Abstract
Perineuronal nets (PNs) are a specialized form of extracellular matrix, surrounding different types of neurons and mainly consist of chondroitin sulfate proteoglycans connected to hyaluronan, stabilized by link protein and cross-linked via tenascin-R. Due to their polyanionic character, caused by the highly charged chondroitin sulfate glycosaminoglycan and hyaluronan components, PNs might be involved in local ion homeostasis. They are able to scavenge and bind redox-active ions and thus reduce the local oxidative potential. We investigated whether netenwrapped neurons are less vulnerable against iron-induced oxidative processes. Oxidative stress is a key factor in the development and progression of neurodegenerative diseases like Alzheimer’s and Parkinson’s disease. Iron is believed to contribute to oxidative stress in Alzheimer brains by catalyzing the generation of free radicals. For examining potential neuroprotective effects of PNs, mice were microinjected with 0.2μl of a 20mM solution of FeCl3 into the barrel field while the control group received an equal volume of 0.9% NaCl. Brains were analyzed after time intervals of 24h and 72h. Neuronal degeneration was visualized using Fluoro-Jade B staining. The presence of PNs was assessed by Wisteria floribunda agglutinin histochemistry or aggrecan immunocytochemistry. The analysis showed a significant lower degeneration rate of net-ensheathed neurons in comparison to neurons without PNs. The results suggest a neuroprotective mechanism associated with the presence of PNs against iron-induced cell death.
Keywords: Perineuronal nets, iron, neuroprotection, oxidative stress
Introduction
The extracellular matrix of the central nervous system provides a highly organized complex of macromolecules that surround glia cells and neurons. A specific form of extracellular matrix is the perineuronal net (PN). PNs are basically composed of large aggregating chondroitin sulfate proteoglycans (CSPGs), mainly aggrecan, connected to hyaluronan, stabilized by link protein and cross-linked via tenascin-R. The lattice-like PN ensheathes cell bodies, proximal dendrites and the axon initial segment of several types of neurons in the mammalian brain [1-4]. PNs mature during postnatal development simultaneously with the synaptogenesis and myelogenesis. Their role is still not completely understood, but their specific characteristics allow suggestions about their functions [5,6]. Based on their inhibitory potential to cell adhesion and the repellent properties of their molecular components against approaching axons and dendrites, PNs might contribute to synaptic stabilization and thus influence neuroplastic potential [1,7,8]. Further tasks could be due to their polyanionic character. The glycosaminoglycan side chains of the CSPGs as well as the hyaluronic acid of the PNs lead to highly negatively charged structures in the direct microenvironment of neurons that might be involved in local ion homeostasis. PNs might also potentially be able to scavenge and bind redox-active iron, and thus reduce the local oxidative potential in the neuronal microenvironment. This may provide some neuroprotection to net-associated neurons against oxidative stress [9-14].
In previous studies we could demonstrate that in Alzheimer’s disease (AD) cortical and subcortical areas with high abundance of PN-enwrapped neurons are less severely affected by neuronal degeneration, and PN-ensheathed neurons are virtually spared from AD related pathologies [15,16]. These results on a neuroprotective capacity of PN-components are extended by demonstrating that net-wearing neurons rarely contain lipofuscin deposits, a major product of advanced iron induced oxidative processes [12]. Furthermore, it could be shown that PNs have a higher capacity to bind iron than any other tissue component in the brain [17]. Iron is believed to contribute to oxidative damage in AD brains by catalyzing the generation of free radicals. Several studies indicate an increased amount of iron in the brain of AD patients, suggesting an abnormality in iron metabolism. This increase in iron may contribute to enhanced oxidative stress in the disease, thereby facilitating neurodegenerative processes [18-22]. It might be suggested that PN, due to their increased iron-binding capacity, can locally reduce oxidative stress and, thus, diminish its deleterious effects on net-associated neurons.
Here we tested the concept that PN-ensheathed neurons are more resistant against iron-induced oxidative stress in vivo induced by injecting iron directly into the mouse brain.
Materials and methods
Animals
Male and female mice (C57BL/6) were bred and housed at the Medizinisch- Experimentelles Zentrum of the Medical Faculty of the University of Leipzig. Animals had free access to food and water and were maintained on an artificial 12 h:12 h light-dark cycle under conditions of constant temperature (22 °C) and humidity. All experimental procedures on animals were carried out in accordance with the European Council Directive of 24 November 1986 (86/609/EEC) and had been approved by the local authorities.
