Abstract
Human induced pluripotent stem cell-derived endothelial cells (hiPSC-ECs) are promising for treatment of vascular diseases. However, hiPSC-ECs purified based on CD31 expression are comprised of arterial, venous, and lymphatic subtypes. It is unclear whether hiPSC-ECs are heterogeneous in nature, and whether there may be functional benefits of enriching for specific subtypes. Therefore, we sought to characterize the hiPSC-ECs and enrich for each subtype, and demonstrate whether such enrichment would have functional significance. The hiPSC-ECs were generated from differentiation of hiPSCs using vascular endothelial growth factor (VEGF)-A and bone morphogenetic protein-4. The hiPSC-ECs were purified based on positive expression of CD31. Subsequently, we sought to enrich for each subtype. Arterial hiPSC-ECs were induced using higher concentrations of VEGF-A and 8-bromoadenosine-3’:5’-cyclic monophosphate in the media, whereas lower concentrations of VEGF-A favored venous subtype. VEGF-C and angiopoietin-1 promoted the expression of lymphatic phenotype. Upon FACS purification based on CD31+ expression, the hiPSC-EC population was observed to display typical endothelial surface markers and functions. However, the hiPSC-EC population was heterogeneous in that they displayed arterial, venous, and to a lesser degree, lymphatic lineage markers. Upon comparing vascular formation in matrigel plugs in vivo, we observed that arterial enriched hiPSC-ECs formed a more extensive capillary network in this model, by comparison to a heterogeneous population of hiPSC-ECs. This study demonstrates that FACS purification of CD31+ hiPSC-ECs produces a diverse population of ECs. Refining the differentiation methods can enrich for subtype-specific hiPSC-ECs with functional benefits of enhancing neovascularization.
Keywords: Heterogeneity, induced pluripotent stem cells, differentiation, endothelial cells, angiogenesis
Introduction
Human pluripotent stem cells including embryonic stem cells (hESCs) and induced pluripotent stem cells (hiPSCs) are a promising source for the generation of healthy endothelial cells (ECs) for repair of vascular diseases [1,2]. Both hiPSCs and hESCs are capable of unlimited self-renewal, as well as differentiation into lineages comprised of all three germ layers. However, as opposed to hESCs, hiPSCs may be used to derive autologous ECs for regenerative medicine applications.
To date, multiple groups have explored the therapeutic efficacy of pluripotent stem cell-derived ECs in pre-clinical models of ischemic cardiovascular disease. These cells appear to enhance angiogenesis, tissue perfusion, and organ function [3-6]. In these studies, the isolation of ECs primarily relies on general surface markers such as KDR, CD31, von Willebrand factor, or CD144 (VE-cadherin). Each of these markers are expressed by arterial, venous, and lymphatic subtypes. Thus it seems likely that previous investigations employing hESCs or hiPSCs have generated ECs that represent a heterogeneous population comprised of endothelial subtypes. However, for therapeutic applications in regenerative medicine, it is unclear whether hiPSC-derived ECs (hiPSC-ECs) are heterogeneous in nature, and whether there may be functional benefits of enriching for specific subtypes.
Therefore, we sought to determine whether hiPSC-ECs were heterogeneous and could be enriched for each subtype, and also if such enrichment would have a functional significance. With our standard differentiation procedure, we find that the hiPSC-ECs that are generated are of all three major subtypes, although arterial and venous hiPSC-ECs predominate. Notably, hiPSC-ECs enriched for arterial lineage generate a more extensive capillary network in vivo than by comparison to a heterogeneous hiPSC-EC population. This observation may be of therapeutic relevance for cell therapy of ischemic diseases.
Methods
Cell culture studies
The hiPSC lines (HUF4, HUF5) were generated using retroviral factors encoding Oct4, Sox2, c-Myc and Klf4 in adult dermal fibroblasts and characterized for their pluripotency previously [3]. The hiPSCs were cultured on mouse embryonic fibroblasts feeder cells in standard WiCell human ESC growth media containing 10 ng/ml basic fibroblast growth factor.
To initiate differentiation, confluent cultures of hiPSCs were incubated with 1mg/ml type IV collagenase for 10 minutes and transferred to ultra low attachment dishes containing differentiation media for 4 days to form embryoid bodies (EBs). The differentiation media used consisted of α-Minimum Eagle’s Medium, 20% fetal bovine serum, L-glutamine, β-mercaptoethanol (0.05mmol/L) and 1% non-essential amino acids supplemented with bone morphogenetic protein-4 (BMP-4, 50 ng/ml, Peprotech) and vascular endothelial growth factor (VEGF-A, 50 ng/ml, Peprotech). For the generation of heterogeneous hiPSC-ECs, the 4-day EBs were reattached to gelatin-coated dishes in the presence of VEGF-A for another 10 days before purification [3]. For arterial EC differentiation, 4-day EBs were seeded on gelatin-coated dishes and cultured for another 10 days in the presence of VEGF-A and 8-bromoadenosine-3’:5’-cyclic monophosphate sodium salt (8Br-cAMP, 0.5mmol/L, Sigma). For venous EC differentiation, the 4-day EBs were differentiated in VEGF-A (10 ng/ml) for 10 additional days. For lymphatic EC differentiation, 4-day EBs were differentiated in BMP-4 (50 ng/ml), VEGF-A (50 ng/ml), VEGF-C (50 ng/ml) and angiopoietin-1 (Ang-1, 20 ng/ml, Peprotech) for 10 additional days. Differentiation media was changed every 2 days for all three types of EC differentiation.
Human coronary arterial ECs (Promocell) and dermal lymphatic microvascular ECs (HMVECs, Lonza) were cultured with endothelial cell growth medium-2MV (EGM-2MV, Lonza).
Fluorescence Activated Cell Sorting (FACS)
On day 14 of differentiation, the ECs were purified using FACS. Differentiated cells were dissociated into single cells with Accutase (Life Technologies) for 20 minutes at 37°C, washed with 1x PBS containing 5% BSA and passed through a 70-μm cell strainer. They were next incubated with PE-conjugated CD31 antibody (eBioscience) for 30 minutes. Isotype-matched antibody served as negative control and 1% propidium iodide was used to stain the nonviable cells. Flow cytometry was performed using BD Digital Vantage cell sorter. The purified hiPSC-ECs were expanded in EGM-2MV media.
Immunofluorescent staining
The purified hiPSC-ECs were fixed with 4% paraformaldehyde for 10 minutes and permeabilized with 0.1% Triton X-100 in 1x PBS for 10 minutes. They were then blocked with either 1% normal goat or donkey serum for 30 minutes, followed by anti-human CD31 (R&D Systems), anti-human CD144 (R&D Systems), anti-human endothelial nitric oxide (eNOS, BD Transduction Laboratories), anti-human von Willebrand factor (vWF, Abcam), anti-EphrinB2 (Thermo Fisher Scientific Inc) and anti-podoplanin (R&D Systems) overnight at 4°C. The cells were washed with 1x PBS and incubated with Alexa Fluor-488 or -594 secondary antibodies (Life Technologies) for 1 hour at room temperature. Cells were washed with 1x PBS and the nuclei were stained with Hoechst 33342 dye.
