Abstract
Growing evidence indicates the role of exosomes in a variety of physiological pathways as conveyors of biological materials from cell-to-cell. However the molecular mechanism(s) of secretion and their interaction with receiving cells are yet unclear. Recently, it is emerging that exosomes are involved in pathological processes as potential carriers in the progression of neurodegenerative pathologies associated with misfolded proteins. In the current review we will discuss some recent findings on the key role of exosomes in the spreading of the aggregated products of α-synuclein from neuron-to-neuron and of inflammatory response propagation from immune cell-to-cell; we will highlight the implication of exosomes in the neurodegeneration and progression of the disease and the their potential interplay with genes related to Parkinson’s disease. Increasing our knowledge on the cell-to-cell transmissions might provide new insights into mechanism of disease onset and progression and identify novel strategies for diagnosis and therapeutic intervention in Parkinson and other neurodegenerative diseases.
Keywords: Exosomes, Parkinson’s disease, α-synuclein, LRRK2, neuronal degeneration
Parkinson’s disease and α-synuclein
Parkinson’s disease (PD) is the second most common neurodegenerative disorder after Alzheimer’s disease, affecting almost 1% of the population over 50 years old. Clinical features of the disease include bradykinesia, resting tremor, muscle tone rigidity, and possible cognitive involvement [1,2]. Pathologically, PD is characterized by selective degeneration of dopaminergic neurons in the substantia nigra pars compact (SNpc) and the presence of Lewy bodies (LBs), primarily composed of fibrillar α-synuclein (α-Syn) and ubiquitinated proteins, in the surviving neurons [3,4].
Although more than 90% of PD cases occur sporadically, the discovery of monogenic hereditable forms of the disease has provided important clues as to the understanding of the molecular mechanisms that lead to neuronal degeneration in this pathology [5]. Different genes have been associated with familial cases of PD, including α-Syn, LRRK2, parkin, DJ-1 and PINK1 [6]. However, the mechanism underlying disease onset and progression are still unclear. Evidences from genetic, pathological and biochemical studies supports critical involvement of α-Syn in the pathogenesis of the disease [7,8].
α-Syn is a small natively unfolded protein widely expressed in neurons at the presynaptic level [9], with a conserved amphipathic N-terminus, an acidic C-terminus and a hydrophobic central region that is required for both oligomerization and fibrillization processes [10]. Although α-Syn biological function is still controversial, it has been suggested to play a role in synaptic vesicles biogenesis and modulation of synaptic transmission [9]. α-Syn (SNCA) gene mutations and single-nucleotide polymorphisms (SNPs) render α-Syn protein more prone to misfolding and accelerate formation of aggregates [5], which have been linked to autosomal-dominant forms of PD [11] and increased susceptibility to sporadic PD [12], respectively. While there is clear evidence that α-Syn plays an important role in the pathogenesis of PD, it is unclear what is/are the toxic form(s) of the protein responsible for neuronal dysfunction and the mechanisms by which these purported toxic forms of α-Syn spreads from neuron to neuron triggering, in turn, neuronal degeneration [13].
Dopaminergic neuronal degeneration
The main pathological hallmark of PD is the progressive degeneration and death of dopaminergic neurons in the SNpc [14], which leads to a severe dopamine depletion in the striatum, responsible for the motor symptoms observed in the disease [15]. In particular, the SNpc is subdivided into ventral and dorsal tiers, and neuronal death in PD mainly occurs in the ventral tier; in this regard, studies on post-mortem PD brains reported that the degeneration affecting the ventral tier account for 80-95% of neurons loss [16-18]. The pattern of cell loss and the selective vulnerability of dopamine (DA) neurons seen in PD do not occur in other neurodegenerative diseases or in normal aging, suggesting that these neurons possess distinct features that render them more susceptible to degeneration [19]. Several mechanisms have been proposed to explain the observed vulnerability of dopaminergic neurons. First, it was suggested that the size and neurochemical phenotype of dopaminergic neurons which can make an elevated number of synaptic connections particularly prone to damage can account for the observed vulnerability [20]. Moreover, the large amounts of the dark-coloured pigment neuromelanin and its ability to interact with transition metals, especially iron, and to mediate intracellular oxidative mechanisms has been suggested to sensitize this population of cells [21,22]. In addition, an unbalanced DA homeostasis (consequent to impaired synthesis, storage, degradation and distribution in the synaptic vesicles) can lead to increased concentration of free cytosolic DA which favors ROS production and oxidative stress [19,23] and, in its oxidized form, can itself covalently modify α-Syn and promote the stabilization of toxic fibrils [24]. Finally, these neurons exhibit enhanced sensitivity to pro-inflammatory mediators compared to other neuronal populations, likely consequent to the high density of microglia cells in this region [25]. All these factors have been reported and suspected to contribute to the dopaminergic neurons vulnerability and degeneration.
