Abstract
Trauma and critical illness are associated with a stress response that results in increased skeletal muscle protein catabolism, which is thought to facilitate the synthesis of acute phase proteins in the liver as well as proteins involved in immune function. What makes burn injury a unique form of trauma is the existence of vast skin lesions, where the majority of afflicted tissue is often surgically excised post injury. Thereafter, recovery is dependent on the formation of a significant quantity of new skin, meaning that the burned patient requires a large and sustained supply of amino acids to facilitate wound healing. Skeletal muscle has the capacity to store surplus glucose and fatty acids within glycogen and triacylglycerol depots respectively, where glycogen and fatty acids can be mobilized during prolonged periods of caloric restriction or heightened metabolic demand (e.g., exercise), to be catabolized in order to maintain cellular ATP availability. Amino acids, on the other hand, are not generally considered to be stored in such a manner within skeletal muscle, i.e., in a temporary pool independent of structural proteins and cellular organelles etc. Subsequently, in response to severe thermal trauma, skeletal muscle assumes the role of an amino acid reserve where muscle protein breakdown and amino acid release from skeletal muscle serves to buffer plasma amino acid concentrations. Interestingly, it seems like aggressive feeding of the severely burned patient may not necessarily supply amino acids in sufficient abundance to normalize skeletal muscle protein metabolism, suggesting that skeletal muscle becomes an essential store of protein in patients suffering from severe burn trauma. In this article, the effects of burn injury on whole body and skeletal muscle protein metabolism will be discussed in an attempt to distill the current understanding of the impact of this debilitating injury on the redistribution of skeletal muscle protein stores.
Keywords: Burn injury, protein turnover, skeletal muscle, muscle protein synthesis, muscle protein breakdown
Introduction
Severe thermal injury triggers a sustained pathophysiological response which includes, but is not limited to, hypermetabolism, chronic inflammation, marked elevations in peripheral catecholamine and cortisol levels, and severe skeletal muscle wasting [1-5]. While many of these responses to thermal injury enable the body to thermoregulate, combat infection and form scar tissue, they themselves can have a deleterious impact on patient recovery and future morbidity. Indeed, burn injury results in profound cachexia [5], something which is likely compounded by the fact that patients spend prolonged periods of time immobilized post burn [6]. While this depletion of skeletal muscle protein stores negatively impacts the functional capacity and metabolic health of patients recovering from severe thermal injury, it is not without purpose. Following burn injury, when large quantities of amino acids are required, primarily to facilitate the formation of new skin, it seems that skeletal muscle fulfills the role of the body’s amino acid depot [7]. Clearly, while the redistribution of protein from muscle to skin may have catastrophic effects on skeletal muscle mass and function, the closure of skin wounds is of paramount importance to recovery from severe thermal trauma; thus, it seems that skeletal muscle is sacrificed as the body fights for survival. That said, while recognizing skeletal muscles role as an amino acid store in the burned patient, there may be opportunities for nutritional and/or pharmacological interventions which stem protein losses while still providing adequate substrates (amino acids) for wound healing. Indeed, several thorough studies have focused on investigating the impact of numerous pharmacological and nutritional interventions on whole body and skeletal muscle protein turnover post burn [8-20]. In this review article, the impact of severe thermal trauma on whole body and skeletal muscle protein turnover, and the role that skeletal muscle plays as an amino acid reserve post burn, will be described and discussed with an aim to offer an accurate description of the impact of thermal trauma on muscle and whole body protein metabolism.
The role of skeletal muscle as an amino acid store
Skeletal muscle represents the bulk of protein within the human body and plays a central role in normal physiological function. Subsequently, changes in muscle mass and/or quality have been implicated in the pathophysiology of numerous diseases (for review see [21]). Unlike glucose and fatty acids, excess amino acids are not stored in independent depots within skeletal muscle in case of temporal reductions in plasma amino acid levels. Rather, excess amino acids in the intracellular compartment of skeletal muscle are incorporated into structural myofibrillar proteins and cellular organelles. Conversely, following even a relatively short period of fasting, skeletal muscle proteins are catabolized in order to buffer plasma amino acid levels. This dynamic relationship between protein synthesis and protein breakdown is generally considered to result in net protein balance in healthy weight stable adults, i.e., protein synthesis is equivalent to breakdown, where skeletal muscle stores turn over at a rate of 1-2 %.day-1 [22,23].
