Abstract
The transient receptor potential (TRP) family comprises a diverse group of cation channels that regulate a variety of intracellular signaling pathways. The TRPV1 (vanilloid 1) channel is best known for its role in nociception and sensory transmission. First studied in the dorsal root ganglia as the receptor for capsaicin, TRPV1 is now recognized to have a broader distribution and function within the central nervous system (CNS). Because it can be activated by a range of potentially noxious stimuli, TRPV1’s polymodal nature and ability to interact with other receptor pathways make it a candidate for a stress response protein. As a result, TRPV1 is emerging as a key mediator of CNS function through modulation of both glial and neuronal activity. Growing evidence has suggested that TRPV1 can mediate a variety of pathways from glial reactivity and cytokine release to synaptic transmission and plasticity. This review highlights the increasing importance of TRPV1 as a regulator of CNS function in response to stress.
Keywords: TRPV1, neurodegeneration, capsaicin, CNS, TRP channel, Huntington’s Disease, neuron, glia, plasticity, synaptic transmission
TRPs: a polymodal family
The transient receptor potential (TRP) family is a diverse group of channels that regulates cation entry and contributes to a vast variety of physiological conditions. There are 28 mammalian TRPs, divided into 6 subfamilies based on homology: canonical (TRPC1-7), vanilloid (TRPV1-6), melastatin (TRPM1-8), ankyrin (TRPA1), polycystin (TRPP1-3) and mucolipin (TRPML1-3). All six members share a common structure of six transmembrane domains with a hydrophobic pore located between the fifth and sixth domains. Situated in the plasma membrane, TRP channels serve as polymodal integrators due to their activation by a variety of stimuli including temperature, osmolality, mechanical force, chemoattractants and ischemia.
One subfamily of the TRP channels is the vanilloid family, named for their responsiveness to various ligands that possess vanillyl moieties. Within the TRPV family, TRPV1 is the best studied particularly due to its role in nociception. Although first identified as the receptor for capsaicin, TRPV1 can also be activated endogenously by voltage, noxious heat (>42°C), pH and lipoxygenase products. Endocannabinoids, including anandamide and N-arachidonoyl dopamine, can activate both TRPV1 and cannabinoid receptors, indicating the importance of cross-talk mechanisms. TRPV1 nonselectively gates cations; however, channel activation results in a 10-fold higher preference for calcium [1]. One of the most potent agonists of TRPV1 is resiniferatoxin (RTX), a plant toxin that exhibits 3-4X greater potency than capsaicin [2]. In contrast, its iodinated form, iodo-resiniferatoxin (I-RTX), is a potent antagonist [3]. The sensitivity of TRPV1 to ligand activation can be modulated by intracellular events and signaling pathways including phosphorylation [4]. For a more extensive review of TRPV1 pharmacology, see Vriens J et al. and Szallasi A et al. [5,6].
The structure of TRPV1
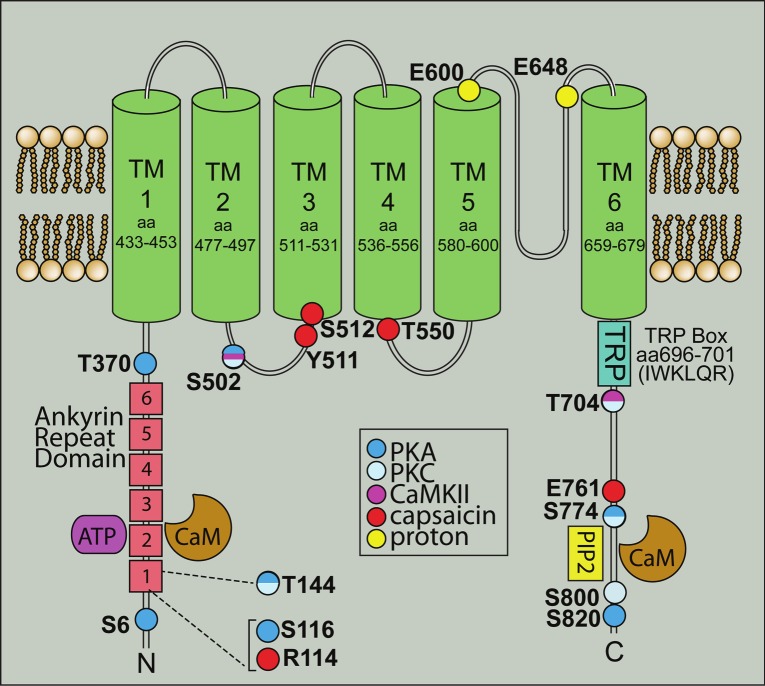
Structural analysis of TRPV1 indicates a compact transmembrane region and a large basket-like intracellular domain [7]. These regions are formed by six transmembrane domains, a structural feature common to all TRPs, and intracellular N- and C-termini. The N-terminal tail contains numerous phosphorylation sites and ankyrin repeats that serve as binding sites for calmodulin and ATP [8]. The C-terminal tail contains a TRP domain as well as binding sites for both calmodulin and PIP2, an endogenous TRPV1 inhibitor [9-11]. Within the extracellular loop domain, the amino acid residues Glu600 and Glu648 can regulate TRPV1 activation by protons, allowing for pH sensitivity [12]. Agonist activation is also mediated intracellularly, as lipophilic capsaicin readily crosses the membrane to bind several sites on TRPV1 [13] (Figure 1). Mutation and deletion studies have identified multiple residues critical for activation. Deletion of Thr550 in transmembrane region 4 can reduce capsaicin sensitivity [14]. Deletion of Arg114 and Glu761 in the N- and C-termini can block capsaicin-induced currents without affecting TRPV1 activation by heat [15]. Mutations of Tyr511 and Ser512 can abolish capsaicin responses, yet leave activation by heat and protons intact [16].
Figure 1.
TRPV1 is a member of the transient receptor potential family. TRPV1 consists of six transmembrane domains with a pore region between the fifth and sixth domain, and long intracellular N- and C- terminal tails. Within the N-terminal tail, six ankyrin repeat domains allow binding of calmodulin and ATP to modulate TRPV1 activation. The C-terminus contains a TRP domain as well as binding sites for PIP2 and calmodulin. Throughout TRPV1 are multiple phosphorylation sites for PKA, PKC and CaMKII, in addition to putative sites for capsaicin and proton binding. TRPV1: transient receptor potential vanilloid 1, TM: transmembrane, aa: amino acid, CaM: calmodulin, ATP: adenosine triphosphate, PIP2: phosphoinositide 4,5-bisphosphate, PKA: protein kinase A, PKC: protein kinase C, CamKII: Ca2+/calmodulin dependent kinase II.
Functional TRPV1 preferentially forms a homotetramer but can also oligermerize with other TRP family subunits including TRPV3 and TRPA1 [7,17,18]. The affinity and specificity of the subunit interactions is determined by amino acids in the transmembrane domains and the C-terminus. In chimeric studies, replacement of the N- and C-termini of TRPV1 with that of TRPV4 did not prevent the formation of TRPV1 tetramers, suggesting a role for the transmembrane region in oligomerization [19]. Deletion studies, however, have shown that the C-terminal TRP domain (684Glu-721Arg) regulates the formation of functional channel tetramers [9]. Removal of this region prevents the oligomerization into stable TRPV1 heteromers.
TRPV1 is a cation channel, and its selectivity filter is believed to lie in the pore domain formed by transmembrane regions 5 and 6. Site-specific analysis has shown that substitutions of Asp646 or Tyr671 in the pore domain can reduce the permeability of divalent cations [20,21]. This cation selectivity is dynamic, not static, and can vary depending on stimulus duration or agonist concentration. Activation can alter the calcium permeability and pore diameter of TRPV1 to allow influx of larger cations [1]. SCAM (substituted cysteine accessibility method) has shown this change in permeability is mediated by amino acid residues in transmembrane domain 6. Within this domain, Leu681 can regulate permeability of large cations, while Tyr671 gates access of smaller cations [22]. The method of channel stimulation can also have a significant effect on calcium permeability - activation by protons produces a smaller calcium current than activation by capsaicin [23].
Regulation of TRPV1
In addition to membrane expression, TRPV1 is also found in the endoplasmic reticulum where it mobilizes calcium from intracellular stores [24,25]. Activation of signaling pathways can translocate TRPV1 from intracellular compartments such as the endoplasmic reticulum to the membrane usually via phosphorylation. For example, PKC activation can lead to membrane insertion of TRPV1 via SNARE-mediated exocytosis [26]. cAMP-dependent activation of PKA can rapidly translocate monomeric TRPV1 from intracellular compartments to form the functional channel at the plasma membrane [27]. Furthermore, nerve growth factor activation of the tyrosine kinase src can phosphorylate TRPV1 at Y200 to increase membrane levels of TRPV1 [28] (Figure 2). Insulin growth factors can also increase membrane expression and potentiation of TRPV1 via PKC-mediated phosphorylation [29].
