Abstract
Plasmodium falciparum-infected erythrocytes often sequester in the placenta of pregnant women, producing placental malaria, a condition that can compromise the health of the developing fetus. Scientists are hopeful that a vaccine can be developed to prevent this condition. Immunological mechanisms responsible for eliminating parasites from the placenta remain unclear, but antibodies to the carboxyl-terminal 19-kDa segment of the merozoite surface protein 1 (MSP1-19), the ring-infected erythrocyte surface antigen (RESA), and an erythrocyte-surface ligand that binds chondroitin sulfate A (CSA-L) have been implicated. In addition, antibodies to sporozoite and liver-stage antigens could reduce initial parasite burdens. This study sought to determine if antibodies to the circumsporozoite protein (CSP), liver-stage antigen 1 (LSA1), RESA, MSP1-19, or CSA-L correlated with either the absence of placental parasites or low placental parasitemias. Using a frequency-matched case-control study design, we compared antibody levels in women (gravidity 1 to 11) with and without placental malaria. Results showed that women who were antibody negative for MSP1-19 were at a higher risk of having placental malaria than women with antibodies (P < 0.007). Furthermore, an association between high levels of antibodies that blocked the binding of infected erythrocytes to CSA and low placental parasitemias was observed (P = 0.02). On the other hand, women with high antibody levels at term to CSP, LSA1, and RESA were more likely to have placental malaria than antibody-negative women. Since antibodies to MSP1-19 and CSA-L were associated with reduced placental malaria, both antigens show promise for inclusion in a vaccine for women of child-bearing age.
During Plasmodium falciparum infection in pregnant women, infected red blood cells (IRBC) often accumulate in the intervillous space of the placenta, creating a situation known as placental malaria. The presence of IRBC frequently induces the infiltration of inflammatory-type cells into the placenta, where they cause pathological alterations (13, 16, 17). As a result, placental malaria increases a woman's risk of having a low-birth-weight baby due both to intrauterine growth retardation or premature delivery (6, 20, 21, 25). Therefore, either prevention or rapid elimination of placental parasites is important. The development of a vaccine that enhances immune responses that mediate parasite clearance is currently being considered.
Studies in pregnant women have reported that anti-P. falciparum antibodies to three asexual-stage antigens appear to be associated with protective immunity. Two studies have shown that pregnant women who lack antibodies to the ring-infected stage antigen (RESA) are more susceptible to P. falciparum infection (3, 22); however, two other studies have not found this association (8, 9). The ability of anti-RESA antibodies to reduce placental parasitemia has not been investigated. In 1996, Fried and Duffy reported that parasites sequestered in the placenta express a ligand that binds specifically to chondroitin sulfate A (CSA) (10). The ligand, CSA-L, is thought to be a variant of P. falciparum erythrocyte membrane protein 1 (10-12). Since antibodies inhibit the binding of IRBC to CSA in vitro (2, 12, 19, 23, 24), they are likely to be protective in vivo. Finally, Branch et al. (7) reported that placental parasite densities were significantly lower in Kenyan mothers who had immunoglobulin G (IgG) antibodies to the carboxyl-terminal 19-kDa segment of the merozoite surface protein 1 (MSP1-19) than mothers who did not. Since late trophozoite and schizont-stage parasites predominate in the placenta, and antibodies to MSP1-19 are known to block merozoite invasion (5, 14, 15), antibodies to MSP1-19 could have a substantial impact on reducing placental parasitemias. Antibodies to the circumsporozoite protein (CSP) and the liver-stage antigen 1 (LSA1) are not effective against asexual-stage parasites sequestered in the placenta, but high titers of antibodies to these antigens could be important in reducing initial parasite burdens. Therefore, the goal of the present study was to determine if antibodies to these antigens correlate with either the absence or low levels of parasites in the placenta at the time of delivery.
MATERIALS AND METHODS
Study population and sample collection.