Prolonged ill health or malnutrition results in changes in muscle protein metabolism which lead to decreased muscle mass. In the context of severe malnutrition, skeletal muscle is catabolized in order to provide metabolic substrates for gluconeogenesis and anaploresis [24]. Indeed, it appears that the body adapts to utilizing fatty acids and ketone bodies for energy production while reducing urinary nitrogen excretion in what seems like an attempt to reduce the quantity of amino acids being siphoned towards gluconeogenesis and transamination, or lost in the urine [24]. Despite this, if adequate nutrition is deprived for long enough, death occurs when the whole body nitrogen store is depleted below a critical threshold. By way of example, during the 2nd world war, Jewish Physicians in the Warsaw Ghettos noted that death from starvation, when uncomplicated by other pathologies, occurred when skeletal muscle was no longer able to provide substrates for gluconeogenesis, in what was termed terminal cachexia [25]. Moreover, depletion of body protein stores is associated with death in chronic illnesses such as AIDS and certain cancers [26,27]. Indeed, when retrospectively evaluating medical records of patients with AIDS in the 100 days preceding death, Kolter and colleagues concluded that death was related to the exhaustion of metabolic substrates, which was attributed to depleted body mass [27]. Moreover, lung cancer is associated with profound muscle wasting, where the degree of cachexia appears to be related to mortality [26]. Subsequently, with the above evidence in mind, it seems that in response to chronic disease or severe malnutrition, skeletal muscle plays an important role in providing substrates (amino acids) for other essential metabolic processes, where the depletion of skeletal muscle protein reserves below a critical level appears to be incompatible with life.
The pathophysiology on thermal trauma
Burn injury, like other forms of trauma results in a sustained inflammatory hypermetabolic state which leads to the dysfunction of numerous organs and physiological systems [1-4,7,13,28-52]. What sets burn injury apart from most other forms of trauma is the existence of large, deep skin wounds. Thus, the severely burned patient is unique in their requirements for amino acids to facilitate wound healing. Profound elevations in skeletal muscle protein breakdown is a hallmark of burn injury [1,7,38], where a persistent catabolic state results in the erosion of lean body mass [45]. Given the purpose of this review, perturbations in metabolic physiology and their pertinence to whole body and skeletal muscle protein turnover following burn injury will be discussed in more detail below.
Increased resting metabolic rate seems synonymous with critical illness, but more specifically, burn injury [2,38,40,52,53]. Indeed, resting energy expenditure in severely burned children has been reported to be up to 100% greater than that of healthy children, where hypermetabolism may persist for several years post burn [2,40]. The mechanism(s) mediating the hypermetabolic phenotype of the severely burned patients remain to be full elucidated, however, evaporative heat loss from open wounds is likely to play a role in at least the acute period post injury, where occlusive dressings have been shown to reduce metabolic rate [30,54]. Further, compromised thermoregulation due to heat radiating from burn and donor wounds likely also contributes to increased metabolic rate post burn [31]. In addition, it seems that chronic adrenergic stimulation is central to the hypermetabolic response in burned patients, where increased cycling of substrates such as fatty acids and glucose appears to increase ATP consumption in burned individuals [50,51,57]. Indeed, ATP consuming reactions like gluconeogenesis, protein synthesis and urea production are double that of healthy individuals in patients with large burns [52]. Most strikingly, while extracellular fatty acid cycling doubles in burn patients relative to unburned controls, the rate of intracellular fatty acid cycling is 15-fold greater in severely burned patients when compared to unburned individuals [52]. In support of the suggestion that adrenergic stimulation and substrate cycling mediates the hypermetabolic response to burn injury, there are numerous reports that blocking this pathway with propranolol treatment attenuates the hypermetabolic response associated with severe burns [10,12,36,55].
In addition to perturbations in fat and carbohydrate metabolism post burn, protein metabolism is severely altered by severe thermal trauma. As mentioned above, increased protein synthesis and urea production in burned patients likely play a causative role in the hypermetabolic response to burn trauma [52]. However, increased protein synthesis and urea production are more than likely the consequence of increased whole body turnover, and more specifically, skeletal muscle catabolism post burn. There are numerous reports of marked elevations in skeletal muscle protein breakdown acutely post burn [28,29,46,56], where skeletal muscle catabolism likely stimulates protein accretion in the burn wound [7].
The impact of thermal trauma on whole body protein metabolism
More than two decades of research performed primarily within the Metabolism Unit at Shriners Hospitals for Children – Galveston, under the direction of Professor Robert R. Wolfe, have contributed enormously to our understanding of the impact of thermal injury on protein metabolism. In the late 1980s, these researchers used stable isotopes of urea, leucine, valine and lysine to describe the dynamic response of protein metabolism to burn injury [38]. It was found that elevated protein turnover post injury was associated with hypermetabolism. Further, they showed that protein breakdown remained elevated for several months post injury [38].
