Abstract
Mild cognitive impairment (MCI) is an etiologically heterogeneous syndrome defined by cognitive impairment in advance of dementia. We previously reported in a retrospective analysis that daily 3 - 9 mg of a fast-release melatonin preparation given p. o. at bedtime for up to 3 years significantly improved cognitive and emotional performance and daily sleep/wake cycle in MCI patients. In a follow up of that study we now report data from another series of 96 MCI outpatients, 61 of who had received daily 3 - 24 mg of a fast-release melatonin preparation p. o. at bedtime for 15 to 60 months. Melatonin was given in addition to the standard medication prescribed by the attending psychiatrist. Patients treated with melatonin exhibited significantly better performance in Mini–Mental State Examination and the cognitive subscale of the Alzheimer’s disease Assessment Scale. After application of a neuropsychological battery comprising a Mattis´ test, Digit-symbol test, Trail A and B tasks and the Rey´s verbal test, better performance was found in melatonin-treated patients for every parameter tested. Abnormally high Beck Depression Inventory scores decreased in melatonin-treated patients, concomitantly with the improvement in the quality of sleep and wakefulness. The comparison of the medication profile in both groups of MCI patients indicated that 9.8% in the melatonin group received benzodiazepines vs. 62.8% in the non-melatonin group. The results further support that melatonin can be a useful add-on drug for treating MCI in a clinic environment.
Keywords: Mild cognitive impairment, Alzheimer´s disease, melatonin, benzodiazepines, neuropsychological tests, retrospective study
Introduction
Normal aging is characterized by a decline of cognitive capacities including reasoning, memory and semantic fluency, which can be detected as early as the 5th decade of life [1]. Although there is a high variability across cognitive domains measured and between individuals in the degree and timing of age-related cognitive losses, there is evidence for a preclinical stage in dementia in which cognitive performance is borderline as compared to normal aging [2]. In community-based studies up 28% of a sample of healthy community-dwelling older show deficits in performance that were not explained by age related changes, education levels, mood or health status [3]. This strongly suggests the existence of early pathological changes which is a transitional state taking place between normal aging and early Alzheimer disease (AD) [4].
Mild cognitive impairment (MCI) is diagnosed in those who have an objective and measurable deficit in cognitive functions, but with a preservation of daily activities. The estimates of annual conversion rates to dementia vary across studies but may be as high 10-15% [5,6], MCI representing a clinically important stage for identifying and treating individuals at risk. Indeed, the degenerative process in AD brain starts 20-30 years before the clinical onset of the disease [7,8]. During this phase, plaques and tangles loads increase and at a certain threshold the first symptom appears [9,10].
Several studies show that melatonin levels are lower in AD patients compared to age-matched control subjects (see for ref. [11]). CSF melatonin levels decrease even in preclinical stages manifest any cognitive impairment, suggesting that the reduction in CSF melatonin may be an early trigger and marker for AD [12,13]. Although it is not known whether the relative melatonin deficiency is either a consequence or a cause of neurodegeneration, it seems clear that the loss in melatonin aggravates the disease and that early circadian disruption can be an important deficit to be considered.
We previously reported a retrospective analysis in which daily 3 - 9 mg of a fast-release melatonin preparation p.o. at bedtime for up to 3 years significantly improved cognitive and emotional performance and daily sleep/wake cycle in 25 MCI patients [14]. We now report data from another series of 96 MCI outpatients, 61 of who had received daily 3 - 24 mg of a fast-release melatonin preparation p.o. at bedtime for 15 to 60 months in comparison to a similar group of 35 MCI patients who did not receive it. In addition, all patients received the individual standard medication considered appropriate by the attending psychiatrist.
Subjects and methods
This is a retrospective study of 96 outpatients complaining of MCI symptoms examined during the period 2007 - 2012 at the facilities of the Centro de Neuropsiquiatría y Neurología de la Conducta, Hospital de Clinicas “José de San Martín”, Facultad de Medicina, Universidad de Buenos Aires. The diagnostic criteria used for MCI were those of Petersen et al. [15]. They included amnesic MCI of a degenerative nature (insidious onset and gradual progression), impaired memory and a score of 24 to 30 on the Mini–Mental State Examination (MMSE). The study was approved by the institutional review board at the Faculty of Medicine, University of Buenos Aires. It was conducted according to the Declaration of Helsinki. All participants were informed on the confidentiality of data and on that by no means they would be personally identified in case of publication of the study.
Sixty-one patients in the sample selected had received daily 3 to 24 mg of a fast-release melatonin preparation (Melatol®, Elisium S.A., Buenos Aires, Argentina) given p. o. at bedtime. The patients were indicated to take melatonin 30 min before the expected time of sleep every day. Melatonin was given in addition to the individual standard medication prescribed by the attending psychiatrist. Specific medication in the case of the melatonin group included memantine / donepezil (30 patients), memantine (9 patients), memantine / atenolol (4 patients), paroxetine (4 patients), memantine / L-thyroxine (4 patients), memantine / donepezil / alprazolam (1 patient), memantine / sertraline / lorazepam (1 patient), memantine / L-thyroxine / atenolol (1 patient), memantine / escitalopram / atenolol (1 patient), memantine / clonazepam (1 patient), donepezil / alprazolam / paroxetine (1 patient), L-thyroxine / levopromazine (1 patient), lorazepam (1 patient), atenolol (1 patient) and escitalopram / clonazepam (1 patient).
Specific medication in the case of MCI patients not taking melatonin included memantine / donepezil / alprazolam (8 patients), memantine / donepezil (6 patients), donepezil / alprazolam (4 patients), donepezil / alprazolam / paroxetine (4 patients), memantine / L-thyroxine (3 patients), memantine / alprazolam (2 patients), memantine / sertraline / lorazepam (1 patient), lorazepam / clonazepam / sertraline (1 patient), fluoxetine / alprazolam / carbamazepine (1 patient) and L-thyroxine / clonazepam (1 patient).
MCI patients with a minimum of 15 and a maximum of 60 months of treatment were included. Duration of treatment and number of patients in the melatonin and non-melatonin groups were as followed: 15 – 24 months (34 and 15 patients, respectively), 25 – 36 months (15 and 13 patients, respectively), 37 – 48 months (9 and 6 patients, respectively) and 49 – 60 months (3 and 1 patients, respectively). Other demographic characteristics of the individuals studied are summarized in Table 1. All patients had undergone clinical examination, routine blood tests and neuropsychological evaluation when first examined and at the end of the period considered for the retrospective analysis.