Between 1997 and 2000, pregnant women who attended the Biyem Assi Hospital, Yaounde, Cameroon, were consecutively recruited at delivery as part of a comprehensive immunological study on placental malaria. The purpose of the study was explained to each woman, and those who gave verbal informed consent were enrolled. The study was approved by the Institutional Review Board of Georgetown University and the Ethical Committee, Ministry of Health, Cameroon, and is covered by single project assurance S-9601-01.
A questionnaire was used to obtain information relevant to the pregnancy, including maternal age, number of previous pregnancies, and use of antimalarial drugs. Following delivery, approximately 5 ml of heparinized maternal venous and intervillous blood was collected. In addition, a small piece (2 cm by 2 cm by 2 cm) of placental tissue was collected. A portion of the tissue was fixed in 10% buffered formalin and processed for histological evaluation.
Detection and quantification of placental parasitemias.
Thick and thin blood smears of maternal intervillous blood and impression smears of placental tissue were prepared, stained with Dif-Quick (Baxter Scientific, Inc., Deerfield, Ill.), and examined for the presence of P. falciparum parasites. Women were considered to have placental malaria if parasites were detected in either impression smears or histological sections of placental tissue. Impression smears of intervillous space blood were used to determine placental parasitemias. Results are expressed as percent parasitemia, based on the number of IRBC per 2,000 erythrocytes.
Study design.
The purpose of this study was to determine if antibodies to specific malarial antigens correlated with a reduction of placental malaria. Many factors, however, influence malarial immunity in pregnant women, including maternal age, gravidity, antimalarial drug use, seasonality of infection, and economic status. To help control for these variables, a frequency-matched case-control study design was employed with a ratio of two cases (n = 117 malaria-positive women) to one control (n = 65 malaria-negative women). Approximately 20% of the women in the case and control groups had had 1, 2, 3, 4, or ≥5 (range, 5 to 11) pregnancies (Table 1). There was no significant difference between the two groups with respect to maternal age, gravidity, antimalarial chemoprophylaxis, or pregnancy outcome (Table 1). Seasonality of conception and delivery and economic status were controlled for by selecting consecutively enrolled women living in the same area of Yaounde.
TABLE 1.
Comparison of women in the case and control groups
| Characteristic | Placental malaria positive (n = 117) | Placental malaria negative (n = 65) | P |
|---|---|---|---|
| Age (yr, mean ± SD) | 25.5 ± 5.5 | 25.2 ± 5.3 | 0.713a |
| No. of pregnancies (% of women) | |||
| 1 | 22.2 | 20.3 | 0.967b |
| 2 | 20.5 | 23.4 | |
| 3 | 18.8 | 18.8 | |
| 4 | 19.7 | 21.9 | |
| 5+ | 18.8 | 15.6 | |
| Use of chemoprophylaxis (% of women) | 75.2 | 80.0 | 0.463b |
| % Full-term deliveries | 98.3 | 94.7 | 0.133b |
| Median optical densities (Q1, Q3)d | |||
| Anti-MA | 1.03 (0.61, 1.65) | 0.50 (0.20, 0.90) | <0.0001c |
| Anti-CSP | 0.55 (0.27, 1.05) | 0.24 (0.12, 0.57) | 0.001c |
| Anti-MSP1-19 | 1.36 (0.35, 2.41) | 1.13 (0.40, 2.09) | 0.729c |
| Anti-RESA | 0.22 (0.07, 0.53) | 0.08 (0.03, 0.39) | 0.018c |
| Anti-CSA-L (%) | 22.0 (7.0, 54.0) | 21.0 (7.0, 41.0) | 0.578c |
| Anti-LSA1 | 0.56 (0.33, 0.98) | 0.32 (0.07, 0.68) | 0.012c |
T-test.
Chi-square test.
Wilcoxon rank-sum test.
Q1 and Q3 are the first and third quartiles, respectively.
Enzyme-linked immunosorbent assay (ELISA) for measuring antibody levels.