In response to burn injury, hepatic gluconeogenesis is elevated in the postprandial state, where alanine [57] and glycerol [50] release from muscle and adipose tissue, respectively, and increased lactate production from peripheral glycolysis [57] are thought to provide substrates for endogenous glucose production. While intracellular fatty acid cycling in adipose tissue and anaerobic glycolysis in skeletal muscle provide glycerol and lactate, respectively, thus preserving endogenous alanine stores, the rate of alanine appearance in severely burned patients is more than double that of healthy individuals [57], suggesting that skeletal muscle catabolism may also play a role in the provision of gluconeogenic precursors. Indeed, when glucose is infused in the postabsorptive state alanine rate of appearance falls in burned patients to levels more or less comparable with healthy individuals [57]. Subsequently, it would appear that in the absence of appropriate glucose provision, alanine appearance in the blood, presumably from skeletal muscle catabolism, serves to provide metabolic substrate for hepatic gluconeogenesis, underscoring the importance of adequate carbohydrate provision for severely burned patients.
While increased alanine turnover in burned patients can be attributed to elevated rates of de novo gluconeogenesis, increased turnover of amino acids such as leucine and arginine have also been reported in severely burned individuals, which are paralleled with increase in whole body protein turnover [58-60]. Importantly, it would appear that whole body protein turnover post burn is the result of both an increase in protein breakdown and protein synthesis. Unlike glucose infusion, which reduced the rate of appearance of alanine from muscle catabolism in burned patients, increasing protein intake acutely post burn, while restoring protein balance to some degree, does not seem to normalize the derangement in skeletal muscle protein metabolism associated with burn injury [61], where protein turnover remained well above levels reported for healthy individuals.
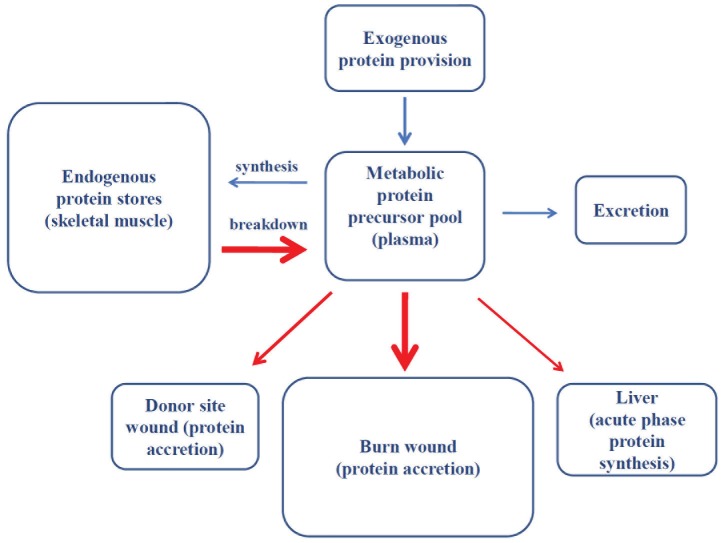
The above observations most likely demonstrate that while increased alanine flux in burned patients may reflect a temporal requirement of the liver for gluconeogenic substrates, chronic increases in whole body protein synthesis and breakdown in severely burned patients permit the redistribution of whole body protein stores, and thus, while transient increases in the nutritional supply of amino acids may mitigate whole body, and in particular skeletal muscle protein losses, they do not resolve the underlying physiological stimulus for elevated protein turnover. In support of this, while burn injury is associated with the erosion of skeletal muscle mass and subsequent changes in body composition [45], we have recently demonstrated that at discharge from hospital, whole body protein turnover is significantly greater in severely burned children compared with healthy children [29]. Interestingly, greater whole body protein turnover in severely burned children seems to result from comparable increases in whole body protein breakdown and synthesis [29]. At first glance these data may be somewhat surprising when considering that severely burned patients remain in a catabolic state with regards to skeletal muscle for several months post injury. However, we believe that this merely reflects redistribution of whole body protein stores, from skeletal muscle to principally skin wounds. This concept is schematically depicted in Figure 1, where we propose that skeletal muscle serves as an endogenous protein source which is mainly disposed of in burn wounds following severe thermal trauma (Figure 1).
Figure 1.
Schematic overview of the proposed redistribution of whole body protein stores in response to severe thermal injury. Red arrows depict the predominant routes of protein redistribution. Bolder arrows depict greater protein efflux/influx.