Table 1.
Basal characteristics of the sample of MCI patients examined
| Variable | Melatonin (N= 61) | Non Melatonin (N= 35) |
|---|---|---|
| Age — yr | 69.2 ± 9.03 | 72.0 ± 9.78 |
| Number of females (%) | 48 (78.7) | 26 (74.2) |
| MMSE score | 26.1 ± 1.8 | 25.8 ± 1.8 |
| ADAS-Cog score | 19.4 ± 7.7 | 16.4 ± 5.1 |
| Mattis′ score | 130.1 ± 6.6 | 133.7 ± 6.2 |
| Digit-symbol | 16.3 ± 9.3 | 19.1 ± 7.7 |
| Trail A task | 120.5 ± 15.6 | 118.6 ± 15.4 |
| Trail B task | 154.9 ± 28.9 | 148.2 ± 33.4 |
| Rey′s verbal test | 71.1 ± 17.1 | 65.4 ± 18.9 |
| Beck inventory | 30.9 ± 9.6 | 28.7 ± 7.9 |
| Wakefulness quality | 5.3 ± 1.3 | 5.4 ± 1.7 |
| Sleep quality | 5.4 ± 2.5 | 5.3 ± 1.2 |
Shown are the means ± SD. Normal scores for the several neuropsychological tests employed are described in Methods. For every parameter tested the differences between groups were not significant as shown by Mann-Whitney U tests.
The neuropsychological evaluation included the MMSE, the cognitive subscale of the Alzheimer’s Disease Assessment Scale (ADAS-Cog) and a neuropsychological battery comprising the Mattis´ test, the Digit-symbol test, Trail A and B tasks and the Rey´s verbal test. A 21-item Beck Depression Inventory and a global assessment of wakefulness and sleep quality were also completed for each patient. Scores for the MMSE rage from 0 to 30, with higher scores indicating better function. Scores for the ADAS-Cog range from 0 to 70, with higher scores indicating poorer function. Mattis´ scores range from 0 to 144 with higher scores indicating better function. Digit-symbol, Trail A and B tasks should be normally completed in less than 10, 120 and 150 seconds, respectively. Normal scores for Rey´s verbal test should be > 85 while those for Beck inventory should be < 20. Scores for wakefulness and sleep quality scales range from 0 to 10, with higher scores indicating better function.
Statistical analysis of results was performed by non-parametric Mann-Whitney U tests. Differences between groups were analyzed for basal characteristics and for differences of neuropsychological evaluation before and after the follow-up period. A Χ2 test was used for comparison of percent use of benzodiazepines in both groups. All tests were made assuming a level of statistical significance of P < 0.05.
Results
Both groups of MCI patients did not differ significantly at admission time in any of the neuropsychological parameters tested (Mann-Whitney U tests) (Table 1).
Figures 1, 2, 3 and Table 2 summarize the results obtained in the MCI patients treated or not with melatonin for 15 - 60 months. Patients receiving melatonin showed significantly better performance in every neuropsychological test assessed. As shown in Figure 3, abnormally high Beck Depression Inventory scores decreased in melatonin-treated patients, concomitantly with an improvement in wakefulness and sleep quality.
Figure 1.
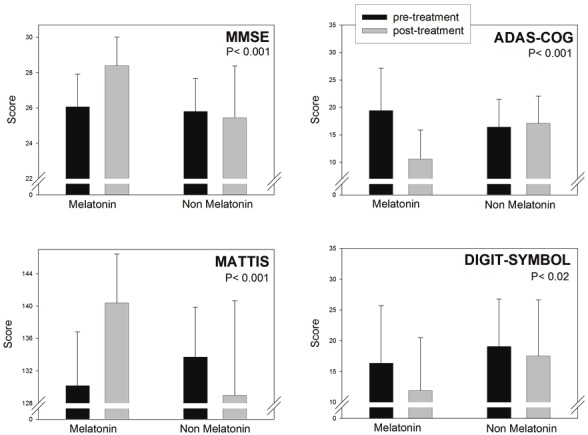
Retrospective analysis of 96 outpatients complaining of MCI symptoms, 61 of which received daily 3 to 24 mg of a fast-release melatonin preparation p.o. at bedtime for 15 to 60 months. Melatonin was given in addition to the individual standard medication prescribed by the attending psychiatrist. The other 35 subjects selected received the medication prescribed by the attending psychiatrist which did not include melatonin. Initial and final neuropsychological evaluation including the MMSE, ADAS-Cog, Mattis´ test and Digit-symbol task are depicted. See Methods for further experimental details. Shown are the means ± SD. P values denote the differences between groups of changes in neuropsychological evaluation before and after the follow-up period (Mann-Whitney U test). Z values are depicted in Table 2.
Figure 2.
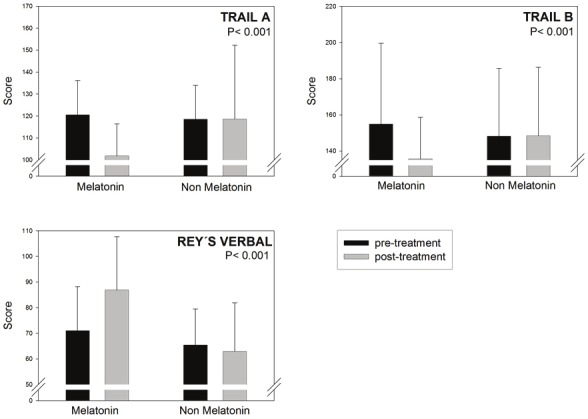
Retrospective analysis of 96 outpatients complaining of MCI symptoms, 61 of which received daily 3 to 24mg of a fast-release melatonin preparation p.o. at bedtime for 15 to 60 months. For details see Legend to Figure 1.Initial and final neuropsychological evaluation of trail A and trail B tasks, and Rey´s verbal test are depicted. Shownare the means ± SD. P values denote the differences between groups of changes in neuropsychological evaluationbefore and after the follow-up period (Mann-Whitney U test). Z values are depicted in Table 2.
Figure 3.