In vivo injection
18 mice at 7-8 weeks of age were deeply anaesthetized by intraperitoneal (IP) injection with a cocktail of ketamine (100 mg/kg, ratiopharm, Ulm, Germany) and xylazine (5 mg/kg, Rompun, Bayer, Germany). Animals were fixed to a stereotaxic apparatus (Stoelting, Wood Dale, Illinois) and a rostrocaudal incision of 1cm length was made using a scalpel. For the injection, Bregma line was exposed clearly and a hole with a diameter of 1mm was drilled. Compounds were injected into the barrel field (-1.7mm from Bregma, 3mm from the midline, 2mm deep from dura) using a 33 gauge needle (33/51/3, Hamilton) attached to a Hamilton syringe (Type 75-RN). Coordinates were determined according to mouse brain atlas of Paxinos and Franklin [23]. Groups of 18 mice each received injections of 0.2μl 20mM FeCl3 or 0.9% NaCl (Braun, Germany) for control. Sequelae of injections were analyzed 24h or 72h post injection.
Tissue preparation
Animals were perfused transcardially under deep CO2-anesthesia with 0.9% NaCl and 4% paraformaldehyd (PFA) in phosphate-buffered saline (PBS). Brains were removed immediately and placed for 24h in 4% PFA for postfixation followed by cryoprotection in 30% sucrose with 0.01% sodium azide in PBS for 48h. 30μm-coronal sections were cut on a freezing microtome and further processed for immunohistochemical and histochemical staining as described below.
Combining Fluoro-Jade B with immunofluorescent labeling
To identify degenerating neurons and to clearly allocate degeneration of neurons ensheathed by PN or of neurons without PN, sections were processed with Fluoro-Jade B in combination with antibodies to aggrecan core protein (HAG7D4, mouse-anti-human aggrecan, 1:10, AbD Serotec, Düsseldorf, Germany) combined with biotinylated NeuN (mouse-anti-NeuN clone A60, ab77315, 1:100, Abcam, Cambridge, UK) or biotinylated Wisteria floribunda agglutinin (WFA, 1:100, Sigma, Taufkirchen, Germany ) combined with NeuN (mouse-anti-NeuN clone A60 MAB 377, 1:100, Millipore, Darmstadt, Germany). Briefly, sections were washed in PBS, preincubated in a blocking solution (2% bovine serum albumin, 0.3% casein, 0.5% donkey normal serum) for 1h followed by a 48h incubation with the primary antibodies at 4°C. After washing, sections were incubated in 0,03% potassium permanganate for 4 min under permanent shaking, washed in distilled water for 2 min and incubated with Cy3-conjugated donkey-antimouse IgG (Dianova, Hamburg, Germany; 1:300) and Cy5-conjugated streptavidin (Dianova, Hamburg, Germany; 1:150) for 3h. Sections were washed, mounted on gelatine coated slides and incubated with 0.001% Fluoro-Jade B (Chemicon, Temecula, CA, USA) in 0.1% acetic acid for 30 min. Slides were washed with distilled water, placed onto a slide warmer until fully dry, immersed in xylene for 2-3 min and coverslipped using DePeX (Serva, Heidelberg, Germany).
Quantification of degeneration
Slides were examined with a Zeiss Fluorescence Microscope (Axiovert 200M, Zeiss, Jena, Germany) and the programme Zeiss Axiovision 4.7 according to Morawski et al. 2004 [12]. For quantification of cell death, the tissue area covered by degenerated cells, which after Fluoro-Jade B staining can clearly be delineated from unaffected tissue (Figure 1), was encircled for each animal on serial sections cut throughout the lesion site at an interval of 120μm. The largest cross-sectional area taken from the animal with the largest lesion size was defined as reference area and copied to all sections. Within this area, counting of Fluoro-Jade B positive neurons, counterstained by NeuN, was performed blinded to the experimental status of the animal within three rectangles of a size of 1.8mm2, randomly placed to the reference area. The contralateral site of injection was used as internal control (Figure 2). The evaluation of Fluoro-Jade B positive neurons was realized clearly above background. Statistical analyzes were performed by paired t-test and Wilcoxon-Range-Test.
Figure 1.