Although it is generally accepted that the loss of midbrain dopaminergic neurons occurs and is largely responsible for the major motor symptoms, this is not the only region showing pathological hallmarks in PD patients. Lewy bodies and cell loss occur in other non-dopaminergic areas of the brain including brainstem cells nuclei, olfactory bulb, limbic system and neocortex [26,27] suggesting a mechanism involving propagation and progression of the pathology, similar to the one observed in prion diseases [28]. This model of a pathological propagation has recently gained particular attention with regard to neurodegenerative diseases linked to misfolded and aggregated proteins.
A few years ago, Braak and co-workers [26] proposed a pathological staging process for PD. In this system, based on the distribution of α-Syn pathology in post-mortem brains, early pathological events first occur in the autonomic nervous system (stage 0), dorsal motor nucleus of the vagus and in the anterior olfactory nucleus (stage 1), then spread the locus coeruleus, SNpc and basal forebrain (stage 2). In the final stages, pathology extends to the neocortex, hippocampus and basal ganglia. Although the Braak staging does not fit all disorder associated with LBs pathology and may be a simplified system, it supports the notion that α-Syn depositions may spread to neighbor cells. There are several lines of evidence supporting cell-to-cell propagation of α-Syn. First, α-Syn exists as a soluble, natively unfolded monomer [29] and/or a folded alpha-helix tetramer [30,31] and, in vitro, it can convert into soluble, oligomeric form and a beta-sheet, fibrillar form, the latter serving as seed or template for the formation of larger aggregates [32]. Of interest, α-Syn dosage is intimately linked to PD, being multiplications of SNCA cause of hereditable, aggressive forms of the disease [33]. Increased cellular concentration of α-Syn can be the results of impaired protein homeostasis [34]. Accordingly, reduced protein clearance due to defects in the proteasome and autophagy machineries may lead to increased concentration of α-Syn and favor the formation of aggregates [35]. In support of a cell-to-cell propagation α-Syn is the striking observation that neural grafts derived from midbrain healthy tissue transplanted into the striatum of individuals affected with PD showed LB pathology after 10-16 years in addition to the expected Lewy pathology observed in the host tissue [36,37].
It has been shown by independent studies that α-Syn toxic forms can be secreted from cells in culture, enter other cells, and seed small intracellular aggregates in the receiving cells [38]. Although the precise mechanism is not yet clear, it is likely that α-Syn is released by the donor cell and uptaken by a recipient cell by energy dependent processes. In this regard, recent studies have reported the crucial role exerted by exosomes as material and structural templates transporters from cell-to-cell and as potential carriers in the dissemination of PD pathology [39,40]. In the next paragraphs we discuss the recent findings on the key role of exosomes in the α-Syn spreading from neuron-to-neuron and in the inflammation propagation from immune cell-to-cell, highlighting their potential implication in the propagation and progression of the PD.
Exosomes biogenesis and physiological functions
Exosomes are microvesicles whose biogenesis occurs within multivesicular bodies (MVBs) in the endosomal system. In details, proteins and signaling complexes that are sequestered to the limiting membrane of MVBs can be selectively incorporated into intraluminal vesicles (ILVs) by invagination of the MVB membrane. Here, MVB can either fuse with the lysosome membrane to degrade ILV and its content, or alternatively with the plasma membrane to release the ILV as exosomes into the extracellular space [40,41].
Exosomes are small vesicles with a size ranging from 30 to 120 nm and appear with a characteristic round or cup-shaped morphology as observed by transmission or cryo-electron microscopy [42]. These vesicles can be released from a variety of cells, both in vitro and in vivo, such as neuronal cells including neurons [43], microglia [44], astrocytes [45] and non-neuronal cells such as lymphocytes and monocytes [46,47]. Recently, it has been shown that exosomes can transport messenger RNA, microRNA, protein and signaling complexes, all of which important in the modulation of gene and protein expression in the receiving cells [40,47]. They contain distinct proteins, some are common across all exosomes and are used as “marker” protein including Alix, tetraspanin (CD63, CD81) and heat shock protein (HSP70, 90) [47,48], while others are specific and depend on the cell type from which they are secreted [49]. In this regard, it has been reported that exosomes released from antigen-presenting cells carry the major histocompatibility complex (MHC) [50] whilst those released from oligondendrocytes carry the myelin proteins [51].
Little is still known about the requirement and regulation of exosomes fusion with the plasma membrane of secreting and receiving cells. Different studies have suggested that exosomes are secreted by a regulated process involving the tethering, docking and fusion of the vesicles at the plasma membrane probably through a specific combination of SNARE proteins, although this combination has not yet been identified [41,46]. It has been demonstrated that exosomes can be taken up by targeting cells; in this regard, different mechanisms have been proposed, including endocytosis, receptor-ligand binding, attachment or fusion with the plasma membrane to deliver their cytosolic content [49,52,53], however the mechanism of interaction and secretion has not yet been well established.
