Abstract
Gene expression changes in response to aging, heat stress, hyperoxia, hydrogen peroxide, and ionizing radiation were compared using microarrays. A set of 18 genes were up-regulated across all conditions, indicating a general stress response shared with aging, including the heat shock protein (Hsp) genes Hsp70, Hsp83 and l(2)efl, the glutathione-S-transferase gene GstD2, and the mitochondrial unfolded protein response (mUPR) gene ref(2)P. Selected gene expression changes were confirmed using quantitative PCR, Northern analysis and GstD-GFP reporter constructs. Certain genes were altered in only a subset of the conditions, for example, up-regulation of numerous developmental pathway and signaling genes in response to hydrogen peroxide. While aging shared features with each stress, aging was more similar to the stresses most associated with oxidative stress (hyperoxia, hydrogen peroxide, ionizing radiation) than to heat stress. Aging is associated with down-regulation of numerous mitochondrial genes, including electron-transport-chain (ETC) genes and mitochondrial metabolism genes, and a sub-set of these changes was also observed upon hydrogen peroxide stress and ionizing radiation stress. Aging shared the largest number of gene expression changes with hyperoxia. The extensive down-regulation of mitochondrial and ETC genes during aging is consistent with an aging-associated failure in mitochondrial maintenance, which may underlie the oxidative stress-like and proteotoxic stress-like responses observed during aging.
Keywords: Oxidative stress, ROS, mitochondria, GST, Nrf2
INTRODUCTION
Heat shock protein (Hsp) genes are induced in response to stresses that cause protein denaturation, through activation of the heat shock factor (HSF) [1]. Up-regulation of Hsp genes is also observed during normal aging [2]. For example, both Hsp70 and Hsp22 are up-regulated during normal Drosophila aging, and this up-regulation requires functional HSF binding sites (Heat Shock Elements, or HSEs) in the promoters of these genes [3-5]. Genome-wide studies of gene expression changes during Drosophila aging have revealed additional features of a stress response, including the up-regulation of additional oxidative stress-response genes, and the dramatic up-regulation of innate immune response genes [6-8]. In addition, Drosophila aging is characterized by a small but across-the-board down-regulation of mitochondrial metabolism and electron transport chain (ETC) genes [6, 8], and this pattern is also observed in aging mammalian tissues [9], and at early adult ages in both Drosophila and C. elegans [10], indicating a conservation of aging mechanisms across species. Both innate immune response genes [6] and Hsp genes [11, 12] have been shown to be predictive biomarkers of individual animal life span when the gene promoters are fused to GFP to create transgenic reporters, thereby supporting the significance of the identified gene expression changes. Here normal aging was compared with multiple stressors to provide further insight into common and unique features.
RESULTS
Gene expression changes common to each stress and to aging
Micro-array analysis was used to identify genes whose expression was altered in response to normal aging, hyperoxia, hydrogen peroxide, ionizing radiation and heat stress. A core set of 18 stress-response genes were up-regulated ≥1.5-fold in response to each of the tested stresses as well as during normal aging (Table 1).
Table 1. Gene expression changes common to aging and each stress.
-
18 genes up-regulated in aging and all other stresses
CG6489 Hsp70 Heat-shock-protein-70 CG3705 aay astray CG32130 stv starvin CG4533 l(2)efl lethal (2) essential for life CG33229 CG33229 CG4181 GstD2 Glutathione S transferase D2 CG3821 Aats-asp Aspartyl-tRNA synthetase CG5966 CG5966 CG11030 CG11030 CG14245 CG14245 CG14246 CG14246 CG15784 CG15784 CG31638 CG31638 CG13941 Arc2 Arc2 CG1242 Hsp83 Heat shock protein 83 CG10360 ref(2)P refractory to sigma P CG32103 CG32103 CG17725 Pepck Phosphoenolpyruvate carboxykinase -
GO enrichment terms for genes upregulated in aging and all other stresses
GO:0035079 polytene chromosome puffing(5) 5.65E-10 GO:0035080 heat shock-mediated polytene chromosome puffing(5) 5.65E-10 GO:0009408 response to heat(7) 8.87E-08 GO:0009266 response to temperature stimulus(7) 5.70E-07 GO:0034605 cellular response to heat(5) 1.12E-06 GO:0001666 response to hypoxia(5) 4.94E-05 GO:0070482 response to oxygen levels(5) 8.15E-05 GO:0009628 response to abiotic stimulus(7) 2.00E-04 -
32 genes down-regulated in aging and all other stresses
CG number Symbol Gene name CG10026 CG10026 CG10467 CG10467 CG14120 CG14120 CG14661 CG14661 CG18302 CG18302 CG18493 CG18493 CG18585 CG18585 CG31148 CG31148 CG3290 CG3290 CG3734 CG3734 CG3940 CG3940 CG5107 CG5107 CG5150 CG5150 CG5804 CG5804 CG6660 CG6660 CG8093 CG8093 CG8147 CG8147 CG9463 CG9463 CG9466 CG9466 CG9468 CG9468 CG9682 CG9682 CG5137 Cyp312a1 Cyp312a1 CG3360 Cyp313a1 Cyp313a1 CG8579 Jon44E Jonah 44E CG11669 Mal-A7 Maltase A7 CG4123 Mipp1 Multiple inositol polyphosphate phosphatase 1 CG6164 Npc2f Niemann-Pick type C-2f CG7754 iotaTry iotaTrypsin CG12388 kappaTry kappaTry CG12350 lambdaTry lambdaTry CG16834 lectin-33A lectin-33A CG4979 sxe2 sex-specific enzyme 2 -
GO enrichment terms for genes down-regulated in aging and all other stresses
GO:0006013 mannose metabolic process(3) 0.018117
These up-regulated genes included the heat shock protein genes Hsp70, Hsp83 (which is the single Drosophila Hsp90-class member), and the small Hsp gene l(2)efl. The up-regulation of Hsp70 and l(2)efl in response to selected stressors was confirmed using quantitative real-time PCR analysis (Figure 1), and in addition Hsp70 was analyzed by Northern blot analysis (Supplemental Figure S1; results summarized in Table 2). Also up-regulated by aging and each stressor were the glutathione S-transferase gene GstD2, the central metabolic regulatory enzyme gene Pepck, and the mitochondrial unfolded protein response (mUPR) gene ref(2)P. Down-regulated genes included several associated with sugar metabolism and proteolysis (Table 1).
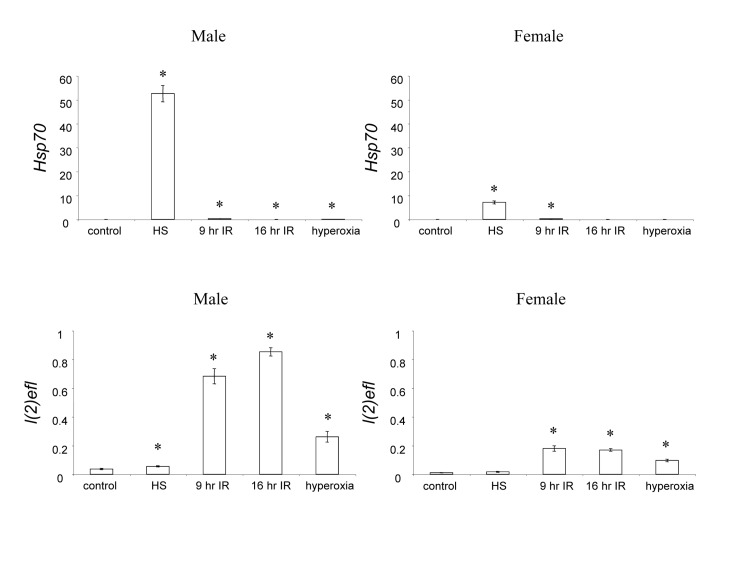
Figure 1. Hsp70 and l(2]ef) RNA levels in response to selected stresses.
Quantitative real-time RT-PCR analysis was used to determine RNA levels for the genes Hsp70 and l(2)efl in response to selected stresses, in both male and female flies, as indicated. HS, heat stress; IR, ionizing radiation. Stress treatment RNA levels were compared to control using unpaired, two-sided t-tests, and statistically significant differences (p < 0.05) are indicated with asterisk.
