Abstract
Porphyromonas gingivalis is a fimbriated mucosal pathogen implicated in chronic periodontitis (CP). The fimbriae are required for invasion of the gingival mucosa and for induction of CP in animal models of periodontitis. CP is associated with infection of immature dendritic cells (DCs) by P. gingivalis in situ and with increased numbers of dermal DCs (DDCs) and mature DCs in the lamina propria. The role of fimbriae in gaining entry into human DCs and how this modulates the inflammatory and effector immune responses, however, have not been explored. To address this, we generated monocyte-derived DCs (MDDCs) in vitro which phenotypically and functionally resemble DDCs. We show here that virulent fimbriated P. gingivalis 381, in contrast to its fimbria-deficient mutant, P. gingivalis DPG3, efficiently gains entry to MDDCs in a manner dependent on active cell metabolism and cytoskeletal rearrangement. In addition, uptake of 381, unlike DPG3, induces DCs to undergo maturation, upregulate costimulatory molecules, and secrete inflammation cytokines interleukin-1β (IL-1β), IL-6, tumor necrosis factor alpha, IL-10, and IL-12. Moreover, MDDCs pulsed with 381 also stimulated a higher autologous mixed lymphocyte reaction and induced a Th1-type response, with gamma interferon (IFN-γ) being the main cytokine. Monocytes used as controls demonstrated fimbria-dependent uptake of 381 as well but produced low levels of inflammatory cytokines compared to MDDCs. When MDDCs were pulsed with recombinant fimbrillin of P. gingivalis (10 μg/ml), maturation of MDDCs was also induced; moreover, matured MDDCs induced proliferation of autologous CD4+ T cells and release of IFN-γ. Thus, these results establish the significance of P. gingivalis fimbriae in the uptake of P. gingivalis by MDDCs and in induction of immunostimulatory Th1 responses.
Porphyromonas gingivalis is an oral pathogen associated with chronic periodontitis (CP), an infection of mucosal tissues that surround the dentition, which causes destruction of the alveolar bone and tooth loss (15). Recent studies have linked CP to increased risk of coronary vascular disease and preterm labor and have also identified P. gingivalis as one of the pathogens that enters the bloodstream after crossing the oral mucosal barrier and probably is instrumental in systemic manifestations (reviewed in reference 5). This is corroborated by several lines of evidence, most notably the presence of P. gingivalis and other species in atheromatous plaques from carotid endarterectomy specimens (24). P. gingivalis expresses a variety of virulence determinants that enable it to perturb the innate defenses and/or invade mucosa (reviewed in references 15, 25, and 40). Among these, fimbriae are important cell surface virulence factors involved in adherence of P. gingivalis to host cells. The fimbriae of P. gingivalis are critical determinants for induction of periodontitis in rats and, when used as immunogens, can reduce periodontal destruction in this model (14). In vitro, fimbriae are required for P. gingivalis to invade epithelial cells (32, 45, 54), endothelial cells (13), and fibroblasts (30) and to activate peritoneal macrophages (50) and THP-1 cells (21) (reviewed in reference 13). Mutation of the fimA gene, encoding fimbrillin, the major subunit of the fimbriae, prevents P. gingivalis adherence to, and invasion of, host cells (22). P. gingivalis fimbriae thus represent important cell structures involved in mucosal pathogenesis and periodontitis by facilitating colonization and invasion of mucosal cells and induction of inflammatory responses (33).
Immature dendritic cells (DCs) reside in the mucosa and are well equipped to capture a diverse array of antigens, apoptotic bodies, and allergens (reviewed in references 8 and 43) which can stimulate their maturation. Maturation of DCs is accompanied by downregulation of antigen capture machinery and upregulation of antigen-presenting molecules and production of cytokines required to prime naive T cells in lymphoid organs (reviewed in references 4, 8, and 49). In the human gingiva, the presence of the epidermal DCs, Langerhans cells, has been documented in many studies (17, 27, 28, 44, 46). Studies performed in our lab have demonstrated that the human gingiva contains two major subpopulations of DCs: immature Langerhans cells restricted to the epidermis and dermal dendritic cells (DDCs) restricted to the lamina propria (27). During CP there is an increase in the number of DDCs and CD83+ mature DCs in the lamina propria. Furthermore, by double immunofluorescence labeling, DCs appear to be undergoing maturation in situ (in CP) and surrounded with large clusters of CD4+ T cells (27). However, the stimuli that lead to their maturation and the type of T-cell response that might be generated in the lymphoid tissues remain ill defined, although P. gingivalis contacts DCs in situ (9). Several mechanistic studies emphasize the important role of the CD4+ T-cell response in the destruction of alveolar bone, a characteristic of CP (3, 51).
In the present study we generated monocyte-derived DCs (MDDCs) in vitro, which are very similar phenotypically and functionally to DDCs (4, 8, 49) that have been identified in human gingiva (27). We observed that fimbriated strain 381, but not afimbriated mutant DPG3, gains efficient entry into MDDCs and stimulates efficient maturation, costimulatory molecule expression, and cytokine production. Furthermore, MDDCs pulsed with 381 or its recombinant fimbrillin (r-Fim) induced a Th1-type response in autologous mixed lymphocyte reactions (MLR) and autologous CD4+ T cells, with gamma interferon (IFN-γ) being the main cytokine.
MATERIALS AND METHODS
Bacterial strains, growth conditions, bacterial labeling and uptake, and r-Fim.