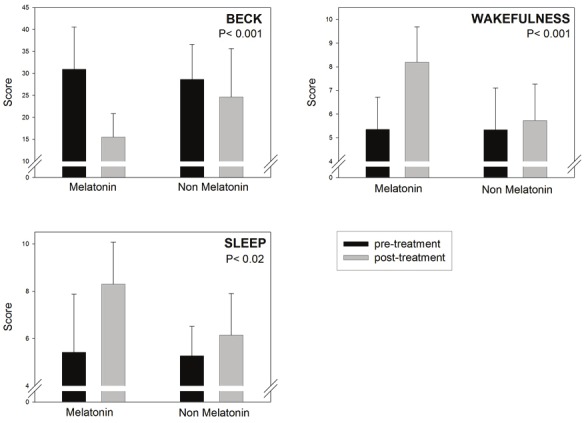
Retrospective analysis of 96 outpatients complaining of MCI symptoms, 61 of which received daily 3 to 24mg of a fast-release melatonin preparation p.o. at bedtime for 15 to 60 months. For details see Legend to Figure 1.Initial and final neuropsychological evaluation of Beck Depression Inventory and a global assessment of wakefulnessand sleep quality are depicted. Shown are the means ± SD. P values denote the differences between groups ofchanges in neuropsychological evaluation before and after the follow-up period (Mann-Whitney U test). Z values aredepicted in Table 2.
Table 2.
Mean values and statistical analysis of the (final – initial) differences in neuropsychological assessment of melatonin-treated (N= 61) and non melatonin MCI patients (N= 35).
| Variable | Melatonin | Non Melatonin | Z (P value) |
|---|---|---|---|
| MMSE score | 2.34 ± 2.09 | -0.36 ± 3.01 | -4.867 (<0.001) |
| ADAS-Cog score | -8.96 ± 8.11 | 0.65 ± 8.84 | -5.733 (<0.001) |
| Mattis′ score | 10.32 ± 11.1 | -4.73 ± 12.9 | -6.591 (<0.001) |
| Digit-symbol | -4.52 ± 8.54 | -1.51 ± 9.57 | -2.318 (<0.02) |
| Trail A task | -18.68 ± 14.3 | 0.14 ± 24.7 | -3.581 (<0.001) |
| Trail B task | -19.31 ± 35.1 | 0.26 ± 30.2 | -3.674 (<0.001) |
| Rey′s verbal test | 15.82 ± 20.4 | -0.14 ± 19.8 | -4.209 (<0.001) |
| Beck inventory | -15.46 ± 9.91 | -5.00 ± 11.2 | -4.519 (<0.001) |
| Wakefulness quality | 2.84 ± 1.99 | 0.39 ± 2.45 | -4.709 (<0.001) |
| Sleep quality | 2.88 ± 2.86 | 0.87 ± 2.66 | -3.266 (< 0.01) |
Shown are the means ± SD. Statistical analysis of results was performed by non-parametric Mann-Whitney U tests.
Table 2 summarizes mean ± SD of differences between final and initial neuropsychological evaluation and Z values obtained after a non parametric Mann-Whitney U test. There were significant differences between melatonin and non-melatonin groups in every neuropsychological parameter tested.
The comparison of the medication profile in both groups of MCI patients indicated that 9.8% (i.e., 6 out of 61 patients) in the melatonin group received benzodiazepines vs. 62.8% (i.e., 22 out of 35 patients) in the non-melatonin group (P < 0.0001, Χ2 test).
Discussion
The foregoing results support the hypothesis [14] that melatonin can be a useful add-on drug for treatment of MCI in a clinical environment. In every neuropsychological task tested, the 61 MCI patients receiving melatonin in addition to their standard medication showed a better performance than their 35 non-melatonin-treated counterparts. This coincided with a decrease in mood-related symptoms as assessed by the Beck inventory and with an improvement in subjective global assessment of the sleep/wake cycle. Generally, the findings agree with our previous observations in a smaller sample of 25 MCI patients who had received 3 - 9 mg/day of a fast-release melatonin preparation p.o. at bedtime for 9 to 18 months [14], except for that the Digit-symbol test remained unchanged in that former sample.
There is published information in the literature indicating that melatonin, as a chronobiotic agent, is effective in treating irregular sleep-wake cycles and sundowning symptoms in AD patients [16-25]. A review of the published results concerning melatonin use in AD [26] yielded eight reports (5 open-label studies, 2 case reports) (N = 89 patients) supporting a possible efficacy of melatonin: sleep quality improved and in patients with AD sundowning was reduced and cognitive decay slowed progression. In six double blind, randomized placebo-controlled trials, a total number of 210 AD patients were examined. Sleep was objectively measured by wrist actigraphy (N = 5) and additionally neuropsychological assessment and sleep quality were subjectively evaluated. Sleep quality increased and sundowning decreased significantly and cognitive performance improved in 4 studies (N = 143) whereas there was absence of effects in 2 studies (N = 67) [26].
Another systematic search of studies published between 1985 and April 2009 on melatonin and sundowning in AD patients was published [27]. All papers on melatonin treatment in dementia were retrieved and the effects of melatonin on circadian rhythm disturbances were scored by means of scoring sundowning/agitated behavior, sleep quality and daytime functioning. A total of 9 papers, including 4 randomized controlled trials (n = 243), and 5 case series (n = 87) were reviewed. Two of the randomized controlled trials found a significant improvement in sundowning/agitated behavior. All five case series found an improvement. The results on sleep quality and daytime functioning were inconclusive [27].
Therefore, whether melatonin has any value in preventing or treating AD remains uncertain. It must be noted that one of the problems with AD patients with fully developed pathology is the heterogeneity of the group examined. Moreover, the reduced hippocampal expression of MT2 melatonin receptors in AD patients [28] and of MT1 receptors in the circadian apparatus at later stages the disease may explain why melatonin treatment is less effective or erratic at this stage [29].
Thus, an early initiation of treatment can be decisive for therapeutic success [30]. In a recent publication, published data concerning melatonin treatment in MCI were analyzed [31]. Five double blind, randomized placebo-controlled trials and 1 open-label retrospective study (N = 651) consistently showed that the administration of daily evening melatonin improves sleep quality and cognitive performance in MCI patients [14,26,32-34]. Together with the results reported herein, one can conclude that melatonin treatment could be effective at early stages of the neurodegenerative disease.