Table 2. Confirmation of selected gene expression changes using qPCR and Northern analysis.
| O2 up | O2 dn | H2O2 up | H2O2 dn | HS up | HS dn | IR up | IR dn | Age up | Age dn | |
|---|---|---|---|---|---|---|---|---|---|---|
| hsp70 | XQL | XN | XQ | XQ | XL | |||||
| hsp22 | XQL | N | XQ | XQ | XL | |||||
| l(2)efl | XQL | Xnt | XQ | XQ | XL | |||||
| Drs | XQL | X | Q | XQf | XL | |||||
| ade3 | XQL | Xnt | Q | XQ | XL | |||||
| CG11089 | XQ | XN | Q | XQ | Xnt | |||||
| GstD2 | X | X | X | X | X | |||||
| GstD1 | X | X | X | Xa |
X GeneChip data (this study); Q q-PCR analysis (this study); N Northern analysis (this study); L Northern analysis (Landis et al 2004 PNAS 101:7663-8); nt not tested.
fold increase >1.2
Gene expression changes unique to each stress
Each stress had gene expression changes that were unique to that stress (listed in Supplemental Table S1) and the enriched GO terms that uniquely characterize each stress are summarized (Table 3). Hyperoxia stress had no enriched GO terms in the uniquely up-regulated genes, and a single enriched GO term, Signal peptide processing (3 genes) among the down-regulated genes. In contrast, there were numerous up-regulated genes unique to hydrogen peroxide stress, and these up-regulated genes were enriched for many GO terms involved in developmental pathways, signaling pathways, and nucleobase metabolism (Table 3). Genes uniquely up-regulated upon heat stress included many of the Hsp60-class, and this list was consequently enriched for the GO term Protein folding (16 genes), whereas down-regulated genes unique to heat stress were enriched for the GO terms Defense response and Melanization defense response (Table 3). Finally, genes uniquely up-regulated in response to ionizing radiation included several proteasome subunit genes (Supplemental Table S1), and this gene list was enriched for the GO terms Protein catabolic process and Macromolecular catabolic process (Table 3), whereas there were no GO terms enriched among down-regulated genes.
Table 3. Features unique to each stress.
GO enrichment terms for genes uniquely up-regulated in hyperoxia None found.
-
GO Enrichment Terms for genes uniquely down-regulated in hyperoxia
GO:0006465 signal peptide processing(3) 0.014095 -
GO enrichment terms for genes uniquely up-regulated in hydrogen peroxide
GO:0032501 multicellular organismal process(153) 2.79E-09 GO:0007275 multicellular organismal development(125) 1.11E-08 GO:0065007 biological regulation(143) 4.03E-08 GO:0050794 regulation of cellular process(128) 7.31E-08 GO:0050789 regulation of biological process(134) 1.20E-07 GO:0009653 anatomical structure morphogenesis(84) 1.62E-07 GO:0048856 anatomical structure development(121) 1.45E-06 GO:0050793 regulation of developmental process(39) 4.68E-06 GO:0032502 developmental process(132) 5.08E-06 GO:0048468 cell development(63) 1.34E-05 GO:0048731 system development(41) 4.96E-05 GO:0009790 embryo development(41) 1.13E-04 GO:0040011 Locomotion(35) 0.001096 GO:0048699 generation of neurons(42) 0.001396 GO:0050896 response to stimulus(106) 0.001425 GO:0051239 regulation of multicellular organismal process(34) 0.001519 GO:0045595 regulation of cell differentiation(25) 0.001776 GO:0030182 neuron differentiation(39) 0.00189 GO:0023052 Signaling(79) 0.002054 GO:0007154 cell communication(80) 0.002305 GO:0048666 neuron development(35) 0.003766 GO:0007165 signal transduction(64) 0.00438 GO:0048513 organ development(62) 0.004542 GO:0022414 reproductive process(54) 0.005853 GO:0003002 Regionalization(33) 0.007295 GO:0022603 regulation of anatomical structure morphogenesis(21) 0.00904 GO:2000026 regulation of multicellular organismal development(26) 0.01046 GO:0009880 embryonic pattern specification(21) 0.012338 GO:0009887 organ morphogenesis(37) 0.013194 GO:0007350 blastoderm segmentation(20) 0.01346 GO:0003006 developmental process involved in reproduction(37) 0.018008 GO:0051093 negative regulation of developmental process(16) 0.018841 GO:0010556 regulation of macromolecule biosynthetic process(49) 0.019198 GO:2000112 regulation of cellular macromolecule biosynthetic process(49) 0.019198 GO:0030154 cell differentiation(86) 0.019291 GO:0048869 cellular developmental process(89) 0.022952 GO:0048667 cell morphogenesis involved in neuron differentiation(29) 0.022954 GO:0007423 sensory organ development(31) 0.023396 GO:0051674 localization of cell(21) 0.024068 GO:0019219 regulation of nucleobase-containing compound metabolic process(51) 0.027513 GO:0048609 multicellular organismal reproductive process(45) 0.029739 GO:0007155 cell adhesion(19) 0.029741 GO:0030030 cell projection organization(33) 0.030221 GO:0003008 system process(43) 0.030877 GO:0051171 regulation of nitrogen compound metabolic process(51) 0.031374 GO:0007389 pattern specification process(33) 0.031638 GO:0048477 Oogenesis(33) 0.034694 GO:0048732 gland development(19) 0.034716 GO:0031326 regulation of cellular biosynthetic process(50) 0.036899 GO:0010468 regulation of gene expression(55) 0.037944 GO:0009889 regulation of biosynthetic process(50) 0.038122 GO:0000003 Reproduction(54) 0.043565 GO:0048870 cell motility(20) 0.048342 GO:0007292 female gamete generation(33) 0.049805 GO enrichment terms for genes uniquely down-regulated in hydrogen peroxide None found.
-
GO enrichment terms for genes uniquely upr-regulated in heat stress
GO:0006457 protein folding(16) 0.018356 -
GO enrichment terms for genes uniquely down-regulated in heat stress
GO:0006582 melanin metabolic process(7) 0.001782 GO:0035006 melanization defense response(6) 0.006478 GO:0006952 defense response(20) 0.00679 -
GO Enrichment terms for genes uniquely up-regulated in Ionizing radiation
GO:0030163 protein catabolic process(16) 8.21E-05 GO:0009057 macromolecule catabolic process(16) 0.049169 GO enrichment terms for genes uniquely down-regulated in Ionizing radiation None found.
Aging is most similar to hyperoxia
As described above, a core set of stress response genes was induced during aging and by each of the stressors tested. Aging shared additional changes in gene expression with each individual stressor (Supplemental Table S2), and was found to be more similar to the stresses most associated with oxidative stress (hyperoxia, hydrogen peroxide, ionizing radiation) than it was to heat stress, based on cluster analysis (Supplemental Figure S2) and by comparison of the GO categories that were enriched in the groups of up-regulated and down-regulated genes (Supplemental Table S3). While aging shared a significant overlap in up-regulated and down-regulated genes with each of the stresses, aging shared the greatest number of gene expression changes with hyperoxia (Table 4).
Table 4. Number of gene expression changes shared by aging and individual stresses.
| Aging change | Number genes | Stress change | Number genes | Number in common | p |
|---|---|---|---|---|---|
| Aging up | 456 | Hyperoxia up | 335 | 165 | 9.8 × 10−170 |
| Aging up | 456 | Ionizing radiation up | 716 | 171 | 2.9 × 10−114 |
| Aging up | 456 | Hydrogen peroxide up | 728 | 133 | 4.3 × 10−70 |
| Aging up | 456 | Heat stress up | 754 | 66 | 1.2 × 10−15 |
| Aging down | 1009 | Hyperoxia down | 556 | 222 | 1.5 × 10−121 |
| Aging down | 1009 | Ionizing radiation down | 674 | 166 | 3.6 × 10−54 |
| Aging down | 1009 | Hydrogen peroxide down | 911 | 132 | 1.1 × 10−18 |
| Aging down | 1009 | Heast stress down | 806 | 89 | 7.3 × 10−7 |
Gene expression changes unique to aging
A number of gene expression changes were found to be unique to aging. These included up-regulation of numerous innate immune response genes, and down-regulation of numerous mitochondrial metabolism genes, including ones encoding components of the ETC (Supplemental Table S1; enriched GO terms listed in Table 4). While up-regulation of innate immune response genes is a feature of aging that is shared with hyperoxia [6] (Supplemental Table S3), the number of up-regulated innate immune response genes was significantly greater for aging, resulting in many changes in this category that were unique to aging. Also uniquely up-regulated during aging were the odorant receptor genes Obp56a and Obp57d.