Protein extraction and western blot analysis
Proteins were extracted from the cells using 1x RIPA lysis buffer with Complete Protease Inhibitor Cocktail and Halt Phosphatase Inhibitor Cocktail (all from Thermo Fisher Scientific Inc). Total protein concentration was quantified using Coomassie Plus (Bradford) Protein Assay (Thermo Fisher Scientific Inc). Equal amount of protein (20ug) were mixed with NuPAGE reducing agent (Life Technologies) and heated for 10 minutes at 70°C. The samples were then run on 15% NuPAGE Novex Bis-Tris mini gels and transferred to polyvinylidene fluoride membrane. Membranes were blocked and incubated overnight at 4°C with primary antibodies, intracellular adhesion molecule-1 (ICAM-1), Jagged-1, DLL-4, Notch-1, Notch-4 (all from R&D Systems). Horseradish peroxidase conjugated secondary antibody was incubated with the membranes for 1 hour at 37°C. The membranes were then washed and developed using ECL Detection Kit (Amersham). Quantification of protein quantities were performed by normalizing to α-actin (R&D Systems, Minneapolis, USA) (n=3).
RNA extraction and quantitative PCR
Total RNA was extracted from the cells using RNeasy Mini Kit (Qiagen) and treated with RNase-free DNase (Qiagen) to remove possible contaminating genomic DNA. Quantitative polymerase chain reaction was performed using Taqman Gene Expression Assays purchased from Applied Biosystems (n=3). The genes that were analyzed are listed in Supplementary Table. The 2-[/delta][/delta] Ct method was used to calculate the gene expression results. All results were expressed as relative fold change, normalized to 18S.
Endothelial functional assays
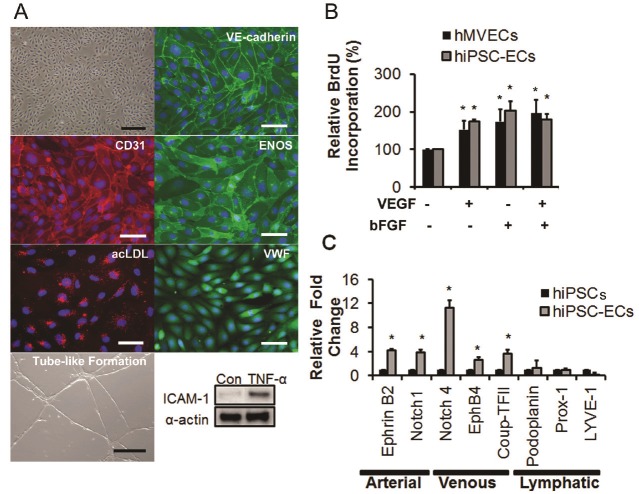
For demonstration of their functional response to tumor necrosis factor-α (TNF-α), HMVECs and hiPSC-derived ECs were serum starved overnight and then incubated with 10ng/ml of TNF-α for 18 hours. The protein lysates were analyzed by Western blotting for ICAM-1. For vascular tube-like formation in vitro, 2.5 x 105 cells were seeded on 24-well plates pre-coated with Matrigel and incubated for 24 hours in 37°C in EGM-2MV. Images were taken using a light microscope. For Dil-labeled acetylated LDL (ac-LDL) uptake, hiPSC-ECs were incubated with 10μg/ml Dil-labeled ac-LDL (Life Technologies) for 4 hours at 37°C. After incubation, they were washed thrice with 1x PBS before being visualized and photographed under fluorescent microscope. Cell proliferation assay (Roche Applied Science) was performed according to the manufacturer’s instruction. Briefly, the hiPSC-ECs were cultured in 96-well plate and were serum-starved for 24 hours. Following that, the hiPSC-ECs were incubated with either bFGF (10 ng/ml) or VEGF (10 ng/ml) or a combination of both for 48 hours. BrdU labeling solution was then added to the culture medium and the cell incubated for 3 hours at 37°C. A fixing-denaturing solution was added and incubated for 30 minutes followed by addition of anti-BrdU-POD working solution for 90 minutes at room temperature. The cells were washed and incubated with substrate solution for 30 minutes. The absorbance measurement was performed at 450 nm using an ELISA plate reader (n=3).
In vivo matrigel angiogenesis assay
Growth factor-reduced matrigel was mixed with bFGF (50ng/ml). Animals were normalized to 3 groups: 1) matrigel and bFGF; 2) heterogeneous hiPSC-ECs in matrigel and bFGF; or 3) arterial enriched hiPSC-ECs in matrigel and bFGF (n>3). For cell transplantation experiments, the matrigel was mixed with 5 x 105 hiPSC-ECs in a final volume of 200 μl and the mixture was subcutaneously injected into the mid-lower abdominal region of SCID mice. After 14 days, the animals were euthanized and dissected to remove the Matrigel plugs. For the purpose of orientation, the abdominal skeletal muscle was removed intact with each matrigel plug. The matrigel plugs were fixed in 4% paraformaldehyde for routine paraffin embedding and hematoxylin & eosin (H&E) staining. All animal studies were approved by the Institutional Animal Care and Use Committee at Stanford University.
Immunohistochemical staining
For determination of total capillary density, the slides were stained with polyclonal rabbit anti mouse CD31 (Abcam), followed by horseradish peroxidase-conjugated secondary antibody. Quantification of capillaries was performed under double-blinded procedure on six different fields at magnification of 20x and expressed in terms of #/mm2 (n≥3). To distinguish humanfrom mouse-specific vessels, sections were immunofluorescently stained with and antihuman-specific CD31 antibody (R&D Systems) and a CD31 antibody (Dianova, Germany) that cross-reacted to both human and mouse vessels. For comparison of human versus mouse vessels, capillary density was quantified in 4 different fields at 40x magnification and expressed in terms of #/mm2.
Mouse anti α-smooth muscle actin (Sigma-Aldrich) was used to identify smooth muscle cells. The erythrocytes in the slides were stained with either biotinylated rat anti-mouse TER-119 (BD Bioscience) or mouse anti-human Glycophorin A (Dako) to confirm the origin of the erythrocytes seen in the micro vessels.
Statistical analysis
All data are expressed as mean ± standard deviation. Statistical analysis between the two groups at each time point was performed by the unpaired t-test. Repeated measurements of samples over time were analyzed by repeated measures of ANOVA with the Holm adjustment. Differences were considered significant at probability values of P< 0.05.