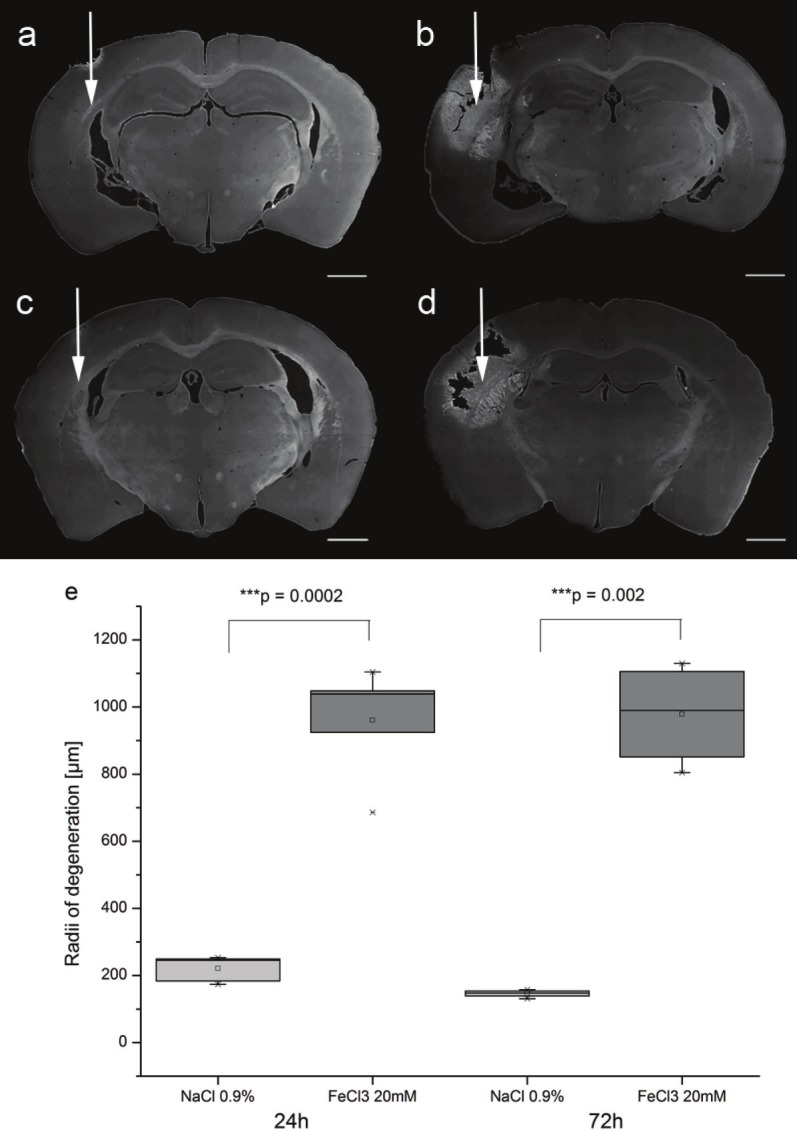
Detection of neuronal degeneration by Fluoro-Jade B. The point of injection is assigned with white arrows. Application of 0.9% NaCl (a=24h, c=72h) induces a small lesion around the injection site in mouse brain. In contrast, injection of 20mM FeCl3 results in a widespread lesion both after 24h (d) and 72h (d) which can be clearly delineated by Fluoro-Jade B staining. To compare the range of degeneration, the widest stained radii after NaCl and FeCl3 injection were measured. Fluoro-Jade B demonstrates significant wider radii for iron-injected sections after 24h and 72h (e). Statistical analyzis performed by paired t-test. Scale bar 1000μm.
Figure 2.
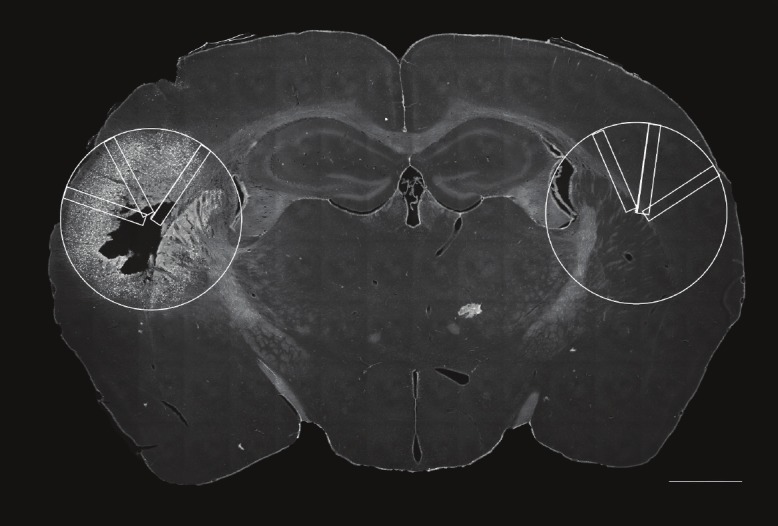
Illustration of evaluationmethod. For examinationof potential protective effectsby perineuronal nets the largestcross-sectional area wasdefined as reference area andcopied to the hemisphere ofinjection as well as its contralateralside. Within this area,counting of Fluoro-Jade B positiveneurons was performedwithin three rectangles of asize of 1.8mm2, randomlyplaced to the reference area.Scale bar 1000μm.
