Abstract
Objective: Measure total tau levels in the circulation of living humans, validate the methods employed and determine if there are consistent differences in total tau levels between normal controls and individuals with mild cognitive impairment (MCI) and/or Alzheimer’s disease (AD). Methods: Employing ELISA methods, validated by Western bolts using three separate tau antibodies, we quantified total tau levels in serially collected serum and plasma samples from individuals longitudinally evaluated for cognitive performance. Results: We identified substantial levels of tau in human circulation using plasma, but not serum. The measurement of authentic tau protein was verified by Western blots using a C-terminal specific antibody, an N-terminal specific antibody and antibody used in the commercially available ELISA kit. We revealed a significant decrease in plasma levels of total tau among subjects with MCI compared to cognitively normal controls, with a further highly significant reduction in AD patients compared to both MCI and normal controls. We also found a significant positive correlation between changing levels of plasma tau and cognitive performance within the entire population and among AD patients. Conclusions: The data suggest that changes in circulating tau levels quantified in plasma samples, but not serum samples, may represent a viable biomarker for tracking the progression of AD and the efficacy of medications in its treatment.
Keywords: Circulating tau levels, mild cognitive impairment, Alzheimer’s disease
Introduction
The search for a reliable biomarker for Alzheimer’s disease (AD) has been ongoing for the last decade or longer. Initially, blood samples were assessed for changes in amyloid-beta (Aβ) - driven by the abundant data that Aβ in the brain was a prime candidate for precipitating AD. It has been reported that there are increased tau and 181-p-tau levels in CSF of MCI subjects converting to AD and that levels are stable in controls and MCI subjects not converting to AD, while the levels of Aβ42 were decreased at baseline in MCI subjects converting to AD [1]. It has been reported that increasing 231-p-tau levels may also be a predictor of conversion from MCI to AD [2].
The initial observations of Blennow’s group [3] between predictable changes in CSF levels of tau and amyloid beta (Aβ) and the risk of AD as well as transition from MCI to AD [4-7] are currently being verified, validated and standardized by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) [8,9]. A number of the research groups - including the ADNI cooperative - have repeatedly reported on tau levels in CSF as a biomarker for AD [10-14] without ever reporting circulating tau levels. There is one report of antibodies to tau in human blood with higher levels to phosporylated-tau occurring in a limited number of patients with AD [15], and recently it was noted by the ADNI group that “methods to analyze tau in blood are at the experimental beginning” [16].
Initial studies focused on measuring tau in the blood of individuals with AD were discouraging [17]. Employing sandwich ELISA methods plasma tau was quantifiable in only 1 of 16 individuals with AD and 7 of 15 normal controls. These authors noted that plasma tau levels were not increased in AD and concluded that circulating tau levels cannot be utilized diagnostically for the disorder [17]. More recently, using carboxylated microsphere bead methods to quantify tau levels in plasma [18], increased level of tau were observed 48 hours after onset of coma after a cardiac arrest, reaching levels as high as 500 pg/ml. Most recently more sensitive ELISA kits for quantifying tau have been developed and used to study serum.
Studies investigating serum samples using this more sensitive ELISA for tau have also been mixed, in that increased levels have been noted in CJD [19] with levels below detection limits in AD and Controls, in multiple sclerosis but being below detection limits in the controls [20], in ischemic stroke [21,22] and in adult [23] and pediatric head trauma [24]. It is of note that in rats with experimental brain injury, serum tau levels are increased from a basal level of 18 pg/ml to 74 pg/ml one hour after insult using these more sensitive ELISA kits [25].
Using the same sensitive ELISA kits noted above, we measured total tau in human plasma, and now report significant differences between cognitively normal control individuals, subjects with MCI and patients with AD. Using the antibody employed in the ELISA kit and antibodies supplied by Dr. L. “Skip” Binder we validated the ELISA methods and results by Western blots.
Materials and methods
Population pool
Inclusion of control, MCI and AD subjects in the current biomarker study was predicated on their participation in the Banner Sun Health Research Institute Brain Donation Program. After an IRB approved consent was attained for participation in the Donation program, subjects received complete medical, neurological, and neuropsychological assessments. All subjects included in the Brain Donation Program were prospectively characterized, diagnosed, and assessed during life and followed to autopsy. AD subjects met NINCDS-ADRDA criteria for a clinical diagnosis of probable or possible AD [26]. The MCI subjects were diagnosed clinically using Petersen criteria [27-29]: all were categorized as single or multi-domain MCI. Control subjects were defined as having no demonstrable cognitively based limitations of activities of daily living including, when applicable, employment. Rigorous criteria were used to exclude anyone with any type of symptomatic or severe brain-related neurologic or psychiatric illnesses. This was done by prospective interview of the participant and careful scrutiny of the medical records.