Results
hiPSC-ECs generated via standard EB differentiation procedure are heterogeneous
We first examined the efficiency of hiPSC lines differentiating into ECs. hiPSCs were differentiated via the EB formation method in the presence of BMP-4 (50ng/ml) and VEGF (50ng/ml) as depicted in Figure 1A. There were cell line-specific differences in the efficiency of endothelial differentiation. Using our standard differentiation procedure, the HUF4 line consistently generated fewer hiPSC-ECs by comparison to the HUF5 line hiPSCs (average yields of 6% and 16% of total differentiated cells respectively (Figure 1B). Therefore for the remainder of the data, we examined hiPSC-ECs derived from the HUF5 line.
Figure 1.
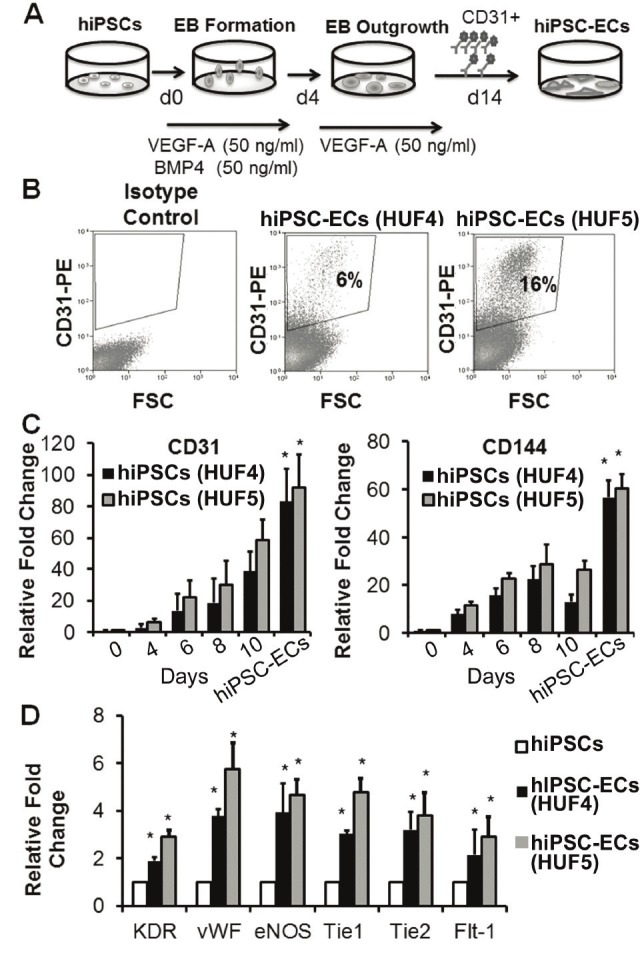
Endothelial differentiation, purification, and characterization of hiPSCs. A. Schematic of endothelial differentiation procedure. B. Efficiency of CD31+ expression for 2 different cell lines. C. Quantitative PCR analysis of endothelial markers during differentiation in 2 different hiPSC lines. D. Expression of endothelial markers in purified hiPSC-ECs. *P<0.05 relative to hiPSCs.
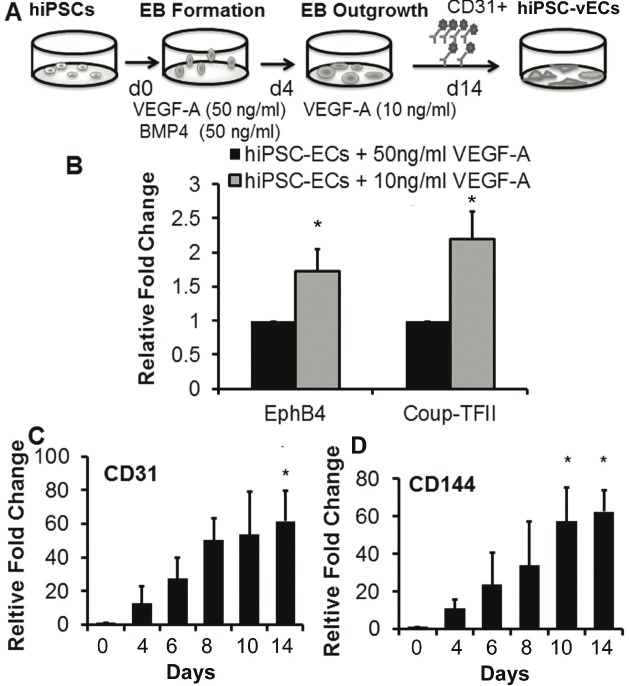
Expression of endothelial markers CD31 and CD144 significantly increased during the course of differentiation and was highest in the purified CD31+ hiPSC-ECs (Figure 1C, *P<0.05 for both HUF4 and HUF5 hiPSC-ECs versus the parental lines), as shown by the quantitative real time gene expression studies. These purified CD31+ hiPSC-ECs also expressed known endothelial markers such as KDR, vWF, eNOS and vascular endothelial growth factor receptor 1 (Flt-1) (Figure 1D, *P<0.05 for both HUF4 and HUF5 hiPSC-ECs versus the parental lines). The robust expression of endothelial markers was associated with very low levels of endogenous Sox2, Oct4 and Nanog gene expression (Supplemental Figure 1, *P<0.05 with respect to the parental hiPSC lines).
The hiPSC-derived ECs were expanded on fibronectin-coated plate and they formed endothelial sheets with cobblestone morphology. They stained positive for CD31, CD144, eNOS and vWF (Figure 2A), took up Dil-labeled ac-LDL and formed a robust network of tubular structures upon seeding on matrigel. Following stimulation with 10ng/ml of inflammatory cytokine, TNF-α for 4 hours, hiPSC-ECs upregulated ICAM-1, resembling the behavior of primary ECs. Functionally, they also proliferated well in response to either bFGF and/or VEGF, to similar levels to that of primary ECs (Figure 2B, *P<0.05 with respect to the untreated group). All of these functions are characteristic of mature endothelial cells.
Figure 2.
Characterization of purified hiPSC-ECs. A. The hiPSC-ECs showed cobblestone morphology and expressed endothelial phenotypic markers. Functionally they could take up ac-LDL, form tube-like structure in matrigel, and upregulate ICAM-1 protein levels in response to TNF-α (*P<0.05 relative to hiPSCs). B. BrdU incorporation assay showed an increase in proliferation in the presence of VEGF and/or bFGF *P<0.05 relative to untreated control group. C. Quantitative gene expression analysis of arterial, venous and lymphatic markers in purified hiPSC-ECs, in comparison to parental hiPSCs. *P<0.05 relative to hiPSCs. Scale bar: 50μm.
When examined by quantitative real time gene expression studies, we found that hiPSC-ECs expressed various markers associated with arterial, venous and or lymphatic ECs (Figure 2C, *P<0.05 with respect to the parental line). They expressed at high levels the arterial markers Ephrin B2, Notch 1 and Notch 4; at moderate level the venous markers CoupTF-II, EphB4; and at a lower level the lymphatic markers podoplanin, Prox-1 and LYVE-1. Therefore, the CD31+ population of hiPSC-ECs represented a heterogeneous mixture of ECs derived from all 3 subtypes.
