Abstract
Physiological aging imposes significant alterations in the repertoire of T cells and all associated functions. Although several studies have reported defects upon antigen-induced activation of T cells during aging, the molecular mechanisms that control T-cell receptor (TCR) downmodulation remain to be fully defined. While previous studies have assessed the role of F-actin in regulating activation-induced TCR internalization, few have delineated the roles of motor proteins, such as non-muscle myosin IIA (NMMIIA). In this study, we describe a series of experiments supporting the hypothesis that effective TCR downmodulation requires not only efficient reorganization of the actin cytoskeleton, but also functional NMMIIA. For the first time, we show that CD4+ T cells from elderly human donors have dysfunctional NMMIIA that contributes to delaying activation-induced TCR internalization and impairing calcium mobilization. Additionally, our results demonstrate that chemical inhibition of NMMIIA in CD4+ T cells from young donors also results in complete abrogation of TCR internalization, strongly supporting the fundamental role of NMMIIA in modulating this event. Recent observations that the generation of an efficient T-cell response requires migration prompted us to investigate whether NMMIIA also plays a regulatory role in CD4+ T-cell migration. We show that chemical inhibition of NMMIIA downmodulates chemotactic migration in CD4+ T cells from both young and elderly donors. Together, these data demonstrate a significant contribution of dysfunctional NMMIIA to TCR-mediated functional defects during aging.
Keywords: chemotaxis, Hsp90, immune senescence, NMMIIA, TCR internalization
Introduction
Aging is a complex process that is accompanied by a decline in various physiological processes.1 In particular, the immune senescence that often accompanies aging of the immune system is characterized by alterations in both innate and adaptive immunity, as well as by the occurrence of chronic inflammatory processes.2, 3, 4 The increased incidence and severity of infectious diseases in the elderly population and their reduced ability to mount an appropriate immune response upon immunization 5, 6 correlate well with relatively weak and nonspecific CD4+ T-cell activation, resulting in a dramatic decrease in the efficacy of responses to vaccination in the elderly.6 Clinically, this is important because the elderly are highly encouraged to get vaccinated for infectious diseases such as influenza and pneumococcal pneumonia.1, 5 On the one hand, minimal alterations in the total number of CD4+ T cells have been noted with age, but on the other, substantial alterations in their signal transduction,3 proliferative responses and migration7, 8, 9 have been documented in murine models. Elegant studies employing a mouse model for aging have shown that CD4+ T cells from aged T-cell receptor (TCR)-transgenic mice fail to form efficient immunological synapses (IS) with antigen-presenting cells (APCs), as seen in CD4+ T cells from young mice.10 Additionally, these data demonstrate that aging in mice leads to decreased recruitment of TCR-associated proteins, such as Lck, Vav and Moesin/Ezrin, to the IS.9, 11 The recruitment of signaling molecules in CD4+ T cells from older mice is approximately 50% of that observed in the young cohorts. Furthermore, CD4+ T cells from aged mice exhibit significant changes in cytoskeletal rearrangement, irrespective of the activation status, which leads to alteration in TCR-rich contact zones and T-cell trafficking.12 Although the role of microcluster formation in the initiation of T-cell activation is well established in both murine models and cell lines, the molecular mechanisms that underlie termination of T-cell activation, such as TCR internalization13 in primary human T cells, remain poorly defined.
The initial contact of CD4+ T cells with APCs14, 15, 16, 17 leads to a series of cytoskeleton reorganizations, including the molecular segregation of T-cell signaling molecules, polymerization and depolymerization of F-actin,16 and development of lamellipodia,18, 19 as posited by the kinetic segregation model of T-cell activation.20, 21 These events, which are tightly controlled in terms of the duration and strength of the response, are followed by termination events, primarily consisting of downmodulation of cell surface TCR expression due to a combination of increased internalization, decreased recycling and increased degradation.
Emerging studies,22, 23, 24 primarily employing cancer cells, have demonstrated the fundamental role of heat shock protein 90 (Hsp90) in regulating microtubules and intermediate filaments of the cytoskeleton.25, 26, 27 However, limited information is available on the role of Hsp90 in cell morphology and migration of T cells and in activation-dependent early events. We therefore assessed the contribution of Hsp90 in early events of T-cell activation and termination.
The data generated previously from our laboratory clearly demonstrate that aging is accompanied by a decline in both the amount and function of Hsp90, which may contribute to the inability of CD4+ T cells from elderly humans to undergo activation-induced proliferation or secrete IL-2. Given that key tyrosine kinases involved in T-cell signaling are Hsp90 client proteins,27 we were interested in determining whether immune senescence impacted the pattern of Hsp90-interacting proteins in CD4+ T cells. We now show that non-muscle myosin IIA (NMMIIA),28, 29, 30 a motor protein composed of six polypeptide chains, is differentially co-precipitated with Hsp90 in lysates obtained from CD4+ T cells from elderly and young donors. NMMIIA is composed of two identical heavy chains (myosin IIA), two essential light chains and two regulatory light chains (RLC or myosin light chain (MLC)). Activation of NMMIIA requires the phosphorylation of Thr18 and Ser19 residues on the RLCs primarily by calcium kinase and Rho-associated coiled coil-containing kinase. In the context of T-cell biology, recent work by Dustin and colleagues28 clearly demonstrated that phosphorylation of RLCs is necessary for the proper assembly of the T-cell signalosome and for the stability of the IS.
Given the emerging role of NMMIIA in regulating many aspects of T-cell biology, we now provide evidence that outlines the contribution of NMMIIA to the observed defects in primary human CD4+ T cells during aging. Our results for the first time demonstrate that altered functional NMMIIA occurs in T cells from the elderly and this alteration in NMMIIA may contribute to defects in early signaling events, including calcium mobilization, TCR internalization and chemotactic migration toward stromal cell-derived factor (SDF)-1α.
Materials and methods
Antibodies and reagents
Antibodies to myosin IIA, MLC2 and MLC2 phosphorylated at Ser19 were from Cell Signaling Technology (Danvers, MA, USA). Antibodies to Hsp90, TCR-α/-β chains and horseradish peroxidase-conjugated goat anti-mouse were from BD Biosciences (San Jose, CA, USA). The β-actin antibody was from Santa Cruz biotechnology (Santa Cruz, CA, USA). Alexa Fluor 488-conjugated mouse anti-rabbit and Alexa Fluor 555-conjugated goat anti-mouse secondary antibodies and Fura-2AM were from Invitrogen (Carlsbad, CA, USA). Mouse anti-human CD3 was purified from an OKT3 hybridoma supernatant. Horseradish peroxidase-conjugated goat anti-rabbit immonoglobulin was from Thermo Fisher Scientific (Rockford, IL, USA). All fine chemicals were obtained from Sigma-Aldrich (Saint Louis, MO, USA), unless otherwise mentioned. Rhodamine-phalloidin was obtained from Cytoskeleton (Denver, CO, USA). SDF-1α was from ProSpec-Tany Technogene (Rehovot, Israel). The electrophoresis supplies were from Bio-Rad (Hercules, CA, USA).
Human subjects
Peripheral blood was obtained by venipuncture from healthy young (21–30 years) and elderly (65–89 years) adults enrolled from the greater Little Rock area. Immunocompromised subjects were excluded from the study, including individuals with asthma and those taking immune-modulating drugs. Subjects on antibiotics or with self-reported symptoms of recent infection (<3 weeks before enrollment) were also excluded. All protocols involving human subjects were approved by the University of Arkansas for Medical Sciences (UAMS) Institutional Review Board, and the appropriate informed consents were obtained. Blood was drawn at the Clinical Research Center at UAMS. The demographics of the volunteers recruited for the study were as follows: the young donor population consisted of 62% males and 38% females with an average age of 25.5 years. Of these individuals, 86% were Caucasians, 10% Asians and the rest minorities. The elderly donor population consisted of 43% males and 57% females with an average age of 75 years, and 93% of these individuals were Caucasians, with the rest minorities.
T-lymphocyte isolation
CD4+ T cells were negatively selected from blood using the EasySep CD4+ T-cell Enrichment Kit according to the manufacturer's recommended protocol (StemCell Tech, Vancouver, Canada). The purity of isolated CD4+ T cells was determined by flow cytometry and was consistently 90%–95%. Lymphocytes were isolated from peripheral blood mononuclear cells following Ficoll/Hypaque-gradient centrifugation of whole blood. In our young donor population, T cells represented 80%–82% of peripheral blood mononuclear cells, with an average of 85% being CD4+ T cells. In the elderly group, T cells represented 81%–83% of peripheral blood mononuclear cells, with 84% being CD4+ T cells. Of the CD4+ T lymphocytes, CD45RA+CCR7+CD45RO− cells represented an average of 54%, and CD45RO+CD45RA− represented an average of 46% in young donors. These were 48% and 52%, respectively, in the elderly donor group. T regulatory cells, identified as CD4+FOXP3+CD25+ cells, represented a mean of 4.82% of the CD4 population in the young donors and a mean of 5.87% of the CD4 population in the elderly donors.
Flow cytometry
For surface staining, CD4+ T cells were incubated with antibody directed to TCR-α/-β (BD Biosciences) followed by incubation with an antibody conjugated to Alexa Fluor 555 for 20 min at 4 °C. At the end of incubation, cells were washed with ice-cold phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (BSA) and fixed with 2% paraformaldehyde. For intracellular staining of F-actin, cells were fixed (2% paraformaldehyde), permeabilized (PBS buffer+0.1% saponin) and stained with rhodamine-phalloidin based on the manufacturer's protocol (Cytoskeleton).