Patient population
Subjects investigated in this biomarker study were a sub-set of individuals participating in our Brain Donation Program at Banner Sun Health Research Institute noted above. Studies were performed on plasma and sera samples from this sub-set of individuals participating in Brain Donation Program (thus allowing us access to the clinical data and diagnosis for each individual gathered under the umbrella of the larger study). Participation in this study was voluntary and at no cost to the subjects.
All subjects entered into the study were thoroughly informed of the details of the study. Each subject signed (and dated) a second separate written IRB approved Informed Consent Statement, which was witnessed in order to participate in this biomarker study. These individuals were rigorously evaluated clinically predicated on their willingness to donate their brain upon demise (Brain Donation Program Participants). Standardized clinical instruments were administered to assess cognitive function.
Study design
Individuals participating in the ongoing longitudinal study provide a blood sample on each day that they are assessed for cognitive performance and consensus diagnosis as part of the Brain Donation Program. The MMSE [30], Rey’s AVLT [31,32], and the clock draw [33] were performed annually as part of the clinical evaluation. Blood samples were collected in purple top tubes, centrifuged and the plasma was stored at -70 °C in 100 μl aliquots within 30 minutes of collection until analysis for total tau levels and other selected biomarkers. On the same day, a red top tube of blood was obtained from each individual and resultant sera was collected and similarly stored until biomarker analysis. Apolipoprotein-E genotyping was not performed and CSF samples were not available for investigation. Tau levels (total human tau) were established in each plasma sample and selected serum samples processed according our protocol using ELISA quantification methods employing ELISA (Immunoassay) kits and standards purchased from Invitrogen Corp., Camarillo, CA.
Of the over 350 individuals we have longitudinally collected both plasma and sera in the last decade we selected all individual having a diagnosis of MCI or AD for investigation (N = 47 and 49 respectively). We randomly selected twice as many age-matched cognitively normal controls for assessment (N = 110). In 38 control individuals we performed test-retest assessment; separate 100ul samples were thawed and analyzed for total tau levels in independent assays. In 29 individuals we performed tissue type and deterioration assessments; we quantified total tau levels in plasma and serum collected simultaneously, and then allowed the thawed plasma sample to sit at 4° C for 24 hours and then reassessed the level of total tau.
Tau ELISA assay
Samples were assayed using the ELISA kit from Invitrogen (#KHB0042) as previously detailed [34]. All reagents used are supplied in the kit. Each ELISA plate was read at 450nm in a Bio-Tex ELx800 plate reader. Reader software (4 parameter algorithm) calculated all data.
Western blots
Plasma samples were stripped of Albumin/IgG using ProteoSeek columns (IgG removal kit #89875, Thermo Sci). We found that by reducing the volume of the final wash that we could reduce the dilution of samples from 1:17 as called for in the kit to a dilution of only 1:9, thus increasing the concentration of protein applied to the gel lanes. Ten to fifteen microliters of resultant eluant were added per well using 4-12% Bis-Tris gels (Invitrogen)., transferred to nitrocellulose .45 micron pore size papers, and papers were immersed in antibody overnight and developed the next day. No fluorescent enhancement was used to visualize stained bands. The non-commercially available Total-tau antibody employed by Invitrogen in making their ELISA kit was quite generously supplied by Invitrogen. Two antibodies, Tau-7 (N-terminal specific) and Tau-12 (C-terminal specific), were generously supplied by Lester ‘Skip’ Binder.
A total of 10 control individuals, 6 individuals with MCI and 8 AD patients were assessed for tau immunoreactive bands by Western blots using all three antibodies. Plasma samples from an additional 3 control individuals were incubated with and without proteinase-K (Promega) after IgG stripping to affirm that tau immunoreactive bands were proteins (employing tau-7 and tau-12 antibodies), likewise samples from an additional 6 control individuals were incubated with and without phosphatase at 37 C for 60 minutes to affirm that higher MW bands stained by tau-12 were likely various forms of p-tau.