P. gingivalis wild-type strain 381 and the corresponding fimA mutant, DPG3, were used in this study and were maintained on anaerobic blood agar (Fischer Scientific Co., Springfield, N.J.) and blood agar supplemented with erythromycin (10 μg/ml), respectively (37). Cultures were maintained at 37°C in an anaerobic glove box (Coy Laboratory Products, Inc., Ann Arbor, Mich.) in an atmosphere of 85% N2-5% H2,-10% CO2 for 3 to 5 days. For MDDC uptake experiments, cultures were transferred from plates into Schaedler broth (Difco, Detroit, Mich.) until the late log phase of growth and bacterial cells were labeled with 4′,6′-diamidino-2-phenylindole (DAPI) (Sigma Chemical Co., St. Louis, Mo.) as described previously (7). Bacteria were added to MDDCs in polypropylene tubes at a 25:1 ratio of bacteria to DCs. After 0.5, 1, 2, and 18 h in culture medium, 50-μl aliquots were removed and added to cytospin chambers. After cytocentrifugation, cyanoacrylate was added as a fixative and cover slips were put in place. See Fig. 1B and C for images of typical microscopic fields. An independent party coded the slides, and measurements (percentage of MDDCs that had taken up at least two bacterial cells per field for 10 fields per slide) were acquired blinded, as described previously (7). The percent viable DCs (typically >90% after 24 h) were monitored by trypan blue exclusion and did not differ between the strains (data not shown). Recombinant fimbrillin (r-Fim) was generated in Escherichia coli DH5α host cells and purified as described previously (48), and purity was confirmed by silver staining, which showed a single component with no detectable contaminants (data not shown)
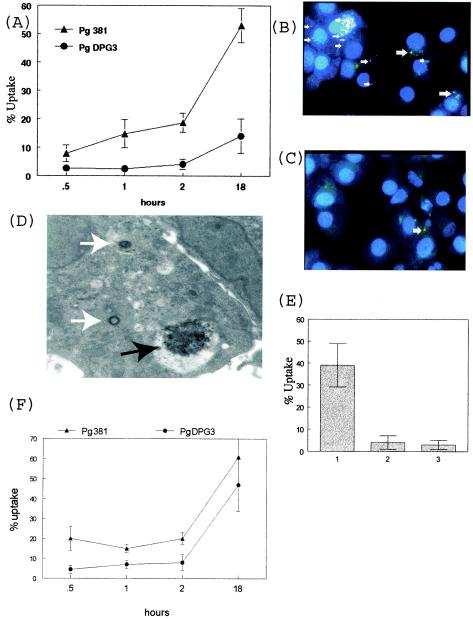
FIG.1.
Fimbria expression correlates with uptake of P. gingivalis by MDDCs. (A) The fimbria-deficient mutant DPG-3 and its fimbriated parent strain, 381, were labeled with DAPI and added at a 25:1 ratio with day 6 MDDCs, and percent uptake ± standard error was quantitated blindly by fluorescence microscopy at 0.5, 1, 2, and 18 h of coculture. (B) Representative images demonstrating uptake of blue DAPI-labeled P. gingivalis 381 (white arrows) by MDDCs at 18 h (magnification, ×100). (C) Minimal uptake of DAPI-labeled DPG-3 at 18 h. (D) Day 6 DCs after 18 h with 381 were subjected to negative staining and transmission electron microscopy. Shown at a magnification of ×12,500 is a DC containing individual P. gingivalis cells located inside MVC (white arrows). Also shown is a large cluster of P. gingivalis outside a vacuole (black arrow). (E) Day 6 DCs were incubated at 37°C alone (bar 1) or with 5 μg of cytochalasin D/ml (bar 2) for 10 min and then extensively washed or incubated on ice at 4°C (bar 3) and then pulsed with DAPI-labeled P. gingivalis 381 for 18 h.. (F) Uptake of P. gingivalis strains by peripheral blood MCs. Results are representative of the results achieved after repeating the experiment a minimum of three times.
DC cultures and multiparameter flow cytometry analysis.
MDDCs were generated as previously described (9, 28). Briefly, MCs were isolated from mononuclear fractions of peripheral blood by negative selection and seeded in the presence of granulocyte-macrophage colony-stimulating factor and interleukin-4 (IL-4) (1 × 105 to 2 × 105 cells/ml) for 6 to 8 days, after which flow cytometry was performed to confirm the immature DC phenotype (CD1a+ CD83−) (see Fig. 2A). Cell surface markers of DCs were evaluated by four-color immunofluorescence staining with the following monoclonal antibodies (MAbs): CD1a- FITC (Biosource), CD40-PE (Coulter/Immunotech), CD80-PE (Becton Dickinson), CD83-PE (Immunotech), CD86- PE (Pharmingen), HLA-DR-PerCP (Becton Dickinson), and CD14- APC (Caltag). After 30 min at 4°C and washing with staining buffer (phosphate-buffered saline [pH 7.2], 2 mM EDTA, 2% fetal bovine serum), cells were fixed in 1% paraformaldehyde. Analysis was performed with a FACScalibur flow cytometer (Becton Dickinson). Marker expression was analyzed as the percentage of positive cells in the relevant population defined by forward-scatter and side-scatter characteristics. Expression levels were evaluated by assessing mean fluorescence intensity (MFI) indices calculated by relating MFI noted with the relevant MAb to that obtained with the isotype control MAb for samples labeled in parallel and acquired using the same setting.
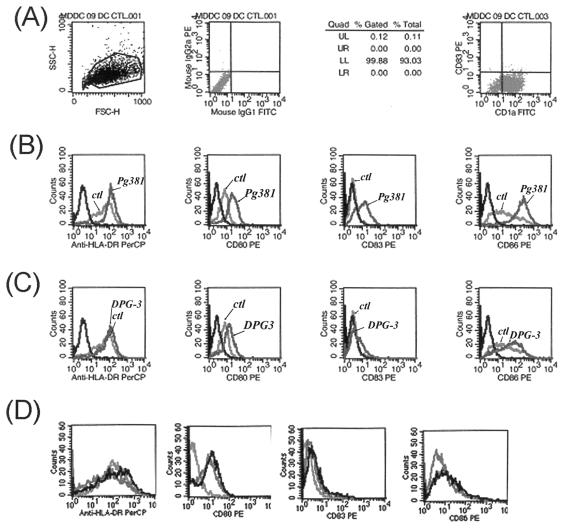
FIG. 2.