High VEGF concentration favors differentiation and specification of hiPSC-ECs into the arterial subtype
Past studies have shown that concentration of VEGF in the media regulates the efficiency of endothelial differentiation in both human and mouse embryonic stem cells (ESCs) [7-9]. Furthermore, in mouse ESCs, the combination of VEGF with 8Br-cAMP enhances the generation of ESC-EC of the arterial subtype [10]. We therefore assessed the role of VEGF and/or 8Br-cAMP on the differentiation of hiPSCs into arterial EC (hiPSC-artEC) subtype. hiPSCs were differentiated in the presence of high concentrations of VEGF (50ng/ml) and 8Br-cAMP (0.5mmol/L) and CD31+ hiPSC-ECs were FACS sorted and their gene expression profiles were analysed (Figure 3A). Gene expression of CD31 and CD144 showed a progressive increase during the 14 days of differentiation (Figure 3B and 3C, *P<0.05). The CD31+ hiPSC-ECs generated from the combination of high VEGF and 8Br-cAMP condition manifested a significantly stronger expression of arterial markers and a reduced expression of venous markers, EphB4 and CoupTFII (Figure 3D, *P<0.05 with respect to parental line, #P<0.05 with respect to CD31+ hiPSC-ECs generated with high VEGF concentration only). At the protein level, hiPSC-ECs generated in the presence of VEGF and 8Br-cAMP expressed higher levels of Ephrin B2 (Figure 3E and 3F), suggesting that the cell population was enriched in the arterial subtype.
Figure 3.
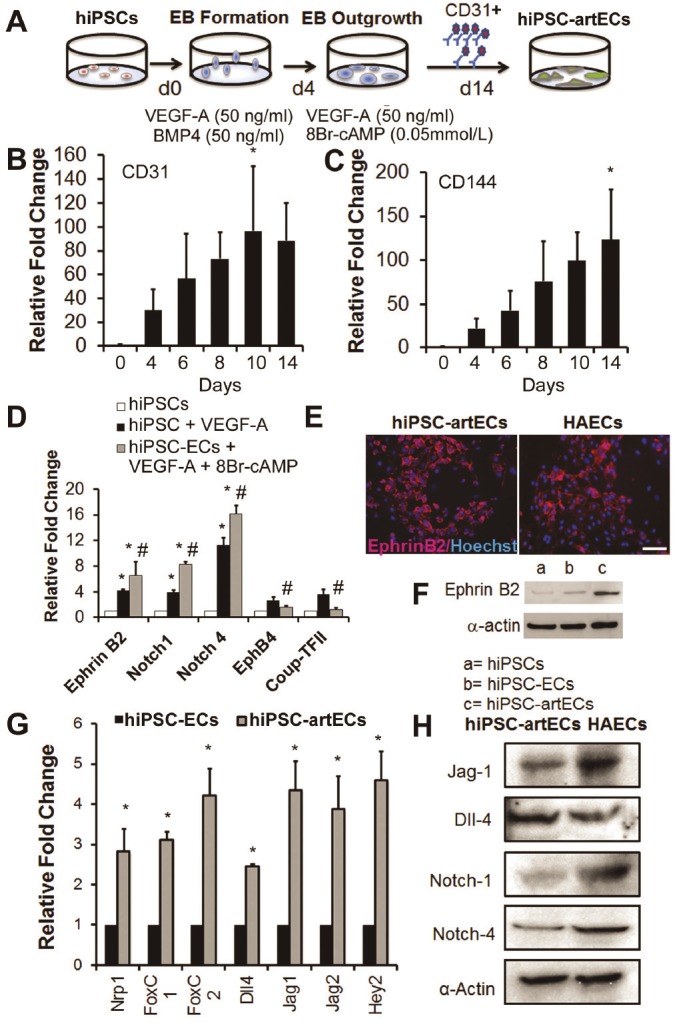
Arterial enrichment of hiPSC-ECs using 8Br-cAMP and VEGF-A. A. Schematic of differentiation procedure to generate arterial EC subtype (hiPSC-artECs). B-C. Gene expression of CD31 and CD144 increased throughout the period of differentiation. *P<0.05 relative to day 0 hiPSCs. D. Upregulated gene expression of arterial markers in the presence of 8Br-cAMP. *P<0.05 relative to hiPSC, #P<0.05 relative to CD31+ hiPSC-ECs generated without 8Br-cAMP. E. CD31+ hiPSC-artECs stained positive for arterial marker, EphrinB2 (red). F. Western blot showing enhancement of EphrinB2 in CD31+ hiPSC-ECs generated using 8Br-cAMP with VEGF-A. G. VEGF-Notch signaling pathway genes were significantly upregulated in the CD31+ hiPSC-artECs, in comparison to heterogeneous hiPSC-ECs. *P<0.05 relative to hiPSC-ECs (heterogeneous). H. Immunoblots validate gene expression analysis showing CD31+ hiPSC-artECs expressed Notch-related markers. Scale bar: 50μm.
Since VEGF-Notch crosstalk has been widely reported to play a central position for vascular morphogenesis [11,12] and in the zebrafish system to regulate the arterial-venous specification [13,14], we analyzed the gene expression of the genes involved in the VEGF-Notch interaction including Nrp1, FoxC1, FoxC2, Dll4, Jag1, Jag2 and Hey2 (Supplemental Figure 2).
Each of these were significantly upregulated in hiPSC-artECs, in comparison to heterogeneous hiPSC-EC (Figure 3G, *P<0.05). This was also reflected in the protein expression results of Jag-1, DLL4, Notch1 and Notch 4 (Figure 3H) indicating a strong arterial identity of these CD31+ hiPSC-artECs.
Whereas high (50ng/ml) VEGF concentration in the media promoted specification of ECs to arterial subtype, a lower concentration (10ng/ml) of VEGF promoted venous specification. In the low VEGF condition, we found that venous gene markers, CoupTFII and EphB4 were upregulated by comparison to hiPSC-ECs using the standard concentration of VEGF during the differentiation process Figure 4A and 4B (*P<0.05, compared to hiPSC-ECs generated under high VEGF-A conditions). However, the expression of CD31 and CD144 were unaffected and continued to become upregulated during the course of differentiation (Figure 4C and 4D, *P<0.05).
Figure 4.
Enrichment of venous EC subtype using low VEGF-A concentration. A. Schematic diagram of hiPSC differentiation procedure to generate hiPSC-derived venous EC (hiPSC-vEC) subtype. B. Comparison of the gene expression of venous markers; EphB4 and Coup-TFII in CD31+ ECs differentiated using high (50ng/ml) or low (10ng/ml) VEGF-A. *P<0.05 relative to hiPSCs induced with VEGF (50ng/ml). Gene expression of CD31 (C) and CD144 (D) increased significantly throughout the differentiation course. *P<0.05 relative to day 0.