Statistical analysis
In the cross sectional analysis the mean level of total tau was compared among the three groups: control, MCI, and AD by using a one way analysis of variance (ANOVA) with post hoc comparison of means depending on Fisher’s protected least significant differences procedure. In addition, for each group total tau was correlated with the MMSE, AVLT, AVLT-A7 and clock draw scores using Pearson’s correlation coefficient.
Longitudinal analysis was conducted using two methods. In the first, slopes were computed by taking the change in tau between successive visits and dividing it by the length of time between visits (estimated slope of the change in total tau between visits). Each slope was assigned to one of seven groups: control to control; CC, control to MCI; CM, control to AD; CA, MCI to AD; MA, MCI to MCI; MM, MCI to control; MC, or AD to AD; AA depending on the diagnosis at the two visits. For example, if a subject was cognitively intact at the first visit but MCI at the second, then that slope was assigned to the CM group. Mean slopes were compared among these 7 groups using a linear mixed model (LMM) since some subjects had three visits and were assigned to different groups on the successive visits. The LMM accounts for the correlation between multiple slopes are computed on the same subject. An alternative analysis used a different LMM to regress total tau on time on study using a random intercept model with fixed effects being the diagnosis at that visit and a potential for interaction between diagnosis and the effect of time on study. Statistical significance is set at p < 0.05 throughout.
Results
Western blot validation of ELISA methods
A sample of Tau isoform ladder standard shows the characteristic six prominent bands and authentic full length h441 tau standard show a prominent band at about 58 kilodalton stained with Invitrogen total-tau antibody (employed in the ELISA kits; Figure 1), tau-7 (not shown) and tau-12 (Figure 2). Bands consistent with authentic tau were found in every plasma sample from control, MCI and AD individuals, (and in rabbit plasma from animals receiving normal control or cholesterol-enriched diet [34], Figure 1), thus validating the ELISA methods for measuring tau in plasma with three separate specific tau antibodies. It is of note that there are both lower MW and higher MW protein bands identified by all three tau antibodies in the human samples. Incubation of plasma samples with proteinase-K eliminated all tau-7 and -12 immunoreactive bands thus verifying that observed immunoreactive bands were different MW proteins. Each plasma sample incubated with phosphatase showed a reduction in higher MW immunoreactive bands and increased intensity of bands consistent with authentic h441 (not shown), thus indicating that p-tau in the samples had likely been converted to non-phosphorylated tau.
Figure 1.
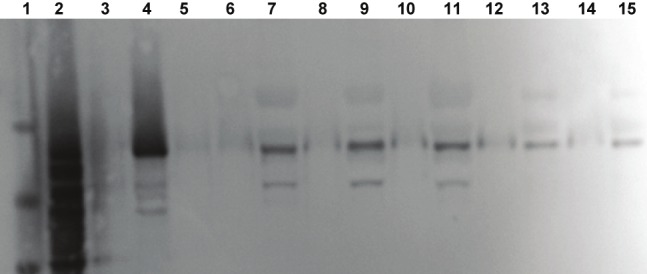
Western blot of standards and plasma samples immunostained with the total-tau antibody employed in the total-tau quantification ELISA kits produced by Invitrogen. Molecular weight standards are in lane 1, tau isoform standards are in lane 2, and authentic human 441 tau is seen in lane 4. No sample (blanks) was added to lanes 3, 5, 6, 8, 10, 12 and 14. A plasma sample - after stripping IgG - from an Alzheimer’s patient was applied to lane 7, from an individual with MCI to lane 9, from a control subject to lane 11, from a rabbit fed control diet to lane 13 and from a rabbit fed a 1% cholesterol diet to lane 15.
Figure 2.
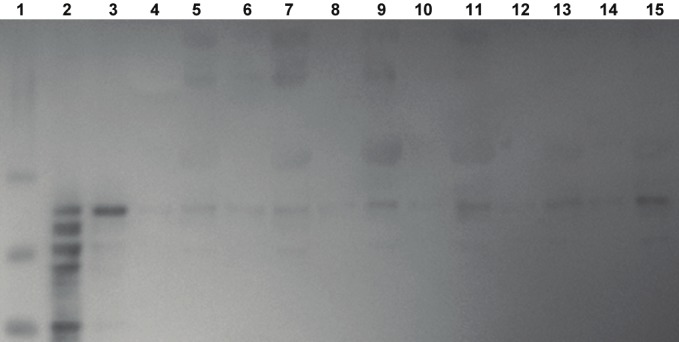
Western blot of standards and plasma samples immunostained with tau-12 antibody (C-terminal) suppliedby Dr. Lester Binder. Molecular weight standards are in lane 1, tau isoform standards are in lane 2, and authentichuman 441 tau is seen in lane 3. No sample (blanks) was added to lanes 4, 6, 8, 10, 12 and 14. A plasma sample - after stripping IgG - from two separate AD patients are seen in lanes 5 and 7, from two separate individuals withMCI are in lanes 9 and 11, and from two separate age-matched control are in lanes 13 and 15.