Down-regulation of mitochondrial genes is a feature of aging that is shared with hydrogen peroxide and ionizing radiation (Supplemental Table S3), but the number of down-regulated mitochondrial genes was greater for aging, resulting in many changes in this category that were unique to aging. Among these many down-regulated mitochondrial genes were ones encoding mitochondrial ribosomal proteins and components of the mitochondrial membrane protein translocases (TIM and TOM), as well as the mitochondrial form of superoxide dismutase (MnSOD or Sod2).
Confirmation of selected gene expression changes
Changes in gene expression caused by one or more stressors were confirmed by quantitative real-time PCR (Figure 1 and Supplemental Figure S3) and by Northern blot analysis (Supplemental Figure S1), and in general an excellent concordance was observed with the micro-array data and with the published literature (Summarized in Table 2). One exception was for expression of the innate immune response gene Drosomycin upon heat stress, which was observed to increase in the micro-array analysis, but to decrease in the qPCR analysis (Table 2). Because bacterial load and Drosomycin gene expression can vary significantly between different flies and vials of flies [13], we conclude that this discrepancy was most likely due to a small difference in bacterial load and Drosomycin gene expression in the control flies used for the qPCR analysis relative to the control flies used for micro-array analysis.
Comparison of the responses to the different stresses reveals preferential induction of certain genes. For example, Hsp70 (Figure 1) and Hsp22 (Supplemental Figure S3) were induced to the greatest extent by heat stress, whereas l(2)efl (Figure 1) and ade3 (Supplemental Figure S3) were induced to a greater extent by ionizing radiation and hyperoxia. In addition significant sexual dimorphism in the magnitude of responses was observed. For example, the induction of Hsp70 (Figure 1) and Hsp22 (Supplemental Figure S3) in response to heat stress was greater in males than in females, and the induction of l(2)efl (Figure 1) and ade3 (Supplemental Figure S3) in response to ionizing radiation was greater in males than in females.
A GstD-GFP reporter construct recapitulates induction during aging
TheGstD1 gene encodes a glutathione-S-transferase, and is induced in adult flies during normal aging and when flies are challenged with oxidative stress produced by hyperoxia and paraquat [6, 7, 14], and was also found to be up-regulated in response to hydrogen peroxide and ionizing radiation stress (Summarized in Table 2). The GstD1 promoter region contains consensus binding motifs for the stress-responsive transcription factors Nrf2 and Foxo (diagrammed in Supplemental Figure S4). A transgenic reporter has been characterized where the regulatory sequences of the GstD1 gene are fused to GFP, and the resulting GstD-GFP reporter is induced in the adult fly by feeding flies with the oxidative stressors paraquat, arsenic or hydrogen peroxide [15]. A clustered point mutation was created to disrupt the antioxidant response element (ARE) in the GstD-GFP reporter to yield a mutant reporter called GstD-deltaARE-GFP (diagrammed in Supplemental Figure S4). These reporters have been used to demonstrate that the GstD-GFP transgene is positively regulated in the adult fly in response to genetically-altered Nrf2 expression, and in response to the cancer chemotherapeutic compound Oltipraz which is known to activate Nrf2, and these regulations required the intact ARE [15]. Quantitative PCR analysis of adult flies indicated that induction of the GstD1 gene by paraquat is reduced in flies hemizygous for the JNKK gene hemipterous, suggesting additional positive regulation of GstD genes by the JNK pathway in response to oxidative stress [16]. The JNK pathway activates the transcription factor Foxo suggesting that the JNK pathway may activate GstD gene expression through the Foxo binding motif located in the GstD gene promoter region [17](diagrammed in Supplemental Figure S4). A GstD1-LacZ reporter has been reported to be up-regulated during normal aging in the enteroendocrine cells (ECs) of the fly intestine, but to be reduced during aging in the intestinal stem cells (ISCs) [18].
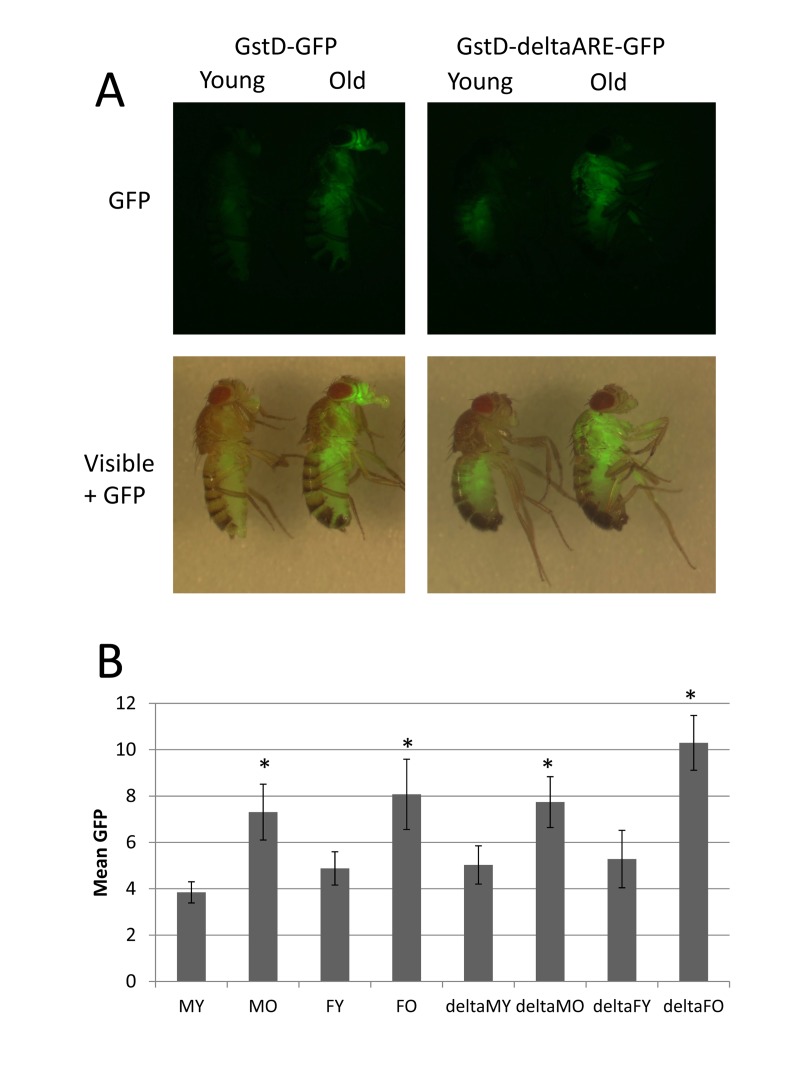
Here the expression of the GstD-GFP and GstD-deltaARE-GFP reporters were examined in whole adult flies during normal aging. The GstD-GFP reporter was expressed at low levels in young flies, and exhibited robust induction throughout the body of the fly during aging, including the head, thorax, abdomen and legs (Figure 2A), consistent with the whole-body micro-array analyses presented above. Induction was apparent even at moderate ages (30 days; Figure 2A) and continued at high levels for the remainder of the life span (data not shown). In contrast, the GstD-deltaARE-GFP reporter was robustly induced in thorax and legs, particularly in flight muscle and leg muscle, whereas induction in the head and abdomen was either greatly reduced or absent. The GstD-deltaARE-GFP reporter was also observed to produce slightly more expression in young flies in the upper abdomen. The mean GFP intensity throughout the body was quantified from captured images of multiple flies using Image J software, and this analysis confirmed the up-regulation of both reporters during aging, in both males and females (Figure 2B). Despite the absence of GFP induction in head and abdomen tissue, the mean intensity of fluorescence produced by the GstD-deltaARE-GFP reporter throughout the fly was comparable to the un-mutated reporter (Figure 2B), as the expression in thorax and legs was relatively greater (see Figure 2A); and this difference may be due to some effect of the different chromosomal insertion sites on the overall expression levels for the reporters. Taken together, these data confirm the up-regulation of the GstD1 gene during aging in the majority of adult tissues, and suggest that efficient expression in the head and abdomen may require the consensus ARE motif (Diagrammed in Supplemental Figure S4).
Figure 2. GstD-GFP transgenic reporters recapitulate GstD1 gene induction during aging.
(A) Expression of the transgenic reporter constructs GstD-GFP and GstD-deltaARE-GFP was visualized in 6 day old (Young) and 30 day old (Old) male and female flies, as indicated, using the fluorescence stereomicroscope. The GFP image and an overlay of the GFP image and the visible light image are presented, as indicated. (B) Quantification of the expression of the GstD-GFP and GstD-deltaARE-GFP reporters in male (M) and female (F) flies, both young (Y) and old (O), as indicated. The data for the Gst-deltaARE-GFP reporter is specified by prefix (delta). Values for old flies were compared to young using unpaired, two-sided t-tests, and statistically significant differences (p < 0.05) are indicated with asterisk.