Figure 2.
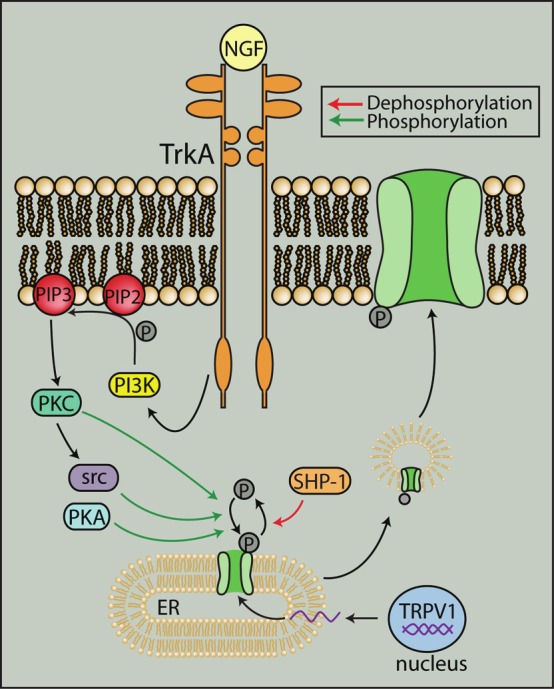
Interaction with other signaling pathways can traffic TRPV1 to the plasma membrane. TrkA stimulation by NGF can cause src-mediated phosphorylation of TRPV1 to traffic TRPV1 from the endoplasmic reticulum to the plasma membrane. Translocation of TRPV1 to the membrane can also be increased through PKA- and PKC-mediated phosphorylation. Dephosphorylation by SHP-1, however, can inhibit translocation. NGF: nerve growth factor, PIP3: phosphatidylinositol 3,4,5-trisphosphate, PI3K: phosphoinositide 3 kinase
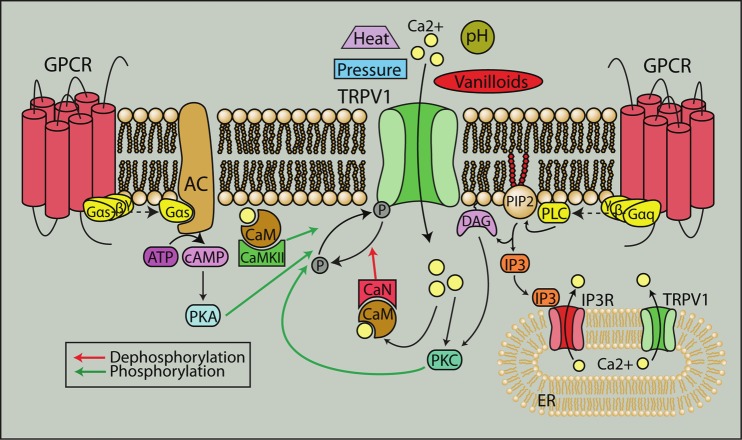
Phosphorylation is also important in modulating the channel, allowing for rapid responses to external stimuli or environmental changes. Generally, phosphorylation sensitizes while dephosphorylation desensitizes the channel. PKC phosphorylation at Ser800 reverses desensitization of TRPV1 from prolonged capsaicin treatment and increases the sensitivity of TRPV1 to agonists [30,31]. Forskolin-mediated activation of PKA can decrease capsaicin-induced desensitization of TRPV1, a phenomenon that is blocked by a PKA inhibitor [32]. PKA can also reduce desensitization by direct phosphorylation of TRPV1 at Ser116 [33]. In addition, PKC or PKA activation through stimulation of multiple receptors including the protease-activated receptor PAR2, bradykinin B1 and B2, purinergic P2 receptors, chemokine receptor CCL3 and endothelin receptors have all been shown to increase sensitivity of the channel [34-38] (Figure 3). Src kinase, CamKII and PI3K can all phosphorylate TRPV1 and increase sensitivity as well [28,29,39]. On the other hand, dephosphorylation by calcineurin/PP2B and increases in intracellular calcium can desensitize the channel [40,41].
Figure 3.
TRPV1 is a polymodal cation channel. TRPV1 can be activated by a variety of noxious stimuli such as heat, pH and pressure, and its interaction with other receptors including G protein-coupled receptors (GPCRs) contributes to its polymodal nature. GPCR activation can directly lead to recruitment of PKC and PKA, through phospholipase C and adenylyl cyclase respectively, to phosphorylate TRPV1 and sensitize the channel. Elevations in intracellular calcium from TRPV1 and GPCR stimulation can activate calcineurin and CaMKII via calmodulin to further modulate TRPV1 activity. GPCR: G-protein coupled receptors, AC: adenylyl cyclase, cAMP: cyclic AMP, CaN: calcineurin, DAG: diacylglycerol, PIP2: phosphoinositide 4,5-bisphosphate, IP3: inositol 1,4,5-trisphosphate, PLC: phospholipase C, ER: endoplasmic reticulum.
TRPV1 in the CNS
Although it is expressed throughout the CNS, TRPV1 is most robust in the sensory neurons of the dorsal root ganglion [42]. Using a combination of knockout mice, radioligand binding and immunocytochemistry, TRPV1 expression within the CNS has been documented. These studies localize TRPV1 mainly to the hippocampus and cortex with additional expression in hypothalamus, olfactory nuclei, dentate gyrus, locus coeruleus, superior colliculus and spinal cord [43,44]. These observations of widespread TRPV1 expression, however, are contested by a TRPV1 reporter mouse that indicates limited expression in the CNS outside of nociceptors in the sensory ganglia. CNS expression was restricted to the posterior caudal hypothalamus, the rostral midbrain, the periaqueductal grey and the hippocampus [45]. Although there is discrepancy regarding the exact distribution of TRPV1, it can be appreciated that CNS expression of TRPV1 indicates a broader function of the channel beyond sensory transmission.
Subcellularly, TRPV1 expression has been found in cell bodies and synapses, predominantly on the post-synaptic dendritic spines of neurons and also in synaptic vesicles [44,46]. TRPV1 is also highly expressed in the cell bodies and neurites of both sensory neurons and neurons differentiated by induction with retinoic acid [47,48]. Retinal ganglion cells also exhibit TRPV1 expression in somas and in discrete pockets in axons [49]. In addition to neurons, TRPV1 protein has also been found in glia including astrocytes and microglia [50,51].
TRPV1 in pain and inflammation
TRPV1 is highly expressed in the dorsal root ganglion (DRG), where it mediates sensory perception especially in nociception where it has been extensively studied. TRPV1 is best characterized as the receptor for capsaicin, an ingredient in chili peppers known to elicit a burning sensation and pain [52]. Within the dorsal root ganglion, TRPV1 expression is localized to the C- and Aδ- fibers. Here, channel activation leads to calcium elevations and subsequent release of neuropeptides including calcitonin-gene-related peptide and substance P [52-54]. TRPV1 can also be activated by noxious heat (>43°C) indicating a role in transducing thermal pain and hyperalgesia [52]. Furthermore, TRPV1-null mice display a reduced response to vanilloid stimulation, thermosensation and hyperalgesia [55].
Inflammation has been linked to TRPV1-mediated nociception. Intradermal injection of capsaicin can cause pain and hyperalgesia in humans in a dose-dependent manner [56]. Following injection of Complete Freund’s Adjuvant (CFA), TRPV1 protein levels increase in the DRG and the channels are transported to the peripheral nociceptive terminals [57]. Oral administration of TRPV1 antagonists reduces capsaicin or CFA-induced pain behavior, hyperalgesia and mechanical allodynia in rodents [58]. Many proinflammatory factors including substance P, nerve growth factor, bradykinin, prostaglandins and ATP can potentiate and sensitize TRPV1 [36,59-61]. Activation of TRPV1 can also mediate the release of inflammatory mediators such as IL-6 [51]. The role of TRPV1 in pain and inflammation has been extensively examined in other reviews [62-66].
TRPV1 in behavior
TRPV1 has been associated with changes induced by drug treatment, addiction, anxiety and depression. Intraperitoneal injections of methamphetamine increase TRPV1 mRNA in the frontal cortex, but not the striatum or hippocampus [67]. Morphine injections also increase TRPV1 expression in the DRG, spinal cord and sciatic nerve through the activation of the MAPK pathway. Inhibition of TRPV1 with SB366791 reduces morphine tolerance and thermal hyperalgesia, suggesting that TRPV1 is involved in the effects of chronic morphine treatments [68].