Exosomes are biologically active entities and are important for a variety of physiological pathways. In particular, the fusion of MVB with the lysosome results in the degradation of unwanted cellular materials, and allows the cell to remove transmembrane proteins essential to down-regulate activated surface receptors, as growth factors or adhesion molecules [54]. Moreover, these vesicles have been shown to play a functional role in neuronal development and regeneration [55], and in the synaptic plasticity of the cortical and hippocampal neurons [56]. In addition, when released from immune cells, they exhibit immune-stimulatory properties important to activate and propagate the inflammation in response to pathogenic stimuli or injury [57,58]. Recently, in the context of pathological processes the role of exosomes has been the subject of intensive research, they have been implicated in the spread of toxic proteins within the brain and in the progression of different neurodegenerative diseases including PD [40].
Exosomes in the cell-to-cell communication and their role in the progression of PD
Cell-to-cell communication exerted by exosomes in the brain, in particular neuron-to-neuron and neuron-to-glia communication, has been intensely studied to investigate their role in the progression of the neurodegenerative disease including PD. In this context, it was demonstrated that α-Syn deposits are released via exosomes and in this way are efficiently able to transfer and propagate α-Syn toxic forms to other cells [40,59-61]. Different studies demonstrated that α-Syn transferred neuron-to-neuron is able to form aggregates in recipient cells [61,62]. Moreover, Emmanouilidou and colleagues reported that secreted α-Syn via exosomes induces cell death of recipient neuronal cells, suggesting that α-Syn deposits spreading to the neighbors neurons causes propagation of the disease [60].
Of interest, immunologists started to focus on exosomes when they discovered that immune cells, B lynmphocytes and dendritic cells, were also able to secrete exosomes [57,63]. Most notably, these exosomes when released by dendritic cells (DCs) expressed the MHC II bound to antigenic peptide, and were able to present MHC-peptide complex to T cells promoting their activation [46,57,58,64]. In addition, a recent study reported that exosomes released from infected macrophages expressed pathogen-associated molecular patterns (PAMPs) and can stimulate macrophages activation by pattern recognition receptor (PRP), and proinflammatory response both in vitro and in vivo [65]. This role of exosomes in antigen-specific immune response results in the spread of antigens or MHC-peptide complex between immune cells, causing the propagation of inflammatory response. Moreover, exosomes can contain proinflammatory mediators such as IL-1β and TNF-α, which are involved in the modulation and propagation of inflammatory response [46,58]. Overall these evidences suggest that exosomes could play an active role in the immune response, thus contributing to the induction, amplification and modulation of inflammatory response.
In this regard, several studies have reported that α-Syn deposits released from neurons can be endocytosed by astrocytes and microglia, the brain immune cells, probably in an attempt to clear toxic species of the protein [41,66,67]. However, excessive α-Syn uptake in these cells could produce glia inclusions, similar to the ones found in PD brains [68], and trigger inflammatory response [67,69]. Indeed, it has been reported that α-Syn released by injured neurons is able to induce and maintain inflammatory response through activation of microglia cells [8,70].
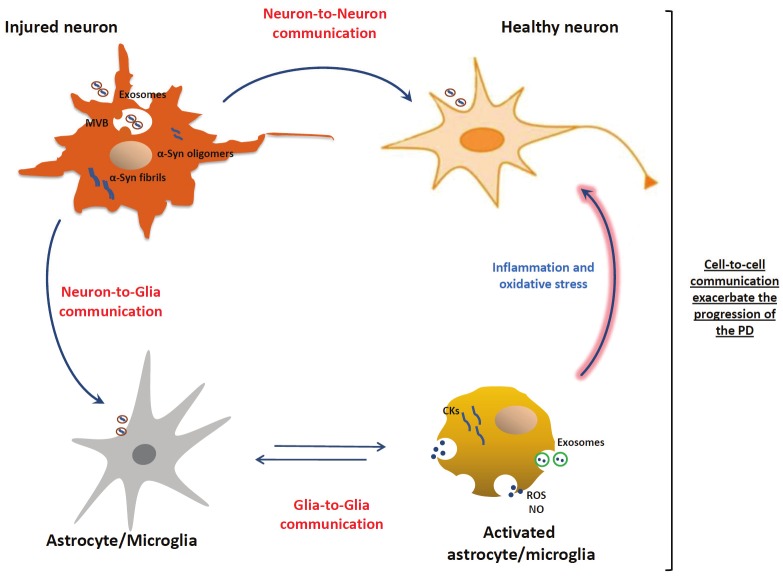
Inflammation is sustained by multiple interactions among cells, and in this context exosomes might act at different stages of the process, including the activation level, through neuron-to-glia communication, or during the propagation of the inflammatory response through glia-to-glia communication, as depicted in Figure 1. To date, it has been widely investigated and discussed the pathogenic transmission of α-Synderived exosomes between neuron-to-neuron and neuron-to-glia cells, however studies on the role of exosomes in glia-to-glia communications and their potential impact in the progression of PD and other neurodegenerative diseases are missing. Although a well-regulated inflammatory process is essential for tissue repair, an excessive or protracted inflammatory response can result in a more severe and chronic neuroinflammatory cycle that promotes the progression of the disease [71]. In fact, it is now established that inflammation is a key hallmark of PD pathogenesis and can contribute and exacerbate the nigral neurodegeneration observed in the disease [72].