Results
Application of FeCl3 leads to a widespread degeneration of brain tissue around the injection site (Figure 1a/c). To analyze the effects of FeCl3-application and assess the potential influences of postoperative survival time on degenerating neurons, we analyzed the cross sectional area covered by Fluoro-Jade B-positive neurons. While there was only a marginal effect of NaCl injections, application of FeCl3 induced a profound degeneration with a maximum radius covered by degenerating neurons three times bigger than after control injections (Figure 1e). Effects obtained 24h or 72h post injections were only marginally different.
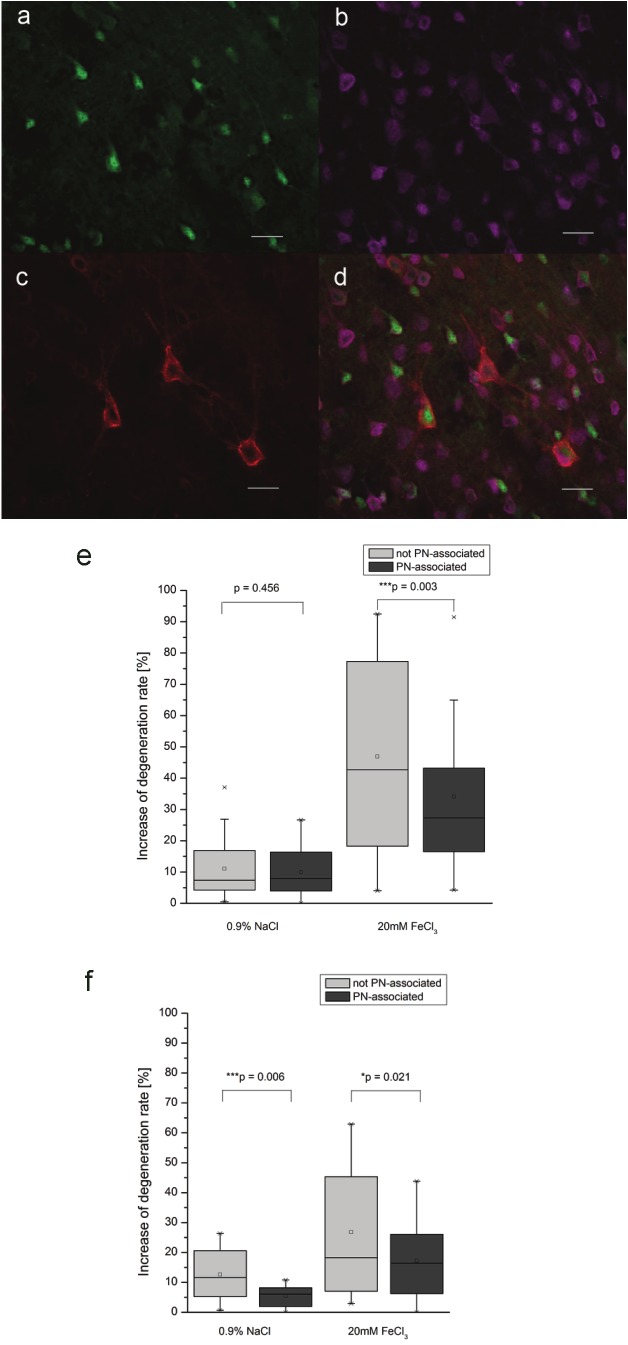
To assess potentially different effects of FeCl3-induced cell death for neurons with and without PN, lectin histochemistry of Wisteria Floribunda Agglutinin (WFA), binding to N-acetylgalactosamines as the main chondroitin sulfate proteoglycan components of PN (Figure 3a-d) [24] was used in combination with NeuN and Fluoro- Jade B staining. Cell counting was performed within the area covered by apoptotic cell death, defined as reference area as described above. Corresponding brain regions of the contralateral site were used as internal controls.
Figure 3.