Quantitative PCR with reverse transcription
Total RNA was isolated using TRIzol Reagent (Invitrogen). Briefly, 4 µg of total RNA for each sample was reverse transcribed, and the resultant cDNA was amplified using a Bio-Rad iCycler PCR system. Reactions were performed in 96-well PCR plates using 12.5 µl of iQ SYBR Green Supermix (Bio-Rad), forward and reverse primers (0.3 nmol each), and cDNA (3 µl) in a final volume of 25 µl. Amplification parameters were denatured at 95 °C for 10 min followed by 40 cycles at 95 °C for 30 s and 60 °C for 70 s. Samples were analyzed in duplicate for the expression of NMMIIA, and human β-actin and GAPDH were used as normalization genes. Fold induction was calculated after normalization using the ΔΔCT method. The dissociation curves indicated that each reaction consisted of a single reaction product. Gene-specific primers were designed by Primer3 software employing human sequences obtained from GenBank. The primer sequences will be provided upon request.
Confocal microscopy
Freshly isolated CD4+ T cells were stained for TCR-α/-β (BD Biosciences) according to the manufacturer's protocol. Briefly, cells were incubated with antibody directed to TCR-α/-β for 20 min and followed by an anti-rabbit Alexa Fluor 555. The cells were then washed and fixed with 2% paraformaldehyde, prior to mounting on poly-ℓ-lysine-coated coverslips. For intracellular protein staining of NMMIIA, CD4+ T cells were fixed, permeabilized (PBS buffer+0.1% saponin), and stained with anti-myosin IIA antibody. Anti-rabbit Alexa Fluor 488 was used as the detection reagent. A series of fluorescence images were captured by a Zeiss confocal LSM 510 META (Carl Zeiss, Thornwood, NY, USA) using a Plan-Apochromat 63/1.4 oil differential interference contrast objective. The images were processed and analyzed with LSM510 and ImageJ software.
Calcium imaging
Intracellular calcium concentrations were measured with the calcium indicator dye Fura-2AM. CD4+ T cells were stimulated with Staphylococcus enterotoxin A-loaded Raji B cells (10 ng/ml) or ionomycin (50 ng/ml). Briefly, CD4+ T cells were loaded with the dye by incubation with 5 µM of Fura-2AM at 37 °C for 30 min in complete media, followed by a 30-min chase. Imaging was performed with an InCa dual-wavelength system (Intracellular Imaging, Cincinnati, OH, USA) with the calcium concentration calculated as the relationship between the ratio of emission at 505 nm to excitation at 340 and 380 nm. Each experimental data point represents the average of calcium concentration calculated from at least 20 individually measured CD4+ T cells from three different fields and six independent donor pairs.
Chemotactic migration assay
CD4+ T cells (3×105) either left untreated or pre-treated with blebbistatin (50 µM, 1 h) were resuspended in 100 µl of medium containing 0.2% BSA and placed into the upper chamber of a transwell migration plate (5 µm pores; Costar, Corning, NY, USA). In the lower chamber, 600 µl of media containing 0.2% BSA with or without 100 nM of SDF-1α was added, and cells were allowed to migrate for 4 h at 37 °C. The number of migrated cells was counted following Trypan blue staining. The percent of migrating cells and migration index were derived from these data.
Western blotting and co-immunoprecipitation
CD4+ T-cell lysates, equalized for protein levels, were resolved using sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE), transferred to nitrocellulose membranes, immunoblotted with specific antibody(s) and detected using chemiluminescence.
For co-immunoprecipitation studies, precleared total cell lysates (250 µg protein) were incubated with an antibody to Hsp90 and protein A/G agarose beads overnight at 4 °C with gentle rocking. Protein A/G beads containing the adsorbed immunoprecipitated complex were washed with radio immunoprecipitation assay buffer, resuspended in 30 µl of 2X SDS sample buffer and heated in a boiling water bath for 5 min. Protein complexes were resolved by SDS–PAGE and subjected to western blot analyses, as detailed above.
Liquid chromatography–tandem mass spectrometry (LC–MS/MS) methods
Protein bands following SDS–PAGE, were excised and subjected to in-gel trypsin digestion as follows. Protein-containing gel slices were destained in 50% methanol, then 100 mM ammonium bicarbonate, followed by reduction in 10 mM Tris(2-carboxyethyl)phosphine and alkylation in 50 mM iodoacetamide. The gel slices were then dehydrated in acetonitrile, followed by the addition of 100 ng sequencing-grade porcine trypsin in 100 mM ammonium bicarbonate (Sigma-Aldrich) and incubated at 37 °C for 12–16 h. The peptide products were then acidified in 0.1% formic acid. Tryptic peptides were separated by reverse-phase high-performance liquid chromatography on a 10-cm C18 column using a NanoLC 2D system (Eksigent, Dublin, CA, USA) and ionized by electrospray upon elution, followed by MS/MS analysis using an LTQ XL mass spectrometer (Thermo Scientific, Waltham, MA, USA). Proteins were identified from MS/MS spectra by database searching using the Mascot search engine (Matrix Science, Boston, MA, USA).
F-actin polymerization assay
Briefly, primary CD4+ T cells were either left untreated or activated with plate-bound αCD3 for 2, 5 or 10 min. At the end of treatment, the cells were subjected to intracellular staining using rhodamine-phalloidin as per the manufacturer's protocol, employing flow cytometry. The data were analyzed using CellQuest Pro software (BD Biosciences).
Statistical analyses
Differences between the means were analyzed using Student's t-tests, and differences were considered significant if P<0.05.
Results
NMMIIA is an abundant protein that coprecipitates with Hsp90 in CD4+ T cells from elderly donors
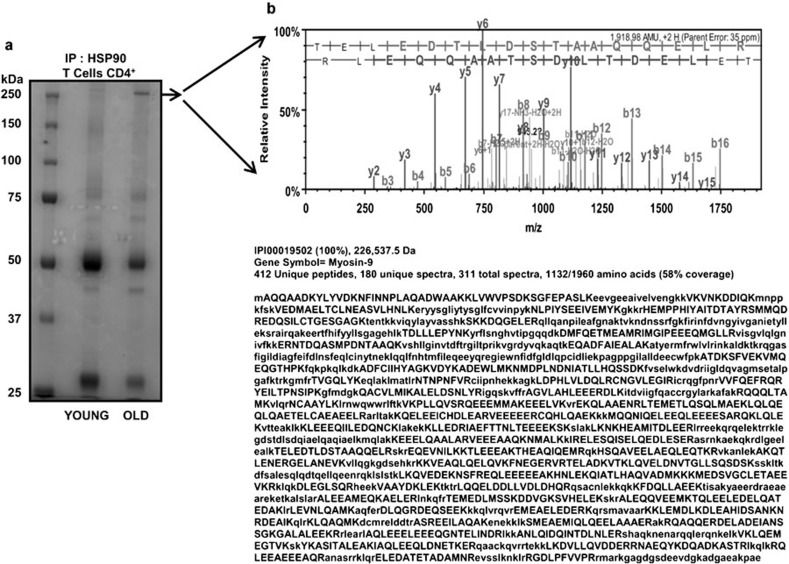
As immune senescence is accompanied by lowered levels and reduced chaperone activity of Hsp90 in T cells,1 we were interested in profiling and identifying Hsp90-associated proteins in T cells during aging. Previous studies employing murine T cells have demonstrated significant changes in the ratio of memory to naive T cells with advancing age, with a preponderance of the memory population. Therefore, we initiated analyses of naive and memory subsets in our T-cell pools. However, our studies employing flow cytometry demonstrated only a minimal increase in memory population with advancing age. In fact, CD4+CD45RO+ memory T cells, negatively selected using specific antibodies against CD8, CD14, CD16, CD19, CD20, CD36, CD56, CD123, TCR-γ/-δ, glycophorin A and CD45RA, were present at levels similar to those observed in samples from young donors. We therefore used the entire population of CD4+ T cells in our assays to evaluate the impact of aging. We initially immunoprecipitated Hsp90 from CD4+ T-cell lysates obtained from young and elderly donors and resolved them using SDS–PAGE, followed by staining with Bio-Safe Coomassie. One of the abundant proteins obtained following immunoprecipitation derived from CD4+ T cells from elderly donors, appearing at 225 kDa (Figure 1a), was excised and subjected to in-gel trypsin digestion. Tryptic peptides were subjected to LC–MS/MS analysis, and proteins were identified from MS/MS spectra by database searching using the Mascot search engine.
Figure 1.
Hsp90-associated proteins detected by co-immunoprecipitation in CD4+ T-cell lysates from the elderly. (a) Hsp90 co-immunoprecipitated proteins obtained from CD4+ T cells from five independent young and elderly donor pairs were resolved by SDS–PAGE and stained with Bio-Safe Coomassie. The predominant band at 225 kDa (more abundent in the elderly) was excised and analyzed by LC–MS/MS. (b) Proteomic profiles (spectrum and amino acid sequence) of peptides that matched the myosin IIA protein sequence obtained from the excised band. Hsp90, heat shock protein 90; LC–MS/MS, liquid chromatography–tandem mass spectrometry; SDS–PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis.