Fimbria expression correlates with MDDC maturation and costimulatory molecule expression. (A) Phenotype of day 6 immature CD1a+ CD83− MDDCs determined by FACS analysis; (B) Upregulation on MDDCs of HLA-DR, CD80, CD83, and CD86 after pulsing with P. gingivalis 381 for 18 h. Shown are isotype controls (ctl) and controls with no bacteria. (C) No upregulation of HLA-DR and CD83 and minimal upregulation of CD80 and CD86 after pulsing with P. gingivalis DPG3 for 18 h. Controls were as described for panel B. (D) No change in expression of HLA-DR, CD80, CD83, and CD86 on MCs pulsed with 381 for 18 h. Results are representative of three separate experiments.
Cytokines from MDDCs.
Culture supernatants were collected from MDDCs pulsed with P. gingivalis 381, DPG3, and r-Fim for 24 h. Culture supernatants were analyzed by flow cytometry using a cytometric bead array (CBA kit; BD Biosciences, SanDiego, Calif.). Based on a standard curve achieved for each cytokine, the CBA software calculates levels in picograms per milliliter.
MLR and CD4+ T-cell proliferation.
For proliferation experiments with P. gingivalis-pulsed MDDCs (see Fig. 4), responder cells were autologous lymphocytes purified from human buffy coats as described previously (9, 28). For proliferation experiments with r-Fim-pulsed MDDCs (see Fig. 5C), responder cells were autologous CD4+ T cells (9, 28) isolated from the mononuclear fraction of buffy coats through positive selection, using anti-CD4 MAb and goat anti-mouse immunoglobulin G-coated microbeads (Miltenyi Biotech GmbH, Gladbach, Germany). Isolation of CD4+ cells was achieved using Minimacs separation columns (Miltenyi Biotech GmbH) as described by the manufacturer. In all r-Fim experiments the isolated cells were 80 to 90% CD4+, as determined by staining with fluorescein isothiocyanate-conjugated anti-CD4 MAb followed by flow cytometry analysis (results not shown). MDDCs were washed extensively after a 24-h pulsing with antigens and cultured at graded doses (5,000, 1,000, and 300 DCs, all per 200 μl) in complete RPMI medium with 10% heat-treated fetal calf serum with autologous lymphocytes (50,000 cells/200 μl). Proliferation was determined after 5 days by uptake of tritiated thymidine (1 μCi/well for the last 16 h).
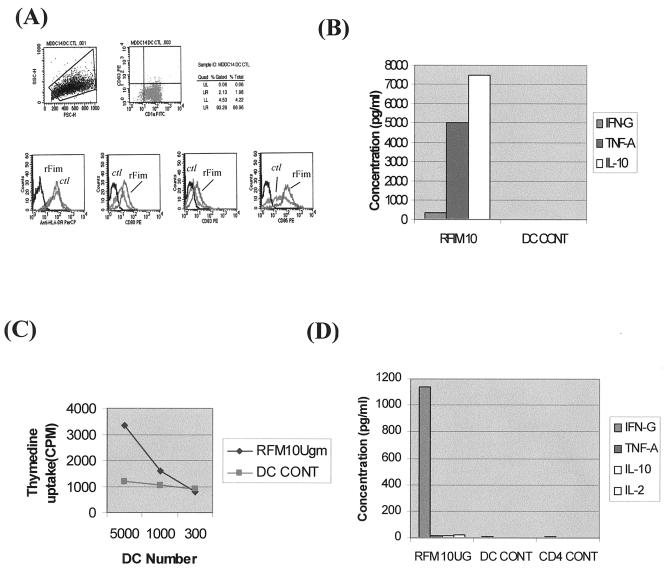
FIG. 4.
r-Fim of P. gingivalis also induces MDDCs to become immunostimulatory. (A) FACS analysis demonstrating upregulation in MFI of, from left to right, HLA-DR, CD80, CD83, and CD86 on MDDCs pulsed with P. gingivalis r-Fim at 10 μg/ml for 18 h or not pulsed with r-Fim. ctl, isotype controls. (B) FACS analysis of IFN-γ, TNF-α, and IL-10 released from MDDCs pulsed with r-Fim (RFIM10) or not pulsed (DC CONT) for 18 h. DC supernatants were analyzed by flow cytometry using the cytometric bead assay (CBA kit; BD Biosciences) as for Fig. 3. (C) MDDCs pulsed with 10 μg of r-Fim/ml for 18 h (RFM10Ugm) or not pulsed with r-Fim (DC CONT) were cocultured in graded doses (5,000, 1,000, and 300 DCs) with 50,000 autologous lymphocytes in AB serum and RPMI for 5 days, and uptake of tritiated thymidine was analyzed. (D) T-cell cytokines. Fifty thousand autologous CD4+ T cells were cocultured with 5,000 MDDCs in AB serum and RPMI, and T-cell supernatants were analyzed for IFN-γ, TNF-α, IL-10, and IL-2 by FACS analysis (CBA kit; BD Biosciences).
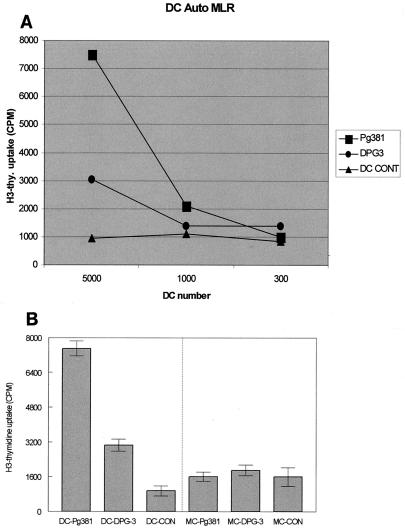
FIG. 5.