DISCUSSION
A core set of stress response genes shared with aging
Changes in gene expression that were common to aging and all tested stresses identified a core set of stress response genes. Pepck encodes an enzyme critical in gluconeogenesis and glyceroneogenesis, and its up-regulation may be part of a basic metabolic adaptation to stress [19]. Interestingly, in mice, over-expression of PEPCK specifically in muscle tissue increases movement, life span and muscle mitochondrial proliferation [20]. Also among the core set of induced genes were Starvin, ref(2)P, and the Hsp genes Hsp70, Hsp83 and l[2]efl. Starvin encodes a co-chaperone involved in autophagy and muscle maintenance and its up-regulation is consistent with its role in protein turnover and the cellular response to proteotoxicity [21]. Similarly, ref(2)P encodes a component of the mitochondrial unfolded protein response (mUPR) pathway, consistent with proteotoxicity in the mito-chondrial compartment. Hsp genes are induced in response to protein denaturation and misfolding through activation of the HSF transcription factor, which in turn binds to HSEs in the Hsp gene promoters and activates transcription [1, 2]. In stressed cells certain Hsps have been shown to function to reduce proteotoxicity by favoring protein re-folding as well as the turnover of damaged proteins through the ubiquitin/proteasome and autophagy pathways [2, 22]. Induction of Drosophila Hsp genes during normal aging and upon oxidative stress has been shown to be dependent upon functional HSEs in the gene promoters, consistent with an increased abundance of misfolded proteins and conse-quent HSF activation under these conditions [4, 5]. The presence of Hsp genes in the set up-regulated by aging and each stressor indicates that protein denaturation/misfolding and HSF activation are common features of aging and each of the tested stresses. Induction of Hsps during aging may be part of a stress response that favors fly function by helping the fly to cope with aging-associated proteotoxicity [3]. Consistent with this idea, increased expression of certain Hsps is associated with increased fly life span [23-25]. In addition, it is also possible that chronic Hsp induction, particularly at late ages, may sometimes be mal-adaptive [2]. Interestingly, 8/18 of the common up-regulated genes and 21/32 of the common down-regulated genes have uncharacterized functions, indicating that there is much yet to be learned about the core stress response.
Table 5. Features unique to aging.
-
GO enrichment terms for genes uniquely up-regulated in aging
GO:0006952 defense response(24) 1.04E-10 GO:0042742 defense response to bacterium(17) 2.52E-09 GO:0009617 response to bacterium(17) 2.61E-08 GO:0050830 defense response to Gram-positive bacterium(11) 1.35E-07 GO:0051707 response to other organism(19) 1.29E-06 GO:0009607 response to biotic stimulus(19) 1.55E-06 GO:0006955 immune response(18) 4.36E-06 GO:0002376 immune system process(20) 1.56E-05 GO:0051704 multi-organism process(21) 7.84E-04 GO:0019731 antibacterial humoral response(8) 0.002999 GO:0009620 response to fungus(7) 0.010042 GO:0006959 humoral immune response(11) 0.015651 GO:0019730 antimicrobial humoral response(10) 0.039404 -
GO enrichment terms for genes uniquely down-regulated in aging
GO:0006091 generation of precursor metabolites and energy(53) 6.12E-32 GO:0045333 cellular respiration(44) 3.06E-29 GO:0015980 energy derivation by oxidation of organic compounds(44)) 2.36E-27 GO:0042773 ATP synthesis coupled electron transport(29) 9.71E-20 GO:0006119 oxidative phosphorylation(30) 1.06E-19 GO:0022900 electron transport chain(30) 1.09E-18 GO:0022904 respiratory electron transport chain(29) 1.88E-18 GO:0042775 mitochondrial ATP synthesis coupled electron transport(27) 4.70E-18 GO:0055114 oxidation-reduction process(62) 8.74E-10 GO:0006084 acetyl-CoA metabolic process(17) 1.56E-09 GO:0006099 tricarboxylic acid cycle(16) 6.58E-09 GO:0046356 acetyl-CoA catabolic process(16) 6.58E-09 GO:0009109 coenzyme catabolic process(16) 1.02E-08 GO:0009060 aerobic respiration(16) 1.57E-08 GO:0051187 cofactor catabolic process(16) 1.57E-08 GO:0006732 coenzyme metabolic process(21) 3.37E-07 GO:0006120 mitochondrial electron transport, NADH to ubiquinone(13) 7.68E-07 GO:0051186 cofactor metabolic process(22) 1.21E-06 GO:0016310 Phosphorylation(47) 1.84E-06 GO:0006793 phosphorus metabolic process(53) 7.47E-06 GO:0006796 phosphate-containing compound metabolic process(53) 7.47E-06 GO:0006839 mitochondrial transport(13) 3.31E-04 GO:0006123 mitochondrial electron transport, cytochrome c to oxygen(7) 0.002098 GO:0009056 catabolic process(46) 0.003113 GO:0044281 small molecule metabolic process(58) 0.003288 GO:0007005 mitochondrion organization(15) 0.003544 GO:0006626 protein targeting to mitochondrion(9) 0.005904 GO:0070585 protein localization in mitochondrion(9) 0.005904 GO:0072655 establishment of protein localization in mitochondrion(9) 0.005904 GO:0007283 Spermatogenesis(22) 0.007147 GO:0048232 male gamete generation(22) 0.007884 GO:0043648 dicarboxylic acid metabolic process(7) 0.010686 GO:0006096 Glycolysis(8) 0.02534 GO:0006122 mitochondrial electron transport, ubiquinol to cytochrome c(6) 0.026633 GO:0044248 cellular catabolic process(36) 0.032559 GO:0006006 glucose metabolic process(10) 0.042632
Each stress has unique gene expression changes
While the pattern of gene expression changes during aging shared features with each of the tested stresses, each stress also had unique features (Summarized in Table 3). For example, hydrogen peroxide stress caused up-regulation of numerous genes involved in developmental pathways and signaling pathways, consistent with the fact that hydrogen peroxide also normally functions as a signaling molecule, during development and in adults, in Drosophila and other metazoans [26-30]. Genes uniquely up-regulated upon heat stress included ones of the Hsp60-class, which are important for protein trafficking and protein import into organelles [31]. Down-regulated genes unique to heat stress were enriched for the GO terms Defense response and Melanization defense response, suggesting that responses to wounding and bacterial challenge may be impaired. Finally, genes uniquely up-regulated in response to ionizing radiation included several proteasome subunit genes, which may indicate a particular requirement for protein turnover, perhaps in response to protein backbone cleavage, or alternatively this might reflect the critical role of the proteasome in DNA repair [32].
Aging has both shared and unique features relative to the tested stresses
While aging shared features with each stress, aging was found to be more similar to the stresses most associated with oxidative stress (hyperoxia, hydrogen peroxide, ionizing radiation) than it was to heat stress. These observations are consistent with the conclusion that aging eukaryotic cells are in a pro-oxidant state [6, 33] associated with up-regulation of oxidative stress-response genes including ones encoding Gsts [34]. In addition to the shared features, a number of gene expression changes were found to be unique to aging (Supplemental Table S1). For example, the gene encoding the mitochondrial form of superoxide dismutase (MnSOD or SOD2) was uniquely down-regulated during aging, and this is of potential interest given the fact that augmenting the expression ofMnSOD can favor life span in adult flies [35, 36] and in C. elegans[37]. Also uniquely up-regulated during aging were the odorant receptor genes Obp56a and Obp57d, which is interesting in light of reports of negative effects of other odorant receptor genes on fly life span [38].
Table 6. Features common to aging and individual stress factors.