Microtiter wells (Polysorp, Nunc) were coated with the following antigens: an extract of 5 × 104 Triton X-100-solubilized, Percoll-enriched, late-stage IRBC (MA extract) or an equivalent number of normal RBC; 0.1 μg of Escherichia coli-expressed CSP containing 30 copies of NANP and 2 NVDP tetrapeptide repeats fused with Leu-Arg, kindly provided by R. Wirtz (Centers for Disease Control); 2 μg of a conserved sequence from LSA1 (LAKEKLQGQQS DLEQERLAKEKLQEQQSDLEQERLAKEKLQ) (Sigma Genosys, The Woodlands, Tex.); 1.0 μg of a 20-mer peptide containing five copies of the conserved B-cell epitope EENV from RESA coupled to bovine serum albumin (AnaSpec, San Jose, Calif.); and 0.1 μg of purified MSP1-19 from the FVO strain produced in Saccharomyces cerevisiae and subsequently purified from the supernatant fluids. Wells were blocked and 100 μl of plasma was added in duplicate to the wells at the following dilutions: 1:200 for CSP and LSA1; 1:100, 1:1,000, and 1:5,000 for the extract of IRBC and normal red blood cells; and 1:100, 1:500, and 1:1,000 for RESA and MSP1-19. Dilutions were selected based on previous titration curves. Following incubation of plasma with antigen, plates were washed, alkaline phosphatase-labeled anti-human IgG was added, followed by substrate (Sigma P104 in diethanolamine buffer). The optical density was read at 405/630 nm. In calculating the results for the MA extract, optical density for the normal red blood cells were subtracted from optical density for the IRBC.
Cytoadherence assay.
A cytoadherence assay was used to measure antibodies that block the binding of IRBC to CSA (1, 2). In brief, chondroitin 4-sulfate proteoglycans (CSPG), purified from human placentas (1), were coated onto plates at 200 ng/ml (2). P. falciparum-IRBC, selected for binding to placenta-derived CSPG, were incubated with 1:10, 1:20, and 1:40 dilutions of plasma for 30 min and then added to the CSPG-coated plates. Following incubation, the plates were washed, stained, and the number of adherent IRBC was counted. Results are expressed as percent inhibition of binding, [1 − (number of IRBC in the test plasma/number of red blood cells in a pool of U.S. control plasma) × 100]. The percent inhibition produced by plasma from 19 nonpregnant Cameroonian women was 4.6% ± 3.7%. The mean + 2 standard deviations (12.1% inhibition of binding) was used as the cutoff for positivity.
Statistical analysis.
The Wilcoxon rank-sum test was used for testing overall antibody levels in women with and without placental malaria. In order to investigate the effect of malaria-specific antibody levels on placental malaria, we categorized antibody levels into four groups: negative; low positive; medium positive; and high positive. A cutoff value for positive ELISA was set at the mean optical density + 3 standard deviations for a pool of U.S. negative control plasma. Categorization of antibodies was as follows: for anti-CSP antibodies, the cutoff was optical density = 0.242; samples in the low positive category had optical densities ranging from 1 to <2 times the cutoff; samples in the medium positive category had optical densities of 2 to <5 times the cutoff; and samples in the high positive category had optical densities of ≥5 times the cutoff. For anti-LSA1 antibodies, the cutoff was optical density = 0.14; low positive = 1 to <3 cutoff, medium positive = 3 to 5 cutoff, and high positive was ≥5 cutoff. For anti-MA extract antibodies, the cutoff was optical density = 0.134; low positive = 1 to <5 cutoff, medium positive = 5 to <15 cutoff, and high positive was ≥15 cutoff. For anti-RESA antibodies, the cutoff was optical density = 0.043; low positive = 1 to <2 cutoff, medium positive = 2 to <6 cutoff, and high positive was ≥6 cutoff. For anti-MSP1-19 antibodies, the cutoff was optical density = either 0.0822 or 0.3483 (depending on the assay); low positive = 1 to <5 cutoff, medium positive = 5 to <10 cutoff, and high positive was ≥10 cutoff. For anti-CSA inhibitory antibodies, the cutoff was 12% inhibition of binding; low positive = 1 to <2 cutoff, medium positive = 2 to <4 cutoff; and high positive was ≥4 cutoff. The Cochran-Armitage trend test was used for comparison of prevalence of placental malaria in different categories of antibody levels. Finally, the Spearman correlation analyses were performed to investigate the nonlinear relationships among placental parasitemia and malaria-specific antibodies.