MDDCs that had taken up fimbriated P. gingivalis became potent immunostimulatory cells. (A) MDDCs pulsed with P. gingivalis strains for 18 h were cocultured in graded doses (5,000, 1,000, and 300 DCs) with 50,000 autologous lymphocytes in AB serum and RPMI for 5 days, and uptake of tritiated thymidine was analyzed by liquid scintillation counting. (B) No lymphocyte proliferation in response to MCs pulsed with 381. Shown is a comparison of lymphocyte proliferation in response to 5,000 P. gingivalis-pulsed DCs or MCs.
Statistical analyses.
Results of in vitro uptake, cytokine levels, and MLR were analyzed by either Student's t test (P < 0.05) or the Kruskall-Wallis test (P < 0.05) (Minitab, State College, Pa.).
RESULTS
Entry of P. gingivalis into MDDCs is fimbria dependent, requires active cell metabolism, and cytoskeletal rearrangement.
Based upon evidence that P. gingivalis fimbriae mediate induction of periodontitis in rats (14) and facilitate entry of P. gingivalis into other host cells (13, 22, 32, 33, 50, 54), we postulated that entry into MDDCs would also be fimbria dependent. Our results show that, indeed, the fimbriated strain 381 rapidly gained access to over 10% of MDDCs after only 1 h of coculture. This increased to over 50% after 18 h (Fig. 1A). Digital images acquired with a 100× objective (Fig. 1B) show multiple DAPI-labeled P. gingivalis 381 cells (white arrows) inside MDDCs. In contrast, the fimbria-deficient mutant DPG-3 did not gain access to MDDCs until about 18 h, when low-level internalization was observed (Fig. 1A and C). Electron microscopy confirmed intracellular localization of P. gingivalis into multivesiculated compartments (MVC), as well as the presence of clusters of P. gingivalis contained within apparent vacuoles in the cytoplasm (Fig. 1D). Capture was dependent on active cell metabolism and cytoskeletal rearrangement, as evidenced by experiments carried out at 4°C and with cytochalasin D pretreatment (Fig. 1E). Expression of fimbriae also correlated with capture by control MCs (Fig. 1F). We also conducted experiments to establish the role of integrins in uptake of 381 by MDDCs (data not shown). It was found that though immature MDDCs express β1 and β2 integrins on their surface, blocking these receptors with MAbs did not alter uptake of 381.
Fimbriated P. gingivalis or r-Fim induce maturation and costimulatory molecule expression on MDDCs.
The pathophysiology of CP in humans involves infiltration of the lamina propria with multiple DC subpopulations that are in the process of maturation or have matured (27). Accordingly, we analyzed the ability of fimbriated and afimbriated P. gingivalis to induce MDDC maturation and costimulatory molecule expression. Preliminary study of the kinetics of maturation and costimulation (data not shown) of MDDCs in response to P. gingivalis established 18 h as a peak; thus, 18 h was consistently used for analysis of maturation and costimulation (as well as cytokine secretion and T-cell proliferation [see Fig. 3 and 4]). As shown in Fig. 2B, 381 induced upregulation of HLA-DR, CD83, and the costimulatory molecules CD80 and CD86. In contrast, DPG3 did not induce upregulation of HLA-DR or CD83 and was a relatively weak inducer of CD80 and CD86 (Fig. 2C). Exposure of MDDCs to r-Fim for 18 h also induced DC maturation and costimulatory molecule expression (see Fig. 5A), although HLA-DR was not upregulated to any extent. Progenitor MCs did not express CD83 or costimulatory molecules after exposure to 381 or DPG3 (Fig. 2D).
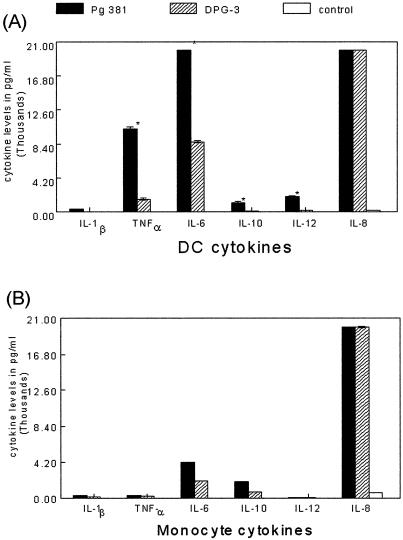
FIG. 3.
Fimbriated P. gingivalis induces potent secretion of inflammatory/dysregulatory cytokines by MDDCs. (A) Supernatants from MDDCs pulsed with P. gingivalis 381 or DPG-3 for 18 h were analyzed in triplicate by flow cytometry using the cytometric bead assay (CBA kit; BD Biosciences). Based on a standard curve for each cytokine, the software calculates levels in picograms per milliliter. The assay was performed in triplicate, and results shown are means ± standard errors (*, P < 0.05, 381 versus DPG3, two-sample Student's t test). (B) Supernatants from MCs pulsed with P. gingivalis 381 or DPG-3 for 18 h were analyzed as described for panel A.
Fimbriated P. gingivalis and r-Fim stimulate MDDCs to secrete an inflammatory and dysregulatory cytokine profile.
The local cytokine response in CP (reviewed in references 12 and 47) includes elevated levels of tumor necrosis factor alpha (TNF-α), IL-8, IL-6, IL-10, IL-12, and IFN-γ. We reasoned that fimbriae would be a significant determinant of the ability of P. gingivalis to induce secretion of inflammatory cytokines/chemokines by MDDCs. The results indicate that indeed, fimbriated P. gingivalis 381 stimulates elevated levels of TNF-α, IL-6, IL-10, IL-12, and IL-8 (Fig. 3A). In contrast, afimbriated DPG-3 stimulated low levels of TNF-α and IL-6 and undetectable levels of IL-10 and IL-12. Interestingly, high levels of the chemokine IL-8 were released regardless of the strain. Relative to MDDCs, progenitor MCs secreted low to undetectable levels of all cytokines, except for IL-8, which was equivalent to that secreted by MDDCs (Fig. 3B). r-Fim induced MDDCs to secrete IFN-γ and very high levels of TNF-α and IL-10 (Fig. 4B).