Tau quantification by ELISA
The mean age (years ± SD) for the control (78.5 ± 7.3) and the MCI (78.7 ± 12.3) populations were somewhat younger than the AD (84.4 ± 7.1) study population. The percent males in the control population was less (39 %) than in the MCI and AD populations, which were similar (MCI = 56 %; AD = 53 %).
Cross sectional analysis of ELISA data
The mean circulating tau level (picograms/ml) is depressed in plasma of patients with AD when compared to both cognitively normal control and individuals with mild cognitive impairment (MCI) (P< 0.0001 and p=0.0002, respectively) (Table 1). The mean Tau levels were also significantly reduced in MCI compared to control (P = 0.048, Table 1).
Table 1.
Mean and standard deviation of total tau levels (picograms/ml plasma) by group.
p = 0.048 compared to control and p = 0.0002 compared to AD;
p = 0.0001 compared to control
There was no correlation found between total tau levels and the neuropsychological tests within each group except for the correlation between tau and AVLT score in the AD group (r = 0.45, P < 0.02). When the three groups are combined total tau is positively correlated with three tests: MMSE, AVLT, and AVLT-A7 (p = 0.01), but not with the clock draw (Table 2).
Table 2.
Pearson’s correlation between total tau levels and neuropsychological test scores.
| Group | MMSE | AVLT | A7 | Clock Draw |
|---|---|---|---|---|
| AD | -0.005 | 0.455* | 0.067 | 0.360 |
| MCI | -0.011 | 0.033 | 0.110 | -0.049 |
| Control | -0.083 | -0.176 | -0.109 | -0.045 |
| Combined | 0.278** | 0.196** | 0.181** | 0.141 |
p = 0.02;
p= 0.01
Longitudinal analyses
Using all visits we classified each subject in the study by their diagnoses at two successive visits and then computed the estimated mean slope of the change in tau per group. Finally, we fitted an alternative LMM by regressing tau on time on study with a random intercept per subject to determine if the tau levels changed significantly over time (P = 0.30) and if this change varied by diagnosis (P = 0.0008). The results are negative in that there is no evidence of a significant change in tau level over time when the subject remains in the same diagnostic category (AA, N = 7; MM, N = 10), although among those individuals maintaining control status (CC) there was a large of enough sample (N=33) to observe a slight but significant increase in circulating tau with increasing age (p = 0.042; between test interval = 1.8 ± 1.8 years (SD)). We have investigated insufficient numbers of CM, CA, MA, and MC individuals to provide viable longitudinal statistical assessment at this time.
Test/retest analysis
The correlation between the level of total tau obtained from the initial assessment from a freshly thawed sample (test) and the value obtained from a second freshly thawed sample (retest) collected simultaneously in each individual was highly significant (r=0.90, p<0.0001 based on n = 38).
Thereafter, we constructed a one way analysis of variance with only random effects to compute reproducibility by computing the intra class correlation coefficient between the test and retest values (ICC). The computed ICC was 0.84 indicating excellent reproducibility.
Tissue type and deterioration analysis
There was no detectable total tau in any serum sample assessed (N = 29), but substantial levels of total tau were identifiable in plasma for the same individuals whether freshly thawed or aged at 4° C for 24 hours. The correlation between the plasma levels of tau identified in the time 0 run and the 24 hour re-run was highly significant ( r=0.93, p<0.0001; n = 29). The tau levels identified at time 0 and 24 hours later were significantly different (p < 0.0001 by a paired t-test); the mean of the paired differences (tau_1 - tau_2) was 130 ± 113 (picograms/ml ± SD), implying the tau levels are significantly greater at time = 0 than at 24 hours by 130 units on average.
Discussion
Substantial levels of tau are found in the blood of humans and they were significantly lower in plasma of AD patients compared with cognitively normal controls. Based on our unpublished data, the circulating levels of tau in elderly normal controls are 2-3 times greater than encountered in the CSF.