As shown in Figure 1b, the protein band detected in lysates from the elderly (resolving at approximately 225 kDa) was identified as NMMIIA. Approximately 412 unique peptides were identified that matched with NMMIIA. From a total of 311 spectra, 180 were unique to the matched protein. Overall, these peptides covered 58% of the full-length NMMIIA protein. The presence of NMMIIA was further confirmed by immunoprecipitation followed by immunoblotting (data not shown). While this is the first demonstration of abundant NMMIIA in Hsp90 immunoprecipitates from CD4+ T cells from elderly donors, NMMIIA has previously been identified in immunoprecipitates of Hsp90 and as an Hsp90-binding partner in proteomic studies in other cell types, such as fibroblasts.31 NMMIIA, a motor protein, is a member of the non-muscle motor protein family and plays vital roles in cell polarization, migration, adhesion and cytokinesis.18, 20, 28
NMMIIA expression remains unaltered in CD4+ T cells during aging
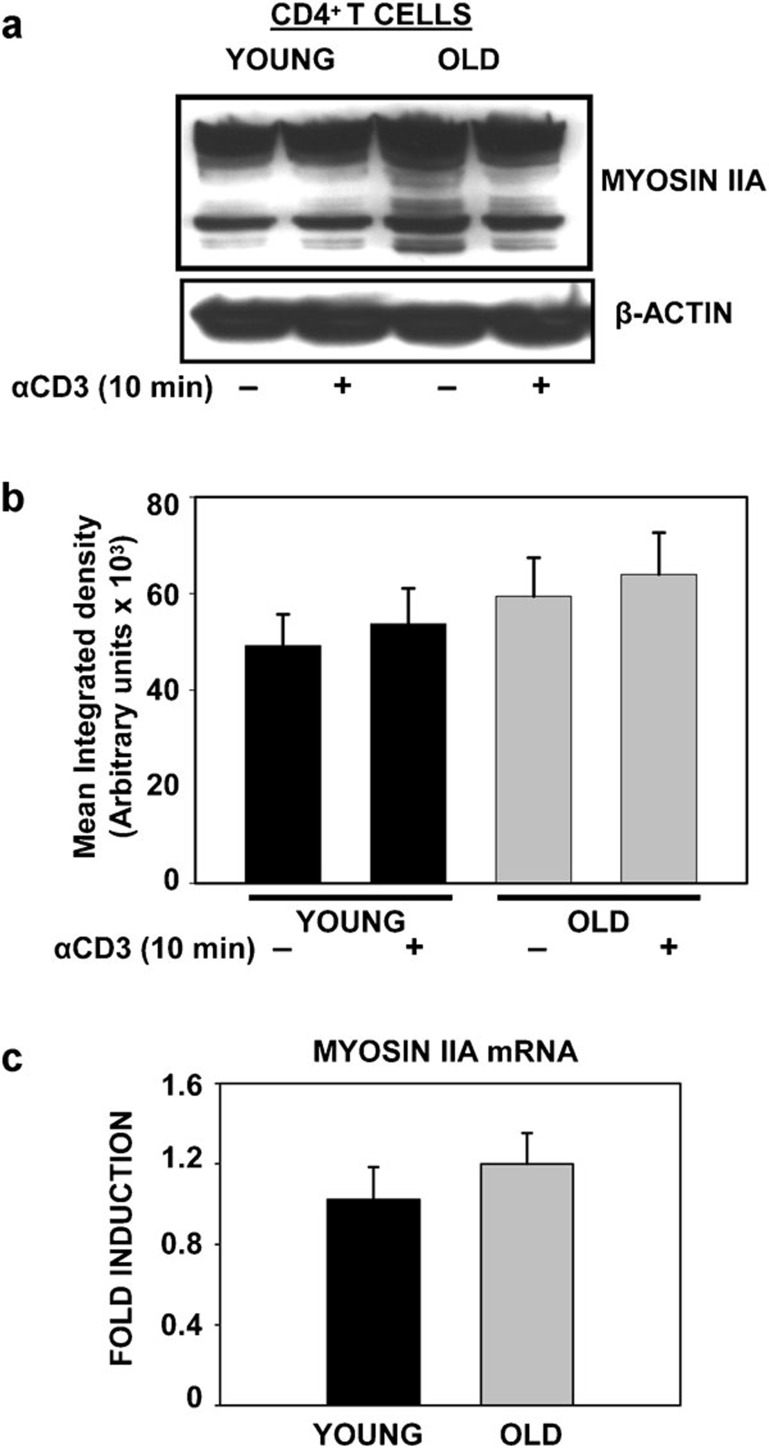
Given the observation that increased association of NMMIIA with Hsp90 occurs in T cells obtained from elderly donors when compared to those from young donors, we next assessed both the constitutive and induced protein levels and mRNA expression of NMMIIA in CD4+ T cells obtained from both young and elderly donors. CD4+ T cells were either left untreated or treated with plate-bound anti-CD3 antibody for 10 min. At the end of the incubation, cells were lysed, and approximately 25 µg of proteins was resolved by SDS–PAGE followed by western blotting with antibody to NMMIIA. As shown in Figure 2a and b, we observed a subtle but consistent increase in constitutive NMMIIA protein expression in CD4+ T cells from elderly donors when compared with those from young donors. Additionally, treatment with anti-CD3 failed to induce any further change in the expression of NMMIIA above constitutive levels in cells from both young and old donors. Interestingly, while the reactivity of NMMIIA with the antibody resulted in distinct bands at about 250 kDa in the lysates of CD4+ T cells from young donors, in contrast, CD4+ T-cell lysates obtained from the elderly demonstrated significant reactivity with several lower molecular weight proteins, which was further accentuated following anti-CD3 treatment. While it is not clear whether these were degradation products or lower molecular weight isoforms, preliminary studies employing phospho-myosin IIA (data not shown) appear to indicate folding intermediates. Future studies will determine the precise nature of these intermediates and the underlying basis for these anti-myosin IIA-reactive bands in cells from the elderly. It is important to note that NMMIIB was not detected in primary CD4+ T cells from young or elderly donors.
Figure 2.
Expression of non-muscle myosin IIA in CD4+ T cells from young and elderly donors. (a) Western blot of NMMIIA in CD4+ T cells from young and elderly donors either left untreated or activated with anti-CD3 beads for 10 min. β-actin was used as a control for equal protein loading. Representative data from one donor pair out of eight pairs tested are provided. (b) The specific band for myosin IIA protein was quantified by densitometry. The values represent the mean integrated density±s.e. obtained from a minimum of eight independent donor pairs. (c) qRT-PCR analysis of myosin IIA mRNA from total RNA collected from CD4+ T cells. The average data from 10 donor pairs are provided. β-actin and GAPDH were used as reference genes and for normalization. The data are presented as fold induction. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; NMMIIA, non-muscle myosin IIA; qRT-PCR, quantitative PCR with reverse transcription.
To determine whether alterations in the transcription of NMMIIA occur with age, we next examined the mRNA expression of NMMIIA by quantitative PCR with reverse transcription in CD4+ T cells from young and elderly human donors. The results presented in Figure 2c demonstrate that NMMIIA mRNA expression level in CD4+ T cells was not affected by the age of the donor. Employing Jurkat T cells and actinomycin D, we next evaluated the half-life of NMMIIA mRNA. Our results indicated a relatively high stability of NMMIIA mRNA, with a turnover rate longer than 72 h (data not shown). Thus, the increased association of NMMIIA with Hsp90 observed upon immunoprecipitation in CD4+ T cells from the elderly could not be attributed to an overall increase in protein or mRNA expression of NMMIIA.
Myosin RLC is constitutively phosphorylated in CD4+ T cells obtained from elderly donors
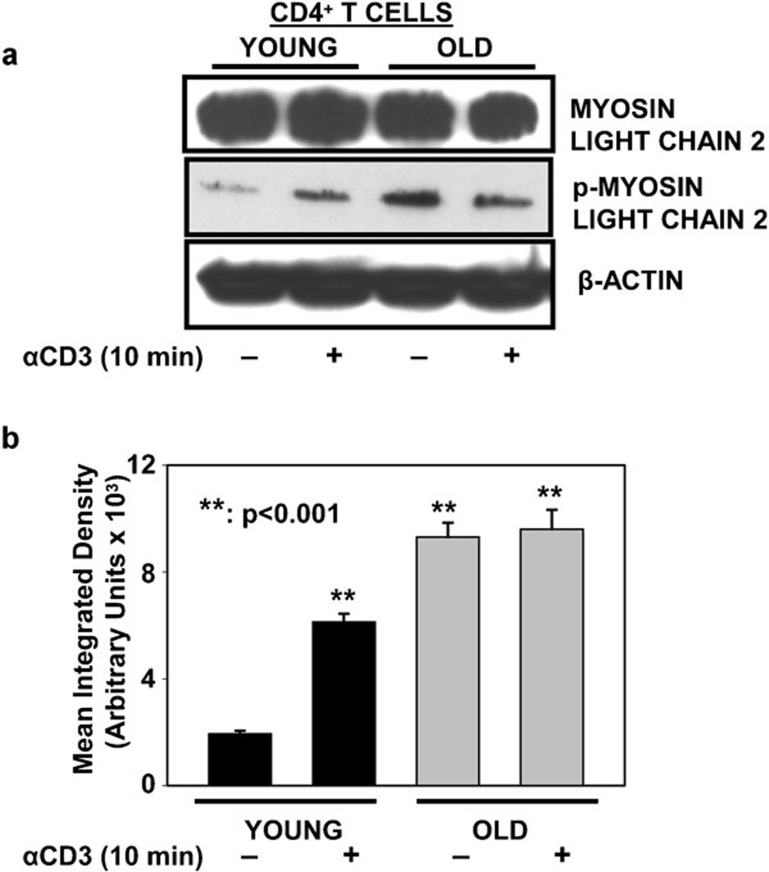
As recent studies have demonstrated a critical role for the phosphorylation of RLCs in the assembly of T-cell signalosome21, 28 during the initiation of T-cell signaling,13, 18, 32 we next analyzed the phosphorylation status of RLC in CD4+ T cells from young and elderly donors, prior to and following activation with immobilized anti-CD3 for 10 min. The cell lysates resolved by SDS–PAGE were immunoblotted using antibodies specific to RLC and phosphorylated RLC (pRLC). As shown in Figure 3, overall levels of RLC were similar in CD4+ T cells obtained from young and elderly donors, prior to and following activation with anti-CD3. In contrast, pRLC was detected only upon treatment with anti-CD3 in CD4+ T cells from young donors, while they were already detectable under basal conditions in lysates obtained from the elderly. Activation with anti-CD3 resulted in no further increase in pRLC in cells from the elderly. Thus, RLC appears to be constitutively phosphorylated in CD4+ T cells from elderly donors and is unaffected by treatment with anti-CD3. To our knowledge, this is the first report of altered activation of NMMIIA in CD4+ T cells from the elderly. As pRLC is important in T-cell signaling, our data for the first time demonstrate altered regulation of pRLC in CD4+ T cells from the elderly, and we believe that this may underlie some of the reported defects in T-cell activation during aging.