There are two reasons why it is convenient the use of melatonin in MCI patients. In the course of the neurodegenerative process, the age-related deterioration in circadian organization becomes significantly exacerbated [29,35] and is responsible of behavioral problems like sundowning [36,37]. Age-related cognitive decline in healthy older adults can be predicted by the fragmentation of the circadian rhythm in locomotor behavior [38]. Hence, replacement of the low melatonin levels occurring in brain [12,13] can be highly convenient in MCI patients. On the other hand, the bulk of information on the neuroprotective properties of melatonin derived from experimental studies (see for ref. [11,39]) turns highly desirable to employ pharmacological doses in MCI patients with the aim of arresting or slowing disease´s progression.
The prevalence of sleep disorder in MCI, subjectively measured, ranged 14 to 59% [40]. Generally sleep disturbances in MCI are approximately double that in controls, and half that in dementia [41]. However, the subjective evaluation of sleep is not always in agreement with objective evaluation by actigraphy. For example, recent studies reported no differences in any sleep parameter actigraphically determined in MCI and control subjects although reduced amplitude and/or phase delay of 24-h rhythm, independently of sleep were found in MCI patients [42,43].
In the present study melatonin treatment was effective to improve subjectively evaluated sleep/wake cycle. The sleep-promoting activity of melatonin in humans has been known for years [44,45] and a number of studies pointed to a beneficial effect of melatonin in a wide variety of sleep disorders (see for ref. [46]). However, controversy continues to surround claims of melatonin’s therapeutic potential. A meta-analysis on the effects of melatonin in sleep disturbances at all age groups (including young adults with presumably normal melatonin levels) failed to document significant and clinically meaningful effects of exogenous melatonin on sleep quality, efficiency and latency [47]. However another meta-analysis involving 17 controlled studies in old subjects has shown that melatonin was effective in increasing sleep efficiency and in reducing sleep onset latency [48]. After the approval by the European Medicines Agency of a prolonged release form of 2 mg melatonin (Circadin®, Neurim, Tel Aviv, Israel) for treatment of insomnia in patients ≥ 55 years of age, a recent consensus of the British Association for Psychopharmacology on evidence-based treatment of insomnia, parasomnia and circadian rhythm sleep disorders concluded that that prolonged release melatonin is the first choice treatment when a hypnotic is indicated in old patients [49].
In addition to sleep promotion, melatonin has a mild sedating effect. This may be the cause for the decrease in Beck´s score seen in the present study. Melatonin has a facilitatory effect on GABAergic transmission [50] which may be responsible for the anticonvulsant, anxiolytic, antihyperalgesic and antinociceptive effects of the methoxyindole. Recently, agomelatine (Servier, Neuilly-sur-Seine, France), the first MT1/MT2 melatonergic agonist displaying also 5-HT2C serotonergic antagonism, has been introduced in the market as a novel antidepressant [46]. Agomelatine is effective in several models of depression as well as in anxiety. It must be noted, however, that both melatonin and ramelteon have been shown to display antidepressant-like effects even though they are not reportedly known to affect serotonergic activity significantly [51-53].
The mechanisms accounting for the therapeutic effect of melatonin in MCI patients remain to be defined. Melatonin treatment mainly promotes slow wave sleep in the elderly [54] and can be beneficial in MCI by augmenting the restorative phases of sleep, including the augmented secretion of GH and neurotrophins. Melatonin was found to protect against the circadian changes produced by β-amyloid25-35 microinjection in the suprachiasmatic nuclei of golden hamsters [55]. The demonstration of the utility of melatonin treatment in correcting the biochemical pathology of AD was given by studies in transgenic murine models of AD (see for ref. [11,39]). As outlined, melatonin acts at different levels relevant to the development and manifestation of AD. The antioxidant, mitochondrial and antiamyloidogenic effects can be seen as a possibility of interfering with the onset of the disease. Therefore, when melatonin treatment begins can be decisive for final response [30].
One important aspect to be considered is the melatonin dose employed, which may be unnecessarily low when one takes into consideration the binding affinities, half-life and relative potencies of the different melatonin agonists on the market. In addition to being generally more potent than the native molecule, melatonin analogs are employed in considerably higher amounts [56]. Licensed doses of ramelteon vary from 8 to 32 mg/day while agomelatine has been licensed for treatment of major depressive disorder at doses of 25 – 50 mg/day. In clinical studies involving healthy human subjects, tasimelteon (Vanda Pharmaceuticals, Washington, DC, USA) was administered at doses of 10 to 100 mg/day [57] while pharmacokinetics, pharmacodynamics and safety of TIK-301 (Tikvah Pharmaceuticals, Atlanta, GA, USA) have been examined in a placebo controlled study using 20 to 100 mg/day [58]. Therefore studies in MCI with melatonin doses in the range of 75 – 100 mg/day are further warranted.
Indeed, melatonin has a high safety profile, it is usually remarkably well tolerated and, in some studies, it has been administered to patients at very large doses. Melatonin (300 mg/day) for up to 3 years decreased oxidative stress in patients with amyotrophic lateral sclerosis [59]. In children with muscular dystrophy, 70 mg/day of melatonin reduced cytokines and lipid peroxidation [60]. Doses of 80 mg melatonin hourly for 4 h were given to healthy men with no undesirable effects other than drowsiness [61]. In healthy women given 300 mg melatonin/day for 4 months there were no side effects [62]. A recent randomized controlled double-blind clinical trial on 50 patients referred for liver surgery indicated that a single preoperative enteral dose of 50 mg/kg melatonin (i.e., an equivalent to 3 g for a 60-kg adult) was safe and well tolerated [63].
Another outcome of the study is that when melatonin is employed much less benzodiazepines are needed to treat sleep disturbances in MCI. In the present series 6 out of 61 patients treated with melatonin received benzodiazepines vs. 22 out of 35 MCI patients not receiving melatonin. Since, as above mentioned, melatonin and benzodiazepines shared some neurochemical (i.e. interaction with GABA-mediated mechanisms in brain [50]) and behavioral properties (e.g., a similar day-dependent anxiolytic activity [64]), melatonin therapy was postulated to be an effective tool to decrease the dose of benzodiazepines needed in patients [16,65-67]. A recent retrospective analysis of a German prescription database identified 512 patients who had initiated treatment with prolonged release melatonin (2 mg) over a 10-month period [68]. From 112 patients in this group who had previously used benzodiazepines, 31% discontinued treatment with benzodiazepines 3-months after beginning prolonged release melatonin treatment. The discontinuation rate was higher in patients receiving two or three melatonin prescription [68]. Therefore melatonin can help to facilitate benzodiazepine discontinuation in older insomniacs. This is particularly important since up to 30 to 40 % of seniors use sedative hypnotic benzodiazepines and related medication showing frequently side effects from hypnotics due to both increased nervous system sensitivity and decreased serum albumin (that binds the drugs) [69,70]. These are major reasons why the older population responds to drugs differently and less predictably than their younger counterparts [71,72].