RESULTS
Description of the study populations.
The data in Table 1 show that women in the case and control groups were similar with respect to maternal age, number of previous pregnancies, antimalarial drug prophylaxis, and pregnancy outcome. Women with placental malaria had asymptomatic infections with placental parasitemias ranging from <0.01% to 40.3%, median 1.0%. Women in the lower third quartile had parasitemias that were ≤0.3% and those in the upper third had parasitemias that were ≥3.1% (Table 1). Among women with placental parasitemias, 12.1% had placental parasitemias that were <0.1%, 37.9% had parasitemias between 0.1 and <1.0%, and 50.0% had parasitemias of 1% or higher.
Comparison of antibody levels in women with and without placental malaria.
Women with placental malaria had significantly higher levels of antibodies to CSP (P = 0.001), LSA1 (P = 0.012), MA (P < 0.0001), and RESA (P = 0.018) than women without placental malaria (Table 1). There was no significant difference between the two groups for antibody levels to MSP1-19 (P = 0.729) or anti-CSA-inhibitory antibodies (P = 0.578) (Table 1).
Association between antibody levels and presence or absence of placental parasites.
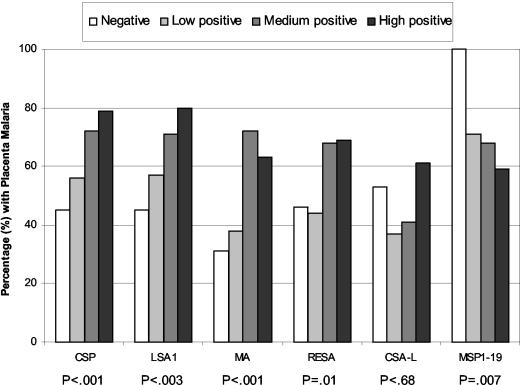
To determine if the presence or absence of antibodies correlated with the absence of placental malaria, women were classified as being antibody negative or antibody positive (low, medium and high levels). The percentage of women with placental malaria in each group was determined (Fig. 1). Results showed that women who were antibody negative for CSP, LSA1, MA, and RESA were less likely to have placental malaria than women who had antibodies. For example, 45 to 46% of the women who were antibody negative for CSP and LSA1 had placental malaria compared to 79 to 80% of the women with high levels of antibody (P < 0.001 and P < 0.003, respectively). A similar situation was found with the asexual-stage antigen extract (MA, P < 0.001) and RESA (P = 0.01). Thus, the presence of antibodies to these antigens suggests they were produced in response to infection, rather than to have prevented its establishment. No significant difference was found in the prevalence of placental malaria in women who had inhibitory CSA-binding antibodies at term compared to those who did not (P = 0.68). On the other hand, women who were negative for MSP1-19 antibodies were at a higher risk of having placental malaria than those who had antibodies (P < 0.007). All women who were antibody negative of MSP1-19 had placental malaria compared to 58% of those in the high-antibody group (Fig. 1). These results suggest that antibodies to anti-MSP1-19 play a role in reducing the prevalence of placental malaria in Cameroonian women.
FIG. 1.
Association between prevalence of placental malaria and antibody levels. The malaria-specific antibody levels were categorized into four groups: negative (N); low positive (L); medium positive (M); and high positive (H). The positive cutoff was the mean + 3 standard deviations of the negative control samples. Further categorization of antibody-positive women is described in the Materials and Methods section. The P values were derived from the Cochran-Armitage trend test.
Association between antibody levels and placental parasitemias.