A possible mechanism for reduced total tau in AD blood would depend on the origin of the peptide deposited in the brain. Animal studies of brain injury suggest that circulating tau emanates from central neurons [25]. If the tau depositing in the AD brain is overproduced there, the most likely mechanism to explain the current data could be reduced clearance of excess central tau to the blood. This would be similar to findings for GDNF where increased levels are found in AD CSF compared to control, but serum levels are significantly lower than found compared to the same control population [35]. Regardless of the mechanism, changing total tau levels in an easily accessible tissue (blood) may constitute a useful biomarker in following the progression of AD or the benefit of medications in a RCT.
The forgoing is supported by the observation that as cognitive performance declines so do circulating total tau levels. We found a highly significant correlation between reduced performance on the MMSE and Rey’s AVLT and decreased circulating total tau levels. On the one hand, the latter correlations may be an artifact of combining the three groups since according to our cross-sectional data (Table 1), tau varies among the groups and it is well known that the neuropsychological tests vary among the groups. On the other hand, the window of change possible within the control and MCI populations may be sufficiently narrow for the clinical scores (i.e., the range for the MMSE may be only 3-5 points) to correlate to circulating tau levels and therefore it would be necessary to combine the populations to observe the widest range of possible cognitive performances.
Our pilot longitudinal studies indicate that there are stable circulating tau levels in AD and MCI patients remaining AD or MCI, although there is a sight, but significant, age-related increase in circulating tau among individuals maintaining a cognitive control status. This could support the premise that tau levels rise during an individual’s life and that in MCI and AD circulating tau may begin entering the brain thus facilitating NFT formation [34]. Longitudinal studies are underway which may be able to confirm the possibility that as an individual makes the transition from cognitive control to MCI and thereafter to AD, that circulating tau levels follow the decrease in cognitive performance in an individual.
Our tissue type and deterioration studies suggested that there is no identifiable tau in human sera employing our methods. We suggest that this might be due to collateral metabolism associated with the clotting cascade, or clot formation, or both. The fact that aged plasma contains less tau than freshly thawed sample suggest that there may also be Ca++ independent tau proteolysis distinct from collateral metabolism (the clotting cascade).
Overall the data suggest that changes in plasma total tau levels may provide a new avenue of identifying the onset of MCI and thereafter AD, thus allowing the ability to follow the disease from beginning to end. Future studies could confirm this hypothesis.
Acknowledgement
This study was funded in part by: the Arizona Biomedical Research Commission (DLS; contract 08-09), the Banner SHRI Brain Donation Program (P30-AG019610). There are no conflicts of interest for any author.
References
- 1.Strozyk D, Launer LJ, Adlard PA, Cherny RA, Tsatsanis A, Volitakis I, Blennow K, Petrovitch H, White LR, Bush AI. Zinc and copper modulate Alzheimer Abeta levels in human cerebrospinal fluid. Nerobiol Aging. 2009;7:1069–1077. doi: 10.1016/j.neurobiolaging.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ewers M, Buerger K, Teipel SJ, Scheltens P, Schroder J, Zinkowski RP, Bouwman FH, Schonknecht P, Schoonenboom NS, Andreasen N, Wallin AK, DeBernardis JF, Kerkman DJ, Heindl B, Blennow K, Hampel H. Multicenter assessment of CSF-phosphorylated tau for the prediction of conversion of MCI. Neurology. 2007;69:2205–2212. doi: 10.1212/01.wnl.0000286944.22262.ff. [DOI] [PubMed] [Google Scholar]