Figure 1.
Exosomes in the cell-to-cell communications and their role in the progression of PD. Exosomes containing α-Syn released by injured neurons can be transmitted from neuron-to-neuron thus leading to α-Syn spreading, and from neuron-to-glia leading to activation of inflammatory response. In turn, exosomes released by activated glial cells, containing inflammatory mediators, can be transmitted from glia-to-glia leading to the propagation of inflammatory response. Consecutively, the propagation of inflammatory mediators and the exacerbated neuroinflammation could contribute to neuronal dysfunctions and to progression of the disease.
Notwithstanding it is established that exosomes are involved in the pathogenesis of neurodegenerative diseases, we are still required to investigate the molecular pathways that regulate their assembly, secretion, interaction with receiving cells and the stimuli that trigger their release both in vitro and in vivo. Increasing our knowledge on the cell-to-cell transmission of α-Syn toxic forms and of inflammatory mediators between brain cells, might provide new insights into mechanism of disease onset and progression and identify novel strategies for diagnosis and therapeutic intervention in PD and other neurodegenerative diseases.
A role of PD related genes in exosome secretion?
As discussed earlier, exosomes derive from MVBs that can either fuse with the lysosome for degradation and with the plasma membrane for exosome release to the extracellular space. A number of genes linked to PD, either carrying causal mutations (LRRK2, ATP13A2 and VPS35) or more common variants acting as risk factors (heterozygous mutations in GBA), have been suggested to play a role in lysosomal-endosomal pathways. In particular, recent studies have provided strong evidence that LRRK2 directly impacts the secretory and endocytic machinery. Shin and colleagues [73] demonstrated that LRRK2 interacts with Rab5b, a regulator of endocytic vesicle trafficking. In addition, LRRK2 has been observed to modulate synaptic vesicle trafficking via interaction with pre-synaptic proteins such as NSF, actin, syntaxin and SV2A [74]. Xiong and colleagues overexpressed wild type LRRK2 in primary neuronal cultures and documented reduced rates of synaptic vesicle endocytosis and exocytosis in hippocampal neurons [75]. Interestingly, LRRK2 has been found to co-localize with MVBs [76]. Although a role for LRRK2 in exosome secretion has not been described yet, based on the strong links between LRRK2 function and endosome-lysosome related pathways, we can speculate that LRRK2 activity is important in exosome secretion during fusion of MVBs with the plasma membrane. In particular, LRRK2 may function as scaffold to assemble components of the fusion machinery such as its partner NSF [74], which has been shown to participate in the fusion between MVBs with the plasma membrane to release exosomes into the extracellular space [77]. In addition, Alegre-Abarrategui and colleagues [76] observed that R1441C LRRK2 pathological mutation induce the formation of skein-like abnormal MVBs. One possibility is that abnormally large MVBs due to pathological LRRK2 activity contain and release an increased number of exosomes, and, as consequence, more α-Syn toxic forms are present in the extracellular space leading thus to the spread of the disease.
It has been reported that variation in tau gene (MAPT), which encoding for a protein involved in the pathogenesis of Alzheimer’s disease [78], can confer genetic risk for PD [79,80]. In this regard, an interesting study reported that under pathological conditions tau protein can interact with α-Syn promoting the oligomerization and the toxicity of these proteins [81]. Taken together these data suggest that tau can accelerate the exosomes-mediated release containing α-Syn toxic forms from injured neurons.
Of interest, Vacuolar Sorting Protein 35 (VPS35), a protein that mutated is cause of late-onset autosomal dominant PD, is an essential component of the retromere complex, which performs the retrograde transport from the endosome to the Golgi apparatus. Sullivan et al. [82] observed that cells with defective retromere activity displayed increased exosomal secretion of amyloid precursor protein (APP). Although experimental models are not yet available, we can predict that pathological mutations of VPS35 may lead to aberrant exosome secretion with consequent accumulation of extracellular α-Syn and cell-to-cell propagation of the pathology. Establishing whether LRRK2, tau, and VPS35 operate within the exosome pathway might provide important clues on the pathological mechanisms of α-Syn spreading and progression of the disease.
Acknowledgements
This work was supported by the PRIN (grant no. 2008-55YP79), the Rientro dei Cervelli Program (lncentivazione alla mobilita di studiosi stranieri e italiani residenti all’estero) from the Italian Ministry of Education, University and Research (EG), the Fondazione CARIPLO (grant no. 2011 0540) and the Micheal J Fox Foundation.