The impact of thermal trauma on skeletal protein metabolism
Assuming that the catabolism of endogenous protein stores (skeletal muscle) primarily supports wound healing in burned individuals (Figure 1), it is perhaps not surprising that muscle wasting is more profound in patients with larger burns. For example, phenylalanine loss across the leg of pediatric patients with burns encompassing <40% of total body surface area was approximately 5 nmol.min-1 100 ml-1 of leg volume-1, whereas phenylalanine loss across the leg of patients with larger burns (>40% of total body surface area) was approximately 10-fold greater (approximately 50 nmol.min-1 100 ml-1 of leg volume-1) [62]. In addition, numerous other factors influence the degree of skeletal muscle catabolism post burn. For example, there are stepwise increases in the magnitude of skeletal muscle catabolism with increasing admission weight, time to wound excision, and extent of post burn hypermetabolism [62]. Also, septic patients are significantly more hypermetabolic and catabolic than those who do not develop sepsis [62]. In addition, it also appears that adults are more catabolic than children in response to burn injury [62,63]. Interestingly, this seems to be the result of a greater compensatory increase in skeletal muscle protein synthesis in response to elevated protein breakdown, rather than a smaller catabolic response in children [63].
Regardless of the mechanism(s) underlying skeletal muscle catabolism in severely burned patients, this adaptive response, while negatively impacting skeletal muscle mass and function, provides amino acids for other essential metabolic processes (Figure 1). Indeed, this concept of redistribution of skeletal muscle protein stores post injury was elegantly demonstrated experimentally in severely burned patients by Gore et al., from our hospital, who employed five compartment modeling of amino acid kinetics in the arterial and venous plasma, vastus lateralis muscle, and skin and burn wounds [7]. Interestingly, the rate of skeletal muscle protein breakdown was very much comparable to the rate of wound protein synthesis. Further, in support of the notion that amino acids derived from skeletal muscle catabolism are disposed of in the burn wound, these investigators were able to show that while there were net losses of phenylalanine from skeletal muscle of these severely burned patients, there was net accretion of phenylalanine in the burn wound despite the fact patients were receiving enteral nutrition throughout the study period [7]. Moreover, the fractional synthesis rate of wound proteins was approximately 5-fold greater than those of skeletal muscle [7], suggesting that amino acid flux into, and presumably their incorporation into constituent proteins of new skin, is greatly accelerated in the burn wound.
A central thesis of this article is that following major burn injury, increased whole body protein turnover reflects the redistribution of protein from skeletal muscle to the evolving burn wound (Figure 1). Interestingly, although there is some disagreement within the literature [56], it seems that skeletal muscle catabolism persists after full wound closure [1,46], suggesting that even when wounds are considered closed there is still dynamic remodeling of the scar which requires additional protein, something which is likely confounded by follow-up surgeries and therefore subsequent trauma. Naturally, this suggests that the provision of exogenous protein may attenuate or even ablate endogenous (skeletal muscle) protein catabolism. Indeed, Wolfe and colleagues highlighted that exogenous protein provision could at least restore leg protein balance in burned patients, something that was lacking in the post-absorptive state [61]. Furthermore, while phenylalanine rate of appearance, a marker of proteolysis, was not related to protein intake in severely burned patients within the initial two weeks of injury, increased dietary protein intake was associated with higher rates of skin protein synthesis [64]. More recently, in the acute phase, approximately two weeks post injury, the provision of high protein nutrition (above 2.5 g.kg-1day-1) appeared to diminish skeletal muscle protein losses in children with severe burns [56].
Although in the acute period post burn, the alterations in skeletal muscle protein turnover, and primarily muscle catabolism may not be readily reversed by increased protein intake, the fact that a less catabolic balance between muscle protein breakdown and synthesis is achieved [61], and that the fractional synthesis rate of skin proteins is increased when dietary protein intake is increased [64], is certainly encouraging. However, it is worth noting these studies were performed within the first two to five weeks post injury. Given that skeletal muscle catabolism persists for at least 9 months post burn [1], this suggests that nutritional support of burn injury, and in particular adequate protein provision, may play an important role in whole body and skeletal muscle protein metabolisms even after wound closure is achieved. Further studies addressing whether protein per se or specific amino acid solutions can augment wound protein accretion or attenuate skeletal muscle catabolism are eagerly awaited.
Summary remarks
Our aim was to distill the current literature pertaining to whole body and skeletal muscle protein metabolism, and to highlight the important role skeletal muscle plays in the pathophysiological response to severe burn injury. Evidence suggests that increased whole body protein turnover following severe thermal trauma reflects the redistribution of the protein compartments in the body, where amino acids derived from skeletal muscle appear to play an important role in protein accretion in skin wounds. While these processes may be an essential adaptation to this catastrophic and unique injury, strategies which attenuate muscle protein breakdown while still providing optimal substrates for wound healing are likely to have beneficial outcomes in burned patients. Exogenous protein or amino acid provision represents a simple intervention which may positively impact the adaption to severe thermal trauma. However, many questions remain as to whether there is an optimal dose and/or composition of amino acids to feed severely burned individuals at various junctures post injury. Answers to the aforementioned questions will likely aid the recovery and rehabilitation of patients afflicted with severe burns.