It must be noted that a retrospective study like that reported herein has several limitations. This non-aleatorized, intervention study includes external groups as control and thus it is subjected to a selection bias since groups are not necessarily homogenous. Although in the present report the basal conditions of melatonin and non-melatonin groups did not differ significantly it is always possible that the degree of initial disease did differ between groups. Another possible bias is observational, because it cannot be ruled out that in an open study like this either the subjects or the attending personnel could influence the final outcome.
In conclusion, the question as to whether melatonin has a therapeutic value in preventing or treating MCI, affecting disease initiation or progression of the neuropathology and the driving mechanisms, deserves further analysis in future studies. Double-blind multicenter studies are urgently needed to further explore and investigate the potential and usefulness of melatonin as an antidementia drug at the early stage of disease. Its effectiveness in symptomatic treatment of sleep, sundowning or cognitive impairment in a naturalistic environment like that of the present study further underlines the need for such decisive studies.
Acknowledgements
This study was supported by grants from the Agencia Nacional de Promoción Científica y Tecnológica, Argentina (PICT 2007-01045 and PICT 2010-1465) and the Universidad de Buenos Aires (ME 048). DPC, DEV and LIB are Research Career Awardees from the Argentine Research Council (CONICET). MFV is a doctoral fellow from CONICET.
References
- 1.Singh-Manoux A, Kivimaki M, Glymour MM, Elbaz A, Berr C, Ebmeier KP, Ferrie JE, Dugravot A. Timing of onset of cognitive decline: results from Whitehall II prospective cohort study. BMJ. 2012;344:d7622. doi: 10.1136/bmj.d7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silveri MC, Reali G, Jenner C, Puopolo M. Attention and memory in the preclinical stage of dementia. J Geriatr Psychiatry Neurol. 2007;20:67–75. doi: 10.1177/0891988706297469. [DOI] [PubMed] [Google Scholar]
- 3.Weaver CJ, Maruff P, Collie A, Masters C. Mild memory impairment in healthy older adults is distinct from normal aging. Brain Cogn. 2006;60:146–155. doi: 10.1016/j.bandc.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Grundman M, Petersen RC, Ferris SH, Thomas RG, Aisen PS, Bennett DA, Foster NL, Jack CR Jr, Galasko DR, Doody R, Kaye J, Sano M, Mohs R, Gauthier S, Kim HT, Jin S, Schultz AN, Schafer K, Mulnard R, van Dyck CH, Mintzer J, Zamrini EY, Cahn-Weiner D, Thal LJ. Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Arch Neurol. 2004;61:59–66. doi: 10.1001/archneur.61.1.59. [DOI] [PubMed] [Google Scholar]
- 5.Dubois B, Albert ML. Amnestic MCI or prodromal Alzheimer's disease? Lancet Neurol. 2004;3:246–248. doi: 10.1016/S1474-4422(04)00710-0. [DOI] [PubMed] [Google Scholar]
- 6.Farias ST, Mungas D, Reed BR, Harvey D, DeCarli C. Progression of mild cognitive impairment to dementia in clinic- vs community-based cohorts. Arch Neurol. 2009;66:1151–1157. doi: 10.1001/archneurol.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies L, Wolska B, Hilbich C, Multhaup G, Martins R, Simms G, Beyreuther K, Masters CL. A4 amyloid protein deposition and the diagnosis of Alzheimer's disease: prevalence in aged brains determined by immunocytochemistry compared with conventional neuropathologic techniques. Neurology. 1988;38:1688–1693. doi: 10.1212/wnl.38.11.1688. [DOI] [PubMed] [Google Scholar]
- 8.Price JL, Morris JC. Tangles and plaques in nondemented aging and "preclinical" Alzheimer's disease. Ann Neurol. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 9.Braak H, Braak E. Staging of Alzheimer's disease-related neurofibrillary changes. Neurobiol Aging. 1995;16:271–278. doi: 10.1016/0197-4580(95)00021-6. [DOI] [PubMed] [Google Scholar]
- 10.Braak H, Braak E. Evolution of neuronal changes in the course of Alzheimer's disease. J Neural Transm Suppl. 1998;53:127–140. doi: 10.1007/978-3-7091-6467-9_11. [DOI] [PubMed] [Google Scholar]
- 11.Pandi-Perumal SR, BaHammam AS, Brown GM, Spence DW, Bharti VK, Kaur C, Hardeland R, Cardinali DP. Melatonin antioxidative defense: therapeutical implications for aging and neurodegenerative processes. Neuroto Res. 2012 doi: 10.1007/s12640-012-9337-4. in press. [DOI] [PubMed] [Google Scholar]
- 12.Wu YH, Feenstra MG, Zhou JN, Liu RY, Torano JS, Van Kan HJ, Fischer DF, Ravid R, Swaab DF. Molecular changes underlying reduced pineal melatonin levels in Alzheimer disease: alterations in preclinical and clinical stages. J Clin Endocrinol Metab. 2003;88:5898–5906. doi: 10.1210/jc.2003-030833. [DOI] [PubMed] [Google Scholar]
- 13.Zhou JN, Liu RY, Kamphorst W, Hofman MA, Swaab DF. Early neuropathological Alzheimer's changes in aged individuals are accompanied by decreased cerebrospinal fluid melatonin levels. J Pineal Res. 2003;35:125–130. doi: 10.1034/j.1600-079x.2003.00065.x. [DOI] [PubMed] [Google Scholar]
- 14.Furio AM, Brusco LI, Cardinali DP. Possible therapeutic value of melatonin in mild cognitive impairment. A retrospective study. J Pineal Res. 2007;43:404–409. doi: 10.1111/j.1600-079X.2007.00491.x. [DOI] [PubMed] [Google Scholar]