In addition to changes in expression levels, TRPV1 can also mediate behavioral effects of drug-induced addiction. In a model of cocaine-induced addiction, the TRPV1 antagonist SB366791 did not affect cocaine self-administration, but did reduce the cocaine-induced reinstatement of cocaine-seeking behavior ; this suggests TRPV1 activity is not necessary for the reward response of cocaine, but may play a role in cocaine relapse [69]. TRPV1-null mice or mice treated with capsazepine, a TRPV1 antagonist, have a higher preference for ethanol and faster recovery from ethanol-induced effects. TRPV1 activation by capsaicin, however, produces opposite effects - lower preference for and slower recovery from ethanol [70]. In mice, nicotine-induced depression can be reduced by TRPV1 agonists, capsaicin and olvanil, as seen in the forced swim test (increased swimming) and tail suspension test (increased mobility time), indicating TRPV1 can counter some nicotine-induced effects [71].
Besides addiction, TRPV1 can mediate anxiety-induced behavior. TRPV1 inhibition by capsazepine injections into the medial prefrontal cortex of rats increase exploration time in the elevated plus maze and the number of licks in the Vogel conflict test, indicating an anxiolytic effect with TRPV1 antagonism [72]. TRPV1-null mice exhibit less anxiety, as determined by the elevated plus maze and increased exploration of the illuminated side of the light-dark test [73]. Interestingly, TRPV1-null mice also show less fear-conditioned responses, which correlate with a decrease in long term potentiation, suggesting that TRPV1 promotes fear [73]. Conversely, intraperitoneal injections of a TRPV1 agonist, olvanil, decreased the time spent in the open arms of the elevated plus maze, further linking TRPV1 activation to induction of anxiety behavior [74].
TRPV1 in glial function
Under conditions of stress and injury glia can become reactive, demonstrating increased hypertrophy, production and secretion of cytokines and changes in gene expression. In addition to neurons, TRPV1 is also found in astrocytes and microglia, and emerging studies have implicated TRPV1 in various aspects of glial function.
TRPV1 is expressed in astrocytes in the spinal cord, retina and various brain regions [44,50,75]. However, immunolabeling indicates that only a subset of astrocytes in the substantia nigra is TRPV1-positive [76]. In TRPV1-null mice, spinal astrocytes and microglia both demonstrate reduced Iba-1 and GFAP immunostaining in response to capsaicin and CFA injections and partial sciatic nerve ligation [77]. This suggests that TRPV1 may mediate gliosis under conditions of inflammatory and neuropathic pain. Injections of capsaicin increase GFAP levels in acutely axotomized retinas that can be blocked with capsazepine [78]. Acid-induced activation of TRPV1 is associated with increases in channel permeability to sodium rather than calcium in cortical astrocytes [79].
TRPV1 also mediates microglial function. The TRPV1 antagonist, WIN-55,212-2 reduces microglial activation but can also stimulate the cannabinoid 1 and 2 receptors to increase hippocampal neurogenesis [80]. WIN-55,212-2 treatments can decrease production of proinflammatory cytokines (TNFα, IL-1β, IL-6) and increase the anti-inflammatory cytokine IL1-RA, suggesting the participation of TRPV1 in hippocampal inflammation [80]. TRPV1 antagonism can reduce increases in microglial IL-6 secretion and intracellular calcium induced by elevations in hydrostatic pressure [51]. Capsaicin treatments can also up-regulate bradykinin B1 receptor levels in rat spinal cord microglia. This up-regulation correlates with increases in spinal cord IL-1β mRNA levels [81]. Moreover, phorbol 12-myristate 13-acetate-stimulated production of reactive oxygen species in microglia can be reduced with TRPV1 antagonists including capsazepine and I-RTX, further suggesting that TRPV1 is involved in microglia-induced inflammation [82].
Emerging studies have suggested TRPV1 might be involved in cell migration. Capsaicin induces dendritic cells of the immune system to migrate to lymph nodes, a result that is absent in TRPV1-null mice [83]. TRPV1 also enhances hepatocyte growth factor-induced migration of hepatoblastoma cells [84]. Capsaicin can also increase migration of corneal epithelial cells by 1.65X in a scratch-wound model of migration through the transactivation of the epidermal growth factor receptor [85]. Furthermore, TRPV1 can interact with the cytoskeleton, suggesting that the channel might be involved in cytoskeletal changes that occur during cell movement. Tubulin dimers can directly bind the C-terminus of TRPV1, an interaction that stabilizes microtubules under depolymerizing conditions [86]. TRPV1 activation by capsaicin or RTX can cause rapid depolymerization of microtubules through an increase in intracellular calcium [87,88]. In addition, TRPV1 has been found in growth cones where it mediates their retraction through microtubule disassembly [89]. TRPV1 has also been shown to localize to filopodial tips where it mediates filopodial initiation and elonglation [46]. By directly interacting with cytoskeletal elements, TPRV1 can mediate glial migration and chemotaxis during stress and injury.
TRPV1 in neuronal function
Growth cones are incredibly dynamic and undergo rapid directional changes in response to a chemical gradient. Localized elevations in calcium at the growth cone can induce extension and turning by activating CaMKII for attraction and calcineurin for repulsion [90,91]. Neurite outgrowth is also believed to be calcium dependent. By interacting with microtubule kinase MARK2, CaMKI is able to induce neurite outgrowth under conditions of increased intracellular calcium [92]. Ionomycin-induced increases in calcium can activate CamKIV, resulting in phosphorylation of cofilin and subsequent remodeling of the actin cytoskeleton and initiation of neurite outgrowth [93]. As a calcium-selective cation channel, TRPV1 is also involved in neurite outgrowth and growth cone dynamics. In retinoic acid-induced differentiation of neuroblastoma cells into neurons, TRPV1 is upregulated in both cell bodies and developing neurites [47]. Activation of TRPV1 can also induce the formation of varicosities along neurites and retraction of growth cones through microtubule disassembly in a dorsal root ganglia cell line [89]. Another member of the TRP family, TRPC5 is expressed in the growth cones of hippocampal neurons, and expression of dominant-negative TRPC5 results in longer neurites and filopodia [94].
In addition to growth cones and neurites, TRPV1 also localizes to synapses, and emerging studies indicate the channel can modulate synaptic transmission. In a DRG cell line, TRPV1 colocalizes with synaptic proteins at filopodia tips, where activation results in vesicle fusion. This activity suggests that TRPV1 modulates neurotransmitter release [46]. For example, capsaicin can activate a subset of neurons in the solitary tract to induce an inward current and an increase in spontaneous activity to facilitate glutamate release [95]. TRPV1, by modulating calcium levels, is required for asynchronous glutamate release from solitary tract neurons [96]. Furthermore, in DRG and spinal cord co-cultures as well as slices from substantia nigra and hypothalamus, capsaicin increases presynaptic calcium to enhance presynaptic activity and glutamate release [97-99]. In addition to glutamate release, TRPV1 has also been implicated in dopamine release. In the ventral tegmental area, capsaicin enhances both the release of dopamine at the nucleus accumbens and also the firing of dopaminergic neurons [100]. In TRPV1-transfected neuroblastoma cells, capsaicin treatments elevate intracellular calcium levels to increase release of [3H] norepinephrine from secretory vesicles [101]. In the peripheral nervous system, capsaicin-induced calcium increases lead to the release of neuropeptides substance P and calcitonin-gene related peptide [53,54].
TRPV1 in synaptic transmission and plasticity
By altering synaptic calcium levels and neurotransmitter release, TRPV1 can modulate synaptic transmission. In spinal cord slices from rats injected with Freund’s complete adjuvant, the TRPV1 antagonist, SB-366791 decreases the frequency but not amplitude of spontaneous and miniature excitatory post-synaptic currents (EPSCs) [102]. In striatal medium spiny neurons and sensory neurons, TRPV1 enhances the frequency of glutamatergic EPSCs that were potentiated by PKC-mediated decrease in desensitization [103,104]. TRPV1-mediated increases in EPSC frequencies have also been observed in the substantia gelatinosa, periaqueductal gray, medial preoptic nucleus, substantia nigra and locus coeruleus [98,105-108].
By modulating synaptic transmission, TRPV1 can influence synaptic plasticity and survival. In hippocampal neurons, TRPV1 activation by capsaicin or 12-(S)-HPETE is necessary to cause long term depression (LTD) by high frequency stimulation. This effect was notably absent in TRPV1-null mice [109]. As a result of this finding, the authors propose a model in which glutamate induces post-synaptic release of 12-(S)-HPETE into the synaptic cleft to activate presynaptic TRPV1 channels. Activated TRPV1 subsequently decreases pre-synaptic glutamate release through a calcium-dependent pathway (Figure 4). Another study found that in the dentate gyrus and in the medium spiny neurons of the nucleus accumbens, post-synaptic activation of TRPV1 by anandamide leads to LTD through calcium-mediated endocytosis of AMPA receptors [110,111] (Figure 4). In the developing superior colliculus, I-RTX blocks the induction of tetanus-induced LTD, while RTX reduces the amplitude of field excitatory postsynaptic potentials [112]. In TRPV1-null mice, there was a reduction in long term potentiation (LTP) compared to wildtype mice in the CA1 region of the hippocampus [73]. These previous studies indicate that TRPV1 facilitates LTD; however, another study found that capsaicin and RTX amplified LTP and suppressed LTD in the CA1 region of the hippocampus [113].
