain reward circuit. Neurochemistry International. 2002;41:333–340. doi: 10.1016/s0197-0186(02)00068-2. [DOI] [PubMed] [Google Scholar]
- Couve A, Kittler JT, Uren JM, Calver AR, Pangalos MN, Walsh FS, Moss SJ. Association of GABA(B) receptors and members of the 14-3-3 family of signaling proteins. Mol Cell Neurosci. 2001;17:317–328. doi: 10.1006/mcne.2000.0938. [DOI] [PubMed] [Google Scholar]
- Couve A, Thomas P, Calver AR, Hirst WD, Pangalos MN, Walsh FS, Smart TG, Moss SJ. Cyclic AMP-dependent protein kinase phosphorylation facilitates GABAB receptor-effector coupling. Nat Neurosci. 2002;5:415–424. doi: 10.1038/nn833. [DOI] [PubMed] [Google Scholar]
- Cruz HG, Berton F, Sollini M, Blanchet C, Pravetoni M, Wickman K, Luscher C. Absence and rescue of morphine withdrawal in GIRK/Kir3 knock-out mice. J Neurosci. 2008;28:4069–4077. doi: 10.1523/JNEUROSCI.0267-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz HG, Ivanova T, Lunn ML, Stoffel M, Slesinger PA, Luscher C. Bidirectional effects of GABAB receptor agonists on the mesolimbic dopamine system. Nat Neurosci. 2004;7:153–159. doi: 10.1038/nn1181. [DOI] [PubMed] [Google Scholar]
- Dobbs LK, Mark GP. Comparison of systemic and local methamphetamine treatment on acetylcholine and dopamine levels in the ventral tegmental area in the mouse. Neuroscience. 2008;156:700–711. doi: 10.1016/j.neuroscience.2008.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobi A, Margolis EB, Wang HL, Harvey BK, Morales M. Glutamatergic and nonglutamatergic neurons of the ventral tegmental area establish local synaptic contacts with dopaminergic and nondopaminergic neurons. J Neurosci. 2010;30:218–229. doi: 10.1523/JNEUROSCI.3884-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt S, Mathe JM, Chergui K, Engberg G, Svensson TH. GABA(B) receptor-mediated modulation of the firing pattern of ventral tegmental area dopamine neurons in vivo. Naunyn Schmiedebergs Arch Pharmacol. 2002;365:173–180. doi: 10.1007/s00210-001-0519-5. [DOI] [PubMed] [Google Scholar]
- Fairfax BP, Pitcher JA, Scott MGH, Calver AR, Pangalos MN, Moss SJ, Couve A. Phosphorylation and Chronic Agonist Treatment Atypically Modulate GABAB Receptor Cell Surface Stability. J Biol Chem. 2004;279:12565–12573. doi: 10.1074/jbc.M311389200. [DOI] [PubMed] [Google Scholar]
- Fowler CE, Aryal P, Suen KF, Slesinger PA. Evidence for association of GABAB receptors with Kir3 channels and RGS4 proteins. J Physiol. 2007;580:51–65. doi: 10.1113/jphysiol.2006.123216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima S, Shen H, Hata H, Ohara A, Ohmi K, Ikeda K, Numachi Y, Kobayashi H, Hall FS, Uhl GR, Sora I. Methamphetamine-induced locomotor activity and sensitization in dopamine transporter and vesicular monoamine transporter 2 double mutant mice. Psychopharmacology. 2007;193:55–62. doi: 10.1007/s00213-007-0749-4. [DOI] [PubMed] [Google Scholar]
- Giorgetti M, Hotsenpiller G, Froestl W, Wolf ME. In vivo modulation of ventral tegmental area dopamine and glutamate efflux by local GABA(B) receptors is altered after repeated amphetamine treatment. Neuroscience. 2002;109:585–595. doi: 10.1016/s0306-4522(01)00510-3. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Wise A, Green A, Koenig JA. Agonist-induced desensitization and endocytosis of heterodimeric GABAB receptors in CHO-K1 cells. Eur J Pharmacol. 2003;481:15–23. doi: 10.1016/j.ejphar.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. Opposing effects of striatonigral feedback pathways on midbrain dopamine cell activity. Brain Res. 1985;333:271–284. doi: 10.1016/0006-8993(85)91581-1. [DOI] [PubMed] [Google Scholar]