Acknowledgements
This work was supported in part by Shriners of North America Grants 84090 and 71006. CP is supported by an Interdisciplinary Rehabilitation Research Postdoctoral Training Grant (H133P110012) from the National Institute of Disability and Rehabilitation Research and Department of Education.
Declaration
The authors have no conflicts of interest to report.
References
- 1.Hart DW, Wolf SE, Mlcak R, Chinkes DL, Ramzy PI, Obeng MK, Ferrando AA, Wolfe RR, Herndon DN. Persistence of muscle catabolism after severe burn. Surgery. 2000;128:312–9. doi: 10.1067/msy.2000.108059. [DOI] [PubMed] [Google Scholar]
- 2.Jeschke MG, Gauglitz GG, Kulp GA, Finnerty CC, Williams FN, Kraft R, Suman OE, Mlcak RP, Herndon DN. Long-term persistance of the pathophysiologic response to severe burn injury. PLoS One. 2011;6:e21245. doi: 10.1371/journal.pone.0021245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kulp GA, Herndon DN, Lee JO, Suman OE, Jeschke MG. Extent and magnitude of catecholamine surge in pediatric burned patients. Shock. 2010;33:369–74. doi: 10.1097/SHK.0b013e3181b92340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norbury WB, Herndon DN, Branski LK, Chinkes DL, Jeschke MG. Urinary cortisol and catecholamine excretion after burn injury in children. J Clin Endocrinol Metab. 2008;93:1270–5. doi: 10.1210/jc.2006-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pereira C, Murphy K, Jeschke M, Herndon DN. Post burn muscle wasting and the effects of treatments. Int J Biochem Cell Biol. 2005;37:1948–61. doi: 10.1016/j.biocel.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Paddon-Jones D, Sheffield-Moore M, Cree M, Hewlings S, Aarsland A, Wolfe R, Ferrando AA. Atrophy and impaired muscle protein synthesis during prolonged inactivity and stress. J Clin Endocrinol Metab. 2006;91:4836–41. doi: 10.1210/jc.2006-0651. [DOI] [PubMed] [Google Scholar]
- 7.Gore DC, Chinkes DL, Wolf SE, Sanford AP, Herndon DN, Wolfe RR. Quantification of protein metabolism in vivo for skin, wound, and muscle in severe burn patients. JPEN J Parenter Enteral Nutr. 2006;30:331–8. doi: 10.1177/0148607106030004331. [DOI] [PubMed] [Google Scholar]
- 8.Ferrando AA, Chinkes DL, Wolf SE, Matin S, Herndon DN, Wolfe RR. A submaximal dose of insulin promotes net skeletal muscle protein synthesis in patients with severe burns. Ann Surg. 1999;229:11–8. doi: 10.1097/00000658-199901000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrando AA, Sheffield-Moore M, Wolf SE, Herndon DN, Wolfe RR. Testosterone administration in severe burns ameliorates muscle catabolism. Crit Care Med. 2001;29:1936–42. doi: 10.1097/00003246-200110000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Hart DW, Wolf SE, Chinkes DL, Lal SO, Ramzy PI, Herndon DN. Beta-blockade and growth hormone after burn. Ann Surg. 2002;236:450–7. doi: 10.1097/00000658-200210000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hart DW, Wolf SE, Ramzy PI, Chinkes DL, Beauford RB, Ferrando AA, Wolfe RR, Herndon DN. Anabolic effects of oxandrolone after severe burn. Ann Surg. 2001;233:556–64. doi: 10.1097/00000658-200104000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herndon DN, Hart DW, Wolf SE, Chinkes DL, Wolfe RR. Reversal of catabolism by betablockade after severe burns. N Engl J Med. 2001;345:1223–9. doi: 10.1056/NEJMoa010342. [DOI] [PubMed] [Google Scholar]
- 13.Herndon DN, Ramzy PI, DebRoy MA, Zheng M, Ferrando AA, Chinkes DL, Barret JP, Wolfe RR, Wolf SE. Muscle protein catabolism after severe burn: effects of IGF-1/IGFBP-3 treatment. Ann Surg. 1999;229:713–20. doi: 10.1097/00000658-199905000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tuvdendorj D, Chinkes DL, Zhang XJ, Suman OE, Aarsland A, Ferrando A, Kulp GA, Jeschke MG, Wolfe RR, Herndon DN. Long-term oxandrolone treatment increases muscle protein net deposition via improving amino acid utilization in pediatric patients 6 months after burn injury. Surgery. 2011;149:645–53. doi: 10.1016/j.surg.