VEGF-C and Ang-1 are required to promote lymphatic hiPSC-EC differentiation from hiPSCs
We generated lymphatic ECs from hiPSCs by differentiating them via the EB method in the presence of 50ng/ml BMP4, VEGF-A, VEGF-C and Ang-1 for 14 days (Figure 5A). As with other subtype enrichment protocols, the lymphatic enrichment protocol showed time-dependent increases in the expression of endothelial genes CD31 and CD144 (Figure 5B and 5C, *P<0.05). Differentiating hiPSCs began to express lymphatic marker podoplanin as early as day 4 and peaked by day 11 whereas they only expressed significant LYVE-1 starting from day 11 onwards (Figure 5D and 5E, *P<0.05). To further characterize the subtype identity of these CD31+ hiPSC-LECs, we demonstrated the co-localization of CD31 and podoplanin (Figure 5F).
Figure 5.
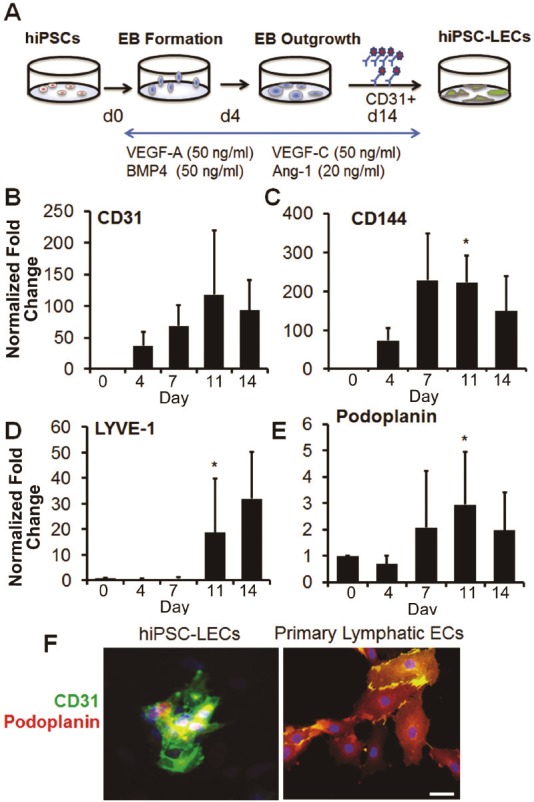
Differentiation of hiPSCs into lymphatic EC subtype using VEGF-C and Ang-1. A. Schematic diagram of hiPSC differentiation procedure to generate hiPSC-derived lymphatic EC (hiPSC-LEC) subtype. Endothelial gene expression of CD31 (B) and CD144 (C) increased during differentiation. Lymphatic markers LYVE-1 (D) and podoplanin (E) increased in gene expression during endothelial differentiation. F. Lymphatic enriched CD31+ (green) hiPSC-LECs stained positive for podoplanin (red). Scale bar: 50μm. *P<0.05 relative to day 0 hiPSCs.
Enriched arterial hiPSC-ECs formed robust large defined capillaries in vivo
In order to determine whether these expanded CD31+ hiPSC-ECs were able to form functional blood capillaries in vivo, we embedded the cells in matrigel and placed the matrigel subcutaneously into immunodeficient mice. Matrigel implants containing heterogenous CD31+ hiPSC-ECs supplemented with bFGF generated more than 2-fold greater total number of capillaries when compared to implants containing cells alone, and more than 6-fold more capillaries compared to bFGF alone (Figure 6A and 6C, *P<0.05). Interestingly, we observed that matrigel implants carrying CD31+ hiPSC-artECs generated a more extensive vascular network. The cells in the network were positive for human CD31, suggesting vessels of human origin (Figure 6B). The human-derived vascular networks formed by hiPSC-artECs were significantly more extensive than those derived from heterogeneous hiPSC-ECs, (Figure 6B and 6D, *P<0.05). In addition, the vessels formed by hiPSC-artECs appeared to be stable and mature, as they were lined with a medial cell layer of murine smooth muscle cells that expressed α-smooth muscle actin (Figure 6E).
Figure 6.
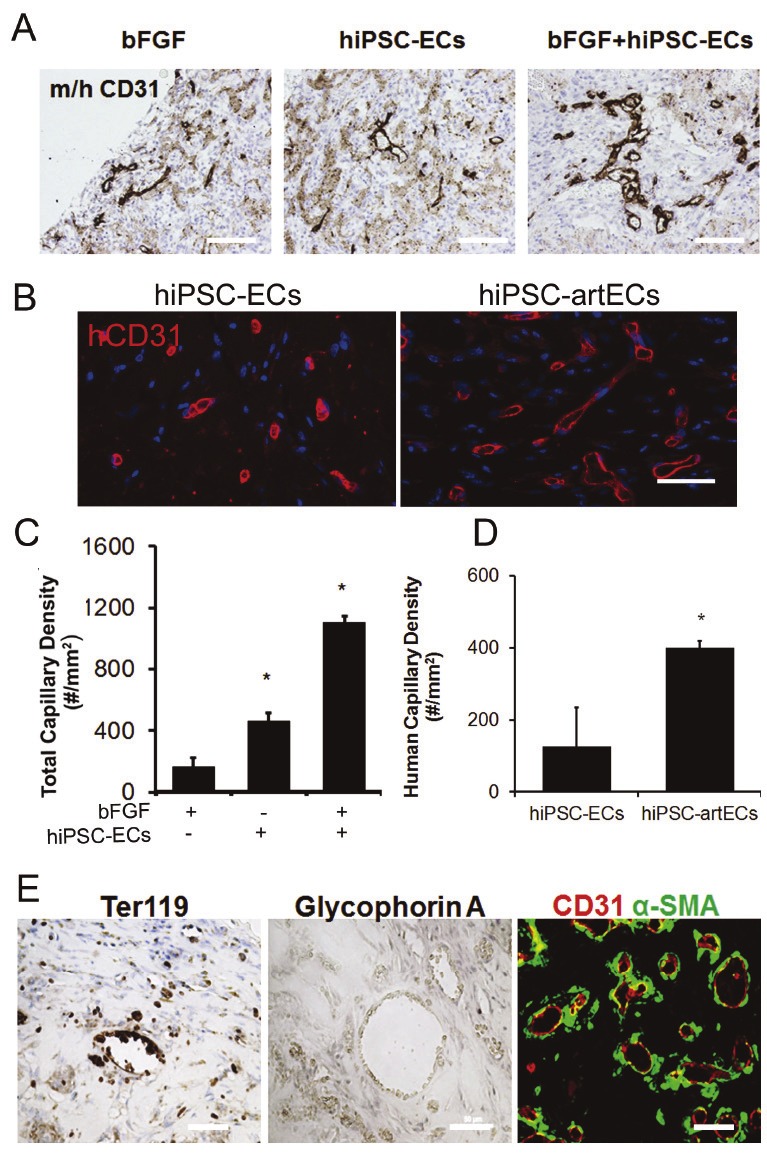
Formation of blood capillaries by hiPSC-ECs in subcutaneous matrigel plugs. A, C. Quantification of total capillary density (*P<0.05 relative to matrigel plug with bFGF only). B, D. Quantification of human-derived capillaries in hiPSC-artEC group (*P<0.05 relative to matrigel with heterogeneous hiPSC-ECs). E. The erythrocytes within neovessels were stained positive for Ter119, a mouse red blood cell marker and not for human blood cell marker glycophorin A. E. Blood capillaries formed by hiPSC-artECs were surrounded by α-SMA positive smooth muscle layer. Scale bar: 50μm.