Figure 4.
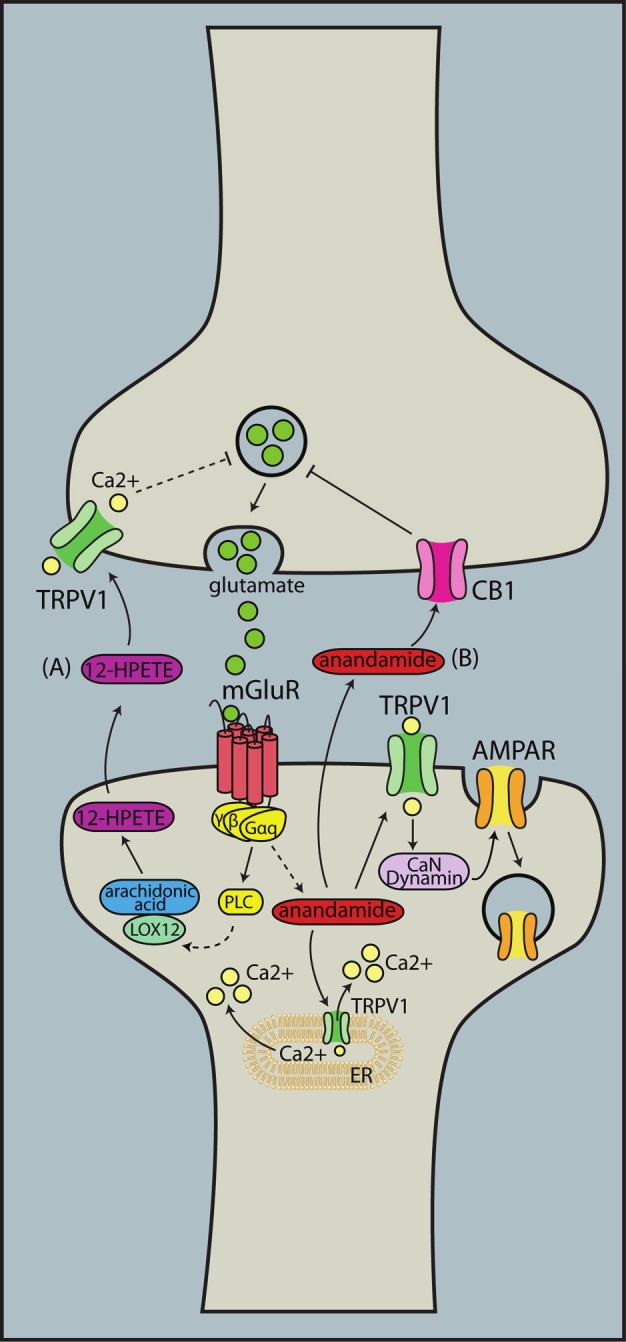
TRPV1 contributes to synaptic plasticity. As a calcium cation channel, TRPV1 has been implicated in synaptic plasticity, especially in facilitating long term depression. (A) Activation of mGluR by glutamate triggers the synthesis and release of 12-(S) HPETE into the extracellular milieu. 12-(S)HPETE activates pre-synaptic TRPV1, and through a calcium-mediated pathway, glutamate release is blocked. (B) mGluR activation can also lead to production and release of anandamide. Anandamide binds postsynaptic TRPV1 to cause endocytosis of AMPA receptors, or to pre-synaptic cannabinoid receptors to inhibit glutamate release. 12-HPETE: 12(S)-hydroperoxyeicosatetraenoic acid, LOX12: 12-Lipoxygenase, CB1: cannabinoid receptor
TRPV1 in neurodegeneration
Associated with increases in intracellular calcium, overactivation of TRPV1 can be toxic to cells. Capsaicin leads to elevated levels of intracellular calcium and subsequent mitochondrial damage and apoptosis in both cultured cortical microglia and mesencephalic neuronal cultures [114,115]. Capsaicin can also induce apoptosis of cultured retinal ganglion cells in a dose-dependent manner [49]. Likewise, intranigral injections of capsaicin lead to death of dopaminergic neurons through calcium-mediated mitochondrial damage. This damage can be reduced by coinjections of capsazepine [115]. Moreover, capsaicin triggers apoptosis in cortical neurons through ERK phosphorylation, activation of caspases and production of reactive oxygen species [116]. TRPV1 has also been found in gliomas, where its activation by capsaicin leads to cell death through calcium-induced mitochondrial damage and p38 activation [117]. The association between TRPV1 and cell death is further supported by the ability of I-RTX to block pressure-induced apoptosis of cultured retinal ganglion cells [49].
Because TRPV1 can initiate calcium-dependent apoptosis of neuronal and glial cell types, TRPV1 has been implicated in neurodegeneration. In an ischemic model of glaucoma induced by high intraocular pressure, both CB1 and TRPV1 protein levels are upregulated. Treatment with a stable anandamide analogue reduces retinal ganglion cell loss, an effect that is diminished with CB1 and TRPV1 antagonists [118]. This suggests that endocannabinoid binding to CB1 or TRPV1 is neuroprotective. Similarly, in a gerbil model of global transient ischemia, capsaicin and the CB1 receptor antagonist rimonabant can both improve locomotor activity, memory and the number of neurons in the CA1 hippocampus. This effect is diminished with capsazepine pre-treatment, suggesting that TPRV1 is neuroprotective during ischemia [119,120]. Capsaicin has also exhibited a neuroprotective function in ouabain-mediated excitotoxicity [121]. This protection may be mediated by rapid agonist-induced desensitization of TRPV1, as TRPV1 antagonism by capsazepine was also neuroprotective and could reduce brain damage.
TRPV1 has also been implicated in Huntington’s disease (HD), a genetic neurodegenerative disorder characterized by cell death in the basal ganglia. HD arises from the expansion of the polyglutamine tract in the huntingtin protein, causing gain-of-function. The symptoms of HD include motor defects as well as cognitive and psychiatric problems. In a 3-nitropropionic acid-induced model of Huntington’s disease, the endocannabinoid ligand AM404 is able to reduce hyperkinesia [122]. This phenomenon can be reversed by capsazepine, but not by the CB1 antagonist SR141716A, suggesting that TRPV1 activation can reduce locomotion. Capsaicin itself is antihyperkinetic and can restore dopamine and GABA transmission in the basal ganglia [122]. Furthermore, intraperitoneal injections of capsaicin into rats reduce ambulation and stereotypic behavior and increase inactivity time during open field testing [123]. These studies indicate that drugs targeting TRPV1 might be beneficial to patients diagnosed with HD.
Conclusion
Emerging studies have shown that TRPV1 has a broader range of function and distribution than once thought. Although it is best characterized for its importance in nociception, expression of TRPV1 in the CNS indicates it may serve a role beyond that in sensory transmission. In combination with its polymodal nature and sensitivity to noxious stimuli, TRPV1 may be an ideal candidate as a stress response protein. By integrating multiple signaling pathways, TRPV1 can modulate intracellular calcium levels to mobilize the cell’s response to stress and injury. As a result, TRPV1 has been implicated in both glial and neuronal function. TRPV1 can mediate gliosis, cytokine levels and cytoskeletal rearrangements. In addition, TRPV1 modulates neurotransmitter release, synaptic transmission, synaptic plasticity and neurodegeneration. Future research will only reveal further roles for TRPV1 as a stress response protein in the brain.
Acknowledgements
Funding provided by an Allergan, Inc. Discovery Research Grant (DJC), the American Health Assistance Foundation (DJC), the Melza M. and Frank Theodore Barr Foundation through the Glaucoma Research Foundation (DJC), NIH EY017427 (DJC), Vanderbilt Pharmacology Training Grant T32GM007628-32 (KWH), Vanderbilt Vision Research Center Training Grant T32EY007135-17) (NJW), Vanderbilt Vision Research Center (P30EY008126), and an Unrestricted Grant from Research to Prevent Blindness to the Vanderbilt University School of Medicine Department of Ophthalmology and Visual Sciences.
Conflicts of interest
KWH, NJW and DJC declare no conflicts of interest.