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolf SE, Thomas S, Dasu MR, Ferrando AA, Chinkes DL, Wolfe RR, Herndon DN. Improved net protein balance, lean mass, and gene expression changes with oxandrolone treatment in the severely burned. Ann Surg. 2003;237:801–10. doi: 10.1097/01.SLA.0000071562.12637.3E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrando AA, Chinkes DL, Wolf SE, Matin S, Herndon DN, Wolfe RR. Acute dichloroacetate administration increases skeletal muscle free glutamine concentrations after burn injury. Ann Surg. 1998;228:249–56. doi: 10.1097/00000658-199808000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gore DC, Wolf SE, Sanford AP, Herndon DN, Wolfe RR. Extremity hyperinsulinemia stimulates muscle protein synthesis in severely injured patients. Am J Physiol Endocrinol Metab. 2004;286:529–34. doi: 10.1152/ajpendo.00258.2003. [DOI] [PubMed] [Google Scholar]
- 18.Gore DC, Wolf SE, Sanford A, Herndon DN, Wolfe RR. Influence of metformin on glucose intolerance and muscle catabolism following severe burn injury. Ann Surg. 2005;241:334–42. doi: 10.1097/01.sla.0000152013.23032.d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herndon DN, Dasu MR, Wolfe RR, Barrow RE. Gene expression profiles and protein balance in skeletal muscle of burned children after beta-adrenergic blockade. Am J Physiol Endocrinol Metab. 2003;285:783–9. doi: 10.1152/ajpendo.00508.2002. [DOI] [PubMed] [Google Scholar]
- 20.Sakurai Y, Aarsland A, Herndon DN, Chinkes DL, Pierre E, Nguyen TT, Patterson BW, Wolfe RR. Stimulation of muscle protein synthesis by long-term insulin infusion in severely burned patients. Ann Surg. 1995;222:294–7. doi: 10.1097/00000658-199509000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006;84:475–82. doi: 10.1093/ajcn/84.3.475. [DOI] [PubMed] [Google Scholar]
- 22.Phillips SM, Glover EI, Rennie MJ. Alterations of protein turnover underlying disuse atrophy in human skeletal muscle. J Appl Physiol. 2009;107:645–54. doi: 10.1152/japplphysiol.00452.2009. [DOI] [PubMed] [Google Scholar]
- 23.Rennie MJ. Anabolic resistance: the effects of aging, sexual dimorphism, and immobilization on human muscle protein turnover. Appl Physiol Nutr Metab. 2009;34:377–81. doi: 10.1139/H09-012. [DOI] [PubMed] [Google Scholar]
- 24.Cahill GJ. Starvation in man. N Engl J Med. 1970;282:668–75. doi: 10.1056/NEJM197003192821209. [DOI] [PubMed] [Google Scholar]
- 25.Winick M, editor. Hunger disease: Studies by the Jewish Physicians in the Warsaw Ghetto. New York, NY: Wiley & Sons; 1979. pp. 115–23. [Google Scholar]
- 26.Kadar L, Albertsson M, Arebert J, Landbert T, Mattsson S. The prognostic value of body protein in patients with lung cancer. Ann N Y Acad Sci. 2000;904:584–91. doi: 10.1111/j.1749-6632.2000.tb06520.x. [DOI] [PubMed] [Google Scholar]
- 27.Kotler D, Tierney A, Wang J. The magnitude of body cell mass depletion determines the timing of death from wasting in AIDS. Am J Clin Nutr. 1989;50:444–7. doi: 10.1093/ajcn/50.3.444. [DOI] [PubMed] [Google Scholar]
- 28.Biolo G, Fleming RY, Maggi SP, Nguyen TT, Herndon DN, Wolfe RR. Inverse regulation of protein turnover and amino acid transport in skeletal muscle of hypercatabolic patients. J Clin Endocrinol Metab. 2002;87:3378–84. doi: 10.1210/jcem.87.7.8699. [DOI] [PubMed] [Google Scholar]
- 29.Børsheim E, Chinkes DL, McEntire SJ, Rodriguez NR, Herndon DN, Suman OE. Whole body protein kinetics measured with a non-invasive method in severely burned children. Burns. 2010;36:1006–12. doi: 10.1016/j.burns.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caldwell FJ, Bowser B, Crabtree J. The effect of occlusive dressings on the energy metabolism of severely burned children. Ann Surg. 1981;193:579–91. doi: 10.1097/00000658-198105000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caldwell FJ, Wallace B, Cone J, Manuel L. Control of the hypermetabolic response to burn injury using environmental factors. Ann Surg. 1992;215:485–90. doi: 10.1097/00000658-199205000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cree MG, Fram RY, Herndon DN, Qian T, Angel C, Green JM, Mlcak R, Aarsland A, Wolfe RR. Human mitochondrial oxidative capacity is acutely impaired after burn trauma. Am J Surg. 2007;196:234–9. doi: 10.1016/j.amjsurg.2007.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finnerty CC, Herndon DN, Przkora R, Pereira CT, Oliveira HM, Queiroz DM, Rocha AM, Jeschke MG. Cytokine expression profile over time in severely burned pediatric patients. Shock. 2006;26:13–9. doi: 10.1097/01.shk.0000223120.26394.7d. [DOI] [PubMed] [Google Scholar]