- Guetg N, Aziz SA, Holbro N, Turecek R, Rose T, Seddik R, Gassmann M, Moes S, Jenoe P, Oertner TG, Casanova E, Bettler B. NMDA receptor-dependent GABAB receptor internalization via CaMKII phosphorylation of serine 867 in GABAB1. Proc Natl Acad Sci USA. 2010;107:13924–13929. doi: 10.1073/pnas.1000909107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Reddy SA, Damuni Z. Purification and characterization of an autophosphorylation-activated protein serine threonine kinase that phosphorylates and inactivates protein phosphatase 2A. The Journal of biological chemistry. 1993;268:11193–11198. [PubMed] [Google Scholar]
- Henry DJ, Greene MA, White FJ. Electrophysiological effects of cocaine in the mesoaccumbens dopamine system: repeated administration. J Pharmacol Exp Ther. 1989;251:833–839. [PubMed] [Google Scholar]
- Huang CS, Shi SH, Ule J, Ruggiu M, Barker LA, Darnell RB, Jan YN, Jan LY. Common molecular pathways mediate long-term potentiation of synaptic excitation and slow synaptic inhibition. Cell. 2005;123:105–118. doi: 10.1016/j.cell.2005.07.033. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA. Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J Physiol. 1992;450:455–468. doi: 10.1113/jphysiol.1992.sp019136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of Addiction. Neuropsychopharm. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya E, Uejima JL, Wihbey KA, Bossert JM, Hope BT, Shaham Y. Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharm. 2009;56(Suppl 1):177–185. doi: 10.1016/j.neuropharm.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyrakh L, Lujan R, Colon J, Karschin C, Kurachi Y, Karschin A, Wickman K. Molecular and Cellular Diversity of Neuronal G-Protein-Gated Potassium Channels. J Neurosci. 2005;25:11468–11478. doi: 10.1523/JNEUROSCI.3484-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulik A, Vida I, Lujan R, Haas CA, Lopez-Bendito G, Shigemoto R, Frotscher M. Subcellular Localization of Metabotropic GABAB Receptor Subunits GABAB1a/b and GABAB2 in the Rat Hippocampus. J Neurosci. 2003;23:11026–11035. doi: 10.1523/JNEUROSCI.23-35-11026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labouèbe G, Lomazzi M, Cruz HG, Creton C, Luján R, Li M, Yanagawa Y, Obata K, Watanabe M, Wickman K, Boyer SB, Slesinger PA, Lüscher C. RGS2 modulates coupling between GABAB receptors and GIRK channels in dopamine neurons of the ventral tegmental area. Nat Neurosci. 2007;12:1559–1568. doi: 10.1038/nn2006. [DOI] [PubMed] [Google Scholar]
- Lavine N, Ethier N, Oak JN, Pei L, Liu F, Trieu P, Rebois RV, Bouvier M, Hebert TE, Van Tol HH. G protein-coupled receptors form stable complexes with inwardly rectifying potassium channels and adenylyl cyclase. J Biol Chem. 2002;277:46010–46019. doi: 10.1074/jbc.M205035200. [DOI] [PubMed] [Google Scholar]
- Lehrmann E, Oyler J, Vawter MP, Hyde TM, Kolachana B, Kleinman JE, Huestis MA, Becker KG, Freed WJ. Transcriptional profiling in the human prefrontal cortex: evidence for two activational states associated with cocaine abuse. Pharmacogenomics J. 2003;3:27–40. doi: 10.1038/sj.tpj.6500146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QS, Pu L, Poo MM. Repeated cocaine exposure in vivo facilitates LTP induction in midbrain dopamine neurons. Nature. 2005;437:1027–1031. doi: 10.1038/nature04050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchesi W, Mizuno K, Giese KP. Novel insights into CaMKII function and regulation during memory formation. Brain Research Bulletin. 