Figure 3.
Constitutively activated NMMIIA is detected in T cells from the elderly. (a) Western blot of MLC2 and pMLC2 in CD4+ T cells from young and elderly donors either left untreated or activated with anti-CD3 beads for 10 min. β-actin was used as a control for equal loading. Representative data from one donor pair out of eight pairs tested are provided. (b) The specific band for pMLC2 protein was quantified by densitometry. The values represent the mean integrated density±s.e. obtained from a minimum of eight independent donor pairs. ** denotes statistical significance at P<0.001. MLC, myosin light chain; NMMIIA, non-muscle myosin IIA; pMLC, phosphorylated myosin light chain.
Altered distribution of NMMIIA but not TCR-α/-β in T cells accompanies aging
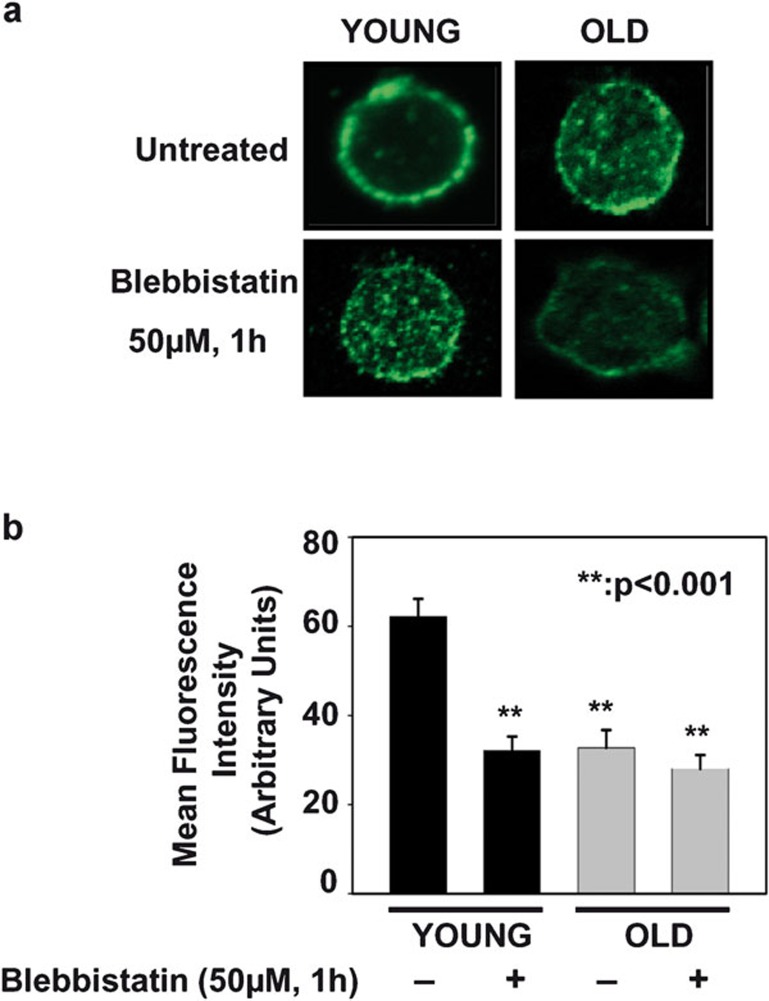
IS are initiated by signaling in discrete TCR microclusters, and these microclusters play an important role in T-cell functional differentiation and effector functions.18, 33, 34 Furthermore, microcluster movement and assembly are associated with centripetal actin flow in coordination with NMMIIA activation.20, 21, 35 The importance of NMMIIA in T-cell signaling and inherent alterations in NMMIIA activation status during aging prompted us to study the cellular localization of NMMIIA in CD4+ T cells from young and elderly donors employing confocal microscopy. The CD4+ T cells from young and elderly donors were either left untreated or pre-treated with blebbistatin, a NMMIIA-specific inhibitor. The CD4+ T cells were then stained with an antibody to either NMMIIA or TCR-α/-β, followed by the appropriate fluorescent secondary antibody. As shown in Figure 4, NMMIIA appeared to be strongly associated with the plasma membrane in CD4+ T cells from the young donors. However, in CD4+ T cells from the elderly, NMMIIA showed a more diffuse and punctate cytosolic localization, with minimal membrane localization. Interestingly, pre-treatment of the cells with blebbistatin induced a punctate/diffuse distribution of NMMIIA in CD4+ T cells from young donors, mimicking the distribution seen constitutively in CD4+ T cells from elderly donors (Figure 4). As blebbistatin is a chemical inhibitor of functional NMMIIA activity, it appears that inhibition of NMMIIA results in the loss of plasma membrane localization of NMMIIA, as seen in T cells from the elderly. This altered NMMIIA localization was in clear contrast to the pattern of TCR localization observed in CD4+ T cells, which was predominantly localized to the plasma membrane both under basal and blebbistatin-treated conditions, regardless of the age of the T-cell donor (Figure 5). Thus, our findings demonstrate that inhibition of NMMIIA by blebbistatin results in punctate, cytosolic rather than plasma membrane distribution of NMMIIA in CD4+ T cells from young donors, mimicking the observation in untreated CD4+ T cells from the elderly, with little or no impact on the cell surface distribution of TCR-α/-β.
Figure 4.
Localization and distribution of myosin IIA in CD4+ T cells from young and elderly donors. (a) Confocal microscopy of myosin IIA distribution in CD4+ T cells from young and elderly donors that were either left untreated (vehicle control) or pre-treated with blebbistatin. Representative data from one donor pair out of a minimum of 10 pairs tested are provided. (b) Cumulative data of intracellular myosin IIA fluorescence intensity in CD4+ T cells from 10 independent donor pairs. The mean fluorescence intensity of myosin IIA localized on the inner face of the plasma membrane is provided. Quantification of the fluorescence signal of myosin IIA in the inner face of the plasma membrane was performed on the complete three-dimensional confocal stack for 30 cells obtained from each of five different fields from a minimum of 10 independent experiments. The data are represented as the mean plasma membrane-associated fluorescence intensity±s.e. ** denotes statistical significance at P<0.001.
Figure 5.
Cell surface TCR-α/-β expression in CD4+ T cells is not affected by age. Confocal microscopy of TCR-α/-β expression in CD4+ T cells from young and elderly donors that were either left untreated (vehicle control) or pre-treated with blebbistatin. Representative data are from one donor pair out of 10 pairs tested. The fluorescence intensity of TCR-α/-β in the plasma membrane was performed on the complete three-dimensional confocal stack for 30 cells obtained from a minimum of five different fields each, from a minimum of 10 independent experiments. TCR, T-cell receptor.