- 3.Olsson A, Vanderstichele H, Andreasen N, De Meyer G, Wallin A, Holmberg B, Rosengren L, Vanmechelen E, Blennow K. Simultaneous measurement of beta-amyloid (1-42), total tau and phosphorylated tau (Thr81) in cerebrospinal fluid by the xMAP technology. Clin Chem. 2005;51:336–345. doi: 10.1373/clinchem.2004.039347. [DOI] [PubMed] [Google Scholar]
- 4.Vanderstichele H, De Meyer G, Shapiro F, Engelborghs S, De Deyn PP, Shaw LM, Trojanowski JQ. Alzheimer's Disease Biomarkers: From concept to clinical utility. In: Galimberti D, Scarpini E, editors. Biomarkers for Early Diagnosis of Alzheimer's Disease. Nova Science Publishers; 2008. pp. 81–122. [Google Scholar]
- 5.de Leon MJ, Mosconi L, Blennow K, DeSanti S, Zinkowski RP, Mehta PD, Pratico D, Tsui W, Saint Louis LA, Sobanska L, Brys M, Li Y, Rich K, Rinne J, Rusinek H. Imaging and CSF studies in the preclinical diagnosis of Alzheimer's disease. Ann N Y Acad Sci. 2007;1097:114–145. doi: 10.1196/annals.1379.012. [DOI] [PubMed] [Google Scholar]
- 6.Hansson O, Zetterberg H, Buchhave P, Andreasson U, Londos E, Minthon L, Blennow K. Prediction of Alzheimer's disease using the CSF Abeta42/Abeta40 ratio in patients with mild cognitive impairment. Dement Geratr Cogn Disord. 2007;23:316–320. doi: 10.1159/000100926. [DOI] [PubMed] [Google Scholar]
- 7.Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer's disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 2006;5:228–234. doi: 10.1016/S1474-4422(06)70355-6. [DOI] [PubMed] [Google Scholar]
- 8.Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Alsen PS, Peterson RC, Blennow K, Soares H, Simon AJ, Lewczuk P, Dean R, Siemers E, Potter W, Lee VM, Trojanowski JQ Initiative AsDN. Cerebrospinal Fluid Biomarker Signature in Alzheimer's Disease Neuroimaging Initiative Subjects. 2009:1–12. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaw LM, Korecka M, Clark CM, Lee VM, Trojanowski JQ. Biomarkers of neurodegeneration for diagnosis and monitoring therapeutics. Nature Reviews Drug Discovery. 2007;6:295–303. doi: 10.1038/nrd2176. [DOI] [PubMed] [Google Scholar]
- 10.Ringman JM, Younkin SG, Pratico D, Seltzer W, Cole GM, Geschwind DH, Rodriguez-Agudelo Y, Schaffer B, Fein J, Sokolow S, Rosario ER, Gylys KH, Varpetian A, Medina LD, Cummings JL. Biochemical markrs in persons with preclinical familial Alzheimer disease. Neurology. 2008 Jul 8;:85–92. doi: 10.1212/01.wnl.0000303973.71803.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trojanowski JQ, Vandeerstichele H, Korecka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, Dean R, Siemers E, Potter WZ, Weiner MW, Jack CR Jr, Jagust W, Toga AW, Lee VM, Shaw LM Alzheimer's Disease Neuroimaging Initiative. Update on the biomarker core of the Alzheimer's Disease Neuroimaging Initiative subjects. Alzheimer's Dement. 2010 May;:230–238. doi: 10.1016/j.jalz.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol. 2010;6:131–44. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- 13.Scheurich A, Urban P, Koch-Khoury N, Fellgiebel A. CSF phospho-tau is independent of age, cognitive status and gender of neurological patients. J Neurol Nerosurge Psychiatry. 2010 Apr;257:609–614. doi: 10.1007/s00415-009-5382-1. [DOI] [PubMed] [Google Scholar]
- 14.De Meyer G, Shapiro F, Vanderstichele H, Vanmechelen E, Engelborghs S, De Deyn PP, Coart E, Hansson O, Minthon L, Zetterberg H, Blennow K, Shaw L, Trojanowski JQ. Diagnosis-independent Alzheimer disease biomarker signature in cognitively normal elderly people. Arch Neorol. 2010;67(8):949–956. doi: 10.1001/archneurol.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenmann H, Meiner Z, Geylis V, Abramsky O, Steinitz M. Detection of circulating antibodies against tau protein in its unphosphorylated and in its neurofibrillary tangles-related phosphorylated state in Alzheimer's disease and healthy subjects. Neurosci Lett. 2006 Dec 20;410:90–93. doi: 10.1016/j.neulet.2006.01.072. [DOI] [PubMed] [Google Scholar]
- 16.Hampel H, Blennow K, Shaw L, Hoessler Y, Zetterberg H, Trojanowski JQ. Total and phosphorylated tau protein as biological markers of Alzheimer's disease. Exp Gerontol. 2010 Jan;45:30–40. doi: 10.1016/j.exger.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ingelsson M, Blomberg M, Benedikz E, Wahlund LO, Karlsson E, Vanmachelen E, Lannfelt L. Tau Immunoreactivity detected in Human Plasma, But no Obvious Increase in Dementia. Dementia and Geriatric Cognitive Disorders. 1999;10:442–445. doi: 10.1159/000017187. [DOI] [PubMed] [Google Scholar]