Quantification of differences by using WFA. Assessing differential effects on neuronal degeneration for neurons with and without PN. Degenerating neurons were identified by labeling of Fluoro-Jade B (a) with NeuN (b). Neurons with and without PN were identified by the presence or absence of WFA labeling (c). Quantification on the overlay of all three signals (d) revealed a significant less frequent degeneration for neurons with PN compared to neurons without PN both 24h (e) and 72h (f) after FeCl3 injection. Statistical analyzis by Wilcoxon Range test, Scale bar 20μm.
Results clearly demonstrate that both 24h and 72h post injection, neurons ensheathed by PNs are significantly less frequently affected by apoptotic cell death than neurons without PN (Figure 3e-f).
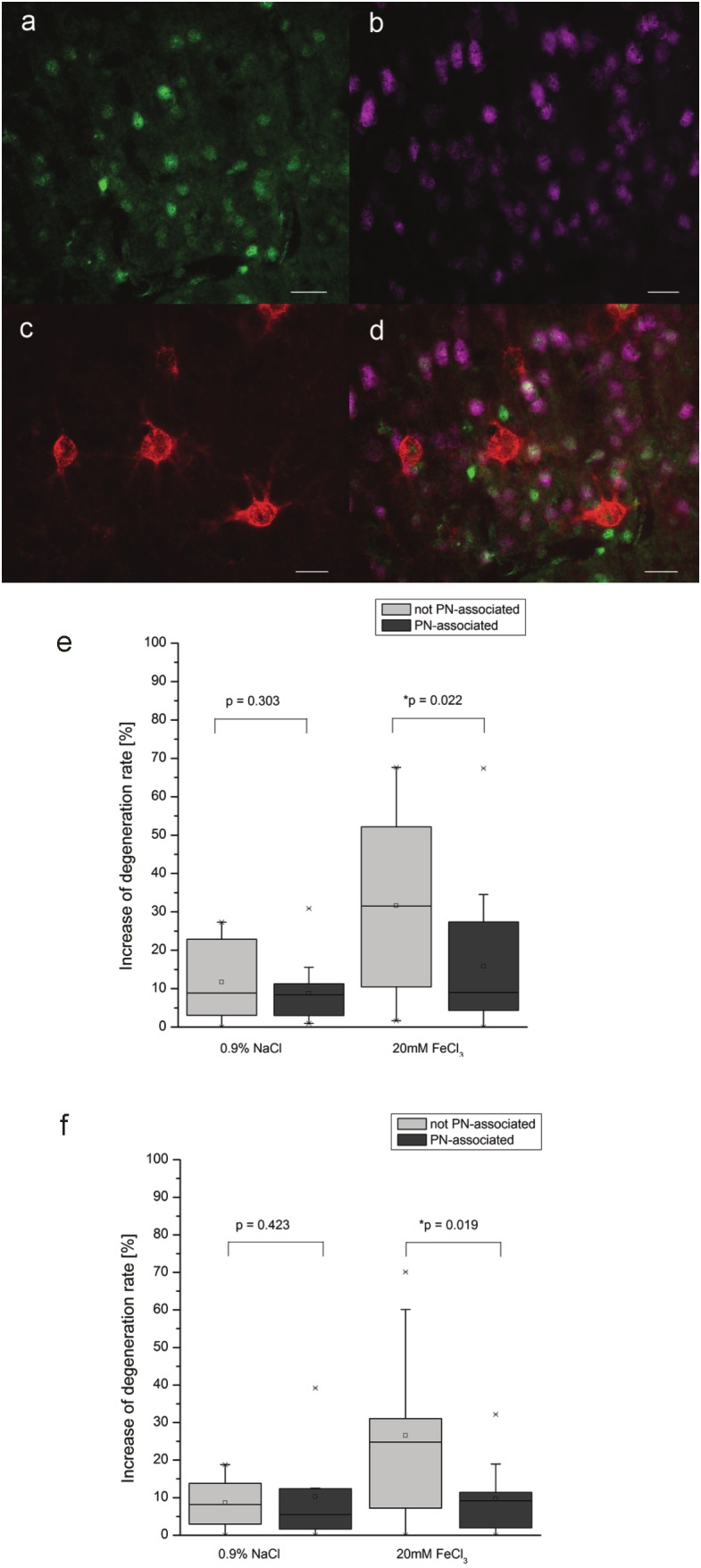
To exclude any sampling bias of PN ensheathed neurons by potential effects of FeCl3 on chondroitin sulfate side chains, results were replicated in second series of experiments, combining Fluoro-Jade B staining with immunocytochemistry for aggrecan (HAG), the main proteoglycan component of PNs. Consistent with results obtained after WFA-labeling, quantitative analyzis revealed a significant lower rate of degeneration for PN-wearing neurons after microinjection of iron (Figure 4a-f).