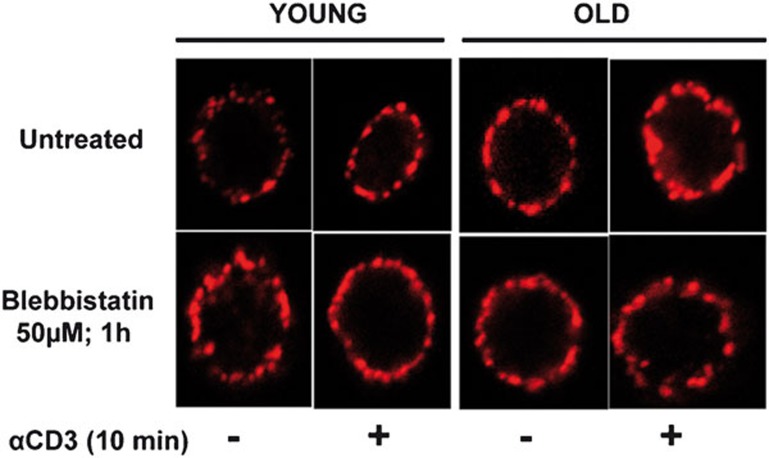
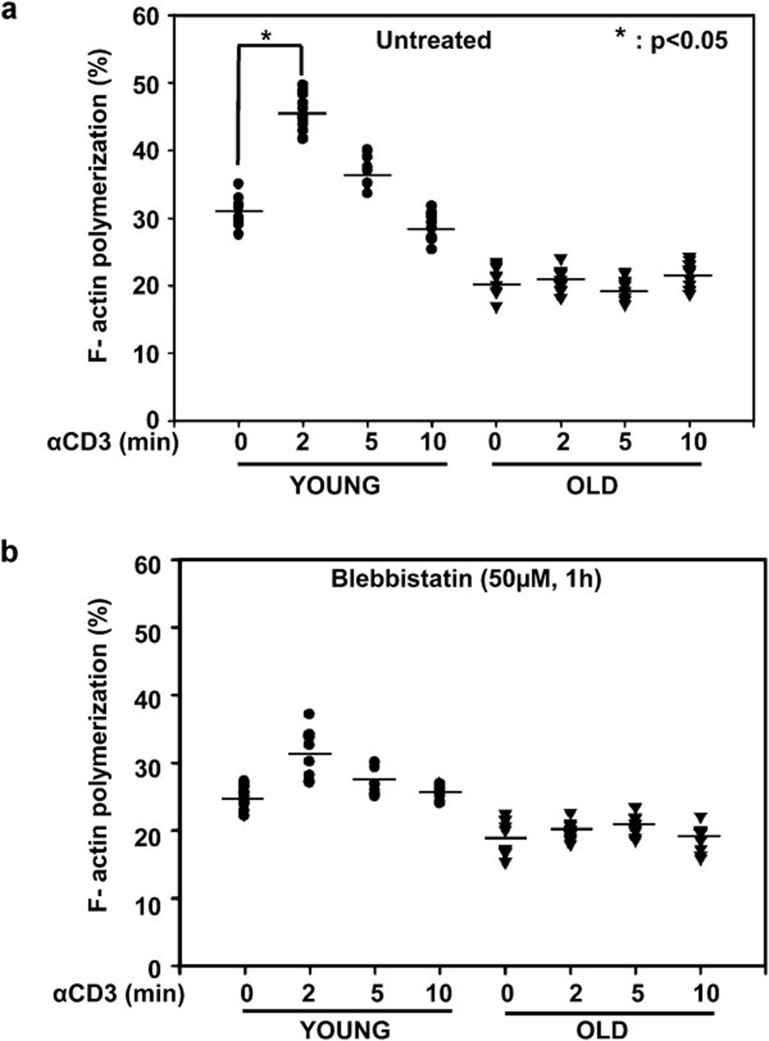
Nonfunctional NMMIIA negatively impacts F-actin polymerization in CD4+ T cells
One of the earliest events during CD4+ T-cell activation is the rapid and transient conversion of monomeric actin, G-actin, into filamentous actin, F-actin. Actin and NMMIIA interact,33 and the actomyosin complex plays an important role not only in the modification of cell shape, but also in crawling and migration in T cells. Although defects in F-actin polymerization have been reported in T cells from murine models of aging,9 limited data exist with regard to actin dynamics in activated human T cells during aging, and the role of NMMIIA in this process remains unknown. Consequently, employing primary CD4+ T cells obtained from young and elderly donors, we evaluated the changes in F-actin polymerization during anti-CD3-induced activation. As shown in Figure 6a, anti-CD3 treatment of CD4+ T cells induced an increase in F-actin that was significantly more pronounced in CD4+ T cells obtained from the young than the elderly donors. In addition, we also observed a significant age-related difference in the kinetics of F-actin polymerization. Specifically, in CD4+ T cells from young donors, the levels of F-actin peaked as early as 2 min and decreased gradually, returning to near basal levels by 10 min of anti-CD3 activation. However, in CD4+ T cells from elderly donors, the levels of F-actin did not change significantly from baseline, irrespective of the time of anti-CD3 activation, indicating a defect in activation-induced F-actin polymerization. To determine if the observed defect in F-actin polymerization is due to nonfunctional NMMIIA, we pre-treated CD4+ T cells with blebbistatin and subsequently activated the cells with anti-CD3 for 2, 5 or 10 min. As shown in Figure 6b, chemical inhibition of NMMIIA resulted in partial inhibition of F-actin polymerization, which was more pronounced in the CD4+ T cells obtained from young donors than those from the elderly. Thus, we conclude that functional inhibition of NMMIIA negatively impacts F-actin polymerization, which may potentially underlie the inability of T cells from the elderly to mediate early cytoskeletal events, including microcluster formation in the initiation of T-cell activation and TCR internalization in the termination of activation.
Figure 6.
Anti-CD3-induced F-actin polymerization in CD4+ T cells during aging. (a) CD4+ T cells were either left untreated or activated with plate-bound anti-CD3 for 2, 5 or 10 min. At the end of activation, cells were fixed, stained with rhodamine phalloidin and analyzed by flow cytometry. The data represent the mean±s.e. derived from 10 independent donor pairs. (b) CD4+ T cells were either pre-treated with blebbistatin or vehicle control, then activated with plate-bound anti-CD3 for 2, 5 or 10 min. F-actin polymerization was determined by flow cytometry. The data represent the mean±s.e. obtained from 10 independent donor pairs. * denotes statistical significance at P<0.05.
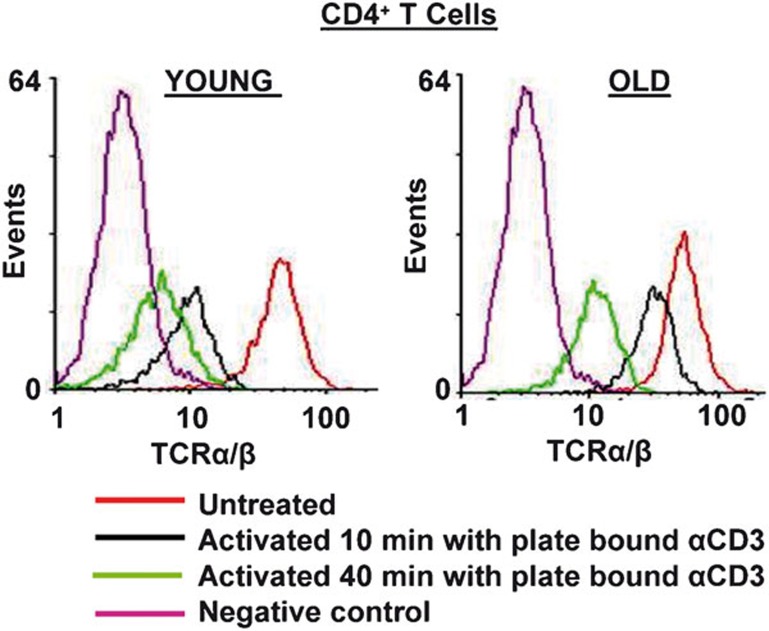
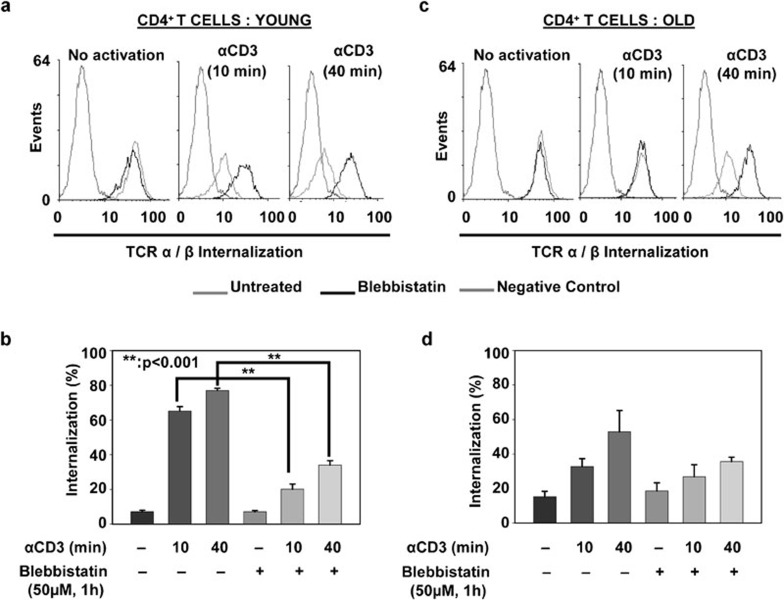
Functional NMMIIA is necessary for activation-induced internalization of TCR-α/-β
T-cell activation by a cognate antigen or anti-CD3 antibody results in the rapid internalization of TCR molecules, resulting in fewer TCR molecules at the surface.13, 17, 36 This downmodulation of the TCR–CD3 complex plays an important role in fine-tuning the avidity and strength of major histocompatibility complex–TCR interaction, and in attenuating TCR signaling. Although recent reports clearly demonstrate a role for NMMIIA in microcluster migration and the stability of the IS, a role for NMMIIA in mediating internalization of TCR-α/-β and receptor downmodulation has not been investigated. Consequently, to elucidate the role of NMMIIA on the internalization of TCR-α/-β receptors, we performed flow cytometry on CD4+ T cells from young and elderly donors, before and after treatment with anti-CD3, for defined periods of time, in the presence and absence of blebbistatin. As shown in Figure 7, the surface expression of TCR-α/-β receptors was similar in untreated T cells from young and elderly donors, demonstrating no change in TCR expression with the age of the donor population. Upon treatment with immobilized anti-CD3, CD4+ T cells from young donors demonstrated a rapid decrease in the surface expression of TCR-α/-β as early as 10 min, accounting for approximately 60% loss in expression, which reached 80% by 40 min, indicating internalization. In contrast, CD4+ T cells from elderly donors demonstrated decreased internalization, with an average 30% decrease in TCR-α/-β surface expression at 10 min following treatment with anti-CD3, approaching a 50% maximum decrease in expression even after 40 min of treatment. To evaluate the contribution of functional activity of NMMIIA during this process, we next pre-treated CD4+ T cells from young and elderly donors with blebbistatin and then either left the cells untreated or activated them with immobilized anti-CD3 antibody for 10 or 40 min. Our results (Figures 7 and 8) demonstrate that treatment with blebbistatin per se does not impact the basal level of TCR-α/-β expression, irrespective of the age of the T-cell donor. However, treatment with blebbistatin significantly inhibited anti-CD3-mediated internalization of TCR-α/-β receptors at both 10 and 40 min in CD4+ T cells obtained from young donors, resulting in an average internalization of approximately 20% at 10 min and 30% at 40 min. In contrast, cells from the elderly failed to show a significant decline upon treatment with blebbistatin (Figures 7 and 8). Based on these results, we conclude that inhibition of the functional activity of NMMIIA negatively impacts anti-CD3-mediated TCR-α/-β internalization/downmodulation and hence cell surface expression in CD4+ T cells from young donors, thus mimicking the overall phenotype observed in anti-CD3-activated but blebbistatin-untreated CD4+ T cells from the elderly.
Figure 7.
Activation-induced internalization of TCR-α/-β is delayed in CD4+ T cells from elderly donors. CD4+ T cells obtained from young and elderly donors were either left untreated or activated with 5 µg/well plate-bound anti-CD3 for 10 or 40 min. The cells were then stained using antibody to TCR-α/-β, followed by Alexa Fluor 555-conjugated secondary antibody, and then fixed with 2% PFA. Representative data are from one donor pair out of five pairs tested. Surface expression of TCR was detected by flow cytometry. Representative data from one donor pair are depicted in the histogram. Cumulative data obtained from a minimum of five independent donor pairs are provided. PFA, paraformaldehyde; TCR, T-cell receptor.