- 15.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 16.Fainstein I, Bonetto A, Brusco LI, Cardinali DP. Effects of melatonin in elderly patients with sleep disturbance. A pilot study. Curr Ther Res. 1997;58:990–1000. [Google Scholar]
- 17.Jean-Louis G, von Gizycki H, Zizi F. Melatonin effects on sleep, mood, and cognition in elderly with mild cognitive impairment. J Pineal Res. 1998;25:177–183. doi: 10.1111/j.1600-079x.1998.tb00557.x. [DOI] [PubMed] [Google Scholar]
- 18.Mishima K, Okawa M, Hozumi S, Hishikawa Y. Supplementary administration of artificial bright light and melatonin as potent treatment for disorganized circadian rest-activity and dysfunctional autonomic and neuroendocrine systems in institutionalized demented elderly persons. Chronobiol Int. 2000;17:419–432. doi: 10.1081/cbi-100101055. [DOI] [PubMed] [Google Scholar]
- 19.Cohen-Mansfield J, Garfinkel D, Lipson S. Melatonin for treatment of sundowning in elderly persons with dementia - a preliminary study. Arch Gerontol Geriatr. 2000;31:65–76. doi: 10.1016/s0167-4943(00)00068-6. [DOI] [PubMed] [Google Scholar]
- 20.Mahlberg R, Kunz D, Sutej I, Kuhl KP, Hellweg R. Melatonin treatment of day-night rhythm disturbances and sundowning in Alzheimer disease: an open-label pilot study using actigraphy. J Clin Psychopharmacol. 2004;24:456–459. doi: 10.1097/01.jcp.0000132443.12607.fd. [DOI] [PubMed] [Google Scholar]
- 21.Brusco LI, Marquez M, Cardinali DP. Melatonin treatment stabilizes chronobiologic and cognitive symptoms in Alzheimer's disease. Neuro Endocrinol Lett. 1998;19:111–115. [PubMed] [Google Scholar]
- 22.Cardinali DP, Brusco LI, Liberczuk C, Furio AM. The use of melatonin in Alzheimer's disease. Neuro Endocrinol Lett. 2002;23(Suppl 1):20–23. [PubMed] [Google Scholar]
- 23.Asayama K, Yamadera H, Ito T, Suzuki H, Kudo Y, Endo S. Double blind study of melatonin effects on the sleep-wake rhythm, cognitive and non-cognitive functions in Alzheimer type dementia. J Nippon Med Sch. 2003;70:334–341. doi: 10.1272/jnms.70.334. [DOI] [PubMed] [Google Scholar]
- 24.Singer C, Tractenberg RE, Kaye J, Schafer K, Gamst A, Grundman M, Thomas R, Thal LJ. A multicenter, placebo-controlled trial of melatonin for sleep disturbance in Alzheimer's disease. Sleep. 2003;26:893–901. doi: 10.1093/sleep/26.7.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pappolla MA, Chyan YJ, Poeggeler B, Frangione B, Wilson G, Ghiso J, Reiter RJ. An assessment of the antioxidant and the antiamyloidogenic properties of melatonin: implications for Alzheimer's disease. J Neural Transm. 2000;107:203–231. doi: 10.1007/s007020050018. [DOI] [PubMed] [Google Scholar]
- 26.Cardinali DP, Furio AM, Brusco LI. Clinical aspects of melatonin intervention in Alzheimer's disease progression. Curr Neuropharmacol. 2010;8:218–227. doi: 10.2174/157015910792246209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Jonghe A, Korevaar JC, van Munster BC, de Rooij SE. Effectiveness of melatonin treatment on circadian rhythm disturbances in dementia. Are there implications for delirium? A systematic review. Int J Geriatr Psychiatry. 2010;25:1201–1208. doi: 10.1002/gps.2454. [DOI] [PubMed] [Google Scholar]
- 28.Savaskan E, Ayoub MA, Ravid R, Angeloni D, Fraschini F, Meier F, Eckert A, Muller-Spahn F, Jockers R. Reduced hippocampal MT2 melatonin receptor expression in Alzheimer's disease. J Pineal Res. 2005;38:10–16. doi: 10.1111/j.1600-079X.2004.00169.x. [DOI] [PubMed] [Google Scholar]
- 29.Wu YH, Swaab DF. Disturbance and strategies for reactivation of the circadian rhythm system in aging and Alzheimer's disease. Sleep Med. 2007;8:623–636. doi: 10.1016/j.sleep.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 30.Quinn J, Kulhanek D, Nowlin J, Jones R, Pratico D, Rokach J, Stackman R. Chronic melatonin therapy fails to alter amyloid burden or oxidative damage in old Tg2576 mice: implications for clinical trials. Brain Res. 2005;1037:209–213. doi: 10.1016/j.brainres.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 31.Cardinali DP, Furio AM, Brusco LI. The use of chronobiotics in the resynchronization of the sleep/wake cycle. Therapeutical application in the early phases of Alzheimer's disease. Recent Pat Endocr Metab Immune Drug Discov. 2011;5:80–90. doi: 10.2174/187221411799015354. [DOI] [PubMed] [Google Scholar]
- 32.Jean-Louis G, Zizi F, von Gizycki H, Taub H. Effects of melatonin in two individuals with Alzheimer's disease. Percept Mot Skills. 1998;87:331–339. doi: 10.2466/pms.1998.87.1.331. [DOI] [PubMed] [Google Scholar]
- 33.Peck JS, LeGoff DB, Ahmed I, Goebert D. Cognitive effects of exogenous melatonin administration in elderly persons: a pilot study. Am J Geriatr Psychiatry. 2004;12:432–436. doi: 10.1176/appi.ajgp.12.4.432. [DOI] [PubMed] [Google Scholar]
- 34.Riemersma-van der Lek RF, Swaab DF, Twisk J, Hol EM, Hoogendijk WJ, van Someren EJ. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: a randomized controlled trial. JAMA. 2008;299:2642–2655. doi: 10.1001/jama.299.22.2642. [DOI] [PubMed] [Google Scholar]