- 34.Fram RY, Cree MG, Wolfe RR, Barr D, Herndon DN. Impaired glucose tolerance in pediatric burn patients at discharge from the acute hospital stay. J Burn Care Res. 2010;31:728–33. doi: 10.1097/BCR.0b013e3181eebe63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gauglitz GG, Herndon DN, Kulp GA, Meyer W Jr, Jeschke MG. Abnormal insulin sensitivity persists up to three years in pediatric patients post-burn. J Clin Endocrinol Metab. 2009;94:1656–64. doi: 10.1210/jc.2008-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herndon DN, Barrow RE, Rutan TC, Minifee P, Jahoor F, Wolfe RR. Effect of propranolol administration on hemodynamic and metabolic responses of burned pediatric patients. Ann Surg. 1988;208:484–92. doi: 10.1097/00000658-198810000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herndon DN, Tompkins RG. Support of the metabolic response to burn injury. Lancet. 2005;363:1895–902. doi: 10.1016/S0140-6736(04)16360-5. [DOI] [PubMed] [Google Scholar]
- 38.Jahoor F, Desai M, Herndon DN, Wolfe RR. Dynamics of the protein metabolic response to burn injury. Metabolism. 1988;37:330–7. doi: 10.1016/0026-0495(88)90132-1. [DOI] [PubMed] [Google Scholar]
- 39.Jeschke MG, Barrow RE, Herndon DN. Extended hypermetabolic response of the liver in severely burned pediatric patients. Arch Surg. 2004;139:641–7. doi: 10.1001/archsurg.139.6.641. [DOI] [PubMed] [Google Scholar]
- 40.Jeschke MG, Chinkes DL, Finnerty CC, Kulp G, Suman OE, Norbury WB, Branski LK, Gauglitz GG, Mlcak RP, Herndon DN FACS. Pathophysiologic response to severe burn injury. Ann Surg. 2008;248:387–401. doi: 10.1097/SLA.0b013e3181856241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jeschke MG, Micak RP, Finnerty CC, Herndon DN. Changes in liver function and size after a severe thermal injury. Shock. 2007;28:172–7. doi: 10.1097/shk.0b013e318047b9e2. [DOI] [PubMed] [Google Scholar]
- 42.Keck M, Herndon DH, Kamolz LP, Frey M, Jeschke MG. Pathophysiology of burns. Wien Med Wochenschr. 2009;159:327–36. doi: 10.1007/s10354-009-0651-2. [DOI] [PubMed] [Google Scholar]
- 43.Klein GL, Chen TC, Holick MF, Langman CB, Price H, Celis MM, Herndon DN. Synthesis of vitamin D in skin after burns. Lancet. 2004;363:291–2. doi: 10.1016/S0140-6736(03)15388-3. [DOI] [PubMed] [Google Scholar]
- 44.Klein GL, Langman CB, Herndon DN. Vitamin D depletion following burn injury in children: a possible factor in post-burn osteopenia. J Trauma. 2002;52:346–50. doi: 10.1097/00005373-200202000-00022. [DOI] [PubMed] [Google Scholar]
- 45.Przkora R, Barrow RE, Jeschke MG, Suman OE, Celis M, Sanford AP, Chinkes DL, Mlcak RP, Herndon DN. Body composition changes with time in pediatric burn patients. J Trauma. 2006;60:968–71. doi: 10.1097/01.ta.0000214580.27501.19. [DOI] [PubMed] [Google Scholar]
- 46.Tuvdendorj D, Chinkes DL, Zhang XJ, Sheffield-Moore M, Herndon DN. Skeletal muscle is anabolically unresponsive to an amino acid infusion in pediatric burn patients 6 months postinjury. Ann Surg. 2011;253:592–7. doi: 10.1097/SLA.0b013e31820d9a63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williams FN, Herndon DN, Suman OE, Lee JO, Norbury WB, Branski LK, Mlcak RP, Jeschke MG. Changes in cardiac physiology after severe burn injury. J Burn Care Res. 2011;32:269–74. doi: 10.1097/BCR.0b013e31820aafcf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolfe RR, Durkot MJ, Allsop JR, Burke JF. Glucose metabolism in severely burned patients. Metabolism. 1979;28:1031–9. doi: 10.1016/0026-0495(79)90007-6. [DOI] [PubMed] [Google Scholar]