- 18.Mortberg E, Zetterberg H, Nordmark J, Blennow K, Catry C, Daecraemer H, Vanmechelen E, Rbertsson S. Plasma tau protein in comatose patients after cardiac arrest treated with therapeutic hypothermia. Acta Anaesthesiol Scand. 2011;55:1132–1138. doi: 10.1111/j.1399-6576.2011.02505.x. [DOI] [PubMed] [Google Scholar]
- 19.Noguchi-Shinohara M, Hamaguchi T, Nozaki I, Sakai K, Yamada M. Serum tau protein as a marker for the diagnosis of Creutzfeldt-Jakob disease. J Neurol. 2011;258:1464–1468. doi: 10.1007/s00415-011-5960-x. [DOI] [PubMed] [Google Scholar]
- 20.Bartosik-Psujek H, Psujek M, Jaworski J, Stelmasiak Z. Total tau and S100b proteins in different types of multiple sclerosis and during immunosuppressive treatment with mitoxantrone. Acta Neurol Scand. 2011;123:252–256. doi: 10.1111/j.1600-0404.2010.01393.x. [DOI] [PubMed] [Google Scholar]
- 21.Wunderlich M, Lins H, Skalej M, Wallesch C, Goertler M. Neuron-specific enolase and tau protein as neurobiochemical markers of neuronal damage are related to early clinical course and long-term otcome in acute ischemic stroke. Clinical Neuology & Neurosurgery. 2006;108:558–563. doi: 10.1016/j.clineuro.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Bielewicz J, Kurzepa J, Czekajska-chehab E, Stelmasiak Z, Bartosik-Psujek H. Does Serum Tau Protein Predict the Outcome of Patients with Ischemic Stroke? J Mol Neurosci. 2011;43:241–245. doi: 10.1007/s12031-010-9403-4. [DOI] [PubMed] [Google Scholar]
- 23.Liliang P-C, Liang C-L, Weng H-C, Lu K, Wang KW, Chen H-J, Chuang J-H. tau Proteins in Serum Predict Outcome After Severe Traumatic Brain Injury. Journal of Surgical Research. 2010;160:302–307. doi: 10.1016/j.jss.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 24.Guzel A, Karasalihoglu S, aylanc H, Temizoz O, Hicdonmez T. Validity of serum tau protein levels in pediatric patients with minor head trauma. American Journal of Emergency Medicine. 2010;28:399–403. doi: 10.1016/j.ajem.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 25.Liliang P-C, Liang C-L, Lu K, Wang K-W, Weng HC, Hsieh C-H, Tsai Y-D, Chen H-J. Relationship between injury severity and serum tau protein levels in traumatic brain injured rats. Resuscitation. 2010;10:1205–1208. doi: 10.1016/j.resuscitation.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 26.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's diseae. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 27.Petersen RC. Mild cognitive impairment: transition between aging and Alzheimer's disease. Neurologia. 2000;15:93–101. [PubMed] [Google Scholar]
- 28.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 29.Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 30.Folstein MF, Folstein SE, McHugh PR. "Mini-Mental State:" A practical method for grading the mental state of patients for the clinician. J Psychiatric Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 31.Rey A. L'examen clinique en psychologie. Paris: Presses Universitaires de France; 1964. [Google Scholar]
- 32.Taylor EM. Psychological appraisal of children with cerebral deficits. Cambridge, MA: Harvard University Press; 1959. [Google Scholar]
- 33.Borod JC, Goodglass H, Kaplan E. Normative data on the Boston Diagnostic Aphasia Examination, Parietal Lobe battery and the Boston Naminig Test. Journal of Clinical Neuropsychology. 1980;2:209–216. [Google Scholar]
- 34.Sparks DL, Ziolkowski C, Lawmaster T, Martin T. Influence of water quality on cholesterolinduced tau pathology: Preliminary data. IJAD. 2011;2011:987023. doi: 10.4061/2011/987023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Straten G, Eschweiler GW, Maetzler W, Laske C, Leyhe T. Glial Cell-Line Derived Neurotrophic Factor (GDNF) Concentrations in Cerebrospinal Fluid and Serum of Patients with Early Alzheimer's Disease and Normal Controls. Journal of Alzheimer's Disease. 2009;18:331–337. doi: 10.3233/JAD-2009-1146. [DOI] [PubMed] [Google Scholar]