-
GO enrichment terms for genes up-regulated in aging and in hyperoxia
GO:0009408 response to heat(16) 2.64E-11 GO:0009266 response to temperature stimulus(17) 1.15E-10 GO:0006950 response to stress(34) 9.95E-08 GO:0035079 polytene chromosome puffing(5) 3.88E-05 GO:0035080 heat shock-mediated polytene chromosome puffing(5) 3.88E-05 GO:0009628 response to abiotic stimulus(17) 8.38E-05 GO:0044271 cellular nitrogen compound biosynthetic process(14) 0.00202 GO:0034605 cellular response to heat(6) 0.002657 GO:0019731 antibacterial humoral response(7) 0.009046 GO:0009156 ribonucleoside monophosphate biosynthetic process(5) 0.032356 GO:0009161 ribonucleoside monophosphate metabolic process(5) 0.032356 GO:0006564 L-serine biosynthetic process(3) 0.04487 -
GO enrichment terms for genes down-regulated in aging and in hyperoxia
GO:0006508 Proteolysis(56) 6.01E-18 GO:0045297 post-mating behavior(7) 9.33E-04 GO:0008152 metabolic process(131) 0.00614 -
GO enrichment terms for genes up-reguated in aging and in hydrogen peroxide
GO:0009408 response to heat(11) 4.75E-06 GO:0035079 polytene chromosome puffing(5) 1.44E-05 GO:0035080 heat shock-mediated polytene chromosome puffing(6) 1.44E-05 GO:0006950 response to stress(27) 2.86E-05 GO:0009266 response to temperature stimulus(11) 7.87E-05 GO:0034605 cellular response to heat(6) 8.32E-04 GO:0044271 cellular nitrogen compound biosynthetic process(13) 0.001277 GO:0009069 serine family amino acid metabolic process(5) 0.005345 GO:0044281 small molecule metabolic process(24) 0.005367 GO:0006564 L-serine biosynthetic process(3) 0.024923 GO:0051707 response to other organism(11) 0.032029 GO:0009607 response to biotic stimulus(11) 0.035404 -
GO enrichment terms for genes down-reguated in aging and in hydrogen peroxide
GO:0006091 generation of precursor metabolites and energy(12) 0.001493 GO:0022900 electron transport chain(9) 0.001518 GO:0055114 oxidation-reduction process(22) 0.002986 GO:0045333 cellular respiration(10) 0.003694 GO:0015980 energy derivation by oxidation of organic compounds(10) 0.008153 GO:0022904 respiratory electron transport chain(8) 0.010626 GO:0042775 mitochondrial ATP synthesis coupled electron transport(7) 0.043253 -
GO enrichment terms for genes up-regulated in aging and in heat stress
GO:0009408 response to heat(18) 1.04E-21 GO:0009266 response to temperature stimulus(18) 1.68E-19 GO:0009628 response to abiotic stimulus(18) 8.84E-13 GO:0006950 response to stress(22) 1.70E-08 GO:0006457 protein folding(11) 1.90E-07 GO:0035079 polytene chromosome puffing(5) 4.29E-07 GO:0035080 heat shock-mediated polytene chromosome puffing(5) 4.29E-07 GO:0034605 cellular response to heat(6) 1.27E-05 GO:0001666 response to hypoxia(6) 0.001193 GO:0070482 response to oxygen levels(6) 0.002157 GO:0042221 response to chemical stimulus(14) 0.031137 -
GO enrichment terms for genes down-regulated in aging and in heat stress
GO:0006508 Proteolysis(25) 1.75E-07 -
GO enrichment terms for genes up-regulated in aging and Ionizing radiation
GO:0006950 response to stress(33) 2.17E-06 GO:0009408 response to heat(12) 5.07E-06 GO:0035079 polytene chromosome puffing(5) 5.04E-05 GO:0035080 heat shock-mediated polytene chromosome puffing(5) 5.04E-05 GO:0009266 response to temperature stimulus(12) 1.05E-04 GO:0034605 cellular response to heat(6) 0.003611 GO:0009069 serine family amino acid metabolic process(5) 0.018171 GO:0044271 cellular nitrogen compound biosynthetic process(13) 0.022327 GO:0033554 cellular response to stress(18) 0.024714 -
GO enrichment terms for genes down-regulated in aging and Ionizing radiation
GO:0006091 generation of precursor metabolites and energy(17) 2.30E-07 GO:0045333 cellular respiration(14) 1.95E-06 GO:0015980 energy derivation by oxidation of organic compounds(14) 6.16E-06 GO:0022900 electron transport chain(11) 4.47E-05 GO:0055114 oxidation-reduction process(26) 2.65E-04 GO:0006119 oxidative phosphorylation(10) 2.91E-04 GO:0022904 respiratory electron transport chain(10) 2.91E-04 GO:0042775 mitochondrial ATP synthesis coupled electron transport(9) 9.52E-04 GO:0042773 ATP synthesis coupled electron transport(9) 0.00164
The aging gene expression pattern indicates a failure in mitochondrial maintenance
The changes in gene expression that were found to be unique to aging included up-regulation of numerous innate immune response genes, and down-regulation of numerous mitochondrial metabolism genes, including ones encoding components of the ETC (Table 4). While up-regulation of innate immune response genes is a feature of aging that is shared with hyperoxia [6] (Supplemental Table S3), the number of up-regulated innate immune response genes was significantly greater for aging, resulting in many changes in this category that were unique to aging. Similarly, down-regulation of mitochondrial and ETC genes is a feature of aging that is shared with ionizing radiation and hydrogen peroxide stress (Supplemental Table S3), but the number of down-regulated mitochondrial and ETC genes was greater for aging, resulting in many changes in this category that were unique to aging. Girardot et al [39] examined gene expression changes during Drosophila aging separately for the head, thorax and abdomen, and found that down-regulation of mitochondrial genes is observed preferentially in the thorax; because the thorax is composed primarily of flight muscle this observation suggests that mitochondrial gene down-regulation may occur preferentially in muscle tissue.
Up-regulation of innate immune response genes during Drosophila aging is in part due to a dramatic increase in microbial load during aging, as eliminating bacteria reduces the response [13]. However, innate immune response genes are still up-regulated during aging in the absence of detectable microbes, suggesting additional mechanisms for activation of these genes during aging. Consistent with this conclusion, innate immune response genes are also up-regulated in response to oxidative stress caused by hyperoxia ([6]; this study), and therefore one possibility is that an aging-related failure in mitochondrial maintenance leads to oxidative stress that can induce innate immune response gene expression. Similarly, studies in mammals reveal that damaged mitochondria also release DNA fragments and formyl-peptides that can induce innate immune response genes [40], and therefore this may be an additional mechanism for innate immune response gene induction during aging that is a consequence of a failure in mitochondrial maintenance. The across-the-board down-regulation of Drosophila mitochondrial genes, ETC genes and mitochondrial metabolism genes observed during aging suggests a possible mechanism for a failure in mitochondrial maintenance during aging (Diagrammed in Supplemental Figure S5). The ETC and mitochondria turn over at a basal rate, and more rapidly in response to signals such as starvation, and a reduced rate of replacement is expected to result in longer-lived structures that will be more susceptible to time-dependent damage and malfunction. This idea is consistent with the observed accumulation of structural-ly abnormal mitochondria during Drosophila aging [41-44], reduced mitochondrial transcription [45], decreased ATP and increased production of ROS [46]. Decreased ATP flux is expected to reduce rates of bulk protein synthesis and turnover, and increased ROS will increase protein damage, consistent with the accumulation of damaged and misfolded proteins (proteotoxicity) and the induction of Hsp genes [2, 22, 24].
Taken together, the data support a model wherein the down-regulation of mitochondrial and ETC genes during aging leads to a failure in mitochondrial maintenance and the accumulation of abnormal mitochondria, which in turns leads to oxidative stress and proteotoxicity; these stresses in turn cause the oxidative-stress-like and proteotoxic-stress-like patterns of gene expression observed during aging (Supplemental Figure S5). Placing oxidative stress down-stream of an aging-associated failure in mitochondrial maintenance is consistent with the observation that oxidative stress correlates with, but does not directly regulate life span in Drosophila [47], and with the implication of mitochondrial malfunction in mammalian aging-related metabolic disorders [48]. Consistent with the importance of mitochondrial maintenance in aging, certain interventions that increase mitochondrial proliferation, such as over-expression of PGC1alpha in gut tissue, have recently been reported to increase life span and tissue function in aging Drosophila [49, 50], and PGC1alpha activity is also implicated in maintaining tissue function during aging in mammals [51]. In contrast, other manipulations that increase Drosophila mitochondrial proliferation, such as increased tissue-general expression of PGC1alpha [50] or cyclin D/Cdk4 [52] had negative consequences for life span and oxidative stress levels, and taken together these studies indicate that effective interventions in mitochondrial maintenance during aging will require tissue-specific targeting. Notably, certain carefully-timed interventions that reduce activity of ETC components have been shown to increase life span in both invertebrates and mammals [53-55], and this might function through a hormetic response to increase production of new mitochondria, or conceivably by inhibiting the activity of abnormal mitochondria. Critical questions for the future include determining the causes and mechanisms for the observed down-regulation of mitochondrial and ETC genes during aging - a pattern shared by Drosophila and mammalian tissues [6, 9]. Possible explanations include the inherently shorter-lived nature of mitochondrial genome sequences relative to nuclear genome sequences, genetic conflicts resulting from the uni-parental inheritance of mitochondrial genomes, and trade-offs between the costly production of new mitochondria and investments in growth, sexual differentiation and reproduction [56-61](Supplemental Figure S5), and these will be interesting areas for future research.