References
- 1.Brundin P, Li JY, Holton JL, Lindvall O, Revesz T. Research in motion: the enigma of Parkinson's disease pathology spread. Nat Rev Neurosci. 2008;9:741–745. doi: 10.1038/nrn2477. [DOI] [PubMed] [Google Scholar]
- 2.Riess O, Kruger R, Schulz JB. Spectrum of phenotypes and genotypes in Parkinson's disease. J Neurol. 2002;249:III/15–20. doi: 10.1007/s00415-002-1303-2. [DOI] [PubMed] [Google Scholar]
- 3.Lees AJ, Hardy J, Revesz T. Parkinson's disease. Lancet. 2009;373:2055–2066. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- 4.Forno LS. Neuropathology of Parkinson's disease. J Neuropathol Exp Neurol. 1996;55:259–272. doi: 10.1097/00005072-199603000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Ali SF, Binienda ZK, Imam SZ. Molecular aspects of dopaminergic neurodegeneration: gene-environment interaction in parkin dysfunction. Int J Environ Res Public Health. 2011;8:4702–4713. doi: 10.3390/ijerph8124702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Houlden H, Singleton AB. The genetics and neuropathology of Parkinson's disease. Acta Neuropathol. 2012;124:325–338. doi: 10.1007/s00401-012-1013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee VM, Trojanowski JQ. Mechanisms of Parkinson's disease linked to pathological alpha-synuclein: new targets for drug discovery. Neuron. 2006;52:33–38. doi: 10.1016/j.neuron.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 8.Lee HJ, Kim C, Lee SJ. Alpha-synuclein stimulation of astrocytes: Potential role for neuroinflammation and neuroprotection. Oxid Med Cell Longev. 2010;3:283–287. doi: 10.4161/oxim.3.4.12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim C, Lee SJ. Controlling the mass action of alpha-synuclein in Parkinson's disease. J Neurochem. 2008;107:303–316. doi: 10.1111/j.1471-4159.2008.05612.x. [DOI] [PubMed] [Google Scholar]
- 10.Giasson BI, Murray IV, Trojanowski JQ, Lee VM. A hydrophobic stretch of 12 amino acid residues in the middle of alpha-synuclein is essential for filament assembly. J Biol Chem. 2001;276:2380–2386. doi: 10.1074/jbc.M008919200. [DOI] [PubMed] [Google Scholar]
- 11.Hardy J, Lewis P, Revesz T, Lees A, Paisan-Ruiz C. The genetics of Parkinson's syndromes: a critical review. Curr Opin Genet Dev. 2009;19:254–265. doi: 10.1016/j.gde.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Nalls MA, Plagnol V, Hernandez DG, Sharma M, Sheerin UM, Saad M, Simon-Sanchez J, Schulte C, Lesage S, Sveinbjornsdottir S, Stefansson K, Martinez M, Hardy J, Heutink P, Brice A, Gasser T, Singleton AB, Wood NW. Imputation of sequence variants for identification of genetic risks for Parkinson's disease: a meta-analysis of genome-wide association studies. Lancet. 2011;377:641–649. doi: 10.1016/S0140-6736(10)62345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marques O, Outeiro TF. Alpha-synuclein: from secretion to dysfunction and death. Cell Death Dis. 2012;3:e350. doi: 10.1038/cddis.2012.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greggio E, Bisaglia M, Civiero L, Bubacco L. Leucine-rich repeat kinase 2 and alph-asynuclein: intersecting pathways in the pathogenesis of Parkinson's disease? Mol Neurodegener. 2011;6:6. doi: 10.1186/1750-1326-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chinta SJ, Andersen JK. Dopaminergic neurons. Int J Biochem Cell Biol. 2005;37:942–946. doi: 10.1016/j.biocel.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson's disease. Brain. 1999;122:1437–1448. doi: 10.1093/brain/122.8.1437. [DOI] [PubMed] [Google Scholar]
- 17.Fearnley JM, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain. 1991;114:2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- 18.Hirsch EC, Faucheux B, Damier P, Mouatt-Prigent A, Agid Y. Neuronal vulnerability in Parkinson's disease. J Neural Transm Suppl. 1997;50:79–88. doi: 10.1007/978-3-7091-6842-4_9. [DOI] [PubMed] [Google Scholar]
- 19.Double KL, Reyes S, Werry EL, Halliday GM. Selective cell death in neurodegeneration: why are some neurons spared in vulnerable regions? Prog Neurobiol. 2010;92:316–329. doi: 10.1016/j.pneurobio.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Hindle JV. Ageing, neurodegeneration and Parkinson's disease. Age Ageing. 2010;39:156–161. doi: 10.1093/ageing/afp223. [DOI] [PubMed] [Google Scholar]
- 21.Korytowski W, Sarna T, Zar ba M. Antioxidant action of neuromelanin: the mechanism of inhibitory effect on lipid peroxidation. Arch Biochem Biophys. 1995;319:142–148. doi: 10.1006/abbi.1995.1276. [DOI] [PubMed] [Google Scholar]