2011;85:2–8. doi: 10.1016/j.brainresbull.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher C, Slesinger PA. Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease. Nat Rev Neurosci. 2010;11:301–315. doi: 10.1038/nrn2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier PJ, Marin I, Grampp T, Sommer A, Benke D. Sustained glutamate receptor activation down-regulates GABAB receptors by shifting the balance from recycling to lysosomal degradation. J Biol Chem. 2010;285:35606–35614. doi: 10.1074/jbc.M110.142406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameli M, Bellone C, Brown MTC, Luscher C. Cocaine inverts rules for synaptic plasticity of glutamate transmission in the ventral tegmental area. Nat Neurosci. 2011;14:414–416. doi: 10.1038/nn.2763. [DOI] [PubMed] [Google Scholar]
- Manzoni OJ, Williams JT. Presynaptic regulation of glutamate release in the ventral tegmental area during morphine withdrawal. J Neurosci. 1999;19:6629–6636. doi: 10.1523/JNEUROSCI.19-15-06629.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan AD, Carroll ME, Loth AK, Stoffel M, Wickman K. Decreased cocaine self-administration in Kir3 potassium channel subunit knockout mice. Neuropsychopharm. 2003;28:932–938. doi: 10.1038/sj.npp.1300100. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Terwilliger RZ, Walker JR, Sevarino KA, Duman RS. Chronic cocaine treatment decreases levels of the G protein subunits Gi alpha and Go alpha in discrete regions of rat brain. J Neurochem. 1990;55:1079–1082. doi: 10.1111/j.1471-4159.1990.tb04602.x. [DOI] [PubMed] [Google Scholar]
- Nobles M, Benians A, Tinker A. Heterotrimeric G proteins precouple with G protein-coupled receptors in living cells. Proc Natl Acad Sci USA. 2005;102:18706–18711. doi: 10.1073/pnas.0504778102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent FS, Penick EC, Kauer JA. Opioids block long-term potentiation of inhibitory synapses. Nature. 2007;446:1086–1090. doi: 10.1038/nature05726. [DOI] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Ultrastructural analysis of local collaterals of rat ventral tegmental area neurons: GABA phenotype and synapses onto dopamine and GABA cells. Synapse. 2009;63:895–906. doi: 10.1002/syn.20668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett CL, Slesinger PA. Advances in Pharmacology. Academic Press; 2010. GABAB Receptor Coupling to G-proteins and Ion Channels; pp. 123–147. [DOI] [PubMed] [Google Scholar]
- Pu L, Liu QS, Poo MM. BDNF-dependent synaptic sensitization in midbrain dopamine neurons after cocaine withdrawal. Nat Neurosci. 2006;9:605–607. doi: 10.1038/nn1687. [DOI] [PubMed] [Google Scholar]
- Riven I, Iwanir S, Reuveny E. GIRK channel activation involves a local rearrangement of a preformed G protein channel complex. Neuron. 2006;51:561–573. doi: 10.1016/j.neuron.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Koob GF. Disruption of cocaine self-administration following 6-hydroxydopamine lesions of the ventral tegmental area in rats. Pharmacology, biochemistry, and behavior. 1982;17:901–904. doi: 10.1016/0091-3057(82)90469-5. [DOI] [PubMed] [Google Scholar]
- Scibelli AC, McKinnon CS, Reed C, Burkhart-Kasch S, Li N, Baba H, Wheeler JM, Phillips TJ. Selective breeding for magnitude of methamphetamine-induced sensitization alters methamphetamine consumption. Psychopharmacology (Berl) 2010 doi: 10.1007/s00213-010-2086-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimosato K, Watanabe S, Kitayama S. Differential effects of trihexyphenidyl on place preference conditioning and locomotor stimulant activity of cocaine and methamphetamine. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:74–80. doi: 10.1007/s002100100433. [DOI] [PubMed] [Google Scholar]