- 35.Thome J, Coogan AN, Woods AG, Darie CC, Hassler F. CLOCK Genes and Circadian Rhythmicity in Alzheimer Disease. J Aging Res. 2011;2011:383091. doi: 10.4061/2011/383091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhong G, Naismith SL, Rogers NL, Lewis SJ. Sleep-wake disturbances in common neurodegenerative diseases: a closer look at selected aspects of the neural circuitry. J Neurol Sci. 2011;307:9–14. doi: 10.1016/j.jns.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 37.Bachman D, Rabins P. "Sundowning" and other temporally associated agitation states in dementia patients. Annu Rev Med. 2006;57:499–511. doi: 10.1146/annurev.med.57.071604.141451. [DOI] [PubMed] [Google Scholar]
- 38.Oosterman JM, van Someren EJ, Vogels RL, Van Harten B, Scherder EJ. Fragmentation of the rest-activity rhythm correlates with age-related cognitive deficits. J Sleep Res. 2009;18:129–135. doi: 10.1111/j.1365-2869.2008.00704.x. [DOI] [PubMed] [Google Scholar]
- 39.Rosales-Corral SA, Acuna-Castroviejo D, Coto-Montes A, Boga JA, Manchester LC, Fuentes-Broto L, Korkmaz A, Ma S, Tan DX, Reiter RJ. Alzheimer's disease: pathological mechanisms and the beneficial role of melatonin. J Pineal Res. 2012;52:167–202. doi: 10.1111/j.1600-079X.2011.00937.x. [DOI] [PubMed] [Google Scholar]
- 40.Beaulieu-Bonneau S, Hudon C. Sleep disturbances in older adults with mild cognitive impairment. Int Psychogeriatr. 2009;21:654–666. doi: 10.1017/S1041610209009120. [DOI] [PubMed] [Google Scholar]
- 41.Geda YE, Smith GE, Knopman DS, Boeve BF, Tangalos EG, Ivnik RJ, Mrazek DA, Edland SD, Petersen RC. De novo genesis of neuropsychiatric symptoms in mild cognitive impairment (MCI) Int Psychogeriatr. 2004;16:51–60. doi: 10.1017/s1041610204000067. [DOI] [PubMed] [Google Scholar]
- 42.Tranah GJ, Blackwell T, Stone KL, Ancoli-Israel S, Paudel ML, Ensrud KE, Cauley JA, Redline S, Hillier TA, Cummings SR, Yaffe K. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann Neurol. 2011;70:722–732. doi: 10.1002/ana.22468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cochrane A, Robertson IH, Coogan AN. Association between circadian rhythms, sleep and cognitive impairment in healthy older adults: an actigraphic study. J Neural Transm. 2012;119:1233–9. doi: 10.1007/s00702-012-0802-2. [DOI] [PubMed] [Google Scholar]
- 44.Vollrath L, Semm P, Gammel G. Sleep induction by intranasal administration of melatonin. Adv Biosci. 1981;29:327–329. [Google Scholar]
- 45.Waldhauser F, Saletu B, Trinchard-Lugan I. Sleep laboratory investigations on hypnotic properties of melatonin. Psychopharmacology (Berl) 1990;100:222–226. doi: 10.1007/BF02244410. [DOI] [PubMed] [Google Scholar]
- 46.Cardinali DP, Srinivasan V, Brzezinski A, Brown GM. Melatonin and its analogs in insomnia and depression. J Pineal Res. 2012;52:365–375. doi: 10.1111/j.1600-079X.2011.00962.x. [DOI] [PubMed] [Google Scholar]
- 47.Buscemi N, Vandermeer B, Hooton N, Pandya R, Tjosvold L, Hartling L, Vohra S, Klassen TP, Baker G. Efficacy and safety of exogenous melatonin for secondary sleep disorders and sleep disorders accompanying sleep restriction: meta-analysis. BMJ. 2006;332:385–393. doi: 10.1136/bmj.38731.532766.F6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brzezinski A, Vangel MG, Wurtman RJ, Norrie G, Zhdanova I, Ben Shushan A, Ford I. Effects of exogenous melatonin on sleep: a meta-analysis. Sleep Med Rev. 2005;9:41–50. doi: 10.1016/j.smrv.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 49.Wilson SJ, Nutt DJ, Alford C, Argyropoulos SV, Baldwin DS, Bateson AN, Britton TC, Crowe C, Dijk DJ, Espie CA, Gringras P, Hajak G, Idzikowski C, Krystal AD, Nash JR, Selsick H, Sharpley AL, Wade AG. British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders. J Psychopharmacol. 2010;24:1577–1601. doi: 10.1177/0269881110379307. [DOI] [PubMed] [Google Scholar]
- 50.Cardinali DP, Pandi-Perumal SR, Niles LP. Melatonin and Its Receptors: Biological Function in Circadian Sleep-Wake Regulation. In: Monti JM, Pandi-Perumal SR, Sinton CM, editors. Neurochemistry of Sleep and Wakefulness. Cambridge UK: Cambridge University Press; 2008. pp. 283–314. [Google Scholar]
- 51.Detanico BC, Piato AL, Freitas JJ, Lhullier FL, Hidalgo MP, Caumo W, Elisabetsky E. Antidepressant-like effects of melatonin in the mouse chronic mild stress model. Eur J Pharmacol. 2009;607:121–125. doi: 10.1016/j.ejphar.2009.02.037. [DOI] [PubMed] [Google Scholar]
- 52.McElroy SL, Winstanley EL, Martens B, Patel NC, Mori N, Moeller D, McCoy J, Keck PE Jr. A randomized, placebo-controlled study of adjunctive ramelteon in ambulatory bipolar I disorder with manic symptoms and sleep disturbance. Int Clin Psychopharmacol. 2010;26:48–53. doi: 10.1097/YIC.0b013e3283400d35. [DOI] [PubMed] [Google Scholar]
- 53.Crupi R, Mazzon E, Marino A, La Spada G, Bramanti P, Cuzzocrea S, Spina E. Melatonin treatment mimics the antidepressant action in chronic corticosterone-treated mice. J Pineal Res. 2010;49:123–129. doi: 10.1111/j.1600-079X.2010.00775.x. [DOI] [PubMed] [Google Scholar]