Fimbriated P. gingivalis or r-Fim induces MDDCs to become immunostimulatory cells.
Our in situ results suggest that the immunopathology of CP involves the formation of immune conjugates between CD83+ mature DCs and CD4+ T cells in diseased lamina propria (27). On the basis of this backdrop and the results presented above, we predicted that MDDCs pulsed with 381, relative to DPG3, would be more potent immunostimulatory cells in vitro. Accordingly, we cocultured 381- or DPG3-pulsed MDDCs (or MCs) with autologous lymphocytes (Fig. 5A) for 5 days. The results indicate that, indeed, MDDCs pulsed with 381 were immunostimulatory for autologous lymphocytes while MDDCs pulsed with afimbriated P. gingivalis were weakly immunostimulatory. MCs pulsed with either P. gingivalis strain (Fig. 5B) did not stimulate an autologous lymphocyte response. MDDCs pulsed with r-Fim were also immunostimulatory for CD4+ T cells, but to a lesser extent than those pulsed with whole intact 381. The T cells proliferated (Fig. 4C) and released >1,000 pg of IFN-γ/ml (Fig. 4D) but did not release TNF-α, IL-10, or IL-2.
DISCUSSION
The present study analyzed the ability of two P. gingivalis strains to gain entry into cultured MDDCs: fimbria-deficient mutant P. gingivalis DPG3 and its fimbriated parent strain, 381. Our results show that fimbriae are essential for this mucosal pathogen to gain entry into MDDCs efficiently (Fig. 1A to C) and that entry requires an intact MDDC cytoskeleton and active cell metabolism (Fig. 1E); moreover, entry culminates in packaging of P. gingivalis into MVC (Fig. 1D). Our in situ studies have previously documented that P. gingivalis gains entry into immature DCs in situ in diseased human gingiva (9), but the role of its virulence characteristics in this regard have not been defined. Studies with nonphagocytic epithelial and endothelial cells have also shown the requirement for P. gingivalis fimbriae in adherence to and invasion of these cells (13, 32, 54). Greater adherence to and invasion of an oral epithelial cell line (KB) was observed with wild-type P. gingivalis strains 33277, 381, and A7436, as compared to fimA mutants DPG3 and MPG1 (37). It has been shown that fimbriated P. gingivalis directs its entry into epithelial and endothelial cells by exploiting host cell signaling pathways (13, 32, 54). Receptors to which P. gingivalis fimbriae can bind have been identified and include β1 integrins (54) and cytokeratin 14 (48) on epithelial cells and β2 integrins, Toll-like receptor 2, and CD14 on macrophages/MCs (39, 50). P. gingivalis fimbrial adhesion to gingival epithelial cells represents a key step in the induction of the invasive process, which is accompanied by a transient increase in cytosolic Ca2+ concentration, activation of JNK, and inactivation of ERK1/ERK2 mitogen-activated protein kinases (26, 53). Studies performed with mouse peritoneal macrophages demonstrated that β2 integrins (CD11/CD18) are used by P. gingivalis fimbriae as cellular receptors for binding; moreover, the β chain (CD18) plays a central role in signaling (50). However, although immature MDDCs express β1 and β2 integrins on their surface, blocking these receptors with MAbs did not alter uptake of 381 (data not shown). However, like epithelial cells, treatment of MDDCs with cytochalasin D completely inhibited the uptake of 381, suggesting the requirement for actin polymerization (28). Further studies will be required to identify the receptor(s) involved in the uptake of fimbriated P. gingivalis 381 by MDDCs.
We showed by fluorescence-activated cell sorter (FACS) analysis that entry by fimbriated 381 upregulates the maturation marker CD83, costimulatory molecules B7.1 (CD80) and B7.2 (CD86), and antigen-presenting molecule HLA-DR on the cell surface (Fig. 2B). This observation is particularly relevant to our original finding that the immunopathology of CP (which is caused by P. gingivalis and other species) involves in situ maturation of DCs (19), including Langerhans cells and dermal DCs (27). The presence of mature DCs in CP has since been corroborated independently from several laboratories (1, 6, 34).
We further show that fimbriated P. gingivalis stimulates MDDCs to secrete inflammatory cytokines TNF-α and IL-6, the immunoregulatory cytokine IL-10, and the chemokine IL-8, while lower levels of IFN-γ, IL-12, and IL-1B were elicited (Fig. 3A). In contrast, DPG3 induced much lower levels of all cytokines except IL-8 (Fig. 3A). MCs were not as responsive as MDDCs, producing lower levels of cytokines, with fewer differences evident between 381 and DPG3 (Fig. 3B). Several studies have shown that P. gingivalis fimbriae induce inflammatory cytokines IL-1α, IL-1β, IL-6, and TNF-α from gingival fibroblasts, epithelial cells, and MCs/macrophages (23, 39, 50). Both IL-1 and TNF-α are important local regulatory factors in bone remodeling (19, 31) and apparently play an important role as immunological mediators in periodontal inflammation and the destruction of alveolar bone (2, 11, 20, 38). Interestingly, in the present study both MDDCs and MCs released comparable levels of the chemokine IL-8 regardless of the bacterial strain (Fig. 3). IL-8 is a potent chemokine which directs migration of neutrophils to the site of inflammation. Induction of IL-8 in gingival epithelial and endothelial cell lines by fimbriated P. gingivalis is controversial. Some investigators have shown increased levels (42), whereas others have shown downregulation of IL-8 (10, 36). Differences in the observations have been attributed to differences in growth conditions of P. gingivalis and in the size of the inocula (36). Understanding the modulation and regulation of chemokines and their receptors by fimbriated and afimbriated P. gingivalis may prove helpful in understanding the pathogenesis of periodontitis.