References
- 1.Chung MK, Guler AD, Caterina MJ. TRPV1 shows dynamic ionic selectivity during agonist stimulation. Nat Neurosci. 2008;11:555–564. doi: 10.1038/nn.2102. [DOI] [PubMed] [Google Scholar]
- 2.Szallasi A, Blumberg PM. Resiniferatoxin, a phorbol-related diterpene, acts as an ultrapotent analog of capsaicin, the irritant constituent in red pepper. Neuroscience. 1989;30:515–520. doi: 10.1016/0306-4522(89)90269-8. [DOI] [PubMed] [Google Scholar]
- 3.Wahl P, Foged C, Tullin S, Thomsen C. Iodo-resiniferatoxin, a new potent vanilloid receptor antagonist. Mol Pharmacol. 2001;59:9–15. doi: 10.1124/mol.59.1.9. [DOI] [PubMed] [Google Scholar]
- 4.Toth A, Blumberg PM, Boczan J. Anandamide and the vanilloid receptor (TRPV1) Vitam Horm. 2009;81:389–419. doi: 10.1016/S0083-6729(09)81015-7. [DOI] [PubMed] [Google Scholar]
- 5.Vriens J, Appendino G, Nilius B. Pharmacology of vanilloid transient receptor potential cation channels. Mol Pharmacol. 2009;75:1262–1279. doi: 10.1124/mol.109.055624. [DOI] [PubMed] [Google Scholar]
- 6.Szallasi A, Cortright DN, Blum CA, Eid SR. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat Rev Drug Discov. 2007;6:357–372. doi: 10.1038/nrd2280. [DOI] [PubMed] [Google Scholar]
- 7.Moiseenkova-Bell VY, Stanciu LA, Serysheva II, Tobe BJ, Wensel TG. Structure of TRPV1 channel revealed by electron cryomicroscopy. Proc Natl Acad Sci U S A. 2008;105:7451–7455. doi: 10.1073/pnas.0711835105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lishko PV, Procko E, Jin X, Phelps CB, Gaudet R. The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity. Neuron. 2007;54:905–918. doi: 10.1016/j.neuron.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Sanz N, Fernandez-Carvajal A, Morenilla-Palao C, Planells-Cases R, Fajardo-Sanchez E, Fernandez-Ballester G, Ferrer-Montiel A. Identification of a tetramerization domain in the C terminus of the vanilloid receptor. J Neurosci. 2004;24:5307–5314. doi: 10.1523/JNEUROSCI.0202-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ufret-Vincenty CA, Klein RM, Hua L, Angueyra J, Gordon SE. Localization of the PIP2 sensor of TRPV1 ion channels. J Biol Chem. 2011;286:9688–9698. doi: 10.1074/jbc.M110.192526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Numazaki M, Tominaga T, Takeuchi K, Murayama N, Toyooka H, Tominaga M. Structural determinant of TRPV1 desensitization interacts with calmodulin. Proc Natl Acad Sci U S A. 2003;100:8002–8006. doi: 10.1073/pnas.1337252100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jordt SE, Tominaga M, Julius D. Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc Natl Acad Sci U S A. 2000;97:8134–8139. doi: 10.1073/pnas.100129497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung J, Hwang SW, Kwak J, Lee SY, Kang CJ, Kim WB, Kim D, Oh U. Capsaicin binds to the intracellular domain of the capsaicin-activated ion channel. J Neurosci. 1999;19:529–538. doi: 10.1523/JNEUROSCI.19-02-00529.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gavva NR, Klionsky L, Qu Y, Shi L, Tamir R, Edenson S, Zhang TJ, Viswanadhan VN, Toth A, Pearce LV, Vanderah TW, Porreca F, Blumberg PM, Lile J, Sun Y, Wild K, Louis JC, Treanor JJ. Molecular determinants of vanilloid sensitivity in TRPV1. J Biol Chem. 2004;279:20283–20295. doi: 10.1074/jbc.M312577200. [DOI] [PubMed] [Google Scholar]
- 15.Jung J, Lee SY, Hwang SW, Cho H, Shin J, Kang YS, Kim S, Oh U. Agonist recognition sites in the cytosolic tails of vanilloid receptor 1. J Biol Chem. 2002;277:44448–44454. doi: 10.1074/jbc.M207103200. [DOI] [PubMed] [Google Scholar]
- 16.Jordt SE, Julius D. Molecular basis for species-specific sensitivity to "hot" chili peppers. Cell. 2002;108:421–430. doi: 10.1016/s0092-8674(02)00637-2. [DOI] [PubMed] [Google Scholar]
- 17.Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, Wright JE, Jerman JC, Walhin JP, Ooi L, Egerton J, Charles KJ, Smart D, Randall AD, Anand P, Davis JB. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature. 2002;418:186–190. doi: 10.1038/nature00894. [DOI] [PubMed] [Google Scholar]
- 18.Staruschenko A, Jeske NA, Akopian AN. Contribution of TRPV1-TRPA1 interaction to the single channel properties of the TRPA1 channel. J Biol Chem. 2010;285:15167–15177. doi: 10.1074/jbc.M110.106153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hellwig N, Albrecht N, Harteneck C, Schultz G, Schaefer M. Homo- and heteromeric assembly of TRPV channel subunits. J Cell Sci. 2005;118:917–928. doi: 10.1242/jcs.01675. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Martinez C, Morenilla-Palao C, Planells-Cases R, Merino JM, Ferrer-Montiel A. Identification of an aspartic residue in the P-loop of the vanilloid receptor that modulates pore properties. J Biol Chem. 2000;275:32552–32558. doi: 10.1074/jbc.M002391200. [DOI] [PubMed] [Google Scholar]
- 21.Mohapatra DP, Wang SY, Wang GK, Nau C. A tyrosine residue in TM6 of the Vanilloid Receptor TRPV1 involved in desensitization and calcium permeability of capsaicin-activated currents. Mol Cell Neurosci. 2003;23:314–324. doi: 10.1016/s1044-7431(03)00054-x. [DOI] [PubMed] [Google Scholar]
- 22.Salazar H, Jara-Oseguera A, Hernandez-Garcia E, Llorente I, Arias-Olguín II, Soriano-Garcia M, Islas LD, Rosenbaum T. Structural determinants of gating in the TRPV1 channel. Nat Struct Mol Biol. 2009;16:704–710. doi: 10.1038/nsmb.1633. [DOI] [PubMed] [Google Scholar]
- 23.Samways DS, Khakh BS, Egan TM. Tunable calcium current through TRPV1 receptor channels. J Biol Chem. 2008;283:31274–31278. doi: 10.1074/jbc.C800131200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marshall IC, Owen DE, Cripps TV, Davis JB, McNulty S, Smart D. Activation of vanilloid receptor 1 by resiniferatoxin mobilizes calcium from inositol 1,4,5-trisphosphate-sensitive stores. Br J Pharmacol. 2003;138:172–176. doi: 10.1038/sj.bjp.0705003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu M, Liu MC, Magoulas C, Priestley JV, Willmott NJ. Versatile regulation of cytosolic Ca2+ by vanilloid receptor I in rat dorsal root ganglion neurons. J Biol Chem. 2003;278:5462–5472. doi: 10.1074/jbc.M209111200. [DOI] [PubMed] [Google Scholar]
- 26.Morenilla-Palao C, Planells-Cases R, Garcia-Sanz N, Ferrer-Montiel A. Regulated exocytosis contributes to protein kinase C potentiation of vanilloid receptor activity. J Biol Chem. 2004;279:25665–25672. doi: 10.1074/jbc.M311515200. [DOI] [PubMed] [Google Scholar]
- 27.Vetter I, Cheng W, Peiris M, Wyse BD, Roberts-Thomson SJ, Zheng J, Monteith GR, Cabot PJ. Rapid, opioid-sensitive mechanisms involved in transient receptor potential vanilloid 1 sensitization. J Biol Chem. 2008;283:19540–19550. doi: 10.1074/jbc.M707865200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005;24:4211–4223. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Buren JJ, Bhat S, Rotello R, Pauza ME, Premkumar LS. Sensitization and translocation of TRPV1 by insulin and IGF-I. Mol Pain. 2005;1:17. doi: 10.1186/1744-8069-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varga A, Bolcskei K, Szoke E, Almasi R, Czeh G, Szolcsanyi J, Petho G. Relative roles of protein kinase A and protein kinase C in modulation of transient receptor potential vanilloid type 1 receptor responsiveness in rat sensory neurons in vitro and peripheral nociceptors in vivo. Neuroscience. 2006;140:645–657. doi: 10.1016/j.neuroscience.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 31.Mandadi S, Tominaga T, Numazaki M, Murayama N, Saito N, Armati PJ, Roufogalis BD, Tominaga M. Increased sensitivity of desensitized TRPV1 by PMA occurs through PKCepsilon-mediated phosphorylation at S800. Pain. 2006;123:106–116. doi: 10.1016/j.pain.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 32.Mohapatra DP, Nau C. Desensitization of capsaicin-activated currents in the vanilloid receptor TRPV1 is decreased by the cyclic AMP-dependent protein kinase pathway. J Biol Chem. 2003;278:50080–50090. doi: 10.1074/jbc.M306619200. [DOI] [PubMed] [Google Scholar]