- 22.Youdim MB, Ben-Shachar D, Riederer P. Is Parkinson's disease a progressive siderosis of substantia nigra resulting in iron and melanin induced neurodegeneration? Acta Neurol Scand Suppl. 1989;126:47–54. doi: 10.1111/j.1600-0404.1989.tb01782.x. [DOI] [PubMed] [Google Scholar]
- 23.Bisaglia M, Mammi S, Bubacco L. Kinetic and structural analysis of the early oxidation products of dopamine: analysis of the interactions with alpha-synuclein. J Biol Chem. 2007;282:15597–15605. doi: 10.1074/jbc.M610893200. [DOI] [PubMed] [Google Scholar]
- 24.Conway KA, Rochet JC, Bieganski RM, Lansbury PT Jr. Kinetic stabilization of the alpha-synuclein protofibril by a dopamine-alpha-synuclein adduct. Science. 2001;294:1346–1349. doi: 10.1126/science.1063522. [DOI] [PubMed] [Google Scholar]
- 25.Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39:151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- 26.Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- 27.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 28.Goedert M, Clavaguera F, Tolnay M. The propagation of prion-like protein inclusions in neurodegenerative diseases. Trends Neurosci. 2010;33:317–325. doi: 10.1016/j.tins.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Fauvet B, Mbefo MK, Fares MB, Desobry C, Michael S, Ardah MT, Tsika E, Coune P, Prudent M, Lion N, Eliezer D, Moore DJ, Schneider B, Aebischer P, El-Agnaf OM, Masliah E, Lashuel HA. alpha-Synuclein in central nervous system and from erythrocytes, mammalian cells, and Escherichia coli exists predominantly as disordered monomer. J Biol Chem. 2012;287:15345–15364. doi: 10.1074/jbc.M111.318949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bartels T, Choi JG, Selkoe DJ. alpha-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature. 2011;477:107–110. doi: 10.1038/nature10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang W, Perovic I, Chittuluru J, Kaganovich A, Nguyen LT, Liao J, Auclair JR, Johnson D, Landeru A, Simorellis AK, Ju S, Cookson MR, Asturias FJ, Agar JN, Webb BN, Kang C, Ringe D, Petsko GA, Pochapsky TC, Hoang QQ. A soluble alpha-synuclein construct forms a dynamic tetramer. Proc Natl Acad Sci USA. 2011;108:17797–17802. doi: 10.1073/pnas.1113260108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, Meaney DF, Trojanowski JQ, Lee VM. Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72:57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lesage S, Brice A. Parkinson's disease: from monogenic forms to genetic susceptibility factors. Hum Mol Genet. 2009;18:R48–59. doi: 10.1093/hmg/ddp012. [DOI] [PubMed] [Google Scholar]
- 34.Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with lewy bodies. Proc Natl Acad Sci USA. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ebrahimi-Fakhari D, Cantuti-Castelvetri I, Fan Z, Rockenstein E, Masliah E, Hyman BT, McLean PJ, Unni VK. Distinct roles in vivo for the ubiquitin-proteasome system and the autophagy-lysosomal pathway in the degradation of alpha-synuclein. J Neurosci. 2011;31:14508–14520. doi: 10.1523/JNEUROSCI.1560-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, Lashley T, Quinn NP, Rehncrona S, Bjorklund A, Widner H, Revesz T, Lindvall O, Brundin P. Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nat Med. 2008;14:501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 37.Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nat Med. 2008;14:504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- 38.Steiner JA, Angot E, Brundin P. A deadly spread: cellular mechanisms of alpha-synuclein transfer. Cell Death Differ. 2011;18:1425–1433. doi: 10.1038/cdd.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aguzzi A, Rajendran L. The transcellular spread of cytosolic amyloids, prions, and prionoids. Neuron. 2009;64:783–790. doi: 10.1016/j.neuron.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 40.Bellingham SA, Guo BB, Coleman BM, Hill AF. Exosomes: vehicles for the transfer of toxic proteins associated with neurodegenerative diseases? Front Physiol. 2012;3:124. doi: 10.3389/fphys.2012.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schneider A, Simons M. Exosomes: vesicular carriers for intercellular communication in neurodegenerative disorders. Cell Tissue Res. 2012 doi: 10.1007/s00441-012-1428-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;Chapter 3:Unit 3.22. doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- 43.Faure J, Lachenal G, Court M, Hirrlinger J, Chatellard-Causse C, Blot B, Grange J, Schoehn G, Goldberg Y, Boyer V, Kirchhoff F, Raposo G, Garin J, Sadoul R. Exosomes are released by cultured cortical neurones. Mol Cell Neurosci. 2006;31:642–648. doi: 10.1016/j.mcn.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 44.Potolicchio I, Carven GJ, Xu X, Stipp C, Riese RJ, Stern LJ, Santambrogio L. Proteomic analysis of microglia-derived exosomes: metabolic role of the aminopeptidase CD13 in neuropeptide catabolism. J Immunol. 2005;175:2237–2243. doi: 10.4049/jimmunol.175.4.2237. [DOI] [PubMed] [Google Scholar]