In the present study P. gingivalis-pulsed MDDCs were cocultured with graded doses of autologous lymphocytes. The lymphocytes demonstrated greater proliferation as compared to those cocultured with DPG3-pulsed MDDCs (Fig. 5A); moreover, the lymphocytes produced higher levels of IFN-γ and lower levels of TNF-α, IL-10,and IL-2, consistent with a Th1 effector response. The r-Fim of P. gingivalis also induced a Th1- type response (Fig. 4). Production of high levels of IFN-γ in response to P. gingivalis 381, suggestive of Th1 response, has also been observed in another recent study (1). To establish the type of Th response, gingival T-cell lines and clones specific to P. gingivalis were generated by pulsing DCs cells with 381. The results show that all T-cell clones were positive for CD4 and the majority produced IFN-γ and a minimal or negligible amount of IL-5 (1). The present study used lymphocytes purified from peripheral blood of a subject with undefined periodontal status. The gingiva contains an array of local T cells specific for other periodontal pathogens (52). Th1/Th2 polarization is also affected by pattern recognition receptors on DCs, the nature of the pathogens, DC subsets, cytokine released by T cells, and other cells in the vicinity (41). A general lack of Th1 or Th2 cytokine polarization best describes the cytokine microenvironment in CP (18). Our published studies of mice in vivo (42) and of human MDDCs in vitro (29) indicate that the lipopolysaccharide of P. gingivalis shifts the DC response away from Th1, towards Th2. Thus, different antigens or structures from the same bacterial species appear to induce diametrically opposed effector responses. This is evocative of the ability of other human pathogens, most notably the helminth parasites, to produce different components that induce different effector responses in an apparent effort to protect themselves from elimination (reviewed in reference 35). MDDCs pulsed with r-Fim induced limited proliferation of autologous CD4+ T cells in the present study and also did not upregulate HLA-DR (Fig. 4C). Thus, further development of fimbria-based vaccines for CP (16) should include basic study of the ability of candidate molecules to be processed and presented in vitro prior to in vivo vaccine studies.
Acknowledgments
This study was supported by Public Health Service grants DE1432803 and DE13154-01 from the National Institute of Dental and Craniofacial Research.
Special thanks to H. Sojar, SUNY—Buffalo, for the purified r-Fim.
Editor: J. T. Barbieri
REFERENCES
- 1.Aroonrerk, N., S. Pichyangkul, K. Yongvanitchit, M. Wisetchang, N. Sa-Ard-Iam, S. Sirisinha, and R. Mahanonda. 2003. Generation of gingival T cell lines/clones specific with Porphyromonas gingivalis pulsed dendritic cells from periodontitis patients. J. Periodontal Res. 38:262-268. [DOI] [PubMed] [Google Scholar]
- 2.Assuma, R., T. Oates, D. Cochran, S. Amar, and D. T. Graves. 1998. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J. Immunol. 160:403-409. [PubMed] [Google Scholar]
- 3.Baker, P. J., L. Howe, J. Garneau, and D. C. Roopenian. 2002. T cell knockout mice have diminished alveolar bone loss after oral infection with Porphyromonas gingivalis. FEMS Immunol. Med. Microbiol. 34:45-50. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 5.Beck, J. D., G. Slade, and S. Offenbacher. 2000. Oral disease, cardiovascular disease and systemic inflammation. Periodontology 2000 23:110-120. [DOI] [PubMed] [Google Scholar]
- 6.Cirrincione, C., N. Pimpinelli, L. Orlando, and P. Romagnoli. 2002. Lamina propria dendritic cells express activation markers and contact lymphocytes in chronic periodontitis. J. Periodontol. 73:45-52. [DOI] [PubMed] [Google Scholar]
- 7.Cutler, C. W., R. R. Arnold, and H. A. Schenkein. 1993. Inhibition of C3 and IgG proteolysis enhances phagocytosis of Porphyromonas gingivalis. J. Immunol. 151:7016-7029. [PubMed] [Google Scholar]
- 8.Cutler, C. W., R. Jotwani, and B. Pulendran. 2001. Dendritic cells: immune saviors or Achilles' heel? Infect. Immun. 69:4703-4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cutler, C. W., R. Jotwani, K. A. Palucka, J. Davoust, D. Bell, and J. Banchereau. 1999. Evidence and a novel hypothesis for the role of dendritic cells and Porphyromonas gingivalis in adult periodontitis. J. Periodontal Res. 34:406-412. [DOI] [PubMed] [Google Scholar]
- 10.Darveau, R. P., C. M. Belton, R. A. Reife, and R. J. Lamont. 1998. Local chemokine paralysis, a novel pathogenic mechanism for Porphyromonas gingivalis. Infect. Immun. 66:1660-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delima, A. J., T. Oates, R. Assuma, Z. Schwartz, D. Cochran, S. Amar, and D. T. Graves. 2001. Soluble antagonists to interleukin-1 (IL-1) and tumor necrosis factor (TNF) inhibits loss of tissue attachment in experimental periodontitis. J. Clin. Periodontol. 28:233-240. [DOI] [PubMed] [Google Scholar]
- 12.De Nardin, E. 2001. The role of inflammatory and immunological mediators in periodontitis and cardiovascular disease. Ann. Periodontol. 6:30-40. [DOI] [PubMed] [Google Scholar]