- 54.Monti JM, Alvarino F, Cardinali DP, Savio I, Pintos A. Polysomnographic study of the effect of melatonin on sleep in elderly patients with chronic primary insomnia. Arch Gerontol Geriatrics. 1999;28:85–98. doi: 10.1016/s0167-4943(98)00129-0. [DOI] [PubMed] [Google Scholar]
- 55.Furio AM, Cutrera RA, Castillo Thea V, Pérez Lloret S, Riccio P, Caccuri RL, Brusco LL, Cardinali DP. Effect of melatonin on changes in locomotor activity rhythm of Syrian hamsters injected with beta amyloid peptide 25-35 in the suprachiasmatic nuclei. Cell Mol Neurobiol. 2002;22:699–709. doi: 10.1023/a:1021805023906. [DOI] [PubMed] [Google Scholar]
- 56.Cardinali DP, Cano P, Jimenez-Ortega V, Esquifino AI. Melatonin and the metabolic syndrome. Physiopathologic and therapeutical implications. Neuroendocrinology. 2011;93:133–142. doi: 10.1159/000324699. [DOI] [PubMed] [Google Scholar]
- 57.Rajaratnam SM, Polymeropoulos MH, Fisher DM, Roth T, Scott C, Birznieks G, Klerman EB. Melatonin agonist tasimelteon (VEC-162) for transient insomnia after sleep-time shift: two randomised controlled multicentre trials. Lancet. 2009;373:482–491. doi: 10.1016/S0140-6736(08)61812-7. [DOI] [PubMed] [Google Scholar]
- 58.Mulchahey JJ, Goldwater DR, Zemlan FP. A single blind, placebo controlled, across groups dose escalation study of the safety, tolerability, pharmacokinetics and pharmacodynamics of the melatonin analog beta-methyl-6-chloromelatonin. Life Sci. 2004;75:1843–1856. doi: 10.1016/j.lfs.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 59.Weishaupt JH, Bartels C, Polking E, Dietrich J, Rohde G, Poeggeler B, Mertens N, Sperling S, Bohn M, Huther G, Schneider A, Bach A, Siren AL, Hardeland R, Bahr M, Nave KA, Ehrenreich H. Reduced oxidative damage in ALS by high-dose enteral melatonin treatment. J Pineal Res. 2006;41:313–323. doi: 10.1111/j.1600-079X.2006.00377.x. [DOI] [PubMed] [Google Scholar]
- 60.Chahbouni M, Escames G, Venegas C, Sevilla B, Garcia JA, Lopez LC, Munoz-Hoyos A, Molina-Carballo A, Acuna-Castroviejo D. Melatonin treatment normalizes plasma pro-inflammatory cytokines and nitrosative/oxidative stress in patients suffering from Duchenne muscular dystrophy. J Pineal Res. 2010;48:282–289. doi: 10.1111/j.1600-079X.2010.00752.x. [DOI] [PubMed] [Google Scholar]
- 61.Waldhauser F, Waldhauser M, Lieberman HR, Deng MH, Lynch HJ, Wurtman RJ. Bioavailability of oral melatonin in humans. Neuroendocrinology. 1984;39:307–313. doi: 10.1159/000123997. [DOI] [PubMed] [Google Scholar]
- 62.Voordouw BC, Euser R, Verdonk RE, Alberda BT, de Jong FH, Drogendijk AC, Fauser BC, Cohen M. Melatonin and melatonin-progestin combinations alter pituitary-ovarian function in women and can inhibit ovulation. J Clin Endocrinol Metab. 1992;74:108–117. doi: 10.1210/jcem.74.1.1727807. [DOI] [PubMed] [Google Scholar]
- 63.Nickkholgh A, Schneider H, Sobirey M, Venetz WP, Hinz U, Pelzl lH, Gotthardt DN, Cekauskas A, Manikas M, Mikalauskas S, Mikalauskene L, Bruns H, Zorn M, Weigand MA, Buchler MW, Schemmer P. The use of high-dose melatonin in liver resection is safe: first clinical experience. J Pineal Res. 2011;50:381–388. doi: 10.1111/j.1600-079X.2011.00854.x. [DOI] [PubMed] [Google Scholar]
- 64.Golombek DA, Pevet P, Cardinali DP. Melatonin effects on behavior: possible mediation by the central GABAergic system. Neurosci Biobehav Rev. 1996;20:403–412. doi: 10.1016/0149-7634(95)00052-6. [DOI] [PubMed] [Google Scholar]
- 65.Dagan Y, Zisapel N, Nof D, Laudon M, Atsmon J. Rapid reversal of tolerance to benzodiazepine hypnotics by treatment with oral melatonin: a case report. Eur Neuropsychopharmacol. 1997;7:157–160. doi: 10.1016/s0924-977x(96)00381-1. [DOI] [PubMed] [Google Scholar]
- 66.Garfinkel D, Zisapel N, Wainstein J, Laudon M. Facilitation of benzodiazepine discontinuation by melatonin: a new clinical approach. Arch Intern Med. 1999;159:2456–2460. doi: 10.1001/archinte.159.20.2456. [DOI] [PubMed] [Google Scholar]
- 67.Siegrist C, Benedetti C, Orlando A, Beltran JM, Tuchscherr L, Noseda CM, Brusco LI, Cardinali DP. Lack of changes in serum prolactin, FSH, TSH, and estradiol after melatonin treatment in doses that improve sleep and reduce benzodiazepine consumption in sleep-disturbed, middle-aged, and elderly patients. J Pineal Res. 2001;30:34–42. doi: 10.1034/j.1600-079x.2001.300105.x. [DOI] [PubMed] [Google Scholar]
- 68.Kunz D, Bineau S, Maman K, Milea D, Toumi M. Benzodiazepine discontinuation with prolonged-release melatonin: hints from a German longitudinal prescription database. Expert Opin Pharmacother. 2012;13:9–16. doi: 10.1517/14656566.2012.638284. [DOI] [PubMed] [Google Scholar]
- 69.Fetveit A. Late-life insomnia: a review. Geriatr Gerontol Int. 2009;9:220–234. doi: 10.1111/j.1447-0594.2009.00537.x. [DOI] [PubMed] [Google Scholar]
- 70.Morin CM, Benca R. Chronic insomnia. Lancet. 2012;379:1129–1141. doi: 10.1016/S0140-6736(11)60750-2. [DOI] [PubMed] [Google Scholar]
- 71.Boyle N, Naganathan V, Cumming RG. Medication and falls: risk and optimization. Clin Geriatr Med. 2010;26:583–605. doi: 10.1016/j.cger.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 72.Faught E. Monotherapy in adults and elderly persons. Neurology. 2007;69:S3–S9. doi: 10.1212/01.wnl.0000302370.01359.8f. [DOI] [PubMed] [Google Scholar]