METHODS
Drosophila culture, microscopy and stress treatments
Drosophila melanogaster flies were cultured on a standard agar/dextrose/corn meal/yeast media at 25°C [62]. The transgenic strains GstD1-GFP and GstD1-deltaARE-GFP were generously provided by Dirk Bohmann [15]. Age-synchronized cohorts of flies were generated by collecting newly-eclosed flies over a period of 48 hours, followed by maintenance in vials at approximately 20 flies per vial, with every-other day transfer to fresh media, until the indicated age time points. Visible images, GFP fluorescence images, and image overlays for flies were generated using the Leica MZFLIII fluorescence stereomicroscope. GFP fluore-scence was quantified using captured GFP images and Image J software, with mean and standard deviation calculated using 6 flies per sample. Flies used for stress treatments, RNA analyses and microarray analyses were generated as follows: males of wild-type strain Oregon-R were crossed to virgins of transgenic laboratory stock w[1118];rtTA(3)E2/TM3 Sb to generate hybrid progeny of genotype w[1118];rtTA(3)E2/+, as was used for previous microarray analyses [63], and 9-10 day-old male flies were used for each stress treatment. Old flies were 61 days of age, which corresponds to approximately the 50% survival point for the cohort [6]. Vials containing 1% sucrose were prepared by adding 1.5 ml of 1% sucrose in deionized water to a Drosophila vial containing a single folded Kimwipe (Kimberly-Clark). For each stress treatment and the sugar-treated controls, replicate vials of 25 flies each were subjected to the treatment, and then the flies from each vial were separately processed for RNA, and each sample was used to generate probe for one micro-array hybridization, such that each treatment is represented by at least three biological replicates. For hyperoxia treatment flies in standard food vials were subjected to 100% oxygen atmosphere for 5 days as previously described [6]. For ionizing radiation treatment flies in standard food vials were irradiated with 5666Rads/hour for 16 hours using a Cesium source (Grammacell 40-Cesium 137, Atomic Energy, Canada) at the USC Norris Cancer Center facility, and then transferred to 1% sucrose vials for two days followed by processing for RNA. For qPCR analysis 9 hour irradiation samples were also generated. Because ionizing radiation is inhibitory to transcription, the two-day recovery period was included to allow the gene expression response to develop; recovery in sucrose vials was employed because the newly-irradiated flies have greatly reduced mobility and will adhere to the surface of a regular food vial. For hydrogen peroxide treatment flies were placed in sucrose vials adjusted to 3% hydrogen peroxide for two days, and then processed for RNA. For heat stress treatment flies were placed in sucrose vials at 37oC for 5.5 hours and then processed for RNA. Controls for the effects of sucrose vials (“sugar-treated controls”) were generated by placing flies in sucrose vials for two days prior to processing for RNA.
RNA isolation and microarray hybridization
An average of 35 μg RNA was isolated from groups of 25 adult male flies using Trizol reagent (Life Technologies, Grand Island) according to the manufacturer's instructions. The RNA was further purified using the RNAqueous kit, and concentration was determined using NanoDrop spectrophotometer. A portion of the RNA (3 μg) was fractionated on 1.0% agarose gels to determine purity. 10 μg of total RNA was then used as substrate to generate biotinylated cRNA according to standard Affymetrix protocol (Childrens Hospital, Los Angeles, CA)[6]. A total of 35 Affymetrix gene chips were analyzed including at least four biological replicates for each experimental condition and control, with the exception of heat stress in which one array was omitted due to poor quality. The old, hyperoxia, and young samples were derived from our previous study [6] in which six arrays were used for the hyperoxia and young conditions and four arrays were used for old flies. Quantitative real-time RT-PCR analyses [64] and Northern blot analyses [65] were performed as previously described, using RNA samples derived independently from those used for the microarrays.
Statistical analysis of microarray data
Gene expres-sion measures were computed based on a non-linear multi-chip model of the perfect match signal [66]. This approach enables the separation of specific and non-specific components of the microarray signal and circumvents the issue of saturation bias in the high-intensity range. The background and concentration parameters were both fit within a single global routine (rather than estimating the background parameter before computing gene expression measures), and the model that best described the observed data selected. Linear modeling and empirical Bayes analysis [67] was performed in the R statistical programming language (http://www.r-project.org/) using the Limma: Linear Models for Microarray Data package [67] to identify genes significantly differentially expressed in response to multiple stressors or during aging; Limma computes an empirical Bayes adjustment for the t-test. Because the identification of genes altered in multiple condiions was a major objective of this study, a nested F-test approach was employed as this can be more powerful at detecting genes altered in multiple contrasts. Multiple testing was corrected for using the Benjamini and Hochberg method, which controls the false discovery rate (FDR) [68] in this framework on a per-gene basis (but not across contrasts). Using this robust method, genes were found to be significantly differentially expressed both by biological and statistical criteria (±1.2 fold change, FDR 1% (p < 0.01); (Supplemental Table S4a, Supplemental Table S4b, Supplemental Table S4c, Supplemental Table S4d, Supplemental Table S4e, Supplemental Table S4f, Supplemental Table S4g, Supplemental Table S4h, Supplemental Table S4i, Supplemental Table S4j, Supplemental Table S4k, Supplemental Table S4l). Gene expression changes of ±1.5 fold were used for subsequent comparisons, as indicated. Hierarchical cluster analysis of the top 1000 differentially expressed genes for each condition based on the F-test p-value from the linear model fit was performed to visualize the gene expression patterns across different stressors, using the R package mclust. The microarray data discussed in this study have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus (GEO) [69] and are accessible through GEO Series numberGSEXXX.
Functional annotation and statistical overrepresentation of Gene Ontology classifications
Statistically over-represented GO categories were identified using Flymine [70], by the calculation of a p-value denoting the probability that the observed numbers of counts could have resulted from randomly distributing a particular GO term between the test and the reference group. Multiple testing was controlled for using the Holm-Bonferoni method.
Statistical significance of overlapping gene sets
The statistical significance of the overlap between various gene sets was evaluated by computing the p-value representing the probability of obtaining the observed number of overlaps by chance under a hypergeometric distribution, using the R function phyper [71].
Identification of enriched GO terms and corrections for effects of sucrose vials
Gene annotations for the AffyDrosGenome1 arrays were updated to the latest information from Flybase using the online tool Flymine [70] for all genes with expression altered ≥1.5 fold. Gene annotations identified by Flymine as matching more than one entry in the current database were resolved where possible, as follows: The Affymetrix probe ID was obtained from the limma files, and the corresponding probe sequence was obtained from the Affymetrix website. The probe sequence was then used to query the current Drosophila genome sequence annotation using the Flybase website and BLAST function to identify the correct gene. Ten probe sequences had ambiguous match that could not be resolved and were not included in the GO term analyses (Supplemental Table S5), and an additional 27 identifiers did not match genes in the current database. To control for any possible effects on gene expression patterns caused by two days maintenance of flies in sucrose vials, GeneChip analysis was performed on flies transferred to sucrose vials for two days in the absence of added stressors as a control. 258 genes were found to be up-regulated and 362 genes were found to be down-regulated relative to controls maintained on normal media (Supplemental Table S6), and these gene sets had no GO terms enriched among the up-regulated genes, and 4 GO terms enriched among the down-regulated genes: Proteolysis, Post-mating behavior, Insemination, and Lipid metabolic process (Supplemental Table S7). The genes that were up-regulated and down-regulated in response to sucrose were subtracted from the lists of genes up-regulated and down-regulated in response to hydrogen peroxide and ionizing radiation treatment to generate the final lists presented in Tables 1, 3, 4, and Supplemental Table S1, Supplemental Tables S2, Supplemental Table S3. While this simple subtraction procedure does not account for possible gene expression changes caused by interactions of sucrose with the stressors, we observe that the major GO term categories enriched in the gene sets up-regulated and down-regulated by hydrogen peroxide and ionizing radiation do not differ significantlywhen the gene expression changes caused by sucrose alone are included or excluded from the analysis (compare Supplemental Table S8 and Supplemental Table S9 where the effects of sucrose are included, to Supplemental Table S10, Supplemental Tables S11 where the effects of sucrose are excluded). In addition, cluster analysis demonstrated that the gene expression changes caused by the hydrogen peroxide stress treatment and ionizing radiation stress treatment were more similar to each other and to aging and hyperoxia than they were to the sucrose-treated control flies (Supplemental Figure S3), providing further evidence that the gene expression changes due to sucrose transfer do not make a significant contribution to the gene expression changes observed in the hydrogen peroxide and ionizing radiation samples.