- 33.Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RWt. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35:721–731. doi: 10.1016/s0896-6273(02)00802-4. [DOI] [PubMed] [Google Scholar]
- 34.Amadesi S, Cottrell GS, Divino L, Chapman K, Grady EF, Bautista F, Karanjia R, Barajas-Lopez C, Vanner S, Vergnolle N, Bunnett NW. Protease-activated receptor 2 sensitizes TRPV1 by protein kinase Cepsilon- and A-dependent mechanisms in rats and mice. J Physiol. 2006;575:555–571. doi: 10.1113/jphysiol.2006.111534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vellani V, Zachrisson O, McNaughton PA. Functional bradykinin B1 receptors are expressed in nociceptive neurones and are upregulated by the neurotrophin GDNF. J Physiol. 2004;560:391–401. doi: 10.1113/jphysiol.2004.067462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tominaga M, Wada M, Masu M. Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc Natl Acad Sci U S A. 2001;98:6951–6956. doi: 10.1073/pnas.111025298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang N, Inan S, Cowan A, Sun R, Wang JM, Rogers TJ, Caterina M, Oppenheim JJ. A proinflammatory chemokine, CCL3, sensitizes the heat- and capsaicin-gated ion channel TRPV1. Proc Natl Acad Sci U S A. 2005;102:4536–4541. doi: 10.1073/pnas.0406030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamamoto H, Kawamata T, Ninomiya T, Omote K, Namiki A. Endothelin-1 enhances capsaicin-evoked intracellular Ca2+ response via activation of endothelin a receptor in a protein kinase Cepsilon-dependent manner in dorsal root ganglion neurons. Neuroscience. 2006;137:949–960. doi: 10.1016/j.neuroscience.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 39.Price TJ, Jeske NA, Flores CM, Hargreaves KM. Pharmacological interactions between calcium/calmodulin-dependent kinase II alpha and TRPV1 receptors in rat trigeminal sensory neurons. Neurosci Lett. 2005;389:94–98. doi: 10.1016/j.neulet.2005.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohapatra DP, Nau C. Regulation of Ca2+-dependent desensitization in the vanilloid receptor TRPV1 by calcineurin and cAMP-dependent protein kinase. J Biol Chem. 2005;280:13424–13432. doi: 10.1074/jbc.M410917200. [DOI] [PubMed] [Google Scholar]
- 41.Koplas PA, Rosenberg RL, Oxford GS. The role of calcium in the desensitization of capsaicin responses in rat dorsal root ganglion neurons. J Neurosci. 1997;17:3525–3537. doi: 10.1523/JNEUROSCI.17-10-03525.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanchez JF, Krause JE, Cortright DN. The distribution and regulation of vanilloid receptor VR1 and VR1 5' splice variant RNA expression in rat. Neuroscience. 2001;107:373–381. doi: 10.1016/s0306-4522(01)00373-6. [DOI] [PubMed] [Google Scholar]
- 43.Roberts JC, Davis JB, Benham CD. [3H] Resiniferatoxin autoradiography in the CNS of wild-type and TRPV1 null mice defines TRPV1 (VR-1) protein distribution. Brain Res. 2004;995:176–183. doi: 10.1016/j.brainres.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 44.Toth A, Boczan J, Kedei N, Lizanecz E, Bagi Z, Papp Z, Edes I, Csiba L, Blumberg PM. Expression and distribution of vanilloid receptor 1 (TRPV1) in the adult rat brain. Brain Res Mol Brain Res. 2005;135:162–168. doi: 10.1016/j.molbrainres.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 45.Cavanaugh DJ, Chesler AT, Jackson AC, Sigal YM, Yamanaka H, Grant R, O'Donnell D, Nicoll RA, Shah NM, Julius D, Basbaum AI. Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J Neurosci. 2011;31:5067–5077. doi: 10.1523/JNEUROSCI.6451-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goswami C, Rademacher N, Smalla KH, Kalscheuer V, Ropers HH, Gundelfinger ED, Hucho T. TRPV1 acts as a synaptic protein and regulates vesicle recycling. J Cell Sci. 2010;123:2045–2057. doi: 10.1242/jcs.065144. [DOI] [PubMed] [Google Scholar]
- 47.El Andaloussi-Lilja J, Lundqvist J, Forsby A. TRPV1 expression and activity during retinoic acid-induced neuronal differentiation. Neurochem Int. 2009;55:768–774. doi: 10.1016/j.neuint.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 48.Puntambekar P, Mukherjea D, Jajoo S, Ramkumar V. Essential role of Rac1/NADPH oxidase in nerve growth factor induction of TRPV1 expression. J Neurochem. 2005;95:1689–1703. doi: 10.1111/j.1471-4159.2005.03518.x. [DOI] [PubMed] [Google Scholar]
- 49.Sappington RM, Sidorova T, Long DJ, Calkins DJ. TRPV1: contribution to retinal ganglion cell apoptosis and increased intracellular Ca2+ with exposure to hydrostatic pressure. Invest Ophthalmol Vis Sci. 2009;50:717–728. doi: 10.1167/iovs.08-2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Doly S, Fischer J, Salio C, Conrath M. The vanilloid receptor-1 is expressed in rat spinal dorsal horn astrocytes. Neurosci Lett. 2004;357:123–126. doi: 10.1016/j.neulet.2003.12.051. [DOI] [PubMed] [Google Scholar]
- 51.Sappington RM, Calkins DJ. Contribution of TRPV1 to microglia-derived IL-6 and NFkappaB translocation with elevated hydrostatic pressure. Invest Ophthalmol Vis Sci. 2008;49:3004–3017. doi: 10.1167/iovs.07-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 53.Huang W, Wang H, Galligan JJ, Wang DH. Transient receptor potential vanilloid subtype 1 channel mediated neuropeptide secretion and depressor effects: role of endoplasmic reticulum associated Ca2+ release receptors in rat dorsal root ganglion neurons. J Hypertens. 2008;26:1966–1975. doi: 10.1097/HJH.0b013e328309eff9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gazzieri D, Trevisani M, Springer J, Harrison S, Cottrell GS, Andre E, Nicoletti P, Massi D, Zecchi S, Nosi D, Santucci M, Gerard NP, Lucattelli M, Lungarella G, Fischer A, Grady EF, Bunnett NW, Geppetti P. Substance P released by TRPV1-expressing neurons produces reactive oxygen species that mediate ethanol-induced gastric injury. Free Radic Biol Med. 2007;43:581–589. doi: 10.1016/j.freeradbiomed.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 55.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 56.Simone DA, Baumann TK, LaMotte RH. Dose-dependent pain and mechanical hyperalgesia in humans after intradermal injection of capsaicin. Pain. 1989;38:99–107. doi: 10.1016/0304-3959(89)90079-1. [DOI] [PubMed] [Google Scholar]
- 57.Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 58.Cui M, Honore P, Zhong C, Gauvin D, Mikusa J, Hernandez G, Chandran P, Gomtsyan A, Brown B, Bayburt EK, Marsh K, Bianchi B, McDonald H, Niforatos W, Neelands TR, Moreland RB, Decker MW, Lee CH, Sullivan JP, Faltynek CR. TRPV1 receptors in the CNS play a key role in broad-spectrum analgesia of TRPV1 antagonists. J Neurosci. 2006;26:9385–9393. doi: 10.1523/JNEUROSCI.1246-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang H, Cang CL, Kawasaki Y, Liang LL, Zhang YQ, Ji RR, Zhao ZQ. Neurokinin-1 receptor enhances TRPV1 activity in primary sensory neurons via PKCepsilon: a novel pathway for heat hyperalgesia. J Neurosci. 2007;27:12067–12077. doi: 10.1523/JNEUROSCI.0496-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- 61.Moriyama T, Higashi T, Togashi K, Iida T, Segi E, Sugimoto Y, Tominaga T, Narumiya S, Tominaga M. Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive mechanism of prostaglandins. Mol Pain. 2005;1:3. doi: 10.1186/1744-8069-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schumacher MA. Transient receptor potential channels in pain and inflammation: therapeutic opportunities. Pain Pract. 2010;10:185–200. doi: 10.1111/j.1533-2500.2010.00358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wong GY, Gavva NR. Therapeutic potential of vanilloid receptor TRPV1 agonists and antagonists as analgesics: Recent advances and setbacks. Brain Res Rev. 2009;60:267–277. doi: 10.1016/j.brainresrev.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 64.Chung MK, Jung SJ, Oh SB. Role of TRP channels in pain sensation. Adv Exp Med Biol. 2011;704:615–636. doi: 10.1007/978-94-007-0265-3_33. [DOI] [PubMed] [Google Scholar]
- 65.Cortright DN, Krause JE, Broom DC. TRP channels and pain. Biochim Biophys Acta. 2007;1772:978–988. doi: 10.1016/j.bbadis.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 66.Knotkova H, Pappagallo M, Szallasi A. Capsaicin (TRPV1 Agonist) therapy for pain relief: farewell or revival? Clin J Pain. 2008;24:142–154. doi: 10.1097/AJP.0b013e318158ed9e. [DOI] [PubMed] [Google Scholar]