- 13.Deshpande, R. G., M. B. Khan, and C. A. Genco. 1998. Invasion of aortic and heart endothelial cells by Porphyromonas gingivalis. Infect. Immun. 66:5337-5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans, R. T., B. Klausen, H. T. Sojar, G. S. Bedi, C. Sfintescu, N. S. Ramamurthy, L. M. Golub, and R. J. Genco. 1992. Immunization with Porphyromonas (Bacteroides) gingivalis fimbriae protects against periodontal destruction. Infect. Immun. 60:2926-2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ezzo, P., and C. W. Cutler. 2003. Microorganisms as risk factors for periodontal disease. Periodontology 2000 32:24-35. [DOI] [PubMed] [Google Scholar]
- 16.Fan, Q., T. Sims, H. Sojar, R. Genco, and R. C. Page. 2001. Fimbriae of Porphyromonas gingivalis induce opsonic antibodies that significantly enhance phagocytosis and killing by human polymorphonuclear leukocytes. Oral Microbiol. Immunol. 16:144-152. [DOI] [PubMed] [Google Scholar]
- 17.Gemmell, E., C. L. Carter, D. N. Hart, K. E. Drysdale, and G. J. Seymour. 2002. Antigen-presenting cells in human periodontal disease tissues. Oral Microbiol. Immunol. 17:388-393. [DOI] [PubMed] [Google Scholar]
- 18.Gemmell, E., K. Yamazaki, and G. J. Seymour. 2002. Destructive periodontitis lesions are determined by the nature of the lymphocytic response. Crit. Rev. Oral Biol. Med. 13:17-34. [DOI] [PubMed] [Google Scholar]
- 19.Gowen, M., D. D. Wood, and R. G. Russell. 1985. Stimulation of the proliferation of human bone cells in vitro by human monocyte products with interleukin-1 activity. J. Clin. Investig. 75:1223-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graves, D. T., A. J. Delima, R. Assuma, S. Amar, T. Oates, and D. Cochran. 1998. Interleukin-1 and tumor necrosis factor antagonists inhibit the progression of inflammatory cell infiltration toward alveolar bone in experimental periodontitis. J. Periodontol. 69:1419-1425. [DOI] [PubMed] [Google Scholar]
- 21.Hajishengallis, G., M. Martin, H. T. Sojar, A. Sharma, R. E. Schifferle, E. DeNardin, M. W. Russell, and R. J. Genco. 2002. Dependence of bacterial protein adhesins on toll-like receptors for proinflammatory cytokine induction. Clin. Diagn. Lab. Immunol. 9:403-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamada, N., K. Watanabe, C. Sasakawa, M. Yoshikawa, F. Yoshimura, and T. Umemoto. 1994. Construction and characterization of a fimA mutant of Porphyromonas gingivalis. Infect. Immun. 62:1696-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanazawa, S., K. Hirose, Y. Ohmori, S. Amano, and S. Kitano. 1988. Bacteroides gingivalis fimbriae stimulate production of thymocyte-activating factor by human gingival fibroblasts. Infect. Immun. 56:272-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haraszthy, V. I., J. J. Zambon, M. Trevisan, M. Zeid, and R. J. Genco. 2000. Identification of periodontal pathogens in atheromatous plaques. J. Periodontol. 71:1554-1560. [DOI] [PubMed] [Google Scholar]
- 25.Holt, S. C., L. Kesavalu, S. Walker, and C. A. Genco. 1999. Virulence factors of Porphyromonas gingivalis. Periodontology 2000 20:168-238. [DOI] [PubMed] [Google Scholar]
- 26.Izutsu, K. T., C. M. Belton, A. Chan, S. Fatherazi, J. P. Kanter, Y. Park, and R. J. Lamont. 1996. Involvement of calcium in interactions between gingival epithelial cells and Porphyromonas gingivalis. FEMS Microbiol. Lett. 144:145-150. [DOI] [PubMed] [Google Scholar]
- 27.Jotwani, R., and C. W. Cutler. 2003. Multiple dendritic cell (DC) subpopulations in human gingiva and association of mature DCs with CD4+ T-cells in situ. J. Dent. Res. 82:736-741. [DOI] [PubMed] [Google Scholar]
- 28.Jotwani, R., A. K. Palucka, M. Al-Quotub, M. Nouri-Shirazi, J. Kim, D. Bell, J. Banchereau, and C. W. Cutler. 2001. Mature dendritic cells infiltrate the T cell-rich region of oral mucosa in chronic periodontitis: in situ, in vivo, and in vitro studies. J. Immunol. 167:4693-4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jotwani, R., B. Pulendran, S. Agrawal, and C. W. Cutler. 2003. Human dendritic cells respond to Porphyromonas gingivalis LPS by promoting TH2 effector response in vitro. Eur. J. Immunol. 33:2980-2986. [DOI] [PubMed] [Google Scholar]
- 30.Kontani, M., S. Kimura, I. Nakagawa, and S. Hamada. 1997. Adherence of Porphyromonas gingivalis to matrix proteins via a fimbrial cryptic receptor exposed by its own arginine-specific protease. Mol. Microbiol. 24:1179-1187. [DOI] [PubMed] [Google Scholar]
- 31.Krakauer, T., J. J. Oppenheim, and H. E. Jasin. 1985. Human interleukin 1 mediates cartilage matrix degradation. Cell. Immunol. 91:92-99. [DOI] [PubMed] [Google Scholar]
- 32.Lamont, R. J., A. Chan, C. M. Belton, K. T. Izutsu, D. Vasel, and A. Weinberg. 1995. Porphyromonas gingivalis invasion of gingival epithelial cells. Infect. Immun. 63:3878-3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lamont, R. J., and Ö. Yilmaz. 2002. In or out: the invasiveness of oral bacteria. Periodontology 2000 30:61-69. [DOI] [PubMed] [Google Scholar]
- 34.Mahanonda, R., N. Sa-Ard-Iam, K. Yongvanitchit, M. Wisetchang, I. Ishikawa, T. Nagasawa, D. S. Walsh, and S. Pichyangkul. 2002. Upregulation of co-stimulatory molecule expression and dendritic cell marker (CD83) on B cells in periodontal disease. J. Periodontal Res. 37:177-183. [DOI] [PubMed] [Google Scholar]