We noted red blood cells within the human CD31+ capillaries formed in the matrigel plugs. Since ECs can form specialized structures in the embryonic vasculature for hematopoiesis, we sought to determine the origin of these red cells by, staining with either mouse or human specific red blood cell markers, TER-119 and glycophorin-A, respectively. The red blood cells stained positive for TER-119 indicating that they were of mouse origin (Figure 6E).
Discussion
The salient findings of this study are: 1) ECs differentiated from hiPSCs are heterogenous in nature, displaying arterial, venous or lymphatic markers, 2) Specification of arterial or venous subtype is influenced by the concentration in the media of VEGF 3) High VEGF concentration and 8Br-cAMP induce arterial CD31+ hiPSCECs, in association with increased expression of the Notch pathway, 4) High VEGF-A and VEGF-C concentration with supplementation of Ang-1 in the medium promotes the specification to lymphatic CD31+ hiPSC-ECs. 5) CD31+ hiPSC-artECs form more extensive and mature capillary networks in vivo.
EC heterogeneity is a well-established concept. It is known that ECs are extraordinarily diverse in their function and gene-expression profile [15-17]. Morphologically, they can be different in size, shape and thickness depending on the vascular beds in which they reside. Specification of arterial or venous phenotype occurs even before blood circulation during embryonic development. In this regard, the Notch signaling pathway is known to be a crucial pathway in specifying the EC phenotype [12].
Heterogeneity of phenotype develops in part through interactions of ECs with the microenvironment such as growth factors, extracellular matrix and mechanical forces. Several growth factors are known to be important in arterial and venous specification during development. BMP-4 is part of the transforming growth factor-beta superfamily and plays a crucial role in the commitment of pluripotent stem cells into mesoderm lineage and following that to the hematopoietic and endothelial lineages. It was previously shown that exposure of pluripotent stem cells to a short BMP-4 treatment induces EC differentiation [18] and hence we have incorporated a short induction of high dosage of BMP-4 in our differentiation protocol. VEGF on the other hand is a widely known key growth factor involved in the development of ECs in vivo, it thus used for robust differentiation toward EC lineage [19-21].
Standard protocols for generating ECs from human pluripotent stem cells use either CD31 or CD144 to isolate the EC population. CD31 is a 130kDa transmembrane molecule of the immunoglobin gene superfamily that is responsible for cell-cell adhesion in ECs [22,23]. On the other hand, CD144 is a 130kDa transmembrane adhesion molecule implicated in the regulation of endothelial tube formation during vasculogenesis and angiogenesis [24]. Since CD31 and CD144 are expressed in ECs of all subtypes, one of the implications of this study is that these two markers alone may not be sufficient for purifying ECs of specific subtypes.
One of the aims of this study was to analyze further the properties of human pluripotent stem cell derived ECs. By observation of morphology, our differentiation protocol (Figure 1A) generated homogenous ECs with cobblestone morphology. However, gene expression analysis showed that these cells expressed varying levels of arterial and venous markers indicating the heterogenous nature of these hiPSC-derived ECs. Previous studies reported differentiation protocols that were used for specific arterial and venous EC induction in mouse ESCs [10,25] or iPSCs [26]. Yurugi-Kobayashi et al reported that addition of cyclic AMP was able to enhance mouse EC specification into arterial EC subtype [10]. Cyclic AMP is known to be an important secondary messenger in mediating many physiological functions including cell growth and differentiation. They have shown that in the mouse ESC system, cyclic AMP promotes arterial EC specification through upregulation of Flk1 and Nrp1 during differentiation and through dual activation of Notch and β-catenin signaling [10,27]. The same group repeated their mouse ESC system differentiation procedure on mouse iPSCs and was able to generate the three EC subtypes from mouse iPSCs. Our differentiation system differs from theirs as we initiated the various EC subtypes differentiation from the beginning of the differentiation and without any enrichment for any mesodermal progenitors. Lanner et al. on the other hand, reported that high VEGF concentration resulted in upregulation of arterial markers in the differentiated mouse ECs [25].
We found that the combined effect of BMP4, high VEGF concentration and cyclic AMP was to enhance arterial specification of hiPSC-ECs, as indicated by the expression of EphrinB2. Furthermore, in these ECs, the expression of venous markers such as EphB4 and CoupTFII was downregulated. Activation of the Notch pathway is a hallmark for arterial EC identity [13,14]. With the arterial specification protocol we observed an upregulation of Notch family members including the receptors Notch 1 and 4 and the ligands, Jag-1 and Dll-4. Unlike arterial EC specification, the molecular mechanisms that govern venous EC specification are still largely unknown. EC differentiation and specification depends highly on growth factor environmental cues which expose ECs to various signaling pathways. Such environmental influence is observed during development in the dorsal aorta and cardinal vein [13]. The ECs in the dorsal aorta are close to the notochord which is a Sonic hedgehog source (Shh). Shh induces high local concentrations of VEGF which in turn induces Notch in the adjacent dorsal aorta. Notch is upstream of the arterial marker EphrinB2, and reinforces the arterial fate of the ECs in the dorsal aorta. Shh signaling has also been shown recently to induce arterialization by repressing the venous EC fate [28]. However, for specification of venous fate, it is possible that lower Shh levels act on the cardinal vein to induce low levels of VEGF, which may induce Coup-TFII expression. Coup-TFII inhibits Notch signaling and thus blocks arterial differentiation [29,30]. Furthermore, the greater distance of the posterior cardinal vein as compared to the dorsal aorta from the VEGF-expressing somites may play a part in the specification of EC into the venous fate [31]. In vitro exposure of mouse ESCs to low VEGF concentrations strongly upregulates expression of CoupTFII [25]. In our study, we primed EC differentiation by using high concentration of BMP4 and VEGF for the first 4 days of differentiation before using a low VEGF concentration for venous specification. VEGF dose dependency in determining arterial-venous EC subtypes seems to be operative in hiPSCs, with low VEGF expression inducing significant expression of EphB4 and CoupTFII.