Next, data were analyzed to determine if high antibody levels to these antigens correlated with low placental parasitemias. Among women with placental malaria, anti-CSA binding activity and antibodies to MA were negatively correlated with placental parasitemia (Spearman correlation coefficient = −0.33, P = 0.02, and −0.19, P = 0.09, respectively). No significant correlation was found between the amount of antibodies to the other antigens and placental parasitemias (CSP −0.04, P = 0.68; LSA1 0.10, P = 0.42; RESA 0.01, P = 0.906; MSP1-19 −0.01, P = 0.302). Thus, overall, antibodies to MSP1-19 were associated with a reduced prevalence of placental malaria, whereas antibodies to CSA-L and MA were associated with reduced placental parasitemias.
Interantibody associations.
Table 2 shows the correlations among antibody levels to the various antigens. Antibody levels to each of the five antigens correlated well with antibodies to the MA extract. A significant correlation was found among any pair of antibodies to CSP, MSP1-19, RESA, and LSA1 with the exception that no correlation was observed between antibodies to RESA and LSA1. However, levels of inhibitory anti-CSA antibodies only correlated with anti-MA, suggesting different kinetics of induction.
TABLE 2.
Correlations between malaria-specific antibody levels
| Antibodies |
Pa
|
||||
|---|---|---|---|---|---|
| CSP | MSP1-19 | RESA | CSA-L | LSA1 | |
| MA | 0.45 (<0.0001) | 0.59 (<0.0001) | 0.51 (<0.0001) | 0.34 (0.0008) | 0.57 (<0.0001) |
| CSP | 0.23 (0.022) | 0.35 (0.0005) | 0.07 (0.4848) | 0.51 (0.0001) | |
| MSP1-19 | 0.19 (0.0304) | 0.17 (0.0943) | 0.31 (0.0036) | ||
| RESA | −0.01 (0.943) | −0.03 (0.8087) | |||
| CSA-L | 0.17 (0.2414) | ||||
Spearman correlation coefficient (P value).
DISCUSSION
This study sought to determine if antibodies to sporozoite, liver-stage or asexual-stage antigens are likely to be important in placental malaria. Results showed that women who lacked antibodies to MSP1-19, but not the other antigens, were more likely to have placental malaria than women who had anti-MSP1-19 antibodies (P < 0.007) (Fig. 1). In addition, a correlation between high levels of antibodies that inhibit IRBC binding to CSA and low placental parasitemias was observed (P = 0.022). A weak association between antibodies to the MA extract and low placental parasitemias was also found (P = 0.09).
On the other hand, women with strong antibody responses to CSP, LSA1, RESA, and the MA extract were more likely to have placental malaria than if they were antibody negative (Fig. 1). It appears that women in Yaounde become infected during pregnancy, thereby boosting their antibody responses, so that by the end of pregnancy, their antibody levels are significantly higher than women who are malaria negative (Table 1). A strong correlation between antibodies to CSP and LSA1 (P = 0.0001), and between CSP and MA (P < 0.0001), RESA (P = 0.0005), and MSP1-19 (P = 0.02), was found. No association however, was found between antibodies to CSP and antibodies that inhibit the binding of IRBC to CSA, suggesting that infection does not always lead to production of anti-CSA-L antibodies.
Previous studies have measured antibodies to CSP, RESA, and the MA extract, but not LSA1, in pregnant women (3, 8, 9, 20, 22). Anti-CSP and anti-MA levels in pregnant and nonpregnant women are reported to be similar (22), and no association between antibody titers and peripheral blood parasite densities has been found (3, 8, 22). Variable results have been obtained in studies evaluating anti-RESA antibodies in pregnant women (3, 8, 9, 22). In a cross-sectional study in Cameroon, Mvondo et al. (22) used glutaraldehyde-fixed infected erythrocytes to measured anti-RESA antibodies in women during the first, second, and third trimesters of pregnancy and in matched nonpregnant controls (22). They found that anti-RESA levels declined during the second trimester, a time when pregnant women are the most susceptible to malaria, and reported an inverse correlation between anti-RESA levels and peripheral blood parasitemias (22). However, Deloron et al. (8), using both glutaraldehyde-fixed cells and the peptide-based ELISA described herein, found neither a decrease in antibodies in Kenyan women during the second trimester nor an association between anti-RESA antibodies and peripheral parasite densities. No explanation for the discrepancies between the two studies is apparent.