Figure 4.
Quantification of differences by using HAG. Detecting differential effects on neuronal degeneration for neurons with and without PN with triple staining of degenerating net-associated neurons with immunocytochemical labeling of Fluoro-Jade B (a), NeuN (b) and HAG (c). Overlay image (d) was used for counting procedures. Net-associated neurons are significantly less severely affected by degeneration after FeCl3 injection. This effect could be seen after 24h (e) as well as 72h (f).Statistical analyzis by Wilcoxon Range test, Scale bar 20μm.
Discussion
Iron is the most abundant transitional metal in the brain and plays an important role in maintaining normal brain function [25]. Iron overload, however, has been detected in various neurodegenerative disorders such as Parkinson’s and Alzheimer’s disease where it might be a driving force of oxidative stress. In the present study we used an in vivo model of iron-induced cell death to assess potential differences in neuronal vulnerability between neurons ensheathed by a PN versus neurons without PN.
Although the injection of FeCl3 into mouse brain leads to a widespread degeneration of brain tissue, we could demonstrate that PN-associated neurons are affected to a much lesser extent than neurons without a PN. These differential effects were clearly present independent as to whether PNs were detected by WFA binding to N-acetylgalactosamine of the chondroitin sulfate proteoglycans or immunocytochemistry of the core protein aggrecan. This clearly indicates some neuroprotective effects associated with PNs.
One of the main components of the PN is the CSPGs. It had been demonstrated previously; that CSPGs have a protective effect on cultured neurons against excitatory amino acid (EAA) induced cell death [26]. This protective effect has been explained by molecular interactions with the binding sites on neuronal membranes, neurotrophic factors as well as an antagonizing action of cellular responses following an activation of EAA receptors [26,27]. In addition, a neuroprotective action of CSPGs has been suggested by buffering divalent cations like Ca2+ which play a critical role in glutamate toxicity [25]. These buffering features of CSPGs are supported by their role on determining the local diffusion properties of calcium in the brain extracellular space [27,28]. These effects on the local ion homeostasis most likely involve electrostatic aspects.[28] The CSPGs as well as the hyaluronan components provide a highly negatively charge to the perineuronal nets. Due to this charge, PNs are able to bind high amounts of cations including iron [17]. This mechanism could, thus, be suggested to act as a buffering system by binding extracellular iron, thereby reducing local oxidative stress in the direct environment of net-associated neurons preventing PN-wearing neurons from degeneration [17,29]. These findings are in agreement with our previous observation that neurons ensheathed by a PN both in normally aged and in Alzheimer’s disease brains are less frequently affected by accumulation of lipofuscin, an intralysosomal pigment generated by iron-catalyzed oxidative processes [17]. Oxidative stress might be one of the fundamental factors contributing to disease progression in neurodegenerative disorders such as Parkinson’s disease and Alzheimer’s disease. Accordingly, PN-associated neurons are virtually protected against the formation of neurofibrillary tangles [15,16,30,31] as well as against amyloid beta toxicity in vitro [32].
Taken together, our results clearly demonstrate neuroprotective properties associated with the presence of perineuronal nets against iron induced cell death. The exact molecular basis of this mechanism and whether it can potentially be used for a neuroprotective strategy remains to be determined.
Acknowledgement
The authors wish to thank Mrs Hildegard Gruschka and Dr. Ulrich Gaertner for their technical support. This work was granted by the European Union and the Free State of Saxony (Project: Neuron 48-038), the German Research Foundation GRK 1097 "INTERNEURO”, the EU-Project “Neuropro” (Grant Agreement No. 223077), COST Action BM1001 “Brain Extracellular Matrix in Health and Disease”, German Research Foundation MO 2249/2-1 within the PP 1608, and the Alzheimer Forschungsinitiative e.V. (AFI #11861) to M. Morawski.