Figure 8.
Inhibition of NMMIIA results in delayed internalization of cell surface TCR-α/-β. (a, c) CD4+ T cells obtained from young and elderly donors were either left untreated or pre-treated with blebbistatin, then activated with 5 µg/well plate-bound anti-CD3 for 10 or 40 min. The cells were then stained using an antibody to TCR-α/-β, followed by Alexa Fluor 555-conjugated secondary antibody, and fixed with 2% PFA. Surface expression of TCR was detected by flow cytometry. Representative data from one donor pair are shown. (b, d), Cumulative data from a minimum of five independent pairs of young and elderly donors. The percentage of internalization was calculated based on the mean fluorescence intensity. The data represent the mean fluorescence intensity±s.e. ** denotes statistical significance at P<0.001. NMMIIA, non-muscle myosin IIA; PFA, paraformaldehyde; TCR, T-cell receptor.
NMMIIA negatively regulates intracellular calcium release upon TCR engagement in CD4+ T cells
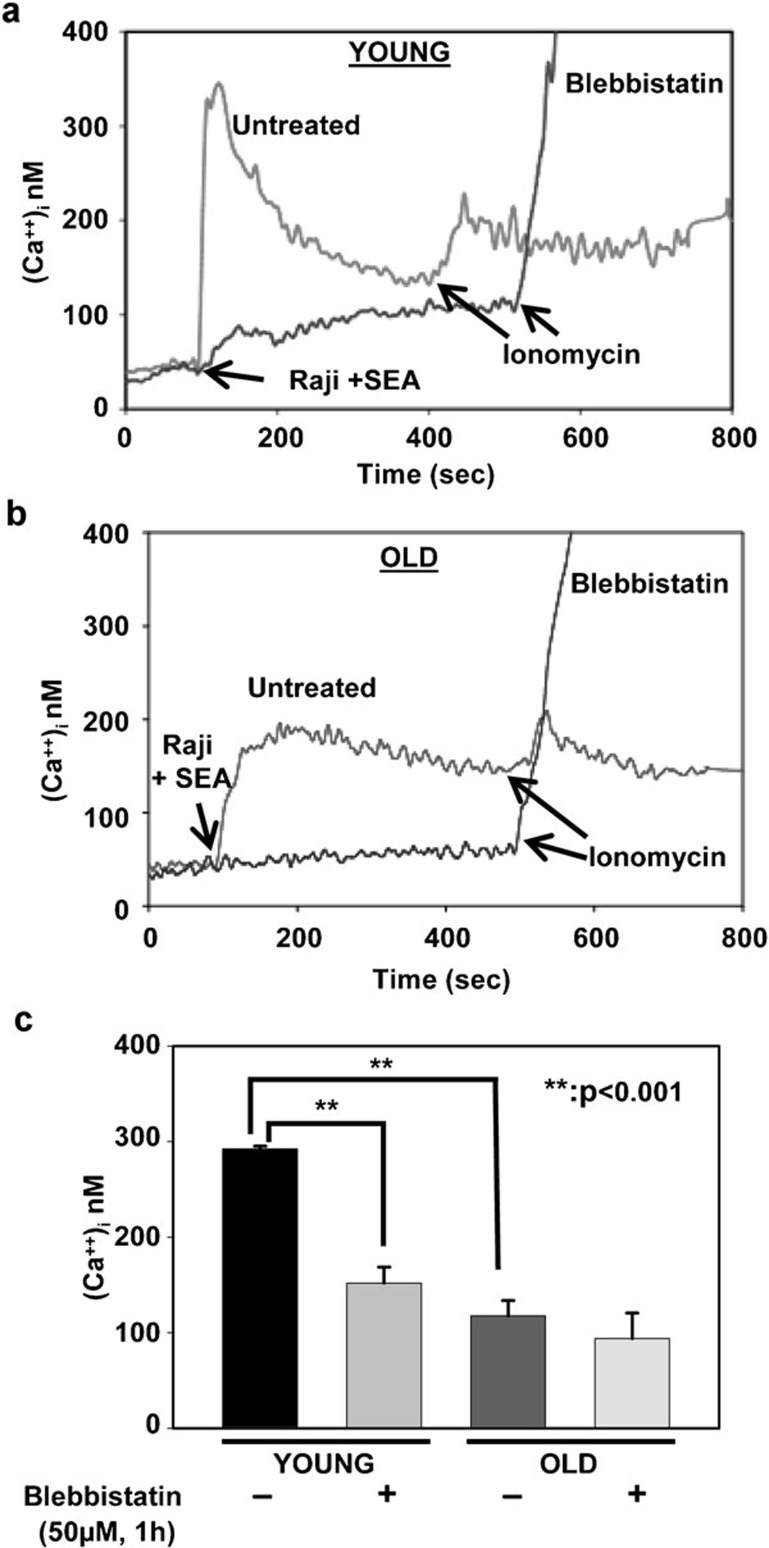
In CD4+ T cells, calcium ions (Ca2+) function as critical second messengers. Calcium signals regulate the activation of lymphocytes, their differentiation and multiple effector functions, as well as the induction of a variety of transcriptional programs.13 In lymphocytes, sustained calcium entry is necessary for complete activation of calcineurin-dependent nuclear factor of activated T-cell pathways. Immune senescence is accompanied by a reduction in calcium release from intracellular stores upon lymphocyte activation. Because a defect in TCR-α/-β internalization was observed in CD4+ T cells from the elderly and upon pre-treatment with blebbistatin in CD4+ T cells from young donors, we next evaluated the impact of inhibition of NMMIIA on activation-induced calcium release from intracellular stores. CD4+ T cells obtained from both young and elderly donors were either pre-treated with blebbistatin or left untreated and subsequently incubated with superantigen-pulsed APCs at a ratio of 1∶1 (Raji B cells pulsed with Staphylococcus enterotoxin A). Microfluorescent imaging of intracellular calcium influx was monitored by loading CD4+ T cells with Fura-2AM. As previously reported in murine studies, our results confirm that CD4+ T cells obtained from elderly human donors, activated by incubation with pulsed APCs, demonstrated lower and sustained calcium release from intracellular stores when compared with similarly treated cells from young donors (Figure 9), where the levels of calcium demonstrated a rapid, significant peak response. Pre-treatment of CD4+ T cells with blebbistatin completely abrogated the release of intracellular calcium upon TCR-α/-β engagement by antigen-pulsed APCs, irrespective of the age of the donor. Interestingly, blebbistatin treatment failed to impact ionomycin-mediated calcium release from CD4+ T cells from both young and elderly donors, implicating a role for NMMIIA function in early events of TCR-α/-β signaling. Thus, a fully functional NMMIIA appears to be an active and necessary participant in calcium release from intracellular stores following TCR engagement, underscoring its role in regulating key steps in early signaling events activated by effective TCR engagement.
Figure 9.
Activation-induced intracellular calcium flux decreased in CD4+ T cells from elderly donors. (a) Microfluorescent imaging of intracellular calcium flux in CD4+ T cells obtained from one representative young donor. (b) Microfluorescent imaging of intracellular calcium flux in CD4+ T cells obtained from a representative elderly donor. CD4+ T cells from both young and elderly donors were either pre-treated with blebbistatin or vehicle control, then incubated with Raji cells pulsed with SEA. Ionomycin was used as a positive control. (c) Mean intracellular concentrations of calcium±s.e. from six independent experiments are presented. ** denotes statistical significance at P<0.001. SEA, Staphylococcus enterotoxin A.
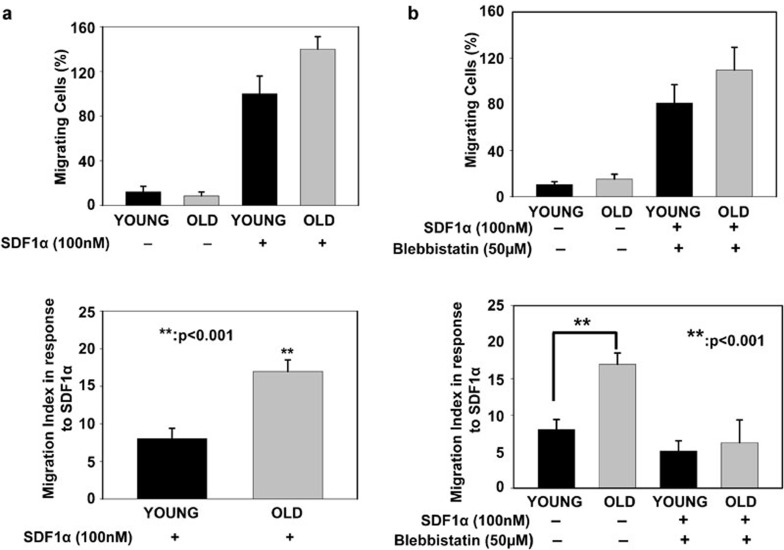
Chemotactic migration of CD4+ T cells in response to SDF-1α is enhanced in CD4+ T cells from the elderly
Continuous recirculation of CD4+ T cells and their eventual migration to tissues upon activation is important for protective immunity against invading pathogens.37, 38 In migrating cells, actin organizes into two basic structures, the lamellipodium and the uropod. Functional NMMIIA is required not only in the generation of the driving force, but also in actomyosin complex formation and F-actin polymerization.20, 21 Therefore, we evaluated the role of NMMIIA in chemotactic migration during aging. Migration39, 40 was evaluated by employing a chemotactic gradient of human SDF (hSDF)-1α. CD4+ T cells from young and elderly donors were allowed to migrate for 4 h in transwell plates that were either left uncoated (PBS–BSA) or coated with 100 nM of hSDF-1α. As shown in Figure 10a, CD4+ T cells from both young and elderly donors migrated minimally in the absence of hSDF-1α. However, in response to hSDF-1α, CD4+ T cells from both young and elderly donors demonstrated robust migration. Significantly higher numbers of CD4+ T cells from elderly donors migrated toward hSDF-1α than those from young donors. This observation is in agreement with data previously reported for CD4+ splenic T cells in aged mice, where an increase in the percentage of transmigration was reported in response to SDF-1α.41 Additionally, increased CXCR4 expression has been demonstrated in T cells from mice during aging, which may account for the increased migration towards hSDF-1α,41 observed in our samples. Inhibition of NMMIIA activity by pre-treatment with blebbistatin resulted in a profound inhibition of the migration toward hSDF-1α in CD4+ T cells from both young and elderly donors (Figure 10b). Taken together, these results suggest that decreased functional NMMIIA in the elderly has a positive impact on the migration of CD4+ T cells. Because complete inhibition of NMMIIA negatively impacts chemotaxis towards hSDF-1α, we believe that the increased migration observed in the elderly may be suggestive of differential complexes recruited by functional and non-functional NMMIIA in CD4+ T-cell migration. Additionally, as previous studies in other cell types have demonstrated both an increase and a decrease in migration of cells upon blebbistatin treatment,42 it is clear that graded NMMIIA levels may regulate migration, with partial versus complete inhibition impacting migration differentially. Future studies will dissect the precise role of NMMIIA in T-cell migration during aging.