- 67.Tian YH, Lee SY, Kim HC, Jang CG. Repeated methamphetamine treatment increases expression of TRPV1 mRNA in the frontal cortex but not in the striatum or hippocampus of mice. Neurosci Lett. 2010;472:61–64. doi: 10.1016/j.neulet.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 68.Chen Y, Geis C, Sommer C. Activation of TRPV1 contributes to morphine tolerance: involvement of the mitogen-activated protein kinase signaling pathway. J Neurosci. 2008;28:5836–5845. doi: 10.1523/JNEUROSCI.4170-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Adamczyk P, Miszkiel J, McCreary AC, Filip M, Papp M, Przegalinski E. The effects of cannabinoid CB1, CB2 and vanilloid TRPV1 receptor antagonists on cocaine addictive behavior in rats. Brain Res. 2012;1444:45–54. doi: 10.1016/j.brainres.2012.01.030. [DOI] [PubMed] [Google Scholar]
- 70.Blednov YA, Harris RA. Deletion of vanilloid receptor (TRPV1) in mice alters behavioral effects of ethanol. Neuropharmacology. 2009;56:814–820. doi: 10.1016/j.neuropharm.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hayase T. Differential effects of TRPV1 receptor ligands against nicotine-induced depression-like behaviors. BMC Pharmacol. 2011;11:6. doi: 10.1186/1471-2210-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aguiar DC, Terzian AL, Guimaraes FS, Moreira FA. Anxiolytic-like effects induced by blockade of transient receptor potential vanilloid type 1 (TRPV1) channels in the medial prefrontal cortex of rats. Psychopharmacology (Berl) 2009;205:217–225. doi: 10.1007/s00213-009-1532-5. [DOI] [PubMed] [Google Scholar]
- 73.Marsch R, Foeller E, Rammes G, Bunck M, Kossl M, Holsboer F, Zieglgansberger W, Landgraf R, Lutz B, Wotjak CT. Reduced anxiety, conditioned fear, and hippocampal long-term potentiation in transient receptor potential vanilloid type 1 receptor-deficient mice. J Neurosci. 2007;27:832–839. doi: 10.1523/JNEUROSCI.3303-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kasckow JW, Mulchahey JJ, Geracioti TD Jr. Effects of the vanilloid agonist olvanil and antagonist capsazepine on rat behaviors. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:291–295. doi: 10.1016/j.pnpbp.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 75.Leonelli M, Martins DO, Kihara AH, Britto LR. Ontogenetic expression of the vanilloid receptors TRPV1 and TRPV2 in the rat retina. Int J Dev Neurosci. 2009;27:709–718. doi: 10.1016/j.ijdevneu.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 76.Marinelli S, Di Marzo V, Florenzano F, Fezza F, Viscomi MT, van der Stelt M, Bernardi G, Molinari M, Maccarrone M, Mercuri NB. N-arachidonoyl-dopamine tunes synaptic transmission onto dopaminergic neurons by activating both cannabinoid and vanilloid receptors. Neuropsychopharmacology. 2007;32:298–308. doi: 10.1038/sj.npp.1301118. [DOI] [PubMed] [Google Scholar]
- 77.Chen Y, Willcockson HH, Valtschanoff JG. Influence of the vanilloid receptor TRPV1 on the activation of spinal cord glia in mouse models of pain. Exp Neurol. 2009;220:383–390. doi: 10.1016/j.expneurol.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leonelli M, Martins DO, Britto LR. TRPV1 receptors are involved in protein nitration and Muller cell reaction in the acutely axotomized rat retina. Exp Eye Res. 2010;91:755–768. doi: 10.1016/j.exer.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 79.Huang C, Hu ZL, Wu WN, Yu DF, Xiong QJ, Song JR, Shu Q, Fu H, Wang F, Chen JG. Existence and distinction of acid-evoked currents in rat astrocytes. Glia. 2010;58:1415–1424. doi: 10.1002/glia.21017. [DOI] [PubMed] [Google Scholar]
- 80.Marchalant Y, Brothers HM, Norman GJ, Karelina K, DeVries AC, Wenk GL. Cannabinoids attenuate the effects of aging upon neuroinflammation and neurogenesis. Neurobiol Dis. 2009;34:300–307. doi: 10.1016/j.nbd.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 81.Talbot S, Dias JP, Lahjouji K, Bogo MR, Campos MM, Gaudreau P, Couture R. Activation of TRPV1 by capsaicin induces functional kinin B (1) receptor in rat spinal cord microglia. J Neuroinflammation. 2012;9:16. doi: 10.1186/1742-2094-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schilling T, Eder C. Importance of the non-selective cation channel TRPV1 for microglial reactive oxygen species generation. J Neuroimmunol. 2009;216:118–121. doi: 10.1016/j.jneuroim.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 83.Basu S, Srivastava P. Immunological role of neuronal receptor vanilloid receptor 1 expressed on dendritic cells. Proc Natl Acad Sci U S A. 2005;102:5120–5125. doi: 10.1073/pnas.0407780102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Waning J, Vriens J, Owsianik G, Stuwe L, Mally S, Fabian A, Frippiat C, Nilius B, Schwab A. A novel function of capsaicin-sensitive TRPV1 channels: involvement in cell migration. Cell Calcium. 2007;42:17–25. doi: 10.1016/j.ceca.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 85.Yang H, Wang Z, Capo-Aponte JE, Zhang F, Pan Z, Reinach PS. Epidermal growth factor receptor transactivation by the cannabinoid receptor (CB1) and transient receptor potential vanilloid 1 (TRPV1) induces differential responses in corneal epithelial cells. Exp Eye Res. 2010;91:462–471. doi: 10.1016/j.exer.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Goswami C, Dreger M, Jahnel R, Bogen O, Gillen C, Hucho F. Identification and characterization of a Ca2+ -sensitive interaction of the vanilloid receptor TRPV1 with tubulin. J Neurochem. 2004;91:1092–1103. doi: 10.1111/j.1471-4159.2004.02795.x. [DOI] [PubMed] [Google Scholar]
- 87.Goswami C, Dreger M, Otto H, Schwappach B, Hucho F. Rapid disassembly of dynamic microtubules upon activation of the capsaicin receptor TRPV1. J Neurochem. 2006;96:254–266. doi: 10.1111/j.1471-4159.2005.03551.x. [DOI] [PubMed] [Google Scholar]
- 88.Han P, McDonald HA, Bianchi BR, Kouhen RE, Vos MH, Jarvis MF, Faltynek CR, Moreland RB. Capsaicin causes protein synthesis inhibition and microtubule disassembly through TRPV1 activities both on the plasma membrane and intracellular membranes. Biochem Pharmacol. 2007;73:1635–1645. doi: 10.1016/j.bcp.2006.12.035. [DOI] [PubMed] [Google Scholar]
- 89.Goswami C, Schmidt H, Hucho F. TRPV1 at nerve endings regulates growth cone morphology and movement through cytoskeleton reorganization. FEBS J. 2007;274:760–772. doi: 10.1111/j.1742-4658.2006.05621.x. [DOI] [PubMed] [Google Scholar]
- 90.Zheng JQ. Turning of nerve growth cones induced by localized increases in intracellular calcium ions. Nature. 2000;403:89–93. doi: 10.1038/47501. [DOI] [PubMed] [Google Scholar]
- 91.Wen Z, Guirland C, Ming GL, Zheng JQ. A CaMKII/calcineurin switch controls the direction of Ca(2+)-dependent growth cone guidance. Neuron. 2004;43:835–846. doi: 10.1016/j.neuron.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 92.Uboha NV, Flajolet M, Nairn AC, Picciotto MR. A calcium- and calmodulin-dependent kinase Ialpha/microtubule affinity regulating kinase 2 signaling cascade mediates calcium-dependent neurite outgrowth. J Neurosci. 2007;27:4413–4423. doi: 10.1523/JNEUROSCI.0725-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Takemura M, Mishima T, Wang Y, Kasahara J, Fukunaga K, Ohashi K, Mizuno K. Ca2+/calmodulin-dependent protein kinase IV-mediated LIM kinase activation is critical for calcium signal-induced neurite outgrowth. J Biol Chem. 2009;284:28554–28562. doi: 10.1074/jbc.M109.006296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Greka A, Navarro B, Oancea E, Duggan A, Clapham DE. TRPC5 is a regulator of hippocampal neurite length and growth cone morphology. Nat Neurosci. 2003;6:837–845. doi: 10.1038/nn1092. [DOI] [PubMed] [Google Scholar]