- 45.Taylor AR, Robinson MB, Gifondorwa DJ, Tytell M, Milligan CE. Regulation of heat shock protein 70 release in astrocytes: role of signaling kinases. Dev Neurobiol. 2007;67:1815–1829. doi: 10.1002/dneu.20559. [DOI] [PubMed] [Google Scholar]
- 46.Bobrie A, Colombo M, Raposo G, Thery C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12:1659–1668. doi: 10.1111/j.1600-0854.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- 47.Simons M, Raposo G. Exosomes--vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21:575–581. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 48.Lee TH, D'Asti E, Magnus N, Al-Nedawi K, Meehan B, Rak J. Microvesicles as mediators of intercellular communication in cancer--the emerging science of cellular 'debris'. Semin Immunopathol. 2011;33:455–467. doi: 10.1007/s00281-011-0250-3. [DOI] [PubMed] [Google Scholar]
- 49.Keller S, Sanderson MP, Stoeck A, Altevogt P. Exosomes: from biogenesis and secretion to biological function. Immunol Lett. 2006;107:102–108. doi: 10.1016/j.imlet.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 50.Thery C, Boussac M, Veron P, Ricciardi-Castagnoli P, Raposo G, Garin J, Amigorena S. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166:7309–7318. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- 51.Kramer-Albers EM, Bretz N, Tenzer S, Winterstein C, Mobius W, Berger H, Nave KA, Schild H, Trotter J. Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: Trophic support for axons? Proteomics Clin Appl. 2007;1:1446–1461. doi: 10.1002/prca.200700522. [DOI] [PubMed] [Google Scholar]
- 52.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 53.van Niel G, Porto-Carreiro I, Simoes S, Raposo G. Exosomes: a common pathway for a specialized function. J Biochem. 2006;140:13–21. doi: 10.1093/jb/mvj128. [DOI] [PubMed] [Google Scholar]
- 54.Dello Sbarba P, Rovida E. Transmodulation of cell surface regulatory molecules via ectodomain shedding. Biol Chem. 2002;383:69–83. doi: 10.1515/BC.2002.007. [DOI] [PubMed] [Google Scholar]
- 55.Marzesco AM, Janich P, Wilsch-Brauninger M, Dubreuil V, Langenfeld K, Corbeil D, Huttner WB. Release of extracellular membrane particles carrying the stem cell marker prominin-1 (CD133) from neural progenitors and other epithelial cells. J Cell Sci. 2005;118:2849–2858. doi: 10.1242/jcs.02439. [DOI] [PubMed] [Google Scholar]
- 56.Lachenal G, Pernet-Gallay K, Chivet M, Hemming FJ, Belly A, Bodon G, Blot B, Haase G, Goldberg Y, Sadoul R. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol Cell Neurosci. 2011;46:409–418. doi: 10.1016/j.mcn.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 57.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 59.Alvarez-Erviti L, Seow Y, Schapira AH, Gardiner C, Sargent IL, Wood MJ, Cooper JM. Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiol Dis. 2011;42:360–367. doi: 10.1016/j.nbd.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH, Stefanis L, Vekrellis K. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci. 2010;30:6838–6851. doi: 10.1523/JNEUROSCI.5699-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E, Lee SJ. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci USA. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hansen C, Angot E, Bergstrom AL, Steiner JA, Pieri L, Paul G, Outeiro TF, Melki R, Kallunki P, Fog K, Li JY, Brundin P. alpha-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest. 2011;121:715–725. doi: 10.1172/JCI43366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 64.Chaput N, Flament C, Viaud S, Taieb J, Roux S, Spatz A, Andre F, LePecq JB, Boussac M, Garin J, Amigorena S, Thery C, Zitvogel L. Dendritic cell derived-exosomes: biology and clinical implementations. J Leukoc Biol. 2006;80:471–478. doi: 10.1189/jlb.0206094. [DOI] [PubMed] [Google Scholar]