Lymphatic differentiation of pluripotent stem cells is not well characterized. During embryonic development, lymphatic ECs are thought to arise from ECs of the cardinal vein that acquire a lymphatic phenotype through the expression of transcription factors Sox18 and Prox1 [32-35]. VEGF-C, which acts on the VEGFR3 receptor, promotes migration of lymphatic endothelial progenitors from the vein [32-34]. Another modulator of lymphatic phenotype is Ang1, which acts through the receptor tyrosine kinase, Tie-2 in regulating lymphatic vessel formation, sprouting, and lymphatic endothelial proliferation [36]. Due to the role of VEGF-C and Ang1 signaling during lymphatic vessel formation, we sought to enhance lymphatic EC specification using these growth factors. Based on gene and protein expression data, there was a marked increase in the expression of lymphatic markers podoplanin and LYVE-1 in hiPSC-ECs with the use of supplemental VEGF-C and Ang1 (Figure 5). In the absence of these lymphatic-promoting factors the levels of lymphatic gene expression was even less than that of hiPSCs (Figure 2). Other groups have also utilized VEGF-C or Ang1 to stimulate lymphatic differentiation of murine ESCs in the presence of other microenvironmental factors such as OP9 stromal feeder cells [37], EB aggregates [38], or in extracellular matrices [39]. Together our results suggest that lymphatic EC specification can be achieved by VEGF-C or Ang1 signaling.
Based on our findings, we have demonstrated that modulation of soluble factors can enrich for specific EC subtypes. Furthermore, we observed that hiPSC-ECs enriched for arterial phenotype generated a more extensive and mature vascular network in vivo than did hiPSC-ECs of a more heterogenous population. However, it is unknown whether sustained delivery of the soluble factors is necessary to retain arterial phenotype, and whether hiPSC-ECs might undergo phenotypic changes upon growth factor withdrawal. It is likely that other microenvironmental factors such as extracellular matrix proteins or mechanical cues are necessary to augment or maintain subtype specification. Furthermore, it remains to be determined whether the enriched arterial subtype is more favorable for therapeutic angiogenesis or if the hiPSC-LECs are superior for treatment of lymphedema. These experiments are interesting and warranted, but beyond the scope of this study.
In summary, this study demonstrates the heterogeneity of hiPSC-ECs and the methods to enrich for subtype specific cells using soluble factors. Furthermore, arterial-enriched hiPSC-ECs enhanced neovascularization compared to heterogeneous cells, suggesting a potential therapeutic benefit in transplanting enriched subtypes.
Acknowledgement
This study was supported by grant to JPC from the National Institutes of Health (U01HL100397). AJR was supported by the National University of Singapore Overseas Postdoctoral Fellowship. NFH was supported by a grant from the National Institutes of Health (HL098688). ETZ and TSP were supported by grants from the National Institutes of Health (1U01HL099775; U01HL100397), and the Maryland Stem Cell Research Fund (2011-MSCRF II-0008-00 (ETZ), 2007-MSCRF II-0379-00 (ETZ), and 2009-MSCRF III-106570 (TSP)).
Disclosure statement
The authors declare that they have no competing financial interests.
Supporting Information
References
- 1.Leeper NJ, Hunter AL, Cooke JP. Stem cell therapy for vascular regeneration: adult, embryonic, and induced pluripotent stem cells. Circulation. 2010;122:517–526. doi: 10.1161/CIRCULATIONAHA.109.881441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Volz KS, Miljan E, Khoo A, Cooke JP. Development of pluripotent stem cells for vascular therapy. Vascular Pharmacol. 2012;56:288–296. doi: 10.1016/j.vph.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rufaihah AJ, Huang NF, Jame S, Lee JC, Nguyen HN, Byers B, De A, Okogbaa J, Rollins M, Reijo-Pera R, Gambhir SS, Cooke JP. Endothelial cells derived from human iPSCS increase capillary density and improve perfusion in a mouse model of peripheral arterial disease. Arterioscler Thromb Vasc Biol. 2011;31:e72–79. doi: 10.1161/ATVBAHA.111.230938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho SW, Moon SH, Lee SH, Kang SW, Kim J, Lim JM, Kim HS, Kim BS, Chung HM. Improvement of postnatal neovascularization by human embryonic stem cell derived endothelial-like cell transplantation in a mouse model of hindlimb ischemia. Circulation. 2007;116:2409–2419. doi: 10.1161/CIRCULATIONAHA.106.687038. [DOI] [PubMed] [Google Scholar]
- 5.Huang NF, Niiyama H, Peter C, De A, Natkunam Y, Fleissner F, Li Z, Rollins MD, Wu JC, Gambhir SS, Cooke JP. Embryonic stem cell-derived endothelial cells engraft into the ischemic hindlimb and restore perfusion. Arterioscler Thromb Vasc Biol. 2010;30:984–991. doi: 10.1161/ATVBAHA.110.202796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Z, Wilson KD, Smith B, Kraft DL, Jia F, Huang M, Xie X, Robbins RC, Gambhir SS, Weissman IL, Wu JC. Functional and transcriptional characterization of human embryonic stem cell-derived endothelial cells for treatment of myocardial infarction. PloS One. 2009;4:e8443. doi: 10.1371/journal.pone.0008443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nourse MB, Halpin DE, Scatena M, Mortisen DJ, Tulloch NL, Hauch KD, Torok-Storb B, Ratner BD, Pabon L, Murry CE. VEGF induces differentiation of functional endothelium from human embryonic stem cells: implications for tissue engineering. Arterioscler Thromb Vasc Biol. 2010;30:80–89. doi: 10.1161/ATVBAHA.109.194233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sone M, Itoh H, Yamahara K, Yamashita JK, Yurugi-Kobayashi T, Nonoguchi A, Suzuki Y, Chao TH, Sawada N, Fukunaga Y, Miyashita K, Park K, Oyamada N, Taura D, Tamura N, Kondo Y, Nito S, Suemori H, Nakatsuji N, Nishikawa S, Nakao K. Pathway for differentiation of human embryonic stem cells to vascular cell components and their potential for vascular regeneration. Arterioscler Thromb Vasc Biol. 2007;27:2127–2134. doi: 10.1161/ATVBAHA.107.143149. [DOI] [PubMed] [Google Scholar]