References
- 1.Morawski M, Brückner G, Arendt T, Matthews RT. Aggrecan: Beyond cartilage and into the brain. Int J Biochem Cell Biol. 2012;44:690–3. doi: 10.1016/j.biocel.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Brückner G, Morawski M, Arendt T. Aggrecan-based extracellular matrix is an integral part of the human basal ganglia circuit. Neuroscience. 2008;151:489–504. doi: 10.1016/j.neuroscience.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 3.Brückner G, Hartig W, Seeger J, Rübsamen R, Reimer K, Brauer K. Cortical perineuronal nets in the gray short-tailed opossum (Monodelphis domestica): a distribution pattern contrasting with that shown in placental mammals. Anat Embryol (Berl) 1998;197:249–262. doi: 10.1007/s004290050135. [DOI] [PubMed] [Google Scholar]
- 4.Celio MR, Blumcke I. Perineuronal nets--a specialized form of extracellular matrix in the adult nervous system. Brain Res Brain Res Rev. 1994;19:128–145. doi: 10.1016/0165-0173(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 5.Zimmermann DR, Dours-Zimmermann MT. Extracellular matrix of the central nervous system: from neglect to challenge. Histochem Cell Biol. 2008;130:635–653. doi: 10.1007/s00418-008-0485-9. [DOI] [PubMed] [Google Scholar]
- 6.Brückner G, Grosche J, Schmidt S, Hartig W, Margolis RU, Delpech B, Seidenbecher CI, Czaniera R, Schachner M. Postnatal development of perineuronal nets in wild-type mice and in a mutant deficient in tenascin-R. J Comp Neurol. 2000;428:616–629. doi: 10.1002/1096-9861(20001225)428:4<616::aid-cne3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 7.Rhodes KE, Fawcett JW. Chondroitin sulphate proteoglycans: preventing plasticity or protecting the CNS? J Anat. 2004;204:33–48. doi: 10.1111/j.1469-7580.2004.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- 9.Brückner G, Hartig W, Kacza J, Seeger J, Welt K, Brauer K. Extracellular matrix organization in various regions of rat brain grey matter. J Neurocytol. 1996;25:333–346. doi: 10.1007/BF02284806. [DOI] [PubMed] [Google Scholar]
- 10.Brückner G, Bringmann A, Koppe G, Hartig W, Brauer K. In vivo and in vitro labelling of perineuronal nets in rat brain. Brain Res. 1996;720:84–92. doi: 10.1016/0006-8993(96)00152-7. [DOI] [PubMed] [Google Scholar]
- 11.Hartig W, Singer A, Grosche J, Brauer K, Ottersen OP, Brückner G. Perineuronal nets in the rat medial nucleus of the trapezoid body surround neurons immunoreactive for various amino acids, calcium-binding proteins and the potassium channel subunit Kv3.1b. Brain Res. 2001;899:123–133. doi: 10.1016/s0006-8993(01)02211-9. [DOI] [PubMed] [Google Scholar]
- 12.Morawski M, Brückner MK, Riederer P, Brückner G, Arendt T. Perineuronal nets potentially protect against oxidative stress. Exp Neurol. 2004;188:309–315. doi: 10.1016/j.expneurol.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 13.Brückner G, Brauer K, Hartig W, Wolff JR, Rickmann MJ, Derouiche A, Delpech B, Girard N, Oertel WH, Reichenbach A. Perineuronal nets provide a polyanionic, glia-associated form of microenvironment around certain neurons in many parts of the rat brain. Glia. 1993;8:183–200. doi: 10.1002/glia.440080306. [DOI] [PubMed] [Google Scholar]
- 14.Reinert T, Morawski M, Arendt T, Butz T. Quantitative microanalysis of perineuronal nets in brain tissue. Nucl Instrum Methods Phys Res, Sect B. 2003;210:395–400. [Google Scholar]
- 15.Brückner G, Hausen D, Hartig W, Drlicek M, Arendt T, Brauer K. Cortical areas abundant in extracellular matrix chondroitin sulphate proteoglycans are less affected by cytoskeletal changes in Alzheimer's disease. Neuroscience. 1999;92:791–805. doi: 10.1016/s0306-4522(99)00071-8. [DOI] [PubMed] [Google Scholar]
- 16.Morawski M, Brückner G, Jäger C, Seeger G, Matthews RT, Arendt T. Involvement of Perineuronal and Perisynaptic Extracellular Matrix in Alzheimer's Disease Neuropathology. Brain Pathol. 2012 doi: 10.1111/j.1750-3639.2011.00557.x. doi: 10.1111/j.1750-3639.2011.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morawski M, Reinert T, Brückner G, Wagner FE, Arendt T, Tröger W. The Binding of Iron to Perineuronal Nets: A Combined Nuclear Microscopy and Mössbauer Study. Hyperfine Interact. 2004;159:285–291. [Google Scholar]