- 65.Bhatnagar S, Shinagawa K, Castellino FJ, Schorey JS. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood. 2007;110:3234–3244. doi: 10.1182/blood-2007-03-079152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee HJ, Suk JE, Bae EJ, Lee JH, Paik SR, Lee SJ. Assembly-dependent endocytosis and clearance of extracellular alpha-synuclein. Int J Biochem Cell Biol. 2008;40:1835–1849. doi: 10.1016/j.biocel.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 67.Lee HJ, Suk JE, Patrick C, Bae EJ, Cho JH, Rho S, Hwang D, Masliah E, Lee SJ. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem. 2010;285:9262–9272. doi: 10.1074/jbc.M109.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Halliday GM, Stevens CH. Glia: initiators and progressors of pathology in Parkinson's disease. Mov Disord. 2011;26:6–17. doi: 10.1002/mds.23455. [DOI] [PubMed] [Google Scholar]
- 69.Vekrellis K, Xilouri M, Emmanouilidou E, Rideout HJ, Stefanis L. Pathological roles of alpha-synuclein in neurological disorders. Lancet Neurol. 2011;10:1015–1025. doi: 10.1016/S1474-4422(11)70213-7. [DOI] [PubMed] [Google Scholar]
- 70.Alvarez-Erviti L, Couch Y, Richardson J, Cooper JM, Wood MJ. Alpha-synuclein release by neurons activates the inflammatory response in a microglial cell line. Neurosci Res. 2011;69:337–342. doi: 10.1016/j.neures.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 71.Gao HM, Hong JS. Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol. 2008;29:357–365. doi: 10.1016/j.it.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Panaro MA, Cianciulli A. Current opinions and perspectives on the role of immune system in the pathogenesis of Parkinson's disease. Curr Pharm Des. 2012;18:200–208. doi: 10.2174/138161212799040574. [DOI] [PubMed] [Google Scholar]
- 73.Shin N, Jeong H, Kwon J, Heo HY, Kwon JJ, Yun HJ, Kim CH, Han BS, Tong Y, Shen J, Hatano T, Hattori N, Kim KS, Chang S, Seol W. LRRK2 regulates synaptic vesicle endocytosis. Exp Cell Res. 2008;314:2055–2065. doi: 10.1016/j.yexcr.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 74.Piccoli G, Condliffe SB, Bauer M, Giesert F, Boldt K, De Astis S, Meixner A, Sarioglu H, Vogt-Weisenhorn DM, Wurst W, Gloeckner CJ, Matteoli M, Sala C, Ueffing M. LRRK2 controls synaptic vesicle storage and mobilization within the recycling pool. J Neurosci. 2011;31:2225–2237. doi: 10.1523/JNEUROSCI.3730-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xiong Y, Coombes CE, Kilaru A, Li X, Gitler AD, Bowers WJ, Dawson VL, Dawson TM, Moore DJ. GTPase activity plays a key role in the pathobiology of LRRK2. PLoS Genet. 2010;6:e1000902. doi: 10.1371/journal.pgen.1000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alegre-Abarrategui J, Christian H, Lufino MM, Mutihac R, Venda LL, Ansorge O, Wade-Martins R. LRRK2 regulates autophagic activity and localizes to specific membrane microdomains in a novel human genomic reporter cellular model. Hum Mol Genet. 2009;18:4022–4034. doi: 10.1093/hmg/ddp346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fader CM, Sanchez DG, Mestre MB, Colombo MI. TI-VAMP/VAMP7 and VAMP3/cellubrevin: two v-SNARE proteins involved in specific steps of the autophagy/multivesicular body pathways. Biochim Biophys Acta. 2009;1793:1901–1916. doi: 10.1016/j.bbamcr.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 78.Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A, Fraser G, Stalder AK, Beibel M, Staufenbiel M, Jucker M, Goedert M, Tolnay M. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009;11:909–913. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Edwards TL, Scott WK, Almonte C, Burt A, Powell EH, Beecham GW, Wang L, Zuchner S, Konidari I, Wang G, Singer C, Nahab F, Scott B, Stajich JM, Pericak-Vance M, Haines J, Vance JM, Martin ER. Genome-wide association study confirms SNPs in SNCA and the MAPT region as common risk factors for Parkinson disease. Ann Hum Genet. 2010;74:97–109. doi: 10.1111/j.1469-1809.2009.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Satake W, Nakabayashi Y, Mizuta I, Hirota Y, Ito C, Kubo M, Kawaguchi T, Tsunoda T, Watanabe M, Takeda A, Tomiyama H, Nakashima K, Hasegawa K, Obata F, Yoshikawa T, Kawakami H, Sakoda S, Yamamoto M, Hattori N, Murata M, Nakamura Y, Toda T. Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson's disease. Nat Genet. 2009;41:1303–1307. doi: 10.1038/ng.485. [DOI] [PubMed] [Google Scholar]
- 81.Giasson BI, Forman MS, Higuchi M, Golbe LI, Graves CL, Kotzbauer PT, Trojanowski JQ, Lee VM. Initiation and synergistic fibrillization of tau and alpha-synuclein. Science. 2003;300:636–640. doi: 10.1126/science.1082324. [DOI] [PubMed] [Google Scholar]
- 82.Sullivan CP, Jay AG, Stack EC, Pakaluk M, Wadlinger E, Fine RE, Wells JM, Morin PJ. Retromer disruption promotes amyloidogenic APP processing. Neurobiol Dis. 2011;43:338–345. doi: 10.1016/j.nbd.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]