- Shoji S, Simms D, McDaniel WC, Gallagher JP. Chronic cocaine enhances gamma-aminobutyric acid and glutamate release by altering presynaptic and not postsynaptic gamma-aminobutyric acidB receptors within the rat dorsolateral septal nucleus. The Journal of pharmacology and experimental therapeutics. 1997;280:129–137. [PubMed] [Google Scholar]
- Sulzer D. How Addictive Drugs Disrupt Presynaptic Dopamine Neurotransmission. Neuron. 2011;69:628–649. doi: 10.1016/j.neuron.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol. 2003;467:60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- Tan KR, Brown M, Labouèbe G, Yvon C, Creton C, Fritschy JM, Rudolph U, Lüscher C. Neural bases for addictive properties of benzodiazepines. Nature. 2010;463:769–774. doi: 10.1038/nature08758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniyama K, Takeda K, Ando H, Tanaka C. Expression of the GABAB receptor in Xenopus oocytes and desensitization by activation of protein kinase C. Adv Exp Med Biol. 1991;287:413–420. doi: 10.1007/978-1-4684-5907-4_36. [DOI] [PubMed] [Google Scholar]
- Terunuma M, Vargas KJ, Wilkins ME, Ramirez OA, Jaureguiberry-Bravo M, Pangalos MN, Smart TG, Moss SJ, Couve A. Prolonged activation of NMDA receptors promotes dephosphorylation and alters postendocytic sorting of GABAB receptors. Proc Natl Acad Sci USA. 2010;107:13918–13923. doi: 10.1073/pnas.1000853107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Vardya I, Drasbek KR, Gibson KM, Jensen K. Plasticity of postsynaptic, but not presynaptic, GABAB receptors in SSADH deficient mice. Exp Neurol. 2010;225:114–122. doi: 10.1016/j.expneurol.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci. 2005;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FJ. Synaptic regulation of mesocorticolimbic dopamine neurons. Annual Review of Neuroscience. 1996;19:405–436. doi: 10.1146/annurev.ne.19.030196.002201. [DOI] [PubMed] [Google Scholar]
- White FJ, Hu XT, Zhang XF, Wolf ME. Repeated administration of cocaine or amphetamine alters neuronal responses to glutamate in the mesoaccumbens dopamine system. J Pharmacol Exp Ther. 1995;273:445–454. [PubMed] [Google Scholar]
- White FJ, Wang RY. Electrophysiological evidence for A10 dopamine autoreceptor subsensitivity following chronic D-amphetamine treatment. Brain Res. 1984;309:283–292. doi: 10.1016/0006-8993(84)90594-8. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Ramamoorthy S, Shen H, Lake R, Samuvel DJ, Kalivas PW. GABA transmission in the nucleus accumbens is altered after withdrawal from repeated cocaine. J Neurosci. 2003;23:3498–3505. doi: 10.1523/JNEUROSCI.23-08-03498.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XF, Hu XT, White FJ, Wolf ME. Increased responsiveness of ventral tegmental area dopamine neurons to glutamate after repeated administration of cocaine or amphetamine is transient and selectively involves AMPA receptors. J Pharmacol Exp Ther. 1997;281:699–706. [PubMed] [Google Scholar]
- Zhao S, Maxwell S, Jimenez-Beristain A, Vives J, Kuehner E, Zhao J, O’Brien C, de Felipe C, Semina E, Li M. Generation of embryonic stem cells and transgenic mice expressing green fluorescence protein in midbrain dopaminergic neurons. Eur J Neurosci. 2004;19:1133–1140. doi: 10.1111/j.1460-9568.2004.03206.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.