In the current study, no association between antibody levels at the time of delivery (third trimester) in Cameroonian women and placental parasitemias was found. Further studies are needed to gain a full appreciation of the impact antibodies to RESA as well as CSP, LSA1, and MA in pregnant women. For example, pregnant women are reported to be more frequently bitten by mosquitoes (18) and to have higher numbers of parasite genotypes in their blood (4) than age-matched nonpregnant women. If a woman became infected early in pregnancy, high levels of antibodies to these antigens could be induced that would be beneficial in preventing reinfection and reducing parasite burdens later in pregnancy. Thus, longitudinal studies in women during pregnancy are needed to assess the full benefit of antibodies to these antigens.
It is striking that all women who lacked antibodies to MSP1-19 had placental malaria compared to 59% of women with high levels of antibody (Fig. 1) suggesting that anti-MSP1-19 antibodies play a role in protection. Antibodies to MSP1-19 can block merozoite invasion, either by inhibiting the binding of merozoites to erythrocytes by blocking processing of MSP-1 or by other mechanisms (5, 14, 15). Since a high proportion of asexual-stage parasites in the placenta are late schizonts that release merozoites, the presence of antibodies that block merozoite invasion would reduce the number of IRBC both in the intervillous space and throughout the body. Although there was no direct correlation between antibody levels and percent placental parasitemia (Spearman correlation coefficient, −0.19; P = 0.30), an exploratory multivariate-regression model that included log-transformed parasitemia as the dependent variable and log-transformed levels of antibodies to all six malaria-specific antigens plus age as independent variables, estimated that as anti-MSP1-19 antibodies increase by 10%, placental parasitemias would decrease by 1.6% (95% confidence interval, −0.3% to 3.5%; P = 0.095). Thus, these data document a beneficial role for MSP1-19 antibodies in placental malaria.
A correlation between high anti-CSA-L inhibitory activity and reduced levels of placental parasites was observed at delivery (P = 0.022). However, no difference in the proportion of antibody-positive and antibody-negative women with placental malaria was found (Fig. 1). Recently, we showed that almost all pregnant women lack anti-CSA-L antibodies at conception (23). In Yaounde, multigravid women begin producing significant anti-adhesion antibodies during the fourth month of gestation, whereas primigravidae do not begin producing these antibodies until around 6 months (23). By term, antibody levels are similar in the two groups. These combined results suggest that in Africa, women become infected during pregnancy produce anti-adhesion antibodies in response to sequestered parasites, and then these antibodies are beneficial in reducing placental parasites either by preventing additional binding or dissociating already bound parasites. As a result, women with high levels of anti-CSA-L antibodies at term have a lower level of parasites than women who do not have antibodies to this antigen (Fig. 1).
Currently, scientists are focusing on a vaccine based on the induction of antibodies that inhibit the binding of IRBC to CSA. Results from the present study suggest that immune responses to MSP1-19 are also important. In addition, a significant correlation between high antibody levels to the asexual-stage parasite extract (MA) and low placental parasitemias was also observed (P = 0.09), suggesting that unidentified antigens may also contribute to parasite clearance. Immune responses for eliminating placental parasites are complex, involving antibodies to multiple antigens as well as acquired cellular and innate immune responses. Thus, development of a vaccine for women of child-bearing age continues to be challenging.
Acknowledgments
This work was supported by grants from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, including UO1 AI 35839 (to D.W.T.—sample collection), UO1AI 43888 (to D.W.T—serological studies), and AI-45086 (to D.C.G.—inhibition-of- binding studies).