- 18.Smith MA, Harris PL, Sayre LM, Perry G. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc Natl Acad Sci U S A. 1997;94:9866–9868. doi: 10.1073/pnas.94.18.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duce JA, Tsatsanis A, Cater MA, James SA, Robb E, Wikhe K, Leong SL, Perez K, Johanssen T, Greenough MA, Cho HH, Galatis D, Moir RD, Masters CL, McLean C, Tanzi RE, Cappai R, Barnham KJ, Ciccotosto GD, Rogers JT, Bush AI. Iron-export ferroxidase activity of beta-amyloid precursor protein is inhibited by zinc in Alzheimer's disease. Cell. 2010;142:857–867. doi: 10.1016/j.cell.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith MA, Nunomura A, Zhu X, Takeda A, Perry G. Metabolic, metallic, and mitotic sources of oxidative stress in Alzheimer disease. Antioxid Redox Signal. 2000;2:413–420. doi: 10.1089/15230860050192198. [DOI] [PubMed] [Google Scholar]
- 21.Honda K, Casadesus G, Petersen RB, Perry G, Smith MA. Oxidative stress and redoxactive iron in Alzheimer's disease. Ann N Y Acad Sci. 2004;1012:179–182. doi: 10.1196/annals.1306.015. [DOI] [PubMed] [Google Scholar]
- 22.Lei P, Ayton S, Finkelstein DI, Spoerri L, Ciccotosto GD, Wright DK, Wong BX, Adlard PA, Cherny RA, Lam LQ, Roberts BR, Volitakis I, Egan GF, McLean CA, Cappai R, Duce JA, Bush AI. Tau deficiency induces parkinsonism with dementia by impairing APP-mediated iron export. Nat Med. 2012;18:291–295. doi: 10.1038/nm.2613. [DOI] [PubMed] [Google Scholar]
- 23.Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. San Diego: Academic Press; 2001. [Google Scholar]
- 24.Giamanco KA, Morawski M, Matthews RT. Perineuronal net formation and structure in aggrecan knockout mice. Neuroscience. 2010;170:1314–1327. doi: 10.1016/j.neuroscience.2010.08.032. [DOI] [PubMed] [Google Scholar]
- 25.Moos T. Brain iron homeostasis. Dan Med Bull. 2002;49:279–301. [PubMed] [Google Scholar]
- 26.Okamoto M, Mori S, Endo H. A protective action of chondroitin sulfate proteoglycans against neuronal cell death induced by glutamate. Brain Res. 1994;637:57–67. doi: 10.1016/0006-8993(94)91217-3. [DOI] [PubMed] [Google Scholar]
- 27.Okamoto M, Mori S, Ichimura M, Endo H. Chondroitin sulfate proteoglycans protect cultured rat's cortical and hippocampal neurons from delayed cell death induced by excitatory amino acids. Neurosci Lett. 1994;172:51–54. doi: 10.1016/0304-3940(94)90660-2. [DOI] [PubMed] [Google Scholar]
- 28.Hrabetova S, Masri D, Tao L, Xiao F, Nicholson C. Calcium diffusion enhanced after cleavage of negatively charged components of brain extracellular matrix by chondroitinase ABC. J Physiol. 2009;587:4029–4049. doi: 10.1113/jphysiol.2009.170092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fiedler A, Reinert T, Morawski M, Brückner G, Arendt T, Butz T. Intracellular iron concentration of neurons with and without perineuronal nets. Nucl Instrum Methods Phys Res, Sect B. 2007;260:153–158. [Google Scholar]
- 30.Morawski M, Brückner G, Jäger C, Seeger G, Arendt T. Neurons associated with aggrecan-based perineuronal nets are protected against tau pathology in subcortical regions in Alzheimer's disease. Neuroscience. 2010;169:1347–1363. doi: 10.1016/j.neuroscience.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 31.Morawski M, Pavlica S, Seeger G, Grosche J, Kouznetsova E, Schliebs R, Brückner G, Arendt T. Perineuronal nets are largely unaffected in Alzheimer model Tg2576 mice. Neurobiol Aging. 2010;31:1254–1256. doi: 10.1016/j.neurobiolaging.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 32.Miyata S, Nishimura Y, Nakashima T. Perineuronal nets protect against amyloid beta-protein neurotoxicity in cultured cortical neurons. Brain Res. 2007;1150:200–206. doi: 10.1016/j.brainres.2007.02.066. [DOI] [PubMed] [Google Scholar]