- 49.Wolfe RR, Herndon DN, Jahoor F, Miyoshi H, Wolfe M. Effect of severe burn injury on substrate cycling by glucose and fatty acids. N Engl J Med. 1987;317:403–8. doi: 10.1056/NEJM198708133170702. [DOI] [PubMed] [Google Scholar]
- 50.Wolfe RR, Herndon DN, Peters EJ, Jahoor F, Desai MH, Holland OB. Regulation of lipolysis in severely burned children. Ann Surg. 1987;206:214–21. doi: 10.1097/00000658-198708000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wolfe RR, Klein S, Herndon DN, Jahoor F. Substrate cycling in thermogenesis and amplification of net substrate flux in human volunteers and burned patients. J Trauma. 1990;30:6–9. doi: 10.1097/00005373-199012001-00004. [DOI] [PubMed] [Google Scholar]
- 52.Yu YM, Tompkins RG, Ryan CM, Young VR. The metabolic basis of the increase of the increase in energy expenditure in severely burned patients. JPEN J Parenter Enteral Nutr. 1999;23:160–8. doi: 10.1177/0148607199023003160. [DOI] [PubMed] [Google Scholar]
- 53.Goran MI, Peters EJ, Herndon DN, Wolfe RR. Total energy expenditure in burned children using the doubly labeled water technique. Am J Physiol. 1990;259:576–85. doi: 10.1152/ajpendo.1990.259.4.E576. [DOI] [PubMed] [Google Scholar]
- 54.Caldwell F, Hammel H, Dolan F. A calorimeter for simultaneous determination of heat production and heat loss in the rat. J Appl Physiol. 1966;21:1665–71. doi: 10.1152/jappl.1966.21.5.1665. [DOI] [PubMed] [Google Scholar]
- 55.Wilmore DW, Long JM, Mason ADJ, Skreen RW, Pruitt BAJ. Catecholamines: mediator of the hypermetabolic response to thermal injury. Ann Surg. 1974;180:653–69. doi: 10.1097/00000658-197410000-00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prelack K, Yu YM, Dylewski M, Lydon M, Sheridan RL, Tompkins RG. The contribution of muscle to whole-body protein turnover throughout the course of burn injury in children. J Burn Care Res. 2010;31:942–8. doi: 10.1097/BCR.0b013e3181f938e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wolfe RR, Jahoor F, Herndon DN, Miyoshi H. Isotopic evaluation of the metabolism of pyruvate and related substrates in normal adult volunteers and severely burned children: effect of dichloroacetate and glucose infusion. Surgery. 1991;110:54–67. [PubMed] [Google Scholar]
- 58.Yu YM, Sheridan RL, Burke JF, Chapman TE, Tompkins RG, Young VR. Kinetics of plasma arginine and leucine in pediatric burn patients. Am J Clin Nutr. 1996;64:60–6. doi: 10.1093/ajcn/64.1.60. [DOI] [PubMed] [Google Scholar]
- 59.Yu YM, Young VR, Castillo L, Chapman TE, Tompkins RG, Ryan CM, Burke JF. Plasma arginine and leucine kinetics and urea production rates in burn patients. Metabolism. 1995;44:659–66. doi: 10.1016/0026-0495(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 60.Yu YM, Ryan CM, Castillo L, Lu XM, Beaumier L, Tompkins RG, Young VR. Arginine and ornithine kinetics in severely burned patients: increased rate of arginine disposal. Am J Physiol Endocrinol Metab. 2000;280:509–17. doi: 10.1152/ajpendo.2001.280.3.E509. [DOI] [PubMed] [Google Scholar]
- 61.Wolfe RR, Goodenough RD, Burke JF, Wolfe MH. Response of protein and urea kinetics in burn patients to different levels of protein intake. Ann Surg. 1983;197:163–71. doi: 10.1097/00000658-198302000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hart DW, Wolf SE, Chinkes DL, Gore DC, Mlcak RP, Beauford RB, Lal S, Wolfe RR, Herndon DN, Obeng MK, Gold WF. Determinants of skeletal muscle catabolism after severe burn. Ann Surg. 2000;232:455–65. doi: 10.1097/00000658-200010000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tuvdendorj D, Chinkes DL, Zhang XJ, Ferrando AA, Elijah IE, Mlcak RP, Finnerty CC, Wolfe RR, Herndon DN. Adult patients are more catabolic than children during acute phase after burn injury: a retrospective analysis on muscle protein kinetics. Intensive Care Med. 2011;37:1317–22. doi: 10.1007/s00134-011-2223-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Patterson BW, Nguyen T, Pierre E, Herndon DN, Wolfe RR. Urea and protein metabolism in burned children: effect of dietary protein intake. Metabolism. 1997;46:573–8. doi: 10.1016/s0026-0495(97)90196-7. [DOI] [PubMed] [Google Scholar]