We acknowledge the collaborative role of the entire malaria research team at the Biotechnology Center, University of Yaounde 1, Yaounde, Cameroon, for their participation in this study. We are particularly indebted to the women who consented to participate.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Achur, R. N., M. Valiyaveettil, A. Alkhalil, C. F. Ockenhouse, and D. C. Gowda. 2000. Characterization of proteoglycans of human placenta and identification of unique chondroitin sulfate proteoglycans of the intervillous spaces that mediate the adherence of Plasmodium falciparum-infected erythrocytes to the placenta. J. Biol. Chem. 275:40344-40356. [DOI] [PubMed] [Google Scholar]
- 2.Alkhalil, A., R. N. Achur, M. Valiyaveettil, C. F. Ockenhouse, and D. C. Gowda. 2000. Structural requirements for the adherence of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate proteoglycans of human placenta. J. Biol. Chem. 275:40357-40364. [DOI] [PubMed] [Google Scholar]
- 3.Astagneau, P., R. W. Steketee, J. J. Wirima, C. O. Khoromana, and P. Millet. 1994. Antibodies to ring-infected erythrocyte surface antigen (Pf155/RESA) protect against P. falciparum parasitemia in highly exposed multigravidas women in Malawi. Acta Trop. 57:317-325. [DOI] [PubMed] [Google Scholar]
- 4.Beck, S., F. P. Mockenhaupt, U. Bienzle, T. A. Eggelte, W. N. Thompson, and S. K. 2001. Multiplicity of Plasmodium falciparum infection in pregnancy. Am. J. Trop. Med. Hyg. 65:631-636. [DOI] [PubMed] [Google Scholar]
- 5.Blackman, M. J., T. J. Scott-Finnigan, S. Shai, and A. A. Holder. 1994. Antibodies inhibit the protease-mediated processing of a malaria merozoite surface protein. J. Exp. Med. 180:389-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brabin, B. J. 1983. An analysis of malaria in pregnancy in Africa. Bull. W. H. O. 61:1005-1016. [PMC free article] [PubMed] [Google Scholar]
- 7.Branch, O. H., V. Udhayakumar, A. W. Hightower, A. J. Oloo, W. A. Hawley, B. L. Nahlen, P. B. Bloland, D. C. Kaslow, and A. Lal. 1998. A longitudinal investigation of IgG and IgM antibody responses to the merozoite surface protein-1 19-kilodalton domain of Plasmodium falciparum in pregnant women and infants: associations with febrile illness, parasitemias, and anemia. Am. J. Trop. Med. Hyg. 58:211-219. [DOI] [PubMed] [Google Scholar]
- 8.Deloron, P., R. W. Steketee, G. H. Campbell, F. Peyron, D. C. Kaseje, and A. D. Brandling-Bennett. 1989. Serological reactivity to the ring-infected erythrocyte surface antigen and circumsporozoite protein in gravid and nulligravid women infected with Plasmodium falciparum. Trans. R. Soc. Trop. Med. Hyg. 83:58-62. [DOI] [PubMed] [Google Scholar]
- 9.Fievet, N., M. Cot, C. Chougnet, B. Maubert, J. Bickii, B. Dubois, j. Y. Le Hesran, Y. Frobert, F. Migot, F. Romain, et al. 1995. Malaria and pregnancy in Cameroonian primigravidae: humoral and cellular immune responses to Plasmodium falciparum blood-stage antigens. Am. J. Trop. Med. Hyg. 53:612-617. [DOI] [PubMed] [Google Scholar]
- 10.Fried, M., and P. E. Duffy. 1996. Adherence of Plasmodium falciparum to CSA in the human placenta. Science 272:1502-1504. [DOI] [PubMed] [Google Scholar]
- 11.Fried, M., and P. E. Duffy. 1998. Maternal malaria and parasite adhesion. J. Mol. Med. 76:162-171. [DOI] [PubMed] [Google Scholar]
- 12.Fried, M., F. Nosten, A. Brockman, B. J. Brabin, and P. E. Duffy. 1998. Maternal antibodies block malaria. Nature 395:851-852. [DOI] [PubMed] [Google Scholar]