- 95.Doyle MW, Bailey TW, Jin YH, Andresen MC. Vanilloid receptors presynaptically modulate cranial visceral afferent synaptic transmission in nucleus tractus solitarius. J Neurosci. 2002;22:8222–8229. doi: 10.1523/JNEUROSCI.22-18-08222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Peters JH, McDougall SJ, Fawley JA, Smith SM, Andresen MC. Primary afferent activation of thermosensitive TRPV1 triggers asynchronous glutamate release at central neurons. Neuron. 2010;65:657–669. doi: 10.1016/j.neuron.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Medvedeva YV, Kim MS, Usachev YM. Mechanisms of prolonged presynaptic Ca2+ signaling and glutamate release induced by TRPV1 activation in rat sensory neurons. J Neurosci. 2008;28:5295–5311. doi: 10.1523/JNEUROSCI.4810-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Marinelli S, Di Marzo V, Berretta N, Matias I, Maccarrone M, Bernardi G, Mercuri NB. Presynaptic facilitation of glutamatergic synapses to dopaminergic neurons of the rat substantia nigra by endogenous stimulation of vanilloid receptors. J Neurosci. 2003;23:3136–3144. doi: 10.1523/JNEUROSCI.23-08-03136.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sasamura T, Sasaki M, Tohda C, Kuraishi Y. Existence of capsaicin-sensitive glutamatergic terminals in rat hypothalamus. Neuroreport. 1998;9:2045–2048. doi: 10.1097/00001756-199806220-00025. [DOI] [PubMed] [Google Scholar]
- 100.Marinelli S, Pascucci T, Bernardi G, Puglisi-Allegra S, Mercuri NB. Activation of TRPV1 in the VTA excites dopaminergic neurons and increases chemical- and noxious-induced dopamine release in the nucleus accumbens. Neuropsychopharmacology. 2005;30:864–870. doi: 10.1038/sj.npp.1300615. [DOI] [PubMed] [Google Scholar]
- 101.Lam PM, Hainsworth AH, Smith GD, Owen DE, Davies J, Lambert DG. Activation of recombinant human TRPV1 receptors expressed in SH-SY5Y human neuroblastoma cells increases [Ca(2+)] (i), initiates neurotransmitter release and promotes delayed cell death. J Neurochem. 2007;102:801–811. doi: 10.1111/j.1471-4159.2007.04569.x. [DOI] [PubMed] [Google Scholar]
- 102.Lappin SC, Randall AD, Gunthorpe MJ, Morisset V. TRPV1 antagonist, SB-366791, inhibits glutamatergic synaptic transmission in rat spinal dorsal horn following peripheral inflammation. Eur J Pharmacol. 2006;540:73–81. doi: 10.1016/j.ejphar.2006.04.046. [DOI] [PubMed] [Google Scholar]
- 103.Sikand P, Premkumar LS. Potentiation of glutamatergic synaptic transmission by protein kinase C-mediated sensitization of TRPV1 at the first sensory synapse. J Physiol. 2007;581:631–647. doi: 10.1113/jphysiol.2006.118620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Musella A, De Chiara V, Rossi S, Prosperetti C, Bernardi G, Maccarrone M, Centonze D. TRPV1 channels facilitate glutamate transmission in the striatum. Mol Cell Neurosci. 2009;40:89–97. doi: 10.1016/j.mcn.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 105.Jiang CY, Fujita T, Yue HY, Piao LH, Liu T, Nakatsuka T, Kumamoto E. Effect of resiniferatoxin on glutamatergic spontaneous excitatory synaptic transmission in substantia gelatinosa neurons of the adult rat spinal cord. Neuroscience. 2009;164:1833–1844. doi: 10.1016/j.neuroscience.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 106.Xing J, Li J. TRPV1 receptor mediates glutamatergic synaptic input to dorsolateral periaqueductal gray (dl-PAG) neurons. J Neurophysiol. 2007;97:503–511. doi: 10.1152/jn.01023.2006. [DOI] [PubMed] [Google Scholar]
- 107.Karlsson U, Sundgren-Andersson AK, Johansson S, Krupp JJ. Capsaicin augments synaptic transmission in the rat medial preoptic nucleus. Brain Res. 2005;1043:1–11. doi: 10.1016/j.brainres.2004.10.064. [DOI] [PubMed] [Google Scholar]
- 108.Marinelli S, Vaughan CW, Christie MJ, Connor M. Capsaicin activation of glutamatergic synaptic transmission in the rat locus coeruleus in vitro. J Physiol. 2002;543:531–540. doi: 10.1113/jphysiol.2002.022863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gibson HE, Edwards JG, Page RS, Van Hook MJ, Kauer JA. TRPV1 channels mediate long-term depression at synapses on hippocampal interneurons. Neuron. 2008;57:746–759. doi: 10.1016/j.neuron.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Grueter BA, Brasnjo G, Malenka RC. Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nat Neurosci. 2010;13:1519–1525. doi: 10.1038/nn.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chavez AE, Chiu CQ, Castillo PE. TRPV1 activation by endogenous anandamide triggers postsynaptic long-term depression in dentate gyrus. Nat Neurosci. 2010;13:1511–1518. doi: 10.1038/nn.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maione S, Cristino L, Migliozzi AL, Georgiou AL, Starowicz K, Salt TE, Di Marzo V. TRPV1 channels control synaptic plasticity in the developing superior colliculus. J Physiol. 2009;587:2521–2535. doi: 10.1113/jphysiol.2009.171900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li HB, Mao RR, Zhang JC, Yang Y, Cao J, Xu L. Antistress effect of TRPV1 channel on synaptic plasticity and spatial memory. Biol Psychiatry. 2008;64:286–292. doi: 10.1016/j.biopsych.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 114.Kim SR, Kim SU, Oh U, Jin BK. Transient receptor potential vanilloid subtype 1 mediates microglial cell death in vivo and in vitro via Ca2+-mediated mitochondrial damage and cytochrome c release. J Immunol. 2006;177:4322–4329. doi: 10.4049/jimmunol.177.7.4322. [DOI] [PubMed] [Google Scholar]
- 115.Kim SR, Lee DY, Chung ES, Oh UT, Kim SU, Jin BK. Transient receptor potential vanilloid subtype 1 mediates cell death of mesencephalic dopaminergic neurons in vivo and in vitro. J Neurosci. 2005;25:662–671. doi: 10.1523/JNEUROSCI.4166-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shirakawa H, Yamaoka T, Sanpei K, Sasaoka H, Nakagawa T, Kaneko S. TRPV1 stimulation triggers apoptotic cell death of rat cortical neurons. Biochem Biophys Res Commun. 2008;377:1211–1215. doi: 10.1016/j.bbrc.2008.10.152. [DOI] [PubMed] [Google Scholar]
- 117.Amantini C, Mosca M, Nabissi M, Lucciarini R, Caprodossi S, Arcella A, Giangaspero F, Santoni G. Capsaicin-induced apoptosis of glioma cells is mediated by TRPV1 vanilloid receptor and requires p38 MAPK activation. J Neurochem. 2007;102:977–990. doi: 10.1111/j.1471-4159.2007.04582.x. [DOI] [PubMed] [Google Scholar]
- 118.Nucci C, Gasperi V, Tartaglione R, Cerulli A, Terrinoni A, Bari M, De Simone C, Agro AF, Morrone LA, Corasaniti MT, Bagetta G, Maccarrone M. Involvement of the endocannabinoid system in retinal damage after high intraocular pressure-induced ischemia in rats. Invest Ophthalmol Vis Sci. 2007;48:2997–3004. doi: 10.1167/iovs.06-1355. [DOI] [PubMed] [Google Scholar]
- 119.Pegorini S, Zani A, Braida D, Guerini-Rocco C, Sala M. Vanilloid VR1 receptor is involved in rimonabant-induced neuroprotection. Br J Pharmacol. 2006;147:552–559. doi: 10.1038/sj.bjp.0706656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pegorini S, Braida D, Verzoni C, Guerini-Rocco C, Consalez GG, Croci L, Sala M. Capsaicin exhibits neuroprotective effects in a model of transient global cerebral ischemia in Mongolian gerbils. Br J Pharmacol. 2005;144:727–735. doi: 10.1038/sj.bjp.0706115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Veldhuis WB, van der Stelt M, Wadman MW, van Zadelhoff G, Maccarrone M, Fezza F, Veldink GA, Vliegenthart JF, Bar PR, Nicolay K, Di Marzo V. Neuroprotection by the endogenous cannabinoid anandamide and arvanil against in vivo excitotoxicity in the rat: role of vanilloid receptors and lipoxygenases. J Neurosci. 2003;23:4127–4133. doi: 10.1523/JNEUROSCI.23-10-04127.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lastres-Becker I, de Miguel R, De Petrocellis L, Makriyannis A, Di Marzo V, Fernandez-Ruiz J. Compounds acting at the endocannabinoid and/or endovanilloid systems reduce hyperkinesia in a rat model of Huntington's disease. J Neurochem. 2003;84:1097–1109. doi: 10.1046/j.1471-4159.2003.01595.x. [DOI] [PubMed] [Google Scholar]
- 123.Di Marzo V, Lastres-Becker I, Bisogno T, De Petrocellis L, Milone A, Davis JB, Fernandez-Ruiz JJ. Hypolocomotor effects in rats of capsaicin and two long chain capsaicin homologues. Eur J Pharmacol. 2001;420:123–131. doi: 10.1016/s0014-2999(01)01012-3. [DOI] [PubMed] [Google Scholar]