- 35.Maizels, R. M., and M. Yazdanbakhsh. 2003. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat. Rev. Immunol. 3:733-744. [DOI] [PubMed] [Google Scholar]
- 36.Nassar, H., H. H. Chou, M. Khlgatian, F. C. Gibson III, T. E. Van Dyke, and C. A. Genco. 2002. Role for fimbriae and lysine-specific cysteine proteinase gingipain K in expression of interleukin-8 and monocyte chemoattractant protein in Porphyromonas gingivalis-infected endothelial cells. Infect. Immun. 70:268-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Njoroge, T., R. J. Genco, H. T. Sojar, N. Hamada, and C. A. Genco. 1997. A role for fimbriae in Porphyromonas gingivalis invasion of oral epithelial cells. Infect. Immun. 65:1980-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oates, T. W., D. T. Graves, and D. L. Cochran. 2002. Clinical, radiographic and biochemical assessment of IL-1/TNF-alpha antagonist inhibition of bone loss in experimental periodontitis. J. Clin. Periodontol. 29:137-143. [DOI] [PubMed] [Google Scholar]
- 39.Ogawa, T., Y. Asai, M. Hashimoto, and H. Uchida. 2002. Bacterial fimbriae activate human peripheral blood monocytes utilizing TLR2, CD14 and CD11a/CD18 as cellular receptors. Eur. J. Immunol. 32:2543-2550. [DOI] [PubMed] [Google Scholar]
- 40.Potempa, J., A. Banbula, and J. Travis. 2000. Role of bacterial proteinases in matrix destruction and modulation of host responses. Periodontology 2000 24:153-192. [DOI] [PubMed] [Google Scholar]
- 41.Pulendran, B., J. Banchereau, E. Maraskovsky, and C. Maliszewski. 2001. Modulating the immune response with dendritic cells and their growth factors. Trends Immunol. 22:41-47. [DOI] [PubMed] [Google Scholar]
- 42.Pulendran, B., P. Kumar, C. W. Cutler, M. Mohamadzadeh, T. Van Dyke, and J. Banchereau. 2001. Lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo. J. Immunol. 167:5067-5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rescigno, M., M. Rittig, S. Citterio, M. K. Matyszak, M. Foti, F. Granucci, M. Martino, U. Fascio, P. Rovere, and P. Ricciardi-Castagnoli. 1999. Interaction of dendritic cells with bacteria, p. 403-419. In M. E. Lotze and A. W. Thomson (ed.), Dendritic cells: biology and clinical applications. Academic Press, Inc., San Diego, Calif.
- 44.Saglie, F. R., J. H. Pertuiset, C. T. Smith, M. G. Nestor, F. A. Carranza, Jr., M. G. Newman, M. T. Rezende, and R. Nisengard. 1987. The presence of bacteria in the oral epithelium in periodontal disease. III. Correlation with Langerhans cells. J. Periodontol. 58:417-422. [DOI] [PubMed] [Google Scholar]
- 45.Sandros, J., C. Karlsson, D. F. Lappin, P. N. Madianos, D. F. Kinane, and P. N. Papapanou. 2000. Cytokine responses of oral epithelial cells to Porphyromonas gingivalis infection. J. Dent. Res. 79:1808-1814. [DOI] [PubMed] [Google Scholar]
- 46.Seguier, S., G. Godeau, M. Leborgne, G. Pivert, and N. Brousse. 2000. Quantitative morphological analysis of Langerhans cells in healthy and diseased human gingiva. Arch. Oral Biol. 45:1073-1081. [DOI] [PubMed] [Google Scholar]
- 47.Seymour, G. J., and E. Gemmell. 2001. Cytokines in periodontal disease: where to from here? Acta Odontol. Scand. 59:167-173. [DOI] [PubMed] [Google Scholar]
- 48.Sojar, H. T., A. Sharma, and R. J. Genco. 2002. Porphyromonas gingivalis fimbriae bind to cytokeratin of epithelial cells. Infect. Immun. 70:96-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steinman, R. M., M. Pack, and K. Inaba. 1997. Dendritic cells in the T-cell areas of lymphoid organs. Immunol. Rev. 156:25-37. [DOI] [PubMed] [Google Scholar]
- 50.Takeshita, A., Y. Murakami, Y. Yamashita, M. Ishida, S. Fujisawa, S. Kitano, and S. Hanazawa. 1998. Porphyromonas gingivalis fimbriae use β2 integrin (CD11/CD18) on mouse peritoneal macrophages as a cellular receptor, and the CD18 beta chain plays a functional role in fimbrial signaling. Infect. Immun. 66:4056-4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Teng, Y. T., H. Nguyen, X. Gao, Y. Y. Kong, R. M. Gorczynski, B. Singh, R. P. Ellen, and J. M. Penninger. 2000. Functional human T-cell immunity and osteoprotegerin ligand control alveolar bone destruction in periodontal infection. J. Clin. Investig. 106(6):R59-R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wassenaar, A., C. Reinhardus, T. Thepen, L. Abraham-Inpijn, and F. Kievits. 1995. Cloning, characterization, and antigen specificity of T-lymphocyte subsets extracted from gingival tissue of chronic adult periodontitis patients. Infect. Immun. 63:2147-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watanabe, K., O. Yilmaz, S. F. Nakhjiri, C. M. Belton, and R. J. Lamont. 2001. Association of mitogen-activated protein kinase pathways with gingival epithelial cell responses to Porphyromonas gingivalis infection. Infect. Immun. 69:6731-6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yilmaz, O., K. Watanabe, and R. J. Lamont. 2002. Involvement of integrins in fimbriae-mediated binding and invasion by Porphyromonas gingivalis. Cell Microbiol. 4:305-314. [DOI] [PubMed] [Google Scholar]