- 13.Galbraith, R. M., W. P. Faulk, G. M. P. Galbraith, T. W. Holbrook, and R. S. Bray. 1980. The human materno-foetal relationship in malaria: I. Identification of pigment and parasites in the placenta. Trans. R. Soc. Trop. Med. Hyg. 74:52-60. [DOI] [PubMed] [Google Scholar]
- 14.Guevara Patino, J. A., A. A. Holder, J. S. McBride, and M. J. Blackman. 1997. Antibodies that inhibit malaria merozoite surface protein-1 processing and erythrocyte invasion are blocked by naturally acquired human antibodies. J. Exp. Med. 186:1689-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holder, A. A., J. A. Guevara Patino, C. Uthaipibull, S. E. Syed, I. T. Ling, T. Scott-Finnigan, and M. Blackman. 1999. Merozoite surface protein 1, immune evasion, and vaccines against asexual blood stage malaria. Parassitologia 41:409-414. [PubMed] [Google Scholar]
- 16.Ismail, M. R., J. Orid, C. Menendez, P. J. Ventura, J. Aponte, E. Kahigwa, R. Hirt, A. Cardesa, and P. L. Alonso. 2000. Placental pathology in malaria: a histological, immunohistochemical, and quantitative study. Hum. Pathol. 31:85-93. [DOI] [PubMed] [Google Scholar]
- 17.Leopardi, O., W. Naughten, L. Salvia, M. Colecchia, A. Matteelli, A. Zucchi, A. Shein, J. A. Muchi, G. Carosi, and M. Ghione. 1996. Malaric placentas. A quantitative study and clinico-pathological correlations. Pathol. Res. Pract. 192:892-898. [DOI] [PubMed] [Google Scholar]
- 18.Lindsay, S., J. Ansell, C. Selman, V. Cox, K. Hamilton, and G. Walraven. 2000. Effect of pregnancy on exposure to malaria mosquitoes. Lancet 355:1972. [DOI] [PubMed]
- 19.Maubert, B., N. Fievet, G. Tami, M. Cot, C. Boudin, and P. Deloron. 1999. Development of antibodies against CSA-adherent Plasmodium falciparum in pregnant women. Infect. Immun. 67:5367-5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGregor, I. A. 1984. Epidemiology, malaria and pregnancy. Am. J. Trop. Med. Hyg. 33:517-525. [DOI] [PubMed] [Google Scholar]
- 21.Menendez, C. 1995. Malaria during pregnancy: a priority area of malaria research and control. Parasitol. Today 11:178-183. [DOI] [PubMed] [Google Scholar]
- 22.Mvondo, J. L., M. A. James, A. J. Sulzer, and C. Campbell. 1992. Malaria and pregnancy in Cameroonian women. Naturally acquired antibody responses to asexual blood-stage antigens and the circumsporozoite protein of Plasmodium falciparum. Trans. R. Soc. Trop. Med. Hyg. 86:486-490. [DOI] [PubMed] [Google Scholar]
- 23.O'Neil-Dunne, I., R. N. Achur, S. T. Agbor-Enoh, M. Valiyaveettil, R. S. Naik, C. F. Ockenhouse, A. Zhou, R. Megnekou, R. Leke, D. W. Taylor, and D. C. Gowda. 2001. Gravidity-dependent production of antibodies that inhibit binding of Plasmodium falciparum-infected erythrocytes to placental chondroitin sulfate proteoglycan during pregnancy. Infect. Immun. 69:7487-7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ricke, C. H., T. Staalsoe, K. Koram, B. D. Akanmori, E. M. Riley, T. G. Theander, and L. Hviid. 2000. Plasma antibodies from malaria-exposed pregnant women recognize variant surface antigens on Plasmodium falciparum-infected erythrocytes in a pariety-dependent manner and block parasite adhesion to chondrointin sulfate. J. Immunol. 165:3309-3316. [DOI] [PubMed] [Google Scholar]
- 25.Steketee, R. W., J. J. Wirima, A. W. Hightower, L. Slutsker, D. L. Heymann, and J. D. Breman. 1996. The effect of malaria and malaria prevention in pregnancy on offspring birthweight, prematurity, and intrauterine growth retardation in rural Malawi. Am. J. Trop. Med. Hyg. 55:33-41. [DOI] [PubMed] [Google Scholar]