Figure 10.
Effects of aging and inhibition of NMMIIA on chemotaxis to a SDF-1α gradient in CD4+ T cells. (a) Percent migration in response to the SDF-1α gradient in untreated CD4+ T cells from young and elderly donors. (b) Percent migration of blebbistatin-pre-treated CD4+ T cells from young and elderly donors in response to the SDF-1α gradient. The data are representative of 12 independent donor pairs. The migration index was derived by calculating the migration of CD4+ T cells subjected to a gradient of SDF-1 in a transwell system normalized to vehicle-treated controls. ** denotes statistical significance at P<0.001. NMMIIA, non-muscle myosin IIA; SDF, stromal cell-derived factor.
Discussion
In this study, we present evidence suggesting a novel role for NMMIIA in T-cell functional deficits observed during aging in humans. Previous reports from our laboratory and those of others have shown that CD4+ T cells from healthy elderly human subjects and aged mice exhibit multiple defects in signal transduction events following TCR ligation.12 These include alterations in tyrosine phosphorylation of TCR-associated ζ-chains,43 calcium release from intracellular stores, polymerization of F-actin11 and induction of nuclear translocation of transcription factors.3, 4 As dynamic properties of CD4+ T cells, such as migration, adhesion and cell division, depend on actin polymerization and NMMIIA-dependent compression and retraction, alterations in the activation status of NMMIA will likely have profound effects not only on migration and adhesion but also on CD4+ T-cell antigen receptor microcluster formation and immunological synapse stabilization. Our data demonstrate a central role for NMMIIA dysregulation in age-associated functional defects observed in CD4+ T cells. These defects range from alterations in actin polymerization and TCR internalization to T-cell migration toward chemotactic gradients. NMMIIA, which is abundantly associated with Hsp90, is constitutively activated by phosphorylation of RLC in CD4+ T cells obtained from elderly donors. This basal phosphorylation of RLC might explain the inability of these cells to undergo any further stimulation upon treatment with common polyclonal T-cell activators. Interestingly, we now provide evidence that pre-treatment of CD4+ T cells from young donors with an NMMIIA-specific inhibitor, blebbistatin, alters NMMIIA distribution in these cells and partially induces phosphorylation of RLC, mimicking the observation in T cells from the elderly. Taken together, these results suggest that inhibition of NMMIIA may contribute to immune dysfunction in the elderly.
The dynamics of TCR downmodulation can directly impinge not only on the termination of TCR signaling and T-cell activation but also on events dictated by coreceptors such as cytotoxic T lymphocyte-associated antigen 4 and programmed death-1.13, 44, 45 As several studies have now attributed the decrease in the number of TCR on the cell surface to a combination of processes involving internalization, recycling and degradation, our studies for the first time show the requirement and involvement of functional NMMIIA in activation-induced TCR internalization. Additionally, we show that the functional inhibition of NMMIIA in CD4+ T cells from young donors results in an almost complete abrogation of the TCR internalization induced by anti-CD3 antibody. This block in TCR internalization is similar to that observed upon anti-CD3 treatment in CD4+ T cells from the elderly, which implicates altered NMMIIA in defective TCR internalization in the elderly.
The ubiquitin ligases, c-Cbl and Cbl-b, play essential roles in the dynamics of TCR downmodulation by regulating vesicle sorting and endosome-to-lysosome trafficking.44, 45, 46, 47 Future studies will be directed at delineating c-Cbl and Cbl-b status in T cells from the elderly.
Contrary to our expectations, defects in NMMIIA function in T cells from the elderly did not drastically impair chemotactic migration towards SDF-1α. In fact, CD4+ T cells from the elderly demonstrated a significant increase in migration index when compared to T cells obtained from young donors. This increased migration toward SDF-1 supports data obtained in murine splenocytes and is indicative of a reciprocal regulation of NMMIIA and chemotactic activity. As treatment of both CD4+ T cells from young and elderly donors with blebbistatin completely abrogated chemotactic migration, we believe that complete inhibition of functional activity of NMMIIA interferes with and abrogates migration in CD4+ T cells from both young and elderly donors. This seemingly paradoxical observation of increased chemotactic migration in CD4+ T cells from the elderly in the context of altered NMMIIA levels may be attributable to a partial but not complete loss of NMMIIA activity in the elderly. Future studies employing graded amounts of NMMIIA may be able to resolve the magnitudes of response to chemokines and the expression of functional NMMIIA. In fact, studies in adherent cells have demonstrated either a reduction or an enhancement in migration upon myosin IIA inhibition. Additionally, alteration in CXCR4 expression (previously reported to be increased), actomyosin complex formation and F-actin polymerization in CD4+ T cells from the elderly may well have contributed to the overall increase in migration observed in the elderly. As CXCR4 expression on the surface of T cells is regulated by NMMIIA, changes in functional NMMIIA in CD4+ T cells from the elderly may indirectly regulate chemotactic migration.
Overall, our findings have uncovered an important contribution of NMMIIA to T-cell functional responses during immune senescence, ranging from its role in activation-mediated TCR internalization to intracellular calcium mobilization to chemotactic migration.
Acknowledgments
This work was supported by grants RO1AG030599 and RO1AG025220 to UP, UAMS Graduate student research fund to SC, and National Center for Research Resources UL1RR029884 to the CCTR. The authors declare no conflicts of interest. We thank Mrs Michela Palmieri at the University of Arkansas for Medical Science for technical assistance in coordinating human subjects for the study and for T-cell isolation. We acknowledge support from the CCTR at UAMS for phlebotomy. Thanks are also due to Dr Steven Barger, Department of Geriatrics at the University of Arkansas for Medical Sciences, for help with assays involving intracellular calcium flux, and Dr Martin Cannon, Department of Microbiology and Immunology at the University of Arkansas for Medical Sciences, for flow cytometry analyses and advice.