SUPPLEMENTARY MATERIAL
Acknowledgments
We thank Christina Curtis and Simon Tavaré for assistance with statistical analyses. This research was supported by a grant from the Department of Health and Human Services to JT (AG011833).
Footnotes
The authors of this manuscript have no conflict of interests to declare.
REFERENCES
- Voellmy R, Boellmann F. Chaperone regulation of the heat shock protein response. Adv Exp Med Biol. 2007;594:89–99. doi: 10.1007/978-0-387-39975-1_9. [DOI] [PubMed] [Google Scholar]
- Tower J. Hsps and aging. Trends Endocrinol Metab. 2009;20:216–222. doi: 10.1016/j.tem.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler JC, Bieschke ET, Tower J. Muscle-specific expression of Drosophila hsp70 in response to aging and oxidative stress. Proc Natl Acad Sci U S A. 1995;92:10408–10412. doi: 10.1073/pnas.92.22.10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler JC, King V, Tower J. Sequence requirements for upregulated expression of Drosophila hsp70 transgenes during aging. Neurobiol Aging. 1999;20:545–553. doi: 10.1016/s0197-4580(99)00088-3. [DOI] [PubMed] [Google Scholar]
- King V, Tower J. Aging-specific expression of Drosophila hsp22. Dev Biol. 1999;207:107–118. doi: 10.1006/dbio.1998.9147. [DOI] [PubMed] [Google Scholar]
- Landis GN, Abdueva D, Skvortsov D, Yang J, Rabin BE, Carrick J, Tavaré S, Tower J. Similar gene expression patterns characterize aging and oxidative stress in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2004;101:7663–7668. doi: 10.1073/pnas.0307605101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou S, Meadows S, Sharp L, Jan LY, Jan YN. Genome-wide study of aging and oxidative stress response in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2000;97:13726–13731. doi: 10.1073/pnas.260496697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletcher SD, Macdonald SJ, Marguerie R, Certa U, Stearns SC, Goldstein DB, Partridge L. Genome-wide transcript profiles in aging and calorically restricted Drosophila melanogaster. Curr Biol. 2002;12:712–723. doi: 10.1016/s0960-9822(02)00808-4. [DOI] [PubMed] [Google Scholar]
- Zahn JM, Sonu R, Vogel H, Crane E, Mazan-Mamczarz K, Rabkin R, Davis RW, Becker KG, Owen AB, Kim SK. Transcriptional profiling of aging in human muscle reveals a common aging signature. PLoS Genet. 2006;2:e115. doi: 10.1371/journal.pgen.0020115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarroll SA, Murphy CT, Zou S, Pletcher SD, Chin CS, Jan YN, Kenyon C, Bargmann CI, Li H. Comparing genomic expression patterns across species identifies shared transcriptional profile in aging. Nat Genet. 2004;36:197–204. doi: 10.1038/ng1291. [DOI] [PubMed] [Google Scholar]
- Yang J, Tower J. Expression of hsp22 and hsp70 transgenes is partially predictive of drosophila survival under normal and stress conditions. J Gerontol A Biol Sci Med Sci. 2009;64:828–838. doi: 10.1093/gerona/glp054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea SL, Wu D, Cypser JR, Vaupel JW, Johnson TE. A stress-sensitive reporter predicts longevity in isogenic populations of Caenorhabditis elegans. Nat Genet. 2005;37:894–898. doi: 10.1038/ng1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren C, Webster P, Finkel SE, Tower J. Increased internal and external bacterial load during Drosophila aging without life-span trade-off. Cell Metab. 2007;6:144–152. doi: 10.1016/j.cmet.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Ling D, Salvaterra PM. Robust RT-qPCR data normalization: validation and selection of internal reference genes during post-experimental data analysis. PLoS One. 2011;6:e17762. doi: 10.1371/journal.pone.0017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykiotis GP, Bohmann D. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev Cell. 2008;14:76–85. doi: 10.1016/j.devcel.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MC, Bohmann D, Jasper H. JNK Signaling Confers Tolerance to Oxidative Stress and Extends Lifespan in Drosophila. Dev Cell. 2003;5:811–816. doi: 10.1016/s1534-5807(03)00323-x. [DOI] [PubMed] [Google Scholar]
- Karpac J, Hull-Thompson J, Falleur M, Jasper H. JNK signaling in insulin-producing cells is required for adaptive responses to stress in Drosophila. Aging Cell. 2009;8:288–295. doi: 10.1111/j.1474-9726.2009.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochmuth CE, Biteau B, Bohmann D, Jasper H. Redox regulation by Keap1 and Nrf2 controls intestinal stem cell proliferation in Drosophila. Cell Stem Cell. 2011;8:188–199. doi: 10.1016/j.stem.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura T, Shimizu H, Nagao T, Ueda R, Ishii S. ATF-2 regulates fat metabolism in Drosophila. Mol Biol Cell. 2007;18:1519–1529. doi: 10.1091/mbc.E06-10-0909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson RW, Hakimi P. Born to run; the story of the PEPCK-Cmus mouse. Biochimie. 2008;90:838–842. doi: 10.1016/j.biochi.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt V, Dick N, Tawo R, Dreiseidler M, Wenzel D, Hesse M, Furst DO, Saftig P, Saint R, Fleischmann BK, Hoch M, Hohfeld J. Chaperone-assisted selective autophagy is essential for muscle maintenance. Curr Biol. 2010;20:143–148. doi: 10.1016/j.cub.2009.11.022. [DOI] [PubMed] [Google Scholar]
- Morimoto RI. Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes Dev. 2008;22:1427–1438. doi: 10.1101/gad.1657108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurapati R, Passananti HB, Rose MR, Tower J. Increased hsp22 RNA levels in Drosophila lines genetically selected for increased longevity. J Gerontol A Biol Sci Med Sci. 2000;55:B552–559. doi: 10.1093/gerona/55.11.b552. [DOI] [PubMed] [Google Scholar]
- Tower J. Heat shock proteins and Drosophila aging. Exp Gerontol. 2011;46:355–362. doi: 10.1016/j.exger.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HD, Kazemi-Esfarjani P, Benzer S. Multiple-stress analysis for isolation of Drosophila longevity genes. Proc Natl Acad Sci U S A. 2004;101:12610–12615. doi: 10.1073/pnas.0404648101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough DR, Cotter TG. Hydrogen peroxide: a Jekyll and Hyde signalling molecule. Cell death & disease. 2011;2:e213. doi: 10.1038/cddis.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ME. H2O2: a dynamic neuromodulator. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2011;17:389–406. doi: 10.1177/1073858411404531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Giordano S, Zhang J. Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem J. 2012;441:523–540. doi: 10.1042/BJ20111451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SG, Woo HA, Kil IS, Bae SH. Peroxiredoxin functions as a peroxidase and a regulator and sensor of local peroxides. J Biol Chem. 2012;287:4403–4410. doi: 10.1074/jbc.R111.283432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owusu-Ansah E, Banerjee U. Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature. 2009;461:537–541. doi: 10.1038/nature08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deocaris CC, Kaul SC, Wadhwa R. On the brotherhood of the mitochondrial chaperones mortalin and heat shock protein 60. Cell stress & chaperones. 2006;11:116–128. doi: 10.1379/CSC-144R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaekers CH, Wouters BG. Regulatory functions of ubiquitin in diverse DNA damage responses. Current molecular medicine. 2011;11:152–169. doi: 10.2174/156652411794859269. [DOI] [PubMed] [Google Scholar]