- 9.Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yurugi T, Naito M, Nakao K. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408:92–96. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- 10.Yurugi-Kobayashi T, Itoh H, Schroeder T, Nakano A, Narazaki G, Kita F, Yanagi K, Hiraoka-Kanie M, Inoue E, Ara T, Nagasawa T, Just U, Nakao K, Nishikawa S, Yamashita JK. Adrenomedullin/cyclic AMP pathway induces Notch activation and differentiation of arterial endothelial cells from vascular progenitors. Arterioscler Thromb Vasc Biol. 2006;26:1977–1984. doi: 10.1161/01.ATV.0000234978.10658.41. [DOI] [PubMed] [Google Scholar]
- 11.Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature. 2005;438:937–945. doi: 10.1038/nature04479. [DOI] [PubMed] [Google Scholar]
- 12.Hellstrom M, Phng LK, Gerhardt H. VEGF and Notch signaling: the yin and yang of angiogenic sprouting. Cell Adh Migr. 2007;1:133–136. doi: 10.4161/cam.1.3.4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, Weinstein BM. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001;128:3675–3683. doi: 10.1242/dev.128.19.3675. [DOI] [PubMed] [Google Scholar]
- 14.Lawson ND, Vogel AM, Weinstein BM. Sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell. 2002;3:127–136. doi: 10.1016/s1534-5807(02)00198-3. [DOI] [PubMed] [Google Scholar]
- 15.Dyer LA, Patterson C. Development of the endothelium: an emphasis on heterogeneity. Semin Thromb Hemost. 2010;36:227–235. doi: 10.1055/s-0030-1253446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res. 2007;100:174–190. doi: 10.1161/01.RES.0000255690.03436.ae. [DOI] [PubMed] [Google Scholar]
- 17.Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007;100:158–173. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 18.Goldman O, Feraud O, Boyer-Di Ponio J, Driancourt C, Clay D, Le Bousse-Kerdiles MC, Bennaceur-Griscelli A, Uzan G. A boost of BMP4 accelerates the commitment of human embryonic stem cells to the endothelial lineage. Stem Cells. 2009;27:1750–1759. doi: 10.1002/stem.100. [DOI] [PubMed] [Google Scholar]
- 19.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 20.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 21.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 22.DeLisser HM, Newman PJ, Albelda SM. Platelet endothelial cell adhesion molecule (CD31) Curr Top Microbiol Immunol. 1993;184:37–45. doi: 10.1007/978-3-642-78253-4_3. [DOI] [PubMed] [Google Scholar]
- 23.DeLisser HM, Newman PJ, Albelda SM. Molecular and functional aspects of PECAM-1/CD31. Immunol Today. 1994;15:490–495. doi: 10.1016/0167-5699(94)90195-3. [DOI] [PubMed] [Google Scholar]
- 24.Vittet D, Buchou T, Schweitzer A, Dejana E, Huber P. Targeted null-mutation in the vascular endothelial-cadherin gene impairs the organization of vascular-like structures in embryoid bodies. Proc Natl Acad Sci U S A. 1997;94:6273–6278. doi: 10.1073/pnas.94.12.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lanner F, Sohl M, Farnebo F. Functional arterial and venous fate is determined by graded VEGF signaling and notch status during embryonic stem cell differentiation. Arterioscler Thromb Vasc Biol. 2007;27:487–493. doi: 10.1161/01.ATV.0000255990.91805.6d. [DOI] [PubMed] [Google Scholar]
- 26.Narazaki G, Uosaki H, Teranishi M, Okita K, Kim B, Matsuoka S, Yamanaka S, Yamashita JK. Directed and systematic differentiation of cardiovascular cells from mouse induced pluripotent stem cells. Circulation. 2008;118:498–506. doi: 10.1161/CIRCULATIONAHA.108.769562. [DOI] [PubMed] [Google Scholar]
- 27.Yamamizu K, Matsunaga T, Uosaki H, Fukushima H, Katayama S, Hiraoka-Kanie M, Mitani K, Yamashita JK. Convergence of Notch and beta-catenin signaling induces arterial fate in vascular progenitors. J Cell Biol. 2010;189:325–338. doi: 10.1083/jcb.200904114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams C, Kim SH, Ni TT, Mitchell L, Ro H, Penn JS, Baldwin SH, Solnica-Krezel L, Zhong TP. Hedgehog signaling induces arterial endothelial cell formation by repressing venous cell fate. Dev Biol. 2010;341:196–204. doi: 10.1016/j.ydbio.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krishnan V, Pereira FA, Qiu Y, Chen CH, Beachy PA, Tsai SY, Tsai MJ. Mediation of Sonic hedgehog-induced expression of COUP-TFII by a protein phosphatase. Science. 1997;278:1947–1950. doi: 10.1126/science.278.5345.1947. [DOI] [PubMed] [Google Scholar]
- 30.You LR, Lin FJ, Lee CT, DeMayo FJ, Tsai MJ, Tsai SY. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature. 2005;435:98–104. doi: 10.1038/nature03511. [DOI] [PubMed] [Google Scholar]
- 31.Swift MR, Weinstein BM. Arterial-venous specification during development. Circ Res. 2009;104:576–588. doi: 10.1161/CIRCRESAHA.108.188805. [DOI] [PubMed] [Google Scholar]
- 32.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 33.Kiefer F, Adams RH. Lymphatic endothelial differentiation: start out with Sox--carry on with Prox. Genome Biol. 2008;9:243. doi: 10.1186/gb-2008-9-12-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maby-El Hajjami H, Petrova TV. Developmental and pathological lymphangiogenesis: from models to human disease. Histochem Cell Biol. 2008;130:1063–1078. doi: 10.1007/s00418-008-0525-5. [DOI] [PubMed] [Google Scholar]
- 35.Kume T. Specification of arterial, venous, and lymphatic endothelial cells during embryonic development. Histol Histopathol. 2010;25:637–646. doi: 10.14670/hh-25.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tammela T, Saaristo A, Lohela M, Morisada T, Tornberg J, Norrmen C, Oike Y, Pajusola K, Thurston G, Suda T, Yla-Herttuala S, Alitalo K. Angiopoietin-1 promotes lymphatic sprouting and hyperplasia. Blood. 2005;105:4642–4648. doi: 10.1182/blood-2004-08-3327. [DOI] [PubMed] [Google Scholar]
- 37.Kono T, Kubo H, Shimazu C, Ueda Y, Takahashi M, Yanagi K, Fujita N, Tsuruo T, Wada H, Yamashita JK. Differentiation of lymphatic endothelial cells from embryonic stem cells on OP9 stromal cells. Arterioscler Thromb Vasc Biol. 2006;26:2070–2076. doi: 10.1161/01.ATV.0000225770.57219.b0. [DOI] [PubMed] [Google Scholar]
- 38.Liersch R, Nay F, Lu L, Detmar M. Induction of lymphatic endothelial cell differentiation in embryoid bodies. Blood. 2006;107:1214–1216. doi: 10.1182/blood-2005-08-3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kreuger J, Nilsson I, Kerjaschki D, Petrova T, Alitalo K, Claesson-Welsh L. Early lymph vessel development from embryonic stem cells. Arterioscler Thromb Vasc Biol. 2006;26:1073–1078. doi: 10.1161/01.ATV.0000217610.58032.b7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.