References
- Ponnappan S, Ponnappan U. Aging and immune function: molecular mechanisms to interventions. Antioxid Redox Signal. 2011;14:1551–1585. doi: 10.1089/ars.2010.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam N, Das S, Mahapatra SK, Chakraborty SP, Kundu PK, Roy S. Age associated oxidative damage in lymphocytes. Oxid Med Cell Longev. 2010;3:275–282. doi: 10.4161/oxim.3.4.12860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnappan S, Uken-Trebilcock G, Lindquist M, Ponnappan U. Tyrosine phosphorylation-dependent activation of NFkappaB is compromised in T cells from the elderly. Exp Gerontol. 2004;39:559–566. doi: 10.1016/j.exger.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Ponnappan U. Regulation of transcription factor NF kappa B in immune senescence. Front Biosci. 1998;3:d152–d168. doi: 10.2741/a271. [DOI] [PubMed] [Google Scholar]
- Leng J, Goldstein DR. Impact of aging on viral infections. Microbes Infect. 2010;12:1120–1124. doi: 10.1016/j.micinf.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WM, Zeijst BA, Boog CJ, Soethout EC. Aging and impaired immunity to influenza viruses: implications for vaccine development. Hum Vaccin. 2011;7:94–98. doi: 10.4161/hv.7.0.14568. [DOI] [PubMed] [Google Scholar]
- Ogino T, Miura S, Komoto S, Hara Y, Hokari R, Tsuzuki Y, et al. Senescence-associated decline of lymphocyte migration in gut-associated lymphoid tissues of rat small intestine. Mech Ageing Dev. 2004;125:191–199. doi: 10.1016/j.mad.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Medina S, del Rio M, Manuel Victor V, Hernanz A, de la Fuente M. Changes with ageing in the modulation of murine lymphocyte chemotaxis by CCK-8S, GRP and NPY. Mech Ageing Dev. 1998;102:249–261. doi: 10.1016/s0047-6374(98)00014-1. [DOI] [PubMed] [Google Scholar]
- Garcia GG, Miller RA. Age-dependent defects in TCR-triggered cytoskeletal rearrangement in CD4+ T cells. J Immunol. 2002;169:5021–5027. doi: 10.4049/jimmunol.169.9.5021. [DOI] [PubMed] [Google Scholar]
- Garcia GG, Miller RA. Age-related changes in lck–Vav signaling pathways in mouse CD4 T cells. Cell Immunol. 2009;259:100–104. doi: 10.1016/j.cellimm.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia GG, Akha AA, Miller RA. Age-related defects in moesin/ezrin cytoskeletal signals in mouse CD4 T cells. J Immunol. 2007;179:6403–6409. doi: 10.4049/jimmunol.179.10.6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamir A, Eisenbraun MD, Garcia GG, Miller RA. Age-dependent alterations in the assembly of signal transduction complexes at the site of T cell/APC interaction. J Immunol. 2000;165:1243–1251. doi: 10.4049/jimmunol.165.3.1243. [DOI] [PubMed] [Google Scholar]
- Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MM, Labno CM, van Seventer GA, Denny MF, Straus DB, Burkhardt JK. Superantigen-induced T cell:B cell conjugation is mediated by LFA-1 and requires signaling through Lck, but not ZAP-70. J Immunol. 2001;167:5708–5718. doi: 10.4049/jimmunol.167.10.5708. [DOI] [PubMed] [Google Scholar]
- Wabnitz GH, Lohneis P, Kirchgessner H, Jahraus B, Gottwald S, Konstandin M, et al. Sustained LFA-1 cluster formation in the immune synapse requires the combined activities of ℓ-plastin and calmodulin. Eur J Immunol. 2010;40:2437–2449. doi: 10.1002/eji.201040345. [DOI] [PubMed] [Google Scholar]
- Becart S, Altman A. SWAP-70-like adapter of T cells: a novel Lck-regulated guanine nucleotide exchange factor coordinating actin cytoskeleton reorganization and Ca2+ signaling in T cells. Immunol Rev. 2009;232:319–333. doi: 10.1111/j.1600-065X.2009.00839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt JJ, Krummel MF. The importance of prolonged binding to antigen-presenting cells for T cell fate decisions. Immunity. 2008;28:143–145. doi: 10.1016/j.immuni.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Chakraborty AK, Dustin ML. Signaling microdomains in T cells. FEBS Lett. 2010;584:4823–4831. doi: 10.1016/j.febslet.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppa JB, Axmann M, Mortelmaier MA, Lillemeier BF, Newell EW, Brameshuber M, et al. TCR-peptide–MHC interactions in situ show accelerated kinetics and increased affinity. Nature. 2010;463:963–967. doi: 10.1038/nature08746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M, Cooper JA. The immunological synapse and the actin cytoskeleton: molecular hardware for T cell signaling. Nat Immunol. 2000;1:23–29. doi: 10.1038/76877. [DOI] [PubMed] [Google Scholar]
- Beemiller P, Krummel MF. Mediation of T-cell activation by actin meshworks. Cold Spring Harb Perspect Biol. 2010;2:a002444. doi: 10.1101/cshperspect.a002444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crevecoeur J, Merville MP, Piette J, Gloire G. Geldanamycin inhibits tyrosine phosphorylation-dependent NF-kappaB activation. Biochem Pharmacol. 2008;75:2183–2191. doi: 10.1016/j.bcp.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Castro JE, Prada CE, Loria O, Kamal A, Chen L, Burrows FJ, et al. ZAP-70 is a novel conditional heat shock protein 90 (Hsp90) client: inhibition of Hsp90 leads to ZAP-70 degradation, apoptosis, and impaired signaling in chronic lymphocytic leukemia. Blood. 2005;106:2506–2512. doi: 10.1182/blood-2005-03-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartis D, Boldizsar F, Kvell K, Szabo M, Palinkas L, Nemeth P, et al. Intermolecular relations between the glucocorticoid receptor, ZAP-70 kinase, and Hsp-90. Biochem Biophys Res Commun. 2007;354:253–258. doi: 10.1016/j.bbrc.2006.12.211. [DOI] [PubMed] [Google Scholar]
- Taiyab A, Rao C. HSP90 modulates actin dynamics: inhibition of HSP90 leads to decreased cell motility and impairs invasion. Biochim Biophys Acta. 2011;1813:213–221. doi: 10.1016/j.bbamcr.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Rajagopal D, Bal V, Mayor S, George A, Rath S. A role for the Hsp90 molecular chaperone family in antigen presentation to T lymphocytes via major histocompatibility complex class II molecules. Eur J Immunol. 2006;36:828–841. doi: 10.1002/eji.200535326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagoz GE, Duarte AM, Ippel H, Uetrecht C, Sinnige T, van Rosmalen M, et al. N-terminal domain of human Hsp90 triggers binding to the cochaperone p23. Proc Natl Acad Sci USA. 2011;108:580–585. doi: 10.1073/pnas.1011867108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilani T, Vasiliver-Shamis G, Vardhana S, Bretscher A, Dustin ML. T cell antigen receptor signaling and immunological synapse stability require myosin IIA. Nat Immunol. 2009;10:531–539. doi: 10.1038/ni.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betapudi V, Gokulrangan G, Chance MR, Egelhoff TT. A proteomic study of myosin II motor proteins during tumor cell migration. J Mol Biol. 2011;407:673–686. doi: 10.1016/j.jmb.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badyal SK, Basran J, Bhanji N, Kim JH, Chavda AP, Jung HS, et al. Mechanism of the Ca2+-dependent interaction between S100A4 and tail fragments of nonmuscle myosin heavy chain IIA. J Mol Biol. 2011;405:1004–1026. doi: 10.1016/j.jmb.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gano J, Simon JA. A proteomic investigation of ligand-dependent HSP90 complexes reveals CHORDC1 as a novel ADP-dependent HSP90-interacting protein. Mol Cell Proteomics. 2010;9:255–270. doi: 10.1074/mcp.M900261-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin ML. The cellular context of T cell signaling. Immunity. 2009;30:482–492. doi: 10.1016/j.immuni.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummel M, Cahalan M. The immunological synapse: a dynamic platform for local signaling. J Clin Immunol. 2010;30:364–372. doi: 10.1007/s10875-010-9393-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin ML. Visualization of cell–cell interaction contacts—synapses and kinapses. Adv Exp Med Biol. 2008;640:164–182. doi: 10.1007/978-0-387-09789-3_13. [DOI] [PubMed] [Google Scholar]
- Burkhardt JK, Carrizosa E, Shaffer MH. The actin cytoskeleton in T cell activation. Annu Rev Immunol. 2008;26:233–259. doi: 10.1146/annurev.immunol.26.021607.090347. [DOI] [PubMed] [Google Scholar]
- Friedman RS, Beemiller P, Sorensen CM, Jacobelli J, Krummel MF. Real-time analysis of T cell receptors in naive cells in vitro and in vivo reveals flexibility in synapse and signaling dynamics. J Exp Med. 2010;207:2733–2749. doi: 10.1084/jem.20091201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth NJ, McQuaid AJ, Sobande T, Kissane S, Agius E, Jackson SE, et al. Different proliferative potential and migratory characteristics of human CD4+ regulatory T cells that express either CD45RA or CD45RO. J Immunol. 2010;184:4317–4326. doi: 10.4049/jimmunol.0903781. [DOI] [PubMed] [Google Scholar]
- Stohlawetz P, Kolussi T, Jahandideh-Kazempour S, Kudlacek S, Graninger W, Willvonseder R, et al. The effect of age on the transendothelial migration of human T lymphocytes. Scand J Immunol. 1996;44:530–534. doi: 10.1046/j.1365-3083.1996.d01-331.x. [DOI] [PubMed] [Google Scholar]
- Garcia GG, Miller RA. Age-related defects in the cytoskeleton signaling pathways of CD4 T cells. Ageing Res Rev. 2011;10:26–34. doi: 10.1016/j.arr.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miletic AV, Graham DB, Sakata-Sogawa K, Hiroshima M, Hamann MJ, Cemerski S, et al. Vav links the T cell antigen receptor to the actin cytoskeleton and T cell activation independently of intrinsic Guanine nucleotide exchange activity. PLoS One. 2009;4:e6599. doi: 10.1371/journal.pone.0006599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruran M, Chen J, Han Y, Bueno-Cannizares C, Misek DE, Lescure PA, et al. T cell chemokine receptor expression in aging. J Immunol. 2003;170:895–904. doi: 10.4049/jimmunol.170.2.895. [DOI] [PubMed] [Google Scholar]
- Jacobelli J, Bennett FC, Pandurangi P, Tooley AJ, Krummel MF. Myosin-IIA and ICAM-1 regulate the interchange between two distinct modes of T cell migration. J Immunol. 2009;182:2041–2050. doi: 10.4049/jimmunol.0803267. [DOI] [PubMed] [Google Scholar]
- Mayya V, Lundgren DH, Hwang SI, Rezaul K, Wu L, Eng JK, et al. Quantitative phosphoproteomic analysis of T cell receptor signaling reveals system-wide modulation of protein–protein interactions. Sci Signal. 2009;2:ra46. doi: 10.1126/scisignal.2000007. [DOI] [PubMed] [Google Scholar]
- Paolino M, Penninger JM. Cbl-b in T-cell activation. Semin Immunopathol. 2010;32:137–148. doi: 10.1007/s00281-010-0197-9. [DOI] [PubMed] [Google Scholar]
- Paolino M, Thien CB, Gruber T, Hinterleitner R, Baier G, Langdon WY, et al. Essential role of E3 ubiquitin ligase activity in Cbl-b-regulated T cell functions. J Immunol. 2011;186:2138–2147. doi: 10.4049/jimmunol.1003390. [DOI] [PubMed] [Google Scholar]
- Balagopalan L, Ashwell BA, Bernot KM, Akpan IO, Quasba N, Barr VA, et al. Enhanced T-cell signaling in cells bearing linker for activation of T-cell (LAT) molecules resistant to ubiquitylation. Proc Natl Acad Sci USA. 2011;108:2885–2890. doi: 10.1073/pnas.1007098108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Jeon MS, Liao L, Yang C, Elly C, Yates 3rd, JR, et al. K33-linked polyubiquitination of T cell receptor-zeta regulates proteolysis-independent T cell signaling. Immunity. 2010;33:60–70. doi: 10.1016/j.immuni.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]