- Salmon AB, Richardson A, Perez VI. Update on the oxidative stress theory of aging: does oxidative stress play a role in aging or healthy aging? Free Radic Biol Med. 2010;48:642–655. doi: 10.1016/j.freeradbiomed.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Imai M, Muraki M, Miyado K, Qin J, Kyuwa S, Yoshikawa Y, Hosoi Y, Saito H, Takahashi Y. GSTT1 is upregulated by oxidative stress through p38-MK2 signaling pathway in human granulosa cells: possible association with mitochondrial activity. Aging. 2011;3:1213–1223. doi: 10.18632/aging.100418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Folk D, Bradley TJ, Tower J. Induced overexpression of mitochondrial Mn-superoxide dismutase extends the life span of adult Drosophila melanogaster. Genetics. 2002;161:661–672. doi: 10.1093/genetics/161.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis C, Landis GN, Folk D, Wehr NB, Hoe N, Waskar M, Abdueva D, Skvortsov D, Ford D, Luu A, Badrinath A, Levine RL, Bradley TJ, et al. Transcriptional profiling of MnSOD-mediated lifespan extension in Drosophila reveals a species-general network of aging and metabolic genes. Genome Biol. 2007;8:R262. doi: 10.1186/gb-2007-8-12-r262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabreiro F, Ackerman D, Doonan R, Araiz C, Back P, Papp D, Braeckman BP, Gems D. Increased life span from overexpression of superoxide dismutase in Caenorhabditis elegans is not caused by decreased oxidative damage. Free Radic Biol Med. 2011;51:1575–1582. doi: 10.1016/j.freeradbiomed.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert S, Zwiener J, Chu X, Vanvoorhies W, Roman G, Pletcher SD. Regulation of Drosophila life span by olfaction and food-derived odors. Science. 2007;315:1133–1137. doi: 10.1126/science.1136610. [DOI] [PubMed] [Google Scholar]
- Girardot F, Lasbleiz C, Monnier V, Tricoire H. Specific age-related signatures in Drosophila body parts transcriptome. BMC Genomics. 2006;7:69. doi: 10.1186/1471-2164-7-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming JE, Miquel J, Bensch KG. Age dependent changes in mitochondria. Basic Life Sci. 1985;35:143–156. doi: 10.1007/978-1-4899-2218-2_7. [DOI] [PubMed] [Google Scholar]
- Miquel J, Lundgren PR, Bensch KG, Atlan H. Effects of temperature on the life span, vitality and fine structure of Drosophila melanogaster. Mech Ageing Dev. 1976;5:347–370. doi: 10.1016/0047-6374(76)90034-8. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Philpott DE, Miquel J. Electron microscope studies on aging Drosophila melanogaster. II. Intramitochondrial crystalloid in fat body cells. J Gerontol. 1970;25:218–221. doi: 10.1093/geronj/25.3.218. [DOI] [PubMed] [Google Scholar]
- Walker DW, Benzer S. Mitochondrial “swirls” induced by oxygen stress and in the Drosophila mutant hyperswirl. Proc Natl Acad Sci U S A. 2004;101:10290–10295. doi: 10.1073/pnas.0403767101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calleja M, Pena P, Ugalde C, Ferreiro C, Marco R, Garesse R. Mitochondrial DNA remains intact during Drosophila aging, but the levels of mitochondrial transcripts are significantly reduced. J Biol Chem. 1993;268:18891–18897. [PubMed] [Google Scholar]
- Schwarze SR, Weindruch R, Aiken JM. Oxidative stress and aging reduce COX I RNA and cytochrome oxidase activity in Drosophila. Free Radic Biol Med. 1998;25:740–747. doi: 10.1016/s0891-5849(98)00153-1. [DOI] [PubMed] [Google Scholar]
- Sanz A, Fernandez-Ayala DJ, Stefanatos RK, Jacobs HT. Mitochondrial ROS production correlates with, but does not directly regulate lifespan in Drosophila. Aging (Albany NY) 2010;2:200–223. doi: 10.18632/aging.100137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury M, Jonscher KR, Friedman JE. Reduced mitochondrial function in obesity-associated fatty liver: SIRT3 takes on the fat. Aging (Albany NY) 2011;3:175–178. doi: 10.18632/aging.100289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J, Hur JH, Walker DW. The role of mitochondria in Drosophila aging. Exp Gerontol. 2011;46:331–334. doi: 10.1016/j.exger.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rera M, Bahadorani S, Cho J, Koehler CL, Ulgherait M, Hur JH, Ansari WS, Lo T, Jr., Jones DL, Walker DW. Modulation of longevity and tissue homeostasis by the Drosophila PGC-1 homolog. Cell Metab. 2011;14:623–634. doi: 10.1016/j.cmet.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon LM, Rebelo AP, Moraes CT. The role of PGC-1 coactivators in aging skeletal muscle and heart. IUBMB Life. 2012;64:231–241. doi: 10.1002/iub.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Icreverzi A, de la Cruz AF, Van Voorhies WA, Edgar BA. Drosophila cyclin D/Cdk4 regulates mitochondrial biogenesis and aging and sensitizes animals to hypoxic stress. Cell Cycle. 2012;11:554–568. doi: 10.4161/cc.11.3.19062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choksi KB, Nuss JE, DeFord JH, Papaconstantinou J. Mitochondrial electron transport chain functions in long-lived Ames dwarf mice. Aging (Albany NY) 2011;3:754–767. doi: 10.18632/aging.100357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur JH, Cho J, Walker DW. Aging: Dial M for Mitochondria. Aging. 2010;2:69–73. doi: 10.18632/aging.100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artal-Sanz M, Tavernarakis N. Opposing function of mitochondrial prohibitin in aging. Aging (Albany NY) 2010;2:1004–1011. doi: 10.18632/aging.100246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tower J. Sex-specific regulation of aging and apoptosis. Mech Ageing Dev. 2006;127:705–718. doi: 10.1016/j.mad.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Hur JH, Walker DW. p53, sex, and aging: lessons from the fruit fly. Aging (Albany NY) 2009;1:881–883. doi: 10.18632/aging.100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waskar M, Landis GN, Shen J, Curtis C, Tozer K, Abdueva D, Skvortsov D, Tavare S, Tower J. Drosophila melanogaster p53 has developmental stage-specific and sex-specific effects on adult life span indicative of sexual antagonistic pleiotropy. Aging (Albany NY) 2009;1:903–936. doi: 10.18632/aging.100099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Ford D, Landis GN, Tower J. Identifying sexual differentiation genes that affect Drosophila life span. BMC Geriatr. 2009;9:56. doi: 10.1186/1471-2318-9-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tower J. The genetic architecture of aging: sexual antagonistic pleiotropy of p53 and foxo. Cell Cycle. 2010;9:3840–3841. doi: 10.4161/cc.9.19.13464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren C, Finkel SE, Tower J. Conditional inhibition of autophagy genes in adult Drosophila impairs immunity without compromising longevity. Exp Gerontol. 2009;44:228–235. doi: 10.1016/j.exger.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieschke ET, Wheeler JC, Tower J. Doxycycline-induced transgene expression during Drosophila development and aging. Mol Gen Genet. 1998;258:571–579. doi: 10.1007/s004380050770. [DOI] [PubMed] [Google Scholar]
- Shen J, Tower J. Drosophila foxo acts in males to cause sexual-dimorphism in tissue-specific p53 life span effects. Exp Gerontol. 2010;45:97–105. doi: 10.1016/j.exger.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford D, Hoe N, Landis GN, Tozer K, Luu A, Bhole D, Badrinath A, Tower J. Alteration of Drosophila life span using conditional, tissue-specific expression of transgenes triggered by doxycyline or RU486/Mifepristone. Exp Gerontol. 2007;42:483–497. doi: 10.1016/j.exger.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdueva D, Skvortsov D, Tavare S. Non-linear analysis of GeneChip arrays. Nucleic Acids Res. 2006;34:e105. doi: 10.1093/nar/gkl435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth GK, Michaud J, Scott HS. Use of within-array replicate spots for assessing differential expression in microarray experiments. Bioinformatics. 2005;21:2067–2075. doi: 10.1093/bioinformatics/bti270. [DOI] [PubMed] [Google Scholar]
- Storey JD, Tibshirani R. Statistical methods for identifying differentially expressed genes in DNA microarrays. Methods Mol Biol. 2003;224:149–157. doi: 10.1385/1-59259-364-X:149. [DOI] [PubMed] [Google Scholar]
- Barrett T, Suzek TO, Troup DB, Wilhite SE, Ngau WC, Ledoux P, Rudnev D, Lash AE, Fujibuchi W, Edgar R. NCBI GEO: mining millions of expression profiles—database and tools. Nucleic Acids Res. 2005;33:D562–566. doi: 10.1093/nar/gki022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyne R, Smith R, Rutherford K, Wakeling M, Varley A, Guillier F, Janssens H, Ji W, McLaren P, North P, Rana D, Riley T, Sullivan J, et al. FlyMine: an integrated database for Drosophila and Anopheles genomics. Genome Biol. 2007;8:R129. doi: 10.1186/gb-2007-8-7-r129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. (2009). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.